Circulating microRNA Profiles in Acute Spinal Cord Injury: Evidence for Distinct Plasma Signatures Compared with Polytrauma Patients
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. NGS-Based miRNA Expression Profiling
2.3. Over-Representation-Analysis Using Reactome
2.4. NGS-Based Selection of Candidate miRNAs
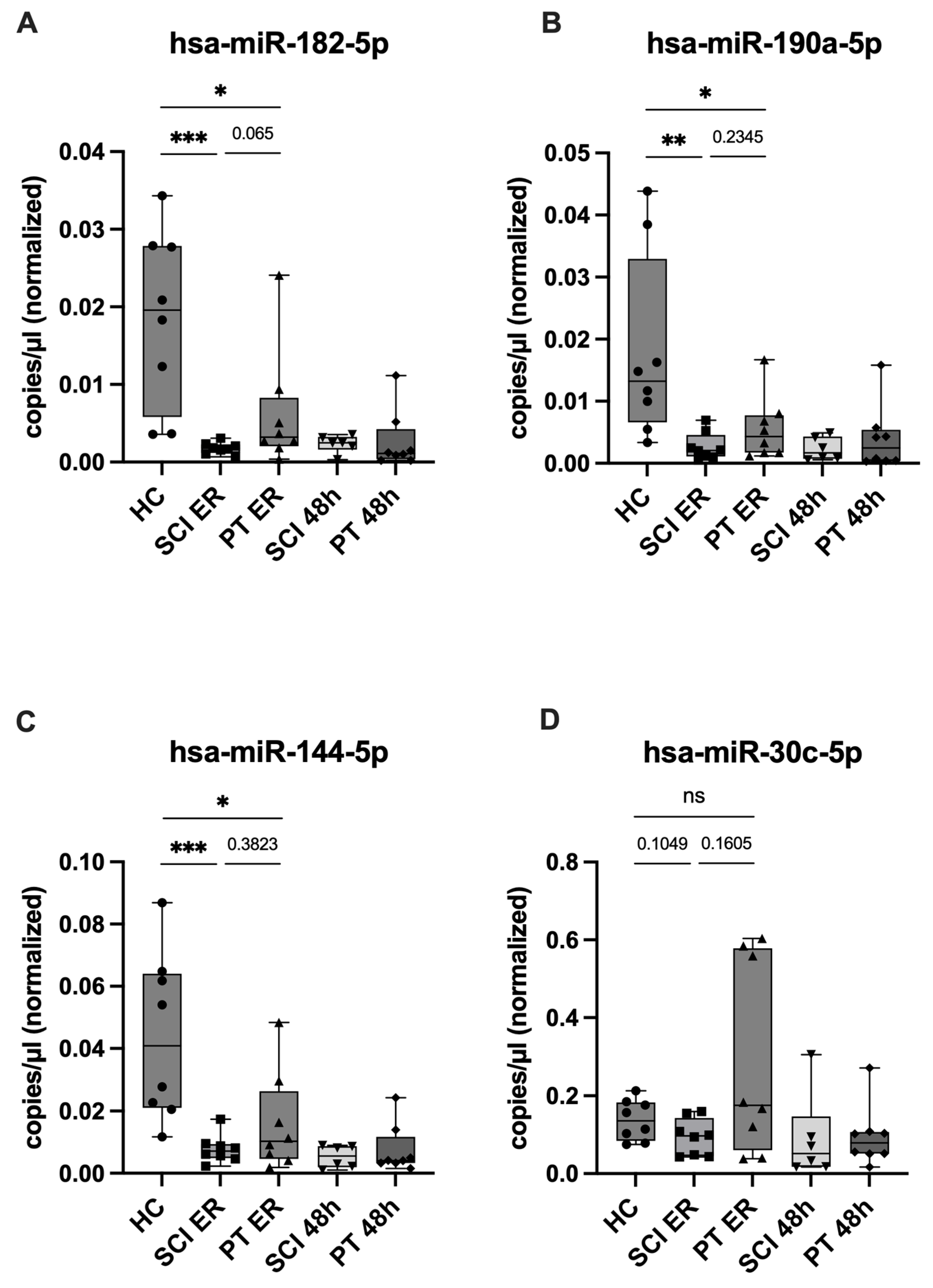
2.5. ddPCR Validation of Candidate miRNAs and Assessment of SCI Specificity
2.6. miRNA Target Analysis Using miRTarBase
2.7. Correlation of miRNA Expression with Neurological Status and Systemic Inflammation
3. Discussion
3.1. Circulating miRNAs as Potential Blood-Based Biomarkers in Acute SCI
3.2. Distinct Regulatory Patterns of Candidate miRNAs in SCI
3.3. Neurotrauma-Specific Regulation Beyond Systemic Trauma Responses
3.4. Oxidative Stress as a Potential Driver of Early Secondary Injury
3.5. Candidate miRNAs Target Genes Linked to Cell Cycle Control, Apoptosis, and Regeneration
3.6. Study Limitations
4. Materials and Methods
4.1. Study Design and Patient Selection
4.2. Blood Sample Collection
4.3. miRNA Next Generation Sequencing
4.4. Analysis of miRNA Expression Using Droplet Digital PCR in SCI and PT Patients
4.5. miRNA Target Prediction and Pathway Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASIA | American Spinal Injury Association |
| BCL | B-cell lymphoma |
| BMP | Bone morphogenetic protein |
| CCN | Cyclin |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| ddPCR | Droplet digital polymerase chain reaction |
| ER | Emergency room |
| FDR | False discovery rate |
| FOX | Forkhead box |
| HC | Healthy control |
| ICU | Intensive care unit |
| IL | Interleukin |
| IQR | Interquartile range |
| I/R | Ischemia/reperfusion |
| miRNA | MicroRNA |
| NGS | Next-generation sequencing |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| ORA | Over representation analysis |
| OXPHOS | Oxidative phosphorylation system |
| PT | Polytrauma |
| ROS | Reactive oxygen species |
| SCI | Spinal cord injury |
| SD | Standard deviation |
| SMAD | Mothers against decapentaplegic homolog |
| STROBE | Strengthening the Reporting of Observational Studies in Epidemiology |
| TGF-β | Transforming growth factor beta |
| TNF-α | Tumor necrosis factor alpha |
References
- Pfeifer, R.; Tarkin, I.S.; Rocos, B.; Pape, H.-C. Patterns of mortality and causes of death in polytrauma patients—Has anything changed? Injury 2009, 40, 907–911. [Google Scholar] [CrossRef]
- Halvachizadeh, S.; Mariani, D.; Pfeifer, R. Impact of trauma on society. Eur. J. Trauma Emerg. Surg. 2025, 51, 155. [Google Scholar] [CrossRef]
- TraumaRegister DGU®. Jahresbericht 2024; Deutsche Gesellschaft für Unfallchirurgie (DGU): Berlin, Germany, 2024; Available online: https://www.auc-online.de/fileadmin/AUC/Dokumente/Register/TraumaRegister_DGU/TR-DGU-Jahresbericht_2024.pdf (accessed on 7 October 2025).
- Malekzadeh, H.; Golpayegani, M.; Ghodsi, Z.; Sadeghi-Naini, M.; Asgardoon, M.; Baigi, V.; Vaccaro, A.R.; Rahimi-Movaghar, V. Direct Cost of Illness for Spinal Cord Injury: A Systematic Review. Glob. Spine J. 2022, 12, 1267–1281. [Google Scholar] [CrossRef]
- Bickenbach, J.; Officer, A.; Shakespeare, T.; von Groote, P.; World Health Organization; The International Spinal Cord Society. International Perspectives on Spinal Cord Injury; World Health Organization: Geneva, Switzerland, 2013; Available online: https://iris.who.int/handle/10665/94190 (accessed on 7 October 2025).
- Jin, L.-Y.; Li, J.; Wang, K.-F.; Xia, W.-W.; Zhu, Z.-Q.; Wang, C.-R.; Li, X.-F.; Liu, H.-Y. Blood–Spinal Cord Barrier in Spinal Cord Injury: A Review. J. Neurotrauma 2021, 38, 1203–1224. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Gao, L.; Liu, R.-H.; Liu, Y.; Zhang, N.-S.; Chen, Z.-Y. microRNAs in Spinal Cord Injury: Potential Roles and Therapeutic Implications. Int. J. Biol. Sci. 2014, 10, 997–1006. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, K.; Wang, Q.; Zheng, Y. MicroRNA-124 improves functional recovery and suppresses Bax-dependent apoptosis in rats following spinal cord injury. Mol. Med. Rep. 2019, 19, 2551–2560. [Google Scholar] [CrossRef]
- Sabirzhanov, B.; Matyas, J.; Coll-Miro, M.; Yu, L.L.; Faden, A.I.; Stoica, B.A.; Wu, J. Inhibition of microRNA-711 limits angiopoietin-1 and Akt changes, tissue damage, and motor dysfunction after contusive spinal cord injury in mice. Cell Death Dis. 2019, 10, 839. [Google Scholar] [CrossRef]
- Pinchi, E.; Frati, A.; Cantatore, S.; D’errico, S.; La Russa, R.; Maiese, A.; Palmieri, M.; Pesce, A.; Viola, R.V.; Frati, P.; et al. Acute Spinal Cord Injury: A Systematic Review Investigating miRNA Families Involved. Int. J. Mol. Sci. 2019, 20, 1841. [Google Scholar] [CrossRef]
- Kwon, B.K.; Bloom, O.; Wanner, I.-B.; Curt, A.; Schwab, J.M.; Fawcett, J.; Wang, K.K. Neurochemical biomarkers in spinal cord injury. Spinal Cord 2019, 57, 819–831. [Google Scholar] [CrossRef]
- Wanner, G.A.; Bloemers, F.; Nau, C.; Wendt, K.; Jug, M.; Komadina, R.; Pape, H.C. Thoracolumbar injuries: Prehospital and emergency management, imaging, classifications and clinical implications. Eur. J. Trauma Emerg. Surg. 2024, 50, 1943–1949. [Google Scholar] [CrossRef]
- Hagebusch, P.; Scheidt, N.; Koch, D.; Klug, A.; Schweigkofler, U.; Faul, P. Biomarkers help identify critically injured patients with only moderate risk of severe injuries in trauma team activation. Eur. J. Trauma Emerg. Surg. 2025, 51, 226. [Google Scholar] [CrossRef] [PubMed]
- Jug, M.; Komadina, R.; Wendt, K.; Pape, H.C.; Bloemers, F.; Nau, C. Thoracolumbar spinal cord injury: Management, techniques, timing. Eur. J. Trauma Emerg. Surg. 2024, 50, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Hartensuer, R.; Weise, A.; Breuing, J.; Bieler, D.; Sprengel, K.; Huber-Wagner, S.; Högel, F. Initial surgical management of spinal injuries in patients with multiple and/or severe injuries– the 2022 update of the German clinical practice guideline. Eur. J. Trauma Emerg. Surg. 2025, 51, 70. [Google Scholar] [CrossRef] [PubMed]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The Reactome Pathway Knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2020, 48, D127–D131. [Google Scholar] [CrossRef]
- Yunta, M.; Nieto-Díaz, M.; Esteban, F.J.; Caballero-López, M.; Navarro-Ruíz, R.; Reigada, D.; Pita-Thomas, D.W.; del Águila, Á.; Muñoz-Galdeano, T.; Maza, R.M. MicroRNA Dysregulation in the Spinal Cord following Traumatic Injury. PLoS ONE 2012, 7, e34534. [Google Scholar] [CrossRef]
- Tigchelaar, S.; Gupta, R.; Shannon, C.P.; Streijger, F.; Sinha, S.; Flibotte, S.; Rizzuto, M.A.; Street, J.; Paquette, S.; Ailon, T.; et al. MicroRNA Biomarkers in Cerebrospinal Fluid and Serum Reflect Injury Severity in Human Acute Traumatic Spinal Cord Injury. J. Neurotrauma 2019, 36, 2358–2371. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Marumo, M.; Ekawa, K.; Daimon, T. Differences in serum and plasma levels of microRNAs and their time-course changes after blood collection. Pract. Lab. Med. 2024, 39, e00376. [Google Scholar] [CrossRef]
- Mompeón, A.; Ortega-Paz, L.; Vidal-Gómez, X.; Costa, T.J.; Pérez-Cremades, D.; Garcia-Blas, S.; Brugaletta, S.; Sanchis, J.; Sabate, M.; Novella, S.; et al. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: A systematic and paired comparative analysis. Sci. Rep. 2020, 10, 5373. [Google Scholar] [CrossRef] [PubMed]
- Fei, M.; Li, Z.; Cao, Y.; Jiang, C.; Lin, H.; Chen, Z. MicroRNA-182 improves spinal cord injury in mice by modulating apoptosis and the inflammatory response via IKKβ/NF-κB. Lab. Investig. 2021, 101, 1238–1253. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.-T.; Yang, X.-Y.; Li, J.-A.; Zan, C.-F.; Xia, P.; Zheng, C.-J.; Qi, Z.-P.; Li, C.-X.; Liu, Z.-G. Key genes expressed in different stages of spinal cord ischemia/reperfusion injury. Neural Regen. Res. 2016, 11, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, H.; Xie, A.; Liao, O.; Ju, F.; Zhou, Y. MicroRNA-144 relieves chronic constriction injury-induced neuropathic pain via targeting RASA1. Biotechnol. Appl. Biochem. 2020, 67, 294–302. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Wu, L.; Duan, X.; Hu, Y.; Sun, Y.; Wei, Y.; Dong, Z.; Wu, C.; Yu, D.; et al. Deletion of MicroRNA-144/451 Cluster Aggravated Brain Injury in Intracerebral Hemorrhage Mice by Targeting 14-3-3ζ. Front. Neurol. 2021, 11, 551411. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Zhang, S.; Wu, J.; Xue, S. Rno-microRNA-30c-5p promotes myocardial ischemia reperfusion injury in rats through activating NF-κB pathway and targeting SIRT1. BMC Cardiovasc. Disord. 2020, 20, 240. [Google Scholar] [CrossRef]
- Sun, M.; Guo, M.; Ma, G.; Zhang, N.; Pan, F.; Fan, X.; Wang, R. MicroRNA-30c-5p protects against myocardial ischemia/reperfusion injury via regulation of Bach1/Nrf2. Toxicol. Appl. Pharmacol. 2021, 426, 115637. [Google Scholar] [CrossRef]
- Wang, X.; Su, X.; Gong, F.; Yin, J.; Sun, Q.; Lv, Z.; Liu, B. MicroRNA-30c abrogation protects against spinal cord ischemia reperfusion injury through modulating SIRT1. Eur. J. Pharmacol. 2019, 851, 80–87. [Google Scholar] [CrossRef]
- Tramullas, M.; Francés, R.; de la Fuente, R.; Velategui, S.; Carcelén, M.; García, R.; Llorca, J.; Hurlé, M.A. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci. Transl. Med. 2018, 10, eaao6299. [Google Scholar] [CrossRef]
- Popa, C.; Popa, F.; Grigorean, V.; Onose, G.; Sandu, A.; Popescu, M.; Burnei, G.; Strambu, V.; Sinescu, C. Vascular dysfunctions following spinal cord injury. J. Med. Life 2010, 3, 275–285. [Google Scholar]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, P.G.; Krishnamurthy, S.; Patel, S.P.; Pandya, J.D.; Rabchevsky, A.G. Temporal Characterization of Mitochondrial Bioenergetics after Spinal Cord Injury. J. Neurotrauma 2007, 24, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; McEwen, M.L.; Nottingham, S.A.; Maragos, W.F.; Dragicevic, N.B.; Sullivan, P.G.; Springer, J.E. The Mitochondrial Uncoupling Agent 2,4-Dinitrophenol Improves Mitochondrial Function, Attenuates Oxidative Damage, and Increases White Matter Sparing in the Contused Spinal Cord. J. Neurotrauma 2004, 21, 1396–1404. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, N.B.; Andrews, S.B. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 2010, 277, 3622–3636. [Google Scholar] [CrossRef]
- Lv, G.; Jia, Z.-Q.; Li, G.; Zhang, Z.-Y.; Li, H.-T.; Wang, J.-Q.; Fan, Z.-K. Time representation of mitochondrial morphology and function after acute spinal cord injury. Neural Regen. Res. 2016, 11, 137–143. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Sahebkar, A. Potential roles of microRNAs in redox state: An update. J. Cell. Biochem. 2019, 120, 1679–1684. [Google Scholar] [CrossRef]
- Narasimhan, M.; Patel, D.; Vedpathak, D.; Rathinam, M.; Henderson, G.; Mahimainathan, L. Identification of Novel microRNAs in Post-Transcriptional Control of Nrf2 Expression and Redox Homeostasis in Neuronal, SH-SY5Y Cells. PLoS ONE 2012, 7, e51111. [Google Scholar] [CrossRef]
- Kukoyi, A.T.; Fan, X.; Staitieh, B.S.; Hybertson, B.M.; Gao, B.; McCord, J.M.; Guidot, D.M. MiR-144 mediates Nrf2 inhibition and alveolar epithelial dysfunction in HIV-1 transgenic rats. Am. J. Physiol.-Cell Physiol. 2019, 317, C390–C397. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Jones, M.A.; Abdelrahman, A.A.; Powell, F.L.; Thounaojam, M.C.; Gutsaeva, D.; Bartoli, M.; Martin, P.M. Inhibiting microRNA-144 potentiates Nrf2-dependent antioxidant signaling in RPE and protects against oxidative stress-induced outer retinal degeneration. Redox Biol. 2020, 28, 101336. [Google Scholar] [CrossRef]
- Zhan, J.; Li, X.; Luo, D.; Yan, W.; Hou, Y.; Hou, Y.; Chen, S.; Luan, J.; Zhang, Q.; Lin, D. Polydatin Attenuates OGD/R-Induced Neuronal Injury and Spinal Cord Ischemia/Reperfusion Injury by Protecting Mitochondrial Function via Nrf2/ARE Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 6687212. [Google Scholar] [CrossRef]
- Lv, R.; Du, L.; Zhang, L.; Zhang, Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019, 217, 119–127. [Google Scholar] [CrossRef]
- Roser, A.-E.; Gomes, L.C.; Halder, R.; Jain, G.; Maass, F.; Tönges, L.; Tatenhorst, L.; Bähr, M.; Fischer, A.; Lingor, P. miR-182-5p and miR-183-5p Act as GDNF Mimics in Dopaminergic Midbrain Neurons. Mol. Ther. Nucleic Acids 2018, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Tian, L.; Zhang, Y.; Zhang, X.; Kang, J.; Dong, H.; Huang, N.; Pan, L.; Ning, B. Apigenin inhibits fibrous scar formation after acute spinal cord injury through TGFβ/SMADs signaling pathway. CNS Neurosci. Ther. 2022, 28, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lei, B.; Li, H.; Zhu, L.; Wang, L.; Tao, H.; Mei, S.; Li, F. MicroRNA-144 is regulated by CP2 and decreases COX-2 expression and PGE2 production in mouse ovarian granulosa cells. Cell Death Dis. 2017, 8, e2597. [Google Scholar] [CrossRef]
- Lagord, C.; Berry, M.; Logan, A. Expression of TGFβ2 but Not TGFβ1 Correlates with the Deposition of Scar Tissue in the Lesioned Spinal Cord. Mol. Cell. Neurosci. 2002, 20, 69–92. [Google Scholar] [CrossRef]
- Jin, M.; Xu, X. MicroRNA-182-5p Inhibits Hypertrophic Scar Formation by Inhibiting the Proliferation and Migration of Fibroblasts via SMAD4 Pathway. Clin. Cosmet. Investig. Dermatol. 2023, 16, 565–580. [Google Scholar] [CrossRef]
- Ueki, Y.; Reh, T.A. Activation of BMP-Smad1/5/8 Signaling Promotes Survival of Retinal Ganglion Cells after Damage In Vivo. PLoS ONE 2012, 7, e38690. [Google Scholar] [CrossRef]
- Farrukh, F.; Davies, E.; Berry, M.; Logan, A.; Ahmed, Z. BMP4/Smad1 Signalling Promotes Spinal Dorsal Column Axon Regeneration and Functional Recovery After Injury. Mol. Neurobiol. 2019, 56, 6807–6819. [Google Scholar] [CrossRef]
- Parikh, P.; Hao, Y.; Hosseinkhani, M.; Patil, S.B.; Huntley, G.W.; Tessier-Lavigne, M.; Zou, H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc. Natl. Acad. Sci. USA 2011, 108, E99–E107. [Google Scholar] [CrossRef]
- Seki, T.; Hida, K.; Tada, M.; Koyanagi, I.; Iwasaki, Y. Role of the bcl-2 Gene after Contusive Spinal Cord Injury in Mice. Neurosurgery 2003, 53, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.-Y.; Zhang, K.-M.; Xu, G.-Q.; Li, G. Bcl-2 in suppressing neuronal apoptosis after spinal cord injury. World J. Emerg. Med. 2011, 2, 38–44. [Google Scholar] [PubMed]
- Cittelly, D.M.; Nesic-Taylor, O.; Perez-Polo, J.R. Phosphorylation of Bcl-xL after spinal cord injury. J. Neurosci. Res. 2007, 85, 1894–1911. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, S.; Knoblach, S.M.; Brandoli, C.; Aden, S.A.; Hoffman, E.P.; Faden, A.I. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann. Neurol. 2003, 53, 454–468. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Stoica, B.A.; Fricke, S.; Di Giovanni, S.; Faden, A.I. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 2007, 130, 2977–2992. [Google Scholar] [CrossRef]
- Tian, D.-S.; Xie, M.-J.; Yu, Z.-Y.; Zhang, Q.; Wang, Y.-H.; Chen, B.; Chen, C.; Wang, W. Cell cycle inhibition attenuates microglia induced inflammatory response and alleviates neuronal cell death after spinal cord injury in rats. Brain Res. 2007, 1135, 177–185. [Google Scholar] [CrossRef]
- Tam, S.; de Borja, R.; Tsao, M.-S.; McPherson, J.D. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab. Investig. 2014, 94, 350–358. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Rupp, R.; Biering-Sørensen, F.; Burns, S.P.; Graves, D.E.; Guest, J.; Jones, L.; Read, M.S.; Rodriguez, G.M.; Schuld, C.; Tansey-Md, K.E.; et al. International Standards for Neurological Classification of Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2021, 27, 1–22. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
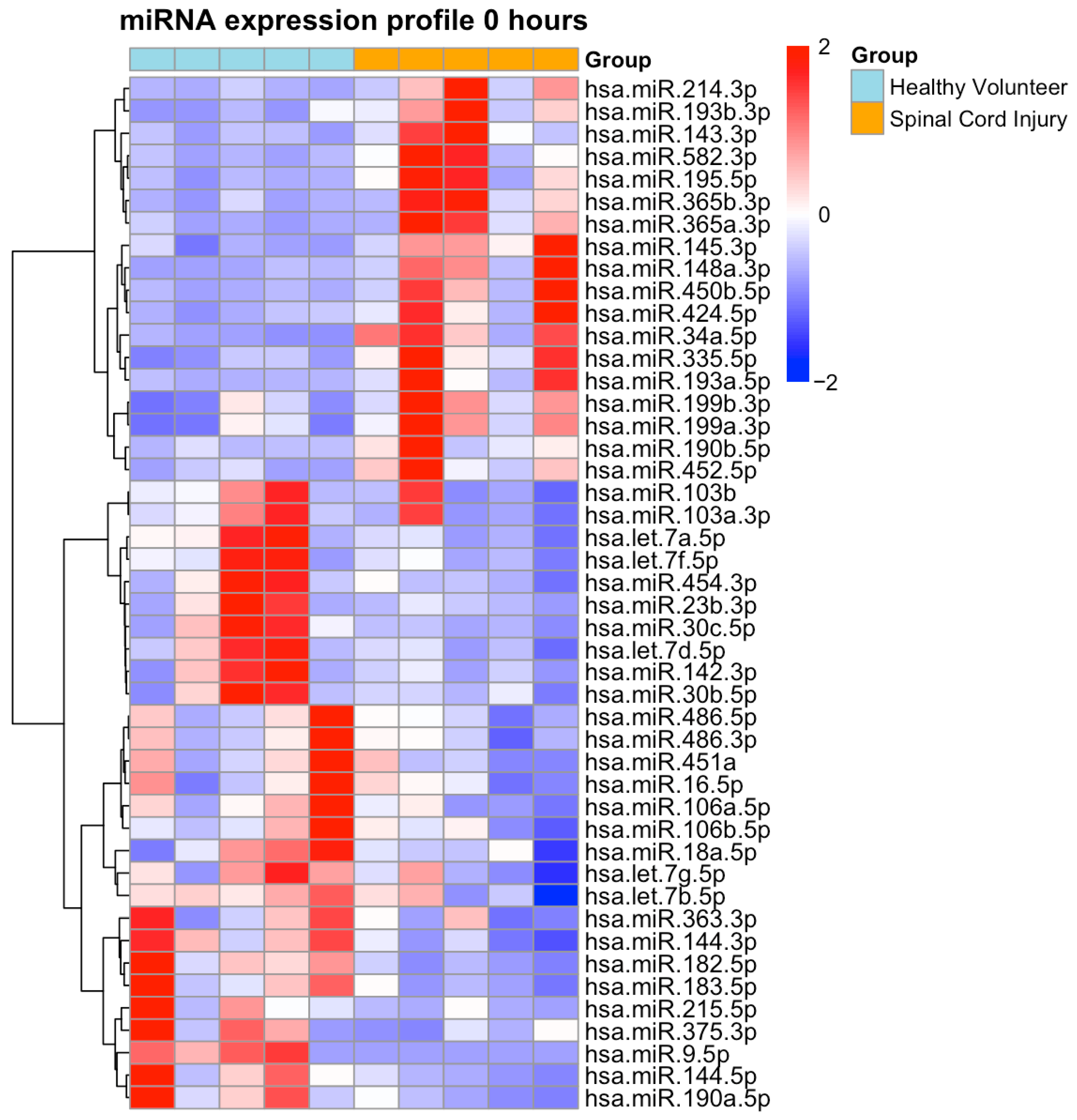
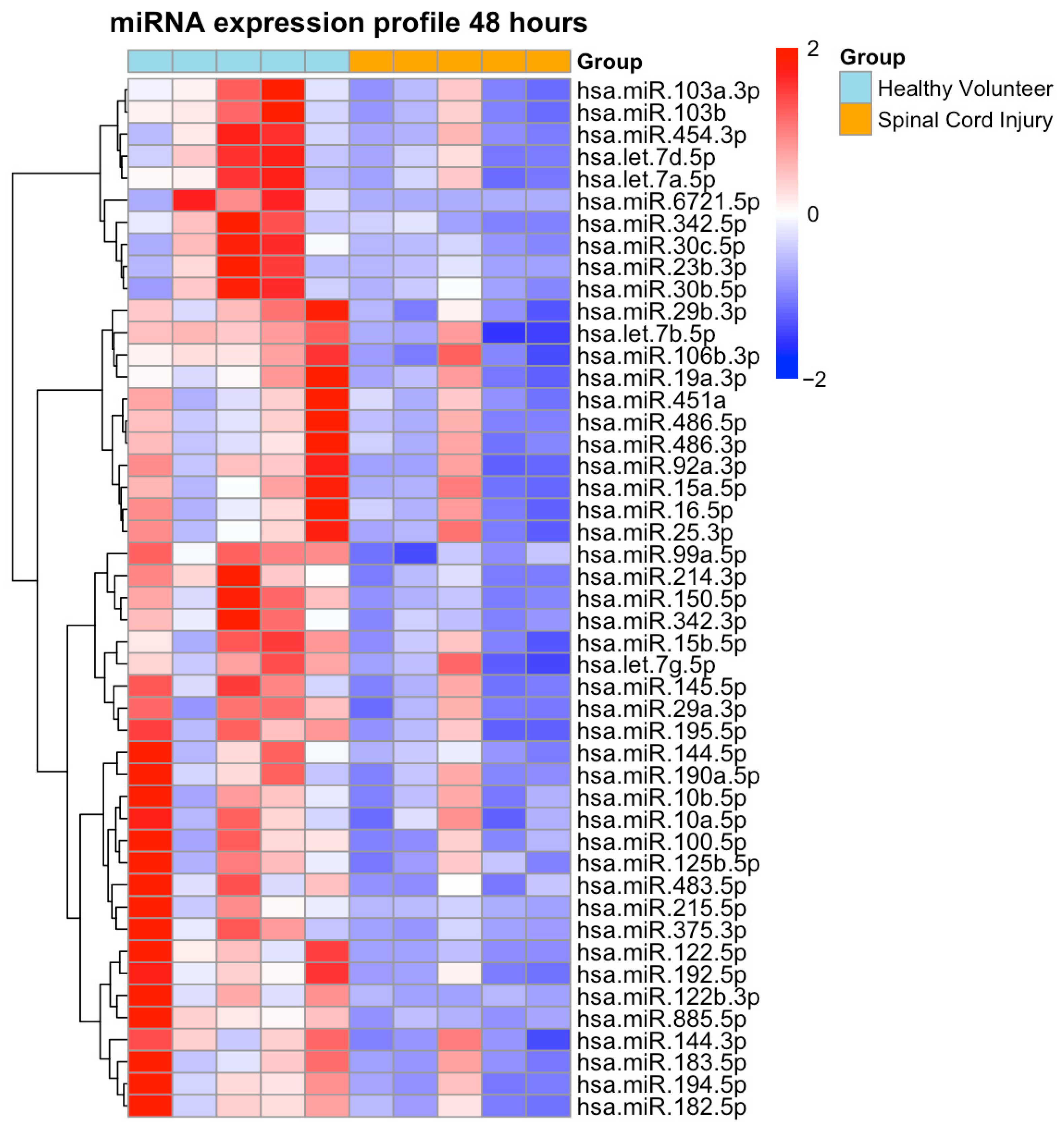
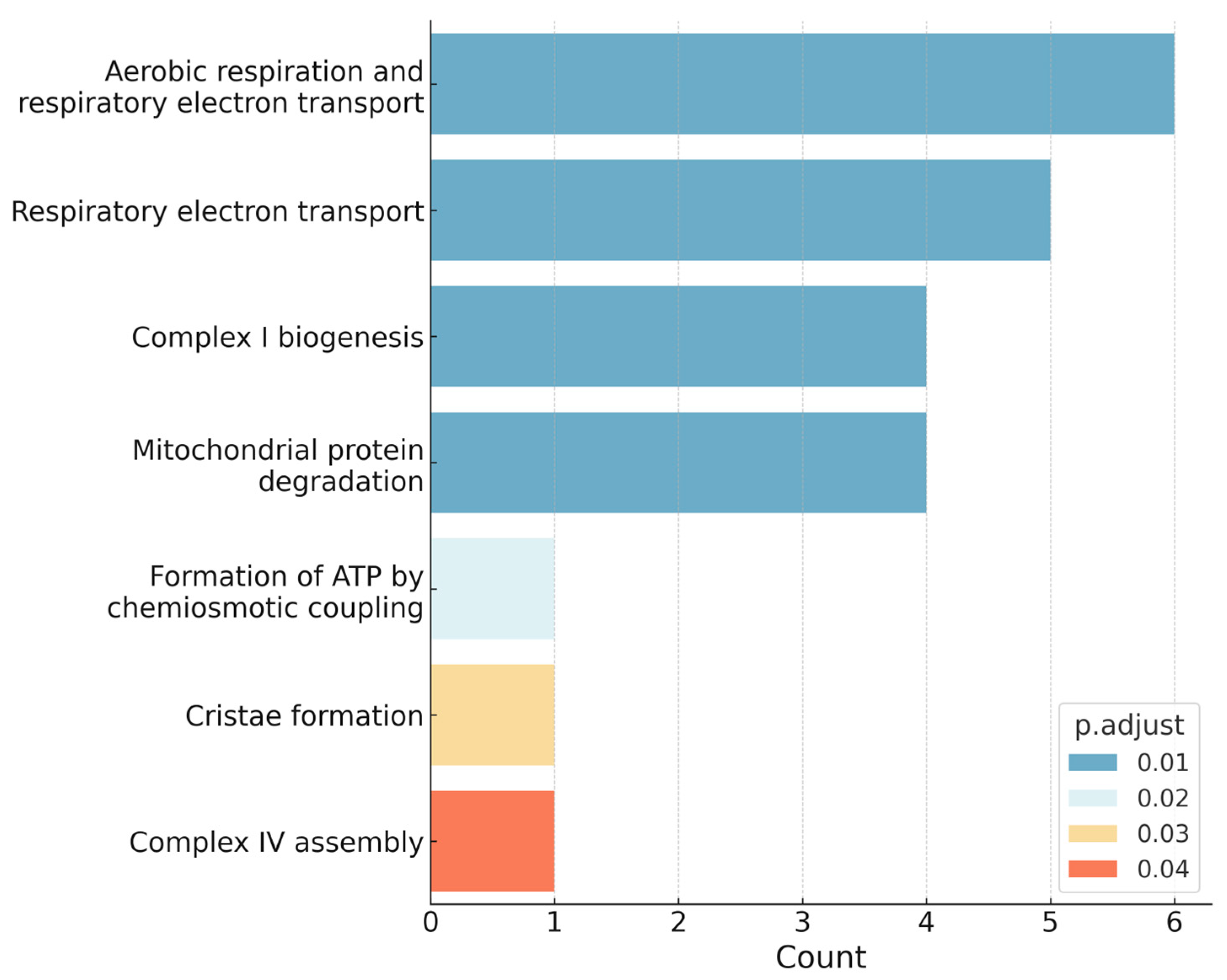
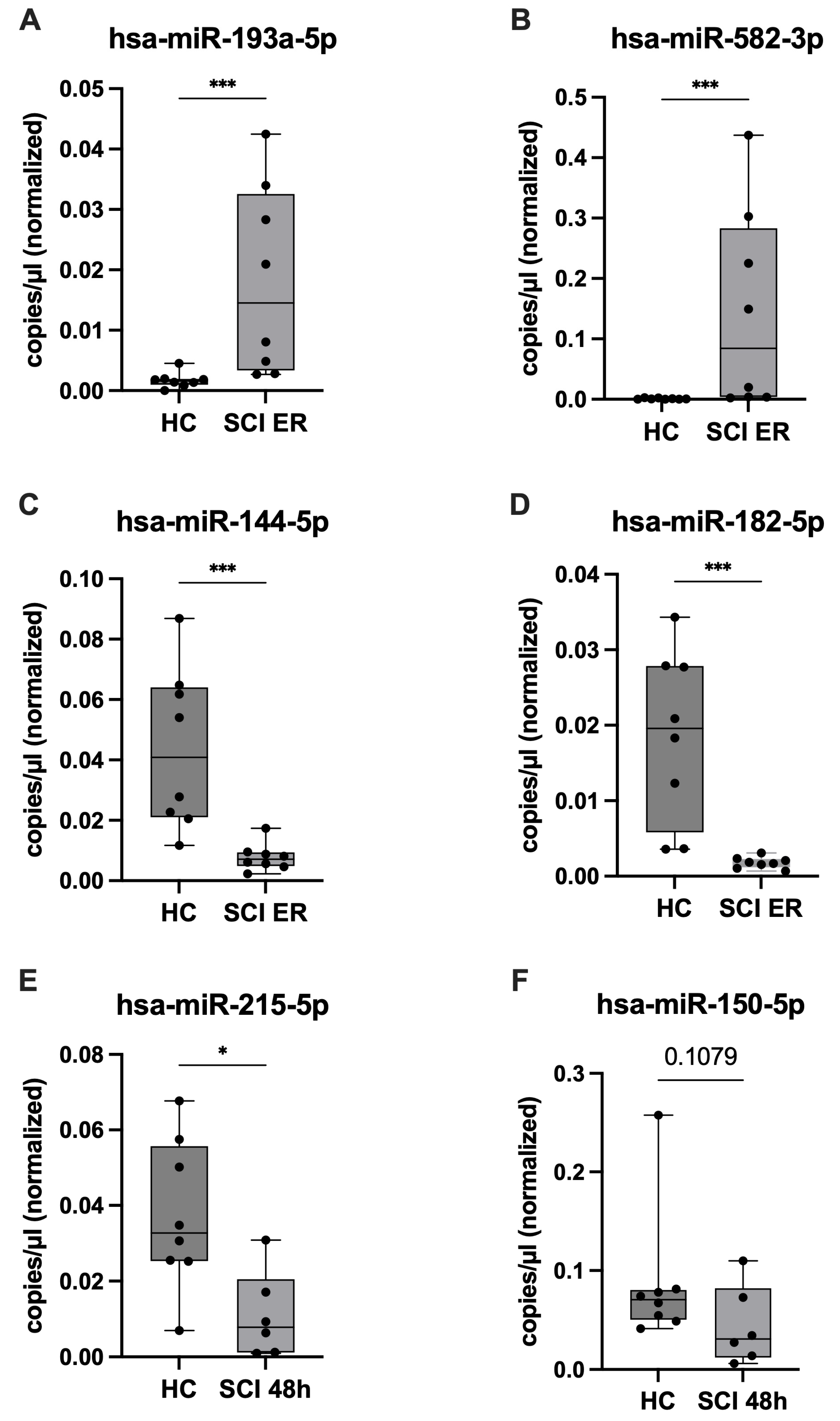
| Characteristics | SCI (n = 8) | PT (n = 8) | p-Value |
|---|---|---|---|
| Male [n] | 6 | 6 | |
| Age [years] (IQR) | 43.5 (32.3–66.5) | 41 (35.5–50.5) | >0.999 |
| ISS [points] (IQR) | 25 (25–25) | 25 (25–26) | 0.446 |
| IL-6 ER [pg/mL] (IQR) | 23 (5.3–31.4) | 60.8 (32.6–134) | 0.094 |
| IL-6 48h [pg/mL] (IQR) | 30.9 (3.2–144) | 101.3 (39.3–215) | 0.187 |
| Leukocytes ER [/nL] (IQR) | 8.2 (5.7–10.2) | 11.3 (8.2–22.3) | 0.130 |
| Leukocytes 48h [/nL] (IQR) | 10.6 (6.8–13.3) | 9.4 (5.7–10.3) | 0.195 |
| Hemoglobin ER [g/dL] (IQR) | 12.4 (10.7–13.3) | 12.1 (10–13.8) | 0.879 |
| Lactate ER [mg/dL] (IQR) | 19.5 (13.8–39) | 40.5 (27–52.3) | 0.078 |
| SBP at scene [mmHg] (IQR) | 85 (72.5–117.5) | 120 (112.5–137) | 0.038 |
| Catecholamines ER [%] | 75 | 25 | 0.132 |
| ICU LOS [days] (IQR) | 8.5 (3.8–16.3) | 5.5 (3.5–8.5) | 0.267 |
| Mechanical ventilation [days] (IQR) | 2 (0.3–16) | 2 (1–2) | 0.694 |
| in-hospital mortality [%] | 0 | 0 |
| miRNA | Healthy Control Mean * (SD) | SCI Mean (SD) | p-Value |
|---|---|---|---|
| ER upregulated | |||
| hsa-miR-34a-5p | 0.0035 (0.0038) | 0.0084 (0.0091) | 0.3807 |
| hsa-miR-335-5p | 0.0170 (0.0083) | 0.0484 (0.0325) | 0.0379 |
| hsa-miR-193a-5p | 0.0017 (0.0013) | 0.0180 (0.0156) | 0.0006 |
| hsa-miR-450b-5p | 0.0008 (0.0009) | 0.0061 (0.0082) | 0.0145 |
| hsa-miR-582-3p | 0.0010 (0.0010) | 0.1430 (0.1658) | 0.0003 |
| ER downregulated | |||
| hsa-miR-144-5p | 0.0438 (0.0267) | 0.0078 (0.0045) | 0.0003 |
| hsa-miR-30c-5p | 0.1379 (0.0520) | 0.0939 (0.0470) | 0.1049 |
| hsa-miR-182-5p | 0.0186 (0.0114) | 0.0018 (0.0007) | 0.0002 |
| hsa-miR-215-5p | 0.0373 (0.0198) | 0.0374 (0.0408) | 0.3823 |
| hsa-miR-190a-5p | 0.0180 (0.0150) | 0.0027 (0.0022) | 0.0011 |
| 48 h downregulated | |||
| hsa-miR-122-5p | 0.0283 (0.0201) | 0.0186 (0.0163) | 0.4136 |
| hsa-miR-215-5p | 0.0373 (0.0198) | 0.0110 (0.0114) | 0.0200 |
| hsa-miR-375-3p | 0.0078 (0.0101) | 0.0035 (0.0054) | 0.1419 |
| hsa-miR-150-5p | 0.0879 (0.0700) | 0.0441 (0.0397) | 0.1079 |
| hsa-miR-885-5p | 0.0112 (0.0051) | 0.0118 (0.0059) | >0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hörauf, J.-A.; Saenger, M.; Störmann, P.; El Saman, A.; Marzi, I.; Henrich, D.; Leppik, L.; Schindler, C.R. Circulating microRNA Profiles in Acute Spinal Cord Injury: Evidence for Distinct Plasma Signatures Compared with Polytrauma Patients. Int. J. Mol. Sci. 2025, 26, 10954. https://doi.org/10.3390/ijms262210954
Hörauf J-A, Saenger M, Störmann P, El Saman A, Marzi I, Henrich D, Leppik L, Schindler CR. Circulating microRNA Profiles in Acute Spinal Cord Injury: Evidence for Distinct Plasma Signatures Compared with Polytrauma Patients. International Journal of Molecular Sciences. 2025; 26(22):10954. https://doi.org/10.3390/ijms262210954
Chicago/Turabian StyleHörauf, Jason-Alexander, Miriam Saenger, Philipp Störmann, André El Saman, Ingo Marzi, Dirk Henrich, Liudmila Leppik, and Cora Rebecca Schindler. 2025. "Circulating microRNA Profiles in Acute Spinal Cord Injury: Evidence for Distinct Plasma Signatures Compared with Polytrauma Patients" International Journal of Molecular Sciences 26, no. 22: 10954. https://doi.org/10.3390/ijms262210954
APA StyleHörauf, J.-A., Saenger, M., Störmann, P., El Saman, A., Marzi, I., Henrich, D., Leppik, L., & Schindler, C. R. (2025). Circulating microRNA Profiles in Acute Spinal Cord Injury: Evidence for Distinct Plasma Signatures Compared with Polytrauma Patients. International Journal of Molecular Sciences, 26(22), 10954. https://doi.org/10.3390/ijms262210954







