Preventive and Protective Effects of Nicotinamide Adenine Dinucleotide Boosters in Aging and Retinal Diseases
Abstract
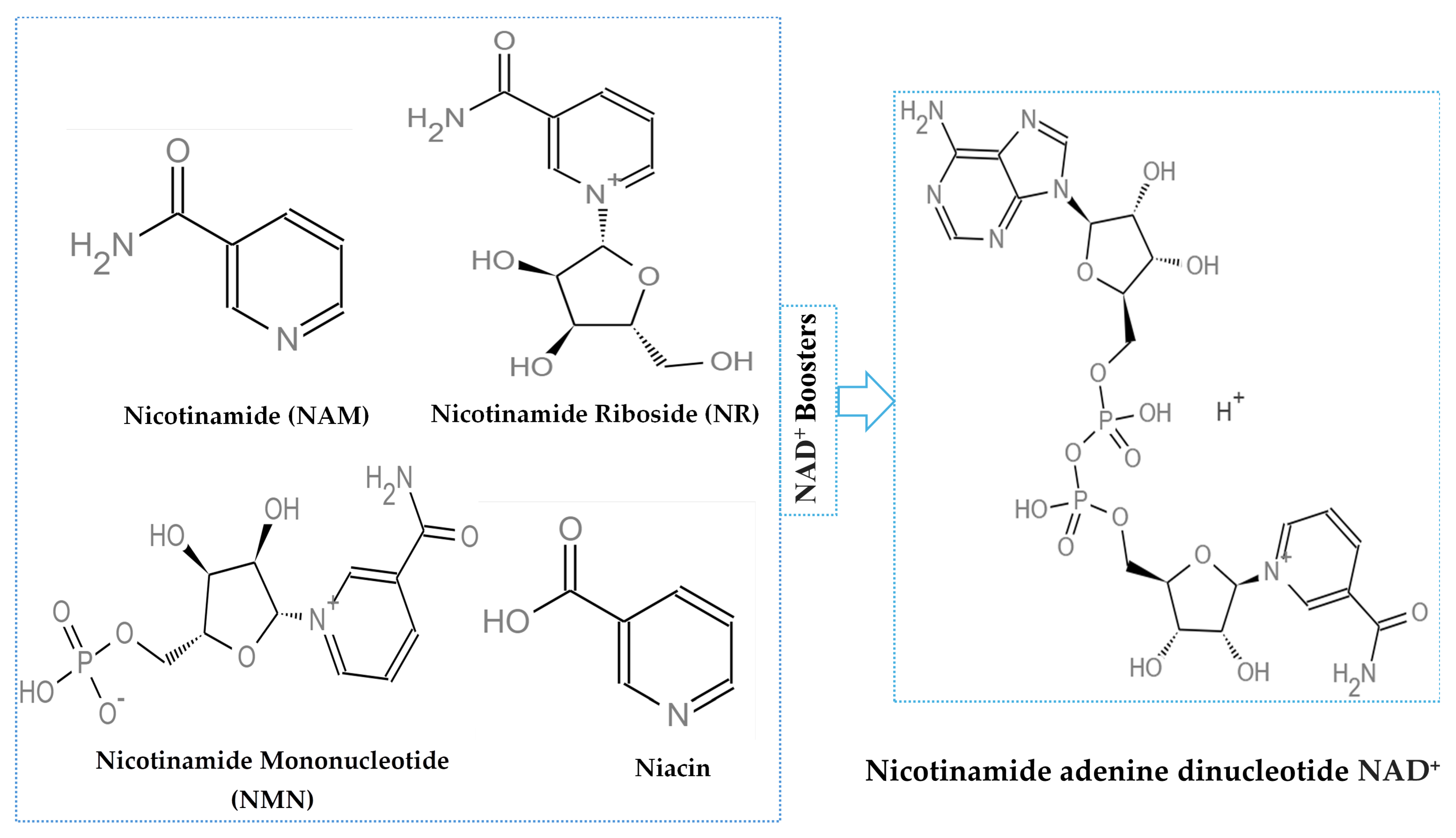
1. Introduction
2. AMD and NAD+ Boosters
3. DR and NAD+ Boosters
4. Clinical Data and Translational Challenges
5. SIRT1, SIRT3, and SIRT6 with Neuronal Protection
6. Natural Products as NAD+ Boosters
7. Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| DR | Diabetic retinopathy |
| NAD+ | Nicotinamide adenine dinucleotide |
| NAMPT | Nicotinamide phosphoribosyl transferase |
| NMNAT | Nicotinamide mononucleotide adenylyl transferase |
| NAM | Nicotinamide |
| NMN | Nicotinamide mononucleotide |
| NR | Nicotinamide riboside |
| RPE | Retinal pigment epithelium |
| CNV | Choroidal neovascularization |
| anti-VEGF | Anti-vascular endothelial growth factor |
| RGC | Retinal ganglion cell |
| BRB | Blood-retinal barrier |
| HO-1 | Heme oxygenase-1 |
| AGEs | Advanced glycation end-products |
| PKC | Protein kinase C |
| SIRT | Sirtuin |
| STZ | Streptozotocin |
| GFAP | Glial fibrillary acidic protein |
| ROS | Reactive oxygen species |
| IP | Intraperitoneal |
| GSPE | Grape seed proanthocyanidin extract |
| PDR | Proliferative DR |
| HIF | Hypoxia inducible factor |
References
- Country, M.W. Retinal metabolism: A comparative look at energetics in the retina. Brain Res. 2017, 1672, 50–57. [Google Scholar] [CrossRef]
- Du, J.; Rountree, A.; Cleghorn, W.M.; Contreras, L.; Lindsay, K.J.; Sadilek, M.; Gu, H.; Djukovic, D.; Raftery, D.; Satrústegui, J. Phototransduction influences metabolic flux and nucleotide metabolism in mouse retina. J. Biol. Chem. 2016, 291, 4698–4710. [Google Scholar] [CrossRef]
- Choi, Y.K. An altered neurovascular system in aging-related eye diseases. Int. J. Mol. Sci. 2022, 23, 14104. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Uusitalo, H.; Blasiak, J.; Felszeghy, S.; Kannan, R.; Kauppinen, A.; Salminen, A.; Sinha, D.; Ferrington, D. Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 2020, 79, 100858. [Google Scholar] [CrossRef]
- Campochiaro, P.A. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog. Retin. Eye Res. 2015, 49, 67–81. [Google Scholar] [CrossRef]
- Julius, A.; Malakondaiah, S.; Subbarayalu, R.; Renuka, R.R.; Pothireddy, R.B. Multi-Target Drug Strategies in the Treatment of Diabetic Retinopathy. SN Compr. Clin. Med. 2025, 7, 170. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Chini, C.C.; Peclat, T.R.; Warner, G.M.; Kashyap, S.; Espindola-Netto, J.M.; de Oliveira, G.C.; Gomez, L.S.; Hogan, K.A.; Tarragó, M.G.; Puranik, A.S. CD38 ecto-enzyme in immune cells is induced during aging and regulates NAD+ and NMN levels. Nat. Metab. 2020, 2, 1284–1304. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Gábriel, R.; Kovács-Valasek, A. Poly (ADP-Ribose) Polymerase-1 (PARP-1) Inhibitors in Diabetic Retinopathy: An Attractive but Elusive Choice for Drug Development. Pharmaceutics 2024, 16, 1320. [Google Scholar] [CrossRef] [PubMed]
- Verdin, E. NAD+ in aging, metabolism, and neurodegeneration. Science 2015, 350, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G.J. Age-associated changes in oxidative stress and NAD+ metabolism in human tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Tovar-Palacio, C.; Noriega, L.G.; Mercado, A. Potential of polyphenols to restore SIRT1 and NAD+ metabolism in renal disease. Nutrients 2022, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Mouchiroud, L.; Houtkooper, R.H.; Moullan, N.; Katsyuba, E.; Ryu, D.; Cantó, C.; Mottis, A.; Jo, Y.-S.; Viswanathan, M.; Schoonjans, K. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 2013, 154, 430–441. [Google Scholar] [CrossRef]
- Lin, J.B.; Apte, R.S. NAD+ and sirtuins in retinal degenerative diseases: A look at future therapies. Prog. Retin. Eye Res. 2018, 67, 118–129. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S.-i. NAD+ intermediates: The biology and therapeutic potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Trammell, S.A.; Schmidt, M.S.; Weidemann, B.J.; Redpath, P.; Jaksch, F.; Dellinger, R.W.; Li, Z.; Abel, E.D.; Migaud, M.E.; Brenner, C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016, 7, 12948. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Fan, B.; Li, Y.; Song, D.; Li, G.Y. PARP-1 Is a Potential Marker of Retinal Photooxidation and a Key Signal Regulator in Retinal Light Injury. Oxidative Med. Cell. Longev. 2022, 2022, 6881322. [Google Scholar] [CrossRef] [PubMed]
- Chini, C.; Hogan, K.A.; Warner, G.M.; Tarragó, M.G.; Peclat, T.R.; Tchkonia, T.; Kirkland, J.L.; Chini, E. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD+ decline. Biochem. Biophys. Res. Commun. 2019, 513, 486–493. [Google Scholar] [CrossRef]
- Miao, X.; Wu, Q.; Du, S.; Xiang, L.; Zhou, S.; Zhu, J.; Chen, Z.; Wang, H.; Pan, X.; Fan, Y.; et al. SARM1 Promotes Neurodegeneration and Memory Impairment in Mouse Models of Alzheimer’s Disease. Aging Dis. 2024, 15, 390–407. [Google Scholar] [CrossRef]
- Khan, A.H.; Chowers, I.; Lotery, A.J. Beyond the Complement Cascade: Insights into Systemic Immunosenescence and Inflammaging in Age-Related Macular Degeneration and Current Barriers to Treatment. Cells 2023, 12, 1708. [Google Scholar] [CrossRef]
- Gomes, A.P.; Price, N.L.; Ling, A.J.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and aging: Signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, L.; Tong, P.; Chen, J.; Wang, C.; Wang, Z.; Liu, J.; Duan, P.; Jiang, Q.; Zhou, Y.; et al. Nicotinamide Riboside Mitigates Retinal Degeneration by Suppressing Damaged DNA-Stimulated Microglial Activation and STING-Mediated Pyroptosis. Investig. Ophthalmol. Vis. Sci. 2025, 66, 14. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Choi, S.; Bikkannavar, P.; Cordeiro, M.F. Microglia: Key Players in Retinal Ageing and Neurodegeneration. Front. Cell. Neurosci. 2022, 16, 804782. [Google Scholar] [CrossRef]
- Croft, T.; Venkatakrishnan, P.; Lin, S.-J. NAD+ metabolism and regulation: Lessons from yeast. Biomolecules 2020, 10, 330. [Google Scholar] [CrossRef]
- Tarragó, M.G.; Chini, C.C.; Kanamori, K.S.; Warner, G.M.; Caride, A.; de Oliveira, G.C.; Rud, M.; Samani, A.; Hein, K.Z.; Huang, R. A potent and specific CD38 inhibitor ameliorates age-related metabolic dysfunction by reversing tissue NAD+ decline. Cell Metab. 2018, 27, 1081–1095.e1010. [Google Scholar] [CrossRef]
- Singh, M.; Negi, R.; Alka; Vinayagam, R.; Kang, S.G.; Shukla, P. Age-Related Macular Degeneration (AMD): Pathophysiology, Drug Targeting Approaches, and Recent Developments in Nanotherapeutics. Medicina 2024, 60, 1647. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Pennesi, M.E.; Neuringer, M.; Courtney, R.J. Animal models of age related macular degeneration. Mol. Asp. Med. 2012, 33, 487–509. [Google Scholar] [CrossRef]
- Ren, C.; Hu, C.; Wu, Y.; Li, T.; Zou, A.; Yu, D.; Shen, T.; Cai, W.; Yu, J. Nicotinamide Mononucleotide Ameliorates Cellular Senescence and Inflammation Caused by Sodium Iodate in RPE. Oxidative Med. Cell. Longev. 2022, 2022, 5961123. [Google Scholar] [CrossRef]
- Du, J.; Yanagida, A.; Knight, K.; Engel, A.L.; Vo, A.H.; Jankowski, C.; Sadilek, M.; Tran, V.T.; Manson, M.A.; Ramakrishnan, A.; et al. Reductive carboxylation is a major metabolic pathway in the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2016, 113, 14710–14715. [Google Scholar] [CrossRef]
- Saini, J.S.; Corneo, B.; Miller, J.D.; Kiehl, T.R.; Wang, Q.; Boles, N.C.; Blenkinsop, T.A.; Stern, J.H.; Temple, S. Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell 2017, 20, 635–647.e637. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Powell, F.L.; Jones, M.A.; Fuller, J.; Joseph, E.; Thounaojam, M.C.; Bartoli, M.; Martin, P.M. Loss of NAMPT in aging retinal pigment epithelium reduces NAD(+) availability and promotes cellular senescence. Aging 2018, 10, 1306–1323. [Google Scholar] [CrossRef]
- Wondmeneh, T.G.; Mohammed, J.A. Prevalence of diabetic retinopathy and its associated risk factors among adults in Ethiopia: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 28266. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.L.; Tham, Y.-C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y. Global prevalence of diabetic retinopathy and projection of burden through 2045: Systematic review and meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Tang, P.; Lv, H. Targeting oxidative stress in diabetic retinopathy: Mechanisms, pathology, and novel treatment approaches. Front. Immunol. 2025, 16, 1571576. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.; Park, S.J.; Song, M. Diabetic Retinopathy (DR): Mechanisms, Current Therapies, and Emerging Strategies. Cells 2025, 14, 376. [Google Scholar] [CrossRef]
- Kurihara, T.; Westenskow, P.D.; Friedlander, M. Hypoxia-inducible factor (HIF)/vascular endothelial growth factor (VEGF) signaling in the retina. Adv. Exp. Med. Biol. 2014, 801, 275–281. [Google Scholar] [CrossRef]
- Kurihara, T. Roles of Hypoxia Response in Retinal Development and Pathophysiology. Keio J. Med. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Zhang, D.; Lv, F.L.; Wang, G.H. Effects of HIF-1α on diabetic retinopathy angiogenesis and VEGF expression. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5071–5076. [Google Scholar] [CrossRef]
- Tomita, Y.; Lee, D.; Tsubota, K.; Kurihara, T. PPARα Agonist Oral Therapy in Diabetic Retinopathy. Biomedicines 2020, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.I.; Han, J.-S.; Park, C.K. Neuroprotective effects of nicotinamide (vitamin B3) on neurodegeneration in diabetic rat retinas. Nutrients 2022, 14, 1162. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.G.; Dinischiotu, A.; Soare, T.; Vlase, E.; Marinescu, G.C. Nicotinamide Mononucleotide (NMN) Works in Type 2 Diabetes through Unexpected Effects in Adipose Tissue, Not by Mitochondrial Biogenesis. Int. J. Mol. Sci. 2024, 25, 2594. [Google Scholar] [CrossRef]
- Yamane, T.; Imai, M.; Bamba, T.; Uchiyama, S. Nicotinamide mononucleotide (NMN) intake increases plasma NMN and insulin levels in healthy subjects. Clin. Nutr. ESPEN 2023, 56, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Meng, X.; Yang, X.; Xie, C.; Tian, C.; Gong, J.; Zhang, J.; Dai, S.; Gao, T. The protection of nicotinamide riboside against diabetes mellitus-induced bone loss via OXPHOS. Bone 2025, 193, 117411. [Google Scholar] [CrossRef]
- Elhassan, Y.S.; Kluckova, K.; Fletcher, R.S.; Schmidt, M.S.; Garten, A.; Doig, C.L.; Cartwright, D.M.; Oakey, L.; Burley, C.V.; Jenkinson, N.; et al. Nicotinamide Riboside Augments the Aged Human Skeletal Muscle NAD(+) Metabolome and Induces Transcriptomic and Anti-inflammatory Signatures. Cell Rep. 2019, 28, 1717–1728.e1716. [Google Scholar] [CrossRef]
- Sun, X.; Huang, W.; Yin, D.; Zhao, X.; Cheng, X.; Zhang, J.; Hao, Y. Nicotinamide riboside activates SIRT3 to prevent paclitaxel-induced peripheral neuropathy. Toxicol. Appl. Pharmacol. 2024, 491, 117066. [Google Scholar] [CrossRef]
- Freeberg, K.A.; Udovich, C.C.; Martens, C.R.; Seals, D.R.; Craighead, D.H. Dietary supplementation with NAD+-boosting compounds in humans: Current knowledge and future directions. J. Gerontol. Ser. A 2023, 78, 2435–2448. [Google Scholar] [CrossRef]
- Henderson, J.D.; Quigley, S.N.; Chachra, S.S.; Conlon, N.; Ford, D. The use of a systems approach to increase NAD+ in human participants. NPJ Aging 2024, 10, 7. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, X.; Xu, K.; Liu, S.; Zhu, X.; Yang, J. The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: An Update. Adv. Nutr. 2023, 14, 1416–1435. [Google Scholar] [CrossRef]
- Figley, M.D.; Gu, W.; Nanson, J.D.; Shi, Y.; Sasaki, Y.; Cunnea, K.; Malde, A.K.; Jia, X.; Luo, Z.; Saikot, F.K.; et al. SARM1 is a metabolic sensor activated by an increased NMN/NAD(+) ratio to trigger axon degeneration. Neuron 2021, 109, 1118–1136.e1111. [Google Scholar] [CrossRef] [PubMed]
- Waller, T.J.; Collins, C.A. An NAD+/NMN balancing act by SARM1 and NMNAT2 controls axonal degeneration. Neuron 2021, 109, 1067–1069. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.L. Is SIRT6 activity neuroprotective and how does it differ from SIRT1 in this regard? Front. Cell. Neurosci. 2017, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Tribble, J.R.; Hagström, A.; Jusseaume, K.; Lardner, E.; Wong, R.C.-B.; Stålhammar, G.; Williams, P.A. NAD salvage pathway machinery expression in normal and glaucomatous retina and optic nerve. Acta Neuropathol. Commun. 2023, 11, 18. [Google Scholar] [CrossRef]
- Chaqour, B.; Rossman, J.B.; Meng, M.; Dine, K.E.; Ross, A.G.; Shindler, K.S. SIRT1-based therapy targets a gene program involved in mitochondrial turnover in a model of retinal neurodegeneration. Sci. Rep. 2025, 15, 13585. [Google Scholar] [CrossRef]
- Mishra, M.; Duraisamy, A.J.; Kowluru, R.A. Sirt1: A Guardian of the Development of Diabetic Retinopathy. Diabetes 2018, 67, 745–754. [Google Scholar] [CrossRef]
- Adu-Agyeiwaah, Y.; Vieira, C.P.; Asare-Bediako, B.; Li Calzi, S.; DuPont, M.; Floyd, J.; Boye, S.; Chiodo, V.; Busik, J.V.; Grant, M.B. Intravitreal Administration of AAV2-SIRT1 Reverses Diabetic Retinopathy in a Mouse Model of Type 2 Diabetes. Transl. Vis. Sci. Technol. 2023, 12, 20. [Google Scholar] [CrossRef]
- Ban, N.; Ozawa, Y.; Osada, H.; Lin, J.B.; Toda, E.; Watanabe, M.; Yuki, K.; Kubota, S.; Apte, R.S.; Tsubota, K. Neuroprotective role of retinal SIRT3 against acute photo-stress. NPJ Aging Mech. Dis. 2017, 3, 19. [Google Scholar] [CrossRef]
- Shi, J.; Liu, M.; Zhu, H.; Jiang, C. SIRT3 mitigates high glucose-induced damage in retinal microvascular endothelial cells via OPA1-mediated mitochondrial dynamics. Exp. Cell Res. 2025, 444, 114320. [Google Scholar] [CrossRef]
- Huang, L.; Yao, T.; Chen, J.; Zhang, Z.; Yang, W.; Gao, X.; Dan, Y.; He, Y. Effect of Sirt3 on retinal pigment epithelial cells in high glucose through Foxo3a/PINK1-Parkin pathway mediated mitophagy. Exp. Eye Res. 2022, 218, 109015. [Google Scholar] [CrossRef]
- Xia, F.; Shi, S.; Palacios, E.; Liu, W.; Buscho, S.E.; Li, J.; Huang, S.; Vizzeri, G.; Dong, X.C.; Motamedi, M.; et al. Sirt6 protects retinal ganglion cells and optic nerve from degeneration during aging and glaucoma. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 1760–1778. [Google Scholar] [CrossRef]
- Ma, L.; Dou, H.L.; Wu, Y.Q.; Huang, Y.M.; Huang, Y.B.; Xu, X.R.; Zou, Z.Y.; Lin, X.M. Lutein and zeaxanthin intake and the risk of age-related macular degeneration: A systematic review and meta-analysis. Br. J. Nutr. 2012, 107, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cho, E.; Willett, W.C.; Sastry, S.M.; Schaumberg, D.A. Intakes of Lutein, Zeaxanthin, and Other Carotenoids and Age-Related Macular Degeneration During 2 Decades of Prospective Follow-up. JAMA Ophthalmol. 2015, 133, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Angelia, M.; Amelia, Y.S.; Pratama, K.G. Mediterranean diet as a modifiable risk factor for age-related macular degeneration: A systematic review and meta-analysis. Tzu Chi Med. J. 2024, 36, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.C.; Li, H.; Yu, M.; Chong, C.C.Y.; Fan, Q.; Tham, Y.C.; Cheung, C.M.G.; Wong, T.Y.; Chew, E.Y.; Cheng, C.Y. Omega-3 Fatty Acids as Protective Factors for Age-Related Macular Degeneration: Prospective Cohort and Mendelian Randomization Analyses. Ophthalmology 2025, 132, 598–609. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; Telis, N.; German, J.B.; Hammock, B.D. Dietary omega-3 fatty acids aid in the modulation of inflammation and metabolic health. Calif. Agric. 2011, 65, 106–111. [Google Scholar] [CrossRef]
- Chew, E.Y. Dietary Intake of Omega-3 Fatty Acids From Fish and Risk of Diabetic Retinopathy. JAMA 2017, 317, 2226–2227. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Vinagre, I.; Cofán, M.; Lázaro, I.; Alé-Chilet, A.; Barraso, M.; Hernandez, T.; Harris, W.S.; Zarranz-Ventura, J.; Ortega, E. Blood omega-3 biomarkers, diabetic retinopathy and retinal vessel status in patients with type 1 diabetes. Eye 2025, 39, 1526–1531. [Google Scholar] [CrossRef]
- Tian, S.; Guo, T.; Qian, F.; Qiu, Z.; Lu, Q.; Li, R.; Zhu, K.; Li, L.; Yu, H.; Li, R.; et al. Fish Oil, Plasma n-3 PUFAs, and Risk of Macro- and Microvascular Complications Among Individuals With Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2025, 110, e1687–e1696. [Google Scholar] [CrossRef]
- Wan, W.; Zhu, W.; Wu, Y.; Long, Y.; Liu, H.; Wan, W.; Wan, G.; Yu, J. Grape Seed Proanthocyanidin Extract Moderated Retinal Pigment Epithelium Cellular Senescence Through NAMPT/SIRT1/NLRP3 Pathway. J. Inflamm. Res. 2021, 14, 3129–3143. [Google Scholar] [CrossRef]
- Guo, C.; Huang, Q.; Wang, Y.; Yao, Y.; Li, J.; Chen, J.; Wu, M.; Zhang, Z.; E, M.; Qi, H.; et al. Therapeutic application of natural products: NAD(+) metabolism as potential target. Phytomed. Int. J. Phytother. Phytopharm. 2023, 114, 154768. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Xu, Y.; Xue, L.; Zhang, W.; Gu, L.; Liu, Q. Resveratrol protects against diabetic retinal ganglion cell damage by activating the Nrf2 signaling pathway. Heliyon 2024, 10, e30786. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Hu, L.; Xu, H.; Fang, J.; Zhong, H. Resveratrol alleviates reactive oxygen species and inflammation in diabetic retinopathy via SIRT1/HMGB1 pathway-mediated ferroptosis. Toxicol. Appl. Pharmacol. 2025, 495, 117214. [Google Scholar] [CrossRef]
- Soufi, F.G.; Mohammad-Nejad, D.; Ahmadieh, H. Resveratrol improves diabetic retinopathy possibly through oxidative stress—Nuclear factor κB—Apoptosis pathway. Pharmacol. Rep. 2012, 64, 1505–1514. [Google Scholar] [CrossRef]
- Li, S.; Gaur, U.; Chong, C.M.; Lin, S.; Fang, J.; Zeng, Z.; Wang, H.; Zheng, W. Berberine Protects Human Retinal Pigment Epithelial Cells from Hydrogen Peroxide-Induced Oxidative Damage through Activation of AMPK. Int. J. Mol. Sci. 2018, 19, 1736. [Google Scholar] [CrossRef]
- Hu, R.Y.; Qi, S.M.; Wang, Y.J.; Li, W.L.; Zou, W.C.; Wang, Z.; Ren, S.; Li, W. Ginsenoside Rg3 Improved Age-Related Macular Degeneration Through Inhibiting ROS-Mediated Mitochondrion-Dependent Apoptosis In Vivo and In Vitro. Int. J. Mol. Sci. 2024, 25, 1414. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Du, X.; Wang, P.; Cui, J.; Xu, J.; Gu, J.; Zhang, T.; Chen, Y. Combination of ginsenoside Rb1 and Rd protects the retina against bright light-induced degeneration. Sci. Rep. 2017, 7, 6015. [Google Scholar] [CrossRef]
- Chang, J.; Wang, Y.; Xu, J.; Du, X.; Cui, J.; Zhang, T.; Chen, Y. Ginsenoside Re Mitigates Photooxidative Stress-Mediated Photoreceptor Degeneration and Retinal Inflammation. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2023, 18, 397–412. [Google Scholar] [CrossRef]
- Lee, H.; Nguyen Hoang, A.T.; Lee, S.J. Ginsenoside protopanaxadiol protects adult retinal pigment epithelial-19 cells from chloroquine by modulating autophagy and apoptosis. PLoS ONE 2022, 17, e0274763. [Google Scholar] [CrossRef]
- Yang, C.C.; Chen, C.Y.; Wu, C.C.; Koo, M.; Yu, Z.R.; Wang, B.J. Panax ginseng Fraction F3 Extracted by Supercritical Carbon Dioxide Protects against Oxidative Stress in ARPE-19 Cells. Int. J. Mol. Sci. 2016, 17, 1717. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yu, H.; Li, M.; Hang, L.; Xu, X. Apigenin Protects Mouse Retina against Oxidative Damage by Regulating the Nrf2 Pathway and Autophagy. Oxidative Med. Cell. Longev. 2020, 2020, 9420704. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, H.; Tai, Z.; Li, T.; Luo, L.; Tong, Z.; Zhu, W. Apigenin and Ethaverine Hydrochloride Enhance Retinal Vascular Barrier In Vitro and In Vivo. Transl. Vis. Sci. Technol. 2020, 9, 8. [Google Scholar] [CrossRef]
- Li, P.; Fang, R.L.; Wang, W.; Zeng, X.X.; Lan, T.; Liu, S.Y.; Hu, Y.J.; Shen, Q.; Wang, S.W.; Tong, Y.H.; et al. Apigenin suppresses epithelial-mesenchymal transition in high glucose-induced retinal pigment epithelial cell by inhibiting CBP/p300-mediated histone acetylation. Biochem. Biophys. Res. Commun. 2024, 717, 150061. [Google Scholar] [CrossRef] [PubMed]
- Shoda, C.; Lee, D.; Miwa, Y.; Yamagami, S.; Nakashizuka, H.; Nimura, K.; Okamoto, K.; Kawagishi, H.; Negishi, K.; Kurihara, T. Inhibition of hypoxia-inducible factors suppresses subretinal fibrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2024, 38, e23792. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Zhang, J.; Xu, G.T.; Zhang, J. Molecular pathogenesis of subretinal fibrosis in neovascular AMD focusing on epithelial-mesenchymal transformation of retinal pigment epithelium. Neurobiol. Dis. 2023, 185, 106250. [Google Scholar] [CrossRef]
- Miao, L.; Cheong, M.S.; Zhou, C.; Farag, M.; Cheang, W.S.; Xiao, J. Apigenin alleviates diabetic endothelial dysfunction through activating AMPK/PI3K/Akt/eNOS and Nrf2/HO-1 signaling pathways. Food Front. 2023, 4, 420–431. [Google Scholar] [CrossRef]
- Cao, X.; Liu, M.; Tuo, J.; Shen, D.; Chan, C.C. The effects of quercetin in cultured human RPE cells under oxidative stress and in Ccl2/Cx3cr1 double deficient mice. Exp. Eye Res. 2010, 91, 15–25. [Google Scholar] [CrossRef]
- Chai, G.R.; Liu, S.; Yang, H.W.; Chen, X.L. Quercetin protects against diabetic retinopathy in rats by inducing heme oxygenase-1 expression. Neural Regen. Res. 2021, 16, 1344–1350. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Y.; Li, M.; Li, J. Quercetin protects against hyperglycemia-induced retinopathy in Sprague Dawley rats by regulating the gut-retina axis and nuclear factor erythroid-2-related factor 2 pathway. Nutr. Res. 2024, 122, 55–67. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Noguchi, J.L.; Seu, M.Y.; Qiao, J.B.; Tan, I.R.; Swaminathan, S.R.; McDonnell, J.F.; Tan, Z.; Bu, P. Kaempferol Protects Against Retinal Photoreceptor Degeneration in a Mouse Model of Light-Induced Retinal Injury. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2023, 39, 80–85. [Google Scholar] [CrossRef]
- Ibuki, M.; Lee, D.; Shinojima, A.; Miwa, Y.; Tsubota, K.; Kurihara, T. Rice Bran and Vitamin B6 Suppress Pathological Neovascularization in a Murine Model of Age-Related Macular Degeneration as Novel HIF Inhibitors. Int. J. Mol. Sci. 2020, 21, 8940. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Kashii, S.; Zhao, L.; Tonchev, A.B.; Katsuki, H.; Akaike, A.; Honda, Y.; Yamashita, J.; Yamashima, T. Vitamin B6 protects primate retinal neurons from ischemic injury. Brain Res. 2002, 940, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res. 2024, 53, D1516–D1525. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noreen, S.; Lim, S.S.; Lee, D. Preventive and Protective Effects of Nicotinamide Adenine Dinucleotide Boosters in Aging and Retinal Diseases. Int. J. Mol. Sci. 2025, 26, 10923. https://doi.org/10.3390/ijms262210923
Noreen S, Lim SS, Lee D. Preventive and Protective Effects of Nicotinamide Adenine Dinucleotide Boosters in Aging and Retinal Diseases. International Journal of Molecular Sciences. 2025; 26(22):10923. https://doi.org/10.3390/ijms262210923
Chicago/Turabian StyleNoreen, Saba, Soon Sung Lim, and Deokho Lee. 2025. "Preventive and Protective Effects of Nicotinamide Adenine Dinucleotide Boosters in Aging and Retinal Diseases" International Journal of Molecular Sciences 26, no. 22: 10923. https://doi.org/10.3390/ijms262210923
APA StyleNoreen, S., Lim, S. S., & Lee, D. (2025). Preventive and Protective Effects of Nicotinamide Adenine Dinucleotide Boosters in Aging and Retinal Diseases. International Journal of Molecular Sciences, 26(22), 10923. https://doi.org/10.3390/ijms262210923






