SLP2/PHB Aggregates in ALS Mouse Models and Patients: Implications Beyond CHCHD10-Associated Motor Neuron Disease
Abstract
1. Introduction
2. Results
2.1. SLP2/PHB Aggregation in FusΔNLS Mice but Not in the Sod1G86R Model
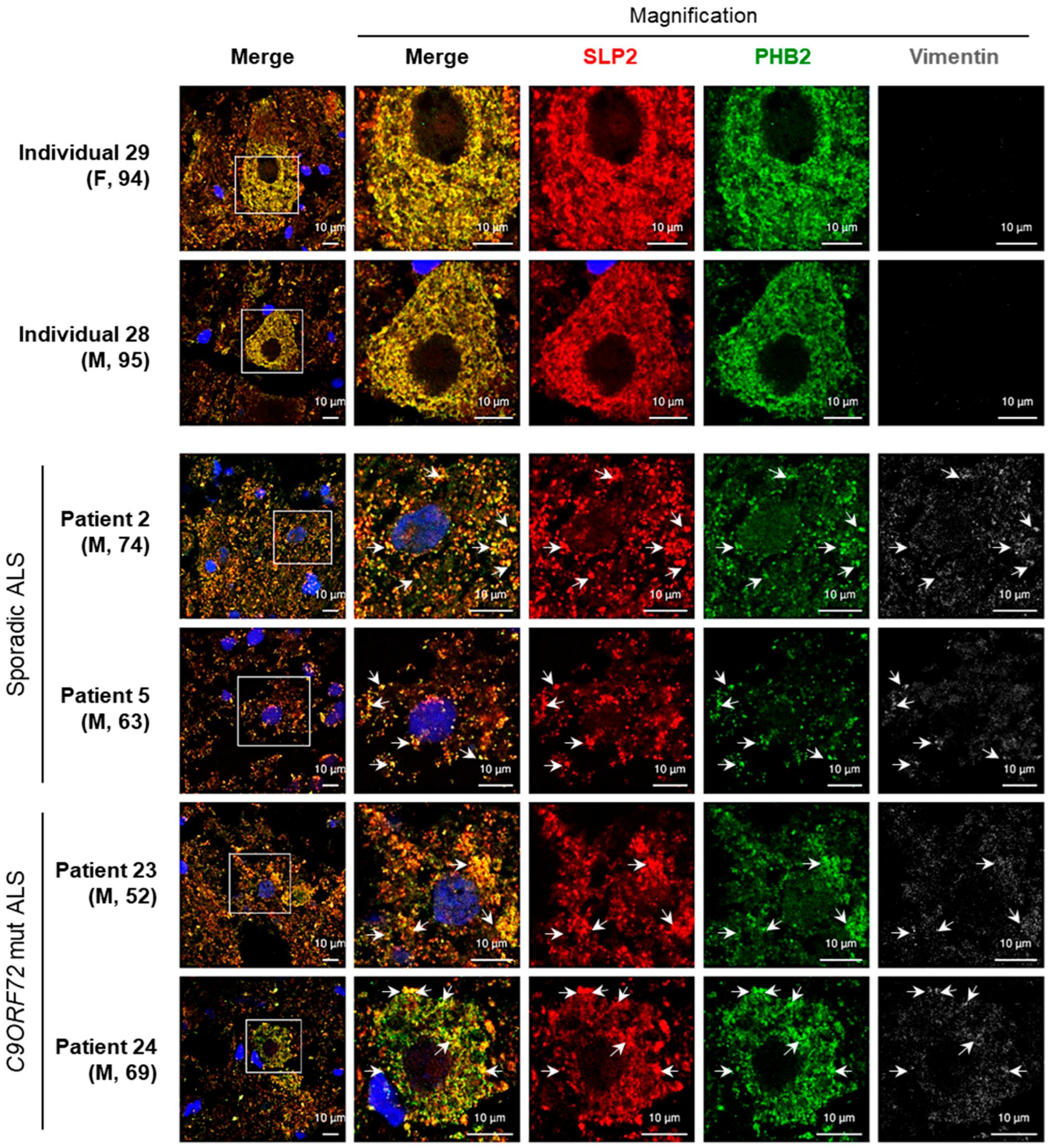
2.2. Aggregates Including SLP2 and PHB2 Are Also Present in ALS Patients
3. Discussion
3.1. SLP2/PHB Aggregation in the FusΔNLS ALS Model
3.2. Detection of SLP2/PHB Aggregates in ALS Patient Tissues
3.3. Absence of SLP2/PHB Aggregates in the Sod1G86R ALS Model
3.4. Age-Dependent Accumulation of SLP2/PHB Aggregates
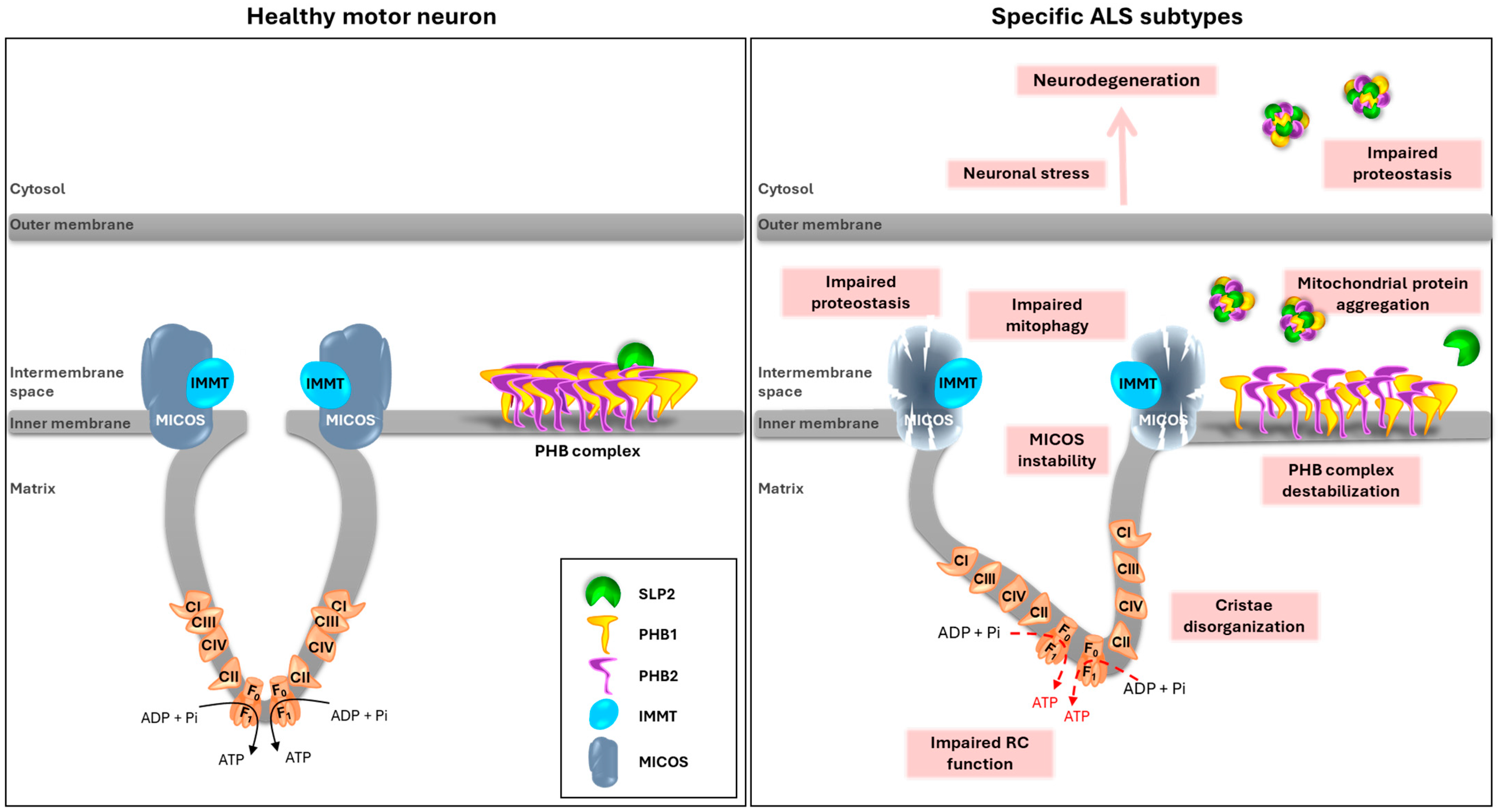
3.5. Integrative Model of SLP2/PHB Aggregation in ALS Pathogenesis
4. Materials and Methods
4.1. Mouse Models
4.2. Immunohistofluorescence on Mouse Tissues
4.3. Immunohistofluorescence on Human ALS Tissues
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALS | Amyotrophic Lateral Sclerosis |
| CA | Cornus Ammonis |
| CHCHD10 | Coiled-coil-Helix-Coiled-coil-Helix Domain containing protein 10 |
| C9ORF72 | Chromosome 9 open reading frame 72 |
| DG | Dentate Gyrus |
| FTD | Frontotemporal Dementia |
| FUS | Fused in Sarcoma |
| MN | motor neuron |
| MND | motor neuron disease |
| PHB | Prohibitin |
| SLP2 | Stomatin-Like Protein 2 |
| SOD1 | Superoxide Dismutase |
| TARDBP | TAR DNA Binding Protein |
References
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Genin, E.C.; Abou-Ali, M.; Paquis-Flucklinger, V. Mitochondria, a Key Target in Amyotrophic Lateral Sclerosis Pathogenesis. Genes 2023, 14, 1981. [Google Scholar] [CrossRef]
- Rizea, R.E.; Corlatescu, A.-D.; Costin, H.P.; Dumitru, A.; Ciurea, A.V. Understanding Amyotrophic Lateral Sclerosis: Pathophysiology, Diagnosis, and Therapeutic Advances. Int. J. Mol. Sci. 2024, 25, 9966. [Google Scholar] [CrossRef] [PubMed]
- Parobkova, E.; Matej, R. Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degenerations: Similarities in Genetic Background. Diagnostics 2021, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; Van Den Berg, L.H. Amyotrophic Lateral Sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Petrov, D.; Mansfield, C.; Moussy, A.; Hermine, O. ALS Clinical Trials Review: 20 Years of Failure. Are We Any Closer to Registering a New Treatment? Front. Aging Neurosci. 2017, 9, 68. [Google Scholar] [CrossRef]
- Benson, B.C.; Shaw, P.J.; Azzouz, M.; Highley, J.R.; Hautbergue, G.M. Proteinopathies as Hallmarks of Impaired Gene Expression, Proteostasis and Mitochondrial Function in Amyotrophic Lateral Sclerosis. Front. Neurosci. 2021, 15, 783624. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Liu, T.; Woo, J.-A.A.; Bukhari, M.Z.; Wang, X.; Yan, Y.; Buosi, S.C.; Ermekbaeva, A.; Sista, A.; Kotsiviras, P.; LePochat, P.; et al. Modulation of Synaptic Plasticity, Motor Unit Physiology, and TDP-43 Pathology by CHCHD10. Acta Neuropathol. Commun. 2022, 10, 95. [Google Scholar] [CrossRef]
- Genin, E.C.; di Borgo, P.P.; Lorivel, T.; Hugues, S.; Farinelli, M.; Mauri-Crouzet, A.; Lespinasse, F.; Godin, L.; Paquis-Flucklinger, V.; Petit-Paitel, A. CHCHD10S59L/+ Mouse Model: Behavioral and Neuropathological Features of Frontotemporal Dementia. Neurobiol. Dis. 2024, 195, 106498. [Google Scholar] [CrossRef]
- Genin, E.C.; Madji Hounoum, B.; Bannwarth, S.; Fragaki, K.; Lacas-Gervais, S.; Mauri-Crouzet, A.; Lespinasse, F.; Neveu, J.; Ropert, B.; Augé, G.; et al. Mitochondrial Defect in Muscle Precedes Neuromuscular Junction Degeneration and Motor Neuron Death in CHCHD10S59L/+ Mouse. Acta Neuropathol. 2019, 138, 123–145. [Google Scholar] [CrossRef]
- Anderson, C.J.; Bredvik, K.; Burstein, S.R.; Davis, C.; Meadows, S.M.; Dash, J.; Case, L.; Milner, T.A.; Kawamata, H.; Zuberi, A.; et al. ALS/FTD Mutant CHCHD10 Mice Reveal a Tissue-Specific Toxic Gain-of-Function and Mitochondrial Stress Response. Acta Neuropathol. 2019, 138, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Shammas, M.K.; Huang, X.; Wu, B.P.; Fessler, E.; Song, I.Y.; Randolph, N.P.; Li, Y.; Bleck, C.K.; Springer, D.A.; Fratter, C.; et al. OMA1 Mediates Local and Global Stress Responses against Protein Misfolding in CHCHD10 Mitochondrial Myopathy. J. Clin. Investig. 2022, 132, e157504. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.L.; Swinkin, E.; Gao, A.; Alminawi, S.; Zhang, M.; McGoldrick, P.; McKeever, P.; Robertson, J.; Rogaeva, E.; Zinman, L. Neuropathologic Description of CHCHD10 Mutated Amyotrophic Lateral Sclerosis. Neurol. Genet. 2020, 6, e394. [Google Scholar] [CrossRef] [PubMed]
- Genin, E.C.; Bannwarth, S.; Ropert, B.; Lespinasse, F.; Mauri-Crouzet, A.; Augé, G.; Fragaki, K.; Cochaud, C.; Donnarumma, E.; Lacas-Gervais, S.; et al. CHCHD10 and SLP2 Control the Stability of the PHB Complex: A Key Factor for Motor Neuron Viability. Brain 2022, 145, 3415–3430. [Google Scholar] [CrossRef]
- Bannwarth, S.; Ait-El-Mkadem, S.; Chaussenot, A.; Genin, E.C.; Lacas-Gervais, S.; Fragaki, K.; Berg-Alonso, L.; Kageyama, Y.; Serre, V.; Moore, D.G.; et al. A Mitochondrial Origin for Frontotemporal Dementia and Amyotrophic Lateral Sclerosis through CHCHD10 Involvement. Brain 2014, 137, 2329–2345. [Google Scholar] [CrossRef]
- Johnson, J.O.; Glynn, S.M.; Gibbs, J.R.; Nalls, M.A.; Sabatelli, M.; Restagno, G.; Drory, V.E.; Chiò, A.; Rogaeva, E.; Traynor, B.J. Mutations in the CHCHD10 Gene Are a Common Cause of Familial Amyotrophic Lateral Sclerosis. Brain 2014, 137, e311. [Google Scholar] [CrossRef]
- Zhang, M.; Xi, Z.; Zinman, L.; Bruni, A.C.; Maletta, R.G.; Curcio, S.A.M.; Rainero, I.; Rubino, E.; Pinessi, L.; Nacmias, B.; et al. Mutation Analysis of CHCHD10 in Different Neurodegenerative Diseases. Brain 2015, 138, e380. [Google Scholar] [CrossRef]
- Chaussenot, A.; Le Ber, I.; Ait-El-Mkadem, S.; Camuzat, A.; De Septenville, A.; Bannwarth, S.; Genin, E.C.; Serre, V.; Augé, G.; Brice, A.; et al. Screening of CHCHD10 in a French Cohort Confirms the Involvement of This Gene in Frontotemporal Dementia with Amyotrophic Lateral Sclerosis Patients. Neurobiol. Aging 2014, 35, 2884.e1–2884.e4. [Google Scholar] [CrossRef]
- Jiao, B.; Xiao, T.; Hou, L.; Gu, X.; Zhou, Y.; Zhou, L.; Tang, B.; Xu, J.; Shen, L. High Prevalence of CHCHD10 Mutation in Patients with Frontotemporal Dementia from China. Brain 2016, 139, e21. [Google Scholar] [CrossRef]
- Da Cruz, S.; Parone, P.A.; Gonzalo, P.; Bienvenut, W.V.; Tondera, D.; Jourdain, A.; Quadroni, M.; Martinou, J.-C. SLP-2 Interacts with Prohibitins in the Mitochondrial Inner Membrane and Contributes to Their Stability. Biochim. Biophys. Acta 2008, 1783, 904–911. [Google Scholar] [CrossRef]
- Dupuis, L.; De Tapia, M.; René, F.; Lutz-Bucher, B.; Gordon, J.W.; Mercken, L.; Pradier, L.; Loeffler, J.-P. Differential Screening of Mutated SOD1 Transgenic Mice Reveals Early Up-Regulation of a Fast Axonal Transport Component in Spinal Cord Motor Neurons. Neurobiol. Dis. 2000, 7, 274–285. [Google Scholar] [CrossRef]
- Ripps, M.E.; Huntley, G.W.; Hof, P.R.; Morrison, J.H.; Gordon, J.W. Transgenic Mice Expressing an Altered Murine Superoxide Dismutase Gene Provide an Animal Model of Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 1995, 92, 689–693. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Oussini, H.E.; Mersmann, S.; Drenner, K.; Wagner, M.; Sun, Y.; Allmeroth, K.; Dieterlé, S.; Sinniger, J.; Dirrig-Grosch, S.; et al. Motor Neuron Intrinsic and Extrinsic Mechanisms Contribute to the Pathogenesis of FUS-Associated Amyotrophic Lateral Sclerosis. Acta Neuropathol. 2017, 133, 887–906. [Google Scholar] [CrossRef]
- Scekic-Zahirovic, J.; Sendscheid, O.; El Oussini, H.; Jambeau, M.; Sun, Y.; Mersmann, S.; Wagner, M.; Dieterlé, S.; Sinniger, J.; Dirrig-Grosch, S.; et al. Toxic Gain of Function from Mutant FUS Protein Is Crucial to Trigger Cell Autonomous Motor Neuron Loss. EMBO J. 2016, 35, 1077–1097. [Google Scholar] [CrossRef] [PubMed]
- Bergemalm, D.; Jonsson, P.A.; Graffmo, K.S.; Andersen, P.M.; Brännström, T.; Rehnmark, A.; Marklund, S.L. Overloading of Stable and Exclusion of Unstable Human Superoxide Dismutase-1 Variants in Mitochondria of Murine Amyotrophic Lateral Sclerosis Models. J. Neurosci. 2006, 26, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Bendotti, C.; Bonetto, V.; Pupillo, E.; Logroscino, G.; Al-Chalabi, A.; Lunetta, C.; Riva, N.; Mora, G.; Lauria, G.; Weishaupt, J.H.; et al. Focus on the Heterogeneity of Amyotrophic Lateral Sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tzeplaeff, L.; Jürs, A.V.; Wohnrade, C.; Demleitner, A.F. Unraveling the Heterogeneity of ALS—A Call to Redefine Patient Stratification for Better Outcomes in Clinical Trials. Cells 2024, 13, 452. [Google Scholar] [CrossRef]
- Höhn, A.; Tramutola, A.; Cascella, R. Proteostasis Failure in Neurodegenerative Diseases: Focus on Oxidative Stress. Oxid. Med. Cell Longev. 2020, 2020, 5497046. [Google Scholar] [CrossRef]
- Lu, B.; Guo, S. Mechanisms Linking Mitochondrial Dysfunction and Proteostasis Failure. Trends Cell Biol. 2020, 30, 317–328. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, Y.; Zhang, S.; Wang, Y.; Pei, X.; Chen, Y.; Zhang, J.; Zhang, Y.; Du, Y.; Hao, S.; et al. Mitochondrial Dysfunction in the Regulation of Aging and Aging-Related Diseases. Cell Commun. Signal 2025, 23, 290. [Google Scholar] [CrossRef]
- Evans, C.S.; Holzbaur, E.L.F. Autophagy and Mitophagy in ALS. Neurobiol. Dis. 2019, 122, 35–40. [Google Scholar] [CrossRef]
- Chua, J.P.; De Calbiac, H.; Kabashi, E.; Barmada, S.J. Autophagy and ALS: Mechanistic Insights and Therapeutic Implications. Autophagy 2022, 18, 254–282. [Google Scholar] [CrossRef]
- Madruga, E.; Maestro, I.; Martínez, A. Mitophagy Modulation, a New Player in the Race against ALS. Int. J. Mol. Sci. 2021, 22, 740. [Google Scholar] [CrossRef]
- Jiao, L.; Du, X.; Li, Y.; Jiao, Q.; Jiang, H. Role of Mitophagy in Neurodegenerative Diseases and Potential Tagarts for Therapy. Mol. Biol. Rep. 2022, 49, 10749–10760. [Google Scholar] [CrossRef]
- Sanjuan-Ruiz, I.; Govea-Perez, N.; McAlonis-Downes, M.; Dieterle, S.; Megat, S.; Dirrig-Grosch, S.; Picchiarelli, G.; Piol, D.; Zhu, Q.; Myers, B.; et al. Wild-Type FUS Corrects ALS-like Disease Induced by Cytoplasmic Mutant FUS through Autoregulation. Mol. Neurodegener. 2021, 16, 61. [Google Scholar] [CrossRef]
- Megat, S.; Mora, N.; Sanogo, J.; Roman, O.; Catanese, A.; Alami, N.O.; Freischmidt, A.; Mingaj, X.; De Calbiac, H.; Muratet, F.; et al. Integrative Genetic Analysis Illuminates ALS Heritability and Identifies Risk Genes. Nat. Commun. 2023, 14, 342, Correction in Nat. Commun. 2023, 14, 8026. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | ALS Form | Pathology | Sexe | Age of Death |
|---|---|---|---|---|
| 1 | ALS | sporadic | M | 55 |
| 2 | ALS | sporadic | M | 74 |
| 3 | ALS | sporadic | M | 53 |
| 4 | ALS | sporadic | M | 69 |
| 5 | ALS | sporadic | M | 63 |
| 6 | ALS | sporadic | F | 62 |
| 7 | ALS | sporadic | M | 57 |
| 8 | ALS | sporadic | F | 66 |
| 9 | ALS | sporadic | M | 65 |
| 10 | ALS | sporadic | ? | ? |
| 11 | ALS | sporadic | ? | ? |
| 12 | ALS-FTD | sporadic | M | 42 |
| 13 | ALS | sporadic | F | 55 |
| 14 | ALS | sporadic | ? | ? |
| 15 | ALS | sporadic | M | 54 |
| 16 | ALS-FTD | sporadic | M | 70 |
| 17 | ALS | C9ORF72 mutation | M | 63 |
| 18 | ALS | C9ORF72 mutation | F | 60 |
| 19 | ALS | familial SOD1 D83G mutation | M | 73 |
| 20 | ALS | familial SOD1 D83G mutation | M | 58 |
| 21 | ALS | C9ORF72 mutation | M | 53 |
| 22 | ALS | TARDBP G348V mutation | M | 47 |
| 23 | ALS | C9ORF72 mutation | M | 52 |
| 24 | ALS | C9ORF72 mutation | M | 69 |
| 25 | ALS | SOD1 mutation | M | 50 |
| 26 | ALS-FTD | C9ORF72 mutation | M | 66 |
| 27 | ALS-FTD | C9ORF72 mutation | F | 77 |
| 28 | Control | Braak stage III and Thal stage 2 Alzheimer lesions + amyloid angiopathy | M | 95 |
| 29 | Control | none | F | 94 |
| 30 | Control | Braak stage III Alzheimer lesions | M | 84 |
| Patient Number | ALS Form | Motor Neurons | % (n) | χ2 |
|---|---|---|---|---|
| 2 | sporadic ALS | without aggregates | 56.52 (13) | p < 0.0001 |
| with aggregates | 43.48 (10) | |||
| 5 | sporadic ALS | without aggregates | 54.90 (28) | p < 0.0001 |
| with aggregates | 45.10 (23) | |||
| 23 | C9ORF72 ALS | without aggregates | 52.38 (11) | p < 0.0001 |
| with aggregates | 47.62 (10) | |||
| 24 | C9ORF72 ALS | without aggregates | 60.53 (23) | p < 0.0001 |
| with aggregates | 39.47 (15) | |||
| 28 | Control | without aggregates | 85.71 (18) | p < 0.0001 |
| with aggregates | 14.29 (3) | |||
| 29 | Control | without aggregates | 82.35 (14) | p < 0.0001 |
| with aggregates | 17.65 (3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genin, E.C.; Lespinasse, F.; Mauri-Crouzet, A.; Dupuis, L.; Paquis-Flucklinger, V. SLP2/PHB Aggregates in ALS Mouse Models and Patients: Implications Beyond CHCHD10-Associated Motor Neuron Disease. Int. J. Mol. Sci. 2025, 26, 10852. https://doi.org/10.3390/ijms262210852
Genin EC, Lespinasse F, Mauri-Crouzet A, Dupuis L, Paquis-Flucklinger V. SLP2/PHB Aggregates in ALS Mouse Models and Patients: Implications Beyond CHCHD10-Associated Motor Neuron Disease. International Journal of Molecular Sciences. 2025; 26(22):10852. https://doi.org/10.3390/ijms262210852
Chicago/Turabian StyleGenin, Emmanuelle C., Françoise Lespinasse, Alessandra Mauri-Crouzet, Luc Dupuis, and Véronique Paquis-Flucklinger. 2025. "SLP2/PHB Aggregates in ALS Mouse Models and Patients: Implications Beyond CHCHD10-Associated Motor Neuron Disease" International Journal of Molecular Sciences 26, no. 22: 10852. https://doi.org/10.3390/ijms262210852
APA StyleGenin, E. C., Lespinasse, F., Mauri-Crouzet, A., Dupuis, L., & Paquis-Flucklinger, V. (2025). SLP2/PHB Aggregates in ALS Mouse Models and Patients: Implications Beyond CHCHD10-Associated Motor Neuron Disease. International Journal of Molecular Sciences, 26(22), 10852. https://doi.org/10.3390/ijms262210852







