In Vitro Evaluation of Sugar-Conjugated Thienopyrimidinone Derivatives with Possible Neuroprotective and Antioxidant Effects
Abstract
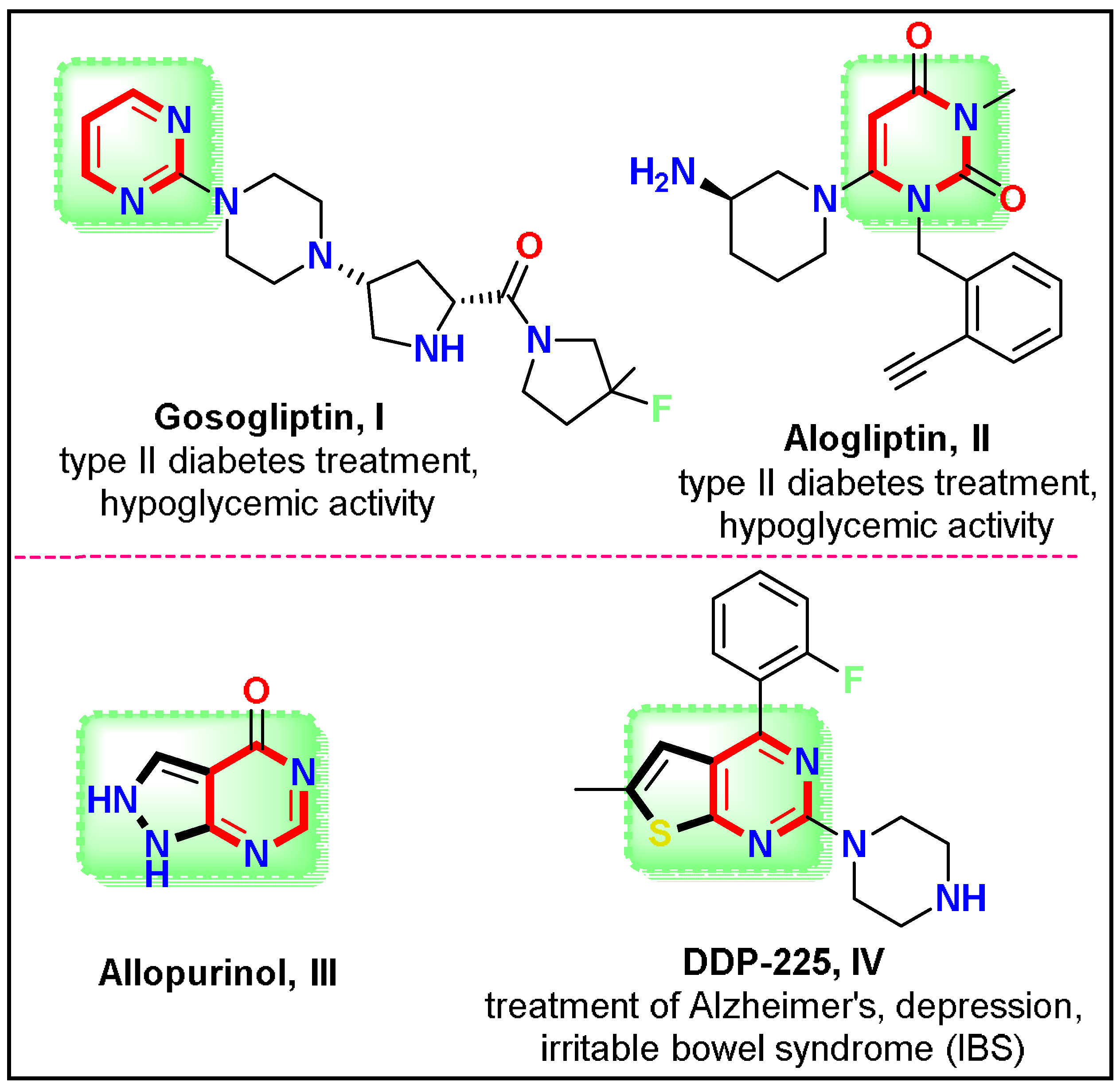
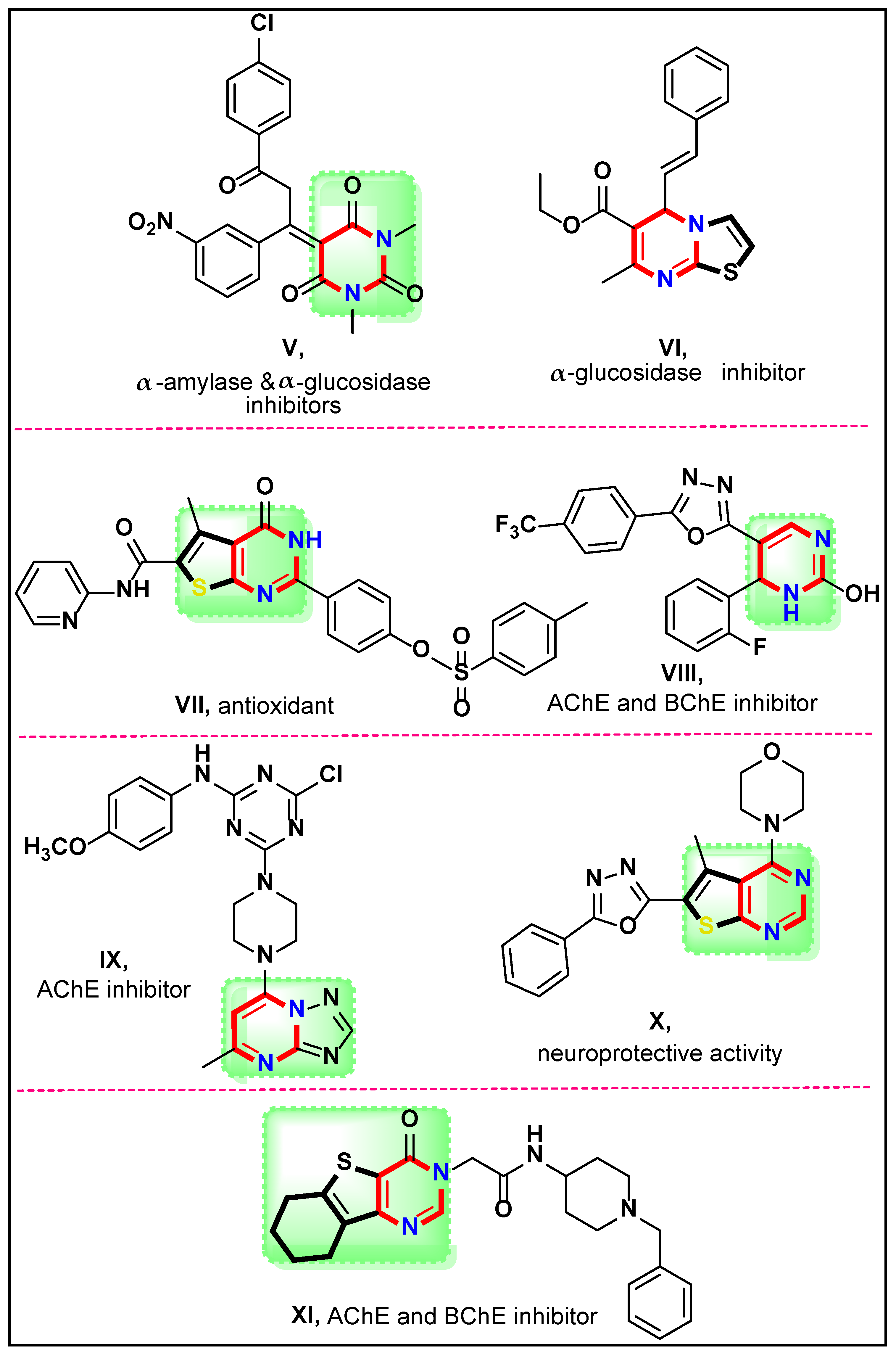
1. Introduction
2. Results and Discussion
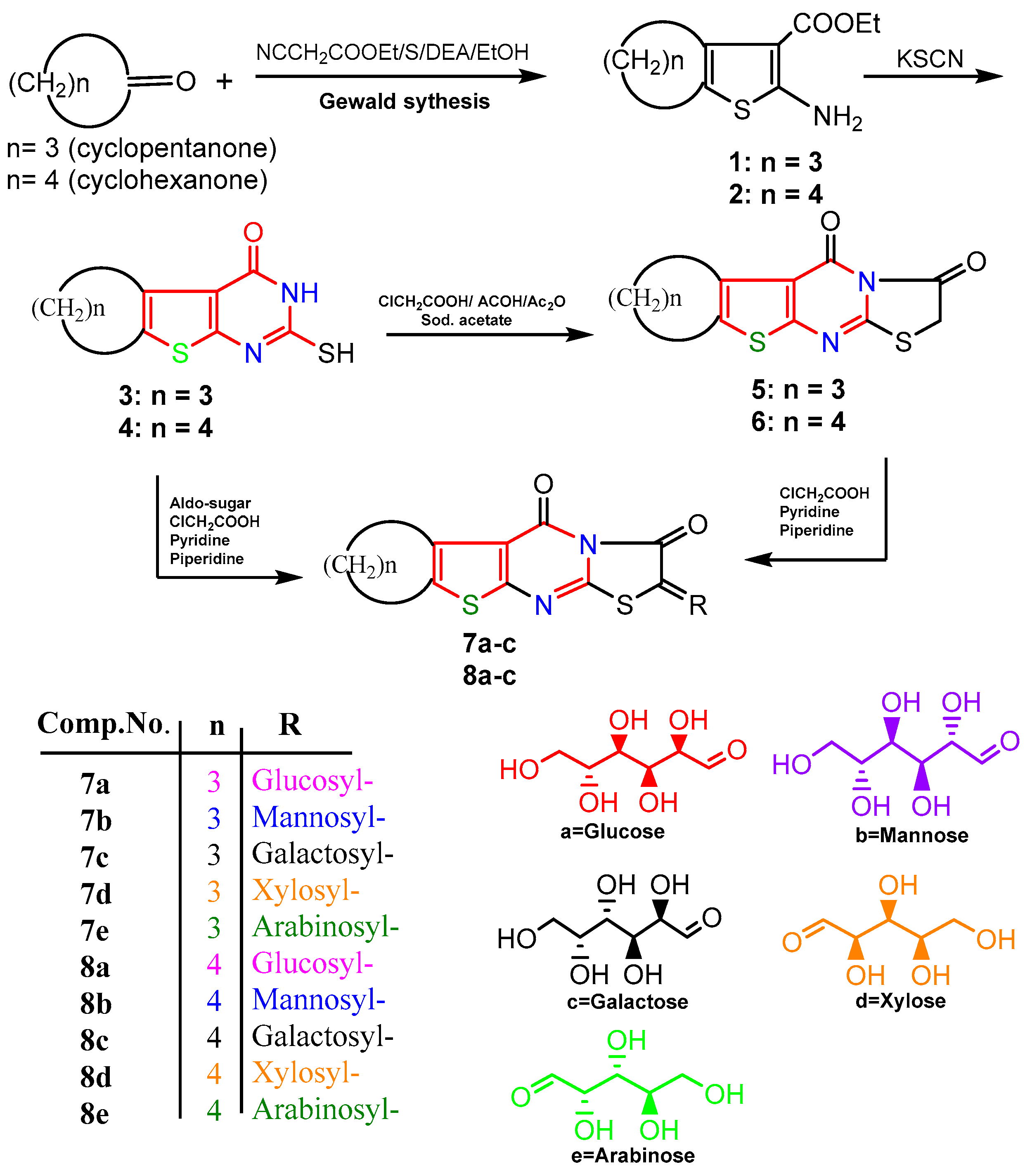
2.1. Chemistry
2.2. Biological Investigations
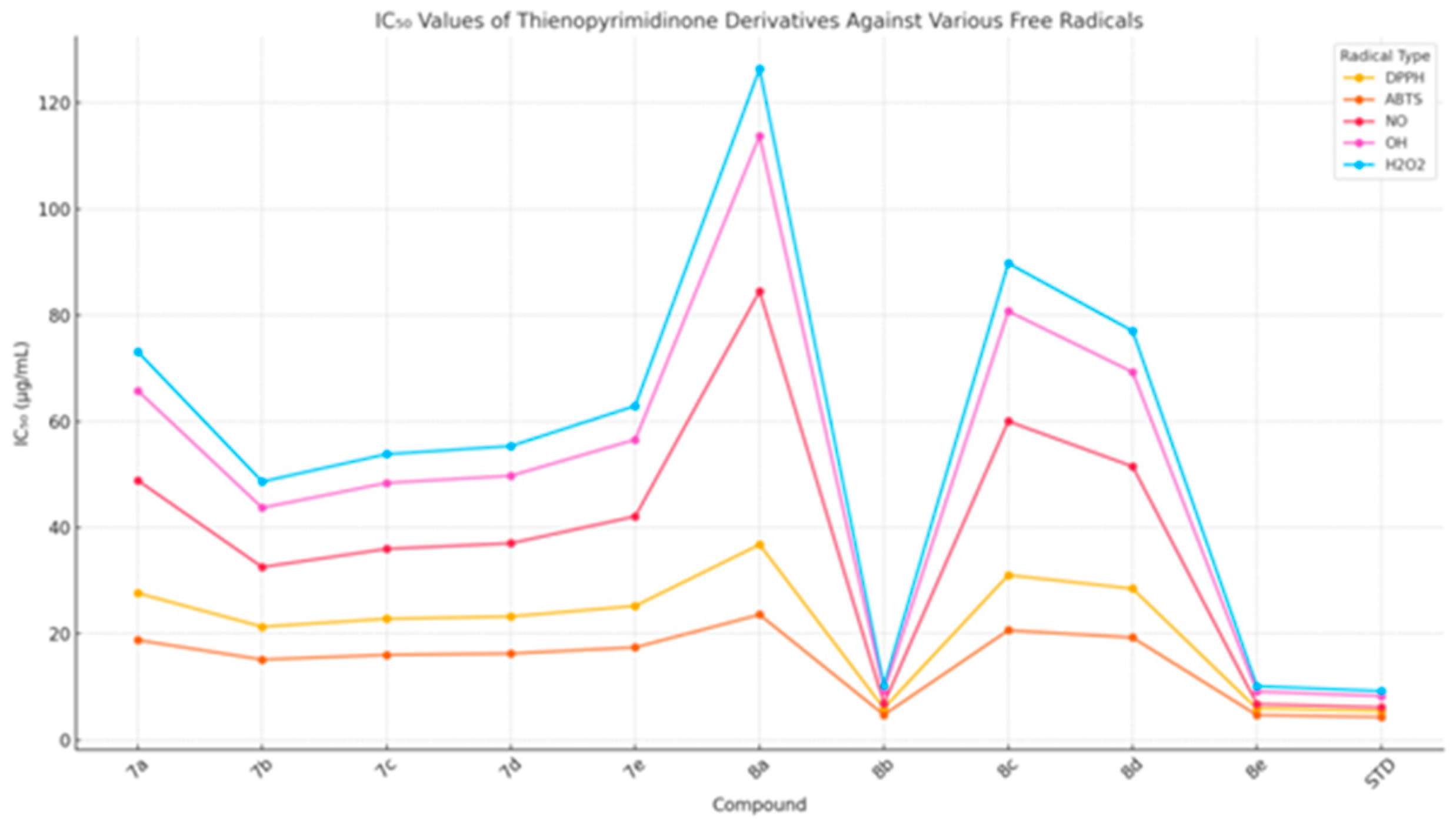
2.2.1. Antioxidant and Radical Scavenging Evaluation
Structure–Activity Relationship (SAR) and Rationalization
2.2.2. Anti-Alzheimer Activity (AChE Inhibition)
2.2.3. Anti-Arthritic Activity
Structure–Activity Relationship (SAR)
2.2.4. Anti-Diabetic Potential
Structure–Activity Relationship (SAR) Observations
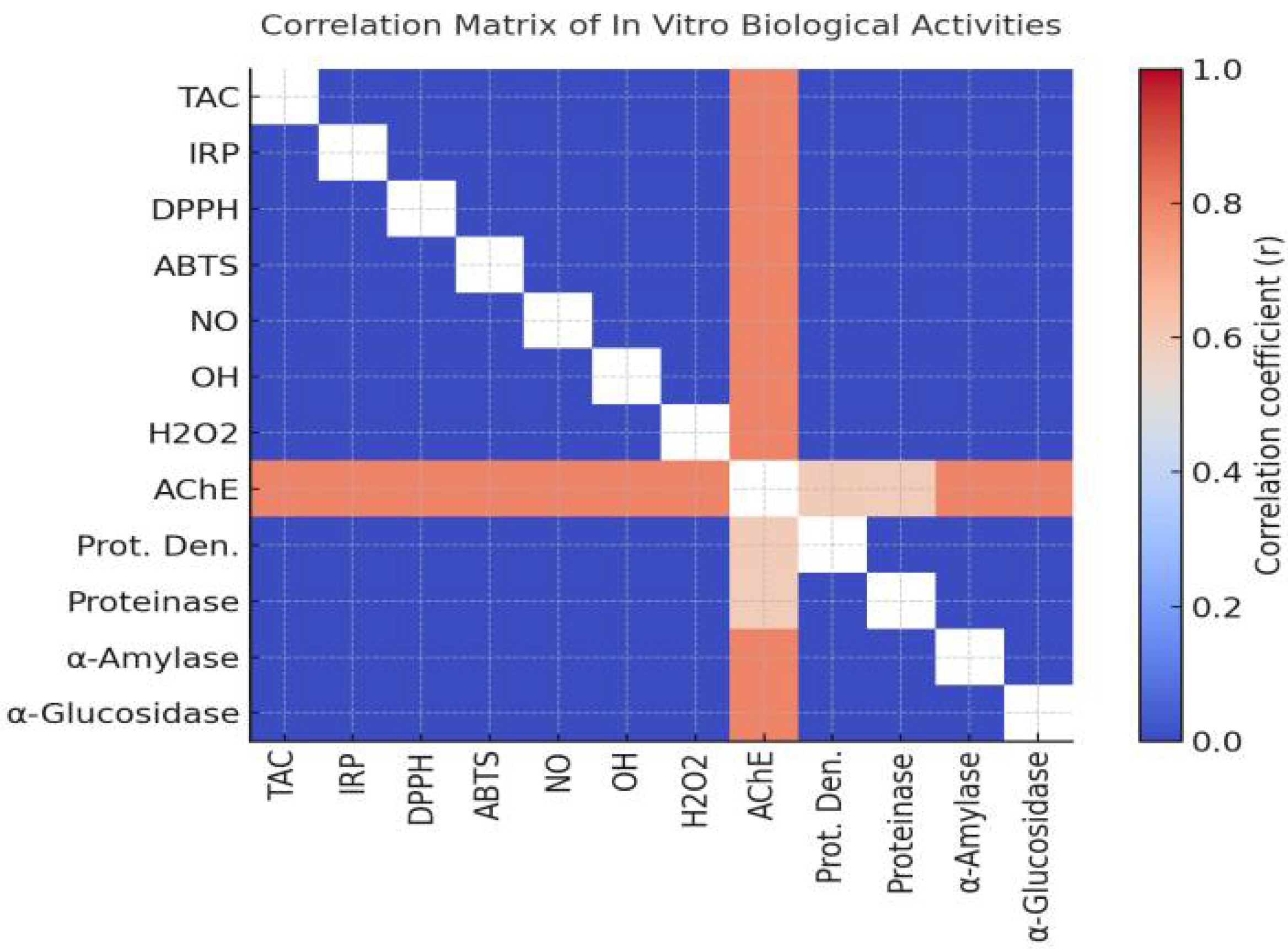
2.2.5. Comparative Overview of Biological Profiles
Interrelationship Among Antioxidant, Anti-Alzheimer’s, Anti-Arthritic, and Anti-Diabetic Activities
2.3. Molecular Docking Simulation
2.3.1. Docking Protocol Validation
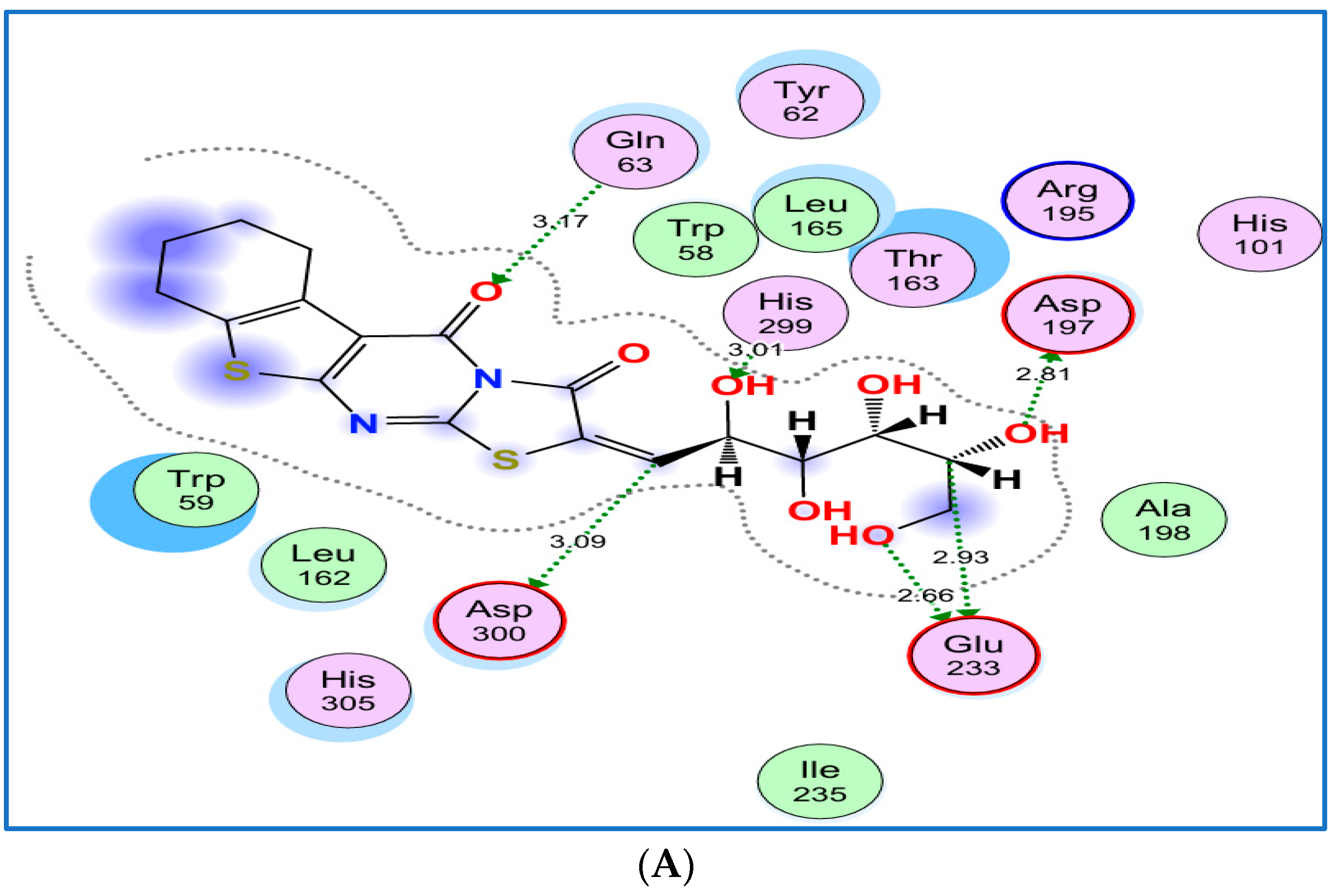
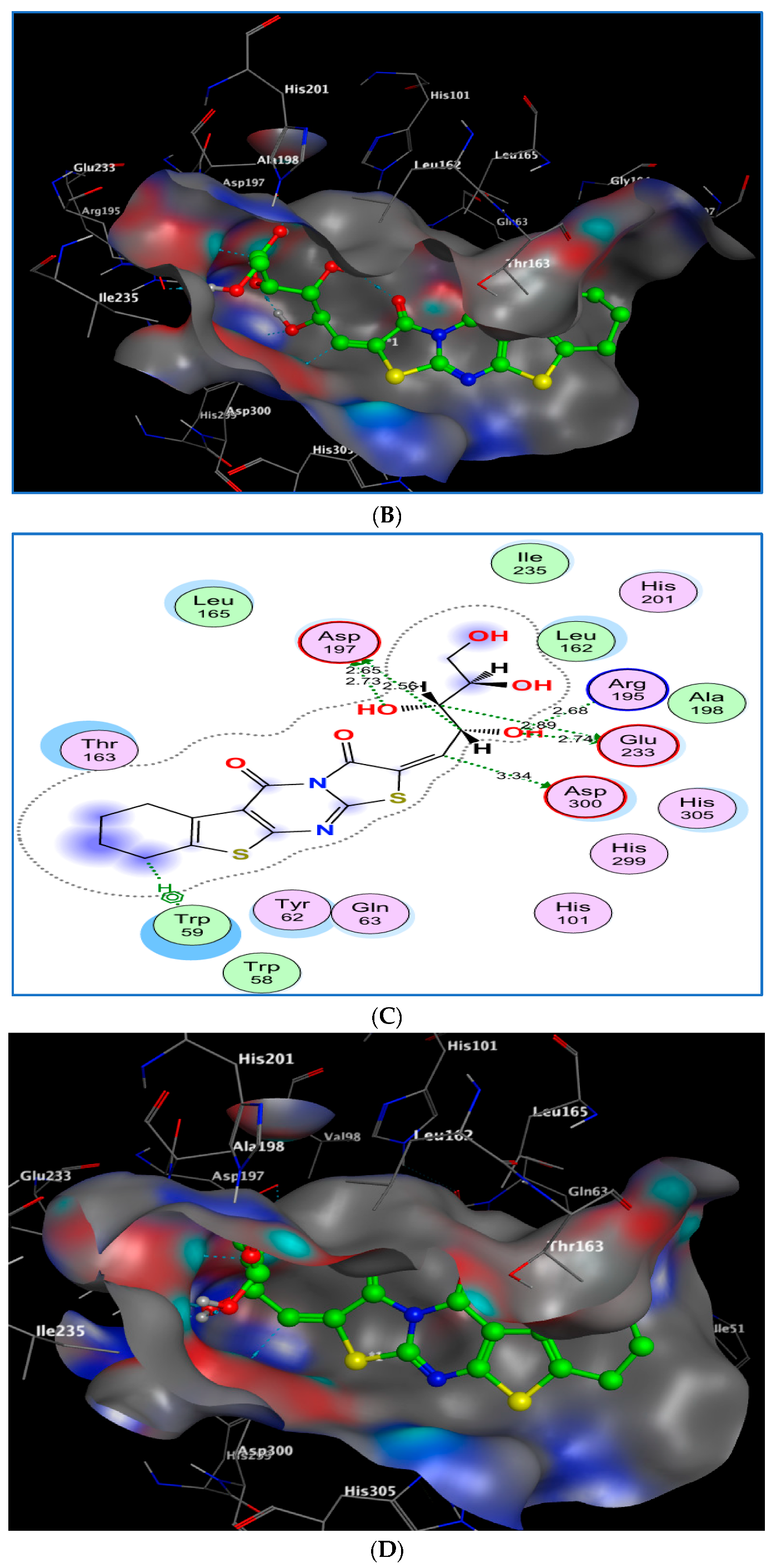
2.3.2. Docking of Compounds 8b and 8e with α-Amylase
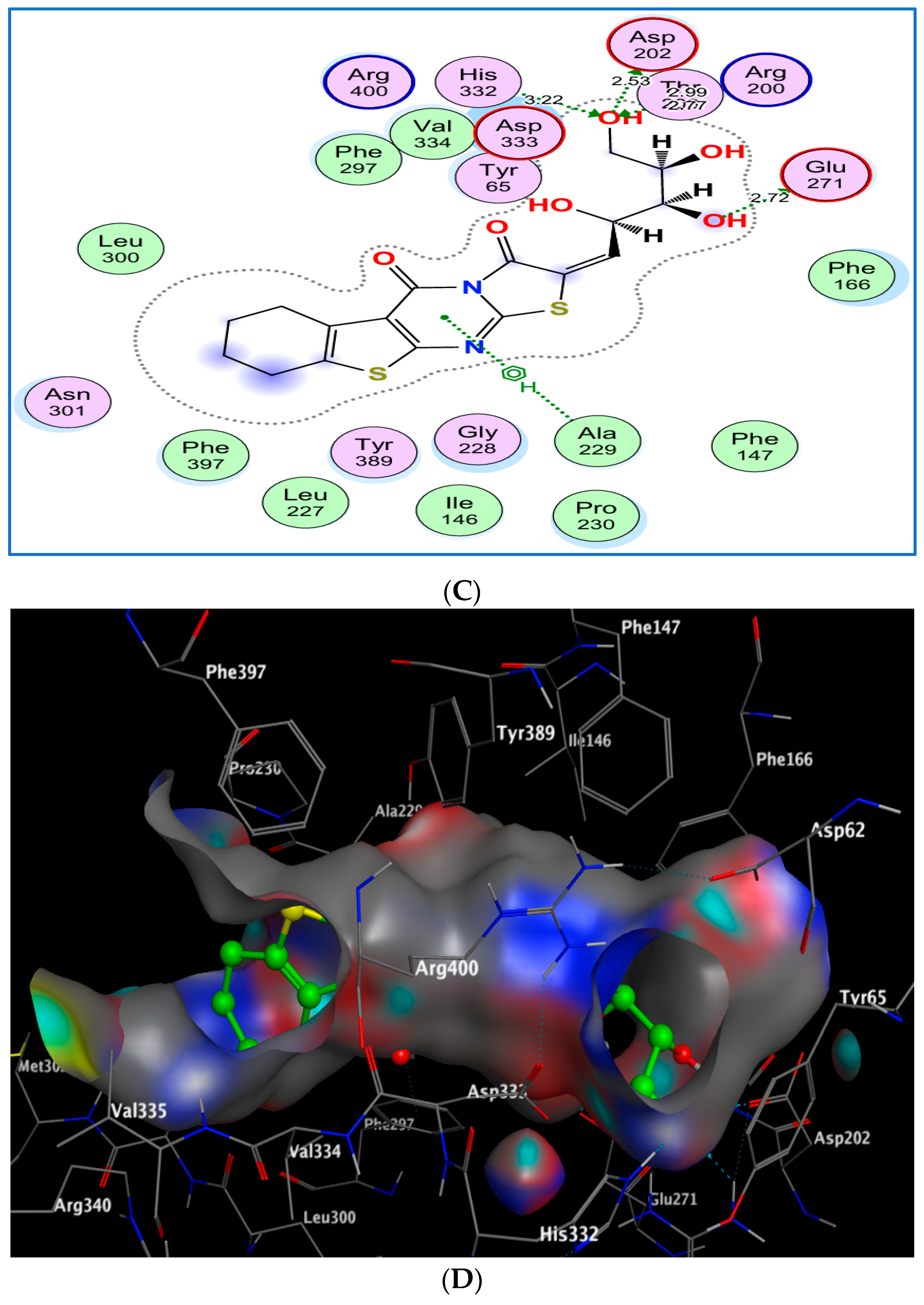
2.3.3. Docking of Compounds 8b and 8e with α-Glucosidase
2.3.4. Interaction Summary and Comparative Insight
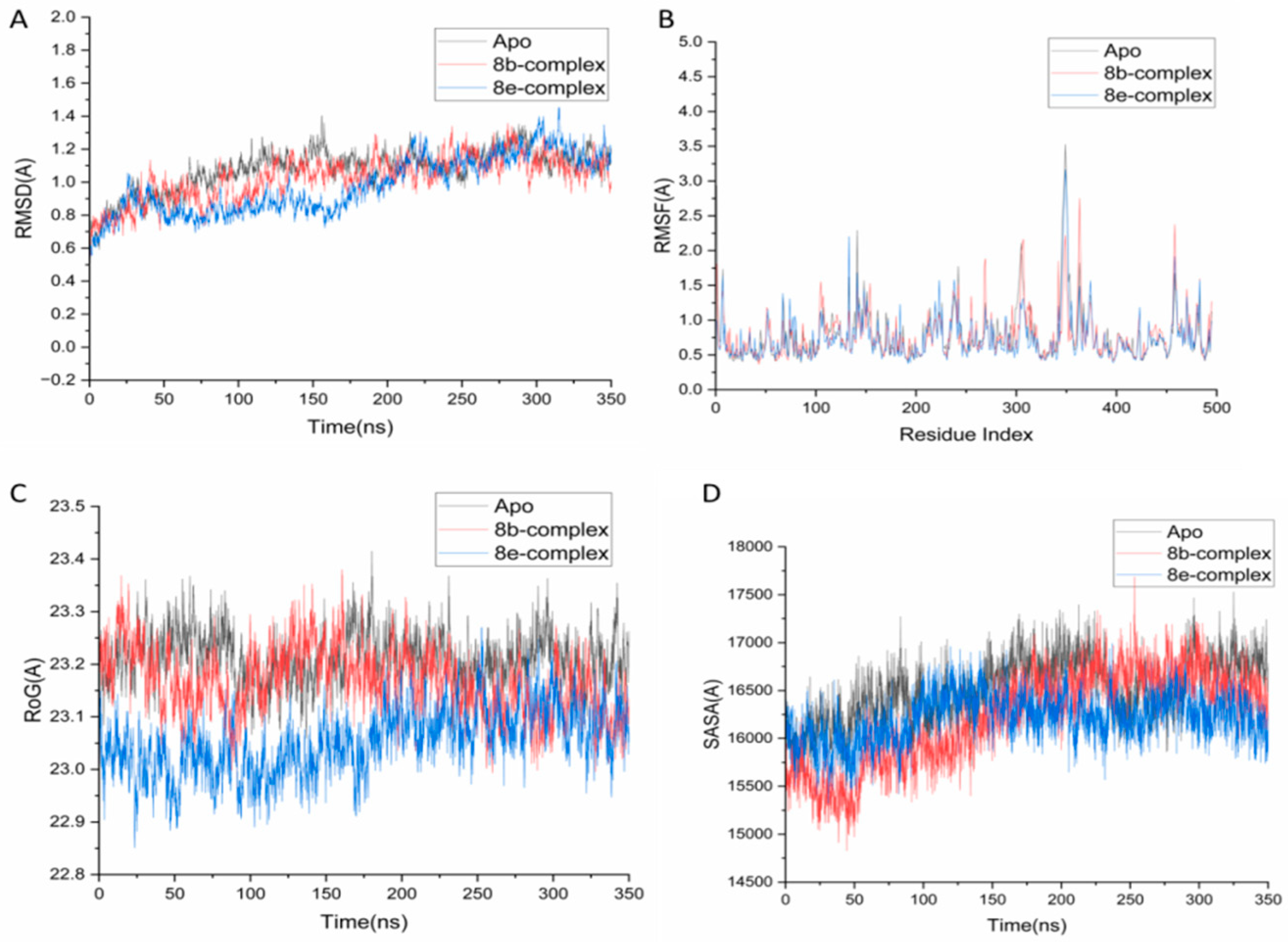
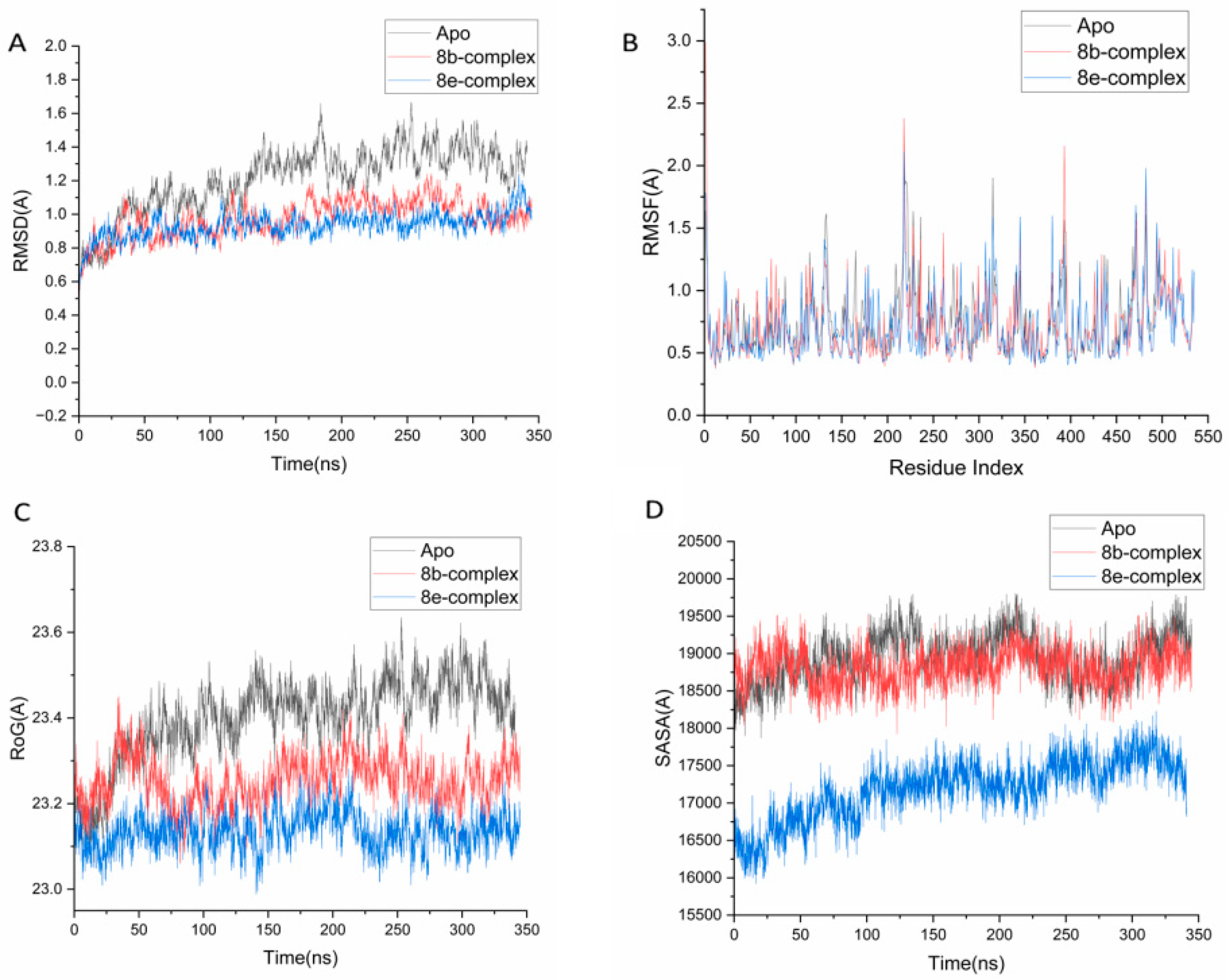
2.4. Molecular Dynamics and System Stability
2.4.1. Protein Flexibility
2.4.2. Structural Compactness
2.4.3. Solvent Accessibility
3. Materials and Methods
3.1. Chemistry
- General Procedure for the Synthesis of Glycoside Derivatives 7, 8 (a–e) [45]
- (E)-2-((2S,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexylidene)-7,8-dihydro-5H,6H-cyclopenta-[4,5]thieno [2,3-d]thiazolo [3,2-a]pyrimidine-3,5(2H)-dione (7a)
- (E)-2-((2R,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexylidene)-7,8-dihydro-5H,6H-cyclopenta [4,5]thieno [2,3-d]thiazolo [3,2-a]pyrimidine-3,5(2H)-dione (7b)
- (E)-2-((2S,3R,4S,5R)-2,3,4,5,6-pentahydroxyhexylidene)-7,8-dihydro-5H,6H-cyclopenta [4,5]thieno [2,3-d]thiazolo [3,2-a]-pyrimidine-3,5(2H)-dione (7c)
- (E)-2-((2S,3R,4R)-2,3,4,5-tetrahydroxypentylidene)-7,8-dihydro-5H,6H-cyclopenta [4,5]thieno [2,3-d]thiazolo [3,2-a]-pyrimidine-3,5(2H)-dione (7d)
- (E)-2-((2R,3S,4R)-2,3,4,5-tetrahydroxypentylidene)-7,8-dihydro-5H,6H-cyclopenta [4,5]thieno [2,3-d]thiazolo [3,2-a]-pyrimidine-3,5(2H)-dione (7e)
- (E)-2-((2S,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexylidene)-6,7,8,9-tetrahydro-5H-benzo [4,5]thieno [2,3-d]thiazolo [3,2-a]-pyrimidine-3,5(2H)-dione (8a)
- (E)-2-((2R,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexylidene)-6,7,8,9-tetrahydro-5H-benzo-[4,5]thieno [2,3-d]thiazolo [3,2-a]pyrimidine-3,5(2H)-dione (8b)
- (E)-2-((2S,3R,4S,5R)-2,3,4,5,6-pentahydroxyhexylidene)-6,7,8,9-tetrahydro-5H-benzo [4,5]-thieno [2,3-d]thiazolo [3,2-a]-pyrimidine-3,5(2H)-dione (8c)
- (E)-2-((2S,3R,4R)-2,3,4,5-tetrahydroxypentylidene)-6,7,8,9-tetrahydro-5H-benzo [4,5]thieno [2,3-d]thiazolo [3,2-a]-pyrimidine-3,5(2H)-dione (8d)
- (E)-2-((2R,3S,4R)-2,3,4,5-tetrahydroxypentylidene)-6,7,8,9-tetrahydro-5H-benzo [4,5]thieno [2,3-d]thiazolo [3,2-a]pyrimidine-3,5(2H)-dione (8e)
3.2. In Vitro Biological Activities
3.2.1. Antioxidant and Scavenging Activity
3.2.2. Anti-Diabetic Activity
3.2.3. Anti-Alzheimer’s Activity
3.2.4. Anti-Arthritic Activity
3.3. Molecular Docking Study
3.4. Molecular Dynamics (MD) Simulations
Post-MD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butterfield, D.A.; Halliwell, B. Oxidative Stress, Dysfunctional Glucose Metabolism and Alzheimer Disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of Reactive Oxygen Species and NF-κB Signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Serio, M. Oxidative Stress and Inflammation as Targets for Novel Preventive and Therapeutic Approaches in Non-Communicable Diseases II. Antioxidants 2022, 11, 824. [Google Scholar] [CrossRef]
- Yu, M.; Wang, S.; Lin, D. Mechanism and Application of Biomaterials Targeting Reactive Oxygen Species and Macrophages in Inflammation. Int. J. Mol. Sci. 2025, 26, 245. [Google Scholar] [CrossRef] [PubMed]
- Repellin, M.; Guerin, H.; Catania, G.; Lollo, G. ROS-Based Nanomedicines for Anti-Inflammatory Therapies. Redox Exp. Med. 2023, 1, REM-23-0021. [Google Scholar] [CrossRef]
- Minhas, P.S.; Jones, J.R.; Latif-Hernandez, A.; Sugiura, Y.; Durairaj, A.S.; Wang, Q.; Mhatre, S.D.; Uenaka, T.; Crapser, J.; Andreasson, K.I.; et al. Restoring Hippocampal Glucose Metabolism Rescues Cognition across Alzheimer’s Disease Pathologies. Science 2024, 385, 6711. [Google Scholar] [CrossRef]
- Mallidi, K.; Gundla, R.; Jeedimalla, N.; Raghupathi, J.K.; Katari, N.K.; Jonnalagadda, S.B. Combination of Ethylene Glycol and TBAB-Mediated Pyrimidine Fused Heterocyclic Derivatives: Synthesis, In Silico, and In Vitro Anti-Diabetic and Anti-Microbial Studies. Results Chem. 2025, 14, 102082. [Google Scholar] [CrossRef]
- Myriagkou, M.; Papakonstantinou, E.; Deligiannidou, G.E.; Patsilinakos, A.; Kontogiorgis, C.; Pontiki, E. Novel Pyrimidine Derivatives as Antioxidant and Anticancer Agents: Design, Synthesis and Molecular Modeling Studies. Molecules 2023, 28, 3913. [Google Scholar] [CrossRef]
- Mallidi, K.; Gundla, R.; Makam, P.; Katari, N.K.; Jonnalagadda, S.B. Dual Active Pyrimidine-Based Carbocyclic Nucleoside Derivatives: Synthesis, and In Silico and In Vitro Anti-Diabetic and Anti-Microbial Studies. RSC Adv. 2024, 14, 9559–9569. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, K.; Gundla, R.; Jeedimalla, N.; Raghupathi, J.K.; Jonnalagadda, S.B.; Katari, N.K. Pyrimidine Fused Heterocyclic Derivatives: Design, In Silico, In Vitro, Anti-Microbial, Antidiabetic and Anti-Biofilm Studies. Results Chem. 2025, 14, 102106. [Google Scholar] [CrossRef]
- Amin, S.; Sheikh, K.A.; Iqubal, A.; Khan, M.A.; Shaquiquzzaman, M.; Tasneem, S.; Khanna, S.; Najmi, A.K.; Akhter, M.; Haque, A.; et al. Synthesis, In Silico Studies and Biological Evaluation of Pyrimidine-Based Thiazolidinedione Derivatives as Potential Anti-Diabetic Agent. Bioorg. Chem. 2023, 134, 106449. [Google Scholar] [CrossRef]
- Wang, S.B.; Deng, X.Q.; Zheng, Y.; Yuan, Y.P.; Quan, Z.S.; Guan, L.P. Synthesis and Evaluation of Anticonvulsant and Antidepressant Activities of 5-Alkoxytetrazolo[1,5-c]Thieno[2,3-e]Pyrimidine Derivatives. Eur. J. Med. Chem. 2012, 56, 139–144. [Google Scholar] [CrossRef]
- El-Said, K.S.; Noser, A.A.; Mohamed, A.E.S. Synthesis of New Chalcones and Pyrimidine Derivatives as Antidiabetic Agents for Type 2 Diabetes Mellitus: In Vitro and In Vivo Studies. Bioorg. Chem. 2025, 163, 108696. [Google Scholar] [CrossRef]
- Dholariya, M.P.; Kapuriya, N.P.; Maliwal, D.; Pissurlenkar, R.; Patel, A.S. Design, Synthesis, and Computational Insights into Pyrazolopyrimidine Derivatives as New Class of α-Amylase Inhibitors for Antidiabetic Therapy. J. Mol. Struct. 2025, 1348, 143393. [Google Scholar] [CrossRef]
- Ali, E.M.; Abdel-Maksoud, M.S.; Oh, C.H. Thieno[2,3-d]Pyrimidine as a Promising Scaffold in Medicinal Chemistry: Recent Advances. Bioorg. Med. Chem. 2019, 27, 1159–1194. [Google Scholar] [CrossRef]
- Marzouk, M.A. Pyrimidine Derivatives as Multifaceted Antidiabetic Agents: A Comprehensive Review of Structure–Activity Relationships, Mechanisms, and Clinical Potential. Eur. J. Med. Chem. 2025, 296, 117859. [Google Scholar] [CrossRef] [PubMed]
- El-Mekabaty, A.; Fouda, A.E.A.S.; Shaaban, I.E. Convenient Synthesis of Functionalized Thieno[2,3-d]Pyrimidine-4-ones and Thieno[2,3-b]Pyridine-4-ones Bearing a Pyridine Moiety with Anticipated Antioxidant Activity. J. Heterocycl. Chem. 2020, 57, 2928–2935. [Google Scholar] [CrossRef]
- Khan, S.; Iqbal, T.; Abass, K.S.; Abdellattif, M.H.; Gomha, S.M.; Zaki, M.E. Pyrimidine-Attached Oxazolidinone Derivatives: Synthesis, Kinetic Profiling and Computational Evaluation for Anti-Alzheimer’s Therapeutics. J. Mol. Struct. 2025, 1348, 143421. [Google Scholar] [CrossRef]
- Pant, S.; Kapri, A.; Nain, S. Pyrimidine Analogues for the Management of Neurodegenerative Diseases. Eur. J. Med. Chem. Rep. 2022, 6, 100095. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhang, Q.; Li, B.Q.; Zhang, D.; Chui, R.H.; Zhang, L.L.; Zhang, Q.; Ma, L.Y. Progress in the Study of Anti-Alzheimer’s Disease Activity of Pyrimidine-Containing Bioactive Molecules. Eur. J. Med. Chem. 2025, 285, 117199. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, S.; Abdelkhalek, A.S.; Rezq, S.; Kull, M.E.A.; Romero, D.G.; Kothayer, H. Magic shotgun approach to anti-inflammatory pharmacotherapy: Synthesis of novel thienopyrimidine monomers/heterodimer as dual COX-2 and 15-LOX inhibitors endowed with potent antioxidant activity. Eur. J. Med. Chem. 2023, 260, 115724. [Google Scholar] [CrossRef]
- Triloknadh, S.; Rao, C.V.; Nagaraju, K.; Hari Krishna, N.; Venkata Ramaiah, C.; Rajendra, W.; Trinath, D.; Suneetha, Y. Design, synthesis, neuroprotective, antibacterial activities and docking studies of novel thieno[2,3-d]pyrimidine-alkyne Mannich base and oxadiazole hybrids. Bioorg. Med. Chem. Lett. 2018, 28, 1663–1669. [Google Scholar] [CrossRef]
- Eissa, K.I.; Kamel, M.M.; Mohamed, L.W.; Galal, M.A.; Kassab, A.E. Design, synthesis, and biological evaluation of thienopyrimidine and thienotriazine derivatives as multitarget anti-Alzheimer agents. Drug Dev. Res. 2022, 83, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Fernandez, M.; Nigro, M.; Travagli, A.; Caldon, F.; Salati, S.; Borea, P.A.; Cadossi, R.; Varani, K.; Gessi, S. PEMFs Restore Mitochondrial and CREB/BDNF Signaling in Oxidatively Stressed PC12 Cells Targeting Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 6495. [Google Scholar] [CrossRef]
- Petrov, V.; Aleksandrova, T.; Pashev, A. Synthetic Approaches to Novel DPP-IV Inhibitors. Molecules 2025, 30, 1043. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, J.M.; Nawaz, F.; Naidu, V.G.M.; Aaghaz, S.; Sahu, M.; Siddiqui, N.; Alam, O. Synthesis, Molecular Docking and Anti-Diabetic Evaluation of 2,4-Thiazolidinedione Based Amide Derivatives. Bioorg. Chem. 2017, 73, 24–36. [Google Scholar] [CrossRef]
- Elmongy, E.; Kedr, M.; Abotaleb, N.; Abbas, S. Design and Synthesis of New Thienopyrimidine Derivatives Along with Their Antioxidant Activity. Egypt. J. Chem. 2021, 64, 6857–6867. [Google Scholar] [CrossRef]
- Galan, M.C.; Benito-Alifonso, D.; Watt, G.M. Carbohydrate chemistry in drug discovery. Org. Biomol. Chem. 2011, 9, 3598–3610. [Google Scholar] [CrossRef]
- Kushwaha, D.; Kushwaha, A.K.; Kumar, R.; Chauhan, D. Recent advances in the synthesis of Glycoconjugated heterocycles: A promising strategy for accessing bioactive compounds. Bioorg. Chem. 2025, 162, 108559. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.V.; Hussein, W.M.; Varamini, P.; Simerska, P.; Toth, I. Glycosylation: An effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 2016, 7, 2492–2500. [Google Scholar] [CrossRef]
- Andreu, A.; Ćorović, M.; Garcia-Sanz, C.; Santos, A.S.; Milivojević, A.; Ortega-Nieto, C.; Mateo, C.; Bezbradica, D.; Palomo, J.M. Enzymatic glycosylation strategies in the production of bioactive compounds. Catalysts 2023, 13, 1359. [Google Scholar] [CrossRef]
- Qadir, T.; Amin, A.; Sharma, P.K.; Jeelani, I.; Abe, H. A review on medicinally important heterocyclic compounds. Open Med. Chem. J. 2022, 16, e187410452202280. [Google Scholar] [CrossRef]
- Hassan, N.A.; Alshamari, A.K.; Hassan, A.A.; Elharrif, M.G.; Alhajri, A.M.; Alanazi, M.S.; Khattab, R.R. Advances on Therapeutic Strategies for Alzheimer’s Disease: From Medicinal Plant to Nanotechnology. Molecules 2022, 27, 4839. [Google Scholar] [CrossRef]
- Alshamari, A.K.; Hassan, N.A.; Alshammari, O.A.O.; Basiony, E.A.; Alshammari, M.Z.; Matalka, S.I.; Hassan, A.A. Synthesis, biological evaluation, molecular docking, molecular dynamics, and ADME studies of novel thiouracil derivatives as dual inhibitors of butyrylcholinesterase and acetylcholinesterase enzymes. J. Mol. Struct. 2025, 1328, 141154. [Google Scholar] [CrossRef]
- Hassan, N.A. Development of Novel Pyrimidine Derivatives for Use as Anticancer Agents. Patent Appl. EG/P/2024/1425, 2024. [Google Scholar]
- Alshamari, A.K.; Elsawalhy, M.; Alhajri, A.M.; Hassan, A.A.; Elharrif, M.G.; Sam, G.; Alamshany, Z.M.; Alshehri, Z.S.; Alshehri, F.F.; Hassan, N.A. Design, Synthesis, Molecular Docking and Biological Evaluation of Donepezil Analogues as Effective Anti-Alzheimer Agents. Egypt. J. Chem. 2024, 67, 473–485. [Google Scholar] [CrossRef]
- Ellithy, S.A.; Abdel-Rahman, A.-H.A.; Hassan, N.A.; Elsawalhy, M.; Abou-Amra, E.S.; Hassan, A.A. Glycosyl Thiourea: Synthesis, Cyclization, Reaction, Molecular Docking, and Evaluation as Potential Acetylcholinesterase Inhibitors. Egypt. J. Chem. 2023, 66, 1759–1777. [Google Scholar] [CrossRef]
- Basiony, E.A.; Hassan, A.A.; Elsawalhy, M.; Abdel-Rahman, A.A.-H.; Mansour, H.; Arafa, R.K.; Hassan, N.A. Rational design, synthesis, biological evaluation, molecular docking, and molecular dynamics of substituted uracil derivatives as potent anti-cancer agents. Bioorg. Chem. 2025, 154, 108066. [Google Scholar] [CrossRef]
- Tashkandi, N.Y.; Al-Amshany, Z.M.; Hassan, N.A. Design, synthesis, molecular docking and antimicrobial activities of novel triazole-ferulic acid ester hybrid carbohydrates. J. Mol. Struct. 2022, 1269, 133832. [Google Scholar] [CrossRef]
- Al-Amshany, Z.M.; Hassan, N.A.; Khattab, R.R.; El-Sayed, A.A.; Tantawy, M.A.; Mostafa, A.; Hassan, A.A. Synthesis and Molecular Docking Study of Novel Pyrimidine Derivatives against COVID-19. Molecules 2023, 28, 739. [Google Scholar] [CrossRef] [PubMed]
- Aboulthana, W.M.; El-Feky, A.M.; Ibrahim, N.E.; Soliman, A.A.F.; Youssef, A.M. Phytochemical Analysis and Biological Study on Sinapis alba L. Seeds Extract Incorporated with Metal Nanoparticles: An In Vitro Approach. Sci. Rep. 2025, 15, 13782. [Google Scholar] [CrossRef]
- Younis, A.; Kassem, A.F.; Aboulthana, W.M.; Sediek, A.A. Green Synthesis, Molecular Docking and In Vitro Biological Evaluation of Novel Hydrazones, Pyrazoles, 1,2,4-Triazoles and 1,3,4-Oxadiazoles. Synth. Commun. 2024, 54, 1984–2002. [Google Scholar] [CrossRef]
- Morsy, N.M.; Abu-Zied, K.M.; Aly, A.S.; Elgamal, A.M. Design, Synthesis, Molecular Docking of Some New Polyhydrobenzothieno Thiazolopyrimidinedione Glycoside Derivatives with Double Anti-microbial–Anti-inflammatory Action. Egypt. J. Chem. 2022, 65, 577–598. [Google Scholar] [CrossRef]
- Aly, A.S.; Abu-Zied, K.M.; Gaafar, A.M. Synthesis and Reactions of Some Novel Azolothienopyrimidines and Thienopyrimido-as-triazines Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 447–474. [Google Scholar] [CrossRef]
- Abu-Zied, K.M. Synthesis and Reactions of Novel Thienopyrimidine and Thiazolothienopyrimidine Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 2179–2191. [Google Scholar] [CrossRef]
- Aly, A.S.; Abu-Zied, K.M.; Gaafar, A.M. Facile Syntheses of Some Thieno [2,3-d]pyrimidine Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2008, 183, 3063–3078. [Google Scholar] [CrossRef]
- Gewald, A.K.; Schinke, E.; Böttcher, H. Heterocyclen aus CH-aciden Nitrilen, VIII. 2-Amino-thiophene aus methylenaktiven Nitrilen, Carbonylverbindungen und Schwefel. Chem. Ber. 1966, 99, 94–100. [Google Scholar] [CrossRef]
- Kermanshah, Z.; Samadanifard, H.; Moghaddam, O.M.; Hejrati, A. Olive leaf and its various health-benefitting effects: A review study. Pak. J. Med. Health Sci. 2020, 14, 1301–1312. [Google Scholar]
- Fahad, N.G.; Imran, N.H.; Kyhoiesh, H.A.K.; Al-Hussainawy, M.K.S. Synthesis, anticancer for prostate cancer cells and antibacterial activity of new diazepine derivatives. Results Chem. 2023, 6, 101049. [Google Scholar] [CrossRef]
- Kyhoiesh, H.A.K.; Al-Adilee, K.J. Synthesis, spectral characterization, antimicrobial evaluation studies and cytotoxic activity of some transition metal complexes with tridentate (N,N,O) donor azo dye ligand. Results Chem. 2021, 3, 100245. [Google Scholar] [CrossRef]
- Kyhoiesh, H.A.K.; Al-Adilee, K.J. Pt(IV) and Au(III) complexes with tridentate-benzothiazole based ligand: Synthesis, characterization, biological applications (antibacterial, antifungal, antioxidant, anticancer and molecular docking) and DFT calculation. Inorg. Chim. Acta 2023, 555, 121598. [Google Scholar] [CrossRef]
- Bahloul, N.; Bellili, S.; Aazza, S.; Chérif, A.; Faleiro, M.L.; Antunes, M.D.; Miguel, M.G.; Mnif, W. Aqueous Extracts from Tunisian Diplotaxis: Phenol Content, Antioxidant and Anti-acetylcholinesterase Activities, and Impact of Exposure to Simulated Gastrointestinal Fluids. Antioxidants 2016, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Suganthy, N.; Sri Ramkumar, V.; Pugazhendhi, A.; Benelli, G.; Archunan, G. Biogenic synthesis of gold nanoparticles from Terminalia arjuna bark extract: Assessment of safety aspects and neuroprotective potential via antioxidant, anticholinesterase, and antiamyloidogenic effects. Environ. Sci. Pollut. Res. Int. 2018, 25, 10418–10433. [Google Scholar] [CrossRef] [PubMed]
- Balraj, B.; Senthilkumar, N.; Potheher, I.V.; Arulmozhi, M. Characterization, Antibacterial, Anti-arthritic and In Vitro Cytotoxic Potentials of Biosynthesized Magnesium Oxide Nanomaterial. Mater. Sci. Eng. B 2018, 231, 121–127. [Google Scholar] [CrossRef]
- Ayman, R.; Radwan, A.M.; Elmetwally, A.M.; Ammar, Y.A.; Ragab, A. Discovery of novel pyrazole and pyrazolo [1,5-a]pyrimidine derivatives as cyclooxygenase inhibitors (COX-1 and COX-2) using molecular modeling simulation. Arch. Pharm. 2023, 356, e2200395. [Google Scholar] [CrossRef]
- Thirumalaisamy, R.; Ameen, F.; Subramanian, A.; Selvankumar, T.; Alwakeel, S.S.; Govarthanan, M. In Vitro and In Silico Anti-inflammatory Activity of Lupeol Isolated from Crateva adansonii and Its Hidden Molecular Mechanism. Int. J. Pept. Res. Ther. 2020, 26, 2179–2189. [Google Scholar] [CrossRef]
- Hwang, H.W. Phenolic Phytochemicals and Their Antioxidant Activities in Maine-Grown Asian, American-Hybrid, and European Plums. Ph.D. Thesis, University of Maine, Orono, ME, USA, 2020; p. 3158. Available online: https://digitalcommons.library.umaine.edu/etd/3158 (accessed on 29 September 2025).
- Cipolla, L.; Peri, F. Carbohydrate-Based Bioactive Compounds for Medicinal Chemistry Applications. Mini-Rev. Med. Chem. 2011, 11, 39–54. [Google Scholar] [CrossRef]
- Yuan, L.; Hua, Y.; Wang, X. Recent Progress of Glycomimetics in Drug Development. Org. Biomol. Chem. 2025, 23, 7671–7680. [Google Scholar] [CrossRef]
- Kozieł, K.; Urbanska, E.M. Kynurenine Pathway in Diabetes Mellitus—Novel Pharmacological Target? Cells 2023, 12, 460. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules 2020, 10, 61. [Google Scholar] [CrossRef]
- Iacobini, C.; Vitale, M.; Pesce, C.; Pugliese, G.; Menini, S. Diabetic complications and oxidative stress: A 20-year voyage back in time and back to the future. Antioxidants 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Moin, S.; Khan, A.Q.; Zafar, A. Redox Signaling in Rheumatoid Arthritis and the Preventive Role of Polyphenols. Clin. Chim. Acta 2016, 463, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Nahoum, V.; Roux, G.; Anton, V.; Rougé, P.; Puigserver, A.; Bischoff, H.; Henrissat, B.; Payan, F. Crystal Structures of Human Pancreatic α-Amylase in Complex with Carbohydrate and Proteinaceous Inhibitors. Biochem. J. 2000, 346, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Saburi, W.; Gai, Z.; Kato, K.; Ojima-Kato, T.; Yu, J.; Komoda, K.; Kido, Y.; Matsui, H.; Mori, H.; et al. Structural Analysis of the α-Glucosidase HaG Provides New Insights into Substrate Specificity and Catalytic Mechanism. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 1382–1391. [Google Scholar] [CrossRef]
- Mirzaei, S.; Eisvand, F.; Hadizadeh, F.; Mosaffa, F.; Ghasemi, A.; Ghodsi, R. Design, Synthesis and Biological Evaluation of Novel 5,6,7-Trimethoxy-N-Aryl-2-Styrylquinolin-4-Amines as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorg. Chem. 2020, 98, 103711. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; El-Rashedy, A.A.; Kamel, S. Synthesis of Novel Heterocyclic Compounds Based on Dialdehyde Cellulose: Characterization, Antimicrobial, Antitumor Activity, Molecular Dynamics Simulation and Target Identification. Cellulose 2021, 28, 8355–8374. [Google Scholar] [CrossRef]
- Machaba, K.E.; Mhlongo, N.N.; Soliman, M.E.S. Induced Mutation Proves a Potential Target for TB Therapy: A Molecular Dynamics Study on LprG. Cell Biochem. Biophys. 2018, 76, 345–356. [Google Scholar] [CrossRef]
- Pan, L.; Patterson, J.C.; Deshpande, A.; Cole, G.; Frautschy, S. Molecular Dynamics Study of Zn(Aβ) and Zn(Aβ)2. PLoS ONE 2013, 8, 70681–70688. [Google Scholar] [CrossRef]
- Wijffels, G.; Dalrymple, B.; Kongsuwan, K.; Dixon, N. Conservation of Eubacterial Replicases. IUBMB Life 2005, 57, 413–419. [Google Scholar] [CrossRef]
- Richmond, T.J. Solvent Accessible Surface Area and Excluded Volume in Proteins: Analytical Equations for Overlapping Spheres and Implications for the Hydrophobic Effect. J. Mol. Biol. 1984, 178, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on Product of Browning Reaction Prepared from Glucose Amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Chakraborthy, G.S. Free Radical Scavenging Activity of Costus speciosus Leaves. Indian J. Pharm. Educ. Res. 2009, 43, 96–98. [Google Scholar]
- Kutlu, T.; Kasim, T.; Bircan, C.; Murat, K. DNA Damage Protecting Activity and In Vitro Antioxidant Potential of the Methanol Extract of Cherry (Prunus avium L). J. Med. Plants Res. 2014, 8, 715–726. [Google Scholar] [CrossRef]
- Amessis-Ouchemoukh, N.; Ouchemoukh, S.; Meziant, N.; Idiri, Y.; Hernanz, D.; Stinco, C.M.; Rodríguez-Pulido, F.J.; Heredia, F.J.; Madani, K.; Luis, J. Bioactive Metabolites Involved in the Antioxidant, Anticancer and Anticalpain Activities of Ficus carica L., Ceratonia siliqua L. and Quercus ilex L. Extracts. Ind. Crops Prod. 2017, 95, 6–17. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The Hydrophilic and Lipophilic Contribution to Total Antioxidant Activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.K. In Vitro Antioxidant and Free Radical Scavenging Activity of Different Parts of Tabebuia pallida Growing in Bangladesh. BMC Res. Notes 2015, 8, 621–628. [Google Scholar]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In Vitro Alpha Amylase Inhibitory Activity of the Leaf Extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A Preparation and Screening Strategy for Glycosidase Inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Soni, D.; Sureshkumar, P. Effect of methanolic root extract of Blepharispermum subsessile DC in controlling arthritic activity. Res. J. Biotechnol. 2016, 11, 65–74. [Google Scholar]
- Oyedapo, O.O.; Famurewa, A.J. Antiprotease and Membrane Stabilizing Activities of Extracts of Fagara Zanthoxyloides, Olax Subscorpioides and Tetrapleura Tetraptera. Int. J. Pharmacogn. 1995, 33, 65–69. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Alminderej, F.M.; Mounier, M.M.; Nossier, E.S.; Saleh, S.M.; Kassem, A.F. New 1, 2, 3-Triazole-Coumarin-Glycoside Hybrids and Their 1,2,4-triazolyl thioglycoside analogs targeting mitochondria apoptotic pathway: Synthesis, anticancer activity and docking simulation. Molecules 2022, 27, 5688. [Google Scholar] [CrossRef]
- Mohi El-Deen, E.M.; Nossier, E.S.; Karam, E.A. New Quinazolin-4(3H)-one Derivatives Incorporating Hydrazone and Pyrazole Scaffolds as Antimicrobial Agents Targeting DNA Gyrase Enzyme. Sci. Pharm. 2022, 90, 52. [Google Scholar] [CrossRef]
- Amr, A.E.G.E.; Mageid, R.E.A.; El-Naggar, M.; Naglah, A.M.; Nossier, E.S.; Elsayed, E.A. Chiral Pyridine-3,5-bis-(L-phenylalaninyl-L-leucinyl) Schiff Base Peptides as Potential Anticancer Agents: Design, Synthesis, and Molecular Docking Studies Targeting Lactate Dehydrogenase-A. Molecules 2020, 25, 1096. [Google Scholar] [CrossRef]
- Alamshany, Z.M.; Algamdi, E.M.; Othman, I.M.; Anwar, M.M.; Nossier, E.S. New Pyrazolopyridine and Pyrazolothiazole-Based Compounds as Anti-Proliferative Agents Targeting c-Met Kinase Inhibition: Design, Synthesis, Biological Evaluation, and Computational Studies. RSC Adv. 2023, 13, 12889–12905. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E. PTRAJ and CPPTRAJ: Software for Processing and Analysis of Molecular Dynamics Trajectory Data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Seifert, E. OriginPro 9.1: Scientific Data Analysis and Graphing Software-Software Review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating Structures and Free Energies of Complex Molecules: Combining Molecular Mechanics and Continuum Models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]

| No. | TAC (mg Gallic Acid/g) | IRP (µg/mL) | DPPH (%) | ABTS (%) | NO (%) | OH (%) | H2O2 (%) |
|---|---|---|---|---|---|---|---|
| 7a | 17.44 ± 0.22 | 10.29 ± 0.22 | 14.04 ± 0.22 | 17.19 ± 0.22 | 8.29 ± 0.22 | 7.21 ± 0.19 | 8.07 ± 0.21 |
| 7b | 21.59 ± 0.25 | 14.44 ± 0.25 | 18.19 ± 0.25 | 21.34 ± 0.25 | 12.44 ± 0.25 | 10.82 ± 0.21 | 12.12 ± 0.24 |
| 7c | 20.40 ± 0.26 | 13.25 ± 0.26 | 17.00 ± 0.26 | 20.15 ± 0.26 | 11.25 ± 0.26 | 9.78 ± 0.22 | 10.96 ± 0.25 |
| 7d | 20.09 ± 0.23 | 12.94 ± 0.23 | 16.69 ± 0.23 | 19.84 ± 0.23 | 10.94 ± 0.23 | 9.51 ± 0.20 | 10.65 ± 0.22 |
| 7e | 18.77 ± 0.21 | 11.62 ± 0.21 | 15.37 ± 0.21 | 18.52 ± 0.21 | 9.62 ± 0.21 | 8.37 ± 0.19 | 9.37 ± 0.21 |
| 8a | 13.95 ± 0.18 | 6.80 ± 0.18 | 10.55 ± 0.18 | 13.70 ± 0.18 | 4.80 ± 0.18 | 4.17 ± 0.15 | 4.67 ± 0.17 |
| 8b | 68.01 ± 0.78 | 60.86 ± 0.78 | 64.61 ± 0.78 | 67.76 ± 0.78 | 58.86 ± 0.78 | 51.18 ± 0.68 | 57.32 ± 0.76 |
| 8c | 15.90 ± 0.20 | 8.75 ± 0.20 | 12.50 ± 0.20 | 15.65 ± 0.20 | 6.75 ± 0.20 | 5.87 ± 0.17 | 6.58 ± 0.20 |
| 8d | 17.02 ± 0.21 | 9.87 ± 0.21 | 13.62 ± 0.21 | 16.77 ± 0.21 | 7.87 ± 0.21 | 6.84 ± 0.19 | 7.66 ± 0.21 |
| 8e | 68.69 ± 0.79 | 61.54 ± 0.79 | 65.29 ± 0.79 | 68.44 ± 0.79 | 59.54 ± 0.79 | 51.77 ± 0.68 | 57.99 ± 0.76 |
| STD | 78.44 ± 0.19 | 67.49 ± 0.17 | 71.24 ± 0.17 | 74.39 ± 0.17 | 65.49 ± 0.17 | 56.95 ± 0.14 | 63.78 ± 0.16 |
| Sample | DPPH | ABTS | NO | OH | H2O2 |
|---|---|---|---|---|---|
| 7a | 27.67 ± 0.29 | 18.83 ± 0.22 | 48.89 ± 0.78 | 65.77 ± 2.12 | 73.10 ± 1.67 |
| 7b | 21.36 ± 0.44 | 15.17 ± 0.15 | 32.58 ± 0.96 | 43.75 ± 0.52 | 48.67 ± 0.85 |
| 7c | 22.84 ± 0.23 | 16.06 ± 0.19 | 36.00 ± 0.45 | 48.42 ± 1.40 | 53.83 ± 1.05 |
| 7d | 23.28 ± 0.49 | 16.31 ± 0.16 | 37.06 ± 1.13 | 49.77 ± 0.65 | 55.36 ± 1.03 |
| 7e | 25.27 ± 0.54 | 17.47 ± 0.17 | 42.12 ± 1.33 | 56.57 ± 0.81 | 62.92 ± 1.25 |
| 8a | 36.82 ± 0.42 | 23.63 ± 0.28 | 84.51 ± 2.12 | 113.71 ± 4.73 | 126.37 ± 4.09 |
| 8b | 6.01 ± 0.12 | 4.78 ± 0.05 | 6.88 ± 0.16 | 9.24 ± 0.05 | 10.28 ± 0.12 |
| 8c | 31.07 ± 0.34 | 20.68 ± 0.24 | 60.02 ± 1.12 | 80.74 ± 2.84 | 89.75 ± 2.32 |
| 8d | 28.53 ± 0.30 | 19.31 ± 0.23 | 51.52 ± 0.85 | 69.29 ± 2.28 | 77.03 ± 1.82 |
| 8e | 5.95 ± 0.12 | 4.73 ± 0.05 | 6.80 ± 0.16 | 9.14 ± 0.05 | 10.17 ± 0.12 |
| STD | 5.45 ± 0.06 | 4.35 ± 0.04 | 6.18 ± 0.09 | 8.31 ± 0.05 | 9.24 ± 0.04 |
| Sample | AChE Inhibition (%) | AChE IC50 (µg/mL) | Protein Denaturation Inhibition (%) | Proteinase Inhibition (%) | Proteinase IC50 (µg/mL) |
|---|---|---|---|---|---|
| 7a | 5.75 ± 0.18 | 49.93 ± 1.96 | 11.62 ± 0.18 | 7.45 ± 0.18 | 62.33 ± 1.77 |
| 7b | 5.14 ± 0.16 | 55.92 ± 2.20 | 15.06 ± 0.20 | 10.89 ± 0.20 | 42.61 ± 0.37 |
| 7c | 5.87 ± 0.19 | 48.95 ± 1.93 | 14.08 ± 0.21 | 9.91 ± 0.21 | 46.88 ± 1.21 |
| 7d | 4.40 ± 0.14 | 65.33 ± 2.57 | 13.82 ± 0.19 | 9.65 ± 0.19 | 48.10 ± 0.46 |
| 7e | 4.11 ± 0.13 | 69.90 ± 2.75 | 12.73 ± 0.18 | 8.56 ± 0.18 | 54.22 ± 0.58 |
| 8a | 5.81 ± 0.18 | 49.44 ± 1.95 | 8.74 ± 0.15 | 4.57 ± 0.15 | 101.84 ± 3.59 |
| 8b | 5.19 ± 0.16 | 55.37 ± 2.18 | 11.80 ± 0.19 | 7.63 ± 0.19 | 65.93 ± 2.02 |
| 8c | 5.93 ± 0.19 | 48.46 ± 1.91 | 10.35 ± 0.17 | 6.18 ± 0.17 | 75.16 ± 2.30 |
| 8d | 5.61 ± 0.18 | 51.23 ± 2.02 | 11.28 ± 0.18 | 7.11 ± 0.18 | 65.40 ± 1.90 |
| 8e | 5.24 ± 0.17 | 54.82 ± 2.16 | 54.07 ± 0.65 | 50.90 ± 0.65 | 9.08 ± 0.11 |
| STD | 54.46 ± 0.16 | 5.26 ± 0.05 | 60.74 ± 0.14 | 56.57 ± 0.14 | 8.20 ± 0.06 |
| Anti-Diabetic Activity | ||||
|---|---|---|---|---|
| Sample | α-Amylase | α-Glucosidase | α-Amylase | α-Glucosidase |
| Inhibition (%) | IC50 (µg/mL) | |||
| 7a | 13.37 ± 0.21 | 31.25 ± 0.65 | 6.62 ± 0.21 | 31.92 ± 0.89 |
| 7b | 17.32 ± 0.24 | 24.10 ± 0.08 | 10.57 ± 0.24 | 19.96 ± 0.34 |
| 7c | 16.19 ± 0.24 | 25.80 ± 0.52 | 9.44 ± 0.24 | 22.37 ± 0.53 |
| 7d | 15.89 ± 0.22 | 26.27 ± 0.09 | 9.14 ± 0.22 | 23.09 ± 0.41 |
| 7e | 14.64 ± 0.20 | 28.51 ± 0.11 | 7.89 ± 0.20 | 26.75 ± 0.52 |
| 8a | 10.05 ± 0.17 | 41.58 ± 0.89 | 3.30 ± 0.17 | 64.26 ± 2.84 |
| 8b | 61.53 ± 0.74 | 6.78 ± 0.02 | 54.78 ± 0.74 | 3.85 ± 0.05 |
| 8c | 11.91 ± 0.19 | 35.08 ± 0.74 | 5.16 ± 0.19 | 41.00 ± 1.33 |
| 8d | 12.97 ± 0.20 | 32.21 ± 0.67 | 6.22 ± 0.20 | 33.99 ± 0.98 |
| 8e | 62.18 ± 0.75 | 6.71 ± 0.02 | 55.43 ± 0.75 | 3.81 ± 0.05 |
| (Acarbose) | 69.85 ± 0.16 | 5.98 ± 0.05 | 63.10 ± 0.16 | 3.34 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamari, A.K.; Aboulthana, W.M.; Mansour, H.; Abu-Zied, K.M.; Alshammari, O.A.O.; Morsy, N.M.; Alsaif, N.O.S.; Alshammari, M.Z.; Nossier, E.S.; Hassan, N.A. In Vitro Evaluation of Sugar-Conjugated Thienopyrimidinone Derivatives with Possible Neuroprotective and Antioxidant Effects. Int. J. Mol. Sci. 2025, 26, 10826. https://doi.org/10.3390/ijms262210826
Alshamari AK, Aboulthana WM, Mansour H, Abu-Zied KM, Alshammari OAO, Morsy NM, Alsaif NOS, Alshammari MZ, Nossier ES, Hassan NA. In Vitro Evaluation of Sugar-Conjugated Thienopyrimidinone Derivatives with Possible Neuroprotective and Antioxidant Effects. International Journal of Molecular Sciences. 2025; 26(22):10826. https://doi.org/10.3390/ijms262210826
Chicago/Turabian StyleAlshamari, Asma K., Wael M. Aboulthana, Hayam Mansour, Khadiga M. Abu-Zied, Odeh A. O. Alshammari, Nesrin M. Morsy, Nuha O. S. Alsaif, Mona Z. Alshammari, Eman S. Nossier, and Nasser A. Hassan. 2025. "In Vitro Evaluation of Sugar-Conjugated Thienopyrimidinone Derivatives with Possible Neuroprotective and Antioxidant Effects" International Journal of Molecular Sciences 26, no. 22: 10826. https://doi.org/10.3390/ijms262210826
APA StyleAlshamari, A. K., Aboulthana, W. M., Mansour, H., Abu-Zied, K. M., Alshammari, O. A. O., Morsy, N. M., Alsaif, N. O. S., Alshammari, M. Z., Nossier, E. S., & Hassan, N. A. (2025). In Vitro Evaluation of Sugar-Conjugated Thienopyrimidinone Derivatives with Possible Neuroprotective and Antioxidant Effects. International Journal of Molecular Sciences, 26(22), 10826. https://doi.org/10.3390/ijms262210826








