Synthesis, Antiprotozoal Activity, and Physicochemical Evaluation of Benzamido–Menadione Derivatives
Abstract
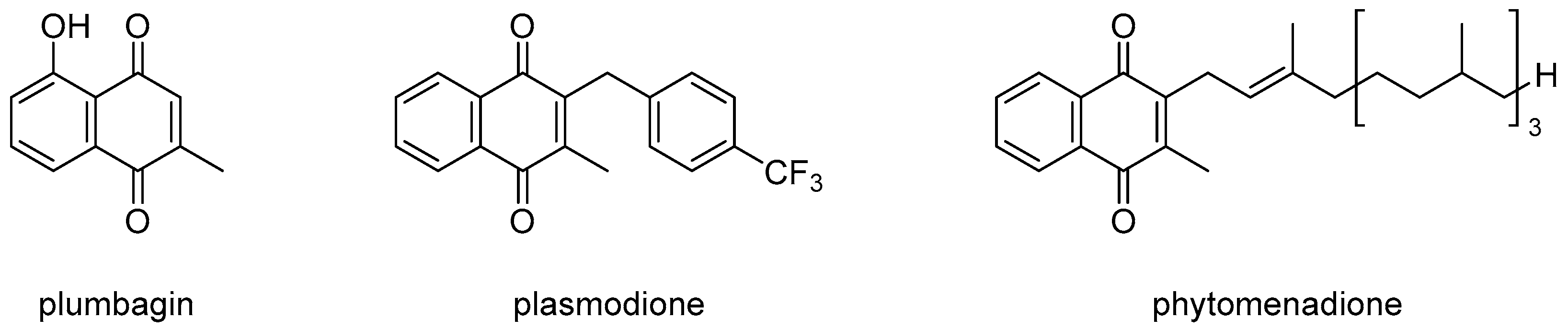
1. Introduction
2. Results and Discussion
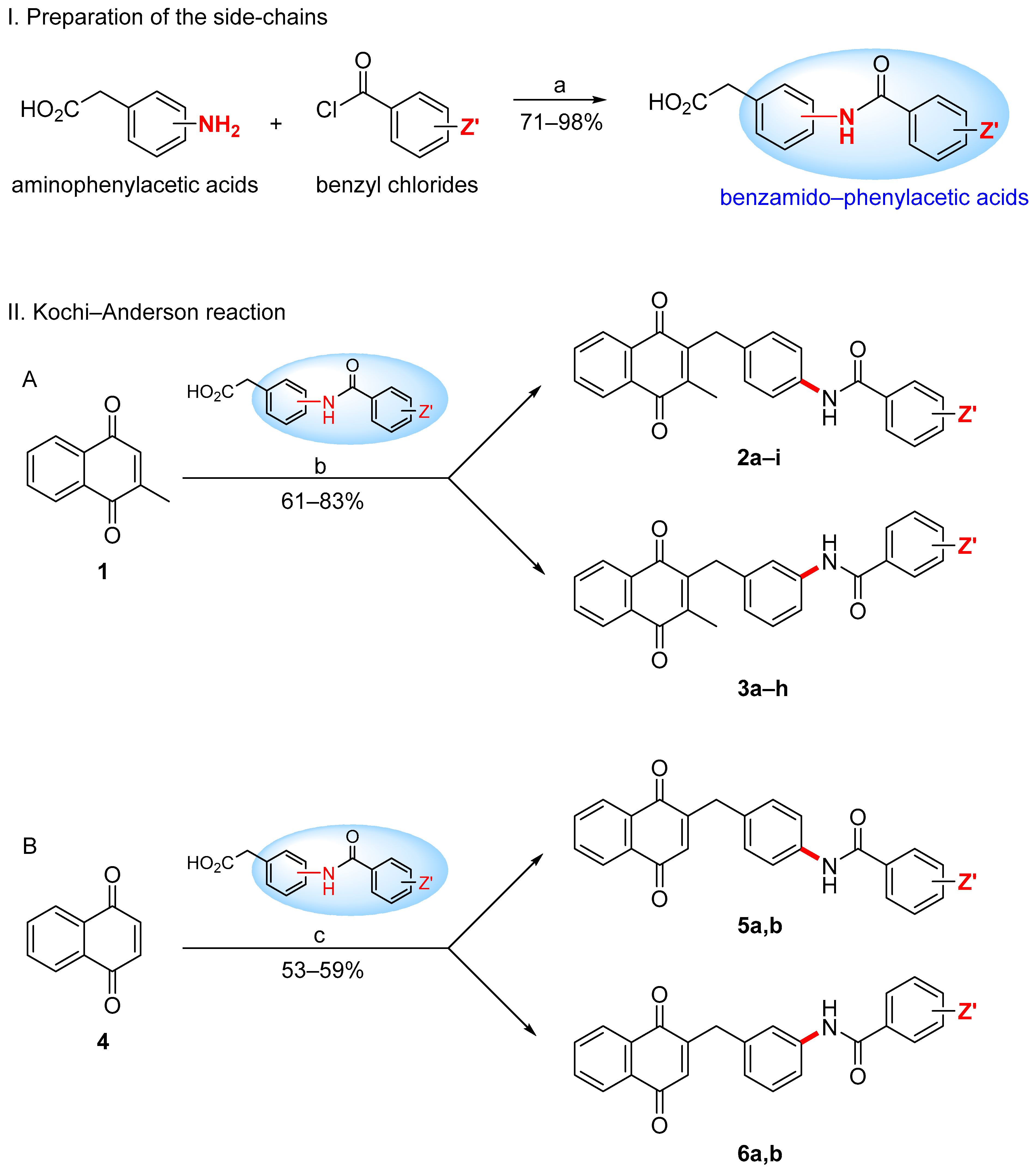
2.1. Synthetic Chemistry
2.2. Biological Evaluation
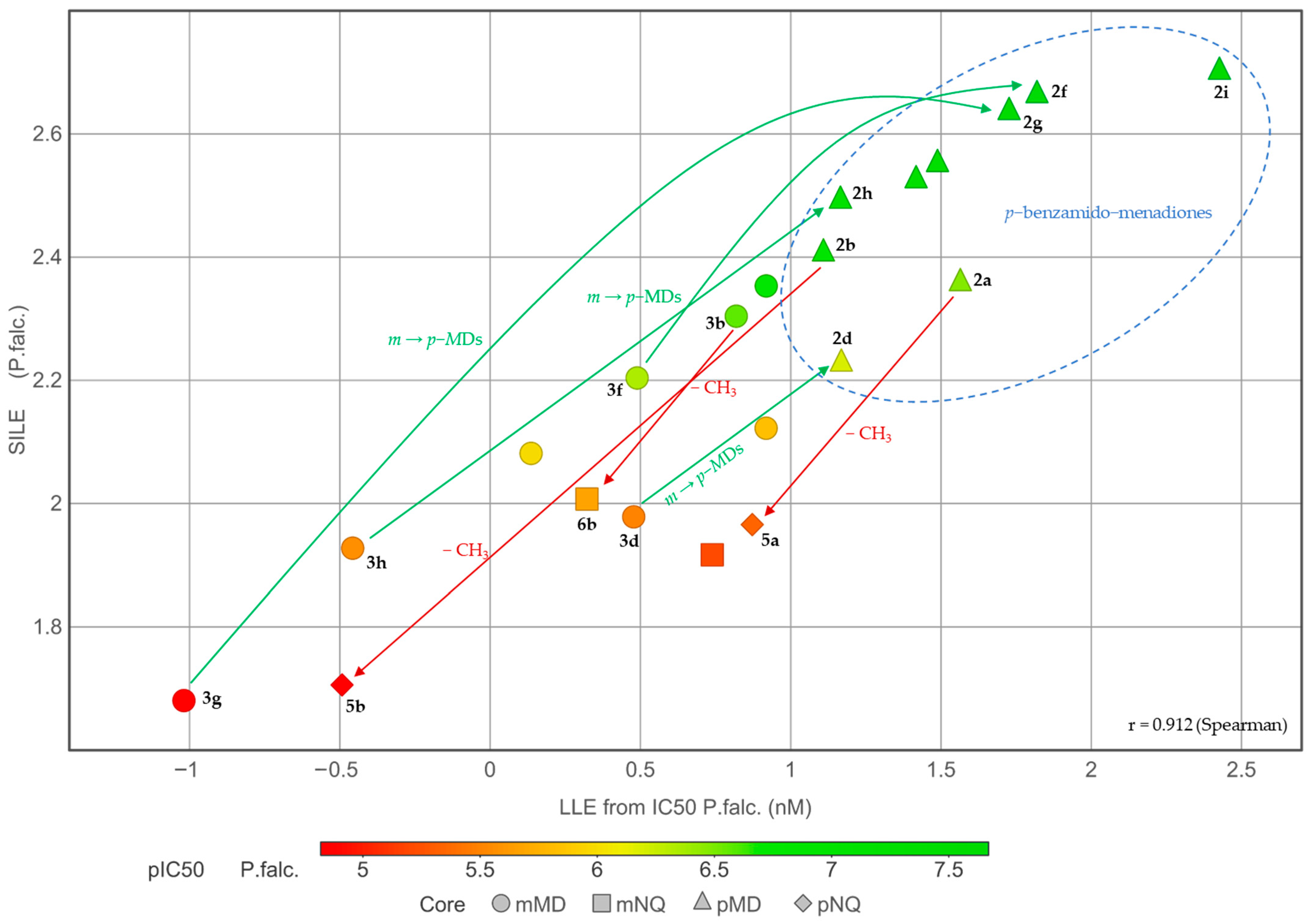
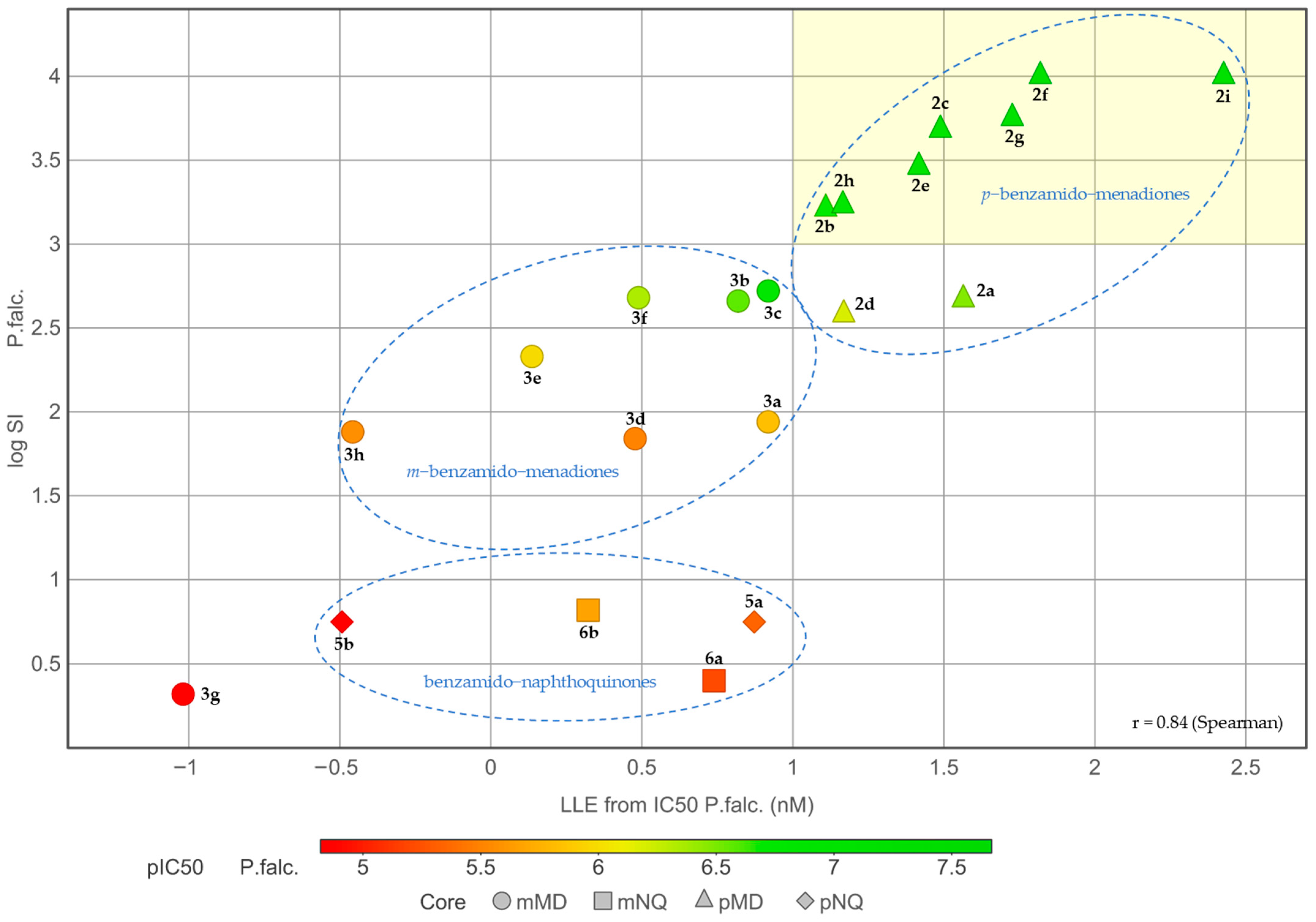
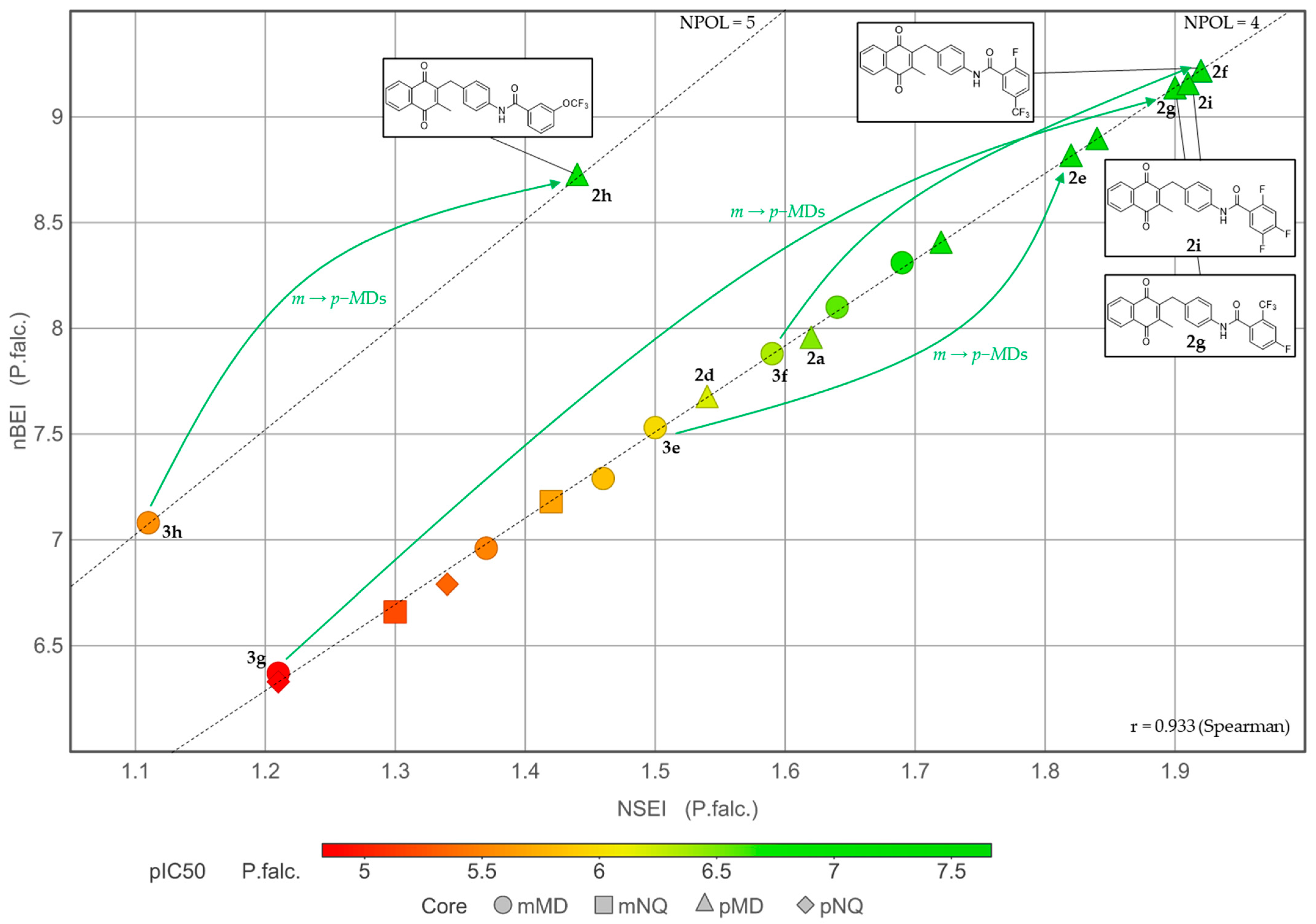
2.3. Physicochemical Investigations
2.4. Structure-Activity Relationships (SAR) of the Antiplasmodial Activity
3. Materials and Methods
3.1. Chemistry
3.1.1. General Information
3.1.2. General Synthetic Procedure for the Preparation of Benzamido-Phenylacetic Acids
3.1.3. General Synthetic Procedure for Benzamido–Menadiones 2a–i and 3a–h (Route A)
3.1.4. General Synthetic Procedure for Benzamido–Naphthoquinones 5a,b and 6a,b (Route B)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AB-MPS | AbbVie’s multi-parameter score |
| BEI | Binding efficiency index |
| FQ | Fit quality |
| H2L | Hit-to-lead |
| LE | Ligand efficiency |
| LELP | Ligand efficiency lipophilicity price |
| LipE | Lipophilic efficiency |
| LLE | Lipophilic ligand efficiency |
| LLEAT | Astex lipophilic ligand efficiency |
| MD | Menadione |
| MW | Molecular weight |
| nBEI | Normalized binding efficiency index |
| NHA | Non-hydrogen atoms |
| NPOL | Number of oxygens and nitrogens in the structure |
| NQ | Naphthoquinone |
| NSEI | Normalized (polar) surface efficiency index |
| PFI | GSK property forecast index |
| Ro5 | Rule of five |
| SEI | Surface efficiency index |
| SFI | Solubility forecast index |
| SILE | Size-independent ligand efficiency |
| TLC | Thin-layer chromatography |
| tPSA | Topological polar surface area |
| WHO | World Health Organization |
References
- Venkatesan, P. WHO world malaria report 2024. Lancet Microbe 2025, 6, 101073. [Google Scholar] [CrossRef]
- WHO Global Malaria Programme. World Malaria Report 2024. Available online: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2024 (accessed on 19 September 2025).
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef]
- Majiene, D.; Kuseliauskyte, J.; Stimbirys, A.; Jekabsone, A. Comparison of the effect of native 1,4-naphthoquinones plumbagin, menadione, and lawsone on viability, redox status, and mitochondrial functions of C6 glioblastoma cells. Nutrients 2019, 11, 1294. [Google Scholar] [CrossRef]
- de Souza, A.S.; Ribeiro, R.C.B.; Costa, D.C.S.; Pauli, F.P.; Pinho, D.R.; de Moraes, M.G.; Silva, F.d.C.d.; Forezi, L.d.S.M.; Ferreira, V.F. Menadione: A platform and a target to valuable compounds synthesis. Beilstein J. Org. Chem. 2022, 18, 381–419. [Google Scholar] [CrossRef] [PubMed]
- Dupouy, B.; Donzel, M.; Roignant, M.; Charital, S.; Keumoe, R.; Yamaryo-Botte, Y.; Feckler, A.; Bundschuh, M.; Bordat, Y.; Rottmann, M.; et al. 3-Benzylmenadiones and their Heteroaromatic Analogs Target the Apicoplast of Apicomplexa Parasites: Synthesis and Bioimaging Studies. ACS Infect. Dis. 2024, 10, 3553–3576. [Google Scholar] [CrossRef] [PubMed]
- Trometer, N.; Pecourneau, J.; Feng, L.; Navarro-Huerta, J.A.; Lazarin-Bidoia, D.; Lautenschlager, S.d.O.S.; Maes, L.; Francisco, A.F.; Kelly, J.M.; Meunier, B.; et al. Synthesis and Anti-Chagas Activity Profile of a Redox-Active Lead 3-Benzylmenadione Revealed by High-Content Imaging. ACS Infect. Dis. 2024, 10, 1808–1838. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, K.; Deregnaucourt, C.; Goetz, A.-A.; Tzanova, T.; Gallo, V.; Arese, P.; Pradines, B.; Adjalley, S.H.; Bagrel, D.; Blandin, S.; et al. The redox cycler plasmodione is a fast-acting antimalarial lead compound with pronounced activity against sexual and early asexual blood-stage parasites. Antimicrob. Agents Chemother. 2016, 60, 5146–5158. [Google Scholar] [CrossRef]
- Sidorov, P.; Desta, I.; Chesse, M.; Horvath, D.; Marcou, G.; Varnek, A.; Davioud-Charvet, E.; Elhabiri, M. Redox Polypharmacology as an Emerging Strategy to Combat Malarial Parasites. ChemMedChem 2016, 11, 1339–1351. [Google Scholar] [CrossRef]
- Mounkoro, P.; Michel, T.; Golinelli-Cohen, M.-P.; Blandin, S.; Davioud-Charvet, E.; Meunier, B. A role for the succinate dehydrogenase in the mode of action of the redox-active antimalarial drug, plasmodione. Free Radic. Biol. Med. 2021, 162, 533–541. [Google Scholar] [CrossRef]
- Iacobucci, I.; Monaco, V.; Hovasse, A.; Dupouy, B.; Keumoe, R.; Cichocki, B.; Elhabiri, M.; Meunier, B.; Strub, J.-M.; Monti, M.; et al. Proteomic Profiling of Antimalarial Plasmodione Using 3-Benz(o)ylmenadione Affinity-Based Probes. ChemBioChem 2024, 25, e202400187. [Google Scholar] [CrossRef]
- Pinto, A.V.; de Castro, S.L. The trypanocidal activity of naphthoquinones: A review. Molecules 2009, 14, 4570–4590. [Google Scholar] [CrossRef]
- Futuro, D.O.; Ferreira, V.F.; Ferreira, P.G.; Nicoletti, C.D.; Borba-Santos, L.P.; Rozental, S.; Silva, F.C.D. The Antifungal Activity of Naphthoquinones: An Integrative Review. An. Da Acad. Bras. De Ciências 2018, 90, 1187–1214. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Beteck, R.M.; Legoabe, L.J. Antimalarial application of quinones: A recent update. Eur. J. Med. Chem. 2021, 210, 113084. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, L.S.P.; Soler, K.A.A.; González, D.L.N.; Núñez, W.E.R.; Cárdenas-Chaparro, A.; Duchowicz, P.R.; Castaño, J.A.G. In Silico Antiprotozoal Evaluation of 1,4-Naphthoquinone Derivatives against Chagas and Leishmaniasis Diseases Using QSAR, Molecular Docking, and ADME Approaches. Pharmaceuticals 2022, 15, 687. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.B.; de Moraes, L.G.C.; Pacheco, P.A.F.; dos Santos, D.G.; Ribeiro, R.M.d.A.C.; Moreira, C.d.S.; da Rocha, D.R. Naphthoquinones as a Promising Class of Compounds for Facing the Challenge of Parkinson’s Disease. Pharmaceuticals 2023, 16, 1577. [Google Scholar] [CrossRef]
- Bielitza, M.; Belorgey, D.; Ehrhardt, K.; Johann, L.; Lanfranchi, D.A.; Gallo, V.; Schwarzer, E.; Mohring, F.; Jortzik, E.; Williams, D.L.; et al. Antimalarial NADPH-Consuming Redox-Cyclers As Superior Glucose-6-Phosphate Dehydrogenase Deficiency Copycats. Antioxid. Redox Signal. 2015, 22, 1337–1351. [Google Scholar] [CrossRef]
- Presser, A.; Blaser, G.; Pferschy-Wenzig, E.-M.; Kaiser, M.; Maeser, P.; Schuehly, W. Pharmacomodulation of the Redox-Active Lead Plasmodione: Synthesis of Substituted 2-Benzylnaphthoquinone Derivatives, Antiplasmodial Activities, and Physicochemical Properties. Int. J. Mol. Sci. 2025, 26, 2114. [Google Scholar] [CrossRef]
- Rodo, E.C.; Feng, L.; Jida, M.; Ehrhardt, K.; Bielitza, M.; Boilevin, J.; Lanzer, M.; Williams, D.L.; Lanfranchi, D.A.; Davioud-Charvet, E. A Platform of Regioselective Methodologies to Access Polysubstituted 2-Methyl-1,4-naphthoquinone Derivatives: Scope and Limitations. Eur. J. Org. Chem. 2016, 2016, 1982–1993. [Google Scholar] [CrossRef]
- Donzel, M.; Karabiyikli, D.; Cotos, L.; Elhabiri, M.; Davioud-Charvet, E. Direct C-H Radical Alkylation of 1,4-Quinones. Eur. J. Org. Chem. 2021, 2021, 3622–3633. [Google Scholar] [CrossRef]
- Naturale, G.; Lamblin, M.; Commandeur, C.; Felpin, F.-X.; Dessolin, J. Direct C-H Alkylation of Naphthoquinones with Amino Acids Through a Revisited Kochi-Anderson Radical Decarboxylation: Trends in Reactivity and Applications. Eur. J. Org. Chem. 2012, 2012, 5774–5788. [Google Scholar] [CrossRef]
- Billamboz, M.; Bailly, F.; Barreca, M.L.; de Luca, L.; Mouscadet, J.-F.; Calmels, C.; Andreola, M.-L.; Witvrouw, M.; Christ, F.; Debyser, Z.; et al. Design, Synthesis, and Biological Evaluation of a Series of 2-Hydroxyisoquinoline-1,3(2H,4H)-diones as Dual Inhibitors of Human Immunodeficiency Virus Type 1 Integrase and the Reverse Transcriptase RNase H Domain. J. Med. Chem. 2008, 51, 7717–7730. [Google Scholar] [CrossRef]
- Nwaka, S.; Ramirez, B.; Brun, R.; Maes, L.; Douglas, F.; Ridley, R. Advancing drug innovation for neglected diseases-criteria for lead progression. PLoS Negl. Trop. Dis. 2009, 3, e440. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, K.; Burrows, J.N.; Duncan, K.; van Huijsduijnen, R.H.; Kaneko, T.; Kita, K.; Mowbray, C.E.; Schmatz, D.; Warner, P.; Slingsby, B.T. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nat. Rev. Drug Discov. 2015, 14, 751–758. [Google Scholar] [CrossRef]
- Samby, K.; Willis, P.A.; Burrows, J.N.; Laleu, B.; Webborn, P.J.H. Actives from MMV Open Access Boxes? A suggested way forward. PLoS Pathog. 2021, 17, e1009384. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Pereira, V.S.d.S.; de Oliveira, C.B.S.; Fumagalli, F.; Emery, F.d.S.; da Silva, N.B.; de Andrade-Neto, V.F. Cytotoxicity, hemolysis and in vivo acute toxicity of 2-hydroxy-3-anilino-1,4-naphthoquinone derivatives. Toxicol. Rep. 2016, 3, 756–762. [Google Scholar] [CrossRef]
- Kombo, D.C.; LaMarche, M.J. The Logic of Chemical Optimization. J. Med. Chem. 2025, 68, 11572–11585. [Google Scholar] [CrossRef]
- Quancard, J.; Bach, A.; Borsari, C.; Craft, R.; Gnamm, C.; Gueret, S.M.; Hartung, I.V.; Koolman, H.F.; Laufer, S.; Lepri, S.; et al. The European Federation for Medicinal Chemistry and Chemical Biology (EFMC) Best Practice Initiative: Hit to Lead. ChemMedChem 2025, 20, e202400931. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Congreve, M.; Murray, C.W.; Carr, R. Fragment-based lead discovery. Nat. Rev. Drug Discov. 2004, 3, 660–672. [Google Scholar] [CrossRef]
- Shultz, M.D. Two Decades under the Influence of the Rule of Five and the Changing Properties of Approved Oral Drugs. J. Med. Chem. 2019, 62, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Tinworth, C.P.; Young, R.J. Facts, Patterns, and Principles in Drug Discovery: Appraising the Rule of 5 with Measured Physicochemical Data. J. Med. Chem. 2020, 63, 10091–10108. [Google Scholar] [CrossRef]
- Beckers, M.; Sturm, N.; Sirockin, F.; Fechner, N.; Stiefl, N. Prediction of Small-Molecule Developability Using Large-Scale In Silico ADMET Models. J. Med. Chem. 2023, 66, 14047–14060. [Google Scholar] [CrossRef]
- Davis, A.M.; Leeson, P.D. Physicochemical Properties. In The Handbook of Medicinal Chemistry: Principles and Practice; Ward, S.E., Davis, A., Eds.; The Royal Society of Chemistry: London, UK, 2023; pp. 1–39. ISBN 978-1-78801-898-2. [Google Scholar]
- Darlami, J.; Sharma, S. The role of physicochemical and topological parameters in drug design. Front. Drug Discov. 2024, 4, 1424402. [Google Scholar] [CrossRef]
- Meanwell, N.A. Improving Drug Design: An Update on Recent Applications of Efficiency Metrics, Strategies for Replacing Problematic Elements, and Compounds in Nontraditional Drug Space. Chem. Res. Toxicol. 2016, 29, 564–616. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.W.; Gallego, R.A.; Edwards, M.P. Lipophilic Efficiency as an Important Metric in Drug Design. J. Med. Chem. 2018, 61, 6401–6420. [Google Scholar] [CrossRef]
- Leeson, P.D.; Bento, A.P.; Gaulton, A.; Hersey, A.; Manners, E.J.; Radoux, C.J.; Leach, A.R. Target-Based Evaluation of “Drug-Like” Properties and Ligand Efficiencies. J. Med. Chem. 2021, 64, 7210–7230. [Google Scholar] [CrossRef]
- Nissink, J.W.M. Simple Size-Independent Measure of Ligand Efficiency. J. Chem. Inf. Model. 2009, 49, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.H.; Tounge, B.A.; Bembenek, S.D. Ligand binding efficiency: Trends, physical basis, and implications. J. Med. Chem. 2008, 51, 2432–2438. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- DeGoey, D.A.; Chen, H.-J.; Cox, P.B.; Wendt, M.D. Beyond the Rule of 5: Lessons Learned from AbbVie’s Drugs and Compound Collection. J. Med. Chem. 2018, 61, 2636–2651. [Google Scholar] [CrossRef]
- Young, R.J.; Green, D.V.S.; Luscombe, C.N.; Hill, A.P. Getting physical in drug discovery II: The impact of chromatographic hydrophobicity measurements and aromaticity. Drug Discov. Today 2011, 16, 822–830. [Google Scholar] [CrossRef]
- McKerrow, J.H.; Lipinski, C.A. The rule of five should not impede anti-parasitic drug development. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.L.; Keserue, G.M.; Leeson, P.D.; Rees, D.C.; Reynolds, C.H. The role of ligand efficiency metrics in drug discovery. Nat. Rev. Drug Discov. 2014, 13, 105–121. [Google Scholar] [CrossRef]
- Mortenson, P.N.; Murray, C.W. Assessing the lipophilicity of fragments and early hits. J. Comput.-Aided Mol. Des. 2011, 25, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Abad-Zapatero, C.; Metz, J.T. Ligand efficiency indices as guideposts for drug discovery. Drug Discov. Today 2005, 10, 464–469. [Google Scholar] [CrossRef]
- Abad-Zapatero, C.; Blasi, D. Ligand Efficiency Indices (LEIs): More than a Simple Efficiency Yardstick. Mol. Inform. 2011, 30, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Abad-Zapatero, C.; Champness, E.J.; Segall, M.D. Alternative variables in drug discovery: Promises and challenges. Future Med. Chem. 2014, 6, 577–593. [Google Scholar] [CrossRef]
- Abad-Zapatero, C. Ligand efficiency indices for effective drug discovery: A unifying vector formulation. Expert Opin. Drug Discov. 2021, 16, 763–775. [Google Scholar] [CrossRef]
| Compd | Structure | Route | Yield (%) | Compd | Structure | Route | Yield (%) |
|---|---|---|---|---|---|---|---|
| 2a | 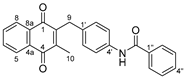 | A | 79 | 3c | 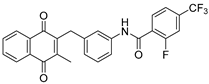 | A | 69 |
| 2b | 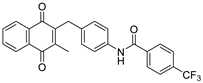 | A | 72 | 3d | 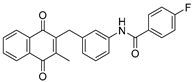 | A | 71 |
| 2c | 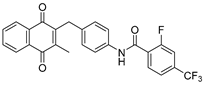 | A | 81 | 3e | 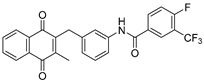 | A | 83 |
| 2d | 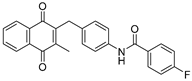 | A | 76 | 3f | 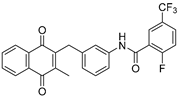 | A | 62 |
| 2e | 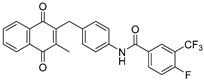 | A | 65 | 3g | 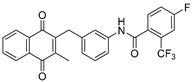 | A | 81 |
| 2f | 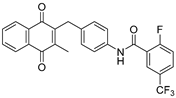 | A | 62 | 3h | 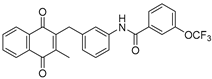 | A | 78 |
| 2g | 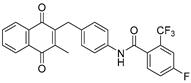 | A | 81 | 5a | 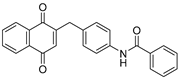 | B | 59 |
| 2h | 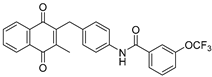 | A | 75 | 5b | 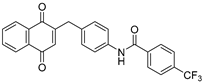 | B | 54 |
| 2i | 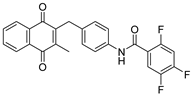 | A | 61 | 6a |  | B | 55 |
| 3a | 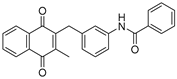 | A | 72 | 6b | 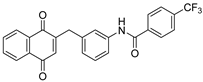 | B | 53 |
| 3b | 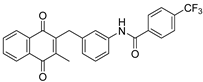 | A | 72 |
| Compd | P.falc. 1 | SI 2 | P.falc. 1 | T.b.rhod. 3 | SI 2 | T.b.rhod. 3 | Cyt L6 4 | Oral Toxicity 6 |
|---|---|---|---|---|---|---|---|---|
| IC50 μM | pIC50 5 | IC50 μM | pIC50 5 | IC50 μM | LD50 mg/kg | |||
| Chl. | 0.009 | 10,000 | 8.03 | 90 | 750 | |||
| Mel. | 0.015 | 12,000 | 7.82 | 180 | 3000 | |||
| Pod. | 0.019 | 100 | ||||||
| 2a | 0.336 | 471 | 6.47 | 64.101 | 2 | 4.19 | 158.17 | 4000 |
| 2b | 0.136 | 1639 | 6.87 | 34.582 | 6 | 4.46 | 222.51 | 1000 |
| 2c | 0.045 | 4762 | 7.35 | 160.627 | 1 | 3.79 | 213.94 | 1000 |
| 2d | 0.663 | 377 | 6.18 | 84.442 | 3 | 4.07 | 250.36 | 4000 |
| 2e | 0.053 | 2914 | 7.27 | 8.911 | 17 | 5.05 | 155.88 | 1000 |
| 2f | 0.021 | 10,000 | 7.67 | 163.537 | 1 | 3.79 | 213.94 | 1000 |
| 2g | 0.026 | 5580 | 7.59 | 32.324 | 4 | 4.49 | 143.26 | 4000 |
| 2h | 0.067 | 1685 | 7.18 | 129.636 | 1 | 3.89 | 112.22 | 57 |
| 2i | 0.023 | 10,000 | 7.64 | 171.796 | 1 | 3.76 | 229.67 | 1600 |
| 3a | 1.487 | 88 | 5.83 | 65.333 | 2 | 4.18 | 130.95 | 4000 |
| 3b | 0.265 | 453 | 6.58 | 27.689 | 4 | 4.56 | 119.82 | 4000 |
| 3c | 0.167 | 525 | 6.78 | 65.344 | 1 | 4.18 | 87.57 | 4000 |
| 3d | 3.252 | 69 | 5.49 | 53.628 | 4 | 4.27 | 225.33 | 4000 |
| 3e | 1.012 | 211 | 5.99 | 50.383 | 4 | 4.30 | 213.94 | 4000 |
| 3f | 0.449 | 476 | 6.35 | 43.195 | 5 | 4.36 | 213.94 | 4000 |
| 3g | 14.462 | 2 | 4.84 | 26.069 | 1 | 4.58 | 30.55 | 1000 |
| 3h | 2.810 | 76 | 5.55 | 94.679 | 2 | 4.02 | 214.86 | 4000 |
| 5a | 4.556 | 6 | 5.34 | 1.900 | 14 | 5.72 | 25.91 | 4000 |
| 5b | 14.975 | 6 | 4.82 | 15.016 | 6 | 4.82 | 83.62 | 1000 |
| 6a | 6.192 | 3 | 5.21 | 3.503 | 4 | 5.46 | 15.59 | 4000 |
| 6b | 2.104 | 7 | 5.68 | 5.234 | 3 | 5.28 | 14.05 | 4000 |
| Variable Name | Definition |
|---|---|
| LE | 1.37 × (pIC50/NHA) |
| SILE | pIC50/NHA0.3 |
| FQ | (pIC50/NHA)/[0.0715 + (7.5328/NHA) + (25.7079/NHA2) − (361.4722/NHA3)] |
| BEI | (pIC50 × 1000)/MW |
| SEI | (pIC50 × 100 Å2)/tPSA |
| LLE (or LipE) | pIC50 − logP (or logD7.4) |
| LLEAT | 0.11 + [(1.37 × LLE)/NHA] = 0.11 + [(LE × LLE)/pIC50] |
| LELP | logP/LE |
| nBEI | pIC50 + log10NHA |
| NSEI | pIC50/NPOL(N+O) |
| PFI (SFI) | logD7.4 + #aromrings |
| AB-MPS | |logD7.4 − 3| + #rotbonds + #aromrings |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presser, A.; Blaser, G.; Pferschy-Wenzig, E.-M.; Cal, M.; Mäser, P.; Schuehly, W. Synthesis, Antiprotozoal Activity, and Physicochemical Evaluation of Benzamido–Menadione Derivatives. Int. J. Mol. Sci. 2025, 26, 10951. https://doi.org/10.3390/ijms262210951
Presser A, Blaser G, Pferschy-Wenzig E-M, Cal M, Mäser P, Schuehly W. Synthesis, Antiprotozoal Activity, and Physicochemical Evaluation of Benzamido–Menadione Derivatives. International Journal of Molecular Sciences. 2025; 26(22):10951. https://doi.org/10.3390/ijms262210951
Chicago/Turabian StylePresser, Armin, Gregor Blaser, Eva-Maria Pferschy-Wenzig, Monica Cal, Pascal Mäser, and Wolfgang Schuehly. 2025. "Synthesis, Antiprotozoal Activity, and Physicochemical Evaluation of Benzamido–Menadione Derivatives" International Journal of Molecular Sciences 26, no. 22: 10951. https://doi.org/10.3390/ijms262210951
APA StylePresser, A., Blaser, G., Pferschy-Wenzig, E.-M., Cal, M., Mäser, P., & Schuehly, W. (2025). Synthesis, Antiprotozoal Activity, and Physicochemical Evaluation of Benzamido–Menadione Derivatives. International Journal of Molecular Sciences, 26(22), 10951. https://doi.org/10.3390/ijms262210951






