Multi-Omics Insights into the Effects of Long-Term Faba Bean Feeding on Muscle Quality and Metabolic Reprogramming in Nile Tilapia (Oreochromis niloticus)
Abstract
1. Introduction
2. Results
2.1. Phenotypic and Compositional Analyses
2.1.1. Growth, Morphometry, and Organ Indices
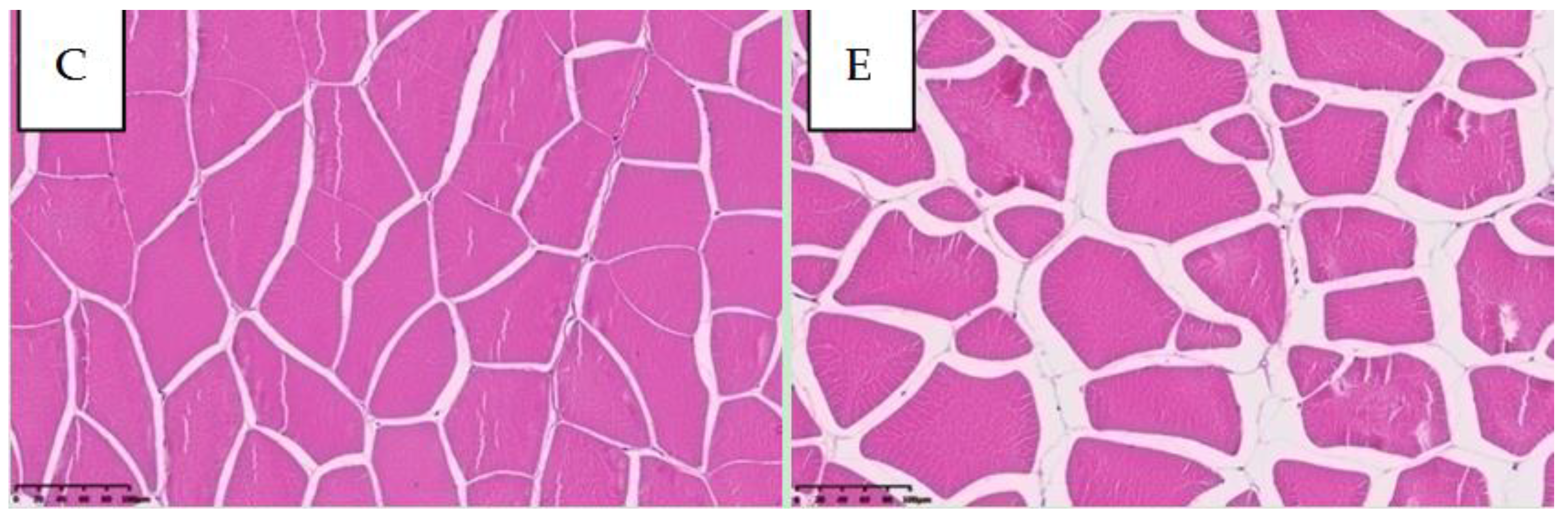
2.1.2. Muscle Histology and Physicochemical Properties
2.1.3. Muscle Textural Properties
2.1.4. Muscle Nutritional Composition
2.2. Muscle Metabolomic Profiling
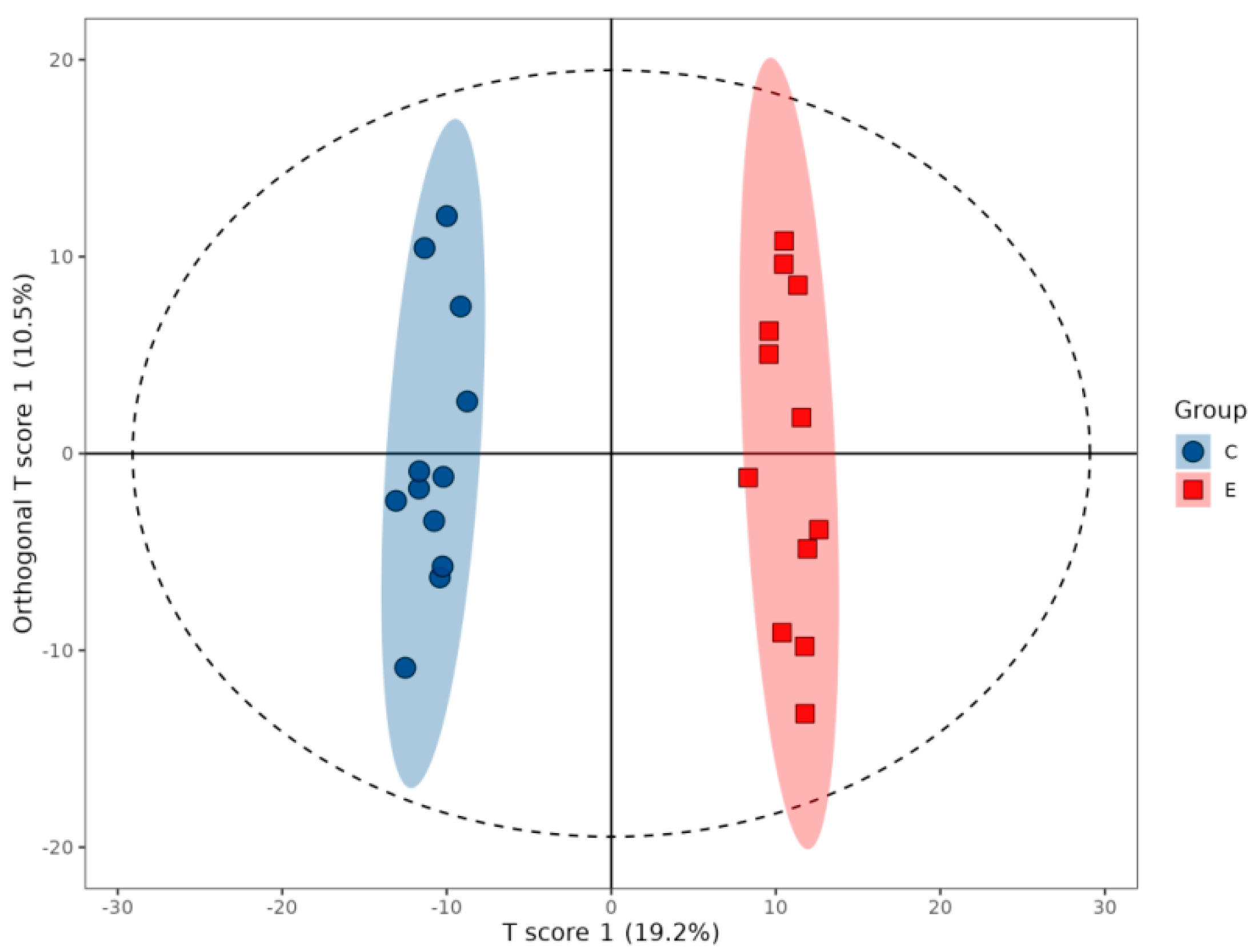
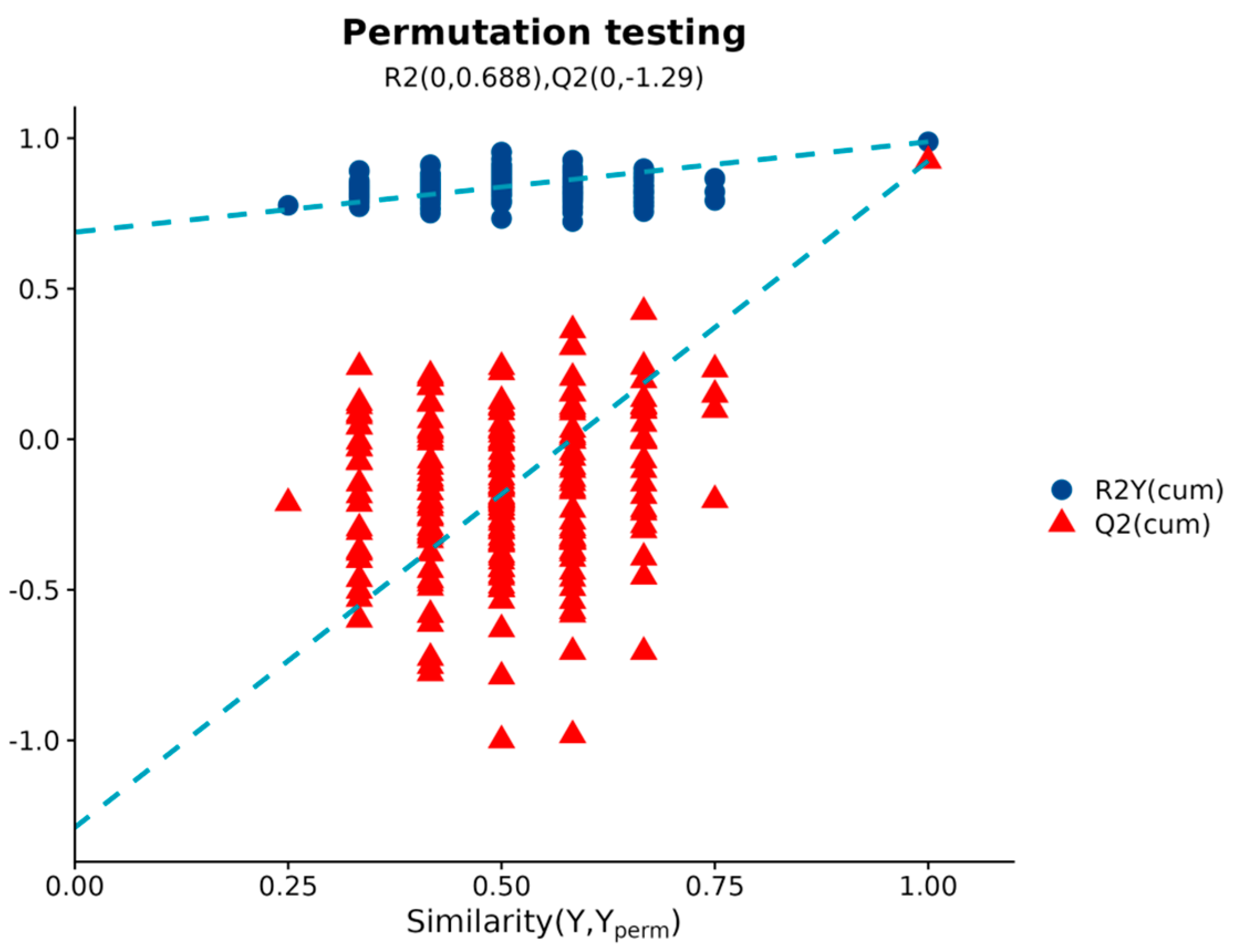
2.2.1. OPLS-DA Modeling and Validation
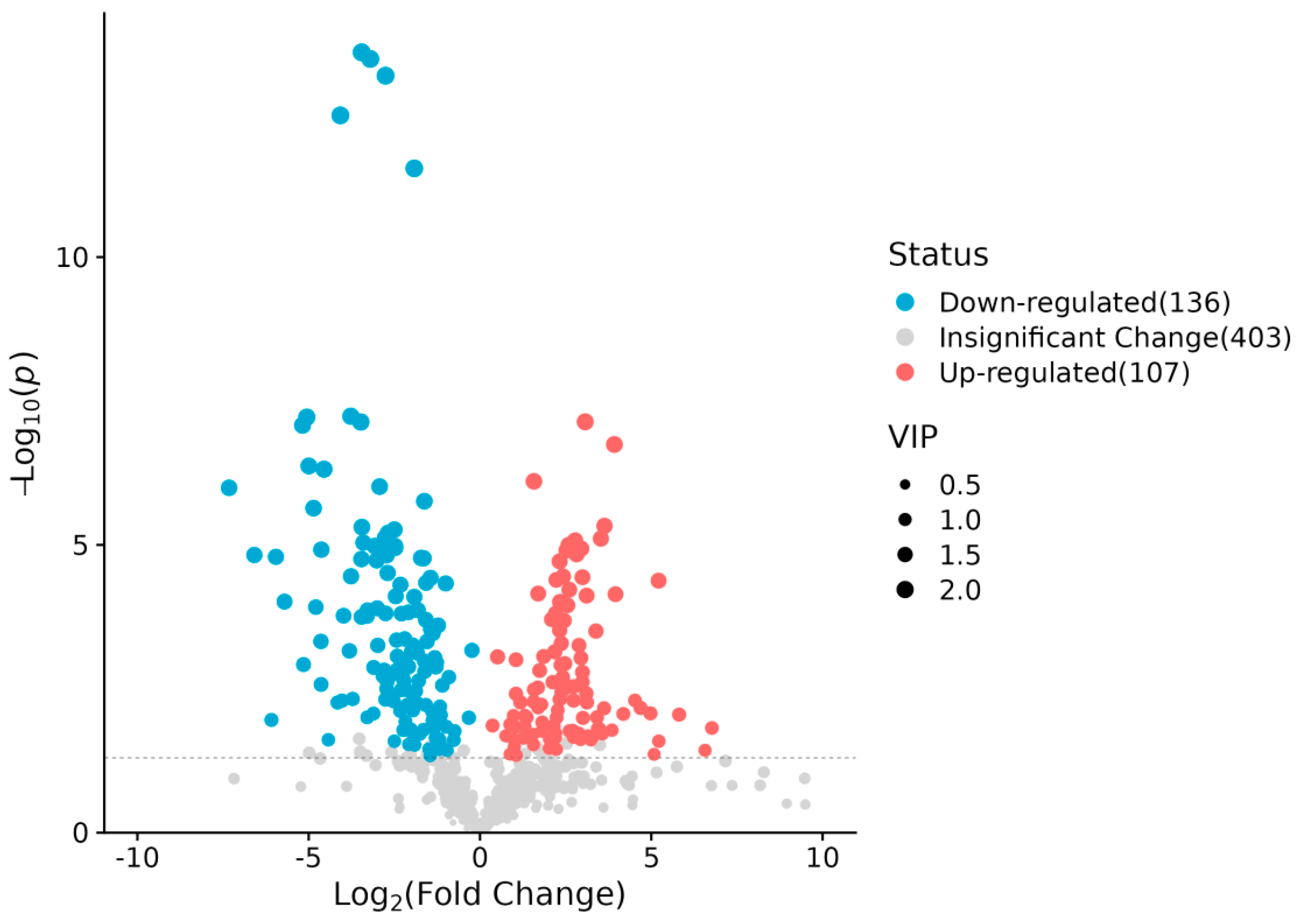
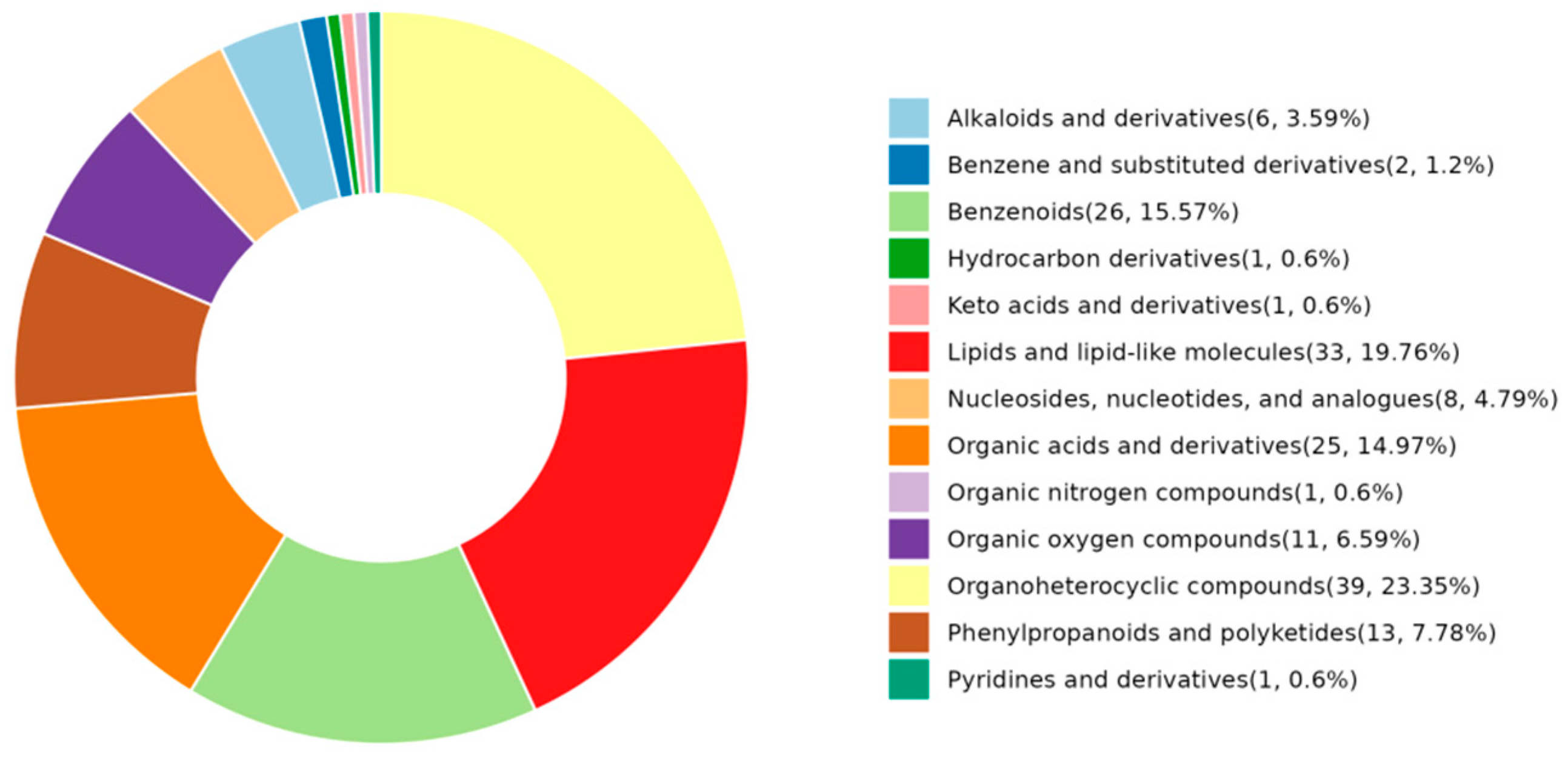
2.2.2. Identification of Differentially Abundant Metabolites
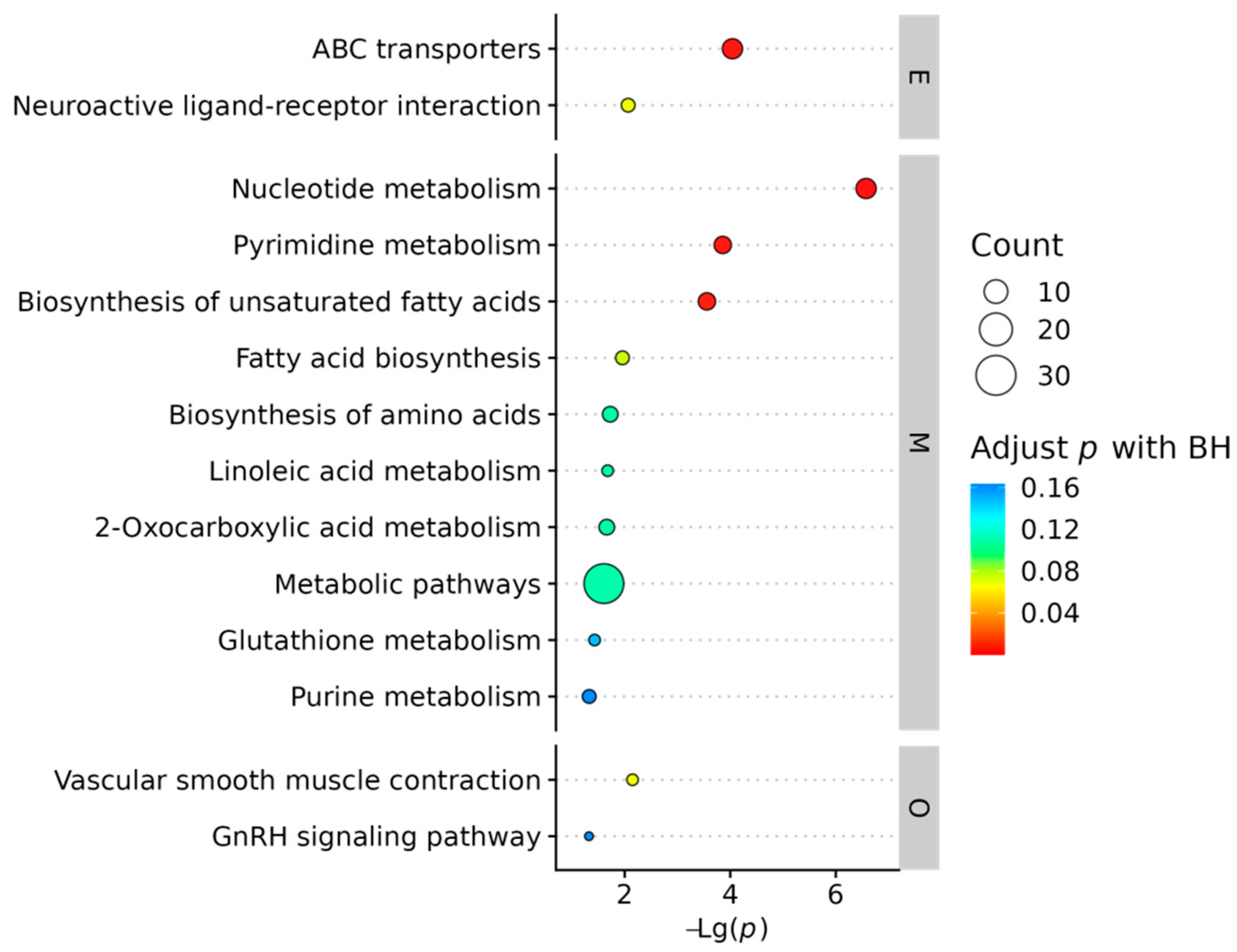
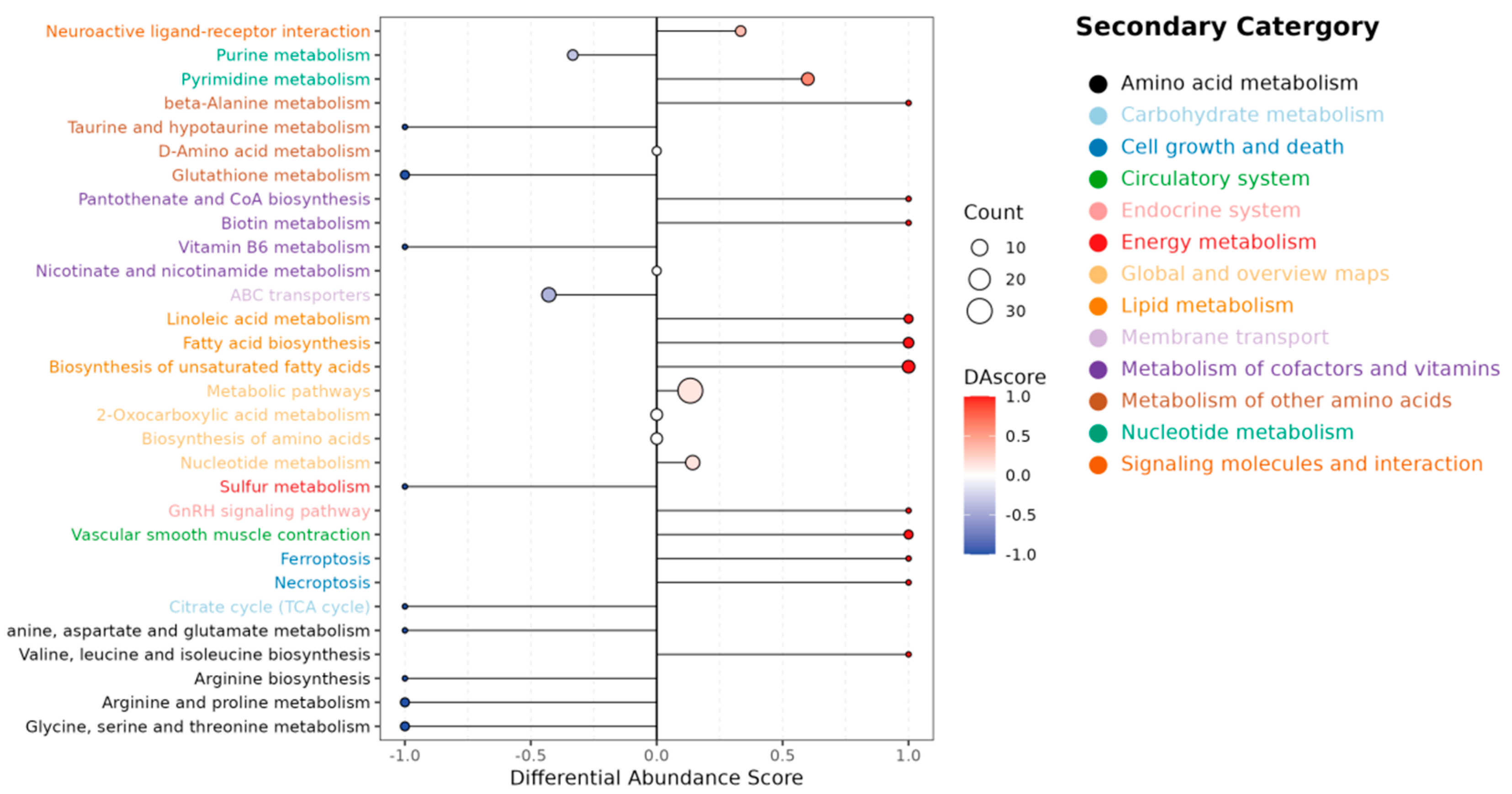
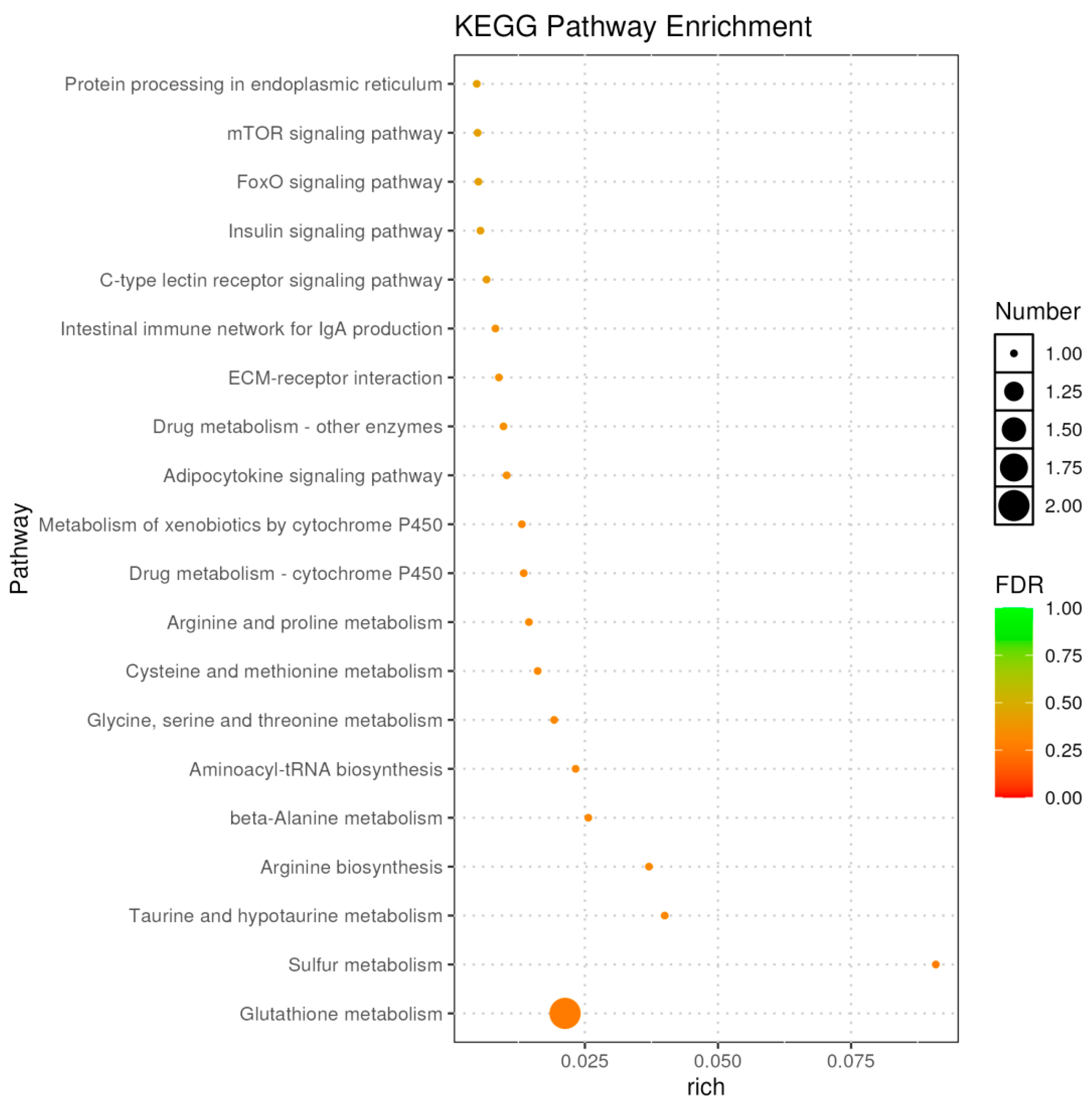
2.2.3. Integrated Metabolic Pathway Analysis
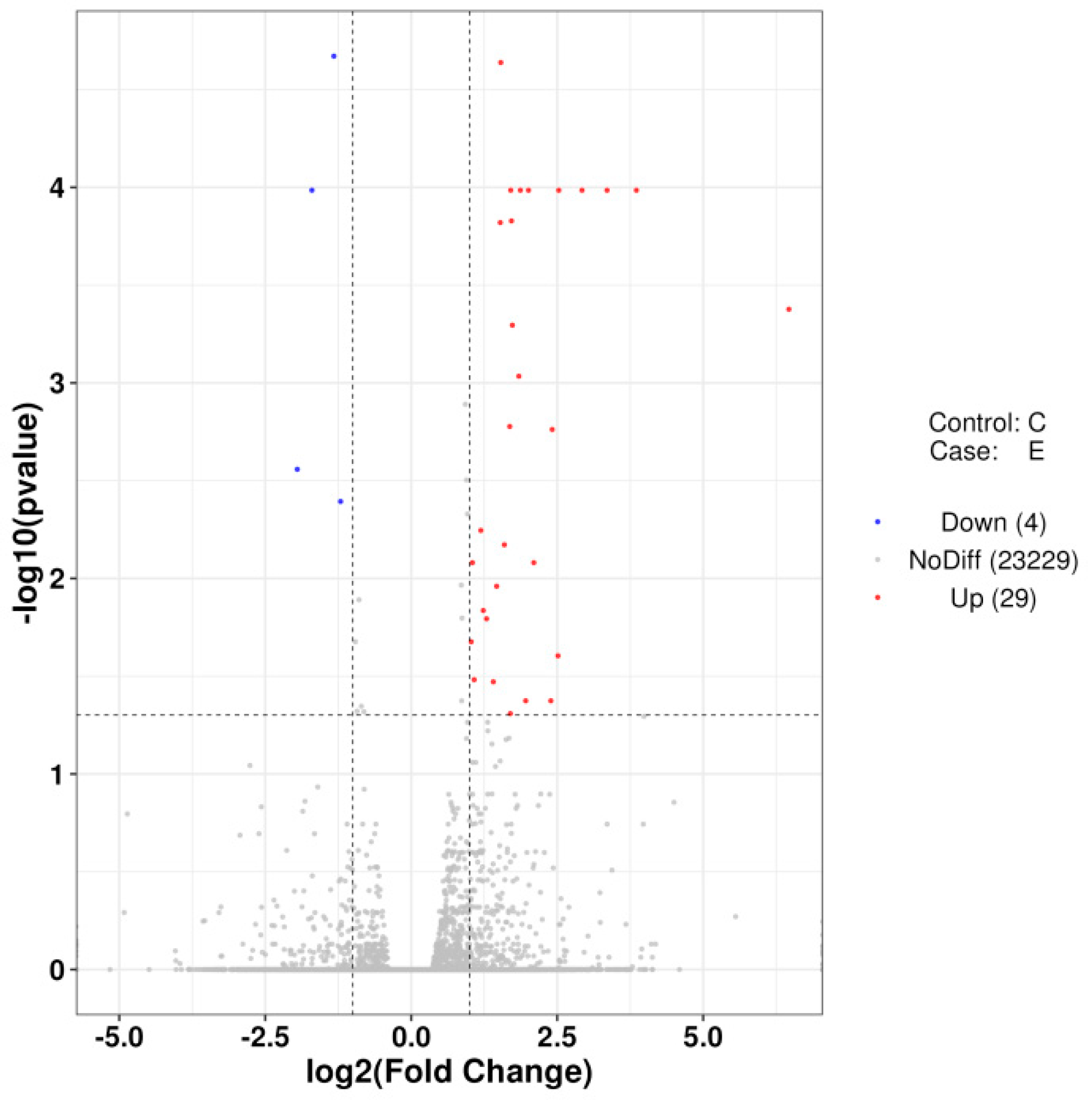
2.3. Muscle Transcriptome Analysis
2.3.1. Sequencing and Data Quality
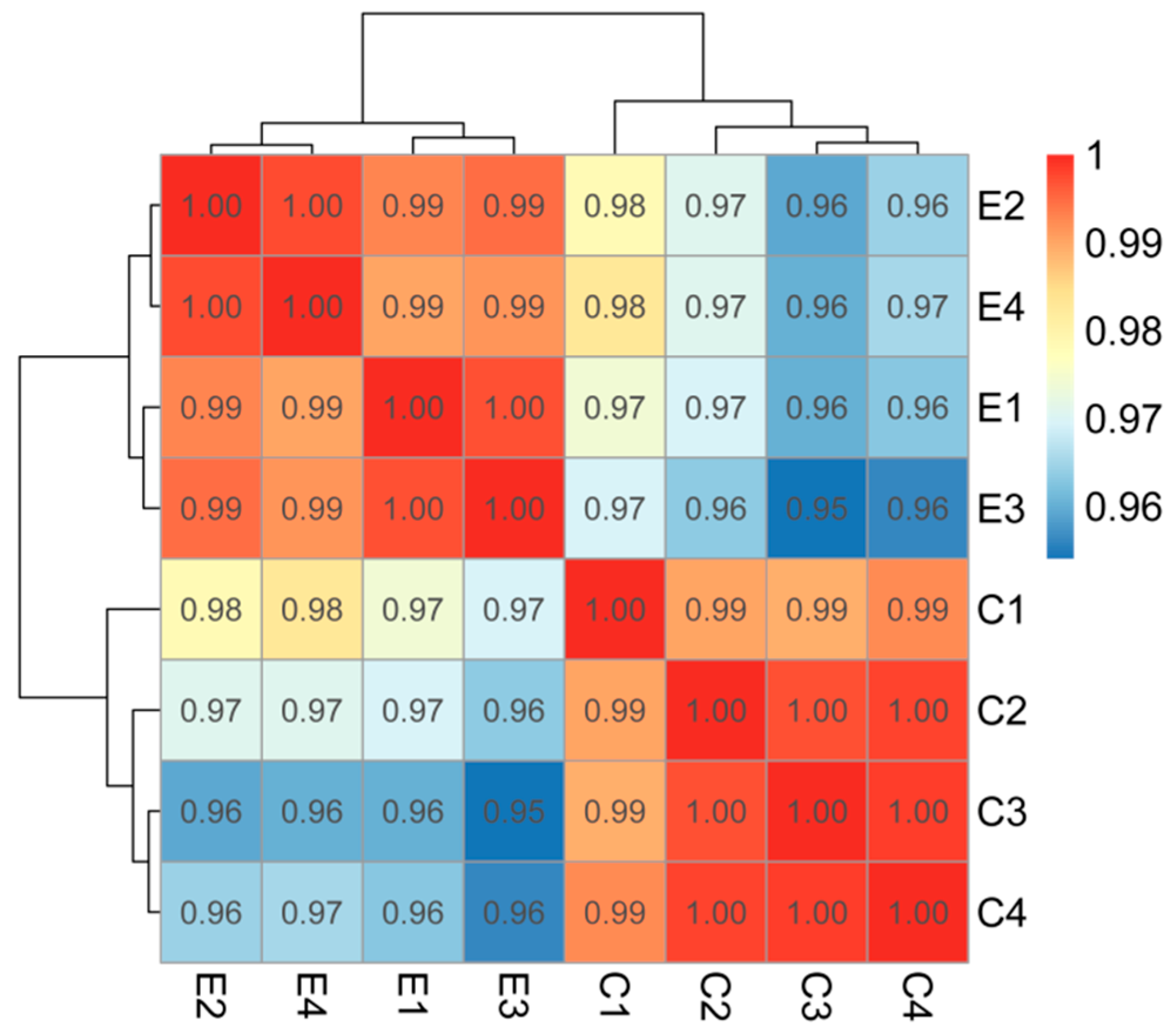
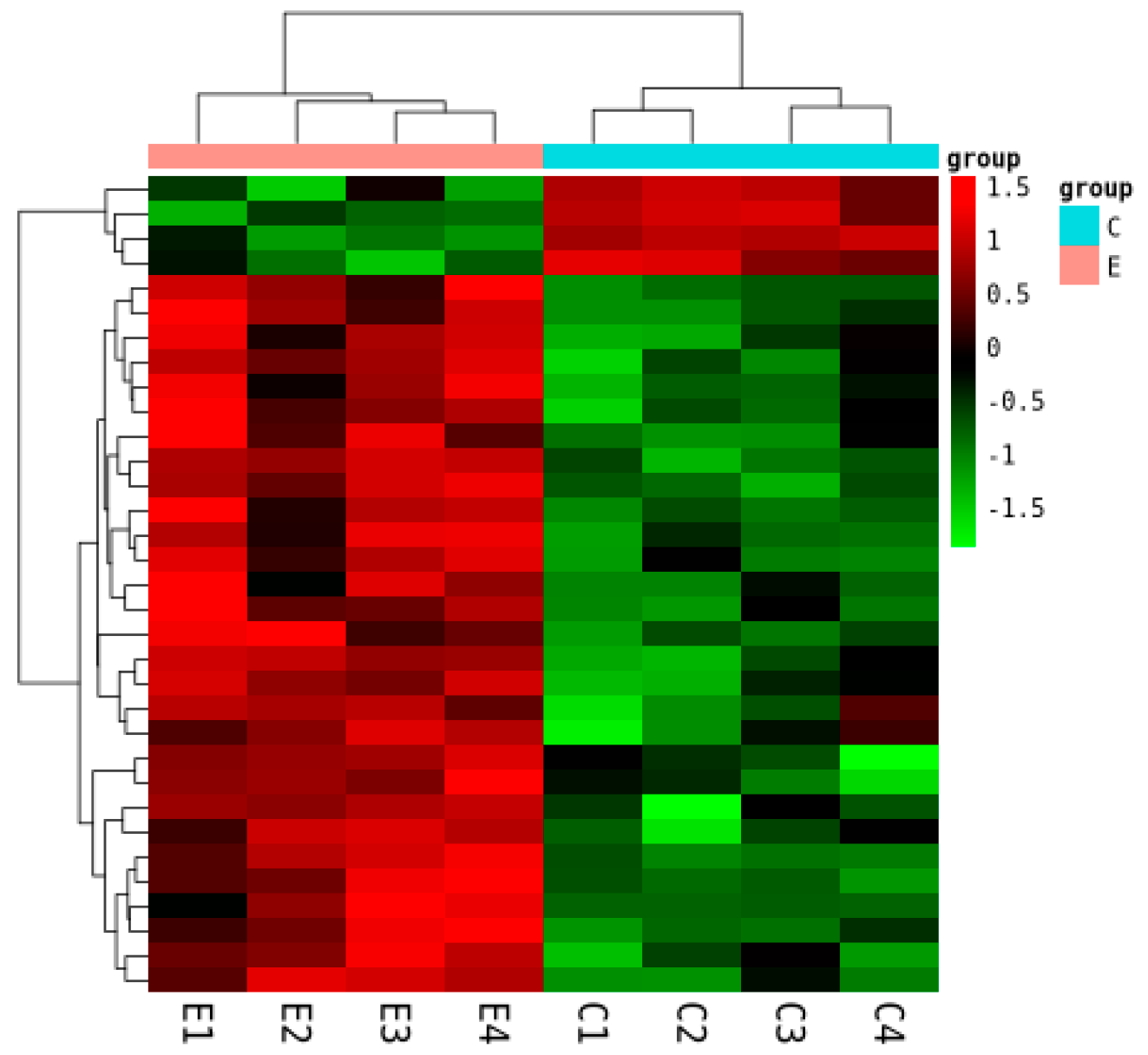
2.3.2. Sample Correlation and Differential Expression
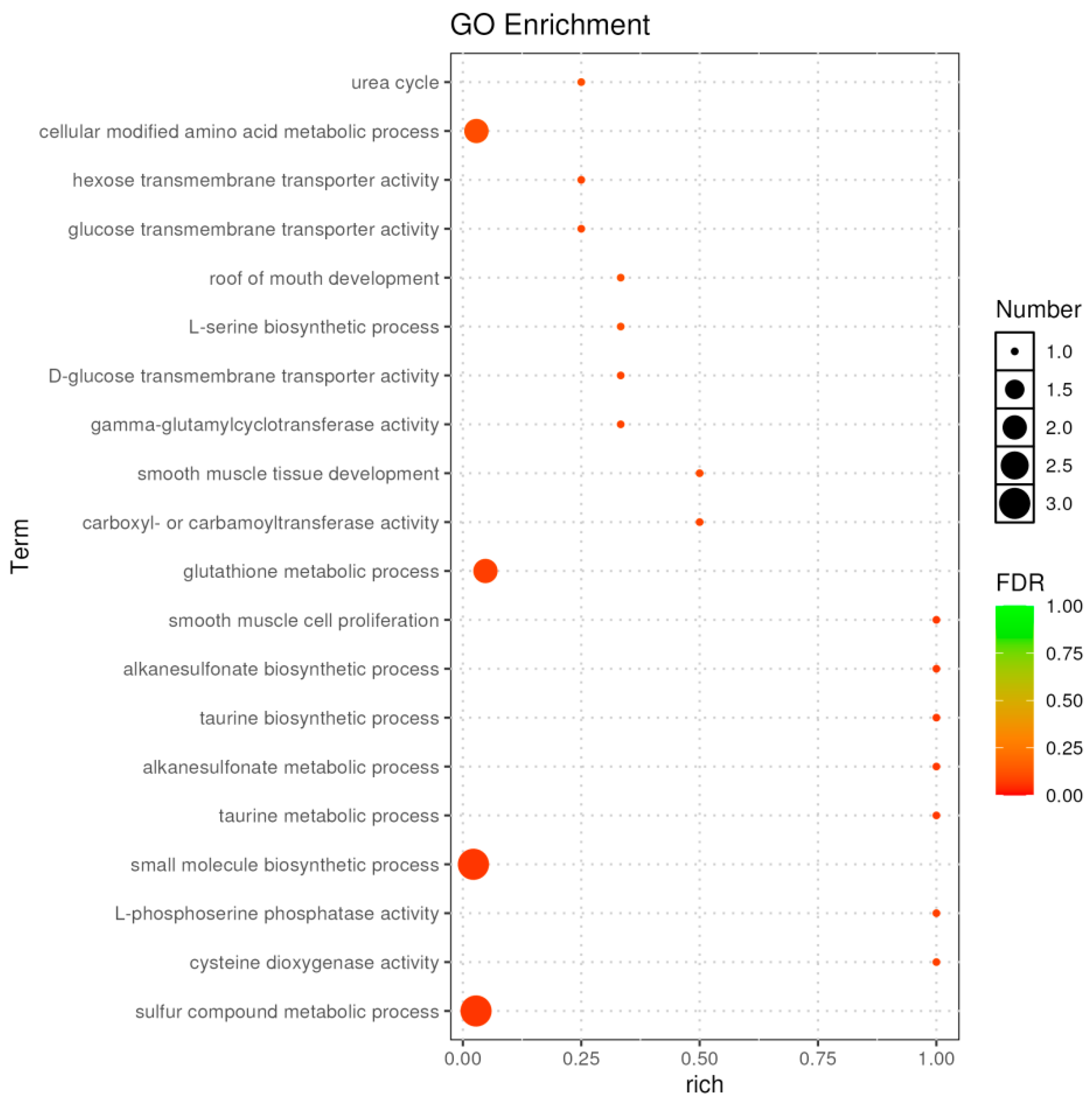
2.3.3. Functional Enrichment of DEGs
2.4. RNA-Seq Data Validation
2.5. Integrated Transcriptomic and Metabolomic Analysis
3. Discussion
3.1. Long-Term Faba Bean Feeding Improves Muscle Composition and Texture
3.2. Altered Muscle Metabolome and Potential Flavor Implications
3.3. Adverse Effects on Growth Performance and Underlying Mechanisms
3.4. Trade-Offs in Muscle Metabolism: Amino Acids, Lipids, and Energy
3.5. A Hypothesized Model for FBD-Induced Muscle Remodeling
3.6. The chac1 Gene as a Key Regulator in FBD-Induced Muscle Remodeling
4. Materials and Methods
4.1. Experimental Diets, Fish Culture, and Tissue Collection
4.1.1. Experimental Design and Fish Rearing
4.1.2. Culture System and Environmental Management
4.1.3. Feeding Regime and Duration
4.2. Assessment of Growth Performance, Nutritional Composition, and Histomorphological Parameters
4.2.1. Quantification of Morphometric Indices
4.2.2. Proximate Composition Analysis of Muscle Tissue
4.2.3. Texture Profile Analysis and Physicochemical Characterization
4.2.4. Histological Analysis and Muscle Fiber Density Determination in Tilapia Muscle Tissue
4.2.5. Statistical Analysis of Growth, Nutritional, and Histological Parameters
4.3. Non-Targeted Metabolomic Analysis
4.3.1. Samle Preparation
4.3.2. UHPLC-MS/MS Analysis
4.3.3. Data Preprocessing and Filtering
4.3.4. Multivariate Statistical Analysis
4.3.5. KEGG Enrichment Analysis
4.4. Transcriptomic Profiling and Differential Gene Expression Analysis
4.4.1. Sample Preparation and RNA Sequencing
4.4.2. Reference Genome and Bioinformatics Pipeline
4.4.3. Functional Enrichment Analysis
4.5. RT-qPCR
4.6. Combination of Transcriptomic and Metabolomic Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srichaiyo, N.; Tongsiri, S.; Hoseinifar, S.H.; Dawood, M.A.; Esteban, M.Á.; Ringø, E.; Van Doan, H. The effect of fishwort (Houttuynia cordata) on skin mucosal, serum immunities, and growth performance of Nile tilapia. Fish Shellfish Immunol. 2020, 98, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Vitule, J.R.; Li, S.; Shuai, F.; Huang, L.; Huang, X.; Fang, J.; Shi, X.; Zhu, Y.; Xu, D.; et al. Introduction of non-native fish for aquaculture in China: A systematic review. Rev. Aquac. 2023, 15, 676–703. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Zhang, X.; Qin, Z.; Zou, C.; Tan, X.; Xie, X.; Liang, S.; Lin, L. Effects of faba beans (Vicia faba L.) on growth performance, textural quality and physiological indices of tilapia (Oreochromis niloticus). Aquaculture 2023, 574, 739640. [Google Scholar] [CrossRef]
- Fu, B.; Peng, K.; Zhao, H.X.; Chen, B.; Wang, G.X.; Cao, J.M.; Huang, W. Research progress on the mechanism of fish embrittlement. Feed Res. 2023, 46, 140–143. [Google Scholar]
- He, X.; Shu, H.; Xu, T.; Yu, M.; Li, H.; Hu, Y.; Mo, J.; Ai, C. Transcriptomics and Metabolomics Explain the Crisping Mechanisms of Broad Bean-Based Crisping Diets on Nile Tilapia (Oreochromis niloticus). Metabolites 2024, 14, 616. [Google Scholar] [CrossRef]
- Tian, J.J.; Fu, B.; Yu, E.M.; Li, Y.-P.; Xia, Y.; Li, Z.-F.; Zhang, K.; Gong, W.-B.; Yu, D.-G.; Wang, G.-J.; et al. Feeding Faba Beans (Vicia faba L.) Reduces Myocyte Metabolic Activity in Grass Carp (Ctenopharyngodon idellus). Front Physiol. 2020, 11, 391. [Google Scholar] [CrossRef]
- Qian, H.C. Effect of Adding Faba Bean Aqueous Extract and Vitamins to Feed on Growth, Meat Quality, and Tissue Health of Tilapia (Oreochromis mossambicus); Shanghai Ocean University: Shanghai, China, 2024. [Google Scholar]
- Peng, K.; Fu, B.; Li, J.; Zhao, H.; Cao, J.; Huang, W.; Chen, B.; Li, X.; Peng, Z.; Wei, M. Effects of replacing soybean meal and rapeseed meal with faba bean meal on growth performance and muscle quality of tilapia (Oreochromis niloticus). Aquac. Rep. 2022, 26, 101328. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Xing, P. Effects of Maternal Nutritional Restriction on Myofiber Characteristics in Offspring Finishing Pigs; Nanjing Agricultural University: Nanjing, China, 2009. [Google Scholar]
- Qin, Z.Q.; Lin, J.B.; Zhu, Q.G.; Zhong, Q.; Liang, P.; Chen, D.; Fan, H.; Wang, M.; Li, J. Effects of feeding embrittlement feed on growth and flesh quality of Tilapia. Freshw. Fish. 2012, 42, 84–87. [Google Scholar]
- Cheng, J.H.; Sun, D.W.; Han, Z.; Zeng, X.-A. Texture and structure measurements and analyses for evaluation of fish and fillet freshness quality: A review. Compr. Rev. Food Sci. Food Saf. 2013, 13, 52–61. [Google Scholar] [CrossRef]
- Hatae, K.; Yoshimatsu, Y.; Matsumoto, J.J. Role of Muscle Fibers in Contributing Firmness of Cooked Fish. J. Food Sci. 2010, 55, 693–696. [Google Scholar] [CrossRef]
- Lin, W.; Zeng, Q.; Zhu, Z.; Song, G. Relation between protein characteristics and TPA texture characteristics of crisp grass carp (Ctenopharyngodon idellus C. et V) and grass carp (Ctenopharyngodon idellus). J. Texture Stud. 2012, 43, 1–11. [Google Scholar] [CrossRef]
- Lin, W.; Zeng, Q.; Zhu, Z. Different changes in mastication between crisp grass carp (Ctenopharyngodon idellus C.et V) and grass carp (Ctenopharyngodon idellus) after heating: The relationship between texture and ultrastructure in muscle tissue. Food Res. Int. 2009, 42, 271–278. [Google Scholar] [CrossRef]
- Lien, E.L.; Richard, C.; Hoffman, D.R. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 26–40. [Google Scholar] [CrossRef]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Bunik, V.I.; Degtyarev, D. Structure-function relationships in the 2-oxo acid dehydrogenase family: Substrate-specific signatures and functional predictions for the 2-oxoglutarate dehydrogenase-like proteins. Proteins 2008, 71, 874–890. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Geesink, G.H. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006, 74, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. Engl. 2014, 53, 7124–7143. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.M. Partial and complete replacement of fish meal by broad bean meal in feeds for Nile tilapia, Oreochromis niloticus, L., fry. Aquac. Res. 2006, 37, 986–993. [Google Scholar] [CrossRef]
- Yokoyama, M.; Takeuchi, T.; Park, G.S.; Nakazoe, J. Hepatic cysteinesulphinate decarboxylase activity in fish. Aquac. Res. 2001, 32, 216–220. [Google Scholar] [CrossRef]
- Chen, S.W.; Chen, Y.X.; Shi, J.; Lin, Y.; Xie, W.-F. The restorative effect of taurine on experimental nonalcoholic steatohepatitis. Dig. Dis. Sci. 2006, 51, 2225–2234. [Google Scholar] [CrossRef]
- Huff Lonergan, E.; Lonergan, S.M. New frontiers in understanding drip loss in pork: Recent insights on the role of postmortem muscle biochemistry. J. Anim. Breed. Genet. 2007, 124, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Li, D.Y.; Zhao, S.M.; Xiong, S.B. Effect of feeding broad bean on growth and flesh quality of channel catfish. J. Huazhong Agric. Univ. 2012, 31, 771–777. [Google Scholar]
- Liu, B.; Wang, G.; Yu, E.; Xie, J.; Yu, D.; Wang, H.; Gong, W. Comparison and evaluation of nutrition composition in muscle of grass carp Ctenopharyngodon idellus fed with broad bean and common compound feed. South China Fish. Sci. 2011, 7, 58–65. [Google Scholar]
- Ahmed, I.; Fatma, S.; Peres, H. Role of branched-chain amino acids on growth, physiology and metabolism of different fish species: A review. Aquac. Nutr. 2021, 27, 1270–1289. [Google Scholar] [CrossRef]
- Elsayed, A.F.M. Is dietary taurine supplementati beneficial for farmed fish and shrimp? A comprehensive review. Rev. Aquac. 2014, 6, 241–255. [Google Scholar] [CrossRef]
- Feng, H.M. Transcriptome and Proteomic Analysis of Primary Hepatocyte Steatosis Treated with Taurine in Grouper (Epinephelus coioides); Jimei University: Xiamen, China, 2020. [Google Scholar]
- Mommsen, T.P. Paradigms of growth in fish. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 207–219. [Google Scholar] [CrossRef]
- Endo, A. The discovery and development of HMG-CoA reductase inhibitors. J. Lipid Res. 1992, 33, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.R.; Currie, P.D. The developmental phases of zebrafish myogenesis. J. Dev. Biol. 2019, 7, 12. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef]
- Koganti, P.; Yao, J.; Cleveland, B.M. Molecular mechanisms regulating muscle plasticity in fish. Animals 2021, 11, 61. [Google Scholar] [CrossRef]
- Yu, E.M.; Zhang, H.F.; Li, Z.F.; Wang, G.-J.; Wu, H.-K.; Xie, J.; Yu, D.-G.; Xia, Y.; Zhang, K.; Gong, W.-B. Proteomic signature of muscle fibre hyperplasia in response to faba bean intake in grass carp. Sci. Rep. 2017, 7, 45950. [Google Scholar] [CrossRef]
- Johnston, I.A.; Alderson, R.; Sandham, C.; Dingwall, A.; Mitchell, D.; Selkirk, C.; Nickell, D.; Baker, R.; Robertson, B.; Whyte, D.; et al. Muscle fibre density in relation to the colour and texture of smoked Atlantic salmon (Salmo salar L.). Aquaculture 2000, 189, 335–349. [Google Scholar] [CrossRef]
- Cameron-Smith, J.F.M.D. Arachidonic acid supplementation enhances in vitro skeletal muscle cell growth via a COX-2-dependent pathway. Am. J. Physiol. 2013, 304, 765–772. [Google Scholar]
- Ricard-Blum, S.; Baffet, G.; Théret, N. Molecular and tissue alterations of collagens in fibrosis. Matrix Biol. 2018, 68, 122–149. [Google Scholar] [CrossRef]
- Chen, B.; Cai, H.; Niu, Y.; Zhang, Y.; Wang, Y.; Liu, Y.; Han, R.; Liu, X.; Kang, X.; Li, Z. Whole transcriptome profiling reveals a lncMDP1 that regulates myogenesis by adsorbing miR-301a-5p targeting CHAC1. Commun. Biol. 2024, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Bower, N.I.; Johnston, I.A. Discovery and characterization of nutritionally regulated genes associated with muscle growth in Atlantic salmon. Physiol. Genom. 2010, 42a, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Balogh, L.M.; Roberts, A.G.; Sbireman, L.M.; Greene, R.J.; Atkins, W.M. The stereochemical course of 4-hydroxy-2-nonenal metabolism by glutathione S-transferases. J. Biol. Chem. 2008, 283, 16702–16710. [Google Scholar] [CrossRef]
- Sahebekhtiari, N.; Fernandez-Guerra, P.; Nochi, Z.; Carlsen, J.; Bross, P.; Palmfeldt, J. Deficiency of the mitochondrial sulfide regulator ETHE1 disturbs cell growth, glutathione level and causes proteome alterations outside mitochondria. Biochim. Et Biophys. acta Mol. Basis Dis. BBA 2019, 1865, 126–135. [Google Scholar] [CrossRef]
- Bachhawat, A.K.; Yadav, S. The glutathione cycle: Glutathione metabolism beyond the γ-glutamyl cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.3-2016; National Food Safety Standard—Determination of Moisture in Foods. China Standards Press: Beijing, China, 2016.
- GB 5009.4-2016; National Food Safety Standard—Determination of Ash in Foods. China Standards Press: Beijing, China, 2016.
- GB 5009.5-2016; National Food Safety Standard—Determination of Protein in Foods. China Standards Press: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard—Determination of Fat in Foods. China Standards Press: Beijing, China, 2016.
- GB/Z 21922-2008; Fundermental terminology and definition of nutritional component in foods. China Standards Press: Beijing, China, 2008.
- GB 5009.268-2016; National Food Safety Standard—Determination of Multiple Elements in Foods. China Standards Press: Beijing, China, 2016.
- GB 5009.124-2016; National Food Safety Standard—Determination of Amino Acids in Foods. China Standards Press: Beijing, China, 2016.
- GB/T 15400-2018; Determination of tryptophan in feeds. China Standards Press: Beijing, China, 2016.
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass spectrometry-based metabolomics: A guide for annotation, quantification and best reporting practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef] [PubMed]

| Parameter (Unit) | Group C | Group E |
|---|---|---|
| Body weight (g) | 1564.6 ± 28.2 ** | 1339.9 ± 49.6 |
| Standard length (cm) | 33.63 ± 0.28 | 32.91 ± 0.33 |
| Total length (cm) | 40.10 ± 0.30 * | 39.03 ± 0.38 |
| Condition factor (%) | 4.17 ± 0.12 ** | 3.75 ± 0.08 |
| Filet yield (%) | 24.28 ± 0.78 | 25.33 ± 0.96 |
| Visceral somatic index (%) | 7.23 ± 0.22 | 8.23 ± 0.40 |
| Hepatic somatic index (%) | 1.60 ± 0.07 * | 1.17 ± 0.13 |
| Intestinal somatic index (%) | 2.35 ± 0.16 | 2.60 ± 0.26 |
| Intestinal fat rate (%) | 1.99 ± 0.21 | 3.09 ± 0.27 * |
| Parameter (Unit) | Group C | Group E |
|---|---|---|
| Muscle fiber density (n/mm2) | 180.89 ± 8.62 | 231.79 ± 11.21 ** |
| Steaming loss (%) | 19.91 ± 0.95 | 40.12 ± 1.84 ** |
| pH | 6.55 ± 0.03 ** | 6.38 ± 0.00 |
| Parameter (Unit) | Group C | Group E |
|---|---|---|
| Hardness (N) | 18.89 ± 0.79 | 42.59 ± 3.88 ** |
| Adhesiveness (N·mm) | 0.44 ± 0.03 ** | 0.28 ± 0.03 |
| Springiness (mm) | 2.67 ± 0.09 ** | 2.33 ± 0.18 |
| Gumminess (N) | 5.29 ± 0.16 | 11.38 ± 1.02 ** |
| Chewiness (mJ) | 14.10 ± 0.59 | 26.48 ± 2.33 ** |
| Parameter (Unit) | Group C | Group E |
|---|---|---|
| Moisture (g/100 g) | 76.88 ± 0.50 | 77.10 ± 0.48 |
| Ash (g/100 g) | 1.17 ± 0.02 ** | 1.02 ± 0.02 |
| Protein (g/100 g) | 20.03 ± 0.43 | 20.05 ± 0.38 |
| Fat (g/100 g) | 1.30 ± 0.15 | 2.02 ± 0.32 * |
| Energy (kJ/100 g) | 408.67 ± 12.62 | 416.33 ± 16.04 |
| Ca (mg/kg) | 98.03 ± 1.56 | 91.33 ± 3.50 |
| P (mg/kg) | 2.01 ± 0.02 * | 1.79 ± 0.06 |
| Collagen (μg/mg) | 0.66 ± 0.07 | 0.98 ± 0.09 * |
| Amino Acid (g/100 g) | Group C | Group E |
|---|---|---|
| Aspartic acid | 1.88 ± 0.04 ** | 1.70 ± 0.03 |
| Threonine | 0.84 ± 0.02 | 0.78 ± 0.01 |
| Serine | 0.75 ± 0.03 | 0.69 ± 0.02 |
| Glutamic acid | 2.98 ± 0.08 * | 2.70 ± 0.06 |
| Proline | 0.66 ± 0.01 | 0.61 ± 0.02 |
| Glycine | 0.95 ± 0.01 | 0.84 ± 0.03 |
| Alanine | 1.15 ± 0.02 | 1.04 ± 0.03 |
| Cystine | 0.18 ± 0.01 | 0.20 ± 0.00 |
| Valine | 0.91 ± 0.03 | 0.84 ± 0.02 |
| Methionine | 0.64 ± 0.02 ** | 0.55 ± 0.02 |
| Isoleucine | 0.85 ± 0.02 * | 0.77 ± 0.02 |
| Leucine | 1.48 ± 0.04 * | 1.34 ± 0.03 |
| Tyrosine | 0.60 ± 0.02 * | 0.54 ± 0.01 |
| Phenylalanine | 0.76 ± 0.02 * | 0.68 ± 0.01 |
| Lysine | 1.76 ± 0.04 * | 1.60 ± 0.03 |
| Histidine | 0.46 ± 0.01 * | 0.42 ± 0.01 |
| Tryptophan | 0.15 ± 0.01 | 0.16 ± 0.01 |
| Arginine | 1.23 ± 0.02 * | 1.12 ± 0.03 |
| Total amino acids (%) | 18.33 ± 0.39 * | 16.59 ± 0.40 |
| Sample | Raw Reads | Clean Reads | Q30 (%) | Total Mapped | Mapped to Exon |
|---|---|---|---|---|---|
| C1 | 41,571,478 | 38,380,328 | 94.8 | 94.45% | 98.11% |
| C2 | 42,124,554 | 38,892,918 | 94.52 | 93.77% | 98.05% |
| C3 | 43,108,144 | 39,972,674 | 94.27 | 93.46% | 98.12% |
| C4 | 42,147,254 | 38,805,278 | 94.71 | 93.74% | 98.18% |
| E1 | 40,683,622 | 37,604,882 | 94.97 | 93.52% | 97.96% |
| E2 | 42,944,138 | 39,714,128 | 94.8 | 94.21% | 98.16% |
| E3 | 41,975,002 | 38,884,452 | 94.68 | 94.05% | 97.96% |
| E4 | 42,402,932 | 39,219,948 | 94.71 | 94.53% | 97.97% |
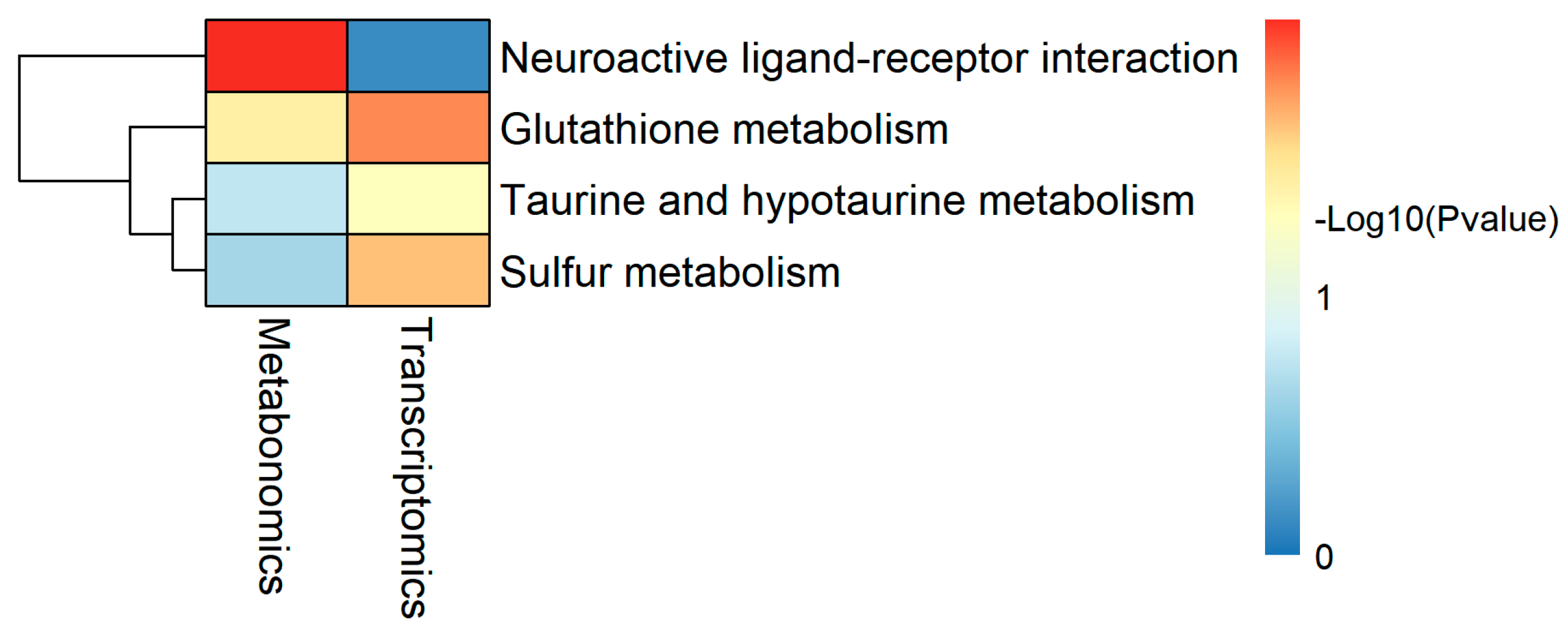
| Shared Pathway | T p-Value | T DEGs | M p-Value | DAMs |
|---|---|---|---|---|
| Glutathione metabolism | 0.015 | chac1↑, LOC100695721↓ | 0.037 | ARA↓, Ornithine↓ |
| Sulfur metabolism | 0.022 | LOC100698874↑ | 0.236 | Taurine↓ |
| Taurine and hypotaurine metabolism | 0.049 | cdo1↑ | 0.178 | Taurine↓ |
| Nutrient Component | C Group (%) | E Group (%) |
|---|---|---|
| Crude Protein | 32.10 | 30.10 |
| Crude Fat | 7.90 | 7.34 |
| Crude Fiber | 4.20 | 9.80 |
| Crude Ash | 9.20 | 7.31 |
| Calcium | 1.58 | 0.51 |
| Available Phosphorus | 0.96 | 0.59 |
| Lysine | 1.90 | 1.47 |
| Methionine | 0.59 | 0.45 |
| Threonine | 1.20 | 1.06 |
| Arginine | 1.92 | 1.54 |
| Tryptophan | 0.28 | 0.39 |
| Primer | Sequence (5′-3′) |
|---|---|
| gapdh-F | TGATGAGCACAGTTCACGCC |
| gapdh-R | GGGATGACTTTGCCGACAGC |
| smox-F | AGGTGGAAAGTTGCGAAAGC |
| smox-R | ATGCTGGGCTGAGTAGTTCC |
| otc-F | CCTTACAGGAGCATTACGGA |
| otc-R | CTTTGAGCCTCTTTTTCTTC |
| psph-F | ATAACAGACCATCCACCTCA |
| psph-R | CTCTCATCAAAACCAGCGTA |
| ass1-F | TCCCTGTGCCTGTGACCCCT |
| ass1-R | ACTCCGTGTTTTCCCCCGAT |
| cmpk2-F | TATCACCCATCTACTCTCAA |
| cmpk2-R | GTAGTCTTACCTGTGGCATC |
| agtr1-F | TCTACACCAGCATCTTCTTC |
| agtr1-R | CTTTCAGCCACATTTCATTT |
| adcy8-F | AGGTCACGGACGAAACACGA |
| adcy8-R | GCGGCGAAGGAAGTCATTGC |
| LOC100534578-F | TCTTTGTTTAGCAGGTGTCC |
| LOC100534578-R | TTCCTCATCTTCATTTTCGT |
| LOC100702411-F | TTTCCGTGAGCCAATCCTTT |
| LOC100702411-R | TTCCCATCCCACAACCTCCT |
| cad-F | F:CACCAGTCAGAATCACGGCT |
| cad-R | R:TTGGGGAACCAGTGAAAGTG |
| egr1-F | AGGTTCTCTCACTCCCCCAT |
| egr1-R | TCTGCTCCACCACTGGCTTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, S.; Sun, Y.; Zhang, X. Multi-Omics Insights into the Effects of Long-Term Faba Bean Feeding on Muscle Quality and Metabolic Reprogramming in Nile Tilapia (Oreochromis niloticus). Int. J. Mol. Sci. 2025, 26, 10819. https://doi.org/10.3390/ijms262210819
Li R, Wang S, Sun Y, Zhang X. Multi-Omics Insights into the Effects of Long-Term Faba Bean Feeding on Muscle Quality and Metabolic Reprogramming in Nile Tilapia (Oreochromis niloticus). International Journal of Molecular Sciences. 2025; 26(22):10819. https://doi.org/10.3390/ijms262210819
Chicago/Turabian StyleLi, Rongni, Saisai Wang, Yansheng Sun, and Xin Zhang. 2025. "Multi-Omics Insights into the Effects of Long-Term Faba Bean Feeding on Muscle Quality and Metabolic Reprogramming in Nile Tilapia (Oreochromis niloticus)" International Journal of Molecular Sciences 26, no. 22: 10819. https://doi.org/10.3390/ijms262210819
APA StyleLi, R., Wang, S., Sun, Y., & Zhang, X. (2025). Multi-Omics Insights into the Effects of Long-Term Faba Bean Feeding on Muscle Quality and Metabolic Reprogramming in Nile Tilapia (Oreochromis niloticus). International Journal of Molecular Sciences, 26(22), 10819. https://doi.org/10.3390/ijms262210819






