Abstract
Bacterial exopolysaccharides have emerged as versatile biopolymers for the design of advanced hydrogels with adjustable physico-chemical, mechanical, and biological properties. Among these, levan, a fructose-based exopolysaccharide synthesized by various microbial species, has attracted increasing attention due to its unique structural features, high biocompatibility, and inherent bioactivity. This review provides a comprehensive overview of hydrogel systems derived from bacterial exopolysaccharides, with a particular focus on levan-based hydrogels. We discuss the molecular structure, synthesis pathways, and physico-chemical characteristics of levan that underpin its hydrogel-forming ability. Emphasis is placed on design strategies, including chemical modification, crosslinking approaches, and composite formation, that enable fine-tuning of mechanical strength, swelling behavior, and degradation kinetics. This review further highlights biomedical applications of levan-based hydrogels, encompassing drug delivery, wound healing, rejuvenation, tissue engineering, regenerative medicine, and bioprinting, while addressing current limitations and future research directions. By elucidating the structure–function relationships and emerging fabrication methodologies, this review underscores the biomedical promise of levan as a sustainable and functional biopolymer for next-generation hydrogel technologies.
1. Introduction
Molecular aggregates formed by hydrophilic polymers and water molecules are biomaterials of major interest [1,2]. Natural compounds and synthetic analogs have been developed and modulated into various architectures to adapt to different applications [3,4,5].
Hydrophilic gels, frequently referred to as hydrogels, are tridimensional networks formed by cross-linked materials with a hydrophilic structure, capable of absorbing high quantities of water or biological fluids [6,7]. Due to the connections between the polymeric chains, the microscopic interactions and their density influence the behavior of the macroscopic molecule [7].
Depending on the source, composition, stimulus responsiveness, physical configuration, and also electronic charge, hydrogels can be classified as follows:
- −
- Based on origin: natural or synthetic;
- −
- Based on the polymers used in synthesis: homopolymeric—a singular repetitive cross-linked structural unit; copolymeric—two different monomers arranged in a random or alternate fashion in the polymeric network; and multipolymeric—a network of two cross-linked polymers and one non-linked monomer, consisting of either natural or synthetic molecules;
- −
- Based on stimulus response: responsive at temperature, pH, ionic strength, and light- and chemical-responsive;
- −
- Based on chemical structure: amorphous, semicrystalline, or crystalline;
- −
- Based on electronic charge: nonionic, ionic, amphoteric, or zwitterionic (repetitions of cationic and anionic groups) [1,4,5,6].
In addition to an insight into the structure at a monomeric level, which forms linked or non-linked polymeric chains, a macromolecular approach to the structural particularities is required in order to infer their stability and potential applications [7]. Moreover, a key point resides in the understanding of the mechanisms behind the transition from a sol to a gel state [6].
A defining feature of hydrophilic gels is represented by the high content of absorbed water, which can surpass 95% of the mass ratio [1]. Another aspect resides in their adjustable porosity, which can be achieved through various techniques such as adjusting the polymer and cross-linker concentrations [8], freeze-drying [9], and cryogelation [10]. Other techniques include solvent casting/particle leaching, aided by the dispersion of porogen particles and leaching via selective solvents [11], or even gas foaming [12].
Due to their unique structure and properties, this group of molecular aggregates formed by hydrophilic polymers and water molecules is of major interest in materials science and engineering [13], with various applications in biomedicine [5,14], cosmetic industry [15], food processing [16], bioremediation technologies [17], and sustainable agriculture [18]. Various natural compounds and synthetic analogs have been recently developed. Their properties and, therefore, their stability can be modulated to adapt to certain media characterized by unfavorable conditions, such as significant pH variations, temperature, chemical stress, etc. [3,4,5].
Hydrogels provide an ideal platform for tridimensional cellular studies [6], being bioengineered for a wide range of biomedical applications, such as tissue engineering, bioprinting, and targeted drug delivery [1,2]. A number of studies have been conducted on such gels, exploiting their biomimetic properties, for example, the development of the extracellular matrix of pancreatic tissue [2], but also for cells of the vascular endothelium [10]. Areas pertaining to regenerative medicine and lesion treatment are indisputable, especially concerning infection control and maintenance of an optimal medium for hemostasis, inflammation, proliferation, and tissue remodeling [19]. Recent approaches focus on the creation of stimulus-responsive cellular constructs, especially in the context of regenerative medicine [1,2,6,20]. Incorporation of bioreceptors such as DNA, enzymes, antibodies, and even cells has led to the development of hydrogel-based sensors for the detection of small molecules, cellular metabolites, and pathogens. Biosensors and composite hydrogels are useful in metabolic diagnosis, cancer screening, and bacterial and viral sensing and capture [21,22,23].
The aim of this paper is to provide a comprehensive analysis of hydrogel design, with a special focus on levan as a promising bacterial exopolysaccharide for biomedical applications. This review integrates current knowledge and highlights emerging trends to emphasize levan-based hydrogels as sustainable, biocompatible, and multifunctional biomaterials for medical bioengineering.
2. Hydrophilic Gels Based on Bacterial Exopolysaccharides
Exopolysaccharides (EPSs) are a group of high-molecular-weight carbohydrate polymers [24] characterized by the presence of a variable number of functional groups, conferring both native properties and the possibility of molecular functionalization [25]. Depending on the repetitive structural unit, they can be classified as homoexopolysaccharides (same monomer linked through glycosidic bonds) and heteroexopolysaccharides (two or more monomers linked through glycosidic bonds) [25,26].
Hydrophilic gels based on bacterial EPSs are formed by cross-linking bacterial biopolymers with high affinity for water, like levan, dextran, cellulose, alginate, hyaluronic acid, gellan, kefiran, and xanthan gum [27,28,29]. These polysaccharides are usually secreted in the extracellular space, sometimes with a tight association with the cell wall, which facilitates scaling at the industrial level, but also higher efficacy for downstream processing [27,30]. The three-dimensional network of bacterial EPS gels is achieved through polymerization and self-assembly during physically or chemically driven cross-linking of biopolymers [31]. Hydrogels can be further modified or blended with other materials as composite hydrogels with enhanced mechanical strength and improved functionality [32,33].
The valuable properties of hydrogels based on bacterial EPSs are high water absorption, biodegradability without forming toxic byproducts, stability in different conditions, biocompatibility, conformational adaptability, the capacity of reverting to their initial state before having absorbed a biological fluid, and also stimulus responsiveness [4,6,19,29,32].
3. Bacterial Levan as a Structural Biopolymer for Hydrogels
Levan is a naturally occurring EPS diversely distributed in bacteria, fungi, and plants [34]. It is widely produced by a wide range of prokaryotes with diverse taxonomy: Gram-positive bacteria belonging to the phyla Actinomycetota and Bacillota, as well as Gram-negative bacteria belonging to Pseudomonadota [35]. Prokaryotic strains originating in a vast range of environments, from common natural settings to extreme habitats, plant or animal sources, as well as reference strains belonging to industrial culture collections, have been previously investigated for levan production. In addition, genomic analysis revealed putative levansucrase-encoding genes that allowed cloning, expression, and purification of enzymes for levan biosynthesis in recombinant or cell-free systems (Table 1).

Table 1.
Bacteria as sources of levan and levansucrase.
The molecular structure of levan defines a homoexopolysaccharide with a backbone of fructose units linked through β-(2,6) glycosidic bonds, alongside a residual glucosyl molecule at the end of the polymeric chain. Short side chains consisting of fructose residues are attached to the main chain via β-(2,1) glycosidic bonds [24,34,66] (Figure 1). This branching is a key structural feature of levan, particularly in microbial levan, which can have a significantly higher molecular weight (28 to 141,000 kDa) and a greater degree of branching [67] compared to plant levan (2 to 33 kDa) [68]. The molecular weight determines polymer conformation and subsequent properties. Low-molecular-weight microbial-derived levans tend to form spherical nanoparticles, while high-molecular-weight levans exhibit microgel characteristics [67,69].
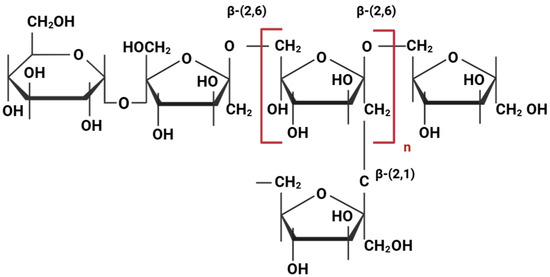
Figure 1.
Chemical structure of levan.
Levan biosynthesis differs significantly between bacteria and plants in terms of molecular weight, enzyme mechanisms, and location. Bacteria synthesize high-molecular-weight levan using enzymes like levansucrase (EC 2.4.1.10) to form a polymer with high viscosity, while plants produce low-molecular-weight levan, often within the vacuole [34]. In bacteria, prior to levan biosynthesis, levansucrase is synthesized in the cytoplasmic space; then, it is accumulated in the periplasm, where it undergoes conformational changes before its secretion through various pathways. Generally, this involves cleavage of a signal peptide in Gram-positive bacteria, yet the majority of Gram-negative bacteria have been described to secrete levansucrase in a signal peptide-independent pathway [70]. Moreover, the genes involved in levan biosynthesis are found in a tricistronic operon. Levansucrase is encoded by the gene sacB, while a protein with endolevanase activity is encoded by yveB. The function of the third protein, encoded by yveA, has not yet been determined, but reports have predicted that it might function as a permease [71,72].
Microbial levan biosynthesis has three key steps: hydrolysis of sucrose from a sucrose-rich substrate, transfructosylation, and polymerization. All reactions are carried out by levansucrase, a fructosyltransferase belonging to the glycoside hydrolase family 68 (GH68). Thus, a sucrose molecule is cleaved by levansucrase into glucose and fructose. Then, a fructose molecule is transferred to a donor sucrose molecule, resulting in the formation of a levan-type trisaccharide, 6-kestotriose, by a bacterial levansucrase type sucrose–fructan 6-fructosyltransferase [73]. After successive fructose residue transfers, the polymeric chain elongates, forming levan molecules while releasing glucose molecules into the medium (polymerization) [66,67,74,75] (Figure 2).
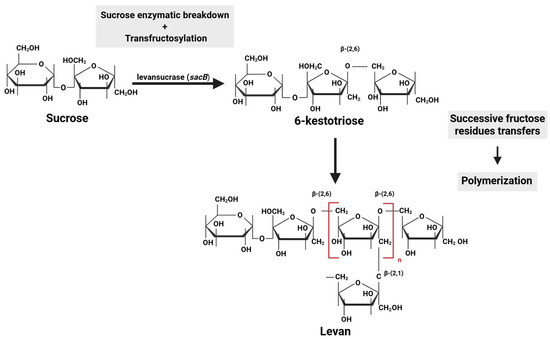
Figure 2.
Microbial levan biosynthesis.
The physico-chemical features of this biopolymer are conferred by its molecular architecture and depend on the source and production conditions [26]. It is soluble in both water and oil, but not in the vast majority of organic solvents, such as ethanol, methanol, isopropanol, toluene, or acetone [68]. Levan is a nonionic fructan with amphiphilic properties, possessing both hydrophilic and hydrophobic groups that allow polymer self-assembly into colloidal dispersions or nanoparticles. The assessment of stability in different pH conditions showed that acid hydrolysis occurs sooner at lower pH conditions, and no hydrolysis was observed for pH 5.5 and higher [76]. Levan has high thermal stability, with previous studies reporting a melting point at 214 °C for levan derived from Bacillus subtilis [58]. Thermal analysis of levan produced by Acetobacter xylinum showed several stages of thermal degradation. Increasing the temperature from 200 to 250 °C induced breakages in the β-(2,1) branch point linkages, followed by breakage of the main chain β-(2,6) linkages [68]. In spite of the high molecular weight, microbial levan has low intrinsic viscosity, ranging from 0.07 to 0.18 dL/g for polymers with molecular weights ranging from 16,000 to 24,000 kDa [77].
The aforementioned characteristics, such as solubility and viscosity, together with its adhesive, matrix-forming, water retention abilities, and compatibility with salts and surfactants, highlight levan as a promising candidate for pharmaceutical coatings and wound bandages [68,75]. Due to its amphiphilic properties, the production of levan-based nanoparticles has been explored to encapsulate hydrophobic materials. The amphiphilic nature of levan can be easily exploited for targeted drug delivery and tissue engineering [78,79]. The particular structure and physico-chemical features conferring thermal stability, biofilm-like matrix formation, and high biocompatibility define relevant functional properties for levan-based hydrogels [25].
3.1. Synthesis of Levan Hydrogels
The molecular and network structure of a hydrogel determines its fundamental nature and defines its functional properties and, therefore, performance in applications. Levan-based hydrogels can mimic the mechanical properties of the in vivo extracellular matrix and modulate the biological properties of host cells, thus being ideal candidates for biomedical purposes. Various synthesis protocols can be adapted for different types of hydrophilic gels with levan, ranging from natural to chemical and hybrid gels.
The synthesis of levan-based hydrogels typically involves physical and/or chemical cross-linking. The process requires a series of successive steps for solubilization and purification of polymers, followed by a phase transition for gelation (Figure 3).
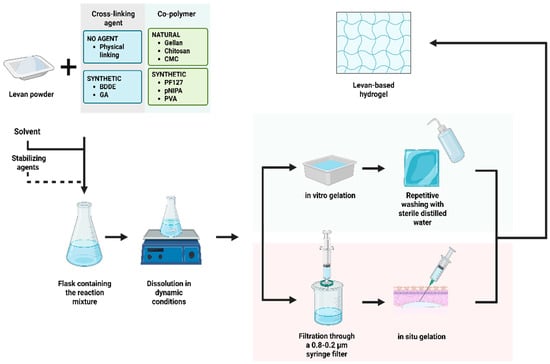
Figure 3.
General steps for obtaining levan-based hydrogels. BDDE—1,4-butanediol diglycidyl ether; CMC—carboxymethyl cellulose; GA—glutaraldehyde; PF127—Pluronic F127; pNIPA—N-isopropyl acrylamide; PVA—polyvinyl alcohol.
Initially, a levan powder is dissolved in dynamic conditions into a basic or neutral solvent, such as sodium nitrate [80], borate buffer [81], or deionized water [82,83]. If the aim is to produce a hybrid gel, an intermediary step is required for the solubilization of the co-polymer and the stabilizing agents into the same solvent [81,82,83].
The next step is the purification of the sol-state mixture. For standard hydrogels, purification consists of repetitive washing with sterile distilled water to remove impurities and/or contaminants [80]. For injectable hydrogels with delayed gelation, the process most commonly involves physical filtration through syringe filters ranging from 0.8 to 0.2 μm [79,82,83].
Lastly, the gel state is induced via different methods, depending on the components and the type of hydrogel. Phase transition for gelation is mostly induced via a thermal stimulus, which determines the interactions between hydrophilic and hydrophobic molecules. The introduction of a hydrophobic compound, such as a methyl or an ethyl group, requires a critical temperature, below the point when the mixture is miscible and above the value when phase separation occurs [6]. Thermo-chemical cross-linking is more suitable for obtaining levan-based hydrogels [84]. The process involves at least one cross-linking agent, such as 1,4-butanediol diglycidyl ether (BDDE) [71] or glutaraldehyde [85].
3.2. Design Strategies to Improve Levan-Based Hydrogels for Biomedical Purposes
Hydrogel tailoring for specific uses allows us to control mechanical strength, swelling behavior, and degradation rates. Desired properties can be achieved by designing polymer composition, chemical modification and functionalization, gelation mechanism, and cross-linking agents. Therefore, a variety of options have been proposed to design levan-based hydrogels suitable for a plethora of biomedical applications, with antioxidant, antitumor, antibacterial, immunomodulatory, biofilm-like matrix formation, and biomimetic properties (Figure 4).
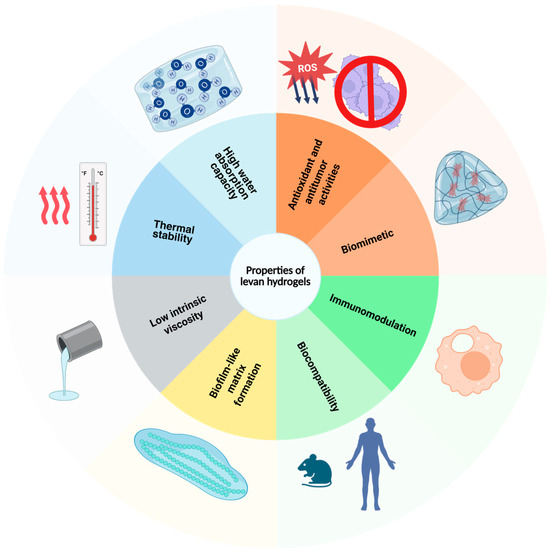
Figure 4.
Properties of levan-based hydrogels for biomedical applications.
The antioxidant activity of levan-based hydrogels stems from the polymer’s strong scavenging abilities against free radicals and induction of anti-inflammatory cytokine production, while the antitumor capacity is a result of the modulation of host cell immune response via caspase activation and induction of apoptosis via the mitochondrial pathway [86,87,88]. Moreover, antibacterial properties are conferred by its polysaccharide backbone, capable of disrupting Gram-negative bacteria’s membrane integrity [89,90]. On top of that, levan-based hydrogels have been shown to possess biocompatible and biomimetic properties due to their porous networks, which favor cell adhesion and proliferation, alongside biomolecule and nutrient transport [91].
Hydrogel composition. Pure levan is not used alone in hydrogels due to inconsistent and difficult control of properties, whereas using levan derivatives or copolymers improves hydrogel properties and functional behavior. Levan methacrylate hydrogels were shown to produce cytocompatible, mechanically stable gels with a moderate degree of swelling. Semisynthetic levan derivatives contain methacrylate groups attached either via ester or urethane linkages to the fructan backbone. Levan methacrylates derived from Bacillus subtilis levan were photo-chemically cross-linked by applying different photoinitiator systems responsive to either UV light or visible light irradiation [81].
Chemical modification and functionalization. Chemically modified forms, such as hydrolized, sulphated, methylated, oxidized, and phosphonated levan derivatives, enable desirable properties of hydrogels, nanofibers, and films for medical applications [26]. Levan-based hydrogels often include copolymers, such as polyvinyl alcohol (PVA), poly-N-isopropyl acrylamide (pNIPA), or Pluronic F127 (PF-127). Levan–PVA hydrogels have a high water solubility and water adsorption ability [92]. Biocompatible methacrylated levan served as a cross-linker in the preparation of levan/pNIPA thermosensitive hydrogels [93]. Other experimental protocols have included PF-127 prepared with carboxymethyl cellulose (CMC) to improve its in vivo stability [82].
Gelation mechanism. Regarding gelation, there are several possibilities, with or without chemical interventions. For example, in the composite levan–gellan hydrogel, gelation occurs by increasing the pH to the alkaline domain, inducing interactions between gellan’s free hydroxyl groups by inter-linking with levan molecules [94]. Besides in vitro gelation through chemical agents, thermal inducers, or inducible polymerization, a specific biological cross-linking involves enzyme-mediated linkage, leading to in situ gelation of injectable hydrogels [79,81,83].
The influence of cross-linking agents. Most hydrogels are formed through cross-linking, either through physical or chemical methods. Physical methods imply linking via a stimulus and are usually reversible, while the rate of the inducer is used to modulate the final properties. Chemical cross-linking presents methods such as the formation of bonds through the addition of a cross-linking agent, polymer functionalization, radical polymerization, enzyme-mediated processes, and even irradiation [95]. Different agents and methods can therefore be used to influence and modify physico-chemical and rheological properties of polysaccharide-based gels, such as mechanical strength, stability, swelling, and water binding [85]. The available literature describes a variety of cross-linking protocols, but the specific influence of certain agents or techniques is insufficiently detailed. Therefore, this section aims to emphasize protocol designs with concrete examples of cross-linkers for particular applications (Table 2).

Table 2.
Examples of cross-linking types and their applications.
Chemical cross-linking. Chemical cross-linking may be achieved through a number of methods, most commonly through a chemical agent, photo-chemical reactions, or Schiff’s base reaction. The work by Selvi et al. [80] showed the influence of BDDE on both native and phosfonated Halomonas-derived levan hydrogels for resveratrol release, with the phosfonated derivative resulting in a firm, homogenous gel. However, an increase in BDDE raises the toxicity of the gels, despite the improvement in their stability and heterogeneity. Similar results were reported by Demirci et al. [96] regarding the stability of hydrogels synthesized for amphotericin B controlled release.
Another candidate is a biocomposite gel of oxidized levan–chitosan, with chitosan acting both as a cross-linking agent and a copolymer. Gel formation occurs through Schiff’s base reaction—oxidized levan aldehyde groups interact with the primary amines of chitosan, resulting in imine bonds, responsible for the three-dimensional gel architecture. The obtained hydrogel showed not only cytocompatibility but also hemocompatibility, besides good swelling capacities and stability [91].
Physical cross-linking. For physical cross-linkage, besides levan, a co-polymer is preferred for increased stability. Injectable hydrogels composed of levan, CMC, and PF127 have been synthesized as an alternative to other commercial dermal fillers, such as hyaluronic acid. CMC was selected to improve in vivo stability, and PF-127 was selected to enhance the cross-linking density by increasing the number of hydrogen bonds. Besides common in vitro testing, promising results on wrinkle mouse models have been obtained [82]. Another excellent compound triad is represented by levan–PVA gels cross-linked with glutaraldehyde (GA) for influenza virus capture. GA and PVA increase the stability and strength of pure levan gels by forming acetal bridges when incubated at room temperature, followed by freeze-drying [92].
3.3. Strategies to Improve Rheological Properties of Levan-Based Hydrogels for Biomedical Purposes
Levan is an unusual carbohydrate, a water-soluble polymer with native properties ranging from biocompatibility and health benefits due to its distinctive rheological features [94]. Rheological testing is often an indispensable tool for analyzing specific characteristics of hydrophilic gels, such as the deformation and flow [99]. Some relevant aspects for biomedical engineering, but not limited to levan-based hydrogels, are swelling, capacity, viscoelasticity, tensile strength, and macroscopic behavior.
Swelling capacity. The consensus regarding the influence of a cross-linker on the swelling ability of a gel is that a high concentration of the cross-linking agent leads to higher-density gels and, thus, a lower water absorption capacity; this behavior applies to levan hydrogels as well [96]. Besides that, for most hydrophilic gels, it is common knowledge that adding an ionic monomer or increasing the number of ionic groups, for example, the addition of salts, increases the swelling degree. This is caused by the presence of a high number of counter-ions, leading to additional osmotic pressure, a pH-independent process, and thus inducing a stimulus-independent behavior [7], and it can be speculated to be applicable to levan hydrogels as well.
Viscoelasticity. Gels are viscoelastic systems and, therefore, possess the capacity for shear thinning and/or thickening. However, they are shear-thinning by default due to a high fluid content. There have been reports of hydrogels based on recombinant proteins or peptides, thus non-polysaccharides, which are capable of shear-thinning and self-healing, though they present disadvantages such as poor mechanical properties and cellular and in vivo toxicity. Hence, biocompatible alternatives with improved stability have been sought through natural carbohydrate-based polymers, including composite gels composed of levan and gellan [94].
Tensile strength. Molecular branches may contribute to the cohesive/adhesive properties of levan [99], while the presence of hydroxyl groups facilitates its interaction with various molecules [95]. Moreover, using levan derivatives has shown good improvements regarding tensile strength, a property in direct relation to shearing. For example, a desirable increased tensile strength of sulfated levan hydrogels, combined with alginate and chitosan, led to the development of both chemically cross-linked and non-linked gels in adhesive-free standing multilayer films for biomedical purposes [99,100].
4. Current and Potential Biomedical Applications of Levan-Based Hydrogels Based on Their Properties
This section aims to describe a few of the current applications associated with levan-based hydrogels. The literature has vast examples, studies, and papers on exopolysaccharide gels; however, only a few focus on levan hydrogels specifically, and their potential is still not fully explored. Compiling all the available information confirms that levan hydrogels and levan-based colloidal systems are promising candidates for the biomedical field [101], with applications ranging from targeted drug delivery/therapeutic agents to regenerative medicine, wound healing, tissue engineering, and even bioprinting ink.
Targeted drug delivery systems. Studies on levan hydrogels as delivery systems for therapeutic substances, such as amphotericin B [96], vancomycin [102], or resveratrol [80], indicate promising results, without affecting the bioavailability of the therapeutic agent. However, there is much more to explore concerning both the hydrogel composition and the form in which the gel is used. Hydrogel-based drug delivery systems are generally represented by nanoparticles [103].
The exciting possibilities of levan nanocarriers also include protein and chemotherapeutic agent delivery systems. Sezer et al. [104] investigated the in vitro release of bovine albumin serum, cementing their suitability as drug carriers. Besides that, levan has an affinity for binding to CD44 receptors. Thus, the potential of paclitaxel-loaded levan nanoparticles has been explored to efficiently deliver chemotherapeutic agents to cancer cells [105]. Moreover, the efficacy of levan–poly (lactic-co-glycolic acid) nanoparticles has been exploited for their delivery of chemotherapeutic and chemopreventive agents [106].
Wound healing and rejuvenation. Other interesting aspects related to the possible applications of levan-based hydrogels are wound healing and the process of rejuvenation. Hydrogels, mainly carbohydrate-based gels, have already been characterized as wound dressing materials due to their non-antigenicity and permeability to water and metabolites whilst isolating the wound site from pathogens [107]. Previous investigations focused on microbial levan as an adjuvant to accelerate the healing of burn injuries. The effects are possibly achieved through the activation of matrix metalloproteinase enzymes, which are crucial during the repair of damaged tissues [108]. Hence, their incorporation in a hydrogel system may be a potential approach for wound treatment. Rejuvenation of soft tissue was also achieved through an injectable levan-based hydrogel, with improved biocompatibility and good in vitro and in vivo stability in comparison to hyaluronic acid hydrogels [82]. Similarly, Hwang et al. improved on the previous hydrogel formulation by adding hydroxyapatite, thus increasing its stability and residence time while keeping the original features, such as biocompatibility and collagen production stimulation [83].
Tissue engineering and bioprinting. Polysaccharide hydrogels have also been investigated for their applications in tissue engineering due to their tridimensional structure and biomimetic abilities, especially in conjunction with cellular matrices, via in situ scaffold formation [109]. By combining both bioprinting technology and tissue engineering, the use of methacrylate levan, a photocurable variety, as bio-ink for bone tissue scaffolds and for a final construct containing pre-osteoblasts, led to favorable cell proliferation and subsequent osteogenesis. This levan-based hydrogel bio-ink formulation highlights its immunomodulatory properties via modulating macrophage phenotype and promoting expression of anti-inflammatory markers [98]. For guided bone regeneration, histopathological studies indicated that the combination of levan hydrogels and conventional bone graft materials led to improved osteoblast formation and neovascularization, rather than the use of deproteinized bovine grafts alone [97]. On top of that, sulfated levan thin-layer films have been investigated for cardiac tissue engineering, not only for their heparin-mimetic capacity, but also for their high rate of functionalization [110]. In the same vein as levan thin-layer films, the current literature has investigated adhesive free-standing multilayer films containing sulfated levan, which promote both myogenic differentiation and mioconductivity [100]. In addition, ternary blend films of chitosan/polyethylene oxide/levan promoted cell proliferation and viability [111], thus pertaining to tissue engineering and bioprinting applications.
5. Perspectives and Challenges
Levan-based hydrogels are still an underexplored frontier because of current experimental limitations. For example, levan-based hydrophilic gel matrices could incorporate not only pre-osteoblasts [97] but also other cell types, which are already incorporated into polysaccharide-based hydrogels: the COS-7 cell line, NIT 3T3 fibroblasts [112], cortical neurons isolated from chick embryos [113], and chondrocytes isolated from porcine cartilages [114]. This leads to the field of bioprinting, where hydrogels are the bio-ink used for three-dimensional cell matrices. However, there are still obstacles related to their optimization in order to accommodate different cell types, specifically maintaining structural fidelity over time [115], as well as a lack of dynamism, which directly impacts biomimicry [116]. Moreover, improvement in mechanical strength by increasing either the polymer’s or cross-linking agent’s concentration may compromise the bioactivity of certain molecules, consequently limiting their biological applications [117]. On top of that, the aspect of uneven and/or cross-linking impacts their mechanical, physico-chemical, and biological activities, requiring additional post-processing [118], such as the inclusion of rheology modifiers [119].
An additional area with tremendous potential would be the development of biosensors and resorbable electronics. The previous literature does not address levan in the hydrogel system, but in different constructs, like a modified levan sensor proved suitable for the voltametric detection of daunorubicin–DNA interactions [120]. Kwon et al. described the use of levan-based films for transient electronic systems, with promising results at both in vivo and in vitro testing, concerning biocompatibility and bioresorption [121].
Unfortunately, creating functional levan hydrogels poses another set of challenges in addition to those mentioned above. Another limitation is the spatial inhomogeneity in hydrophilic gels, caused by high concentrations of the monomer unit and the concentration of polymers at the level of the linking region. However, it can be managed through temperature and concentration adjustments, or incorporation of synthetic components, to achieve stabilized and uniform cross-linked networks [7,10]. Nevertheless, the addition or increase in concentration of a synthetic component may lead to toxicity issues [80,82]. A fundamental problem of polymeric materials resides in the precise control of the polymer structure [122], with particular importance in the context of bioinspired materials. Finally, an obstacle that is not related to the technical side itself is represented by the scalability and reproducibility of these gels for commercialization purposes, implicitly, the approval by regulatory agencies [123].
Author Contributions
Conceptualization, A.I.P. and R.C.; methodology, A.I.P., R.C., and A.F.; investigation, A.I.P., R.C., and A.F.; resources, A.I.P., R.C., and A.F.; writing—original draft preparation, A.I.P. and R.C.; writing—review and editing, A.I.P., R.C., and A.F.; visualization, A.I.P., R.C., and A.F.; supervision, R.C. and A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Fu, J. Hydrogel properties and applications. J. Mater. Chem. B 2019, 7, 1523–1525. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of hydrogels: Linking the nano to the microscale. Adv. Colloid. Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Segneanu, A.-E.; Bejenaru, L.E.; Bejenaru, C.; Blendea, A.; Mogoşanu, G.D.; Biţă, A.; Boia, E.R. Advancements in hydrogels: A comprehensive review of natural and synthetic innovations for biomedical applications. Polymers 2025, 17, 2026. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Okay, O. General properties of hydrogels. Hydrogel sensors and actuators. In Hydrogel Sensors and Actuators: Engineering and Technology; Springer Series on Chemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6, pp. 1–14. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater. 2019, 94, 195–203. [Google Scholar] [CrossRef]
- Henderson, T.M.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682–2695. [Google Scholar] [CrossRef]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, R.; Li, B.; Li, K.; Hao, Y. A controlled light-induced gas-foaming porous hydrogel with adhesion property for infected wound healing. Int. J. Biol. Macromol. 2024, 261, 129751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Mitura, S.; Sionkowska, A.; Jaiswal, A. Biopolymers for hydrogels in cosmetics: Review. J. Mater. Sci. Mater. Med. 2020, 31, 50. [Google Scholar] [CrossRef]
- Nath, P.C.; Debnath, S.; Sridhar, K.; Inbaraj, B.S.; Nayak, P.K.; Sharma, M. A comprehensive review of food hydrogels: Principles, formation mechanisms, microstructure, and its applications. Gels 2023, 9, 1. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef]
- Ali, K.; Asad, Z.; Agbna, G.H.D.; Saud, A.; Khan, A.; Zaidi, S.J. Progress and innovations in hydrogels for sustainable agriculture. Agronomy 2024, 14, 2815. [Google Scholar] [CrossRef]
- Alberts, A.; Tudorache, D.I.; Niculescu, A.G.; Grumezescu, A.M. Advancements in wound dressing materials: Highlighting recent progress in hydrogels, foams, and antimicrobial dressings. Gels 2025, 11, 123. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozatic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel based sensors for biomedical applications: An updated review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Dsouza, A.; Constantinidou, C.; Arvanitis, T.N.; Haddleton, D.M.; Charmet, J.; Hand, R.A. Multifunctional composite hydrogels for bacterial capture, growth/elimination, and sensing applications. ACS Appl. Mater. Interfaces 2022, 14, 47323–47344. [Google Scholar] [CrossRef]
- Mukherjee, S.; Strakova, P.; Richtera, L.; Adam, V.; Ashrafi, A. Biosensors-based approaches for other viral infection detection. Advanced Biosensors for Virus Detection. In Advanced Biosensors for Virus Detection; Academic Press: Cambridge, MA, USA, 2022; pp. 391–405. [Google Scholar] [CrossRef]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides producing bacteria: A review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef]
- Domżał-Kędzia, M.; Ostrowska, M.; Lewińska, A.; Łukaszewicz, M. Recent developments and applications of microbial Levan, a versatile polysaccharide-based biopolymer. Molecules 2023, 28, 5407. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sieber, V.; Rehm, B. Bacterial exopolysaccharides: Biosynthesis pathways and engineering strategies. Front. Microbiol. 2015, 6, 496. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jung, Y.; Lee, N.; Lee, I.; Lee, J.H. Nature-derived polysaccharide-based composite hydrogels for promoting wound healing. Int. J. Mol. Sci. 2023, 24, 16714. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.A.G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent advancements in microbial polysaccharides: Synthesis and applications. Polymers 2021, 13, 4136. [Google Scholar] [CrossRef]
- Nasution, H.; Harahap, H.; Dalimunthe, N.F.; Ginting, M.H.S.; Jaafar, M.; Tan, O.O.H.; Aruan, H.K.; Herfananda, A.L. Hydrogel and Effects of Crosslinking Agent on Cellulose-Based Hydrogels: A Review. Gels 2022, 8, 568. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef]
- Khattak, S.; Qin, X.T.; Huang, L.H.; Xie, Y.Y.; Jia, S.R.; Zhong, C. Preparation and characterization of antibacterial bacterial cellulose/chitosan hydrogels impregnated with silver sulfadiazine. Int. J. Biol. Macromolec. 2021, 189, 483–493. [Google Scholar] [CrossRef]
- Tomulescu, C.; Stoica, R.; Sevcenco, C.; Căşărică, A.; Moscovici, M.; Vamanu, A. Levan-A mini review. Sci. Bull. Series F Biotechnol. 2016, 20, 309–317. [Google Scholar]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Moussa, T.A.; Al-Qaysi, S.A.; Thabit, Z.A.; Kadhem, S.B. Microbial levan from Brachybacterium phenoliresistens: Characterization and enhancement of production. Process Biochem. 2017, 57, 9–15. [Google Scholar] [CrossRef]
- Moosavi-Nasab, M.; Layegh, B.; Aminlari, L.; Hashemi, M.B. Microbial production of levan using date syrup and investigation of its properties. World Acad. Sci. Eng. Technol. 2010, 44, 1248–1254. [Google Scholar]
- Xu, L.; Wu, D.; Xu, H.; Zhao, Z.; Chen, Q.; Li, H.; Chen, L. Characterization, production optimization, and fructanogenic traits of levan in a new Microbacterium isolate. Int. J. Biol. Macromolec. 2023, 250, 126330. [Google Scholar] [CrossRef]
- Cai, G.; Liu, Y.; Li, X.; Lu, J. New levan-type exopolysaccharide from Bacillus amyloliquefaciens as an antiadhesive agent against enterotoxigenic Escherichia coli. J. Agric. Food Chem. 2019, 67, 8029–8034. [Google Scholar] [CrossRef]
- Abou-Taleb, K.; Abdel-Monem, M.; Yassin, M.; Draz, A. Production, purification and characterization of levan polymer from Bacillus lentus V8 strain. Br. Microbiol. Res. J. 2015, 5, 22–32. [Google Scholar] [CrossRef]
- Wahyuningrum, D.; Hertadi, R. Isolation and characterization of levan from moderate halophilic bacteria Bacillus licheniformis BK AG21. Procedia Chem. 2015, 16, 292–298. [Google Scholar] [CrossRef][Green Version]
- Pei, F.; Ma, Y.; Chen, X.; Liu, H. Purification and structural characterization and antioxidant activity of levan from Bacillus megaterium PFY-147. Int. J. Biol. Macromolec. 2020, 161, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, R.; Qian, H.; Mu, W.; Miao, M.; Jiang, B. Biosynthesis of levan by levansucrase from Bacillus methylotrophicus SK 21.002. Carbohydr. Polym. 2014, 101, 975–981. [Google Scholar] [CrossRef]
- Haddar, A.; Hamed, M.; Bouallegue, A.; Bastos, R.; Coelho, E.; Coimbra, M.A. Structural elucidation and interfacial properties of a levan isolated from Bacillus mojavensis. Food Chem. 2021, 343, 128456. [Google Scholar] [CrossRef]
- Nasir, A.; Ahmad, W.; Sattar, F.; Ashfaq, I.; Lindemann, S.R.; Chen, M.H.; Van den Ende, W.; Ӧner, E.T.; Kirtel, O.; Khaliq, S.; et al. Production of a high molecular weight levan by Bacillus paralicheniformis, an industrially and agriculturally important isolate from the buffalo grass rhizosphere. Antonie Van. Leeuwenhoek 2022, 115, 1101–1112. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E.; Farrag, A.R.H.; Esawy, M.A. In vivo assessment of possible probiotic properties of Bacillus subtilis and prebiotic properties of levan. Biocatal. Agri. Biotechnol. 2018, 13, 190–197. [Google Scholar] [CrossRef]
- Hamdy, A.A.; Elattal, N.A.; Amin, M.A.; Ali, A.E.; Mansour, N.M.; Awad, G.E.; Awad, H.M.; Esawy, M.A. Possible correlation between levansucrase production and probiotic activity of Bacillus sp. isolated from honey and honey bee. World J. Microbiol. Biotechnol. 2017, 33, 69. [Google Scholar] [CrossRef] [PubMed]
- Bouallegue, A.; Chaari, F.; Casillo, A.; Corsaro, M.M.; Bachoal, R.; Ellouz-Chaabouni, M. Levan produced by Bacillus subtilis AF17: Thermal, functional and rheological properties. J. Food Meas. Charact. 2022, 16, 440–447. [Google Scholar] [CrossRef]
- Han, Y.W.; Clarke, M.A. Production and characterization of microbial levan. J. Agricult. Food Chemistry. 1990, 38, 393–396. [Google Scholar] [CrossRef]
- Mendonça, C.M.N.; Oliveira, R.C.; Freire, R.K.B.; Piazentin, A.C.M.; Pereira, W.A.; Gudiña, E.J.; Evtuguin, D.V.; Converti, A.; Santos, J.H.P.M.; Nunes, C.; et al. Characterization of levan produced by a Paenibacillus sp. isolated from Brazilian crude oil. Int. J. Biol. Macromol. 2021, 186, 788–799. [Google Scholar] [CrossRef]
- Han, J.; Feng, H.; Wang, X.; Liu, Z.; Wu, Z. Levan from Leuconostoc citreum BD1707: Production optimization and changes in molecular weight distribution during cultivation. BMC Biotechnol. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Ahmad, W.; Nasir, A.; Sattar, F.; Ashfaq, I.; Chen, M.H.; Hayat, A.; Rehman, M.U.; Zhao, S.; Khaliq, S.; Ghauri, M.A.; et al. Production of bimodal molecular weight levan by a Lactobacillus reuteri isolate from fish gut. Folia Microbiol. 2022, 67, 21–31. [Google Scholar] [CrossRef]
- Ua-Arak, T.; Jakob, F.; Vogel, R.F. Fermentation pH modulates the size distributions and functional properties of Gluconobacter albidus TMW 2.1191 levan. Front. Microbiol. 2017, 8, 807. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef]
- Hövels, M.; Kosciow, K.; Kniewel, J.; Jakob, F.; Deppenmeier, U. High yield production of levan-type fructans by Gluconobacter japonicus LMG 1417. Int. J. Biol. Macromolec. 2020, 164, 295–303. [Google Scholar] [CrossRef]
- Srikanth, R.; Siddartha, G.; Reddy, C.H.S.; Harish, B.S.; Ramaiah, M.J.; Uppuluri, K.B. Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum NCIM 2526 and its statistical optimization. Carbohydr. Polym. 2015, 123, 8–16. [Google Scholar] [CrossRef]
- Silbir, S.; Dagbagli, S.; Yegin, S.; Baysal, T.; Goksungur, Y. Levan production by Zymomonas mobilis in batch and continuous fermentation systems. Carbohydr. Polym. 2014, 99, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xu, W.; Ni, D.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Preparation of a novel water-soluble gel from Erwinia amylovora levan. Int. J. Biol. Macromolec. 2019, 122, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Al-Qaysi, S.A.; Al-Haideri, H.; Al-Shimmary, S.M.; Abdulhameed, J.M.; Alajrawy, O.I.; Al-Halbosiy, M.M.; Moussa, T.A.A.; Farahat, M.G. Bioactive levan-type exopolysaccharide produced by Pantoea agglomerans ZMR7: Characterization and optimization for enhanced production. J. Microbiol. Biotechnol. 2021, 31, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yu, S.; Zhang, T.; Jiang, B.; Mu, W. Efficient biosynthesis of levan from sucrose by a novel levansucrase from Brenneria goodwinii. Carbohydr. Polym. 2017, 157, 1732–1740. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Tabernero, A.; Marcelo, G.; Sebastián, V.; Arruebo, M.; Santamaría, J.; Martín Del Valle, E. Differences in levan nanoparticles depending on their synthesis route: Microbial vs cell-free systems. Int. J. Biol. Macromol. 2019, 137, 62–68. [Google Scholar] [CrossRef]
- Nasir, D.Q.; Wahyuningrum, D.; Hertadi, R. Screening and characterization of levan secreted by halophilic bacterium of Halomonas and Chromohalobacter genuses originated from Bledug Kuwu mud crater. Procedia Chem. 2015, 16, 272–278. [Google Scholar] [CrossRef]
- Erdal Altıntaş, Ö.; Toksoy Öner, E.; Çabuk, A.; Aytar Çelik, P. Biosynthesis of Levan by Halomonas elongata 153B: Optimization for enhanced production and potential Biological activities for Pharmaceutical Field. J. Polym. Environ. 2023, 31, 440–1455. [Google Scholar] [CrossRef]
- Tohme, S.; Hacıosmanoğlu, G.G.; Eroğlu, M.S.; Kasavi, C.; Genç, S.; Can, Z.S.; Öner, E.T. Halomonas smyrnensis as a cell factory for co-production of PHB and levan. Int. J. Biol. Macromolec. 2018, 118, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Jathore, N.R.; Bule, M.V.; Tilay, A.V.; Annapure, U.S. Microbial levan from Pseudomonas fluorescens: Characterization and medium optimization for enhanced production. Food Sci. Biotechnol. 2012, 21, 1045–1053. [Google Scholar] [CrossRef]
- Abaramak, G.; Kırtel, O.; Öner, E.T. Fructanogenic halophiles: A new perspective on extremophiles. In Physiological and Biotechnological Aspects of Extremophiles; Academic Press: Cambridge, MA, USA, 2020; pp. 123–130. [Google Scholar] [CrossRef]
- Miranda-Molina, A.; Castrejón-Carrillo, S.; Zavala-Padilla, G.T.; Antúnez-Mojica, M.; Alvarez, L.; Rodríguez-Alegría, M.E.; Munguía, A.L. Branching and molecular weight in levan: A detailed analysis of structural variability and enzymatic hydrolysis susceptibility. Carbohydr. Polym. 2025, 352, 123236. [Google Scholar] [CrossRef]
- Srikanth, R.; Reddy, C.H.S.; Siddartha, G.; Ramaiah, M.J.; Uppuluri, K.B. Review on production, characterization and applications of microbial levan. Carbohydr. Polym. 2015, 120, 102–114. [Google Scholar] [CrossRef]
- Hundschell, C.S.; Jakob, F.; Wagemans, A.M. Molecular weight dependent structure of the exopolysaccharide levan. Int. J. Biol. Macromol. 2020, 161, 398–405. [Google Scholar] [CrossRef] [PubMed]
- González-Garcinuño, Á.; Tabernero, A.; Sánchez-Álvarez, J.M.; Galán, M.A.; Martin Del Valle, E.M. Effect of bacteria type and sucrose concentration on levan yield and its molecular weight. Microb. Cell Fact. 2017, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Daguer, J.P.; Geissmann, T.; Petit-Glatron, M.F.; Chambert, R. Autogenous modulation of the Bacillus subtilis sacB–levB–yveA levansucrase operon by the levB transcript. Microbiology 2004, 150, 3669–3679. [Google Scholar] [CrossRef][Green Version]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessières, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef]
- Velázquez-Hernández, M.L.; Baizabal-Aguirre, V.M.; Bravo-Patiño, A.; Cajero-Juárez, M.; Chávez-Moctezuma, M.P.; Valdez-Alarcón, J.J. Microbial fructosyltransferases and the role of fructans. J. Appl. Microbiol. 2009, 106, 1763–1778. [Google Scholar] [CrossRef]
- Kumar, C.G.; Sripada, S.; Poornachandra, Y. Status and future prospects of fructooligosaccharides as nutraceuticals. In Role of Materials Science in Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 451–503. [Google Scholar] [CrossRef]
- Wang, S.; Wu, B.; Todhanakasem, T. Expanding the horizons of levan: From microbial biosynthesis to applications and advanced detection methods. World J. Microbiol. Biotechnol. 2024, 40, 214. [Google Scholar] [CrossRef]
- Runyon, J.R.; Nilsson, L.; Ulmius, M.; Castro, A.; Ionescu, R.; Andersson, C.; Schmidt, C. Characterizing changes in levan physicochemical properties in different pH environments using asymmetric flow field-flow fractionation. Anal. Bioanal. Chem. 2014, 406, 1597–1605. [Google Scholar] [CrossRef]
- Arvidson, S.A.; Rinehart, B.T.; Gadala-Maria, F. Concentration regimes of solutions of levan polysaccharide from Bacillus sp. Carbohydr. Polym. 2006, 65, 144–149. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Chung, B.H. Self-assembled levan nanoparticles for targeted breast cancer imaging. Chem. Commun. 2015, 51, 107–110. [Google Scholar] [CrossRef]
- Xie, C.; Liu, G.; Wang, L.; Yang, Q.; Liao, F.; Yang, X.; Xiao, B.; Duan, L. Synthesis and properties of injectable hydrogel for tissue filling. Pharmaceutics 2024, 16, 430. [Google Scholar] [CrossRef]
- Selvi, S.S.; Hasköylü, M.E.; Genç, S.; Toksoy Öner, E. Synthesis and characterization of levan hydrogels and their use for resveratrol release. J. Bioact. Compat. Polym. 2021, 36, 464–480. [Google Scholar] [CrossRef]
- Berg, A.; Oner, E.T.; Combie, J.; Schneider, B.; Ellinger, R.; Weisser, J.; Wirwa, R.; Schnabelrauch, M. Formation of new, cytocompatible hydrogels based on photochemically crosslinkable levan methacrylates. Int. J. Biol. Macromolec. 2018, 107, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.I.; Hwang, Y.; Sahu, A.; Min, K.; Sung, D.; Tae, G.; Chang, J.H. An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomat. Sci. 2018, 6, 2627–2638. [Google Scholar] [CrossRef]
- Hwang, Y.; Lee, J.S.; An, H.; Oh, H.; Sung, D.; Tae, G.; Choi, W.I. Hydroxyapatite-embedded levan composite hydrogel as an injectable dermal filler for considerable enhancement of biological efficacy. J. Ind. Eng. Chem. 2021, 104, 491–499. [Google Scholar] [CrossRef]
- Tekin, A.; Tornacı, S.; Boyacı, D.; Li, S.; Calligaris, S.; Maalej, H.; Toksoy Öner, E. Hydrogels of levan polysaccharide: A systematic review. Int. J. Biol. Macromol. 2025, 315, 144430. [Google Scholar] [CrossRef]
- Srivastava, N.; Choudhury, A.R. Recent advances in composite hydrogels prepared solely from polysaccharides. Colloids Surf. B Biointerfaces 2021, 205, 111891. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.M.; Gamal-Eldeen, A.M.; Helmy, W.A.; Esawy, M.A. Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr. Polym. 2012, 89, 314–322. [Google Scholar] [CrossRef]
- Leibovici, J.; Stark, Y. Increase in cell permeability to a cytotoxic agent by the polysaccharide levan. Cell Molec Biol. 1985, 31, 337–341. [Google Scholar]
- Taylan, O.; Yilmaz, M.T.; Dertli, E. Partial characterization of a levan type exopolysaccharide (EPS) produced by Leuconostoc mesenteroides showing immunostimulatory and antioxidant activities. Int. J. Biol. Macromol. 2019, 136, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Su, Q.; Ma, Y.; Zhao, S.; Zhang, H.; Gao, X. Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Qamar, S.A.; Riasat, A.; Jahangeer, M.; Fatima, R.; Bilal, M.; Iqbal, H.M.; Mu, B.Z. Prospects of microbial polysaccharides-based hybrid constructs for biomimicking applications. J. Basic Microbiol. 2022, 62, 1319–1336. [Google Scholar] [CrossRef]
- Veerapandian, B.; Selvaraj, T.K.R.; Shanmugam, S.R.; Sarwareddy, K.K.; Mani, K.P.; Venkatachala, P. In-vitro drug release and stability assessment of tailored levan–chitosan biocomposite hydrogel. Iran. Polym. J. 2024, 33, 11–23. [Google Scholar] [CrossRef]
- Kim, S.J.; Bae, P.K.; Choi, M.; Keem, J.O.; Chung, W.; Shin, Y.B. Fabrication and application of Levan–PVA hydrogel for effective influenza virus capture. ACS Appl. Mater. Interfaces 2020, 12, 29103–29109. [Google Scholar] [CrossRef]
- Osman, A.; Oner, E.T.; Eroglu, M.S. Novel levan and pNIPA temperature sensitive hydrogels for 5-ASA controlled release. Carbohydr. Polym. 2017, 165, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Choudhury, A.R. Synthesis and rheological characterization of a novel shear thinning levan gellan hydrogel. Int. J. Biol. Macromolec. 2020, 159, 922–930. [Google Scholar] [CrossRef]
- González-Garcinuño, Á.; Tabernero, A.; del Valle, E.M.M. Hydrogels based on levan. In Polysaccharide Hydrogels for Drug Delivery and Regenerative Medicine; Elsevier: Amsterdam, The Netherlands, 2024; pp. 175–186. [Google Scholar] [CrossRef]
- Demirci, T.; Hasköylü, M.E.; Eroğlu, M.S.; Hemberger, J.; Öner, E.T. Levan-based hydrogels for controlled release of Amphotericin B for dermal local antifungal therapy of Candidiasis. Eur. J. Pharma. Sci. 2020, 145, 105255. [Google Scholar] [CrossRef]
- Akkulah, C.Y.; Erginer, M.; Cumbul, A.; Kirtel, O.; Bayram, F.; Öner, E.T. Enhanced effects of levan hydrogels and bovine grafts on guided bone regeneration: In-vitro and in-vivo analysis. Int. J. Biol. Macromolec. 2025, 292, 139129. [Google Scholar] [CrossRef] [PubMed]
- Waidi, Y.O.; Wagh, V.S.; Mishra, S.; Jhunjhunwala, S.; Dastager, S.G.; Chatterjee, K. Vat-Based 3D-Bioprinted Scaffolds from Photocurable Bacterial Levan for Osteogenesis and Immunomodulation. Biomacromolecules 2025, 26, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Combie, J.; Öner, E.T. From healing wounds to resorbable electronics, levan can fill bioadhesive roles in scores of markets. Bioinspir. Biomim. 2018, 14, 011001. [Google Scholar] [CrossRef] [PubMed]
- Gomes, T.D.; Caridade, S.G.; Sousa, M.P.; Azevedo, S.; Kandur, M.Y.; Öner, E.T.; Alves, N.M.; Mano, J.F. Adhesive free-standing multilayer films containing sulfated levan for biomedical applications. Acta Biomater. 2018, 69, 183–195. [Google Scholar] [CrossRef]
- de Siqueira, E.C.; Rebouças, J.S.; Pinheiro, I.O.; Formiga, F.R. Levan-based nanostructured systems: An overview. Int. J. Pharm. 2020, 580, 119242. [Google Scholar] [CrossRef]
- Sezer, A.D.; Kazak Sarılmışer, H.; Rayaman, E.; Çevikbaş, A.; Öner, E.T.; Akbuğa, J. Development and characterization of vancomycin-loaded levan-based microparticular system for drug delivery. Pharm. Dev. Technol. 2015, 22, 627–634. [Google Scholar] [CrossRef]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-assembled hydrogel nanoparticles for drug delivery applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef]
- Sezer, A.D.; Kazak, H.; Öner, E.T.; Akbuğa, J. Levan-based nanocarrier system for peptide and protein drug delivery: Optimization and influence of experimental parameters on the nanoparticle characteristics. Carbohydr. Polym. 2011, 84, 358–363. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, E.; Oh, H.; Choi, W.I.; Koo, H. Levan nanoparticles with intrinsic CD44-targeting ability for tumor-targeted drug delivery. Int. J. Biol. Macromolec. 2023, 234, 123634. [Google Scholar] [CrossRef]
- Bahadori, F.; Eskandari, Z.; Ebrahimi, N.; Bostan, M.S.; Eroğlu, M.S.; Oner, E.T. Development and optimization of a novel PLGA-Levan based drug delivery system for curcumin, using a quality-by-design approach. Eur. J. Pharm. Sci. 2019, 138, 105037. [Google Scholar] [CrossRef]
- Kennedy, J.F.; Methacanon, P.; Lloyd, L.L.; Paterson, M.; Knill, C.J. Carbohydrate polymers as wound management aids. Carbohydr. Polym. 1997, 4, 422. [Google Scholar] [CrossRef]
- Hamada, M.A.; Hassan, R.A.; Abdou, A.M.; Elsaba, Y.M.; Aloufi, A.S.; Sonbol, H.; Korany, S.M. Bio_fabricated levan polymer from Bacillus subtilis MZ292983. 1 with antibacterial, antibiofilm, and burn healing properties. Appl. Sci. 2022, 12, 6413. [Google Scholar] [CrossRef]
- Upadhyay, R. Use of polysaccharide hydrogels in drug delivery and tissue engineering. Adv. Tissue Eng. Regen. Med. 2017, 2, 145–151. [Google Scholar] [CrossRef]
- Erginer, M.; Akcay, A.; Coskunkan, B.; Morova, T.; Rende, D.; Bucak, S.; Baysal, N.; Ozisik, R.; Eroglu, M.S.; Agirbasli, M.; et al. Sulfated levan from Halomonas smyrnensis as a bioactive, heparin-mimetic glycan for cardiac tissue engineering applications. Carbohydr. Polym. 2016, 149, 289–296. [Google Scholar] [CrossRef]
- Bostan, M.S.; Mutlu, E.C.; Kazak, H.; Sinan Keskin, S.; Oner, E.T.; Eroglu, M.S. Comprehensive characterization of chitosan/PEO/levan ternary blend films. Carbohydr Polym. 2014, 102, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, J.; Qi, X.; Zhong, Y.; Zuo, G.; Pan, X.; Su, T.; Zhang, J.; Dong, W. Synthesis and characterization of a multi-sensitive polysaccharide hydrogel for drug delivery. Carbohydr. Polym. 2017, 177, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Zuidema, J.M.; Pap, M.M.; Jaroch, D.B.; Morrison, F.A.; Gilbert, R.J. Fabrication and characterization of tunable polysaccharide hydrogel blends for neural repair. Acta Biomater. 2011, 7, 1634–1643. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, C.; Lai, R.C.; Su, K.; Zhang, F.; Wang, D.A. An improved injectable polysaccharide hydrogel: Modified gellan gum for long-term cartilage regeneration in vitro. J. Mater. Chem. 2009, 19, 1968–1977. [Google Scholar] [CrossRef]
- Aghajani, M.; Garshasbi, H.R.; Naghib, S.M.; Mozafari, M.R. 3D Printing of Hydrogel Polysaccharides for Biomedical Applications: A Review. Biomedicines 2025, 13, 731. [Google Scholar] [CrossRef]
- Shokrani, H.; Shokrani, A.; Saeb, M.R. Methods for biomaterials printing: A short review and perspective. Methods 2022, 206, 1–7. [Google Scholar] [CrossRef]
- Qiu, J.; Ma, S.; Qu, X. Challenges and Innovative Strategies in 3D Printing of Natural Biomolecular Hydrogels. Nano Sel. 2025, 6, e202400149. [Google Scholar] [CrossRef]
- Damiri, F.; Fatimi, A.; Liu, Y.; Musuc, A.M.; Fajardo, A.R.; Gowda, B.H.J.; Vora, L.K.; Shavandi, A.; Okoro, O.V. Recent advances in 3D bioprinted polysaccharide hydrogels for biomedical applications: A comprehensive review. Carbohydr. Polym. 2025, 348, 122845. [Google Scholar] [CrossRef]
- Lapomarda, A.; Pulidori, E.; Cerqueni, G.; Chiesa, I.; De Blasi, M.; Geven, M.A.; Montemurro, F.; Duce, C.; Mattioli-Belmonte, M.; Tiné, M.R.; et al. Pectin as rheology modifier of a gelatin-based biomaterial ink. Materials 2021, 14, 3109. [Google Scholar] [CrossRef] [PubMed]
- Congur, G.; Eksin, E.; Erdem, A. Levan modified DNA biosensor for voltammetric detection of daunorubicin-DNA interaction. Sens. Actuators B Chem. 2021, 326, 128818. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Lee, J.S.; Ko, G.J.; Sunwoo, S.H.; Lee, S.; Jo, Y.J.; Choi, C.H.; Hwang, S.W.; Kim, T.I. Biosafe, eco-friendly levan polysaccharide toward transient electronics. Small 2018, 14, 1801332. [Google Scholar] [CrossRef]
- Wani, S.; Shaikh, S.; Sayyed, R. Microbial biopolymers in biomedical field. MedCrave Online J. Cell Sci. Rep. 2016, 3, 65–67. [Google Scholar] [CrossRef][Green Version]
- De France, K.J.; Xu, F.; Hoare, T. Structured macroporous hydrogels: Progress, challenges, and opportunities. Adv. Healthcare Mat. 2018, 7, 1700927. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).