Molecular and Clinical Insights into TP53-Mutated MDS and AML
Abstract
1. Introduction
2. Molecular Events and Related Changes in TP53-Mutated MDS/AML
2.1. P53 as “The Guardian of the Genome”
2.2. Alternations of TP53 Gene Locus and p53 Function in Myeloid Neoplasms
2.3. Concurrent Mutations and Chromosomal Aberrations
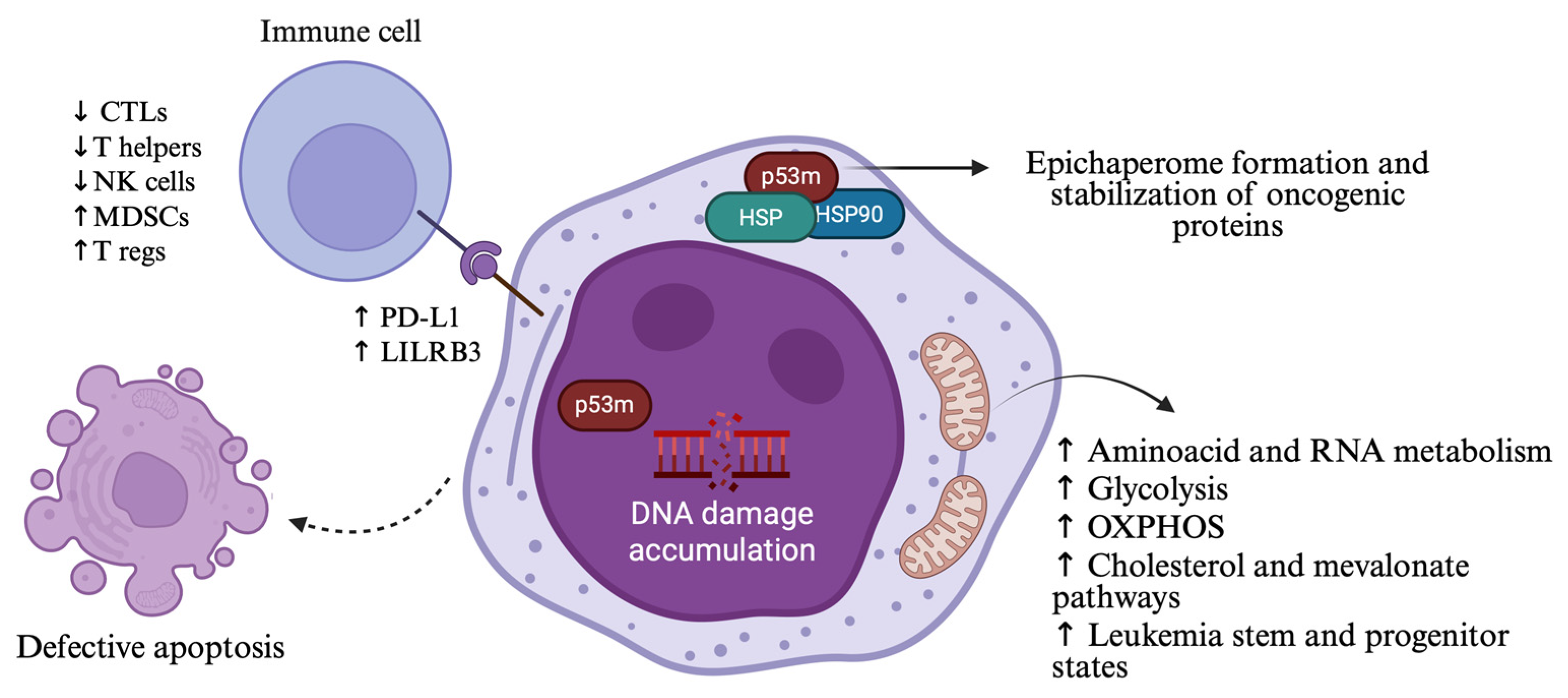
2.4. TP53 Mutations and Changes in Cellular Functions
2.5. TP53-Mutated Clones and the Driving Force of Cancer Treatments
2.6. The Effect of TP53 Mutations in Immune Microenvironment
3. From Classification to Clinical Outcomes: Understanding TP53-Mutated MDS/AML
3.1. Classification Systems
3.2. Role of Allelic Status and Variant Allele Frequency of TP53 Mutations in Patient Outcomes
4. Treatment Armamentarium and Novel Strategies for TP53-Mutated MDS/AML
4.1. Hypomethylating Agents (HMAs) and Their Combination with Venetoclax Take Precedence over Intensive Chemotherapy
4.2. Allogeneic Stem Cell Transplantation (Allo-BMT)
4.3. The Role of Immunotherapy in TP53-Mutated MDS and AML
4.3.1. Immune Checkpoint Inhibitors (ICI)
4.3.2. Anti-CD47 Targeting Strategies
4.3.3. Sabatolimab—A TIM-3 Inhibitor
4.3.4. CD123 × CD3 Bi-Specific Antibodies
4.3.5. Chimeric Antigen Receptor (CAR)-T Cell Therapies
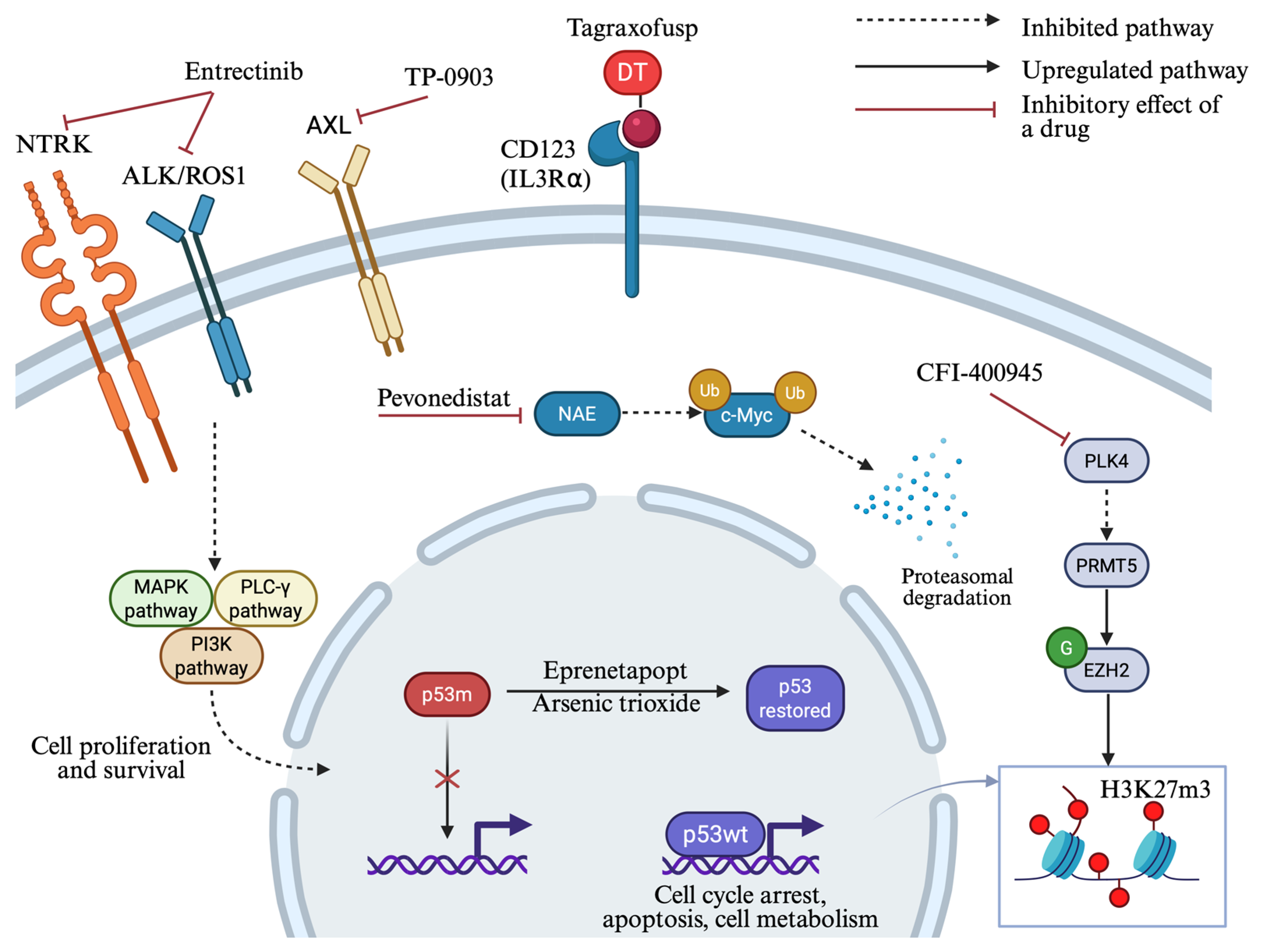
4.4. p53 Targeting Strategies
4.5. Ongoing Studies of Novel Molecular Targets
4.5.1. Tropomyosin Receptor Kinase (TRK) Inhibition
4.5.2. AXL Inhibition
4.5.3. Pevonedistat—An NEDD8-Activating Enzyme Inhibitor
4.5.4. PLK4 Inhibition
4.5.5. STING Agonists
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, D.P. P53, Guardian of the Genome. Nature 1992, 358, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Wattel, E.; Preudhomme, C.; Hecquet, B.; Vanrumbeke, M.; Quesnel, B.; Dervite, I.; Morel, P.; Fenaux, P. P53 Mutations Are Associated with Resistance to Chemotherapy and Short Survival in Hematologic Malignancies. Blood 1994, 84, 3148–3157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zawacka, J.E. P53 Biology and Reactivation for Improved Therapy in MDS and AML. Biomark. Res. 2024, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating Morphologic, Clinical, and Genomic Data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Li, W. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. In Leukemia; Li, W., Ed.; Exon Publications: Brisbane, Australia, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK586208 (accessed on 30 October 2025).
- Grob, T.; Al Hinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L.; Biemond, B.J.; Breems, D.A.; Maertens, J.; van Marwijk Kooy, M.; et al. Molecular Characterization of Mutant TP53 Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Abel, H.J.; Oetjen, K.A.; Miller, C.A.; Ramakrishnan, S.M.; Day, R.B.; Helton, N.M.; Fronick, C.C.; Fulton, R.S.; Heath, S.E.; Tarnawsky, S.P.; et al. Genomic Landscape of TP53-Mutated Myeloid Malignancies. Blood Adv. 2023, 7, 4586–4598. [Google Scholar] [CrossRef]
- Shah, M.V.; Arber, D.A.; Hiwase, D.K. TP53-Mutated Myeloid Neoplasms: 2024 Update on Diagnosis, Risk-Stratification, and Management. Am. J. Hematol. 2025, 100 (Suppl. 4), 88–115. [Google Scholar] [CrossRef]
- Singhal, D.; Kutyna, M.M.; Hahn, C.N.; Shah, M.V.; Hiwase, D.K. Therapy-Related Myeloid Neoplasms: Complex Interactions among Cytotoxic Therapies, Genetic Factors, and Aberrant Microenvironment. Blood Cancer Discov. 2024, 5, 400–416. [Google Scholar] [CrossRef]
- Sallman, D.A.; McLemore, A.F.; Aldrich, A.L.; Komrokji, R.S.; McGraw, K.L.; Dhawan, A.; Geyer, S.; Hou, H.-A.; Eksioglu, E.A.; Sullivan, A.; et al. TP53 Mutations in Myelodysplastic Syndromes and Secondary AML Confer an Immunosuppressive Phenotype. Blood 2020, 136, 2812–2823. [Google Scholar] [CrossRef]
- Badar, T.; Knutson, K.L.; Foran, J.; Gangat, N.; Pavelko, K.D.; Kaufmann, S.H.; Litzow, M.R.; Murthy, H.; Cogen, D.; Ushman, M.; et al. T-Cell Immune Cluster Analysis Using CyTOF Identifies Unique Subgroups of Patients with Acute Myeloid Leukemia. Blood Adv. 2025, 9, 239–243. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Bewersdorf, J.P.; Hasle, V.; Shallis, R.M.; Thompson, E.; de Menezes, D.L.; Rose, S.; Boss, I.; Halene, S.; Haferlach, T.; et al. Integrated Genetic, Epigenetic, and Immune Landscape of TP53 Mutant AML and Higher Risk MDS Treated with Azacitidine. Ther. Adv. Hematol. 2024, 15, 20406207241257904. [Google Scholar] [CrossRef]
- Toledo, F.; Wahl, G.M. Regulating the P53 Pathway: In Vitro Hypotheses, in Vivo Veritas. Nat. Rev. Cancer 2006, 6, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why Are There Hotspot Mutations in the TP53 Gene in Human Cancers? Cell Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bahaj, W.; Kewan, T.; Gurnari, C.; Durmaz, A.; Ponvilawan, B.; Pandit, I.; Kubota, Y.; Ogbue, O.D.; Zawit, M.; Madanat, Y.; et al. Novel Scheme for Defining the Clinical Implications of TP53 Mutations in Myeloid Neoplasia. J. Hematol. Oncol.J Hematol Oncol 2023, 16, 91. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, S.; Miller, P.G.; Sharma, R.; McConkey, M.; Leventhal, M.; Krivtsov, A.V.; Giacomelli, A.O.; Wong, W.; Kim, J.; Chao, S.; et al. A Dominant-Negative Effect Drives Selection of TP53 Missense Mutations in Myeloid Malignancies. Science 2019, 365, 599–604. [Google Scholar] [CrossRef]
- Stengel, A.; Meggendorfer, M.; Walter, W.; Baer, C.; Nadarajah, N.; Hutter, S.; Kern, W.; Haferlach, T.; Haferlach, C. Interplay of TP53 Allelic State, Blast Count, and Complex Karyotype on Survival of Patients with AML and MDS. Blood Adv. 2023, 7, 5540–5548. [Google Scholar] [CrossRef]
- Bernard, E.; Nannya, Y.; Hasserjian, R.P.; Devlin, S.M.; Tuechler, H.; Medina-Martinez, J.S.; Yoshizato, T.; Shiozawa, Y.; Saiki, R.; Malcovati, L.; et al. Implications of TP53 Allelic State for Genome Stability, Clinical Presentation and Outcomes in Myelodysplastic Syndromes. Nat. Med. 2020, 26, 1549–1556, Correction in Nat. Med. 2021, 27, 927; Correction in Nat. Med. 2021, 27, 562. [Google Scholar] [CrossRef]
- Rücker, F.G.; Dolnik, A.; Blätte, T.J.; Teleanu, V.; Ernst, A.; Thol, F.; Heuser, M.; Ganser, A.; Döhner, H.; Döhner, K.; et al. Chromothripsis Is Linked to TP53 Alteration, Cell Cycle Impairment, and Dismal Outcome in Acute Myeloid Leukemia with Complex Karyotype. Haematologica 2018, 103, e17–e20. [Google Scholar] [CrossRef]
- Korbel, J.O.; Campbell, P.J. Criteria for Inference of Chromothripsis in Cancer Genomes. Cell 2013, 152, 1226–1236. [Google Scholar] [CrossRef]
- Vadakekolathu, J.; Skuli, S.; Boocock, D.J.; Coveney, C.; Ikpo, E.G.; Wang, B.; Fenu, E.M.; Abbas, H.A.; Lai, C.E.; Carroll, M.P.; et al. Multi-Omic Analyses of TP53-Mutated Acute Myeloid Leukemia Identify Prognostic Metabolic Signatures. Blood 2024, 144, 2911. [Google Scholar] [CrossRef]
- Naji, N.S.; Pasca, S.; Chatzilygeroudi, T.; Toledano-Sanz, P.; Rimando, J.; An, Y.; Hemani, Y.; Perkins, B.; Zeng, X.; Talbot, C.; et al. C-C Motif Chemokine Receptor-like 2 Promotes the Interferon-γ Signaling Response in Myeloid Neoplasms with Erythroid Differentiation and Mutated TP53. Haematologica, 2025. [Google Scholar] [CrossRef]
- Vadakekolathu, J.; Lai, C.; Reeder, S.; Church, S.E.; Hood, T.; Lourdusamy, A.; Rettig, M.P.; Aldoss, I.; Advani, A.S.; Godwin, J.; et al. TP53 Abnormalities Correlate with Immune Infiltration and Associate with Response to Flotetuzumab Immunotherapy in AML. Blood Adv. 2020, 4, 5011–5024. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, A.V.; Mahdevar, M.; Mirzaei, S.; Entezari, M.; Hashemi, M.; Hushmandi, K.; Peymani, M. Introduction of Mutant TP53 Related Genes in Metabolic Pathways and Evaluation Their Correlation with Immune Cells, Drug Resistance and Sensitivity. Life Sci. 2022, 303, 120650. [Google Scholar] [CrossRef] [PubMed]
- Skuli, S.J.; Bakayoko, A.; Kruidenier, M.; Manning, B.; Pammer, P.; Salimov, A.; Riley, O.; Brake-Sillá, G.; Dopkin, D.; Bowman, M.; et al. Chemoresistance of TP53 Mutant Acute Myeloid Leukemia Requires the Mevalonate Byproduct, Geranylgeranyl Pyrophosphate, for Induction of an Adaptive Stress Response. Leukemia 2025, 39, 2087–2098. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.; Schimmer, R.R.; Koch, C.; Schneiter, F.; Fullin, J.; Lysenko, V.; Pellegrino, C.; Klemm, N.; Russkamp, N.; Myburgh, R.; et al. Targeting the Mevalonate or Wnt Pathways to Overcome CAR T-Cell Resistance in TP53-Mutant AML Cells. EMBO Mol. Med. 2024, 16, 445–474. [Google Scholar] [CrossRef]
- Rodina, A.; Wang, T.; Yan, P.; Gomes, E.D.; Dunphy, M.P.S.; Pillarsetty, N.; Koren, J.; Gerecitano, J.F.; Taldone, T.; Zong, H.; et al. The Epichaperome Is an Integrated Chaperome Network That Facilitates Tumour Survival. Nature 2016, 538, 397–401. [Google Scholar] [CrossRef]
- Carter, B.Z.; Mak, P.Y.; Muftuoglu, M.; Tao, W.; Ke, B.; Pei, J.; Bedoy, A.D.; Ostermann, L.B.; Nishida, Y.; Isgandarova, S.; et al. Epichaperome Inhibition Targets TP53-Mutant AML and AML Stem/Progenitor Cells. Blood 2023, 142, 1056–1070. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, W. P53 in Ferroptosis Regulation: The New Weapon for the Old Guardian. Cell Death Differ. 2022, 29, 895–910. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y. P53 Involvement in Clonal Hematopoiesis of Indeterminate Potential. Curr. Opin. Hematol. 2019, 26, 235–240. [Google Scholar] [CrossRef]
- Warren, J.T.; Link, D.C. Clonal Hematopoiesis and Risk for Hematologic Malignancy. Blood 2020, 136, 1599–1605. [Google Scholar] [CrossRef]
- Morton, L.M.; Dores, G.M.; Schonfeld, S.J.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Curtis, R.E. Association of Chemotherapy for Solid Tumors with Development of Therapy-Related Myelodysplastic Syndrome or Acute Myeloid Leukemia in the Modern Era. JAMA Oncol. 2019, 5, 318–325. [Google Scholar] [CrossRef]
- Leone, G.; Fianchi, L.; Pagano, L.; Voso, M.T. Incidence and Susceptibility to Therapy-Related Myeloid Neoplasms. Chem. Biol. Interact. 2010, 184, 39–45. [Google Scholar] [CrossRef]
- Csizmar, C.M.; Saliba, A.N.; Swisher, E.M.; Kaufmann, S.H. PARP Inhibitors and Myeloid Neoplasms: A Double-Edged Sword. Cancers 2021, 13, 6385. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.L.; Greipp, P.T.; Rangan, A.; Jatoi, A.; Nguyen, P.L. Myeloid Malignancies in Cancer Patients Treated with Poly(ADP-Ribose) Polymerase (PARP) Inhibitors: A Case Series. Blood Cancer J. 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.E.; Khalife-Hachem, S.; Grinda, T.; Kfoury, M.; Garciaz, S.; Pasquier, F.; Vargaftig, J.; Uzunov, M.; Belhabri, A.; Bertoli, S.; et al. Therapy-Related Myeloid Neoplasms Following Treatment with PARP Inhibitors: New Molecular Insights. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Alkhateeb, H.B.; Mohty, R.; Greipp, P.; Bansal, R.; Hathcock, M.; Rosenthal, A.; Murthy, H.; Kharfan-Dabaja, M.; Bisneto Villasboas, J.C.; Bennani, N.; et al. Therapy-Related Myeloid Neoplasms Following Chimeric Antigen Receptor T-Cell Therapy for Non-Hodgkin Lymphoma. Blood Cancer J. 2022, 12, 113. [Google Scholar] [CrossRef]
- Gazeau, N.; Beauvais, D.; Tilmont, R.; Srour, M.; Ferrant, E.; Safar, V.; Fouillet, L.; Flandrin-Gresta, P.; Gower, N.; Chauvet, P.; et al. Myeloid Neoplasms after CD19-Directed CAR T Cells Therapy in Long-Term B-Cell Lymphoma Responders, a Rising Risk over Time? Leukemia 2025, 39, 1714–1722. [Google Scholar] [CrossRef]
- Lal, R.; Lind, K.; Heitzer, E.; Ulz, P.; Aubell, K.; Kashofer, K.; Middeke, J.M.; Thiede, C.; Schulz, E.; Rosenberger, A.; et al. Somatic TP53 Mutations Characterize Preleukemic Stem Cells in Acute Myeloid Leukemia. Blood 2017, 129, 2587–2591. [Google Scholar] [CrossRef]
- Barakos, G.P.; Hatzimichael, E. Microenvironmental Features Driving Immune Evasion in Myelodysplastic Syndromes and Acute Myeloid Leukemia. Dis. Basel Switz. 2022, 10, 33. [Google Scholar] [CrossRef]
- Rodriguez-Meira, A.; Norfo, R.; Wen, S.; Chédeville, A.L.; Rahman, H.; O’Sullivan, J.; Wang, G.; Louka, E.; Kretzschmar, W.W.; Paterson, A.; et al. Single-Cell Multi-Omics Identifies Chronic Inflammation as a Driver of TP53-Mutant Leukemic Evolution. Nat. Genet. 2023, 55, 1531–1541. [Google Scholar] [CrossRef]
- Takizawa, H.; Boettcher, S.; Manz, M.G. Demand-Adapted Regulation of Early Hematopoiesis in Infection and Inflammation. Blood 2012, 119, 2991–3002. [Google Scholar] [CrossRef]
- Basiorka, A.A.; McGraw, K.L.; Eksioglu, E.A.; Chen, X.; Johnson, J.; Zhang, L.; Zhang, Q.; Irvine, B.A.; Cluzeau, T.; Sallman, D.A.; et al. The NLRP3 Inflammasome Functions as a Driver of the Myelodysplastic Syndrome Phenotype. Blood 2016, 128, 2960–2975. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Xu, Y.; Schultz, R.D.; Chen, H.; Xie, J.; Deng, M.; Liu, X.; Gui, X.; John, S.; Lu, Z.; et al. LILRB3 Supports Acute Myeloid Leukemia Development and Regulates T-Cell Antitumor Immune Responses through the TRAF2-cFLIP-NF-κB Signaling Axis. Nat. Cancer 2021, 2, 1170–1184. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.V.; Kutyna, M.; Shah, S.; Tran, E.N.H.; Baranwal, A.; Ladon, D.; Al-Kali, A.; Brown, A.; Chen, D.; Greipp, P.; et al. Comparison of World Health Organization and International Consensus Classification Guidelines for Myeloid Neoplasms Harboring TP53-Mutations Using an Independent International Cohort. Blood 2023, 142, 3243. [Google Scholar] [CrossRef]
- Hart, S.A.; Lee, L.A.; Seegmiller, A.C.; Mason, E.F. Diagnosis of TP53-Mutated Myeloid Disease by the ICC and WHO Fifth Edition Classifications. Blood Adv. 2025, 9, 445–454. [Google Scholar] [CrossRef]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and Management of AML in Adults: 2022 Recommendations from an International Expert Panel on Behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Bao, Z.; Li, B.; Qin, T.; Xu, Z.; Qu, S.; Jia, Y.; Li, C.; Pan, L.; Gao, Q.; Jiao, M.; et al. Molecular Characteristics and Clinical Implications of TP53 Mutations in Therapy-Related Myelodysplastic Syndromes. Blood Cancer J. 2025, 15, 58. [Google Scholar] [CrossRef]
- Kaur, A.; Rojek, A.E.; Symes, E.; Nawas, M.T.; Patel, A.A.; Patel, J.L.; Sojitra, P.; Aqil, B.; Sukhanova, M.; McNerney, M.E.; et al. Real World Predictors of Response and 24-Month Survival in High-Grade TP53-Mutated Myeloid Neoplasms. Blood Cancer J. 2024, 14, 99. [Google Scholar] [CrossRef]
- Ozga, M.; Nicolet, D.; Mrózek, K.; Yilmaz, A.S.; Kohlschmidt, J.; Larkin, K.T.; Blachly, J.S.; Oakes, C.C.; Buss, J.; Walker, C.J.; et al. Sex-Associated Differences in Frequencies and Prognostic Impact of Recurrent Genetic Alterations in Adult Acute Myeloid Leukemia (Alliance, AMLCG). Leukemia 2024, 38, 45–57. [Google Scholar] [CrossRef]
- Shah, M.V.; Hung, K.; Baranwal, A.; Kutyna, M.M.; Al-Kali, A.; Toop, C.; Greipp, P.; Brown, A.; Shah, S.; Khanna, S.; et al. Evidence-Based Risk Stratification of Myeloid Neoplasms Harboring TP53 Mutations. Blood Adv. 2025, 9, 3370–3380. [Google Scholar] [CrossRef]
- Jambhekar, A.; Ackerman, E.E.; Alpay, B.A.; Lahav, G.; Lovitch, S.B. Comparison of TP53 Mutations in Myelodysplasia and Acute Leukemia Suggests Divergent Roles in Initiation and Progression. Blood Neoplasia 2024, 1, 100004. [Google Scholar] [CrossRef]
- Dutta, S.; Pregartner, G.; Rücker, F.G.; Heitzer, E.; Zebisch, A.; Bullinger, L.; Berghold, A.; Döhner, K.; Sill, H. Functional Classification of TP53 Mutations in Acute Myeloid Leukemia. Cancers 2020, 12, 637. [Google Scholar] [CrossRef]
- Puzo, C.J.; Hager, K.M.; Rinder, H.M.; Weinberg, O.K.; Siddon, A.J. Overall Survival in TP53-Mutated AML and MDS. Ann. Hematol. 2024, 103, 5359–5369. [Google Scholar] [CrossRef] [PubMed]
- Lontos, K.; Saliba, R.M.; Kanagal-Shamanna, R.; Özcan, G.; Ramdial, J.; Chen, G.; Kadia, T.; Short, N.J.; Daver, N.G.; Kantarjian, H.; et al. TP53-Mutant Variant Allele Frequency and Cytogenetics Determine Prognostic Groups in MDS/AML for Transplantation. Blood Adv. 2025, 9, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Nawas, M.T.; Kosuri, S. Utility or Futility? A Contemporary Approach to Allogeneic Hematopoietic Cell Transplantation for TP53-Mutated MDS/AML. Blood Adv. 2024, 8, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Yoshizato, T.; Nannya, Y.; Atsuta, Y.; Shiozawa, Y.; Iijima-Yamashita, Y.; Yoshida, K.; Shiraishi, Y.; Suzuki, H.; Nagata, Y.; Sato, Y.; et al. Genetic Abnormalities in Myelodysplasia and Secondary Acute Myeloid Leukemia: Impact on Outcome of Stem Cell Transplantation. Blood 2017, 129, 2347–2358. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellström-Lindberg, E.; Santini, V.; Gattermann, N.; Germing, U.; Sanz, G.; List, A.F.; Gore, S.; Seymour, J.F.; et al. Azacitidine Prolongs Overall Survival Compared with Conventional Care Regimens in Elderly Patients with Low Bone Marrow Blast Count Acute Myeloid Leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 562–569. [Google Scholar] [CrossRef]
- Bories, P.; Prade, N.; Lagarde, S.; Cabarrou, B.; Largeaud, L.; Plenecassagnes, J.; Luquet, I.; De Mas, V.; Filleron, T.; Cassou, M.; et al. Impact of TP53 Mutations in Acute Myeloid Leukemia Patients Treated with Azacitidine. PLoS ONE 2020, 15, e0238795. [Google Scholar] [CrossRef]
- Welch, J.S.; Petti, A.A.; Miller, C.A.; Fronick, C.C.; O’Laughlin, M.; Fulton, R.S.; Wilson, R.K.; Baty, J.D.; Duncavage, E.J.; Tandon, B.; et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N. Engl. J. Med. 2016, 375, 2023–2036. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Döhner, H.; Pratz, K.W.; DiNardo, C.D.; Wei, A.H.; Jonas, B.A.; Pullarkat, V.A.; Thirman, M.J.; Récher, C.; Schuh, A.C.; Babu, S.; et al. Genetic Risk Stratification and Outcomes among Treatment-Naive Patients with AML Treated with Venetoclax and Azacitidine. Blood 2024, 144, 2211–2222. [Google Scholar] [CrossRef]
- Kim, K.; Maiti, A.; Loghavi, S.; Pourebrahim, R.; Kadia, T.M.; Rausch, C.R.; Furudate, K.; Daver, N.G.; Alvarado, Y.; Ohanian, M.; et al. Outcomes of TP53-Mutant Acute Myeloid Leukemia with Decitabine and Venetoclax. Cancer 2021, 127, 3772–3781. [Google Scholar] [CrossRef]
- Goldfinger, M.; Mantzaris, I.; Shastri, A.; Saunthararajah, Y.; Gritsman, K.; Sica, R.A.; Kornblum, N.; Shah, N.; Levitz, D.; Rockwell, B.; et al. A Weekly Low-Dose Regimen of Decitabine and Venetoclax Is Efficacious and Less Myelotoxic in a Racially Diverse Cohort. Blood 2024, 144, 2360–2363. [Google Scholar] [CrossRef]
- Hiwase, D.; Hahn, C.; Tran, E.N.H.; Chhetri, R.; Baranwal, A.; Al-Kali, A.; Sharplin, K.; Ladon, D.; Hollins, R.; Greipp, P.; et al. TP53 Mutation in Therapy-Related Myeloid Neoplasm Defines a Distinct Molecular Subtype. Blood 2023, 141, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Short, N.J.; Montalban-Bravo, G.; Hwang, H.; Ning, J.; Franquiz, M.J.; Kanagal-Shamanna, R.; Patel, K.P.; DiNardo, C.D.; Ravandi, F.; Garcia-Manero, G.; et al. Prognostic and Therapeutic Impacts of Mutant TP53 Variant Allelic Frequency in Newly Diagnosed Acute Myeloid Leukemia. Blood Adv. 2020, 4, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Grenet, J.; Jain, A.G.; Burkart, M.; Waksal, J.; Famulare, C.; Numan, Y.; Stahl, M.; Mckinnell, Z.; Ball, B.; Ma, X.; et al. Comparing Outcomes between Liposomal Daunorubicin/Cytarabine (CPX-351) and HMA + Venetoclax As Frontline Therapy in Acute Myeloid Leukemia. Blood 2021, 138, 32. [Google Scholar] [CrossRef]
- Othman, J.; Wilhelm-Benartzi, C.; Dillon, R.; Knapper, S.; Freeman, S.D.; Batten, L.M.; Canham, J.; Hinson, E.L.; Wych, J.; Betteridge, S.; et al. A Randomized Comparison of CPX-351 and FLAG-Ida in Adverse Karyotype AML and High-Risk MDS: The UK NCRI AML19 Trial. Blood Adv. 2023, 7, 4539–4549. [Google Scholar] [CrossRef]
- Zugasti, I.; Lopez-Guerra, M.; Castaño-Díez, S.; Esteban, D.; Avendaño, A.; Pomares, H.; Perez, A.; García-Ávila, S.; Conejo, I.P.; de la Fuente Montes, C.; et al. Hypomethylating Agents plus Venetoclax for High-Risk MDS and CMML as Bridge Therapy to Transplant: A GESMD Study. Exp. Hematol. Oncol. 2025, 14, 61. [Google Scholar] [CrossRef]
- Baranwal, A.; Langer, K.J.; Gannamani, V.; Rud, D.; Cibich, A.; Saygin, C.; Nawas, M.; Badar, T.; Kharfan-Dabaja, M.A.; Ayala, E.; et al. Factors Associated with Survival after Allogeneic Transplantation for Myeloid Neoplasms Harboring TP53 Mutations. Blood Adv. 2025, 9, 3395–3407. [Google Scholar] [CrossRef]
- Shahzad, M.; Tariq, E.; Chaudhary, S.G.; Anwar, I.; Iqbal, Q.; Fatima, H.; Abdelhakim, H.; Ahmed, N.; Balusu, R.; Hematti, P.; et al. Outcomes with Allogeneic Hematopoietic Stem Cell Transplantation in TP53-Mutated Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. Leuk. Lymphoma 2022, 63, 3409–3417. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Saber, W.; Mar, B.G.; Redd, R.; Wang, T.; Haagenson, M.D.; Grauman, P.V.; Hu, Z.-H.; Spellman, S.R.; Lee, S.J.; et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N. Engl. J. Med. 2017, 376, 536–547. [Google Scholar] [CrossRef]
- Sinanidis, I.; Hochman, M.J.; Tsai, H.-L.; Randall, M.P.; Bonilla, B.; Varadhan, R.; Ambinder, A.J.; Jones, R.J.; DeZern, A.E.; Karantanos, T. Favorable Outcomes in MDS and Oligoblastic AML-MR after Reduced-Intensity Conditioning Allogeneic Bone Marrow Transplantation with Post-Transplantation Cyclophosphamide. Bone Marrow Transplant. 2024, 59, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Pasca, S.; Haldar, S.D.; Ambinder, A.; Webster, J.A.; Jain, T.; Dalton, W.B.; Prince, G.T.; Ghiaur, G.; DeZern, A.E.; Gojo, I.; et al. Outcome Heterogeneity of TP53-Mutated Myeloid Neoplasms and the Role of Allogeneic Hematopoietic Cell Transplantation. Haematologica 2024, 109, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Badar, T.; Atallah, E.; Shallis, R.; Saliba, A.N.; Patel, A.; Bewersdorf, J.P.; Grenet, J.; Stahl, M.; Duvall, A.; Burkart, M.; et al. Survival of TP53-Mutated Acute Myeloid Leukemia Patients Receiving Allogeneic Stem Cell Transplantation after First Induction or Salvage Therapy: Results from the Consortium on Myeloid Malignancies and Neoplastic Diseases (COMMAND). Leukemia 2023, 37, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.M.; Komrokji, R.S.; Yun, S.; Al Ali, N.; Chan, O.; Song, J.; Hussaini, M.; Talati, C.; Sweet, K.L.; Lancet, J.E.; et al. Baseline and Serial Molecular Profiling Predicts Outcomes with Hypomethylating Agents in Myelodysplastic Syndromes. Blood Adv. 2021, 5, 1017–1028. [Google Scholar] [CrossRef]
- Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; Devine, S.M.; et al. Impact of Conditioning Intensity and Genomics on Relapse After Allogeneic Transplantation for Patients with Myelodysplastic Syndrome. JCO Precis. Oncol. 2021, 5, PO.20.00355. [Google Scholar] [CrossRef]
- Senapati, J.; Garcia-Manero, G.; DiNardo, C.D.; Deshmukh, I.; Borthakur, G.; Kadia, T.M.; Jabbour, E.; Short, N.J.; Abbas, H.A.; Pemmaraju, N.; et al. Barriers to Allogeneic Stem Cell Transplantation in Responding Patients with TP53-Mutated Acute Myeloid Leukemia. Bone Marrow Transplant. 2025, 60, 910–913. [Google Scholar] [CrossRef]
- Hourigan, C.S.; Dillon, L.W.; Gui, G.; Logan, B.R.; Fei, M.; Ghannam, J.; Li, Y.; Licon, A.; Alyea, E.P.; Bashey, A.; et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia with Genomic Evidence of Residual Disease. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 1273–1283. [Google Scholar] [CrossRef]
- Byrne, M.T.; Kurian, T.J.; Patel, D.A.; Tamari, R.; Hong, S.; Abdelhakim, H.; Klein, V.; Rojas, P.; Madhavan, R.; Kent, A.; et al. Non-Relapse Mortality in TP53-Mutated MDS/AML—A Multi-Center Collaborative Study. Blood 2021, 138, 2922. [Google Scholar] [CrossRef]
- University of Birmingham. A Double-Blind, Phase III, Randomised Study to Compare the Efficacy and Safety of Oral Azacitidine (CC-486) Versus Placebo in Subjects with Acute Myeloid Leukaemia or Myelodysplastic Syndromes as Maintenance After Allogeneic Haematopoietic Stem Cell Transplantation. 2024. Available online: https://clinicaltrials.gov/study/NCT04173533 (accessed on 20 September 2025).
- AbbVie. A Randomized, Open Label Phase 3 Study Evaluating Safety and Efficacy of Venetoclax in Combination with Azacitidine After Allogeneic Stem Cell Transplantation in Subjects with Acute Myeloid Leukemia (AML) (VIALE-T). 2025. Available online: https://clinicaltrials.gov/study/NCT04161885 (accessed on 20 September 2025).
- DeFilipp, Z.M. A Phase Ib Study of Oral Decitabine/Cedazuridine as Maintenance Therapy Following Allogeneic Hematopoietic Cell Transplantation for Patients with Myeloid Neoplasms. 2025. Available online: https://clinicaltrials.gov/study/NCT04980404 (accessed on 20 September 2025).
- Garcia, J. A Phase 1 Study of Adding Venetoclax to a Reduced Intensity Conditioning Regimen and to Maintenance in Combination with a Hypomethylating Agent After Allogeneic Hematopoietic Cell Transplantation for Patients with High Risk AML, MDS, and MDS/MPN Overlap Syndromes. 2025. Available online: https://clinicaltrials.gov/study/NCT03613532 (accessed on 20 September 2025).
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T.; et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef]
- Ravandi, F.; Assi, R.; Daver, N.; Benton, C.B.; Kadia, T.; Thompson, P.A.; Borthakur, G.; Alvarado, Y.; Jabbour, E.J.; Konopleva, M.; et al. Idarubicin, Cytarabine, and Nivolumab in Patients with Newly Diagnosed Acute Myeloid Leukaemia or High-Risk Myelodysplastic Syndrome: A Single-Arm, Phase 2 Study. Lancet Haematol. 2019, 6, e480–e488. [Google Scholar] [CrossRef]
- Garcia, J.S.; Flamand, Y.; Penter, L.; Keng, M.; Tomlinson, B.K.; Mendez, L.M.; Koller, P.; Cullen, N.; Arihara, Y.; Pfaff, K.; et al. Ipilimumab plus Decitabine for Patients with MDS or AML in Posttransplant or Transplant-Naïve Settings. Blood 2023, 141, 1884–1888. [Google Scholar] [CrossRef]
- Bouligny, I.M.; Montalban-Bravo, G.; Sasaki, K.; Daver, N.; Jabbour, E.; Alvarado, Y.; DiNardo, C.D.; Ravandi, F.; Borthakur, G.; Bose, P.; et al. A Phase II Trial of Azacitidine with Ipilimumab, Nivolumab, or Ipilimumab and Nivolumab in Previously Untreated Myelodysplastic Syndrome. Haematologica 2025, 110, 1628–1633. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Boss, I.; Beach, C.L.; Copeland, W.B.; Thompson, E.; Fox, B.A.; Hasle, V.E.; Hellmann, A.; Taussig, D.C.; Tormo, M.; et al. A Randomized Phase 2 Trial of Azacitidine with or without Durvalumab as First-Line Therapy for Older Patients with AML. Blood Adv. 2022, 6, 2219–2229. [Google Scholar] [CrossRef]
- University of Southern California. A Phase I/II Multicenter Study Combining Guadecitabine, a DNA Methyltransferase Inhibitor, with Atezolizumab, an Immune Checkpoint Inhibitor, in Patients with Intermediate or High-Risk Myelodysplastic Syndrome or Chronic Myelomonocytic Leukemia. 2025. Available online: https://clinicaltrials.gov/study/NCT02935361 (accessed on 20 September 2025).
- Daver, N.G.; Vyas, P.; Kambhampati, S.; Al Malki, M.M.; Larson, R.A.; Asch, A.S.; Mannis, G.; Chai-Ho, W.; Tanaka, T.N.; Bradley, T.J.; et al. Tolerability and Efficacy of the Anticluster of Differentiation 47 Antibody Magrolimab Combined with Azacitidine in Patients with Previously Untreated AML: Phase Ib Results. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Senapati, J.; Maiti, A.; Loghavi, S.; Kadia, T.M.; DiNardo, C.D.; Pemmaraju, N.; Jabbour, E.; Montalban-Bravo, G.; Tang, G.; et al. Phase I/II Study of Azacitidine (AZA) with Venetoclax (VEN) and Magrolimab (Magro) in Patients (Pts) with Newly Diagnosed (ND) Older/Unfit or High-Risk Acute Myeloid Leukemia (AML) and Relapsed/Refractory (R/R) AML. Blood 2022, 140, 141–144. [Google Scholar] [CrossRef]
- Gilead Statement on the Discontinuation of Magrolimab Study in AML with TP53 Mutations. Available online: https://www.gilead.com/company/company-statements/2023/gilead-statement-on-the-discontinuation-of-magrolimab-study-in-aml-with-tp53-mutations (accessed on 20 September 2025).
- Gilead Sciences. A Phase 3, Randomized, Open-Label Study Evaluating the Safety and Efficacy of Magrolimab in Combination with Azacitidine Versus Physician’s Choice of Venetoclax in Combination with Azacitidine or Intensive Chemotherapy in Previously Untreated Patients with TP53 Mutant Acute Myeloid Leukemia. 2025. Available online: https://clinicaltrials.gov/study/NCT04778397 (accessed on 20 September 2025).
- Gilead Statement on Discontinuation of Phase 3 ENHANCE-3 Study in AML. Available online: https://www.gilead.com/company/company-statements/2024/gilead-statement-on-discontinuation-of-phase-3-enhance-3-study-in-aml (accessed on 20 September 2025).
- Daver, N.; Vyas, P.; Huls, G.; Döhner, H.; Maury, S.; Novak, J.; Papayannidis, C.; Martínez Chamorro, C.; Montesinos, P.; Niroula, R.; et al. The ENHANCE-3 Study: Venetoclax and Azacitidine plus Magrolimab or Placebo for Untreated AML Unfit for Intensive Therapy. Blood 2025, 146, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Przespolewski, A.; Abaza, Y.; Byrne, M.; Fong, A.P.; Jin, F.; Forgie, A.J.; Tsiatis, A.C.; Guan, S.; Erba, H.P. Evorpacept (ALX148), a CD47-Blocking Myeloid Checkpoint Inhibitor, in Combination with Azacitidine and Venetoclax in Patients with Acute Myeloid Leukemia (ASPEN-05): Results from Phase 1a Dose Escalation Part. Blood 2022, 140, 9046–9047. [Google Scholar] [CrossRef]
- ALX Oncology Inc. A Phase 1/2 Study of Evorpacept (ALX148) in Combination with Venetoclax and Azacitidine in Patients with Acute Myeloid Leukemia (AML) (ASPEN-05). 2024. Available online: https://clinicaltrials.gov/study/NCT04755244 (accessed on 20 September 2025).
- ALX Oncology Inc. A Phase 1/2 Study of Evorpacept (ALX148) in Combination with Azacitidine in Patients with Higher Risk Myelodysplastic Syndrome (MDS) (ASPEN-02). 2025. Available online: https://clinicaltrials.gov/study/NCT04417517 (accessed on 20 September 2025).
- Daver, N.G.; Stevens, D.A.; Hou, J.-Z.; Yamauchi, T.; Moshe, Y.; Fong, C.Y.; Marzocchetti, A.; Adamec, R.; Patel, M.; Lambert, S.; et al. Lemzoparlimab (Lemzo) with Venetoclax (Ven) and/or Azacitidine (Aza) in Patients (Pts) with Acute Myeloid Leukemia (AML) or Myelodysplastic Syndromes (MDS): A Phase 1b Dose Escalation Study. J. Clin. Oncol. 2022, 40, TPS7067. [Google Scholar] [CrossRef]
- AbbVie. A Phase 1b Dose Escalation Study of Lemzoparlimab in Combination with Venetoclax and/or Azacitidine in Subjects with Acute Myeloid Leukemia (AML) or Myelodysplastic Syndrome (MDS). 2024. Available online: https://clinicaltrials.gov/study/NCT04912063 (accessed on 20 September 2025).
- Akeso. A Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase 2 Study of AK117 or Placebo in Combination with Azacitidine in Patients with Newly Diagnosed Higher-Risk Myelodysplastic Syndromes. 2025. Available online: https://clinicaltrials.gov/study/NCT06196203 (accessed on 20 September 2025).
- Zeidan, A.M.; Tong, H.; Xiao, Z.; Baratam, P.; Abboud, R.; Benton, C.B.; Zeidner, J.F.; Borate, U.; Chai-Ho, W.; Lu, Y.; et al. Trial in Progress: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Phase 2 Study of AK117/Placebo in Combination with Azacitidine in Patients with Newly Diagnosed Higher-Risk Myelodysplastic Syndromes (AK117-205). Blood 2024, 144, 6705. [Google Scholar] [CrossRef]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.; et al. Efficacy and Safety of Sabatolimab (MBG453) in Combination with Hypomethylating Agents (HMAs) in Patients (Pts) with Very High/High-Risk Myelodysplastic Syndrome (vHR/HR-MDS) and Acute Myeloid Leukemia (AML): Final Analysis from a Phase Ib Study. Blood 2021, 138, 244. [Google Scholar] [CrossRef]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.J.W.M.; et al. Phase Ib Study of Sabatolimab (MBG453), a Novel Immunotherapy Targeting TIM-3 Antibody, in Combination with Decitabine or Azacitidine in High- or Very High-Risk Myelodysplastic Syndromes. Am. J. Hematol. 2024, 99, E32–E36. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. A Randomized, Double-Blind, Placebo-Controlled Phase II Multi-Center Study of Intravenous MBG453 Added to Hypomethylating Agents in Adult Subjects with Intermediate, High or Very High Risk Myelodysplastic Syndrome (MDS) as Per IPSS-R Criteria. 2025. Available online: https://clinicaltrials.gov/study/NCT03946670 (accessed on 20 September 2025).
- Novartis Pharmaceuticals. A Randomized, Double-Blind, Placebo-Controlled Phase III Multi-Center Study of Azacitidine with or Without MBG453 for the Treatment of Patients with Intermediate, High or Very High Risk Myelodysplastic Syndrome (MDS) as Per IPSS-R, or Chronic Myelomonocytic Leukemia-2 (CMML-2). 2025. Available online: https://clinicaltrials.gov/study/NCT04266301 (accessed on 20 September 2025).
- Novartis Pharmaceuticals. A Phase II Multi-Center, Single Arm, Safety and Efficacy Study of MBG453 in Combination with Azacitidine and Venetoclax for the Treatment of Acute Myeloid Leukemia (AML) in Adult Patients Unfit for Chemotherapy. 2025. Available online: https://clinicaltrials.gov/study/NCT04150029 (accessed on 20 September 2025).
- Novartis Pharmaceuticals. A Single-Arm, Open-Label, Phase II Study of Sabatolimab in Combination with Azacitidine and Venetoclax in Adult Participants with High or Very High Risk Myelodysplastic Syndromes (MDS) as Per IPSS-R Criteria. 2025. Available online: https://clinicaltrials.gov/study/NCT04812548 (accessed on 20 September 2025).
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as Salvage Immunotherapy for Refractory Acute Myeloid Leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Maris, M.; Lin, T.L.; Patel, P.; Madanat, Y.F.; Cogle, C.R.; Borthakur, G.; Huebner, D.; Khaskhely, N.; Bonham, L.; et al. Updated Results from a Phase 1 Study of APVO436, a Novel Bispecific Anti-CD123 x Anti-CD3 AdaptirTM Molecule, in Relapsed/Refractory Acute Myeloid Leukemia and Myelodysplastic Syndrome. Blood 2022, 140, 6204–6205. [Google Scholar] [CrossRef]
- Aptevo Therapeutics. A Phase 1b/2 Open-Label Study of APVO436 in Combination with Venetoclax and Azacitidine in Patients with Newly Diagnosed Acute Myeloid Leukemia (AML). 2025. Available online: https://clinicaltrials.gov/study/NCT06634394 (accessed on 20 September 2025).
- Lane, A.A.; Garcia, J.S.; Raulston, E.G.; Garzon, J.L.; Galinsky, I.; Baxter, E.W.; Leonard, R.; DeAngelo, D.J.; Luskin, M.R.; Reilly, C.R.; et al. Phase 1b Trial of Tagraxofusp in Combination with Azacitidine with or without Venetoclax in Acute Myeloid Leukemia. Blood Adv. 2024, 8, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Lane, A. Phase 1 Study of SL-401 in Combination with Azacitidine and Venetoclax in Relapsed/Refractory Acute Myeloid Leukemia (AML) and in Treatment-Naive Subjects with AML Not Eligible for Standard Induction and in Subjects with Blastic Plasmacytoid Dendritic Cell Neoplasm (BPDCN) or SL-401 in Combination with Azacitidine in Subjects with High-Risk Myelodysplastic Syndrome (MDS). 2025. Available online: https://clinicaltrials.gov/study/NCT03113643 (accessed on 20 September 2025).
- Zhang, H.; Zhu, H.-H. Breakthroughs of CAR T-Cell Therapy in Acute Myeloid Leukemia: Updates from ASH 2024. Exp. Hematol. Oncol. 2025, 14, 57. [Google Scholar] [CrossRef]
- Liu, F.; Cao, Y.; Pinz, K.; Ma, Y.; Wada, M.; Chen, K.; Ma, G.; Shen, J.; Tse, C.O.; Su, Y.; et al. First-in-Human CLL1-CD33 Compound CAR T Cell Therapy Induces Complete Remission in Patients with Refractory Acute Myeloid Leukemia: Update on Phase 1 Clinical Trial. Blood 2018, 132, 901. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Sun, L.; Li, Y.; Zhang, S.; He, G.; Yi, H.; Wada, M.; Pinz, K.G.; Chen, K.H.; et al. First-In-Human CLL1-CD33 Compound Car (CCAR) T Cell Therapy in Relapsed and Refractory Acute Myeloid Leukemia. EHA 2020—Abstract S149. Available online: https://library.ehaweb.org/eha/2020/eha25th/294969/fang.liu.first-in-human.cll1-cd33.compound.car.28ccar29.t.cell.therapy.in.html (accessed on 20 September 2025).
- Xu, K.L. Application of Anti Tim-3/CD123 CAR-T Cell Therapy in Relapsed and Refractory Acute Myeloid Leukemia (rr/AML). 2023. Available online: https://clinicaltrials.gov/study/NCT06125652 (accessed on 20 September 2025).
- He, X.; Feng, Z.; Ma, J.; Ling, S.; Cao, Y.; Gurung, B.; Wu, Y.; Katona, B.W.; O’Dwyer, K.P.; Siegel, D.L.; et al. Bispecific and Split CAR T Cells Targeting CD13 and TIM3 Eradicate Acute Myeloid Leukemia. Blood 2020, 135, 713–723. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yu, K.-R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.-H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173, 1439–1453.e19. [Google Scholar] [CrossRef]
- Tuval, A.; Strandgren, C.; Heldin, A.; Palomar-Siles, M.; Wiman, K.G. Pharmacological Reactivation of P53 in the Era of Precision Anticancer Medicine. Nat. Rev. Clin. Oncol. 2024, 21, 106–120. [Google Scholar] [CrossRef]
- Cluzeau, T.; Sebert, M.; Rahmé, R.; Cuzzubbo, S.; Lehmann-Che, J.; Madelaine, I.; Peterlin, P.; Bève, B.; Attalah, H.; Chermat, F.; et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone Des Myélodysplasies (GFM). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar] [CrossRef]
- Sallman, D.A.; DeZern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Aprea Therapeutics. A Phase III Multicenter, Randomized, Open Label Study of APR-246 in Combination with Azacitidine Versus Azacitidine Alone for the Treatment of (Tumor Protein) TP53 Mutant Myelodysplastic Syndromes. 2025. Available online: https://clinicaltrials.gov/study/NCT03745716 (accessed on 20 September 2025).
- Garcia-Manero, G.; Goldberg, A.D.; Winer, E.S.; Altman, J.K.; Fathi, A.T.; Odenike, O.; Roboz, G.J.; Sweet, K.; Miller, C.; Wennborg, A.; et al. Eprenetapopt Combined with Venetoclax and Azacitidine in TP53-Mutated Acute Myeloid Leukaemia: A Phase 1, Dose-Finding and Expansion Study. Lancet Haematol. 2023, 10, e272–e283. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tamari, R.; DeZern, A.E.; Byrne, M.T.; Gooptu, M.; Chen, Y.-B.; Deeg, H.J.; Sallman, D.; Gallacher, P.; Wennborg, A.; et al. Eprenetapopt Plus Azacitidine After Allogeneic Hematopoietic Stem-Cell Transplantation for TP53-Mutant Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; Daver, N.G. Eprenetapopt in the Post-Transplant Setting: Mechanisms and Future Directions. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3994–3997. [Google Scholar] [CrossRef]
- Mohell, N.; Alfredsson, J.; Fransson, Å.; Uustalu, M.; Byström, S.; Gullbo, J.; Hallberg, A.; Bykov, V.J.N.; Björklund, U.; Wiman, K.G. APR-246 Overcomes Resistance to Cisplatin and Doxorubicin in Ovarian Cancer Cells. Cell Death Dis. 2015, 6, e1794. [Google Scholar] [CrossRef]
- Song, H.; Wu, J.; Tang, Y.; Dai, Y.; Xiang, X.; Li, Y.; Wu, L.; Wu, J.; Liang, Y.; Xing, Y.; et al. Diverse Rescue Potencies of P53 Mutations to ATO Are Predetermined by Intrinsic Mutational Properties. Sci. Transl. Med. 2023, 15, eabn9155. [Google Scholar] [CrossRef]
- The University of Hong Kong. A Phase 2 Study of Oral Arsenic Trioxide (Arsenol®)-Based Low-Intensity Treatment for Previously Untreated or Relapsed/Refractory TP53-Mutated Myeloid Malignancies. 2025. Available online: https://clinicaltrials.gov/study/NCT06778187 (accessed on 20 September 2025).
- Ma, J.; Sun, S.; Xie, Y.; Zhou, S.; Wu, M.; Zuo, X.; Xie, M.; Wang, X. Decitabine with Etoposide Is Effective in TP53 Mutated Myeloid Tumors via Overcoming Differentiation Block. Blood Cancer J. 2025, 15, 19. [Google Scholar] [CrossRef]
- Nechiporuk, T.; Kurtz, S.E.; Nikolova, O.; Liu, T.; Jones, C.L.; D’Alessandro, A.; Culp-Hill, R.; d’Almeida, A.; Joshi, S.K.; Rosenberg, M.; et al. The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells. Cancer Discov. 2019, 9, 910–925. [Google Scholar] [CrossRef]
- Tognon, C.; Mishra, S.; Kaempf, A.; Park, B.; Joshi, S.K.; Huang, A.; Kurtz, S.E.; Sax, L.; Eide, C.A.; Johnson, K.; et al. Phase 1 Trial Testing the Novel Combination Therapy of Entrectinib and ASTX727 in TP53 Mutated Relapsed/Refractory Acute Myeloid Leukemia Patients. Blood 2024, 144, 1498. [Google Scholar] [CrossRef]
- Ben-Batalla, I.; Schultze, A.; Wroblewski, M.; Erdmann, R.; Heuser, M.; Waizenegger, J.S.; Riecken, K.; Binder, M.; Schewe, D.; Sawall, S.; et al. Axl, a Prognostic and Therapeutic Target in Acute Myeloid Leukemia Mediates Paracrine Crosstalk of Leukemia Cells with Bone Marrow Stroma. Blood 2013, 122, 2443–2452. [Google Scholar] [CrossRef]
- Eisenmann, E.D.; Stromatt, J.C.; Fobare, S.; Huang, K.M.; Buelow, D.R.; Orwick, S.; Jeon, J.Y.; Weber, R.H.; Larsen, B.; Mims, A.S.; et al. TP-0903 Is Active in Preclinical Models of Acute Myeloid Leukemia with TP53 Mutation/Deletion. Cancers 2022, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Eisenmann, E.D.; Swords, R.; Huang, Y.; Orwick, S.; Buelow, D.; Abbott, N.; Phelps, M.; Zeidner, J.; Foster, M.C.; Lin, T.L.; et al. A Phase 1b/2 Study of TP-0903 and Decitabine Targeting Mutant TP53 and/or Complex Karyotype in Patients with Untreated Acute Myeloid Leukemia ≥Age 60 Years. Cancer Res. Commun. 2025, 5, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Swords, R.T.; Coutre, S.; Maris, M.B.; Zeidner, J.F.; Foran, J.M.; Cruz, J.; Erba, H.P.; Berdeja, J.G.; Tam, W.; Vardhanabhuti, S.; et al. Pevonedistat, a First-in-Class NEDD8-Activating Enzyme Inhibitor, Combined with Azacitidine in Patients with AML. Blood 2018, 131, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.N.; Kaufmann, S.H.; Stein, E.M.; Patel, P.A.; Baer, M.R.; Stock, W.; Deininger, M.; Blum, W.; Schiller, G.J.; Olin, R.L.; et al. Pevonedistat with Azacitidine in Older Patients with TP53-Mutated AML: A Phase 2 Study with Laboratory Correlates. Blood Adv. 2023, 7, 2360–2363. [Google Scholar] [CrossRef]
- Short, N.J.; Muftuoglu, M.; Ong, F.; Nasr, L.; Macaron, W.; Montalban-Bravo, G.; Alvarado, Y.; Basyal, M.; Daver, N.; Dinardo, C.D.; et al. A Phase 1/2 Study of Azacitidine, Venetoclax and Pevonedistat in Newly Diagnosed Secondary AML and in MDS or CMML after Failure of Hypomethylating Agents. J. Hematol. Oncol. 2023, 16, 73. [Google Scholar] [CrossRef]
- Murthy, G.S.G.; Saliba, A.N.; Szabo, A.; Harrington, A.; Abedin, S.; Carlson, K.; Michaelis, L.; Runaas, L.; Baim, A.; Hinman, A.; et al. A Phase I Study of Pevonedistat, Azacitidine, and Venetoclax in Patients with Relapsed/Refractory Acute Myeloid Leukemia. Haematologica 2024, 109, 2864–2872. [Google Scholar] [CrossRef]
- Man, C.-H.; Lam, W.; Dang, C.-C.; Zeng, X.-Y.; Zheng, L.-C.; Chan, N.N.-M.; Ng, K.-L.; Chan, K.-C.; Kwok, T.-H.; Ng, T.C.-C.; et al. Inhibition of PLK4 Remodels Histone Methylation and Activates the Immune Response via the cGAS-STING Pathway in TP53-Mutated AML. Blood 2023, 142, 2002–2015. [Google Scholar] [CrossRef]
- Ayoub, E.; Marupudi, A.; Nishida, Y.; Heinz Montoya, R.; Mohanty, V.; Walter, W.; Bray, M.R.; Patsilevas, T.; Boettcher, S.; Issa, G.C.; et al. Polyploidy Is Necessary for Apoptotic Cell Death in TP53-Mut AML in Response to Polo-like-Kinase 4 (PLK4) Inhibition and Results in Caspase 3 Cleavage. Blood 2022, 140, 5941–5943. [Google Scholar] [CrossRef]
- Murphy, T.; Mason, J.M.; Leber, B.; Bray, M.R.; Chan, S.M.; Gupta, V.; Khalaf, D.; Maze, D.; McNamara, C.J.; Schimmer, A.D.; et al. Preclinical Characterization and Clinical Trial of CFI-400945, a Polo-like Kinase 4 Inhibitor, in Patients with Relapsed/Refractory Acute Myeloid Leukemia and Higher-Risk Myelodysplastic Neoplasms. Leukemia 2024, 38, 502–512. [Google Scholar] [CrossRef]
- Treadwell Therapeutics, Inc. Phase 1b/2 Clinical Study of the Safety, Tolerability, and Pharmacokinetic and Pharmacodynamic Profiles of CFI-400945 as a Single Agent or in Combination with Azacitidine in Patients with AML, MDS or CMML. 2025. Available online: https://clinicaltrials.gov/study/NCT04730258 (accessed on 20 September 2025).
- Jonas, B.A.; Yee, K.; Koller, P.B.; Brandwein, J.; Mims, A.S.; Michelson, G.C.; Nguyen, L.; Bray, M.R.; Roberts-Thomson, E.L.; Borthakur, G. Preliminary Results from a Phase 2 Open-Label, Multicenter, Dose Optimization Clinical Study of the Safety, Tolerability, and Pharmacokinetic (PK) and Pharmacodynamic (PD) Profiles of Cfi-400945 As a Single Agent or in Combination with Azacitidine or Decitabine in Patients with Acute Myeloid Leukemia, Myelodysplastic Syndrome or Chronic Myelomonocytic Leukemia (TWT-202). Blood 2022, 140, 9076–9077. [Google Scholar] [CrossRef]
- Song, X.; Peng, Y.; Wang, X.; Chen, Q.; Lan, X.; Shi, F. The Stimulator of Interferon Genes (STING) Agonists for Treating Acute Myeloid Leukemia (AML): Current Knowledge and Future Outlook. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2023, 25, 1545–1553. [Google Scholar] [CrossRef]
- Baba, T.; Yoshida, T.; Tanabe, Y.; Nishimura, T.; Morishita, S.; Gotoh, N.; Hirao, A.; Hanayama, R.; Mukaida, N. Cytoplasmic DNA Accumulation Preferentially Triggers Cell Death of Myeloid Leukemia Cells by Interacting with Intracellular DNA Sensing Pathway. Cell Death Dis. 2021, 12, 322. [Google Scholar] [CrossRef]
- Adam, M.; Yu, J.; Plant, R.; Shelton, C.; Schmidt, H.; Yang, J. Sting Agonist GSK3745417 Induces Apoptosis, Antiproliferation, and Cell Death in a Panel of Human AML Cell Lines and Patient Samples. Blood 2022, 140, 11829. [Google Scholar] [CrossRef]
- Montesinos, P.; Al-Ali, H.; Alonso-Dominguez, J.M.; Jentzsch, M.; Jongen-Lavrencic, M.; Martelli, M.P.; Röllig, C.; Sica, S.; Iadevaia, R.; Yablonski, K.; et al. Abstract CT124: A First-in-Clinic Phase 1 Study of GSK3745417 STING Agonist in Relapsed/Refractory Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Cancer Res. 2023, 83, CT124. [Google Scholar] [CrossRef]
- GlaxoSmithKline. A Phase 1, Open Label Study of Intravenous GSK3745417 to Evaluate Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Determine RP2D and Schedule in Participants with Relapsed or Refractory Myeloid Malignancies Including Acute Myeloid Leukemia (AML) and High-Risk Myelodysplastic Syndrome (HR-MDS). 2025. Available online: https://clinicaltrials.gov/study/NCT05424380 (accessed on 20 September 2025).
- Baer, M. Phase 1 Clinical Trial of the STING Agonist CRD3874-SI in Patients with Relapsed/Refractory Acute Myeloid Leukemia. 2024. Available online: https://clinicaltrials.gov/study/NCT06626633 (accessed on 20 September 2025).
- Brem, E.A.; Shieh, K.; Juarez, D.; Buono, R.; Jeyakumar, D.; O’Brien, S.; Taylor, T.H.; Fruman, D.A. A Phase 1 Study Adding Pitavastatin to Venetoclax Therapy in AML and CLL/SLL: A Mechanism-Based Drug Repurposing Strategy. Blood Neoplasia 2024, 1, 100036. [Google Scholar] [CrossRef]
- Maslah, N.; Rety, S.; Bonnamy, M.; Aguinaga, L.; Huynh, T.; Parietti, V.; Giraudier, S.; Fenaux, P.; Cassinat, B. Niclosamide Combined to Azacitidine to Target TP53-Mutated MDS/AML Cells. Leukemia 2024, 38, 1630–1633. [Google Scholar] [CrossRef] [PubMed]

| Type of Aberration | Chromosomes Involved | Notes | Reference |
|---|---|---|---|
| Chromosomal loss | 5 (del5q), 7, 12, 16, 17 (del17p), 18, and 20q | Recurrent abnormalities in TP53-mutated MDS/AML | [6,7] |
| Chromosomal gain | 21, 22, 1p, and 8 | [7] | |
| Chromothripsis | 5, 17, and 21 | 35% in TP53-mutated MNs with CK | [7,19] |
| Complex karyotype | >3 concurrent cytogenetic abnormalities | 84% of patients with TP53-mutated AML/MDS-EB | [6] |
| Study | Intervention | Outcome |
|---|---|---|
| Baranwal, et al. [70] | Allo-BMT in patients with TP53-mutated MNs | Median survival: 1.03 year OS at 3 years: 25.1% |
| Shahzad, et al. [71] | Systematic review and meta-analysis of 8 studies investigating allo-BMT in TP53-mutated AML | Pooled OS: 21% (median follow-up at 3 years) Pooled RR: 58.9% (at a median of 1.75 years) |
| Pasca, et al. [74] | Allo-BMT vs. no allo-BMT in patients with TP53-mutated MNs | Median survival 18.9 months vs. 4.1 months, respectively |
| Senapati, et al. [78] | Allo-BMT vs. no allo-BMT in patients with TP53-mutated AML | Median OS: 13.6 vs. 7.6 months, respectively, Median RFS: 9.3 vs. 4.5 months, respectively |
| Intervention | Phase | Outcome | Trial Identifier |
|---|---|---|---|
| Eprenetapopt (APR-246) combined with venetoclax and azacitidine for the treatment of TP53-mutated MNs | I | ORR: 64%, CR: 38% | NCT04214860 |
| Eprenetapopt (APR-246) ± azacitidine for the treatment of TP53-mutated MDS | III | Not reaching primary endpoint (12-month CR rate 35 vs. 22% with azacitidine alone) | NCT03745716 |
| Magrolimab vs. placebo with azacitidine and venetoclax for patients with untreated AML unfit for intensive therapy (ENHANCE-3 trial, discontinued) | III | Median OS 7.4 months vs. 6.9 months, respectively in the lower-benefit group (including TP53-mutated AML patients) | NCT05079230 |
| Flotetuzumab in Primary Induction Failure (PIF) or Early Relapse (ER) AML (VOYAGE study) | I/II | CR rate: 47% among patients with TP53-mutated R/R AML, median OS: 10.3 months in responding patients | NCT02152956 |
| Tagraxofusp combined with azacitidine ± venetoclax in AML | Ib | 54% of patients with TP53 mutations achieved CR/CRi/MLFS | NCT03113643 |
| Sabatolimab combined with azacitidine or decitabine in patients with HR/vHR-MDS and AML | Ib | ORR: 71.4%, mDOR: 21.5 months in patients with HR/vHR-MDS and TP53 mutations | NCT03066648 |
| Intervention | Mechanism of Action | Phase/Status | Trial Identifier |
|---|---|---|---|
| Entrectinib combined with ASTX727 (decitabine and cedazuridine) for the treatment of TP53-mutated R/R AML | NTRK/ALK/ROS1 inhibitor | I—active, not recruiting | NCT05396859 |
| CFI-400945 ± azacitidine in patients with AML, MDS or CMML | PLK4 inhibitor | Ib/II—active, not recruiting | NCT04730258 |
| CRD3874-SI in patients with R/R AML | Synthetic STING agonist | I—active, not recruiting | NCT06626633 |
| Oral-ATO combined with ascorbic acid and investigator choice of low-intensity therapy (HMAs ± venetoclax) for previously untreated or R/R TP53-mutated MDS, AML, or CMML | Mutant p53 reactivation (ATO) | I—active, currently recruiting | NCT06778187 |
| SL-401 (Tagraxofusp) combined with azacitidine ± venetoclax in patients with AML, high-risk MDS, or BPDCN | Recombinant IL-3 protein fused to a truncated diphtheria toxin payload | I—active, currently recruiting | NCT03113643 |
| APVO436 combined with azacitidine and venetoclax in patients with newly diagnosed AML | CD123 × CD3 bi-specific antibody | Ib/II—active, currently recruiting | NCT06634394 |
| AK117 (Ligufalimab) or placebo combined with azacitidine in patients with newly diagnosed high-risk MDS | Next-generation CD47 blocker | II—active, currently recruiting | NCT06196203 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgantzinos, E.; Karantanos, T. Molecular and Clinical Insights into TP53-Mutated MDS and AML. Int. J. Mol. Sci. 2025, 26, 10818. https://doi.org/10.3390/ijms262210818
Georgantzinos E, Karantanos T. Molecular and Clinical Insights into TP53-Mutated MDS and AML. International Journal of Molecular Sciences. 2025; 26(22):10818. https://doi.org/10.3390/ijms262210818
Chicago/Turabian StyleGeorgantzinos, Erotokritos, and Theodoros Karantanos. 2025. "Molecular and Clinical Insights into TP53-Mutated MDS and AML" International Journal of Molecular Sciences 26, no. 22: 10818. https://doi.org/10.3390/ijms262210818
APA StyleGeorgantzinos, E., & Karantanos, T. (2025). Molecular and Clinical Insights into TP53-Mutated MDS and AML. International Journal of Molecular Sciences, 26(22), 10818. https://doi.org/10.3390/ijms262210818








