KBN2202, a Salicylic Acid Derivative, Preserves Neuronal Architecture, Enhances Neurogenesis, Attenuates Amyloid and Inflammatory Pathology, and Restores Recognition Memory in 5xFAD Mice at an Advanced Stage of AD Pathophysiology
Abstract
1. Introduction
2. Results
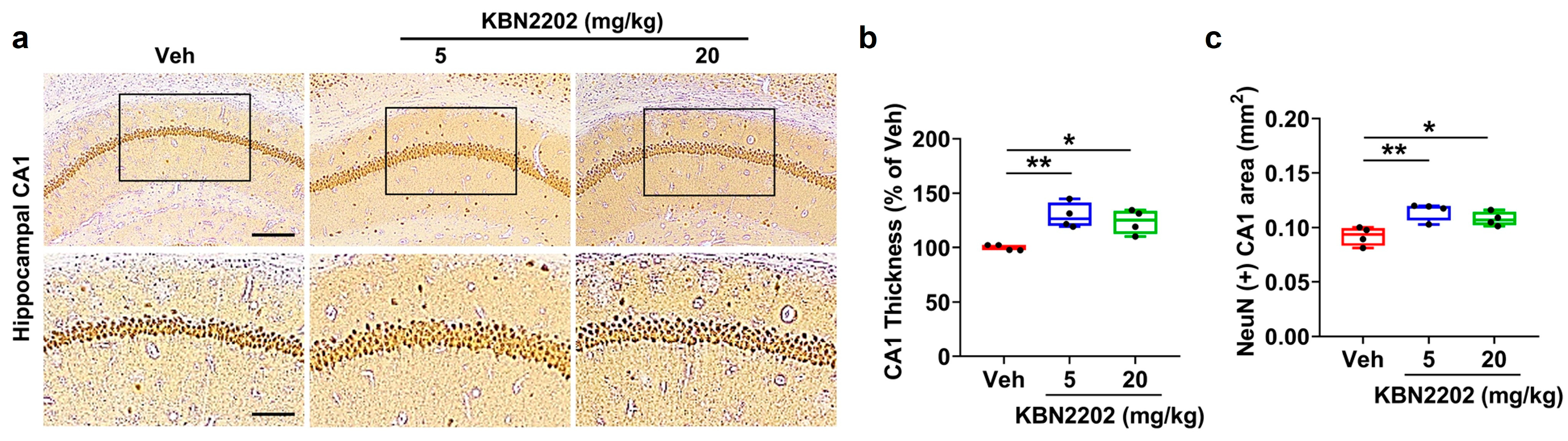
2.1. Neuroprotective Effect of KBN2202 on Hippocampal CA1 Structure
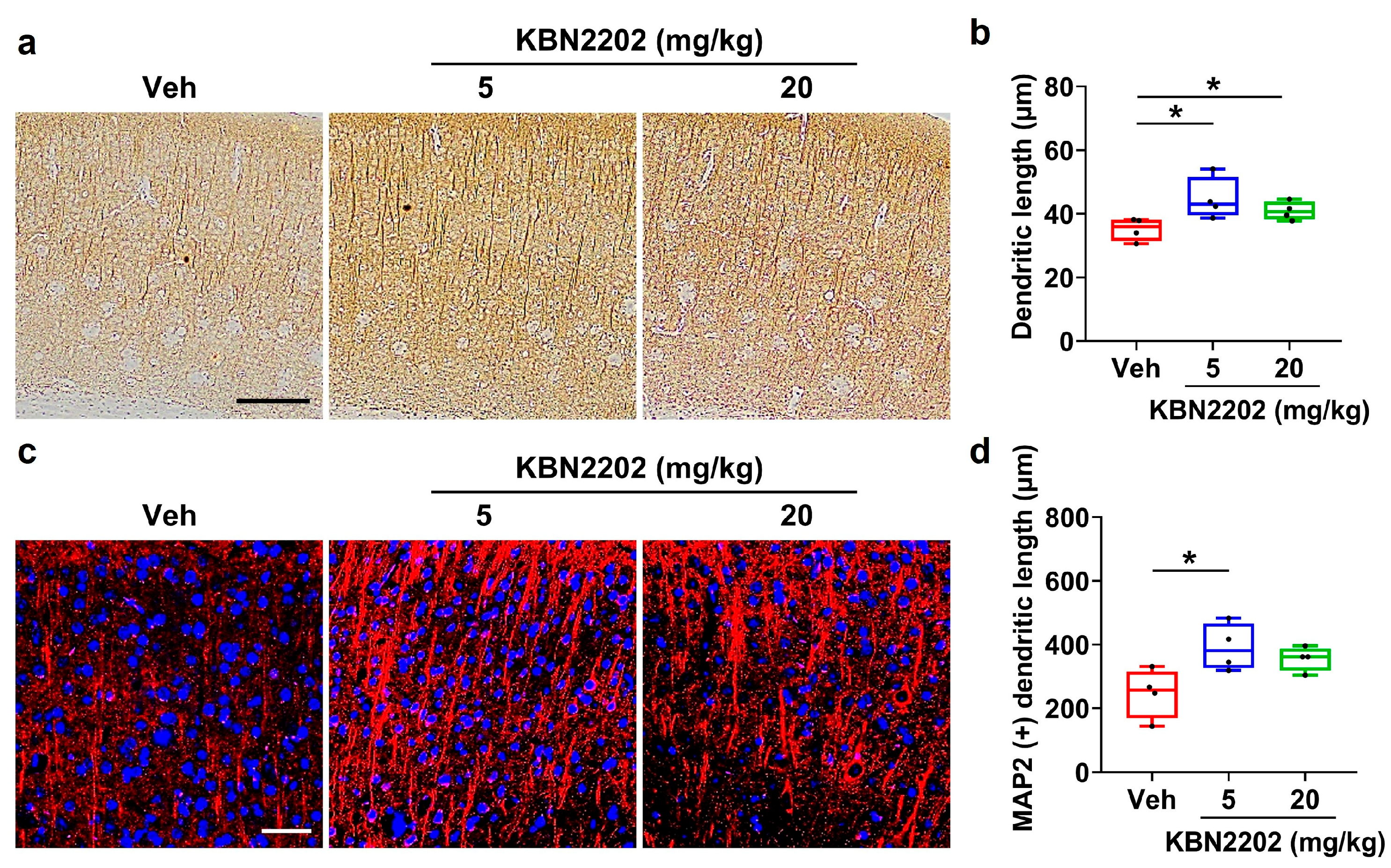
2.2. Preservation of Cortical Dendritic Architecture
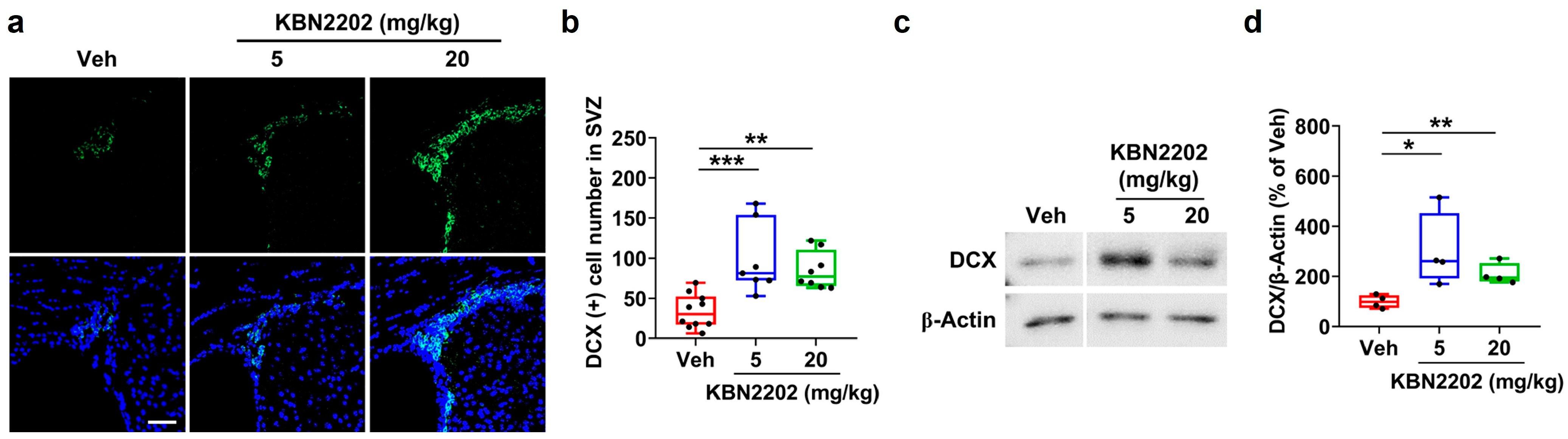
2.3. Enhancement of Adult Neurogenesis in the Subventricular Zone by KBN2202
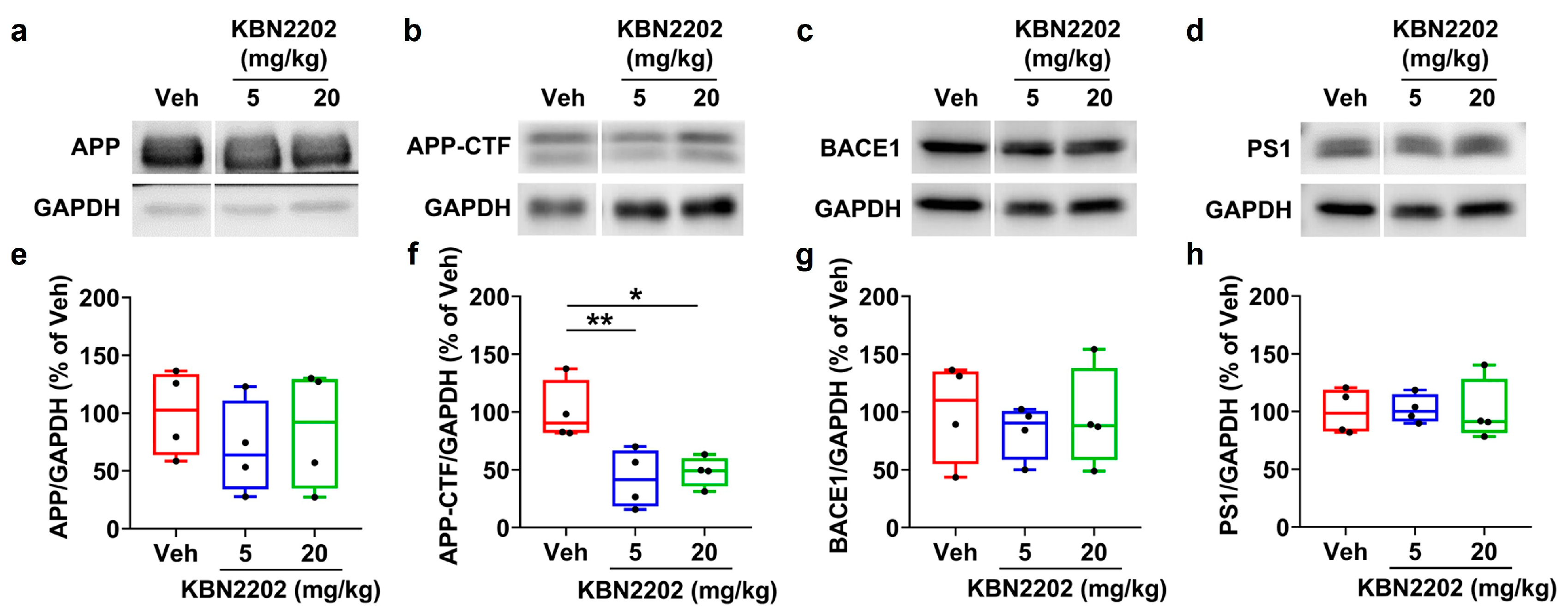
2.4. Effects of KBN2202 on Amyloidogenic Protein Expression
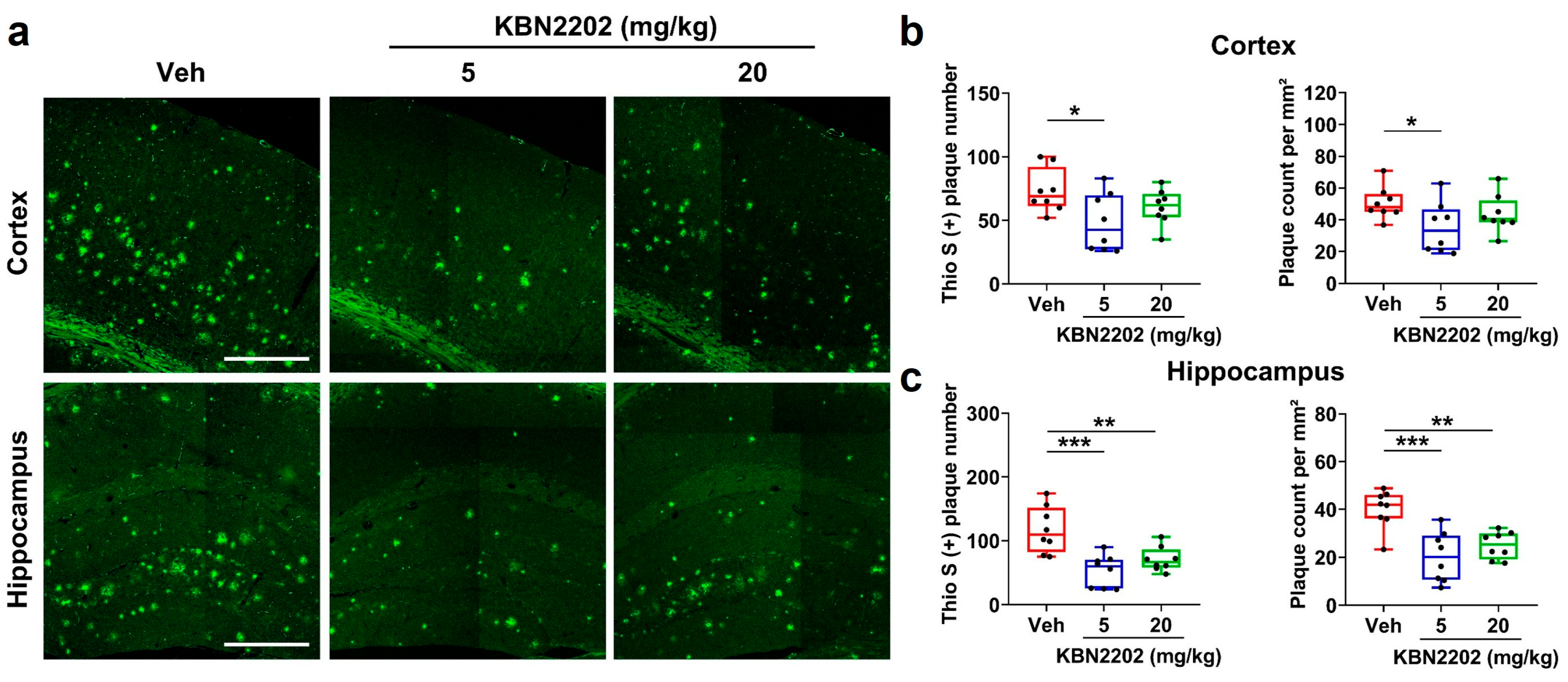
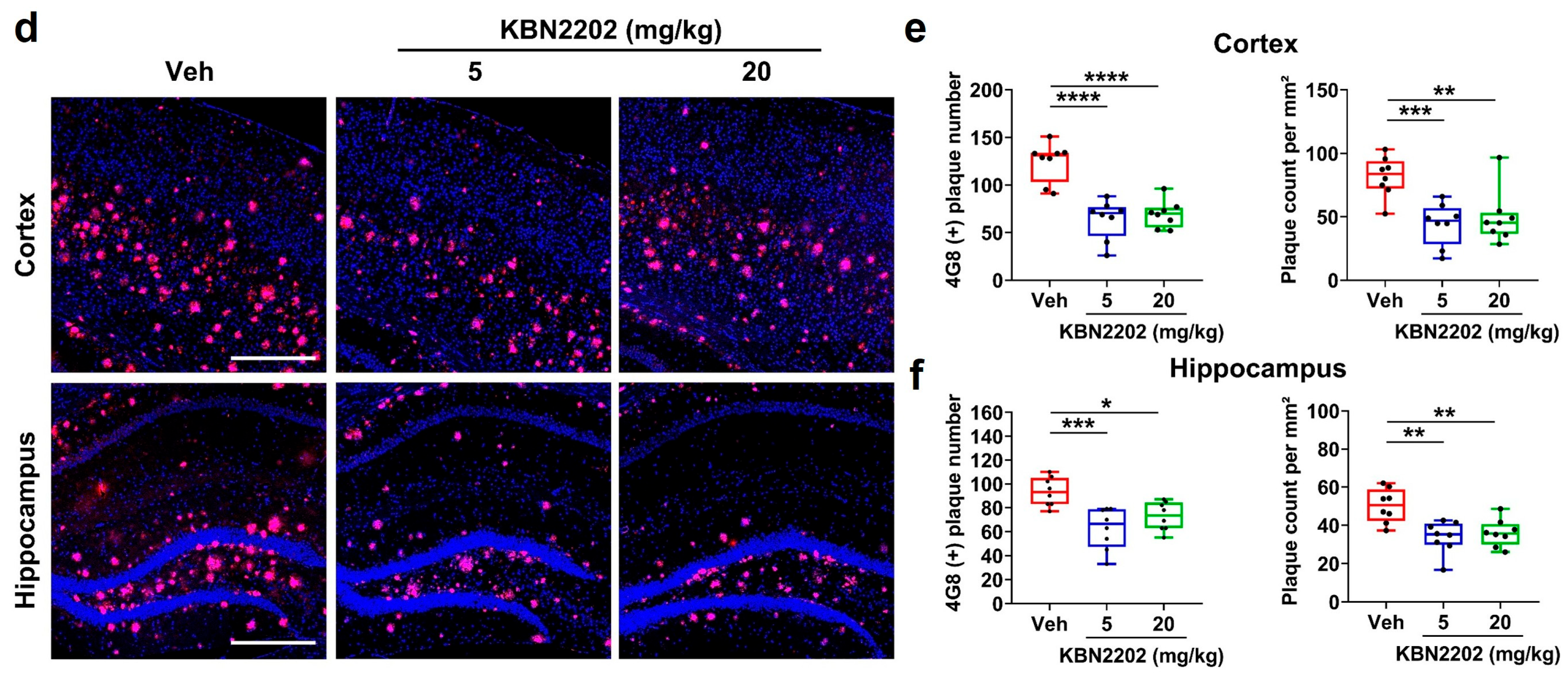
2.5. Reduction in Cortical and Hippocampal Aβ Plaque Burden by KBN2202
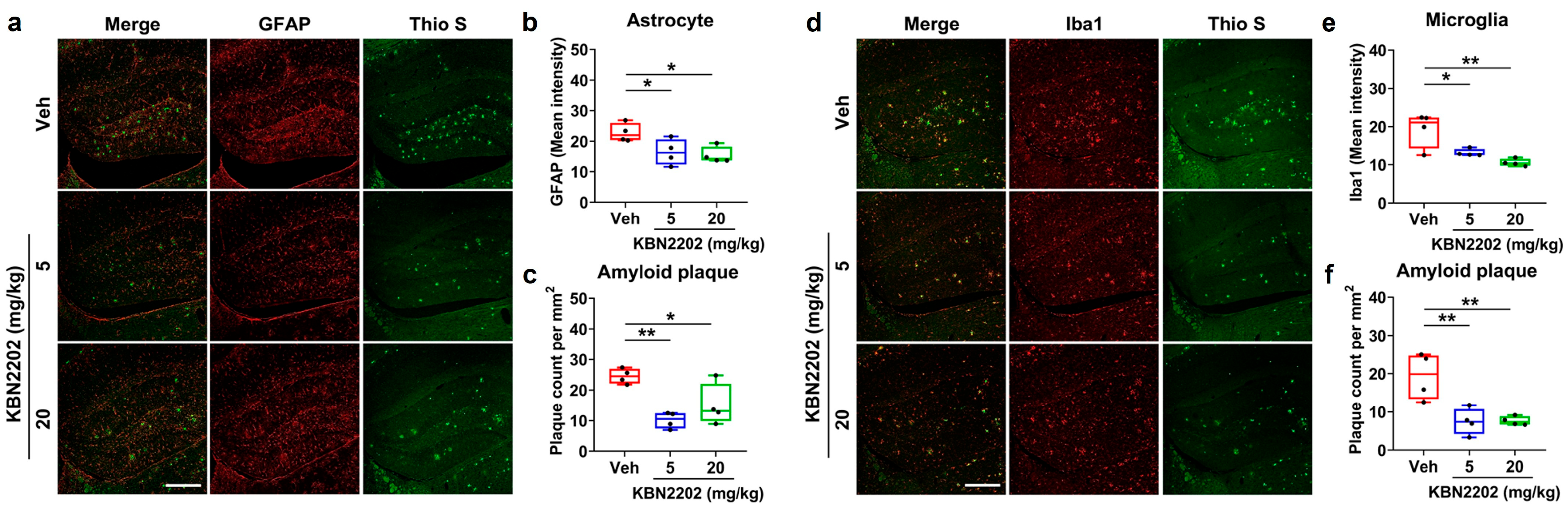
2.6. Attenuation of Hippocampal Neuroinflammation and Amyloid Pathology by KBN2202
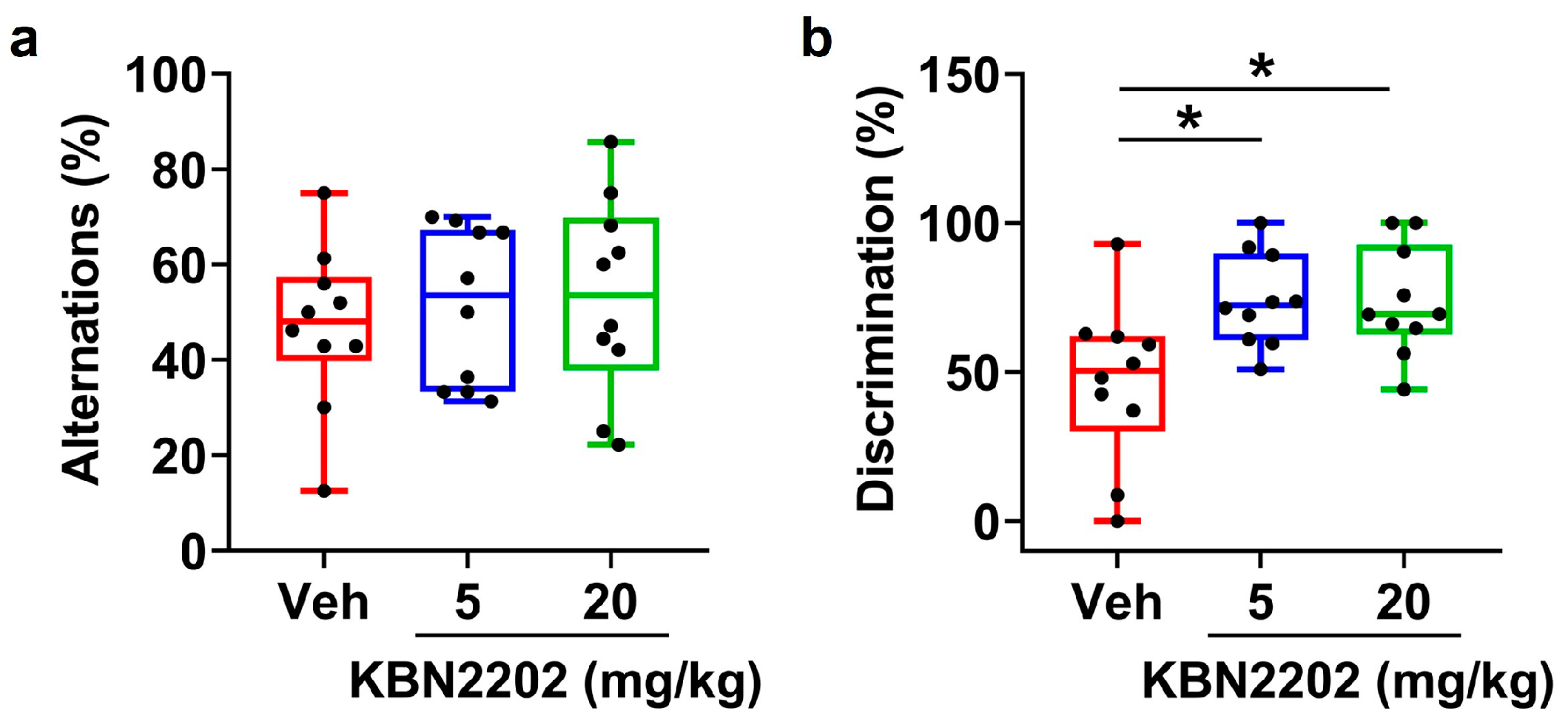
2.7. Improvement of Recognition Memory but Not Working Memory by KBN2202
3. Discussion
4. Materials and Methods
4.1. Animals and Housing Conditions
4.2. KBN2202 Treatment
4.3. Behavioral Assessment
4.3.1. Y-Maze
4.3.2. NOR
4.4. Tissue Preparation and Immunohistochemistry
4.4.1. Tissue Preparation
4.4.2. DAB Staining
4.4.3. Immunohistochemistry
4.4.4. Image Acquisition
4.5. Protein Extraction and Western Blot Analysis
4.5.1. Protein Extraction
4.5.2. Western Blot Analysis
4.5.3. Densitometric Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| APP-CTFs | Amyloid precursor protein C-terminal fragments |
| BACE1 | β-site APP cleaving enzyme 1 |
| BSA | Bovine serum albumin |
| DAB | 3,3’-Diaminobenzidine |
| DAPI | 4’,6-diamidino-2-phenylindole |
| ECL | Enhanced chemiluminescence |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GFAP | Glial fibrillary acidic protein |
| HRP | Horseradish peroxidase |
| Iba1 | Ionized calcium-binding adaptor molecule 1 |
| LPS | Lipopolysaccharide |
| MAP2 | Microtubule-associated protein 2 |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| NC | Nitrocellulose |
| NOR | Novel object recognition |
| OCT | Optimal cutting temperature |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PS1 | Presenilin-1 |
| PVDF | Polyvinylidene difluoride |
| RIPA | Radio immuno precipitation assay |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide |
| Thio S | Thioflavin S |
References
- De Strooper, B.; Karran, E. The Cellular Phase of Alzheimer’s Disease. Cell 2016, 164, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef] [PubMed]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef]
- Fruholz, I.; Meyer-Luehmann, M. The intricate interplay between microglia and adult neurogenesis in Alzheimer’s disease. Front. Cell Neurosci. 2024, 18, 1456253. [Google Scholar] [CrossRef]
- Cummings, J.; Zhou, Y.; Lee, G.; Zhong, K.; Fonseca, J.; Cheng, F. Alzheimer’s disease drug development pipeline: 2023. Alzheimers Dement. 2023, 9, e12385. [Google Scholar] [CrossRef]
- Karran, E.; De Strooper, B. The amyloid hypothesis in Alzheimer disease: New insights from new therapeutics. Nat. Rev. Drug Discov. 2022, 21, 306–318. [Google Scholar] [CrossRef]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- Mintun, M.A.; Lo, A.C.; Duggan Evans, C.; Wessels, A.M.; Ardayfio, P.A.; Andersen, S.W.; Shcherbinin, S.; Sparks, J.; Sims, J.R.; Brys, M.; et al. Donanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2021, 384, 1691–1704. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.M.; Gabr, M.T. Multitarget therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2019, 14, 437–440. [Google Scholar] [CrossRef]
- Gong, C.X.; Dai, C.L.; Liu, F.; Iqbal, K. Multi-Targets: An Unconventional Drug Development Strategy for Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 837649. [Google Scholar] [CrossRef]
- Hossain, M.S.; Hussain, M.H. Multi-Target Drug Design in Alzheimer’s Disease Treatment: Emerging Technologies, Advantages, Challenges, and Limitations. Pharmacol. Res. Perspect. 2025, 13, e70131. [Google Scholar] [CrossRef]
- De La Cruz, J.P.; Guerrero, A.; Gonzalez-Correa, J.A.; Arrebola, M.M.; Sanchez de la Cuesta, F. Antioxidant effect of acetylsalicylic and salicylic acid in rat brain slices subjected to hypoxia. J. Neurosci. Res. 2004, 75, 280–290. [Google Scholar] [CrossRef]
- Gepdiremen, A.; Hacimuftuoglu, A.; Duzenli, S.; Oztas, S.; Suleyman, H. Effects of salicylic acid in glutamate- and kainic acid-induced neurotoxicity in cerebellar granular cell culture of rats. Pharmacol. Res. 2000, 42, 547–551. [Google Scholar] [CrossRef]
- Borah, A.; Mohanakumar, K.P. Salicylic acid protects against chronic L-DOPA-induced 6-OHDA generation in experimental model of parkinsonism. Brain Res. 2010, 1344, 192–199. [Google Scholar] [CrossRef]
- Ferger, B.; Teismann, P.; Earl, C.D.; Kuschinsky, K.; Oertel, W.H. Salicylate protects against MPTP-induced impairments in dopaminergic neurotransmission at the striatal and nigral level in mice. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 360, 256–261. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Grishchenko, M.V.; Lushchekina, S.V.; Astakhova, T.Y.; Serebryakova, O.G.; Timokhina, E.N.; Zhilina, E.F.; et al. Conjugates of Tacrine and Salicylic Acid Derivatives as New Promising Multitarget Agents for Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 2285. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Heo, J.H.; Kim, M.; Choi, M.R.; Lee, S.R. Effect of a salicylic acid derivative, KBN-2202, on changes of Alzheimer’s disease-related factors in murine neuroblastoma Neuro2a cells. In Proceedings of the Society for Neuroscience Annual Meeting, Chicago, IL, USA, 5–9 October 2024. [Google Scholar]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Forner, S.; Kawauchi, S.; Balderrama-Gutierrez, G.; Kramar, E.A.; Matheos, D.P.; Phan, J.; Javonillo, D.I.; Tran, K.M.; Hingco, E.; da Cunha, C.; et al. Systematic phenotyping and characterization of the 5xFAD mouse model of Alzheimer’s disease. Sci. Data 2021, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- West, M.J.; Kawas, C.H.; Stewart, W.F.; Rudow, G.L.; Troncoso, J.C. Hippocampal neurons in pre-clinical Alzheimer’s disease. Neurobiol. Aging 2004, 25, 1205–1212. [Google Scholar] [CrossRef]
- Baloyannis, S.J. Dendritic pathology in Alzheimer’s disease. J. Neurol. Sci. 2009, 283, 153–157. [Google Scholar] [CrossRef]
- Knobloch, M.; Mansuy, I.M. Dendritic spine loss and synaptic alterations in Alzheimer’s disease. Mol. Neurobiol. 2008, 37, 73–82. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef]
- Capilla-Gonzalez, V.; Herranz-Perez, V.; Garcia-Verdugo, J.M. The aged brain: Genesis and fate of residual progenitor cells in the subventricular zone. Front. Cell Neurosci. 2015, 9, 365. [Google Scholar] [CrossRef]
- Moreno-Jimenez, E.P.; Flor-Garcia, M.; Terreros-Roncal, J.; Rabano, A.; Cafini, F.; Pallas-Bazarra, N.; Avila, J.; Llorens-Martin, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Bussiere, T.; Friend, P.D.; Sadeghi, N.; Wicinski, B.; Lin, G.I.; Bouras, C.; Giannakopoulos, P.; Robakis, N.K.; Morrison, J.H.; Perl, D.P.; et al. Stereologic assessment of the total cortical volume occupied by amyloid deposits and its relationship with cognitive status in aging and Alzheimer’s disease. Neuroscience 2002, 112, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Games, D.; Adams, D.; Alessandrini, R.; Barbour, R.; Berthelette, P.; Blackwell, C.; Carr, T.; Clemens, J.; Donaldson, T.; Gillespie, F.; et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 1995, 373, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar]
- Golde, T.E.; DeKosky, S.T.; Galasko, D. Alzheimer’s disease: The right drug, the right time. Science 2018, 362, 1250–1251. [Google Scholar] [CrossRef]
- Shukla, V.; Zheng, Y.L.; Mishra, S.K.; Amin, N.D.; Steiner, J.; Grant, P.; Kesavapany, S.; Pant, H.C. A truncated peptide from p35, a Cdk5 activator, prevents Alzheimer’s disease phenotypes in model mice. FASEB J. 2013, 27, 174–186. [Google Scholar] [CrossRef]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The beta-Secretase BACE1 in Alzheimer’s Disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Jin, K.; Peel, A.L.; Mao, X.O.; Xie, L.; Cottrell, B.A.; Henshall, D.C.; Greenberg, D.A. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 343–347. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2019. Alzheimer’s Dement. 2019, 5, 272–293. [Google Scholar] [CrossRef]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef]
- Cao, J.; Hou, J.; Ping, J.; Cai, D. Advances in developing novel therapeutic strategies for Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 64. [Google Scholar] [CrossRef]
- Keskin, A.D.; Kekus, M.; Adelsberger, H.; Neumann, U.; Shimshek, D.R.; Song, B.; Zott, B.; Peng, T.; Forstl, H.; Staufenbiel, M.; et al. BACE inhibition-dependent repair of Alzheimer’s pathophysiology. Proc. Natl. Acad. Sci. USA 2017, 114, 8631–8636. [Google Scholar] [CrossRef]
- Jawhar, S.; Trawicka, A.; Jenneckens, C.; Bayer, T.A.; Wirths, O. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Abeta aggregation in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Aging 2012, 33, 196.e29-40. [Google Scholar] [CrossRef]
- Klessig, D.F.; Tian, M.; Choi, H.W. Multiple Targets of Salicylic Acid and Its Derivatives in Plants and Animals. Front. Immunol. 2016, 7, 206. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Dandapani, M.; Hardie, D.G. AMPK: Mediating the metabolic effects of salicylate-based drugs? Trends Endocrinol. Metab. 2013, 24, 481–487. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Kim, J.C.; Choi, M.R.; Song, J.; Kim, M.; Chang, S.-H.; Kim, J.S.; Park, J.-S.; Lee, S.-R. KBN2202, a Salicylic Acid Derivative, Preserves Neuronal Architecture, Enhances Neurogenesis, Attenuates Amyloid and Inflammatory Pathology, and Restores Recognition Memory in 5xFAD Mice at an Advanced Stage of AD Pathophysiology. Int. J. Mol. Sci. 2025, 26, 10942. https://doi.org/10.3390/ijms262210942
Lee S-Y, Kim JC, Choi MR, Song J, Kim M, Chang S-H, Kim JS, Park J-S, Lee S-R. KBN2202, a Salicylic Acid Derivative, Preserves Neuronal Architecture, Enhances Neurogenesis, Attenuates Amyloid and Inflammatory Pathology, and Restores Recognition Memory in 5xFAD Mice at an Advanced Stage of AD Pathophysiology. International Journal of Molecular Sciences. 2025; 26(22):10942. https://doi.org/10.3390/ijms262210942
Chicago/Turabian StyleLee, Sun-Young, Jong Chul Kim, Mi Ran Choi, Jiseo Song, Moonhang Kim, Seok-Hwan Chang, Jong Sung Kim, Joon-Suk Park, and Sang-Rae Lee. 2025. "KBN2202, a Salicylic Acid Derivative, Preserves Neuronal Architecture, Enhances Neurogenesis, Attenuates Amyloid and Inflammatory Pathology, and Restores Recognition Memory in 5xFAD Mice at an Advanced Stage of AD Pathophysiology" International Journal of Molecular Sciences 26, no. 22: 10942. https://doi.org/10.3390/ijms262210942
APA StyleLee, S.-Y., Kim, J. C., Choi, M. R., Song, J., Kim, M., Chang, S.-H., Kim, J. S., Park, J.-S., & Lee, S.-R. (2025). KBN2202, a Salicylic Acid Derivative, Preserves Neuronal Architecture, Enhances Neurogenesis, Attenuates Amyloid and Inflammatory Pathology, and Restores Recognition Memory in 5xFAD Mice at an Advanced Stage of AD Pathophysiology. International Journal of Molecular Sciences, 26(22), 10942. https://doi.org/10.3390/ijms262210942





