Tart Cherry (Prunus cerasus) Extract Exerts High Intracellular ROS Scavenging Activity and Repression of ARE (Antioxidant Response Element) Pathway in Human Hepatocytes
Abstract
1. Introduction
2. Results
2.1. Determination of Chemical Antioxidant Activity with a Cell-Free ORAC Assay
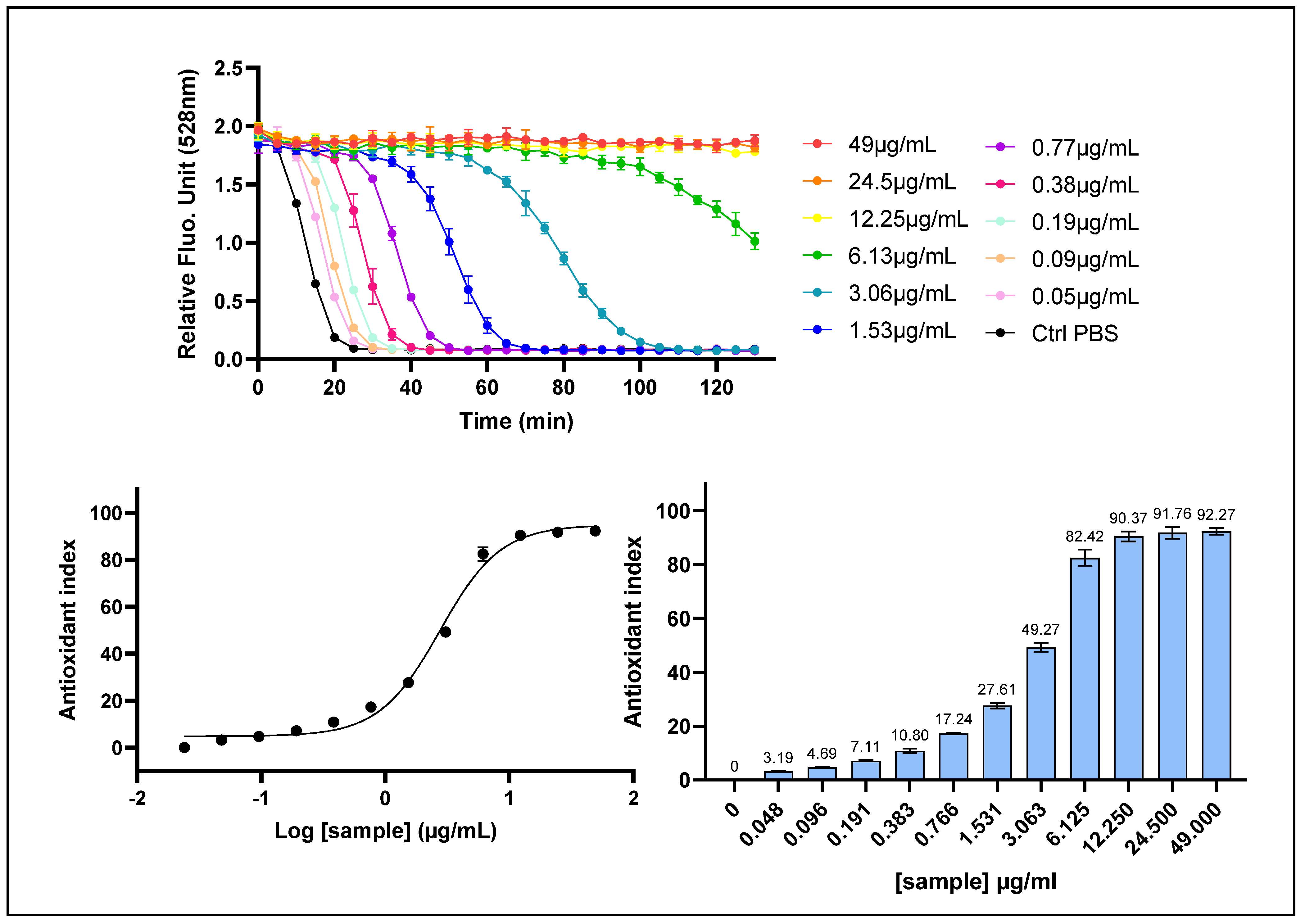
2.2. Determination of Intracellular ROS Scavenging Activity with the AOP1 Cell-Based Assay
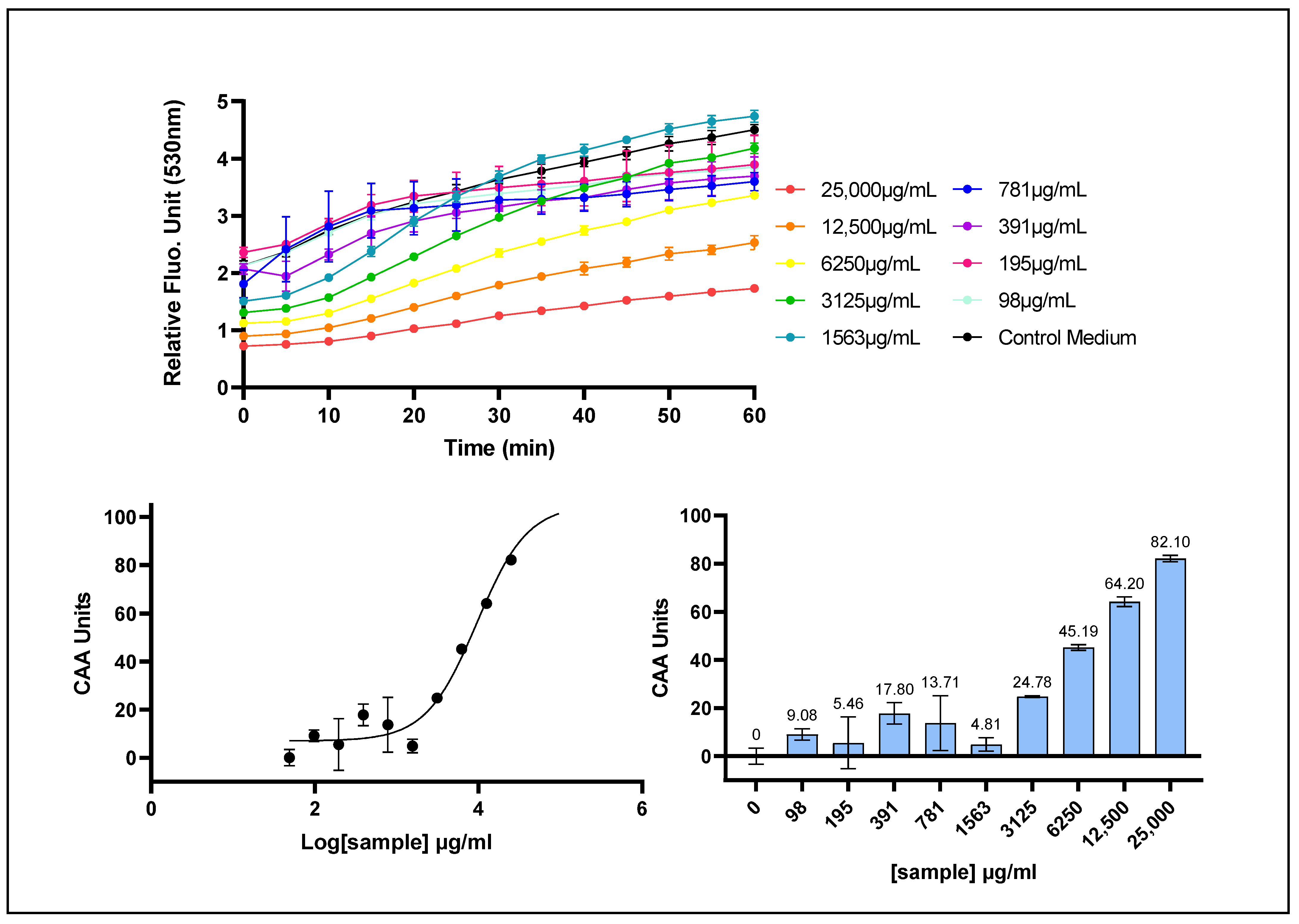
2.3. Determination ROS Scavenging Activity at Cell Membrane with the CAA (AAPH/DCFH-DA) Assay
2.4. Determination of ARE-Dependent Transcriptional Activity with a Hepatocyte-Based ARE Driven-Luciferase Reporter Assay
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Cell Lines
4.3. Cell Culture Conditions
4.4. Preparation of Samples
4.5. Chemical Radical Scavenging Activity with ORAC Assay
4.6. Intracellular ROS Scavenging Activity with AOP1 Assay
4.7. ROS Scavenging Activity at Cell Membrane with CAA Assay (AAPH/DCFH-DA)
4.8. Transcriptional Activity of the Nrf2-Regulated ARE Pathway with HepG2-ARE-Luc Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| ARE | Antioxidant Response Element |
| AOP1 | Anti Oxidant Power 1 |
| CAA | Cellular Antioxidant Assay |
| Keap1 | Kelch-like ECH-associated protein 1 |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
References
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid. Med. Cell Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- de Almeida, A.J.P.O.; de Oliveira, J.C.P.L.; da Silva Pontes, L.V.; de Souza Júnior, J.F.; Gonçalves, T.A.F.; Dantas, S.H.; de Almeida Feitosa, M.S.; Silva, A.O.; de Medeiros, I.A. ROS: Basic Concepts, Sources, Cellular Signaling, and Its Implications in Aging Pathways. Oxid. Med. Cell Longev. 2022, 2022, 1225578. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, Vegetable, and Legume Intake, and Cardiovascular Disease and Deaths in 18 Countries (PURE): A Prospective Cohort Study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Liu, W.; Hu, B.; Dehghan, M.; Mente, A.; Wang, C.; Yan, R.; Rangarajan, S.; Tse, L.A.; Yusuf, S.; Liu, X.; et al. Fruit, Vegetable, and Legume Intake and the Risk of All-Cause, Cardiovascular, and Cancer Mortality: A Prospective Study. Clin. Nutr. 2021, 40, 4316–4323. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Afshin, A.; Ashbaugh, C.; Bisignano, C.; Brauer, M.; Ferrara, G.; Garcia, V.; Haile, D.; Hay, S.I.; He, J.; et al. Health Effects Associated with Vegetable Consumption: A Burden of Proof Study. Nat. Med. 2022, 28, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Heiss, C.; Borges, G.; Crozier, A. Berry (Poly)Phenols and Cardiovascular Health. J. Agric. Food Chem. 2014, 62, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry Anthocyanins as Novel Antioxidants in Human Health and Disease Prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical Profile and Antioxidant Capacities of Tart Cherry Products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency Cherries Reduce the Oxidative Stress and Inflammatory Responses to Repeated Days High-Intensity Stochastic Cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Kelly, M.E.; Bielinski, D.F.; Fisher, D.R. Tart Cherry Extracts Reduce Inflammatory and Oxidative Stress Signaling in Microglial Cells. Antioxidants 2016, 5, 33. [Google Scholar] [CrossRef]
- Mansoori, S. Effects of Tart Cherry and Its Metabolites on Aging and Inflammatory Conditions: Efficacy and Possible Mechanisms. Ageing Res. Rev. 2021, 66, 101254. [Google Scholar] [CrossRef]
- Balentine, D.A.; Dwyer, J.T.; Erdman, J.W.; Ferruzzi, M.G.; Gaine, P.C.; Harnly, J.M.; Kwik-Uribe, C.L. Recommendations on Reporting Requirements for Flavonoids in Research. Am. J. Clin. Nutr. 2015, 101, 1113–1125. [Google Scholar] [CrossRef]
- Gironde, C.; Rigal, M.; Dufour, C.; Furger, C. AOP1, a New Live Cell Assay for the Direct and Quantitative Measure of Intracellular Antioxidant Effects. Antioxidants 2020, 9, 471. [Google Scholar] [CrossRef]
- Derick, S.; Gironde, C.; Perio, P.; Reybier, K.; Nepveu, F.; Jauneau, A.; Furger, C. LUCS (Light-Up Cell System), a Universal High Throughput Assay for Homeostasis Evaluation in Live Cells. Sci. Rep. 2017, 7, 18069. [Google Scholar] [CrossRef]
- Furger, C. Live Cell Assays for the Assessment of Antioxidant Activities of Plant Extracts. Antioxidants 2021, 10, 944. [Google Scholar] [CrossRef]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a Phytocomplex of Bilberry (Vaccinium myrtillus) and Spirulina (Spirulina platensis), Exerts Both Direct Antioxidant Activity and Modulation of ARE/Nrf2 Pathway in HepG2 Cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Mannino, G.; Maffei, M.E. Metabolomics-Based Profiling, Antioxidant Power, and Uropathogenic Bacterial Anti-Adhesion Activity of SP4TM, a Formulation with a High Content of Type-A Proanthocyanidins. Antioxidants 2022, 11, 1234. [Google Scholar] [CrossRef]
- Dufour, C.; Villa-Rodriguez, J.A.; Furger, C.; Lessard-Lord, J.; Gironde, C.; Rigal, M.; Badr, A.; Desjardins, Y.; Guyonnet, D. Cellular Antioxidant Effect of an Aronia Extract and Its Polyphenolic Fractions Enriched in Proanthocyanidins, Phenolic Acids, and Anthocyanins. Antioxidants 2022, 11, 1561. [Google Scholar] [CrossRef]
- Chambon, M.; Ho, R.; Baghdikian, B.; Herbette, G.; Bun-Llopet, S.-S.; Garayev, E.; Raharivelomanana, P. Identification of Antioxidant Metabolites from Five Plants (Calophyllum Inophyllum, Gardenia Taitensis, Curcuma Longa, Cordia Subcordata, Ficus Prolixa) of the Polynesian Pharmacopoeia and Cosmetopoeia for Skin Care. Antioxidants 2023, 12, 1870. [Google Scholar] [CrossRef]
- Rehrl, J.; Sepperer, T.; Häsler Gunnarsdottir, S.; Schnabel, T.; Oostingh, G.J.; Schuster, A. Antioxidant Potential of Tree Bark Extracts: Insight from the Multi-Level Output of the Antioxidant Power 1 Assay. PLoS ONE 2025, 20, e0328790. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Furger, C.; Gironde, C.; Rigal, M.; Dufour, C.; Guillemet, D. Cell-Based Antioxidant Properties and Synergistic Effects of Natural Plant and Algal Extracts Pre and Post Intestinal Barrier Transport. Antioxidants 2022, 11, 565. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Gironde, C.; Rigal, M.; Furger, C.; Le Roux, E. Bioactivity of Grape Pomace Extract and Sodium Selenite, Key Components of the OenoGrape Advanced Complex, on Target Human Cells: Intracellular ROS Scavenging and Nrf2/ARE Induction Following In Vitro Intestinal Absorption. Antioxidants 2024, 13, 1392. [Google Scholar] [CrossRef]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human Metabolism and Elimination of the Anthocyanin, Cyanidin-3-Glucoside: A (13)C-Tracer Study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef]
- Keane, K.M.; Bell, P.G.; Lodge, J.K.; Constantinou, C.L.; Jenkinson, S.E.; Bass, R.; Howatson, G. Phytochemical Uptake Following Human Consumption of Montmorency Tart Cherry (L. Prunus Cerasus) and Influence of Phenolic Acids on Vascular Smooth Muscle Cells in Vitro. Eur. J. Nutr. 2016, 55, 1695–1705. [Google Scholar] [CrossRef]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dayalan Naidu, S.; Dinkova-Kostova, A.T. Regulating Nrf2 Activity: Ubiquitin Ligases and Signaling Molecules in Redox Homeostasis. Trends Biochem. Sci. 2025, 50, 179–205. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of Activation of the Transcription Factor Nrf2 by Redox Stressors, Nutrient Cues, and Energy Status and the Pathways through Which It Attenuates Degenerative Disease. Free Radic. Biol. Med. 2015, 88 Pt B, 108–146. [Google Scholar] [CrossRef]
- Sihvola, V.; Levonen, A.-L. Keap1 as the Redox Sensor of the Antioxidant Response. Arch. Biochem. Biophys. 2017, 617, 94–100. [Google Scholar] [CrossRef]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Stress-Sensing Mechanisms and the Physiological Roles of the Keap1-Nrf2 System during Cellular Stress. J. Biol. Chem. 2017, 292, 16817–16824. [Google Scholar] [CrossRef] [PubMed]
- Horie, Y.; Suzuki, T.; Inoue, J.; Iso, T.; Wells, G.; Moore, T.W.; Mizushima, T.; Dinkova-Kostova, A.T.; Kasai, T.; Kamei, T.; et al. Molecular Basis for the Disruption of Keap1-Nrf2 Interaction via Hinge & Latch Mechanism. Commun. Biol. 2021, 4, 576. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, S.; Chan, J.Y.; Zhang, D.D. Keap1 Controls Postinduction Repression of the Nrf2-Mediated Antioxidant Response by Escorting Nuclear Export of Nrf2. Mol. Cell Biol. 2007, 27, 6334–6349. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, T.; Zhao, F.; Lau, A.; Birch, C.M.; Zhang, D.D. KPNA6 (Importin {alpha}7)-Mediated Nuclear Import of Keap1 Represses the Nrf2-Dependent Antioxidant Response. Mol. Cell Biol. 2011, 31, 1800–1811. [Google Scholar] [CrossRef]
- Liu, P.; Saad, S.; Mascarenhas, J.B.; Kerins, M.; Dodson, M.; Sun, X.; Wang, T.; Ooi, A.; Garcia, J.G.N.; Zhang, D.D. RPA1 Binding to NRF2 Switches ARE-Dependent Transcriptional Activation to ARE-NRE-Dependent Repression. Proc. Natl. Acad. Sci. USA 2018, 115, E10352–E10361. [Google Scholar] [CrossRef]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol Enhances the Efficacy of Chemotherapy by Inhibiting the Nrf2-Mediated Defense Mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef]
- Vartanian, S.; Ma, T.P.; Lee, J.; Haverty, P.M.; Kirkpatrick, D.S.; Yu, K.; Stokoe, D. Application of Mass Spectrometry Profiling to Establish Brusatol as an Inhibitor of Global Protein Synthesis. Mol. Cell Proteom. 2016, 15, 1220–1231. [Google Scholar] [CrossRef]
- Tang, X.; Wang, H.; Fan, L.; Wu, X.; Xin, A.; Ren, H.; Wang, X.J. Luteolin Inhibits Nrf2 Leading to Negative Regulation of the Nrf2/ARE Pathway and Sensitization of Human Lung Carcinoma A549 Cells to Therapeutic Drugs. Free Radic. Biol. Med. 2011, 50, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Kweon, M.-H.; Adhami, V.M.; Lee, J.-S.; Mukhtar, H. Constitutive Overexpression of Nrf2-Dependent Heme Oxygenase-1 in A549 Cells Contributes to Resistance to Apoptosis Induced by Epigallocatechin 3-Gallate. J. Biol. Chem. 2006, 281, 33761–33772. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zhang, F.; Sun, Z.; Zhou, W.; Li, Z.-Y.; You, Q.-D.; Guo, Q.-L.; Hu, R. Drug Resistance Associates with Activation of Nrf2 in MCF-7/DOX Cells, and Wogonin Reverses It by down-Regulating Nrf2-Mediated Cellular Defense Response. Mol. Carcinog. 2013, 52, 824–834. [Google Scholar] [CrossRef]
- Liu, J.; Qin, X.; Ma, W.; Jia, S.; Zhang, X.; Yang, X.; Pan, D.; Jin, F. Corilagin Induces Apoptosis and Autophagy in NRF2-addicted U251 Glioma Cell Line. Mol. Med. Rep. 2021, 23, 320. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Ananda Sadagopan, S.K.; Dharmalingam, P.; Ganapasam, S. Luteolin, a Bioflavonoid Inhibits Azoxymethane-Induced Colorectal Cancer through Activation of Nrf2 Signaling. Toxicol. Mech. Methods 2014, 24, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Tang, H.; Cao, L.; Zhang, J.; Li, J.; Ma, D.; Guo, C. Epigallocatechin-3-O-Gallate Ameliorates Oxidative Stress-Induced Chondrocyte Dysfunction and Exerts Chondroprotective Effects via the Keap1/Nrf2/ARE Signaling Pathway. Chem. Biol. Drug Des. 2022, 100, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a Plant Derived Small Molecule, Exerts Potent Anti-Inflammatory and Chondroprotective Effects through the Activation of ROS/ERK/Nrf2 Signaling Pathways in Human Osteoarthritis Chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox Basis of Exercise Physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Wruck, C.J.; Streetz, K.; Pavic, G.; Götz, M.E.; Tohidnezhad, M.; Brandenburg, L.-O.; Varoga, D.; Eickelberg, O.; Herdegen, T.; Trautwein, C.; et al. Nrf2 Induces Interleukin-6 (IL-6) Expression via an Antioxidant Response Element within the IL-6 Promoter. J. Biol. Chem. 2011, 286, 4493–4499. [Google Scholar] [CrossRef] [PubMed]


| Analysis | Method | Unit | Value |
|---|---|---|---|
| pH | Powder—diluted 1:5 in deionized water | 3.35 | |
| Total acidity (W) pH 7.0 | IFU 3 | g/kg | 38.86 |
| Total acidity (Z) pH 8.1 | anhydrous, IFU 3 | g/kg | 42.859 |
| Dry matter | Drying at 105 °C | % | 99.44 |
| Moisture | Drying at 105 °C | % | 0.56 |
| Density (measurement) | Bulk density | g/mL | 0.529 |
| Color | 520 nm, pH 1.0 | 2581.02 | |
| Color | 420 nm, pH 1.0 | 896.86 | |
| Color | Ratio 520 nm/420 nm | 2.878 | |
| Anthocyanins | as Cya-3-glu (spectrometric by pH-difference) | g/kg | 32.943 |
| Anthocyanins | as Cya-3-glu (HPLC) | g/kg | 29.5 |
| Polyphenols | as Catechin (Folin–Ciocalteu) | g/kg | 196.907 |
| Yeasts | Pour Plate | cfu/g | <5 |
| Molds | Pour Plate | cfu/g | 5 |
| TVC (total viable count) | cfu/g | 55 | |
| Coliforms | Product | cfu/g | 0 |
| E. coli | cfu/g | 0 | |
| Enterobacteriaceae | cfu/g | 0 | |
| Sucrose | IFU 56 | g/kg | 0 |
| Glucose | IFU 55 | g/kg | 88.75 |
| Fructose | IFU 55 | g/kg | 75.88 |
| Total sugars | g/kg | 164.63 | |
| L-Malic acid | IFU 21 | g/kg | 24.84 |
| Citric acid | IFU 22 | g/kg | 0.05 |
| D-Isocitric acid | IFU 54 | mg/kg | 40.39 |
| Galacturonic acid | HPLC | mg/kg | 269.00 |
| Sodium (Na) | ICP-MS | mg/kg | 67.7 |
| Potassium (K) | ICP-MS | mg/kg | 3064.21 |
| Calcium (Ca) | ICP-MS | mg/kg | 454.44 |
| Phosphorus | as phosphate (ICP-MS) | mg/kg | 1641.628 |
| Magnesium (Mg) | ICP-MS | mg/kg | 227.99 |
| L-Malic acid (amount) | g | 0.639 | |
| D-Isocitric acid (amount) | mg | 1.039 | |
| Ash | g | 0.226 | |
| Potassium (amount) | mg | 78.853 | |
| Calcium (amount) | mg | 11.694 | |
| Magnesium (amount) | mg | 5.867 | |
| Phosphate (amount) | mg | 42.245 | |
| Sulfate (amount) | mg | 6.176 | |
| Sum of free amino acids | HPLC | mg/kg | 4373 |
| Hydroxycinnamic acids | Chlorogenic acid (HPLC) | mg/kg | 1297 |
| Hydroxycinnamic acids | Neochlorogenic acid (HPLC) | mg/kg | 927 |
| Flavonols | Rutin (Quercetin-3-Rut) (HPLC) | mg/kg | 2710 |
| Flavonols | Isoquercitrin (Quercetin-3-Glu) (HPLC) | mg/kg | 179 |
| Flavonols | Kaempferol-3-Rut (HPLC) | mg/kg | 442 |
| Flavonols | Isorhamnetin-3-Rut (HPLC) | mg/kg | 1043 |
| Flavonols | Quercetin (HPLC) | mg/kg | 32.4 |
| Flavonols | Ratio Rutin/Isoquercitrin | 15.14 | |
| Proanthocyanidin | Procyanidin B2 (HPLC) | mg/kg | 3965 |
| Energy (kcal) | kcal | kcal/100 g | 372 |
| Energy (kJ) | kJ | kJ/100 g | 1577 |
| Fat | total fat | g/100 g | 0 |
| Carbohydrates | total carbohydrates | g/100 g | 88.87 |
| Protein | N × 6.25 | g/100 g | 1.4 |
| Dietary fiber | total | g/100 g | 5.36 |
| Assay (Mechanism) | EC50 (µg/mL) [95% CI] | EC10 (µg/mL) | EC90 (µg/mL) | R2 |
|---|---|---|---|---|
| AOP1 (intracell ROS) | 72.02 [57.95–112.15] | 15.80 [8.631–23.56] | 328.3 [183.4–1218] | 0.9787 |
| ARE-luciferase | ND (repression effect) | ND (repression effect) | ND (repression effect) | ND |
| CAA (mb ROS) | 9545 [7552–12,820] | 1982 [1254–2857] | 45,970 [27,120–101,200] | 0.9697 |
| ORAC (cell-free) | 2.755 [2.447–3.096] | 0.798 [0.589–1.045] | 9.513 [7.374–12.97] | 0.9866 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dufour, C.; Rigal, M.; Gironde, C.; Plattner, S.; Furger, C. Tart Cherry (Prunus cerasus) Extract Exerts High Intracellular ROS Scavenging Activity and Repression of ARE (Antioxidant Response Element) Pathway in Human Hepatocytes. Int. J. Mol. Sci. 2025, 26, 10827. https://doi.org/10.3390/ijms262210827
Dufour C, Rigal M, Gironde C, Plattner S, Furger C. Tart Cherry (Prunus cerasus) Extract Exerts High Intracellular ROS Scavenging Activity and Repression of ARE (Antioxidant Response Element) Pathway in Human Hepatocytes. International Journal of Molecular Sciences. 2025; 26(22):10827. https://doi.org/10.3390/ijms262210827
Chicago/Turabian StyleDufour, Cécile, Mylène Rigal, Camille Gironde, Stephan Plattner, and Christophe Furger. 2025. "Tart Cherry (Prunus cerasus) Extract Exerts High Intracellular ROS Scavenging Activity and Repression of ARE (Antioxidant Response Element) Pathway in Human Hepatocytes" International Journal of Molecular Sciences 26, no. 22: 10827. https://doi.org/10.3390/ijms262210827
APA StyleDufour, C., Rigal, M., Gironde, C., Plattner, S., & Furger, C. (2025). Tart Cherry (Prunus cerasus) Extract Exerts High Intracellular ROS Scavenging Activity and Repression of ARE (Antioxidant Response Element) Pathway in Human Hepatocytes. International Journal of Molecular Sciences, 26(22), 10827. https://doi.org/10.3390/ijms262210827






