Perspective for Modulation of Hypothalamic Neurogenesis: Integrating Anatomical Insights with Exercise and Dietary Interventions
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Anatomy of the Hypothalamus and the Third Ventricle
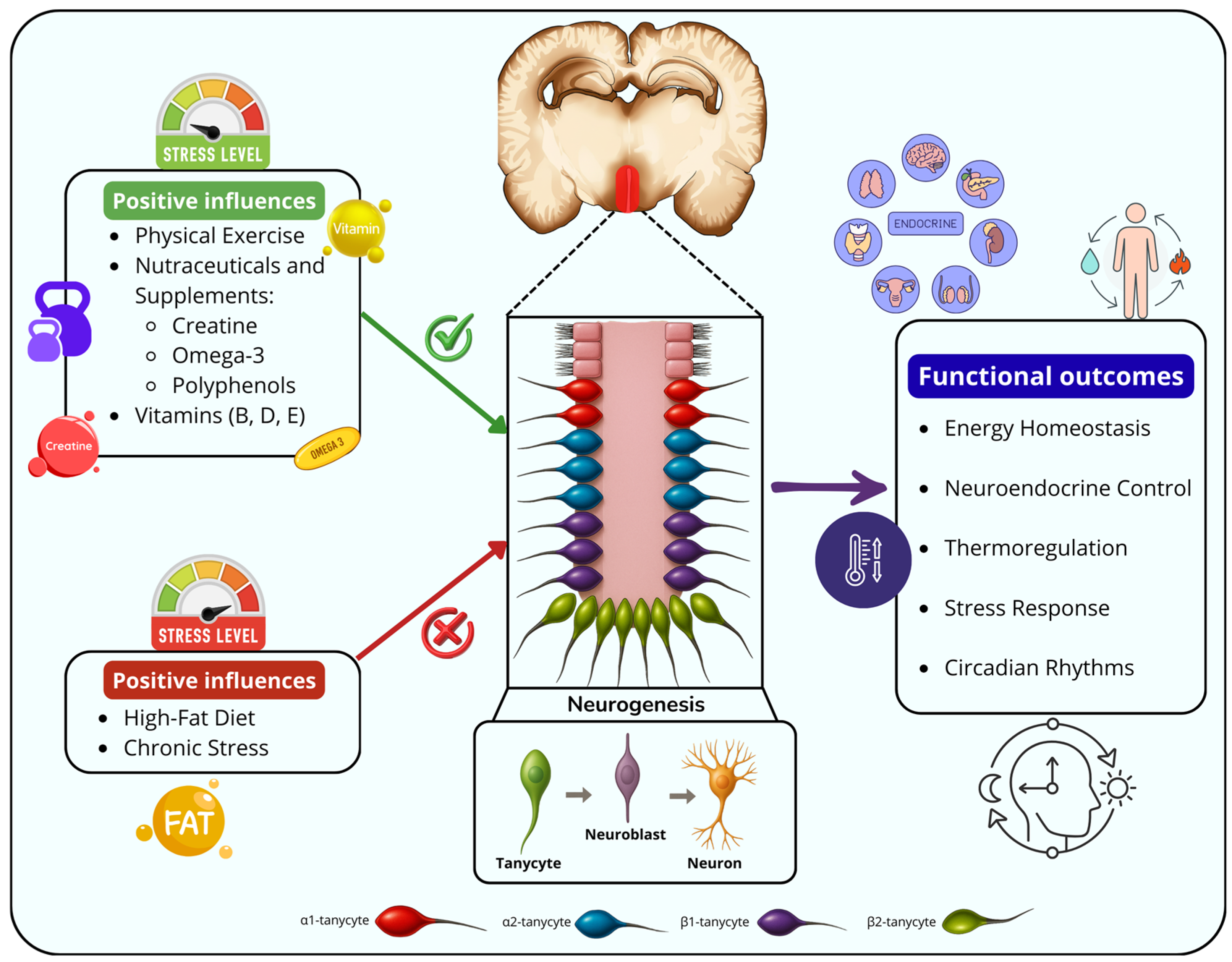
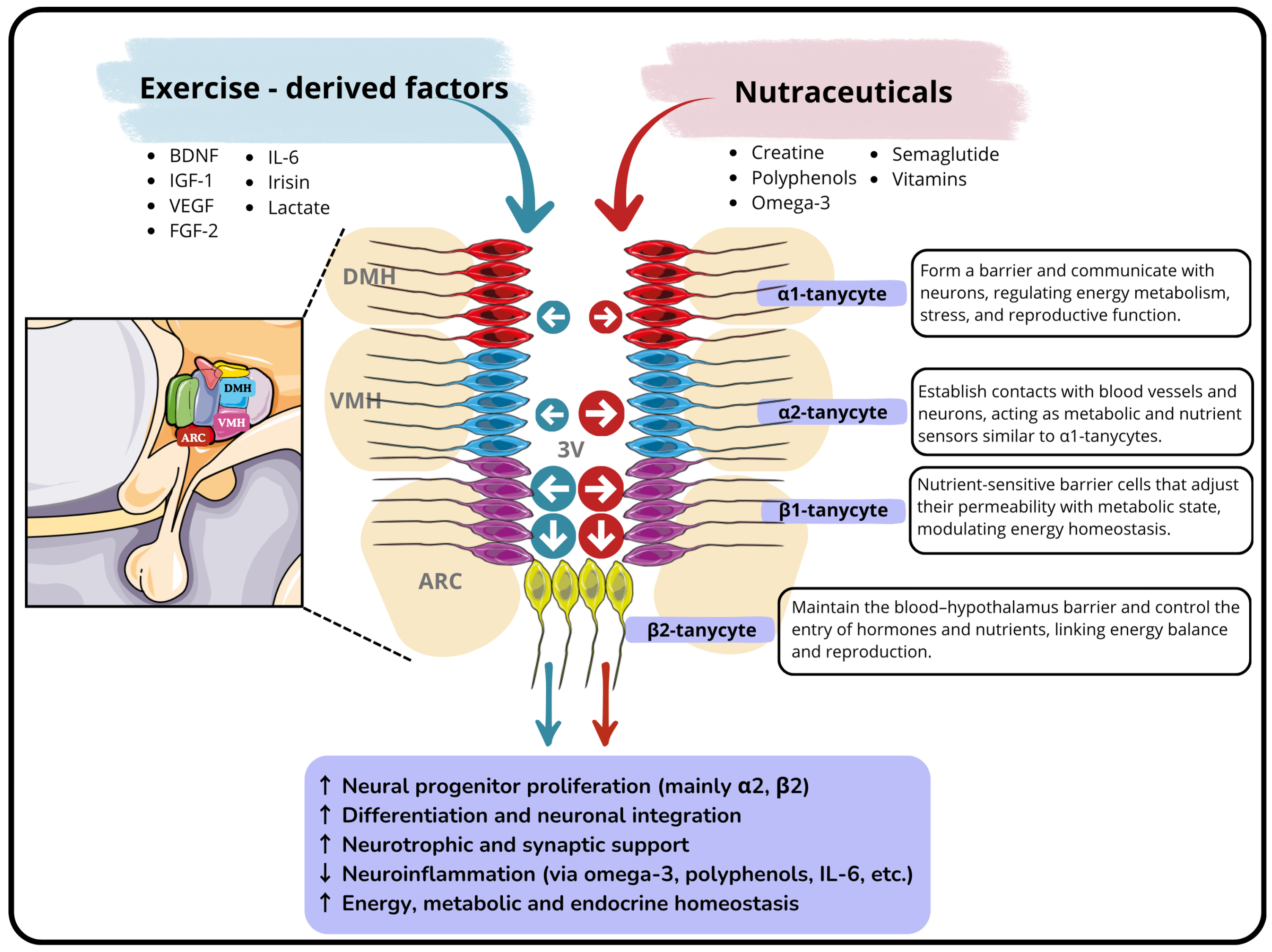
3.2. Hypothalamic Neurogenic Niche: The Tanycytes as Hypothalamic Stem Cells
3.3. Metabolic Regulation in the Hypothalamus and Its Relationship with Neurogenesis
3.3.1. Regulation of Appetite and Energy Expenditure
3.3.2. Thermoregulatory Control
3.3.3. Hydric Homeostasis and Osmoregulation in the Hypothalamus
3.3.4. Hypothalamic Regulation of Stress, Circadian Rhythms, and Sleep–Wake Cycles
3.4. Effects of Physical Exercise on Hypothalamic Neurogenesis
3.5. Nutraceuticals, Sports Supplements and Their Effects on the Neurogenic Niches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sánchez-Gomar, I.; Geribaldi-Doldán, N.; Santos-Rosendo, C.; Sanguino-Caneva, C.; Carrillo-Chapman, C.; Fiorillo-Moreno, O.; Camacho, J.L.V.; Quiroz, E.N.; Verástegui, C. Exploring the Intricacies of Neurogenic Niches: Unraveling the Anatomy and Neural Microenvironments. Biomolecules 2024, 14, 335. [Google Scholar] [CrossRef]
- Ribeiro, F.F.; Xapelli, S. An Overview of Adult Neurogenesis. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1331. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, P.; Chen, Q.; Zhang, Y.; Tang, Y.; Jin, W.; Yu, L. Neurodegenerative diseases and immune system: From pathogenic mechanism to therapy. Neural Regen. Res. 2025. [Google Scholar] [CrossRef]
- Ming, G.-L.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Ming, G.-L.; Song, H. Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef]
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular—Subventricular Zone. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef]
- Seri, B.; García-Verdugo, J.M.; McEwen, B.S.; Alvarez-Buylla, A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 2001, 21, 7153–7160. [Google Scholar] [CrossRef]
- Haan, N.; Goodman, T.; Najdi-Samiei, A.; Stratford, C.M.; Rice, R.; El Agha, E.; Bellusci, S.; Hajihosseini, M.K. Fgf10-expressing tanycytes add new neurons to the appetite/energy-balance regulating centers of the postnatal and adult hypothalamus. J. Neurosci. 2013, 33, 6170–6180. [Google Scholar] [CrossRef]
- Robins, S.; Stewart, I.; McNay, D.; Taylor, V.; Giachino, C.; Goetz, M.; Ninkovic, J.; Briancon, N.; Maratos-Flier, E.; Flier, J.; et al. α-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 2013, 4, 2049. [Google Scholar] [CrossRef] [PubMed]
- Robins, S.C.; Trudel, E.; Rotondi, O.; Liu, X.; Djogo, T.; Kryzskaya, D.; Bourque, C.W.; Kokoeva, M.V. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS ONE 2013, 8, e78236. [Google Scholar] [CrossRef]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical sites of adult neurogenesis in the mammalian brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef]
- Burbridge, S.; Stewart, I.; Placzek, M. Development of the neuroendocrine hypothalamus. Compr. Physiol. 2016, 6, 623–643. [Google Scholar] [CrossRef]
- Concetti, C.; Peleg-Raibstein, D.; Burdakov, D. Hypothalamic MCH Neurons: From Feeding to Cognitive Control. Function 2024, 5, 59. [Google Scholar] [CrossRef]
- Morris, J.F. Functional Anatomy of the Hypothalamus and Pituitary. In Oxford Textbook of Endocrinology and Diabetes, 3rd ed.; Oxford Academic: Oxford, UK, 2022. [Google Scholar] [CrossRef]
- Samodien, E.; Chellan, N. Hypothalamic neurogenesis and its implications for obesity-induced anxiety disorders. Front. Neuroendocr. 2021, 60, 100871. [Google Scholar] [CrossRef]
- Lechan, R.M.; Toni, R. Functional Anatomy of the Hypothalamus and Pituitary. In Endotext; NCBI Bookshelf: Bethesda, MD, USA, 2016. [Google Scholar]
- Makrygianni, E.A.; Chrousos, G.P. Neural Progenitor Cells and the Hypothalamus. Cells 2023, 12, 1822. [Google Scholar] [CrossRef]
- Sharif, A.; Fitzsimons, C.P.; Lucassen, P.J. Neurogenesis in the adult hypothalamus: A distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 179. [Google Scholar] [CrossRef]
- Goodman, T.; Hajihosseini, M.K. Hypothalamic tanycytes—Masters and servants of metabolic, neuroendocrine, and neurogenic functions. Front. Neurosci. 2015, 9, 387. [Google Scholar] [CrossRef]
- Bolborea, M.; Dale, N. Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends Neurosci. 2013, 36, 91–100. [Google Scholar] [CrossRef] [PubMed]
- van Praag, H. Neurogenesis and exercise: Past and future directions. NeuroMolecular Med. 2008, 10, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Kalra, E.K. Nutraceutical—Definition and introduction. AAPS PharmSciTech 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Zeisel, S.H. Regulation of “Nutraceuticals”. Science 1999, 285, 1853–1855. [Google Scholar] [CrossRef]
- Mecocci, P.; Tinarelli, C.; Schulz, R.J.; Polidori, M.C. Nutraceuticals in cognitive impairment and Alzheimer’s disease. Front. Pharmacol. 2014, 5, 147. [Google Scholar] [CrossRef]
- Reza-Zaldívar, E.E.; Jacobo-Velázquez, D.A. Comprehensive Review of Nutraceuticals against Cognitive Decline Associated with Alzheimer’s Disease. ACS Omega 2023, 8, 35499–35522. [Google Scholar] [CrossRef]
- Rabiei, Z.; Rafieian-Kopaei, M.; Heidarian, E.; Saghaei, E.; Mokhtari, S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus basalis of meynert in rat. Neurochem. Res. 2014, 39, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Geribaldi-Doldan, N.; Szele, F.G.; Isoda, H. Grape skin extract modulates neuronal stem cell proliferation and improves spatial learning in senescence-accelerated prone 8 mice. Aging 2021, 13, 18131–18149. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Geribaldi-Doldán, N.; Wu, Q.; Davies, J.; Szele, F.G.; Isoda, H. Microalgae Aurantiochytrium Sp. Increases Neurogenesis and Improves Spatial Learning and Memory in Senescence-Accelerated Mouse-Prone 8 Mice. Front. Cell Dev. Biol. 2021, 8, 600575. [Google Scholar] [CrossRef]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and an-ti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytotherapy Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Khosravi-Boroujeni, H.; Sarrafzadegan, N.; Mohammadifard, N.; Sajjadi, F.; Maghroun, M.; Asgari, S.; Rafieian-Kopaei, M.; Azadbakht, L. White rice consumption and CVD risk factors among Iranian population. J. Health Popul. Nutr. 2013, 31, 252–261. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Willis, M.S.; Wians, F.H. The role of nutrition in preventing prostate cancer: A review of the proposed mechanism of action of various dietary substances. Clin. Chim. Acta 2003, 330, 57–83. [Google Scholar] [CrossRef]
- Kruger, C.; Murphy, M.; DeFreitas, Z.; Pfannkuch, F.; Heimbach, J. An innovative approach to the determination of safety for a dietary ingredient derived from a new source: Case study using a crystalline lutein product. Food Chem. Toxicol. 2002, 40, 1535–1549. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.; Asgary, S.; Moshtaghian, J.; Rafieian, M.; Adelnia, A.; Shamsi, F. Liver-protective effects of hydroalcoholic extract of allium hirtifolium boiss. In rats with alloxan-induced diabetes mellitus. ARYA Atheroscler. 2010, 6, 11–15. [Google Scholar]
- Gordji-Nejad, A.; Matusch, A.; Kleedörfer, S.; Patel, H.J.; Drzezga, A.; Elmenhorst, D.; Binkofski, F.; Bauer, A. Single dose creatine improves cognitive performance and induces changes in cerebral high energy phosphates during sleep deprivation. Sci. Rep. 2024, 14, 4937. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Ostojic, S.M.; Prokopidis, K.; Stock, M.S.; Harmon, K.K.; Faulkner, P. “Heads Up” for Creatine Supplementation and its Potential Applications for Brain Health and Function. Sports Med. 2023, 53, 49–65, Erratum in Sports Med. 2024, 54, 235–236. [Google Scholar] [CrossRef]
- Gomes, F.T.d.S.; de Andrade, A.V.D.; Melo, P.K.M.; Júnior, R.R.d.S.; de Souza, D.L.S.; Tavares, É.A.F.; de Sena, I.G.; Fernandes, T.A.A.d.M.; Morais, P.L.A.d.G.; Fonseca, I.A.T.; et al. The Effects of the Association Between a High-Fat Diet and Physical Exercise on BDNF Expression in the Hippocampus: A Comprehensive Review. Life 2025, 15, 945. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef]
- Schättin, A.; Baur, K.; Stutz, J.; Wolf, P.; de Bruin, E.D. Effects of physical exercise combined with nutritional supplements on aging brain related structures and functions: A systematic review. Front. Aging Neurosci. 2016, 8, 161. [Google Scholar] [CrossRef]
- Quan, H.; Koltai, E.; Suzuki, K.; Aguiar, A.S.; Pinho, R.; Boldogh, I.; Berkes, I.; Radak, Z. Exercise, redox system and neurodegenerative diseases. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165778. [Google Scholar] [CrossRef] [PubMed]
- Dadhania, V.P.; Trivedi, P.P.; Vikram, A.; Tripathi, D.N. Nutraceuticals against Neurodegeneration: A Mechanistic Insight. Curr. Neuropharmacol. 2016, 14, 627–640. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pinilla, F. The combined effects of exercise and foods in preventing neurological and cognitive disorders. Prev. Med. 2011, 52, S75–S80. [Google Scholar] [CrossRef]
- Pratchayasakul, W.; Arunsak, B.; Suparan, K.; Sriwichaiin, S.; Chunchai, T.; Chattipakorn, N.; Chattipakorn, S.C. Combined caloric restriction and exercise provides greater metabolic and neurocognitive benefits than either as a monotherapy in obesity with or without estrogen deprivation. J. Nutr. Biochem. 2022, 110, 109125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Song, Z.; Zhang, X. Exercise, Diet, and Brain Health: From the Perspective of Gut Microbiota Regulation. Nutrients 2025, 17, 1686. [Google Scholar] [CrossRef]
- Klein, C.; Jonas, W.; Iggena, D.; Empl, L.; Rivalan, M.; Wiedmer, P.; Spranger, J.; Hellweg, R.; Winter, Y.; Steiner, B. Exercise prevents high-fat diet-induced impairment of flexible memory expression in the water maze and modulates adult hippocampal neurogenesis in mice. Neurobiol. Learn. Mem. 2016, 131, 26–35. [Google Scholar] [CrossRef]
- Chou, T.C.; Scammell, T.E.; Gooley, J.J.; Gaus, S.E.; Saper, C.B.; Lu, J. Critical Role of Dorsomedial Hypothalamic Nucleus in a Wide Range of Behavioral Circadian Rhythms. J. Neurosci. 2003, 23, 10691–10702. [Google Scholar] [CrossRef]
- Saper, C.B.; Cano, G.; Scammell, T.E. Homeostatic, circadian, and emotional regulation of sleep. J. Comp. Neurol. 2005, 493, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, Y.; Zhao, J.; Ma, X. Possible regulatory mechanisms of typical and atypical absence seizures through an equivalent projection from the subthalamic nucleus to the cortex: Evidence in a computational model. J. Theor. Biol. 2025, 602–603, 112059. [Google Scholar] [CrossRef]
- Pop, M.G.; Crivii, C.; Opincariu, I. Anatomy and Function of the Hypothalamus. In Hypothalamus in Health and Diseases; Books on Demand: Norderstedt, Germany, 2018. [Google Scholar] [CrossRef]
- Stocker, S.D.; Wenner, M.M.; Farquhar, W.B.; Browning, K.N. Activation of the organum vasculosum of the lamina terminalis produces a sympathetically mediated hypertension. Hypertension 2021, 79, 139–149. [Google Scholar] [CrossRef]
- Sandgren, J.A.; Linggonegoro, D.W.; Zhang, S.Y.; Sapouckey, S.A.; Claflin, K.E.; Pearson, N.A.; Leidinger, M.R.; Pierce, G.L.; Santillan, M.K.; Gibson-Corley, K.N.; et al. Angiotensin AT1Areceptors expressed in vasopressin-producing cells of the supraoptic nucleus contribute to osmotic control of vasopressin. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R770–R780. [Google Scholar] [CrossRef]
- Jensen, D.; Ebmeier, K.; Suri, S.; Rushworth, M.; Klein-Flügge, M. Nuclei-specific hypothalamus networks predict a dimensional marker of stress in humans. Neurosci. Appl. 2023, 2, 2426. [Google Scholar] [CrossRef]
- Fassini, A.; Scopinho, A.A.; Alves, F.H.; Fortaleza, E.A.; Corrêa, F.M. The medial preoptic area modulates autonomic function under resting and stress conditions. Neuroscience 2017, 364, 164–174. [Google Scholar] [CrossRef]
- Whiting, A.C.; Oh, M.Y.; Whiting, D.M. Deep brain stimulation for appetite disorders: A review. Neurosurg. Focus 2018, 45, E9. [Google Scholar] [CrossRef]
- Hsu, T.I.; Nguyen, A.; Gupta, N.; Godbole, N.; Perisetla, N.; Hatter, M.J.; Beyer, R.S.; Bui, N.E.; Jagan, J.; Yang, C.; et al. Effectiveness of Deep Brain Stimulation in Treatment of Anorexia Nervosa and Obesity: A Systematic Review. World Neurosurg. 2022, 168, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, J.; Tang, K. The paraventricular nucleus of the hypothalamus: Development, function, and human diseases. Endocrinology 2018, 159, 3458–3472. [Google Scholar] [CrossRef] [PubMed]
- Morita-Takemura, S.; Wanaka, A. Blood-to-brain communication in the hypothalamus for energy intake regulation. Neurochem. Int. 2019, 128, 135–142. [Google Scholar] [CrossRef]
- Vertes, R.; Albo, Z.; Di Prisco, G.V. Theta-rhythmically firing neurons in the anterior thalamus: Implications for mnemonic functions of Papez’s circuit. Neuroscience 2001, 104, 619–625. [Google Scholar] [CrossRef]
- Yoo, S.; Blackshaw, S. Regulation and function of neurogenesis in the adult mammalian hypothalamus. Prog. Neurobiol. 2018, 170, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.; Nestler, E.; Hyman, S. Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin. In Molecular Neu-ropharmacology: A Foundation for Clinical Neuroscience; McGraw-Hill: New York, NY, USA, 2009. [Google Scholar]
- Fujita, A.; Bonnavion, P.; Wilson, M.H.; Mickelsen, L.E.; Bloit, J.; de Lecea, L.; Jackson, A.C. Hypothalamic tuberomammillary nucleus neurons: Electrophysiological diversity and essential role in arousal stability. J. Neurosci. 2017, 37, 9574–9592. [Google Scholar] [CrossRef]
- Scammell, T.E.; Jackson, A.C.; Franks, N.P.; Wisden, W.; Dauvilliers, Y. Histamine: Neural circuits and new medications. Sleep 2019, 42, 183. [Google Scholar] [CrossRef]
- Flores-Clemente, C.; Nicolás-Vázquez, M.I.; Jiménez, E.M.; Hernández-Rodríguez, M. Inhibition of astrocytic histamine n-methyltransferase as a possible target for the treatment of alzheimer’s disease. Biomolecules 2021, 11, 1408. [Google Scholar] [CrossRef]
- Rossi, M.A. Control of energy homeostasis by the lateral hypothalamic area. Trends Neurosci. 2023, 46, 738–749. [Google Scholar] [CrossRef]
- Kurt, G.; Kodur, N.; Quiles, C.R.; Reynolds, C.; Eagle, A.; Mayer, T.; Brown, J.; Makela, A.; Bugescu, R.; Seo, H.D.; et al. Time to drink: Activating lateral hypothalamic area neurotensin neurons promotes intake of fluid over food in a time-dependent manner. Physiol. Behav. 2022, 247, 113707. [Google Scholar] [CrossRef]
- Fakhoury, M.; Salman, I.; Najjar, W.; Merhej, G.; Lawand, N. The Lateral Hypothalamus: An Uncharted Territory for Processing Peripheral Neurogenic Inflammation. Front. Neurosci. 2020, 14, 101. [Google Scholar] [CrossRef]
- Fattahi, M.; Rahimpour, M.; Riahi, E. Opioid reward and deep brain stimulation of the lateral hypothalamic area. Vitam. Horm. 2025, 127, 245–281. [Google Scholar] [CrossRef]
- Ishii, Y.; Bouret, S.G. Embryonic birthdate of hypothalamic leptin-activated neurons in mice. Endocrinology 2012, 153, 3657–3667. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Fu, S.; Wan, Y.; Mao, J.; Cui, K.; Jiang, H. The Integrated Function of the Lateral Hypothalamus in Energy Homeostasis. Cells 2025, 14, 1042. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Santiago, A.M.; Thomas, M.P.; Routh, V.H. Metabolic regulation of lateral hypothalamic glucose-inhibited orexin neurons may influence midbrain reward neurocircuitry. Mol. Cell. Neurosci. 2014, 62, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Prokofeva, K.; Saito, Y.C.; Niwa, Y.; Mizuno, S.; Takahashi, S.; Hirano, A.; Sakurai, T. Structure and Function of Neuronal Circuits Linking Ventrolateral Preoptic Nucleus and Lateral Hypothalamic Area. J. Neurosci. 2023, 43, 4075–4092. [Google Scholar] [CrossRef]
- Sherin, J.E.; Shiromani, P.J.; McCarley, R.W.; Saper, C.B. Activation of Ventrolateral Preoptic Neurons During Sleep. Science (1979) 1996, 271, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, E.; Fuller, P.M. The Sleep-Promoting Ventrolateral Preoptic Nucleus: What Have We Learned over the Past 25 Years? Int. J. Mol. Sci. 2022, 23, 2905. [Google Scholar] [CrossRef]
- Song, J.; Choi, S.-Y. Arcuate Nucleus of the Hypothalamus: Anatomy, Physiology, and Diseases. Exp. Neurobiol. 2023, 32, 371–386. [Google Scholar] [CrossRef]
- Jais, A.; Brüning, J.C. Arcuate Nucleus-Dependent Regulation of Metabolism—Pathways to Obesity and Diabetes Mellitus. Endocr. Rev. 2022, 43, 314–328. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Fukuda, M.; Tong, Q.; Xu, Y. The ventromedial hypothalamic nucleus: Watchdog of whole-body glucose homeostasis. Cell Biosci. 2022, 12, 71. [Google Scholar] [CrossRef]
- Routh, V.H. Glucosensing neurons in the ventromedial hypothalamic nucleus (VMN) and hypoglycemia-associated autonomic failure (HAAF). Diabetes Metab. Res. Rev. 2003, 19, 348–356. [Google Scholar] [CrossRef]
- Durán, D.A.B.; Guzmán, S.J.B.; Hernández, A.T.; Gómez, A.B.S. Obese female Zucker rats (fa/fa) exhibit dendritic retraction in neurons in the ventromedial hypothalamic nucleus. J. Chem. Neuroanat. 2021, 113, 101919. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Hu, F. AMPK in the Ventromedial Nucleus of the Hypothalamus: A Key Regulator for Thermogenesis. Front. Endocrinol. 2020, 11, 578830. [Google Scholar] [CrossRef]
- Vargas, Y.; de Oca, M.P.-M.; Sánchez-Jaramillo, E.; Jaimes-Hoy, L.; Sánchez-Islas, E.; Uribe, R.M.; Joseph-Bravo, P.; Charli, J.-L. Sex-dependent and -independent regulation of thyrotropin-releasing hormone expression in the hypothalamic dorsomedial nucleus by negative energy balance, exercise, and chronic stress. Brain Res. 2022, 1796, 148083. [Google Scholar] [CrossRef]
- Ambler, M.; Hitrec, T.; Wilson, A.; Cerri, M.; Pickering, A. Neurons in the Dorsomedial Hypothalamus Promote, Prolong, and Deepen Torpor in the Mouse. J. Neurosci. 2022, 42, 4267–4277. [Google Scholar] [CrossRef]
- Li, L.; Zhang, M.-Q.; Sun, X.; Liu, W.-Y.; Huang, Z.-L.; Wang, Y.-Q. Role of Dorsomedial Hypothalamus GABAergic Neurons in Sleep–Wake States in Response to Changes in Ambient Temperature in Mice. Int. J. Mol. Sci. 2022, 23, 1270. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat. Rev. Neurosci. 2018, 19, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Tsuno, Y.; Mieda, M. Circadian rhythm mechanism in the suprachiasmatic nucleus and its relation to the olfactory system. Front. Neural Circuits 2024, 18, 1385908. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Gong, Y.; Eckel-Mahan, K.L.; Sun, Z. Central Circadian Clock Regulates Energy Metabolism. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; pp. 79–103. [Google Scholar] [CrossRef]
- Xie, Z.; Gu, H.; Huang, M.; Cheng, X.; Shang, C.; Tao, T.; Li, D.; Xie, Y.; Zhao, J.; Lu, W.; et al. Mechanically evoked defensive attack is controlled by GABAergic neurons in the anterior hypothalamic nucleus. Nat. Neurosci. 2022, 25, 72–85. [Google Scholar] [CrossRef]
- Klemfuss, H.; Seiden, L.S. Water deprivation increases anterior hypothalamic norepinephrine metabolism in the rat. Brain Res. 1985, 341, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.G.S.; De Jesus, O. Hypothalamic Dysfunction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hong, C.Y.; Din, J.S.; Chang, H.; Bang, J.Y.; Kim, J.C. Anterior hypothalamic nucleus drives distinct defensive responses through cell-type-specific activity. iScience 2025, 28, 112097. [Google Scholar] [CrossRef]
- Ferris, C.F.; Melloni, R.H., Jr.; Koppel, G.; Perry, K.W.; Fuller, R.W.; Delville, Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 1997, 17, 4331–4340. [Google Scholar] [CrossRef]
- Gobrogge, K.L.; Liu, Y.; Young, L.J.; Wang, Z. Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. USA 2009, 106, 19144–19149. [Google Scholar] [CrossRef]
- Iremonger, K.J.; Power, E.M. The paraventricular nucleus of the hypothalamus: A key node in the control of behavioural states. J. Physiol. 2025, 603, 2231–2243. [Google Scholar] [CrossRef]
- Daviu, N.; Füzesi, T.; Rosenegger, D.G.; Rasiah, N.P.; Sterley, T.-L.; Peringod, G.; Bains, J.S. Paraventricular nucleus CRH neurons encode stress controllability and regulate defensive behavior selection. Nat. Neurosci. 2020, 23, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Marraudino, M.; Garcia-Segura, L.; Panzica, G. The hypothalamic paraventricular nucleus as a central hub for the estrogenic modulation of neuroendocrine function and behavior. Front. Neuroendocr. 2022, 65, 100974. [Google Scholar] [CrossRef]
- Gao, H.-R.; Zhuang, Q.-X.; Li, B.; Li, H.-Z.; Chen, Z.-P.; Wang, J.-J.; Zhu, J.-N. Corticotropin releasing factor excites neurons of posterior hypothalamic nucleus to produce tachycardia in rats. Sci. Rep. 2016, 6, 20206. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Nakamura, S.; Watanabe, S.; Ueki, S. Effect of l-glutamate, injected into the posterior hypothalamus, on blood pressure and heart rate in unanesthetized and unrestrained rats. Neuropharmacology 1985, 24, 445–451. [Google Scholar] [CrossRef]
- Nyhuis, T.J.; Masini, C.V.; Day, H.E.; Campeau, S. Evidence for the integration of stress-related signals by the rostral posterior hypothalamic nucleus in the regulation of acute and repeated stress-evoked hypothalamo-pituitary-adrenal response in rat. J. Neurosci. 2016, 36, 795–805, Erratum in J. Neurosci. 2016, 36, 5183. [Google Scholar] [CrossRef]
- Ishiwata, T.; Greenwood, B.N. Changes in thermoregulation and monoamine release in freely moving rats during cold exposure and inhibition of the ventromedial, dorsomedial, or posterior hypothalamus. J. Comp. Physiol. B 2018, 188, 541–551. [Google Scholar] [CrossRef]
- Çavdar, S.; Onat, F.; Aker, R.; ŞehïrLï, Ü.; Şan, T.; Yananli, H.R. The afferent connections of the posterior hypothalamic nucleus in the rat using horseradish peroxidase. Am. J. Anat. 2001, 198, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Abrahamson, E.E.; Moore, R.Y. The posterior hypothalamic area: Chemoarchitecture and afferent connections. Brain Res. 2001, 889, 1–22. [Google Scholar] [CrossRef]
- Stettner, G.M.; Kubin, L. Antagonism of orexin receptors in the posterior hypothalamus reduces hypoglossal and cardiorespiratory excitation from the perifornical hypothalamus. J. Appl. Physiol. 2013, 114, 119–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Myers, B.; Carvalho-Netto, E.; Wick-Carlson, D.; Wu, C.; Naser, S.; Solomon, M.B.; Ulrich-Lai, Y.M.; Herman, J.P. GABAergic signaling within a limbic-hypothalamic circuit integrates social and anxiety-like behavior with stress reactivity. Neuropsychopharmacology 2016, 41, 1530–1539. [Google Scholar] [CrossRef]
- Thornhill, J.A.; Halvorson, I. Electrical stimulation of the posterior and ventromedial hypothalamic nuclei causes specific activation of shivering and nonshivering thermogenesis. Can. J. Physiol. Pharmacol. 1994, 72, 89–96. [Google Scholar] [CrossRef]
- Wang, S.C.; Parpura, V.; Wang, Y.-F. Astroglial Regulation of Magnocellular Neuroendocrine Cell Activities in the Supraoptic Nucleus. Neurochem. Res. 2021, 46, 2586–2600. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Das, J.M. Neuroanatomy, Nucleus Supraoptic. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Tsuneoka, Y. Molecular neuroanatomy of the mouse medial preoptic area with reference to parental behavior. Anat. Sci. Int. 2019, 94, 39–52. [Google Scholar] [CrossRef]
- Pal, T.; McQuillan, H.J.; Wragg, L.; E Brown, R.S. Hormonal Actions in the Medial Preoptic Area Governing Parental Behavior: Novel Insights from New Tools. Endocrinology 2024, 166, bqae152. [Google Scholar] [CrossRef]
- Zhao, C.; Riters, L.V. The Medial Preoptic Area and Its Projections to the Ventral Tegmental Area and the Periaqueductal Gray Are Acti-vated in Response to Social Play Behavior in Juvenile Rats. Behav. Neurosci. 2023, 137, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, M.; Yoshida, K.; Coppari, R.; E Bass, C.; Mochizuki, T.; Lowell, B.B.; Saper, C.B. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007, 10, 1131–1133. [Google Scholar] [CrossRef]
- Gvilia, I.; Xu, F.; McGinty, D.; Szymusiak, R. Homeostatic regulation of sleep: A role for preoptic area neurons. J. Neurosci. 2006, 26, 9426–9433. [Google Scholar] [CrossRef]
- Marciante, A.B.; Wang, L.A.; Farmer, G.E.; Cunningham, J.T. Selectively inhibiting the median preoptic nucleus attenuates angiotensin II and hyperosmotic- induced drinking behavior and vasopressin release in adult male rats. eNeuro 2019, 6, 473. [Google Scholar] [CrossRef]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2019, 24, 67–87. [Google Scholar] [CrossRef]
- van Praag, H.; Schinder, A.F.; Christie, B.R.; Toni, N.; Palmer, T.D.; Gage, F.H. Functional neurogenesis in the adult hippocampus. Nature 2002, 415, 1030–1034. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.A.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science (1979) 2021, 374, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Bedont, J.L.; Newman, E.A.; Blackshaw, S. Patterning, specification, and differentiation in the developing hypothalamus. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 445–468. [Google Scholar] [CrossRef]
- Zhou, X.; Zhong, S.; Peng, H.; Liu, J.; Ding, W.; Sun, L.; Ma, Q.; Liu, Z.; Chen, R.; Wu, Q.; et al. Cellular and molecular properties of neural progenitors in the developing mammalian hypothalamus. Nat. Commun. 2020, 11, 4063. [Google Scholar] [CrossRef]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef]
- Prevot, V.; Dehouck, B.; Sharif, A.; Ciofi, P.; Giacobini, P.; Clasadonte, J. The versatile tanycyte: A hypothalamic integrator of reproduction and energy metabolism. Endocr. Rev. 2018, 39, 333–368. [Google Scholar] [CrossRef]
- Monroe, B.G. A comparative study of the ultrastructure of the median eminence, infundibular stem and neural lobe of the hypophysis of the rat. Z. Zellforsch. Mikrosk. Anat. 1967, 76, 405–432. [Google Scholar] [CrossRef]
- Knigge, K.M.; Scott, D.E. Structure and function of the median eminence. Am. J. Anat. 1970, 129, 223–243. [Google Scholar] [CrossRef]
- Recabal, A.; Caprile, T.; García-Robles, M.d.L.A. Hypothalamic neurogenesis as an adaptive metabolic mechanism. Front. Neurosci. 2017, 11, 190. [Google Scholar] [CrossRef] [PubMed]
- Markakis, E.A.; Palmer, T.D.; Randolph-Moore, L.; Rakic, P.; Gage, F.H. Novel Neuronal Phenotypes from Neural Progenitor Cells. J. Neurosci. 2004, 24, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tamamaki, N.; Noda, T.; Kimura, K.; Itokazu, Y.; Matsumoto, N.; Dezawa, M.; Ide, C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005, 192, 251–264. [Google Scholar] [CrossRef] [PubMed]
- A Lee, D.; Bedont, J.L.; Pak, T.; Wang, H.; Song, J.; Miranda-Angulo, A.; Takiar, V.; Charubhumi, V.; Balordi, F.; Takebayashi, H.; et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012, 15, 700–702. [Google Scholar] [CrossRef]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science (1979) 2005, 310, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Trubert, C.; Terrien, J.; Pifferi, F.; Leroy, D.; Loyens, A.; Migaud, M.; Baroncini, M.; Maurage, C.; Fontaine, C.; et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J. Comp. Neurol. 2018, 526, 1419–1443. [Google Scholar] [CrossRef]
- Rodríguez, E.; Guerra, M.; Peruzzo, B.; Blázquez, J.L. Tanycytes: A rich morphological history to underpin future molecular and physiological investigations. J. Neuroendocr. 2019, 31, e12690. [Google Scholar] [CrossRef]
- Rodriguez, E.; Blazquez, J.; Pastor, F.; Pelaez, B.; Pena, P.; Peruzzo, B.; Amat, P. Hypothalamic Tanycytes: A Key Component of Brain–Endocrine Interaction. Int. Rev. Cytol. 2005, 247, 89–164. [Google Scholar] [CrossRef]
- Cheng, M.-F. Hypothalamic neurogenesis in the adult brain. Front. Neuroendocrinol. 2013, 34, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martín, M.; Cifuentes, M.; Grondona, J.M.; López-Ávalos, M.D.; Gómez-Pinedo, U.; García-Verdugo, J.M.; Fernández-Llebrez, P. IGF-I stimulates neurogenesis in the hypothalamus of adult rats. Eur. J. Neurosci. 2010, 31, 1533–1548. [Google Scholar] [CrossRef]
- Campbell, J.N.; Macosko, E.Z.; Fenselau, H.; Pers, T.H.; Lyubetskaya, A.; Tenen, D.; Goldman, M.; Verstegen, A.M.; Resch, J.M.; McCarroll, S.A.; et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017, 20, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Chaker, Z.; George, C.; Petrovska, M.; Caron, J.-B.; Lacube, P.; Caillé, I.; Holzenberger, M. Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiol. Aging 2016, 41, 64–72. [Google Scholar] [CrossRef]
- McNay, D.E.; Briançon, N.; Kokoeva, M.V.; Maratos-Flier, E.; Flier, J.S. Remodeling of the arcuate nucleus energy-balance circuit is inhibited in obese mice. J. Clin. Investig. 2012, 122, 142–152. [Google Scholar] [CrossRef]
- Bobbo, V.C.; Engel, D.F.; Jara, C.P.; Mendes, N.F.; Haddad-Tovolli, R.; Prado, T.P.; Sidarta-Oliveira, D.; Morari, J.; Velloso, L.A.; Araujo, E.P. Interleukin-6 actions in the hypothalamus protects against obesity and is involved in the regulation of neurogenesis. J. Neuroinflammation 2021, 18, 192. [Google Scholar] [CrossRef]
- Morrison, S.F. Central control of body temperature. F1000Research 2016, 5, 880. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Katakura, M.; Sugimoto, N.; Hara, T.; Hashimoto, M.; Shido, O. Neural progenitor cell proliferation in the hypothalamus is involved in acquired heat tolerance in long-term heat-acclimated rats. PLoS ONE 2017, 12, e0178787. [Google Scholar] [CrossRef]
- Niwa, A.; Nishibori, M.; Hamasaki, S.; Kobori, T.; Liu, K.; Wake, H.; Mori, S.; Yoshino, T.; Takahashi, H. Voluntary exercise induces neurogenesis in the hypothalamus and ependymal lining of the third ventricle. Brain Struct. Funct. 2016, 221, 1653–1666. [Google Scholar] [CrossRef]
- Infantes-López, M.I.; Nieto-Quero, A.; Chaves-Peña, P.; Zambrana-Infantes, E.; Cifuentes, M.; Márquez, J.; Pedraza, C.; Pérez-Martín, M. New insights into hypothalamic neurogenesis disruption after acute and intense stress: Implications for microglia and inflammation. Front. Neurosci. 2023, 17, 1190418, Erratum in Front. Neurosci. 2023, 17, 1335034. [Google Scholar] [CrossRef]
- Solak, H.; Gormus, Z.I.S.; Koca, R.O.; Gunes, C.E.; Iyisoy, M.S.; Kurar, E.; Kutlu, S. The effect of neuropeptide Y1 receptor agonist on hypothalamic neurogenesis in rat experimental depression model. Metab. Brain Dis. 2024, 40, 39. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.; Lazcano, I.; Sánchez-Jaramillo, E.; Uribe, R.M.; Jaimes-Hoy, L.; Joseph-Bravo, P.; Charli, J.-L. Tanycytes and the Control of Thyrotropin-Releasing Hormone Flux Into Portal Capillaries. Front. Endocrinol. 2019, 10, 401. [Google Scholar] [CrossRef]
- Langlet, F. Tanycyte Gene Expression Dynamics in the Regulation of Energy Homeostasis. Front. Endocrinol. 2019, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Kannangara, H.; Cullen, L.; Miyashita, S.; Korkmaz, F.; Macdonald, A.; Gumerova, A.; Witztum, R.; Moldavski, O.; Sims, S.; Burgess, J.; et al. Emerging roles of brain tanycytes in regulating blood–hypothalamus barrier plasticity and energy homeostasis. Ann. N. Y. Acad. Sci. 2023, 1525, 61–69. [Google Scholar] [CrossRef]
- Kim, N.; Kim, S.; Park, S.; Kim, E.-K. Adenosine transmission from hypothalamic tanycytes to AGRP/NPY neurons regulates energy homeostasis. Exp. Mol. Med. 2025, 57, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Li, S.; Xu, J.; Guo, X.; Wu, H.; Chen, Z.; Qiao, L.; Helfer, G.; Lu, F.; Liu, C.; et al. Hypothalamic Rax+ tanycytes contribute to tissue repair and tumorigenesis upon oncogene activation in mice. Nat. Commun. 2021, 12, 2288. [Google Scholar] [CrossRef] [PubMed]
- Safahani, M.; Aligholi, H.; Noorbakhsh, F.; Djalali, M.; Pishva, H.; Mousavi, S.M.M.; Alipour, F.; Gorji, A.; Koohdani, F. Resveratrol promotes the arcuate nucleus architecture remodeling to produce more anorexigenic neurons in high-fat-diet–fed mice. Nutrition 2018, 50, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.A.; Yoo, S.; Pak, T.; Salvatierra, J.; Velarde, E.; Aja, S.; Blackshaw, S. Dietary and sex-specific factors regulate hypothalamic neurogenesis in young adult mice. Front. Neurosci. 2014, 8, 157. [Google Scholar] [CrossRef]
- Bless, E.P.; Reddy, T.; Acharya, K.D.; Beltz, B.S.; Tetel, M.J. Oestradiol and diet modulate energy homeostasis and hypothalamic neurogenesis in the adult female mouse. J. Neuroendocr. 2014, 26, 805–816. [Google Scholar] [CrossRef]
- Gouazé, A.; Brenachot, X.; Rigault, C.; Krezymon, A.; Rauch, C.; Nédélec, E.; Lemoine, A.; Gascuel, J.; Bauer, S.; Pénicaud, L.; et al. Cerebral Cell Renewal in Adult Mice Controls the Onset of Obesity. PLoS ONE 2013, 8, e72029. [Google Scholar] [CrossRef]
- Batailler, M.; Droguerre, M.; Baroncini, M.; Fontaine, C.; Prevot, V.; Migaud, M. DCX-expressing cells in the vicinity of the hypothalamic neurogenic niche: A comparative study between mouse, sheep, and human tissues. J. Comp. Neurol. 2014, 522, 1966–1985. [Google Scholar] [CrossRef]
- Jörgensen, S.K.; Karnošová, A.; Mazzaferro, S.; Rowley, O.; Chen, H.-J.C.; Robbins, S.J.; Christofides, S.; Merkle, F.T.; Maletínská, L.; Petrik, D. An analogue of the Prolactin Releasing Peptide reduces obesity and promotes adult neurogenesis. EMBO Rep. 2024, 25, 351–377. [Google Scholar] [CrossRef]
- Klein, C.; Jonas, W.; Wiedmer, P.; Schreyer, S.; Akyüz, L.; Spranger, J.; Hellweg, R.; Steiner, B. High-fat Diet and Physical Exercise Differentially Modulate Adult Neurogenesis in the Mouse Hypothalamus. Neuroscience 2019, 400, 146–156. [Google Scholar] [CrossRef]
- Feighan, K.M.; Nesan, D.; Kurrasch, D.M. Gestational bisphenol A exposure alters energy homeostasis and adult hypothalamic neurogenesis in female mice. Sci. Rep. 2024, 14, 16082. [Google Scholar] [CrossRef]
- Lévy, F.; Batailler, M.; Meurisse, M.; Keller, M.; Cornilleau, F.; Moussu, C.; Poissenot, K.; Migaud, M. Differential effects of oxytocin on olfactory, hippocampal and hypothalamic neurogenesis in adult sheep. Neurosci. Lett. 2019, 713, 134520. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.D.; Kucherepa, N.N.; Fisher, K.R.; Halina, W.G.; Partlow, G.D. Neurogenesis of oxytocin-containing neurons in the paraventricular nucleus (PVN) of the female pig in 3 reproductive states: Puberty gilts, adult gilts and lactating sows. Brain Res. 2006, 1102, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zetter, M.A.; Hernández, V.S.; Hernández-Pérez, O.R.; Jáuregui-Huerta, F.; Krabichler, Q.; Grinevich, V. Morphological Signatures of Neurogenesis and Neuronal Migration in Hypothalamic Vasopressinergic Magnocellular Nuclei of the Adult Rat. Int. J. Mol. Sci. 2024, 25, 6988. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Katakura, M.; Inoue, T.; Hara, T.; Hashimoto, M.; Shido, O. Aging attenuates acquired heat tolerance and hypothalamic neurogenesis in rats. J. Comp. Neurol. 2015, 523, 1190–1201. [Google Scholar] [CrossRef]
- Shido, O.; Matsuzaki, K. Involvement of neurogenesis in the hypothalamic area in establishing long-term heat acclimation in rats. Temp. Multidiscip. Biomed. J. 2015, 2, 362–367. [Google Scholar] [CrossRef][Green Version]
- Hill, J.W. Gene Expression and the Control of Food Intake by Hypothalamic POMC/CART Neurons. Open Neuroendocrinol. J. 2010, 3, 21–27. [Google Scholar][Green Version]
- Sohn, J.-W.; Elmquist, J.K.; Williams, K.W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013, 36, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Paeger, L.; Karakasilioti, I.; Altmüller, J.; Frommolt, P.; Brüning, J.; Kloppenburg, P. Antagonistic modulation of NPY/AgRP and POMC neurons in the arcuate nucleus by noradrenalin. Elife 2017, 6, e25770. [Google Scholar] [CrossRef] [PubMed]
- Rafiei, N.; Mitchell, C.S.; Tedesco, C.R.; Chen, J.; Choi, E.A.; Roughley, S.; Jean-Richard-Dit-Bressel, P.; Kumar, N.N.; McNally, G.P.; Herzog, H.; et al. Chemogenetic activation of arcuate nucleus NPY and NPY/AgRP neurons increases feeding behaviour in mice. Neuropeptides 2024, 107, 102454. [Google Scholar] [CrossRef]
- Al Massadi, O.; López, M.; Tschöp, M.; Diéguez, C.; Nogueiras, R. Current Understanding of the Hypothalamic Ghrelin Pathways Inducing Appetite and Adiposity. Trends Neurosci. 2017, 40, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, S.; Sato, T.; Kangawa, K.; Nakazato, M. The Homeostatic Force of Ghrelin. Cell Metab. 2018, 27, 786–804. [Google Scholar] [CrossRef]
- Balland, E.; Dam, J.; Langlet, F.; Caron, E.; Steculorum, S.; Messina, A.; Rasika, S.; Falluel-Morel, A.; Anouar, Y.; Dehouck, B.; et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014, 19, 293–301. [Google Scholar] [CrossRef]
- Collden, G.; Balland, E.; Parkash, J.; Caron, E.; Langlet, F.; Prevot, V.; Bouret, S.G. Neonatal overnutrition causes early alterations in the central response to peripheral ghrelin. Mol. Metab. 2015, 4, 15–24. [Google Scholar] [CrossRef]
- Elizondo-Vega, R.; Cortés-Campos, C.; Barahona, M.J.; Carril, C.; Ordenes, P.; Salgado, M.; Oyarce, K.; García-Robles, M.d.L.A. Inhibition of hypothalamic MCT1 expression increases food intake and alters orexigenic and anorexigenic neuropeptide expression. Sci. Rep. 2016, 6, 33606. [Google Scholar] [CrossRef] [PubMed]
- López, M. Hypothalamic AMPK as a possible target for energy balance-related diseases. Trends Pharmacol. Sci. 2022, 43, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef]
- Williams, L.M. Hypothalamic dysfunction in obesity. Proc. Nutr. Soc. 2012, 71, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Valdearcos, M.; Xu, A.W.; Koliwad, S.K. Hypothalamic Inflammation in the Control of Metabolic Function. Annu. Rev. Physiol. 2015, 77, 131–160. [Google Scholar] [CrossRef]
- Palkar, R.; Lippoldt, E.K.; McKemy, D.D. The molecular and cellular basis of thermosensation in mammals. Curr. Opin. Neurobiol. 2015, 34, 14–19. [Google Scholar] [CrossRef]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Medina-Gómez, G.; López, M. Hypothalamus and thermogenesis: Heating the BAT, browning the WAT. Mol. Cell. Endocrinol. 2016, 438, 107–115. [Google Scholar] [CrossRef]
- Song, K.; Wang, H.; Kamm, G.B.; Pohle, J.; Reis, F.d.C.; Heppenstall, P.; Wende, H.; Siemens, J. The TRPM2 channel is a hypothalamic heat sensor that limits fever and can drive hypothermia. Science 2016, 353, 1393–1398. [Google Scholar] [CrossRef]
- Hu, F.; Xu, Y.; Liu, F. Hypothalamic roles of mTOR complex I: Integration of nutrient and hormone signals to regulate energy homeostasis. Am. J. Physiol. Metab. 2016, 310, E994–E1002. [Google Scholar] [CrossRef]
- Benevento, M.; Alpár, A.; Gundacker, A.; Afjehi, L.; Balueva, K.; Hevesi, Z.; Hanics, J.; Rehman, S.; Pollak, D.D.; Lubec, G.; et al. A brainstem–hypothalamus neuronal circuit reduces feeding upon heat exposure. Nature 2024, 628, 826–834. [Google Scholar] [CrossRef]
- Ambroziak, W.; Nencini, S.; Pohle, J.; Zuza, K.; Pino, G.; Lundh, S.; Araujo-Sousa, C.; Goetz, L.I.L.; Schrenk-Siemens, K.; Manoj, G.; et al. Thermally induced neuronal plasticity in the hypothalamus mediates heat tolerance. Nat. Neurosci. 2025, 28, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Sladek, C.D.; Johnson, A.K. Integration of thermal and osmotic regulation of water homeostasis: The role of TRPV channels. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R669–R678. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Gagnon, K.B. Water Homeostasis and Cell Volume Maintenance and Regulation. Curr. Top. Membr. 2018, 81, 3–52. [Google Scholar]
- Kageyama, K.; Iwasaki, Y.; Daimon, M. Hypothalamic regulation of corticotropin-releasing factor under stress and stress resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-R.; Li, L.; Zhao, J.-H.; Ge, X.-T.; Ren, W.-J.; Zhou, Y.; Chen, Y.; Zhang, F.; Gao, H.; Jia, T.-Z. Study on the Regulation of Hypothalamic-Pituitary-Adrenal Axis (HPA Axis) in Rats with Kidney-Yin Deficiency Syndrome by the Raw and Salt-Water Processing of Phellodendri chinensis Cortex. Int. J. Pharmacol. 2024, 20, 455–471. [Google Scholar] [CrossRef]
- Bielefeld, P.; Abbink, M.R.; Davidson, A.R.; Reijner, N.; Abiega, O.; Lucassen, P.J.; Korosi, A.; Fitzsimons, C.P. Early life stress decreases cell proliferation and the number of putative adult neural stem cells in the adult hypothalamus. Stress 2021, 24, 189–195. [Google Scholar] [CrossRef]
- Van Drunen, R.; Dai, Y.; Wei, H.; Fekry, B.; Noori, S.; Shivshankar, S.; Bravo, R.; Zhao, Z.; Yoo, S.-H.; Justice, N.; et al. Cell-specific regulation of the circadian clock by BMAL1 in the paraventricular nucleus: Implications for regulation of systemic biological rhythms. Cell Rep. 2024, 43, 114380. [Google Scholar] [CrossRef]
- Nicola, A.C.; Ferreira, L.B.; Mata, M.M.; Vilhena-Franco, T.; Leite, C.M.; Martins, A.B.; Antunes-Rodrigues, J.; Poletini, M.O.; Dornelles, R.C.M. Vasopressinergic Activity of the Suprachiasmatic Nucleus and mRNA Expression of Clock Genes in the Hypothalamus-Pituitary-Gonadal Axis in Female Aging. Front. Endocrinol. 2021, 12, 652733. [Google Scholar] [CrossRef]
- Kostin, A.; Alam, A.; McGinty, D.; Alam, N. Adult hypothalamic neurogenesis and sleep–wake dysfunction in aging. Sleep 2021, 44, zsaa173. [Google Scholar] [CrossRef]
- Chung, S.; Weber, F.; Zhong, P.; Tan, C.L.; Nguyen, T.N.; Beier, K.T.; Hörmann, N.; Chang, W.-C.; Zhang, Z.; Do, J.P.; et al. Identification of preoptic sleep neurons using retrograde labelling and gene profiling. Nature 2017, 545, 477–481. [Google Scholar] [CrossRef]
- Saper, C.B.; Fuller, P.M. Wake–sleep circuitry: An overview. Curr. Opin. Neurobiol. 2017, 44, 186–192. [Google Scholar] [CrossRef]
- Chen, K.-S.; Xu, M.; Zhang, Z.; Chang, W.-C.; Gaj, T.; Schaffer, D.V.; Dan, Y. A Hypothalamic Switch for REM and Non-REM Sleep. Neuron 2018, 97, 1168–1176.e4. [Google Scholar] [CrossRef]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D.; et al. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Karamacoska, D.; Butt, A.; Leung, I.H.K.; Childs, R.L.; Metri, N.-J.; Uruthiran, V.; Tan, T.; Sabag, A.; Steiner-Lim, G.Z. Brain function effects of exercise interventions for cognitive decline: A systematic review and meta-analysis. Front. Neurosci. 2023, 17, 1127065. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- Kennedy, M.B. Synaptic signaling in learning and memory. Cold Spring Harb. Perspect. Biol. 2016, 8, a016824. [Google Scholar] [CrossRef]
- Safdar, A.; Tarnopolsky, M.A. Exosomes as mediators of the systemic adaptations to endurance exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029827. [Google Scholar] [CrossRef] [PubMed]
- Novelli, G.; Calcaterra, G.; Casciani, F.; Pecorelli, S.; Mehta, J.L. ‘Exerkines’: A Comprehensive Term for the Factors Produced in Response to Exercise. Biomedicines 2024, 12, 1975. [Google Scholar] [CrossRef]
- Trinchero, M.F.; Buttner, K.A.; Cuevas, J.N.S.; Temprana, S.G.; Fontanet, P.A.; Monzón-Salinas, M.C.; Ledda, F.; Paratcha, G.; Schinder, A.F. High Plasticity of New Granule Cells in the Aging Hippocampus. Cell Rep. 2017, 21, 1129–1139. [Google Scholar] [CrossRef]
- Gandy, K.; Kim, S.; Sharp, C.; Dindo, L.; Maletic-Savatic, M.; Calarge, C. Pattern separation: A potential marker of impaired hippocampal adult neurogenesis in major depressive disorder. Front. Neurosci. 2017, 11, 571. [Google Scholar] [CrossRef]
- van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005, 25, 8680–8685. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Hong, D.K.; Kang, B.S.; Lee, S.H.; Choi, S.; Kim, H.-J.; Lee, S.M.; Suh, S.W. Engineered Mesenchymal Stem Cells Over-Expressing BDNF Protect the Brain from Traumatic Brain Injury-Induced Neuronal Death, Neurological Deficits, and Cognitive Impairments. Pharmaceuticals 2023, 16, 436. [Google Scholar] [CrossRef]
- Zare, N.; Bishop, D.J.; Levinger, I.; Febbraio, M.A.; Broatch, J.R. Exercise intensity matters: A review on evaluating the effects of aerobic exercise intensity on muscle-derived neuroprotective myokines. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2025, 11, e70056. [Google Scholar] [CrossRef]
- Jiang, H.; Kimura, Y.; Inoue, S.; Li, C.; Hatakeyama, J.; Wakayama, M.; Takamura, D.; Moriyama, H. Effects of different exercise modes and intensities on cognitive performance, adult hippocampal neurogenesis, and synaptic plasticity in mice. Exp. Brain Res. 2024, 242, 1709–1719. [Google Scholar] [CrossRef]
- Zhao, R.; Tian, X.; Xu, H.; Wang, Y.; Lin, J.; Wang, B. Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway. Aging Cell 2025, 24, e70061. [Google Scholar] [CrossRef] [PubMed]
- Bednarczyk, M.R.; Aumont, A.; Décary, S.; Bergeron, R.; Fernandes, K.J. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus 2009, 19, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Chae, C.H.; Jung, S.L.; An, S.H.; Park, B.Y.; Kim, T.W.; Wang, S.W.; Kim, J.H.; Lee, H.C.; Kim, H.T. Swimming exercise stimulates neuro-genesis in the subventricular zone via increase in synapsin i and nerve growth factor levels. Biol. Sport 2014, 31, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Nishii, A.; Amemiya, S.; Kubota, N.; Nishijima, T.; Kita, I. Adaptive changes in the sensitivity of the dorsal raphe and hypothalamic paraventricular nuclei to acute exercise, and hippocampal neurogenesis may contribute to the antidepressant effect of regular treadmill running in rats. Front. Behav. Neurosci. 2017, 11, 235. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.L.; Lemus, M.; Reichenbach, A.; Selathurai, A.; Oldfield, B.J.; Andrews, Z.B.; Watt, M.J. Hypothalamic neurogenesis is not required for the improved insulin sensitivity following exercise training. Diabetes 2014, 63, 3647–3658. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Cai, J.; Gu, B.; Lv, Y.; Zhao, L. The frequency-dependent aerobic exercise effects of hypothalamic GABAergic expression and cardiovascular func-tions in aged rats. Front. Aging Neurosci. 2017, 9, 212. [Google Scholar] [CrossRef]
- Rodrigues, Q.T.; Drummond, L.R.; Lima, P.M.A.; Machado, F.S.M.; Campos, H.O.; Szawka, R.E.; Leite, L.H.R.; Coimbra, C.C. Exercise performance effect of central dopamine is mediated by hypothalamic neuronal activation. Behav. Brain Res. 2025, 480, 115406. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, M.J.; Alviña, K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front. Aging Neurosci. 2021, 13, 649929. [Google Scholar] [CrossRef]
- Skouras, A.Z.; Antonakis-Karamintzas, D.; Tsantes, A.G.; Triantafyllou, A.; Papagiannis, G.; Tsolakis, C.; Koulouvaris, P. The Acute and Chronic Effects of Resistance and Aerobic Exercise in Hemostatic Balance: A Brief Review. Sports 2023, 11, 74. [Google Scholar] [CrossRef]
- Zuo, C.; Yin, Y.; Zheng, Z.; Mu, R.; Liang, Y.; Wang, S.; Ye, C. Unlocking the full potential of resistance training: A comparative analysis of low- and high-intensity effects on neurotrophic growth factors and homocysteine. Discov. Appl. Sci. 2025, 7, 108. [Google Scholar] [CrossRef]
- Gomes, F.G.N.; Fernandes, J.; Campos, D.V.; Cassilhas, R.C.; Viana, G.M.; D’aLmeida, V.; Rêgo, M.K.d.M.; Buainain, P.I.; Cavalheiro, E.A.; Arida, R.M. The beneficial effects of strength exercise on hippocampal cell proliferation and apoptotic signaling is impaired by anabolic androgenic steroids. Psychoneuroendocrinology 2014, 50, 106–117. [Google Scholar] [CrossRef]
- Nokia, M.S.; Lensu, S.; Ahtiainen, J.P.; Johansson, P.P.; Koch, L.G.; Britton, S.L.; Kainulainen, H. Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J. Physiol. 2016, 594, 1855–1873. [Google Scholar] [CrossRef]
- Shi, J.-X.; Wang, Z.-Y.; Wang, S.-W.; Shen, Q.; Tan, X. Exercise-mediated muscle-hypothalamus crosstalk: Improvement for cognitive dysfunction caused by disrupted circadian rhythm. Life Sci. 2025, 373, 123657. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Xiao, Q.; Zhu, L.; Tang, H.; Peng, W. Icariin targets p53 to protect against ceramide-induced neuronal senescence: Implication in Alzheimer’s disease. Free. Radic. Biol. Med. 2024, 224, 204–219. [Google Scholar] [CrossRef]
- He, R.; He, F.; Hu, Z.; He, Y.; Zeng, X.; Liu, Y.; Tang, L.; Xiang, J.; Li, J.; He, B.; et al. Analysis of Potential Mechanism of Herbal Formula Taohong Siwu Decoction against Vascular Dementia Based on Network Pharmacology and Molecular Docking. BioMed Res. Int. 2023, 2023, 1235552. [Google Scholar] [CrossRef]
- Ellison, C.; White, H.; McElhone, S. A content analysis of magazine diets in relation to the Eatwell plate. J. Hum. Nutr. Diet. 2011, 24, 386–387. [Google Scholar] [CrossRef]
- Kronenberg, G.; Harms, C.; Sobol, R.W.; Cardozo-Pelaez, F.; Linhart, H.; Winter, B.; Balkaya, M.; Gertz, K.; Gay, S.B.; Cox, D.; et al. Folate deficiency induces neurodegeneration and brain dysfunction in mice lacking uracil DNA glycosylase. J. Neurosci. 2008, 28, 7219–7230. [Google Scholar] [CrossRef]
- Smith, A.D. Hippocampus as a mediator of the role of vitamin B-12 in memory. Am. J. Clin. Nutr. 2016, 103, 959–960. [Google Scholar] [CrossRef]
- Michel, A.; Kokten, T.; Saber-Cherif, L.; Umoret, R.; Alberto, J.-M.; Helle, D.; Julien, A.; Daval, J.-L.; Guéant, J.-L.; Bossenmeyer-Pourié, C.; et al. Folate and Cobalamin Deficiencies during Pregnancy Disrupt the Glucocorticoid Response in Hypothalamus through N-Homocysteinilation of the Glucocorticoid Receptor. Int. J. Mol. Sci. 2023, 24, 9847. [Google Scholar] [CrossRef]
- Cuppini, R.; Ciaroni, S.; Cecchini, T.; Ambrogini, P.; Ferri, P.; Del Grande, P.; Papa, S. α-Tocopherol controls cell proliferation in the adult rat dentate gyrus. Neurosci. Lett. 2001, 303, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Oliva, R.; Geribaldi-Doldán, N.; Domínguez-García, S.; Carrascal, L.; Verástegui, C.; Nunez-Abades, P.; Castro, C. Vitamin D deficiency as a potential risk factor for accelerated aging, impaired hippocampal neurogenesis and cognitive decline: A role for Wnt/β-catenin signaling. Aging 2020, 12, 13824–13844. [Google Scholar] [CrossRef]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the brain: Genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Di Somma, C.; Scarano, E.; Barrea, L.; Zhukouskaya, V.V.; Savastano, S.; Mele, C.; Scacchi, M.; Aimaretti, G.; Colao, A.; Marzullo, P. Vitamin D and neurological diseases: An endocrine view. Int. J. Mol. Sci. 2017, 18, 2482. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.-P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Borsini, A.; Stangl, D.; Jeffries, A.R.; Pariante, C.M.; Thuret, S. The role of omega-3 fatty acids in preventing glucocorticoid-induced reduction in human hippocampal neurogenesis and increase in apoptosis. Transl. Psychiatry 2020, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, L.F.R.; Souza, G.F.P.; Morari, J.; Barbosa, G.O.; Solon, C.; Moura, R.F.; Victório, S.C.; Ignácio-Souza, L.M.; Razolli, D.S.; Carvalho, H.F.; et al. N-3 fatty acids induce neurogenesis of predominantly POMC-expressing cells in the hypothalamus. Diabetes 2016, 65, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Izquierdo, T.; Nemzer, B.; Shu, C.; Huynh, L.; Argumedo, R.; Keller, R.; Pietrzkowski, Z. Modulatory effect of coffee fruit extract on plasma levels of brain-derived neurotrophic factor in healthy subjects. Br. J. Nutr. 2013, 110, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Shukitt-Hale, B.; Bielinski, D.F.; Lau, F.C.; Willis, L.M.; Carey, A.N.; Joseph, J.A. The beneficial effects of berries on cognition, motor behaviour and neuronal function in ageing. Br. J. Nutr. 2015, 114, 1542–1549. [Google Scholar] [CrossRef]
- Casadesus, G.; Shukitt-Hale, B.; Stellwagen, H.M.; Zhu, X.; Lee, H.-G.; Smith, M.A.; Joseph, J.A. Modulation of hippocampal plasticity and cognitive behavior by short-term blueberry supplementation in aged rats. Nutr. Neurosci. 2004, 7, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Lou, S.; Gong, D.; Yang, M.; Qiu, Q.; Luo, J.; Chen, T. Curcumin Improves Neurogenesis in Alzheimer’s Disease Mice via the Upregulation of Wnt/β-Catenin and BDNF. Int. J. Mol. Sci. 2024, 25, 5123. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef]
- Moriya, J.; Chen, R.; Yamakawa, J.-I.; Sasaki, K.; Ishigaki, Y.; Takahashi, T. Resveratrol improves hippocampal atrophy in chronic fatigue mice by enhancing neurogenesis and inhibiting apoptosis of granular cells. Biol. Pharm. Bull. 2011, 34, 354–359. [Google Scholar] [CrossRef]
- Azargoonjahromi, A.; Abutalebian, F.; Hoseinpour, F. The role of resveratrol in neurogenesis: A systematic review. Nutr. Rev. 2025, 83, e257–e272. [Google Scholar] [CrossRef]
- Ong, J.; Wu, Q.; Sasaki, K.; Isoda, H.; Szele, F.G. Nutraceuticals: Using food to enhance brain health by modulating postnatal neurogenesis in animal models and patient populations. Stem Cells Transl. Med. 2025, 14, 6. [Google Scholar] [CrossRef]
- Santos, C.L.; Vizuete, A.F.K.; Weber, F.B.; Thomaz, N.K.; Bobermin, L.D.; Gonçalves, C.-A.; Quincozes-Santos, A. Age-dependent effects of resveratrol in hypothalamic astrocyte cultures. NeuroReport 2023, 34, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Erfanizadeh, M.; Noorafshan, A.; Namavar, M.R.; Karbalay-Doust, S.; Talaei-Khozani, T. Curcumin mitigates the sleep-deprivation impacts on rat hypothalamic paraventricular nucleus. IBRO Neurosci. Rep. 2023, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Leem, Y.-H.; Kato, M.; Chang, H. Regular exercise and creatine supplementation prevent chronic mild stress-induced decrease in hippocampal neurogenesis via Wnt/GSK3β/β-catenin pathway. J. Exerc. Nutr. Biochem. 2018, 22, 1. [Google Scholar] [CrossRef]
- Bender, A.; Klopstock, T. Creatine for neuroprotection in neurodegenerative disease: End of story? Amino Acids 2016, 48, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Leem, Y.-H. The potential role of creatine supplementation in neurodegenerative diseases. Phys. Act. Nutr. 2023, 27, 48–54. [Google Scholar] [CrossRef]
- Smith, A.N.; Morris, J.K.; Carbuhn, A.F.; Herda, T.J.; Keller, J.E.; Sullivan, D.K.; Taylor, M.K. Creatine as a Therapeutic Target in Alzheimer’s Disease. Curr. Dev. Nutr. 2023, 7, 102011. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Widmer, H.R.; Andres, R.H.; Wallimann, T. Creatine supplementation improves neural progenitor cell survival in Huntington’s disease. Brain Circ. 2016, 2, 133–137. [Google Scholar] [CrossRef]
- Antonio, J.; Pereira, F.; Curtis, J.; Rojas, J.; Evans, C. The Top 5 Can’t-Miss Sport Supplements. Nutrients 2024, 16, 3247. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC consensus statement: Dietary supplements and the high-performance athlete. Br. J. Sports Med. 2018, 52, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance—An umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2020, 54, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Mishra, A.; Singh, S.; Shukla, S. Caffeine improves memory and cognition via modulating neural progenitor cell survival and decreasing oxidative stress in Alzheimer’s rat model. Curr. Alzheimer Res. 2023, 20, 175–189. [Google Scholar] [CrossRef]
- Stazi, M.; Lehmann, S.; Sakib, M.S.; Pena-Centeno, T.; Büschgens, L.; Fischer, A.; Weggen, S.; Wirths, O. Long-term caffeine treatment of Alzheimer mouse models ameliorates behavioural deficits and neuron loss and promotes cellular and molecular markers of neurogenesis. Cell. Mol. Life Sci. 2021, 79, 55. [Google Scholar] [CrossRef]
- Han, M.-E.; Park, K.-H.; Baek, S.-Y.; Kim, B.-S.; Kim, J.-B.; Kim, H.-J.; Oh, S.-O. Inhibitory effects of caffeine on hippocampal neurogenesis and function. Biochem. Biophys. Res. Commun. 2007, 356, 976–980. [Google Scholar] [CrossRef]
- Wentz, C.T.; Magavi, S.S. Caffeine alters proliferation of neuronal precursors in the adult hippocampus. Neuropharmacology 2009, 56, 994–1000. [Google Scholar] [CrossRef]
- Mao, Z.; Ouyang, S.; Zhang, Q.; Wu, Y.; Wang, G.; Tu, L.; Luo, Z.; Li, W.; Kurihara, H.; Li, Y.; et al. New insights into the effects of caffeine on adult hippocampal neurogenesis in stressed mice: Inhibition of CORT-induced microglia activation. FASEB J. 2020, 34, 10998–11014. [Google Scholar] [CrossRef]
- Stazi, M.; Zampar, S.; Nadolny, M.; Büschgens, L.; Meyer, T.; Wirths, O. Combined long-term enriched environment and caffeine supplementation improve memory function in C57Bl6 mice. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 269–281. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Winter, K.; von Haefen, C.; Bührer, C. Caffeine Protects Against Anticonvulsant-Induced Impaired Neurogenesis in the Developing Rat Brain. Neurotox. Res. 2018, 34, 173–187. [Google Scholar] [CrossRef]
- Houghton, V.; Du Preez, A.; Lefèvre-Arbogast, S.; de Lucia, C.; Low, D.Y.; Urpi-Sarda, M.; Ruigrok, S.R.; Altendorfer, B.; González-Domínguez, R.; Andres-Lacueva, C.; et al. Caffeine Compromises Proliferation of Human Hippocampal Progenitor Cells. Front. Cell Dev. Biol. 2020, 8, 806. [Google Scholar] [CrossRef]
- Warrier, A.A.; Azua, E.N.; Kasson, L.B.; Allahabadi, S.; Khan, Z.A.; Mameri, E.S.; Swindell, H.W.; Tokish, J.M.; Chahla, J. Performance-Enhancing Drugs in Healthy Athletes: An Umbrella Review of Systematic Reviews and Meta-analyses. Sports Health A Multidiscipl. Approach 2024, 16, 695–705. [Google Scholar] [CrossRef]
- Yang, J.; Palsule, G.; Jiao, X.; Messenger, J.S.; Hart, R.P.; Kiledjian, M. Creatine mitigates neurogenesis impairment caused by defective DcpS decapping. Sci. Rep. 2025, 15, 17915. [Google Scholar] [CrossRef]
- Pazini, F.L.; Cunha, M.P.; Azevedo, D.; Rosa, J.M.; Colla, A.; de Oliveira, J.; Ramos-Hryb, A.B.; Brocardo, P.S.; Gil-Mohapel, J.; Rodrigues, A.L.S. Creatine Prevents Corticosterone-Induced Reduction in Hippocampal Proliferation and Differentiation: Possible Implication for Its Antidepressant Effect. Mol. Neurobiol. 2017, 54, 6245–6260. [Google Scholar] [CrossRef]
- Vercalsteren, E.; Karampatsi, D.; Buizza, C.; Paul, G.; Lundberg, J.O.; Nyström, T.; Darsalia, V.; Patrone, C. Prevention of Metabolic Impairment by Dietary Nitrate in Overweight Male Mice Improves Stroke Outcome. Nutrients 2025, 17, 2434. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary Nitrate Supplementation and Exercise Performance. Sports Med. 2014, 44 (Suppl. S1), S35–S45. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, L.; Zhang, Z.; Wang, Y.; Lu, M.; LaPointe, M.; Chopp, M. A nitric oxide donor induces neurogenesis and reduces functional deficits after stroke in rats. Ann. Neurol. 2001, 50, 602–611. [Google Scholar] [CrossRef]
- Suhett, L.G.; Santos, R.d.M.M.; Silveira, B.K.S.; Leal, A.C.G.; de Brito, A.D.M.; de Novaes, J.F.; Della Lucia, C.M. Effects of curcumin supplementation on sport and physical exercise: A systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 946–958. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, Q.; Mitchell, E.S.; Xiu, J.; Duan, Y.; Li, C.; Tiwari, J.K.; Hu, Y.; Cao, X.; Zhao, Z. Curcumin enhances neurogenesis and cognition in aged rats: Implications for transcriptional interactions related to growth and synaptic plasticity. PLoS ONE 2012, 7, e31211. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Tae, G.S.; Hee, R.P.; Park, M.; Kim, M.S.; Hyung, S.K.; Hae, Y.C.; Mattson, M.P.; Lee, J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J. Biol. Chem. 2008, 283, 14497–14505. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wu, Q.; Wang, C.; Deng, P.; Li, J.; Zhai, Z.; Li, Y. Curcumin reverses cognitive deficits through promoting neurogenesis and synapse plasticity via the upregulation of PSD95 and BDNF in mice. Sci. Rep. 2025, 15, 1135. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Park, H.R.; Lee, J.Y.; Kim, J.; Yang, S.; Lee, C.; Kim, K.; Kim, H.S.; Chang, S.-C.; Lee, J. Low-dose curcumin enhances hippocampal neurogenesis and memory retention in young mice. Arch. Pharmacol. Res. 2023, 46, 423–437. [Google Scholar] [CrossRef]
- Yang, X.; Song, D.; Chen, L.; Xiao, H.; Ma, X.; Jiang, Q.; Cheng, O. Curcumin promotes neurogenesis of hippocampal dentate gyrus via Wnt/β-catenin signal pathway following cerebral ischemia in mice. Brain Res. 2021, 1751, 147197. [Google Scholar] [CrossRef]
- Chen, J.; Wei, C.; Zhang, Y.; Chen, Q.; Li, X.; Zhang, W.; Zhang, X.; Han, F. Curcumin exerts neuroprotective effects on proliferation of neural stem cells in vitro and APP/PS1 mouse model in vivo. Sci. Rep. 2025, 15, 27045. [Google Scholar] [CrossRef]
- Mattova, S.; Simko, P.; Urbanska, N.; Kiskova, T. Bioactive Compounds and Their Influence on Postnatal Neurogenesis. Int. J. Mol. Sci. 2023, 24, 16614. [Google Scholar] [CrossRef]
- Miguel, A.M.C.S.; Roche, E.; Herranz-López, M.; Miguel, M.C.S.; Mielgo-Ayuso, J.; Fernández-Lázaro, D. Impact of Melatonin Supplementation on Sports Performance and Circulating Biomarkers in Highly Trained Athletes: A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 1011. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Zhou, W.; Ji, S.; Li, X.; Li, G.; Liu, G.; Wang, F.; Hao, A. Effect of melatonin on neuronal differentiation requires CBP/p300-mediated acetylation of histone H3 lysine 14. Neuroscience 2017, 364, 45–59. [Google Scholar] [CrossRef]
- Ghareghani, M.; Sadeghi, H.; Zibara, K.; Danaei, N.; Azari, H.; Ghanbari, A. Melatonin Increases Oligodendrocyte Differentiation in Cultured Neural Stem Cells. Cell. Mol. Neurobiol. 2017, 37, 1319–1324. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Lv, Q.; Chen, X.; Deng, W.; Shi, K.; Pan, L. Effects and mechanisms of melatonin on the proliferation and neural differentiation of PC12 cells. Biochem. Biophys. Res. Commun. 2016, 478, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ottenhof, T.; Rzeczkowska, P.A.; Niles, L.P. Epigenetic targets for melatonin: Induction of histone H3 hyperacetylation and gene expression in C17.2 neural stem cells. J. Pineal Res. 2008, 45, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef]
- Rennie, K.; De Butte, M.; Pappas, B.A. Melatonin promotes neurogenesis in dentate gyrus in the pinealectomized rat. J. Pineal Res. 2009, 47, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Motta-Teixeira, L.C.; Machado-Nils, A.V.; Battagello, D.S.; Diniz, G.B.; Andrade-Silva, J.; Silva, S., Jr.; Matos, R.A.; Amaral, F.G.D.; Xavier, G.F.; Bittencourt, J.C.; et al. The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm. Behav. 2018, 105, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.; Vega-Rivera, N.M.; Benítez-King, G.; Castro-García, M.; Ortíz-López, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58. [Google Scholar] [CrossRef]
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal Res. 2013, 54, 222–231. [Google Scholar] [CrossRef]
- Ortiz-López, L.; Pérez-Beltran, C.; Ramírez-Rodríguez, G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Ortiz-López, L.; Granados-Juárez, A.; Estrada-Camarena, E.M.; Ramírez-Rodríguez, G.B. Melatonin Reverses the Depression-associated Behaviour and Regulates Microglia, Fractalkine Expression and Neurogenesis in Adult Mice Exposed to Chronic Mild Stress. Neuroscience 2020, 440, 316–336. [Google Scholar] [CrossRef]
- Abidin, N.Z.; Ooi, C.H.; Nosaka, K.; Rathakrishnan, V.; Chan, S.Y.; A Karim, N.K. Effects of Resveratrol Supplementation on Delayed Onset Muscle Soreness and Muscle Recovery: A Systematic Review. Malays. J. Med Sci. 2024, 31, 77–102. [Google Scholar] [CrossRef]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef]
- Thomas, J.; Garg, M.L.; Smith, D.W. Dietary resveratrol supplementation normalizes gene expression in the hippocampus of streptozotocin-induced diabetic C57Bl/6 mice. J. Nutr. Biochem. 2014, 25, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Parihar, V.K.; Hattiangady, B.; Mishra, V.; Shuai, B.; Shetty, A.K. Resveratrol Prevents Age-Related Memory and Mood Dysfunction with Increased Hippocampal Neurogenesis and Microvasculature and Reduced Glial Activation. Sci. Rep. 2015, 5, 8075. [Google Scholar] [CrossRef]
- Park, H.R.; Kong, K.H.; Yu, B.P.; Mattson, M.P.; Lee, J. Resveratrol inhibits the proliferation of neural progenitor cells and hippocampal neurogenesis. J. Biol. Chem. 2012, 287, 42588–42600. [Google Scholar] [CrossRef]
- Crupi, R.; Marino, A.; Cuzzocrea, S. n-3 fatty acids: Role in neurogenesis and neuroplasticity. Curr. Med. Chem. 2013, 20, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Arribalzaga, S.; Gutiérrez-Abejón, E.; Azarbayjani, M.A.; Mielgo-Ayuso, J.; Roche, E. Omega-3 Fatty Acid Supplementation on Post-Exercise Inflammation, Muscle Damage, Oxidative Response, and Sports Performance in Physically Healthy Adults—A Systematic Review of Randomized Controlled Trials. Nutrients 2024, 16, 2044. [Google Scholar] [CrossRef] [PubMed]
- Beltz, B.S.; Tlusty, M.F.; Benton, J.L.; Sandeman, D.C. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci. Lett. 2007, 415, 154–158. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef]
- Kawakita, E.; Hashimoto, M.; Shido, O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006, 139, 991–997. [Google Scholar] [CrossRef]
- Rodríguez-Iglesias, N.; Nadjar, A.; Sierra, A.; Valero, J. Susceptibility of Female Mice to the Dietary Omega-3/Omega-6 Fatty-Acid Ratio: Effects on Adult Hippocampal Neurogenesis and Glia. Int. J. Mol. Sci. 2022, 23, 3399. [Google Scholar] [CrossRef]
- Dyall, S.C.; Michael, G.J.; Michael-Titus, A.T. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J. Neurosci. Res. 2010, 88, 2091–2102. [Google Scholar] [CrossRef]
- Huguet, G.; Puig-Parnau, I.; Serrano, J.C.E.; Martin-Gari, M.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Tibau, J.; Kádár, E. Hippocampal neurogenesis and Arc expression are enhanced in high-fat fed prepubertal female pigs by a diet including omega-3 fatty acids and Bifidobacterium breve CECT8242. Eur. J. Nutr. 2023, 62, 2463–2473. [Google Scholar] [CrossRef]
- Cesak, O.; Vostalova, J.; Vidlar, A.; Bastlova, P.; Student, V. Carnosine and Beta-Alanine Supplementation in Human Medicine: Narrative Review and Critical Assessment. Nutrients 2023, 15, 1770. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, T.E.; Pence, B.D.; Petr, G.; Ossyra, J.M.; Mach, H.C.; Bhattacharya, T.K.; Perez, S.; Martin, S.A.; McCusker, R.H.; Kelley, K.W.; et al. Voluntary wheel running, but not a diet containing (−)-epigallocatechin-3-gallate and β-alanine, improves learning, memory and hippocampal neurogenesis in aged mice. Behav. Brain Res. 2014, 272, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bátiz, L.F.; Castro, M.A.; Burgos, P.V.; Velásquez, Z.D.; Muñoz, R.I.; Lafourcade, C.A.; Troncoso-Escudero, P.; Wyneken, U. Exosomes as novel regulators of adult neurogenic niches. Front. Cell. Neurosci. 2016, 9, 501. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Arellano, J.I.; Duque, A.; Rakic, P. Comment on “Impact of neurodegenerative diseases on human adult hippocampal neurogenesis”. Science 2022, 376, 7083. [Google Scholar] [CrossRef] [PubMed]
- Terreros-Roncal, J.; Moreno-Jiménez, E.P.; Flor-García, M.; Rodríguez-Moreno, C.B.; Trinchero, M.F.; Márquez-Valadez, B.; Cafini, F.; Rábano, A.; Llorens-Martín, M. Response to Comment on “Impact of neurodegenerative diseases on human adult hippocampal neurogenesis”. Science 2022, 376, 7270. [Google Scholar] [CrossRef]
- Wei, L.-C.; Shi, M.; Chen, L.-W.; Cao, R.; Zhang, P.; Chan, Y. Nestin-containing cells express glial fibrillary acidic protein in the proliferative regions of central nervous system of postnatal developing and adult mice. Dev. Brain Res. 2002, 139, 9–17. [Google Scholar] [CrossRef]
- Shimogori, T.; A Lee, D.; Miranda-Angulo, A.; Yang, Y.; Wang, H.; Jiang, L.; Yoshida, A.C.; Kataoka, A.; Mashiko, H.; Avetisyan, M.; et al. A genomic atlas of mouse hypothalamic development. Nat. Neurosci. 2010, 13, 767–775. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef]
- Feng, S.; Wu, C.; Zou, P.; Deng, Q.; Chen, Z.; Li, M.; Zhu, L.; Li, F.; Liu, T.C.-Y.; Duan, R.; et al. High-intensity interval training ameliorates Alzheimer’s disease-like pathology by regulating astrocyte phenotype-associated AQP4 polarization. Theranostics 2023, 13, 3434–3450. [Google Scholar] [CrossRef]
- Okamoto, M.; Mizuuchi, D.; Omura, K.; Lee, M.; Oharazawa, A.; Yook, J.S.; Inoue, K.; Soya, H. High-intensity Intermittent Training Enhances Spatial Memory and Hippocampal Neurogenesis Associated with BDNF Signaling in Rats. Cereb. Cortex 2021, 31, 4386–4397. [Google Scholar] [CrossRef] [PubMed]
- Hugues, N.; Pellegrino, C.; Rivera, C.; Berton, E.; Pin-Barre, C.; Laurin, J. Is high-intensity interval training suitable to promote neuroplasticity and cognitive functions after stroke? Int. J. Mol. Sci. 2021, 22, 3003. [Google Scholar] [CrossRef] [PubMed]
| Hypothalamic Nucleus | Main Function | Signaling Pathways |
|---|---|---|
| Tuberomammillary nucleus (TMN) | Control of arousal, learning, memory and wakefulness Functional inactivation produces somnolence [62,63] | Only source of histamine in brain; synthesized from Histidine by Histidine decarboxylase Acts on histamine receptors (H1–H4): H1 → Neuronal depolarization → Promotes wakefulness H2 → Modulates behavior H3 → Inhibitory autoreceptor/heteroreceptor → Regulates multiple neurotransmitters H4 → Mainly outside the central nervous system [64,65] |
| Lateral hypothalamic area (LHA) | Regulation of feeding, drinking, energy balance, thermogenesis, and motivated behaviors Lesions disrupt feeding, drinking, and body weight regulation Electrical stimulation promotes ingestive behaviors [66,67,68,69] | Distinct cell types exert opposing influences: Orexin neurons: ↑ food-seeking, arousal, and thermogenesis Melanin-concentrating hormone (MCH) neurons: ↑ feeding and fat storage and ↓ activity Leptin receptor and neurotensin-expressing neurons: ↓ hunger and ↑ exploration Glucose-inhibited glutamic acid decarboxylase 65 (GAD65) neurons: modulate feeding in response to energy status Cells expressing solute carrier family 12 member 8 (Slc12a8) influence systemic metabolism via sympathetic pathways [70,71,72] |
| Ventrolateral preoptic nucleus (VLPO) | Inhibiting subcortical arousal centers → Promotion and maintenance of sleep Lesions → insomnia Activation of γ-aminobutyric acid (GABA) → ↑ wakefulness [73,74] | Inhibit primarily GABAergic and galaninergic neurons projecting to orexin neurons in the LHA Some neurons co-express GABA and galanin Others express excitatory markers → integrates inputs from multiple brain regions → modulate arousal [73,75] |
| Arcuate nucleus (ARC) | Regulation of feeding behavior, energy expenditure, glucose homeostasis, cardiovascular function, and fertility [76,77] | Is richly endowed with hormone receptors and specialized neuroendocrine cells with distinct physiological roles: Orexigenic agouti-related peptide and neuropeptide Y (NPY) neurons → project to multiple hypothalamic targets → ↑ feeding and ↓ energy expenditure Anorexigenic pro-opiomelanocortin and cocaine- and amphetamine-regulated transcript (CART) neurons → release alpha-melanocyte-stimulating hormone (α-MSH) → ↓ appetite and ↑ metabolism These two populations function antagonistically. Ghrelin → excites orexigenic neurons and inhibits anorexigenic neurons Leptin and insulin → inhibit orexigenic neurons and activate anorexigenic neurons [77] |
| Ventromedial hypothalamic nucleus (VMH) | Satiety center of the central nervous system, playing a crucial role in regulating food intake, glucose homeostasis, body weight and thermogenesis Bilateral lesions → overeating and obesity Electrical stimulation of its neurons → ↓ food consumption [78,79,80] | Sends sympathetic and parasympathetic signals to visceral organs AMP-activated protein kinase (AMPK) signaling, estrogen receptor alpha (ERα) activation, and melanocortin signaling → Modulates brown and white adipose tissue Senses glucose through specialized neurons and regulates glucagon and cortisol [78,81] |
| Dorsomedial hypothalamic nucleus (DMH) | Plays a central role in regulating feeding rhythms, food intake, brown adipose tissue thermogenesis, cardiorespiratory activity, neuroendocrine responses, stress avoidance, arousal, locomotor activity, and torpor [82,83,84] | Specific DMH neurons sense energy balance and corticosterone levels, relaying this information to the LHA to coordinate adaptive responses. Thyrotropin-releasing hormone (TRH) neurons → project to various hypothalamic regions → depolarize orexin neurons → ↑ arousal and locomotor activity, and inhibit MCH neurons via local GABAergic interneurons Leptin receptor-expressing neurons → ↑ energy expenditure and ↓ body weight [82,84] |
| Suprachiasmatic nucleus (SCN) | Central pacemaker of the circadian timing system, regulating most circadian rhythms → sleep–wake cycles, appetite, autonomic functions, and neuroendocrine activity It modulates hormone secretion and diurnal behaviors based on light input received through the retinohypothalamic tract [85,86] | Most neurons are GABA-positive Core region enriched with vasoactive intestinal polypeptide neurons and shell region contains arginine vasopressin (AVP) neurons Receives inhibitory GABAergic input from the DMH → regulate food-anticipatory activity Regulates glucocorticoid rhythms through projections to the PVN, modulating corticotropin-releasing hormone (CRH) release Connects to the ARC to influence feeding and energy balance through α-MSH neurons [87] |
| Anterior hypothalamic nucleus (AHN) | Modulation of heat loss, metabolic heat, defensive attacks, social aggression, and thirst/fluid balance [88,89,90,91] | Vasopressin-expressing neurons (via V1a receptor) → stress responses, social behavior Noradrenergic activity → thirst regulation, fluid homeostasis, autonomic adjustments [92,93] |
| Paraventricular nucleus (PVN) | Control arousal, defensive behavior, pain perception, the hypothalamic–pituitary–adrenal axis, hypothalamic–pituitary–thyroid axis, growth hormone axis, hydromineral balance, uterine contraction, milk letdown, appetite, and metabolism Dysfunctions → obesity, insomnia, anxiety, depression, and chronic pain [94,95] | Diverse neuronal populations producing distinct hormones: CRH, TRH, oxytocin, AVP, glutamate/neuropeptides Beyond direct endocrine control, sends glutamatergic and neuropeptidergic projections to multiple brain regions [94,96] |
| Posterior hypothalamic nucleus (PHN) | Regulates heat production, sympathetic activity, heart rate, blood pressure, alertness, and defensive behaviors Local inhibition decreases body temperature and heart rate under cold stress [97,98,99,100] | Receives afferents from the insular cortex, septal nuclei, amygdala, subiculum, bed nucleus of stria terminalis, central gray, parabrachial nucleus, nucleus of the solitary tract and brainstem reticular nuclei Contains a mix of glutamate-releasing, GABA-releasing, peptide-producing (such as orexin, MCH, and NPY), and catecholamine-releasing neurons Expresses a wide range of receptors including GABA-A, N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), orexin, corticotropin-releasing factor, muscarinic acetylcholine, adrenergic, and dopamine receptors [97,101,102,103,104,105] |
| Supraoptic nucleus (SON) | Play essential roles in water homeostasis, lactation, and reproductive behaviors [106,107] | Composed of magnocellular neuroendocrine cells (MNCs) → including vasopressin- and oxytocin-producing neurons → regulate vasopressin secretion Receives projections from the MPO, ARC, and parabrachial nuclei [106,107] |
| Medial preoptic nucleus (MPO) | Play a central role in sexual behavior, parental care, aggression, social interactions and social behaviors [108,109,110] | Integrates hormonal signals: oxytocin, prolactin, estrogen, and progesterone Relies on specific neuronal subtypes: galanin-, neurotensin-, and GABA-expressing neurons Hormone-sensitive neurons convert endocrine signals into activity patterns → project to ventral tegmental area and periaqueductal gray Spatial organization defines hormonal integration [58,108] |
| Median preoptic nucleus (MnPO) | Contributes to body fluid balance, integrates thermal and immune signals, mediating fever and regulate sleep–wake states [111,112,113] | Expresses angiotensin type 1 receptors, allowing angiotensin II to increase neuronal excitability → regulate thirst, blood pressure, and AVP release via MnPO → PVN/SON projections Prostaglandin E2 acts on prostaglandin E2 receptor subtype 3-expressing (EP3R) neurons → mediate fever Vesicular glutamate transporter 2 (Vglut2) glutamatergic neurons → wakefulness Vesicular GABA transporter (Vgat) GABAergic neurons → non-rapid eye movement sleep [111,112,113] |
| Hypothalamic Nucleus | Author and Year (Ref) | Animal Species | Neurogenic Markers | Treatment | Conclusion |
|---|---|---|---|---|---|
| Lateral hypothalamic area (LHA) | Chaker et al., 2016 [137] | CAG-tdTomato/0 mice ♀ | BrdU+/NeuN+ Tom+/NeuN+ | Tamoxifen induction of Cre recombinase | Although the LHA is functionally important, to our knowledge, evidence for adult neurogenesis in this region is limited to a single study reporting widespread neurogenesis across multiple hypothalamic nuclei, including the LHA. |
| Arcuate nucleus (ARC) | Safahani et al., 2018 [150] | C57BL/6 mice ♂ | BrdU+/POMC+ BrdU+/NPY+ | High-fat diet (HFD) exposure | Although only the most relevant articles are highlighted here, evidence for adult neurogenesis in the ARC is extensive and it seems to be mainly modulated by dietary factors such as an HFD and exercise. |
| Lee et al., 2014 [151] | C57BL/6 mice ♀/♂ | BrdU+/Hu+ | HFD exposure | ||
| Bless et al., 2014 [152] | C57BL6 mice ♀ | BrdU+/Hu+ | HFD exposure | ||
| Gouazé et al., 2013 [153] | C57BL/6 mice ♂ | BrdU+/NeuN+ BrdU+/POMC+ | HFD exposure | ||
| Chaker et al., 2016 [137] | CAG-tdTomato/0 mice ♀ | BrdU+/NeuN+ Tom+/NeuN+ | Tamoxifen induction of Cre recombinase | ||
| Batailler et al., 2014 [154] | C57BL/6 mice ♀/♂ | Nestin+/Sox2+/DCX+ | None | ||
| Jörgensen et al., 2023 [155] | C57BL/6 mice ♂ | BrdU+/NeuN+ | HFD exposure, Prolactin-Releasing Peptide administration, and Liraglutide administration | ||
| Klein et al., 2019 [156] | C57BL/6 mice ♀ | BrdU+/HuD+ BrdU+/POMC+ | HFD and exercise exposure | ||
| Ventromedial hypothalamic nucleus (VMH) | Bless et al., 2014 [152] | C57BL/6 mice ♀ | BrdU+/Hu+ | HFD exposure | Similar to the ARC, extensive studies have demonstrated adult neurogenesis in the VMH, which appears to be modulated by dietary and hormonal factors. Certain subpopulations of newborn neurons have been identified as responsive to hormones such as estrogens and leptin, indicating functional integration into metabolic circuits. |
| Chaker et al., 2016 [137] | CAG-tdTomato/0 mice ♀ | BrdU+/NeuN+ Tom+/NeuN+ | Tamoxifen induction of Cre recombinase | ||
| Batailler et al., 2014 [154] | C57BL/6 mice ♀/♂ | Nestin+/Sox2+/DCX+ | None | ||
| Feighan et al., 2024 [157] | C57BL/6 mice ♀/♂ | BrdU+/NeuN+ | Gestational Bisphenol A (BPA) and HFD offspring exposure | ||
| Levy et al., 2019 [158] | Ile de France ewes ♀ | Ki67+/DCX+ Sox2+/DCX+ | Steroid-primed and oxytocin infusion | ||
| Jörgensen et al., 2023 [155] | C57BL/6 mice ♂ | BrdU+/NeuN+ | HFD exposure, Prolactin-Releasing Peptide administration, and Liraglutide administration | ||
| Dorsomedial hypothalamic nucleus (DMH) | Bless et al., 2014 [152] | C57BL/6 mice ♀ | BrdU+/Hu+ | HFD exposure | While less extensively studied than the ARC or VMH, evidence for adult neurogenesis in the DMH has been reported. |
| Chaker et al., 2016 [137] | CAG-tdTomato/0 mice ♀ | BrdU+/NeuN+ Tom+/NeuN+ | Tamoxifen induction of Cre recombinase | ||
| Feighan et al., 2024 [157] | C57BL/6 mice ♀/♂ | BrdU+/NeuN+ | Gestational Bisphenol A (BPA) and HFD offspring exposure | ||
| Jörgensen et al., 2023 [155] | C57BL/6 mice ♂ | BrdU+/NeuN+ | HFD exposure, Prolactin-Releasing Peptide administration, and Liraglutide administration | ||
| Paraventricular nucleus (PVN) | Feighan et al., 2024 [157] | C57BL/6 mice ♀/♂ | BrdU+/NeuN+ | Gestational Bisphenol A (BPA) and HFD offspring exposure | Evidence for adult neurogenesis in the PVN is limited but consistent across species. New neurons can be generated under various physiological and environmental conditions, such as gestational BPA exposure, HFD, and WD. |
| Raymond et al., 2006 [159] | Yorkshire pigs ♀ | PCNA+/OT+ | None | ||
| Zhang et al., 2024 [160] | Wistar and Sprague–Dawley rats ♀/♂ | BrdU+/NPII+ DCX+/NPII+ | Chronic intermittent water-deprivation (WD) | ||
| Posterior hypothalamic nucleus (PHN) | Chaker et al., 2016 [137] | CAG-tdTomato/0 mice ♀ | BrdU+/NeuN+ Tom+/NeuN+ | Tamoxifen induction of Cre recombinase | To our knowledge, evidence for adult neurogenesis in the PHN is limited, and no studies have specifically investigated this nucleus. |
| Preoptic area of anterior hypothalamus (POA/AH) | Raymond et al., 2006 [159] | Yorkshire pigs ♀ | PCNA+/OT+ | None | While the specific nuclei within the PO/AH have not been individually investigated for adult neurogenesis, evidence consistently supports the occurrence of this process in the PO/AH as a whole, modulated by factors such as heat exposure and WD. |
| Matsuzaki et al., 2015 [161] | Wistar rats ♂ | BrdU+/NeuN+ | Heat exposure | ||
| Shido and Matsuzaki, 2015 [162] | Wistar rats ♂ | BrdU+/NeuN+ | Heat exposure | ||
| Matsuzaki et al., 2017 [141] | Wistar rats ♂ | BrdU+/NeuN+ | Heat exposure | ||
| Zhang et al., 2024 [160] | Wistar and Sprague–Dawley rats ♀/♂ | BrdU+/NPII+ DCX+/NPII+ | WD |
| Compound | Proposed Mechanism of Action | Effects on Exercise Performance | Author and Year (ref) | Dose and Duration | Neurogenic Evidence |
|---|---|---|---|---|---|
| Caffeine | Adenosine receptor antagonism, ↑ endorphin release [251] | ↑ Performance → ↑ Muscle endurance ↑ Strength ↑ Anaerobic power ↑ Aerobic capacity [251,252] | Tiwari et al., 2023 [253] | 10 mg/kg intra-peritoneally (i.p.), once daily for 28 days | ↑ BrdU+/DCX+ ↑ BrdU+/NeuN+ Niche: Hippocampus |
| Stazi et al., 2021 [254] | 300 mg/L in drinking water for 4 months | ↓ Neuron loss ↑ DCX+ Niche: Hippocampus | |||
| Han et al., 2007 [255] | 0.3 g/L in drinking water for 4 weeks | ↓ Proliferation of neural stem cells (NSCs) Niche: Hippocampus | |||
| Wentz et al., 2009 [256] | 20–60 mg/kg per day in saline (0.02 mL/g body weight) for 7 days | Intermediate doses: ↓ BrdU+ highest dose: ↑ BrdU+ ↔ Differentiation and survival Niche: Hippocampus | |||
| Mao et al., 2020 [257] | 10–20 mg/kg, oral (intragastric) daily for 4 weeks | ↑ BrdU+/DCX+ Niche: Hippocampus | |||
| Stazi et al., 2023 [258] | 300 mg/L in drinking water, chronic administration for 4 months | ↔ DCX+ cells Niche: Hippocampus | |||
| Endesfelder et al., 2018 [259] | 10 mg/kg, i.p., daily for 3 consecutive days | ↑ Ki67+/NeuN+ ↑ DCX+ Niche: Hippocampus | |||
| Houghton et al., 2020 [260] | 0.1–1.0 mM | Low dose: ↔ DAPI+, Nestin+, SOX2+ High dose: ↓ DAPI+, Nestin+, SOX2+ ↔Ki67+/CC3+ In vitro | |||
| Creatine | ↑ Brain creatine stores → ↑ phosphocreatine resynthesis [248,251] | ↑ Lean body mass ↑ Muscle strength ↑ Performance in short, high-intensity, repetitive activities Anti-inflammatory and antioxidant benefits [251,261] | Leem et al., 2018 [244] | Oral via food, 4% of pellet, 4 weeks | ↑Ki-67+/DCX+ Niche: Hippocampus |
| Yang et al., 2025 [262] | 100 μM | ↑ HuC/D+ ↑ neurite length In vitro | |||
| Pazini et al., 2017 [263] | 10 mg/kg, oral gavage, once daily for 21 days | ↑Ki-67+ ↑NeuroD+ versus corticosterone Niche: Hippocampus | |||
| Nitrate | ↑ nitric oxide → ↑ AMPK, glucose uptake, insulin and endothelial function. ↓ oxidative stress and fat synthesis [251,264] | ↑ Endurance performance → ↑ Muscle oxygenation ↑ Mitochondrial efficiency ↑ Contractile function. ↑ Type II muscle fiber function [251,265] | Vercalsteren et al., 2025 [264] | 0.1 mmol/kg/day, oral via drinking water, 18 weeks | ↔ Ki67+ ↔ DCX+ Niche: subventricular zone (SVZ) |
| Zhang et al., 2001 [266] | 0.1–0.4 mg/kg/day, intravenous or i.p., for 7 days | ↑ BrdU+ cells Niche: Hippocampus, SVZ | |||
| Curcumin | Anti-inflammatory, ↓ oxidative stress [251,267] | ↑ Recovery ↓ Inflammation ↓ Oxidative stress ↑ Cardiovascular response ↑ Thermoregulatory response ↑ Psychological response [267] | Dong et al., 2012 [268] | Oral via chow, 480 mg/kg for 6–12 weeks | ↑ BrdU+ Niche: Hippocampus |
| Kim et al., 2008 [269] | 0.1, 0.5, 1, 10, 20, and 50 μm 500 nmol/kg, i.p., once daily for 4 days | ↑ BrdU+ ↑ BrdU+/NeuN+ Niche: Hippocampus, In vitro | |||
| Li et al., 2025 [270] | 10–20 mg/mL in 0.9% saline, administered by gavage for 7 days | ↑ BrdU+ ↑ dendritic growth. Niche: Hippocampus | |||
| Lee et al., 2023 [271] | Oral, once daily at 0.4, 2, or 10 mg/kg for 14 days | ↑ BrdU+ ↑ BrdU+/NeuN+ ↑ DCX+ [251,267] Niche: Hippocampus | |||
| Yang et al., 2021 [272] | 50, 100 mg/kg/day i.p. | ↑ Brdu+ ↑ DCX+ ↑ Brdu+/NeuN+ Niche: Hippocampus. | |||
| Lou et al., 2024 [237] | 100 mg/kg, intragastric, 14 days | ↑ BrdU+ ↑ BrdU+/DCX+ Niche: Hippocampus. | |||
| Chen et al., 2025 [273] | 0 µM, 0.5 µM, 2.5 µM, 12.5 µM, 62.5 µM 100 mg/kg and 300 mg/kg | ↑ Proliferation of NSCs ↑ EdU+/NeuN+ Niche: Hippocampus, In vitro | |||
| Melatonin | Regulating circadian rhythms and the sleep–wake cycle. Neuroprotective, antioxidant, anti-inflammatory, and anti-apoptotic effects [274] | ↓ Oxidative stress ↓ Inflammation ↓ Muscle damage ↓ Liver damage ↑ Recovery of muscle function [275] | Li et al., 2017 [276] | 100 nM for 1, 3, 5, or 7 days | ↓ Nestin+/DAPI+ ↑ Tuj1+ ↑ MAP2+ ↔ GFAP In vitro |
| Ghareghani et al., 2017 [277] | 0.05, 0.1, 0.5, 1, 5 and 10 μM | Low dose: ↑ Viability of NSCs ↑ MBP+ ↑ GFAP+ In vitro | |||
| Liu et al., 2016 [278] | 10 μM | ↑ PC12 cell proliferation ↑ Neurite outgrowth ↑ MAP2+ In vitro | |||
| Sharma et al., 2008 [279] | 1.0 and 10 nM | ↑ Neurite-like extensions ↑ mRNA expression of Nestin ↑ mRNA expression of β-III-tubulin In vitro | |||
| Ramirez-Rodriguez et al., 2011 [280] | 8 mg/kg/day, i.p. for 14 days | ↑ DCX+ ↑ Dendritic maturation Niche: Hippocampus | |||
| Rennie et al., 2009 [281] | 0.51 mg/kg/day, oral (drinking water), for 7 days | ↑ DCX+ ↑ BrdU+/NeuN+ Niche: Hippocampus | |||
| Motta-Teixeira et al., 2018 [282] | 0.5 mg/kg/day, oral (drinking water), for 7 days | ↑ Ki-67+ versus maternal melatonin deprivation Niche: Hippocampus | |||
| Ramírez-Rodríguez et al., 2012 [283] | 8 mg/kg/day, oral, for 3, 6, 9, or 12 months | ↑ Ki67+ ↑ pH3H+ ↑ BrdU+ ↑ DCX+ Niche: Hippocampus | |||
| Liu et al., 2013 [284] | 0.02 mg/mL/day, oral for 12 days | ↑ BrdU+/NeuN+ Niche: Hippocampus | |||
| Ortiz-López et al., 2016 [285] | Chronic blockade of melatonin membrane receptors | Chronic blockade of melatonin membrane receptors: ↓ Ki67+ ↓ DCX+ Niche: Hippocampus | |||
| Vega-Rivera et al., 2020 [286] | 2.5 mg/kg/day, i.p., for 4 weeks | ↑ Ki-67+ ↑ BrdU+ ↑ DCX+ ↑ Dendritic complexity versus chronic mild stress Niche: Hippocampus | |||
| Resveratrol | Protective effects against vascular and neurodegenerative diseases, atherosclerosis, oxidative damage, and some cancers. Antioxidant activity [274] | ↓ Delayed onset muscle soreness ↓ Muscle damage ↓ Inflammation ↓ Oxidative stress ↑ Recovery [287] | Dasgupta et al., 2007 [288] | 10 μM | ↑ Proliferation ↑ neurite outgrowth In vitro |
| Thomas et al., 2014 [289] | 50 mg/kg/day mixed with diet for 6 weeks | ↑ Neurogenesis ↑ synaptic plasticity ↑ Hdac4, Hat1, Wnt7a, ApoE versus diabetic control Niche: Hippocampus | |||
| Kodali et al., 2015 [290] | 40 mg/kg/day dissolved in 0.5 mL of 2% ethyl alcohol for 4 weeks | ↑ BrdU+ ↑ DCX+ ↔NeuN+ Niche: Hippocampus | |||
| Park et al., 2012 [291] | 0.1, 1, 10, 20, 50 μM 1 or 10 mg/kg/day, i.p., for 14 days | Higher doses: In vitro: ↓ Proliferation of NSCs ↓ Survival of NSCs In vivo: ↓ BrdU+ ↓ DCX+ ↓ NeuN+/BrdU+ Niche: Hippocampus, In vitro | |||
| omega-3 fatty acids (PUFAs) | Amongst PUFAs: α-linolenic acid, eicosapentaenoic acid (EPA) Docosahexaenoic acid (DHA) → Anti-oxidative stress, anti-inflammatory and antiapoptotic effects [292] | ↑ Muscle recovery ↓ Inflammation ↓ Oxidative stress ↓ Muscle damage ↑ Muscle protein synthesis [251,293] | Borsini et al., 2020 [232] | 10 µM for 7 days | EPA and DHA: ↑ DCX+ DHA: ↑ Map2+ EPA: ↓ CC3+ apoptosis Against IFN-α, EPA and DHA: ↑ DCX+ ↑ Map2+ In vitro |
| Beltz et al., 2007 [294] | 27.2 mg/g oral 25 days | ↑ BrdU+ Niche: Hippocampus | |||
| He et al., 2009 [295] | In vitro: 5 µM DHA In vivo: DHA by diet for 10–12 weeks | DHA, in vitro: ↑ Tuj1+ ↑ BrdU+ ↑ Neurite length DHA, in vivo: ↑ BrdU+ Niche: Hippocampus, In vitro | |||
| Kawakita et al., 2006 [296] | DHA 10 µM for 4–7 days | DHA, in vitro: ↑ Tuj1+ ↓ BrdU+ ↑ Neurite length DHA, in vivo: ↑ BrdU+/NeuN+ Niche: Hippocampus, In vitro | |||
| Rodríguez-Iglesias et al., 2022 [297] | Ω6/Ω3 ratio 6.7 for 10 weeks | ↑ Nestin+ ↑ GFAP+ ↑ DCX+ ↑ BrdU+/DCX+ ↑ DCX+ dendritic extension Niche: Hippocampus | |||
| Dyall et al., 2010 [298] | 270 mg/kg/day, EPA to DHA ratio (1.5:1) for 12 weeks | ↑ DCX+ versus age-related decline Niche: Hippocampus. | |||
| Huguet et al., 2023 [299] | 2 g DHA/100 g fat for 10 weeks | ↑ DCX+ Niche: Hippocampus, Hyphotalamus | |||
| β-alanine | Anti-inflammatory, antioxidant, antiglycation, anticarbonylation, calcium-regulatory, immunomodulatory, and chelating properties [251,300] | ↑ Performance for continuous and intermittent exercise [251] | Gibbons et al., 2014 [301] | 417 mg/kg/day for 28 days | ↔ BrdU ↔ gene expression Niche: Hippocampus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choquet de Isla, J.; Bández-Ruiz, M.; Rosety-Rodríguez, I.; Pérez-López, I.; Rosety-Rodríguez, M.Á.; Verástegui-Escolano, C.; Sánchez-Gomar, I.; Geribaldi-Doldán, N. Perspective for Modulation of Hypothalamic Neurogenesis: Integrating Anatomical Insights with Exercise and Dietary Interventions. Int. J. Mol. Sci. 2025, 26, 10914. https://doi.org/10.3390/ijms262210914
Choquet de Isla J, Bández-Ruiz M, Rosety-Rodríguez I, Pérez-López I, Rosety-Rodríguez MÁ, Verástegui-Escolano C, Sánchez-Gomar I, Geribaldi-Doldán N. Perspective for Modulation of Hypothalamic Neurogenesis: Integrating Anatomical Insights with Exercise and Dietary Interventions. International Journal of Molecular Sciences. 2025; 26(22):10914. https://doi.org/10.3390/ijms262210914
Chicago/Turabian StyleChoquet de Isla, Javier, Manuel Bández-Ruiz, Ignacio Rosety-Rodríguez, Inmaculada Pérez-López, Miguel Ángel Rosety-Rodríguez, Cristina Verástegui-Escolano, Ismael Sánchez-Gomar, and Noelia Geribaldi-Doldán. 2025. "Perspective for Modulation of Hypothalamic Neurogenesis: Integrating Anatomical Insights with Exercise and Dietary Interventions" International Journal of Molecular Sciences 26, no. 22: 10914. https://doi.org/10.3390/ijms262210914
APA StyleChoquet de Isla, J., Bández-Ruiz, M., Rosety-Rodríguez, I., Pérez-López, I., Rosety-Rodríguez, M. Á., Verástegui-Escolano, C., Sánchez-Gomar, I., & Geribaldi-Doldán, N. (2025). Perspective for Modulation of Hypothalamic Neurogenesis: Integrating Anatomical Insights with Exercise and Dietary Interventions. International Journal of Molecular Sciences, 26(22), 10914. https://doi.org/10.3390/ijms262210914








