A Review of Formulation Strategies for Cyclodextrin-Enhanced Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
Abstract
1. Introduction
2. Fundamentals of Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)
2.1. Solid Lipid Nanoparticles (SLNs)
- Biocompatibility and biodegradability;
- Protection of encapsulated drugs from degradation;
- Avoidance of burst release associated with some other nanocarriers;
- Ease of surface modification for targeted delivery.
2.2. Nanostructured Lipid Carriers (NLCs)
- Higher drug loading capacity;
- Improved long-term physical stability;
- Reduced crystallinity and lower risk of polymorphic transitions;
- Greater flexibility in drug release kinetics.
2.3. Preparation Methods
2.3.1. Hot High-Pressure Homogenization (Hot HPH)
2.3.2. Cold High-Pressure Homogenization (Cold HPH)
2.3.3. Microemulsion Technique
2.3.4. Emulsification–Solvent Evaporation
2.3.5. Emulsification–Solvent Diffusion
2.3.6. Solvent Injection (Nanoprecipitation)
2.3.7. Coacervation (Fatty Acid Coacervation)
2.3.8. Membrane Contactor Method
2.3.9. Ultrasonication (High-Shear Homogenization)
2.3.10. Spray Drying
2.3.11. Supercritical Fluid Techniques
2.3.12. Phase Inversion Temperature (PIT) Method
2.3.13. Double Emulsion (W/O/W)
2.3.14. Electrospray (Electrohydrodynamic Atomization)
2.3.15. Microfluidic Mixing
2.3.16. Microfluidization
2.3.17. Spontaneous Emulsification
2.3.18. High-Speed Homogenization
2.3.19. Microwave-Assisted Synthesis (MAS)
2.3.20. Ultrasound-Assisted Synthesis (UAS)
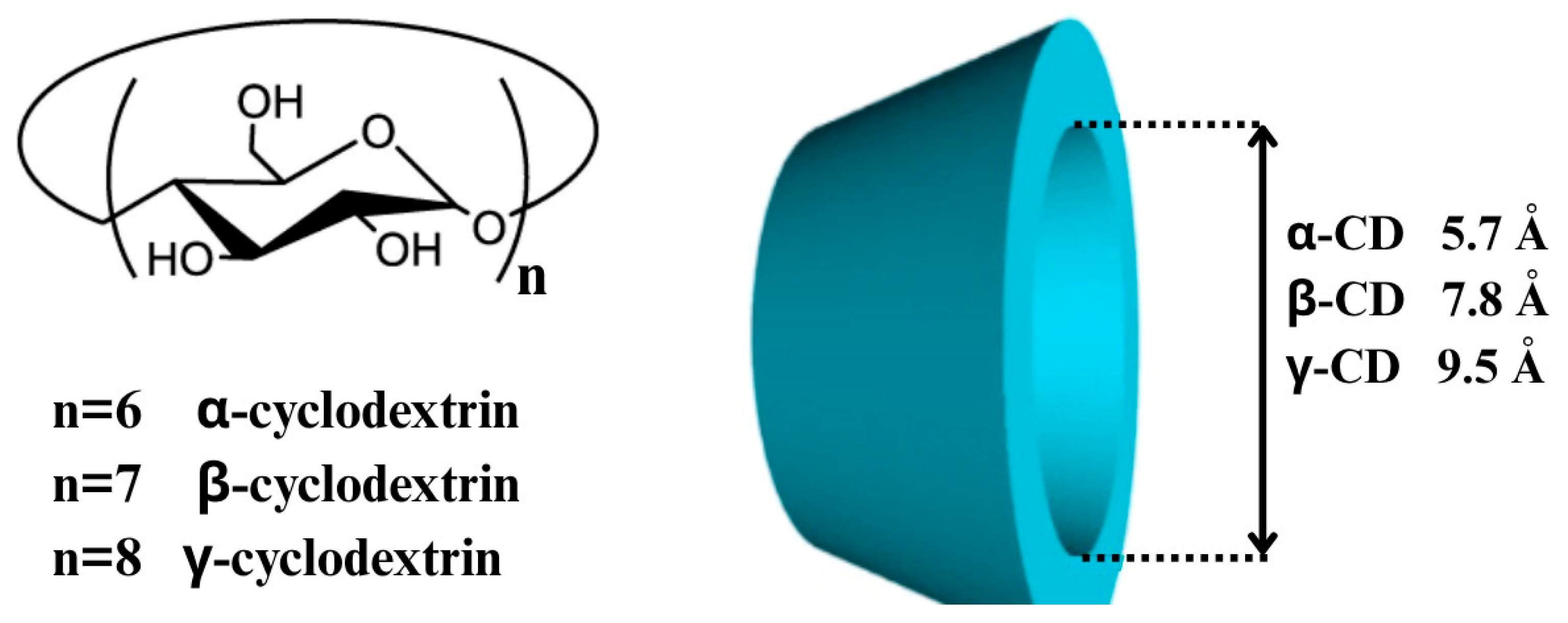
3. Cyclodextrins: Properties and Pharmaceutical Relevance
3.1. Pharmaceutical Utility
3.2. Modified Cyclodextrins and Regulatory Status
3.3. Integration with Nanocarrier Systems
4. Formulation Strategies for Cyclodextrin-Modified SLNs and NLCs
4.1. Pre-Formulation Studies
- Phase solubility behavior of the drug with various CDs to determine complexation efficiency and stability constants [112];
- Thermal stability of the CD–drug complex using Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA);
- Compatibility with lipids through miscibility studies;
- Complex characterization via FT-IR, X-ray Diffraction (XRD), and NMR to confirm inclusion [113].
4.2. Preparation Methods for Cyclodextrin-Modified SLNs and NLCs
4.2.1. Pre-Complexation Method
4.2.2. Co-Encapsulation Method
4.2.3. Surface Functionalization Method
4.3. Drug Selection Criteria for CD-Modified SLNs and NLCs
5. Characterization Techniques for Cyclodextrin-Modified SLNs and NLCs
5.1. Particle Size and Polydispersity Index (PDI)
5.2. Zeta Potential
- High absolute values (±30 mV or more) indicate good stability [133].
- CD modification may shift the zeta potential depending on the CD type (neutral, cationic, or anionic) and its mode of incorporation (surface adsorption vs. internal complexation) [134].
- For example, anionic derivatives, such as SBE-β-CD, can increase the negative charge of the nanoparticles [135].
5.3. Entrapment Efficiency (EE%) and Drug Loading (DL%)
- EE% = [(Total drug − Free drug)/Total drug] × 100
5.4. Thermal Analysis
5.4.1. Differential Scanning Calorimetry (DSC)
- Disappearance or shift of the drug’s melting peak may indicate successful encapsulation or complexation;
- Reduced enthalpy values suggest a decrease in lipid crystallinity, often enhanced by the presence of CDs [140].
5.4.2. Thermogravimetric Analysis (TGA)
5.5. Crystallinity and Structural Analysis
5.5.1. X-Ray Diffraction (XRD)
5.5.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
- Shifts in characteristic peaks (e.g., carbonyl, hydroxyl) indicate hydrogen bonding or hydrophobic interactions.
- Used to confirm inclusion complex formation and detect chemical compatibility [11].
5.5.3. Proton Nuclear Magnetic Resonance (1H-NMR)
5.6. Morphology and Surface Topography
5.7. In Vitro Drug Release Studies
5.8. Stability Studies
6. Therapeutic Applications of Cyclodextrin-Enhanced Lipid Nanoparticles
7. Challenges and Limitations of Cyclodextrin–Lipid Hybrid Systems
7.1. Formulation Complexity
7.2. Drug Loading Efficiency
7.3. Manufacturing and Scale-Up
7.4. Stability Impact of Cyclodextrins
7.5. Toxicological Considerations
7.6. Regulatory Considerations
7.7. Cost and Scalability
8. Future Outlook
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| BCS | Biopharmaceutics Classification System |
| CD | Cyclodextrin |
| SLN | Solid Lipid Nanoparticle |
| NLC | Nanostructured Lipid Carrier |
| FT-IR | Fourier Transform Infrared |
| NMR | Nuclear Magnetic Resonance |
| ZP | Zeta Potential |
| HPH | High-Pressure Homogenization |
| PVA | Polyvinyl Alcohol |
| PIT | Phase Inversion Temperature |
| HLB | Hydrophilic–Lipophilic Balance |
| MAS | Microwave-Assisted Synthesis |
| UAS | Ultrasound-Assisted Synthesis |
| SBE-β-CD | Sulfobutylether-β-Cyclodextrin |
| HP-β-CD | Hydroxypropyl-β-Cyclodextrin |
| HP-γ-CD | Hydroxypropyl-γ-Cyclodextrin |
| RAMEB | Randomly Methylated β-Cyclodextrin |
| DIMEB | 2,6-Di-O-methyl-β-Cyclodextrin |
| IV | Intravenous |
| DSC | Differential Scanning Calorimetry |
| TGA | Thermogravimetric Analysis |
| XRD | X-ray Diffraction |
| EE | Encapsulation Efficiency |
| EEn | Emulsification Energy |
| PSD | Particle Size Distribution |
| MW | Molecular Weight |
| PDI | Polydispersity Index |
| DLS | Dynamic Light Scattering |
| DL | Drug Loading |
| UV–VIS | Ultraviolet–Visible |
| HPLC | High-Performance Liquid Chromatography |
| USP | United States Pharmacopeia |
| UWL | Unstirred Water Layer |
| FDA | Food and Drug Administration |
| EMA | European Medicines Agency |
| CMC | Chemistry, Manufacturing, and Controls |
| GRAS | Generally Recognized as Safe |
| AI | Artificial Intelligence |
| SEM | Scanning Electron Microscopy |
| TEM | Transmission Electron Microscopy |
References
- Pouton, C.W. Formulation of poorly water-soluble drugs for oral administration: Physicochemical and physiological issues and the lipid formulation classification system. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.; Mithu, M.S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86, 104709. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef]
- Viegas, C.; Patrício, A.B.; Prata, J.M.; Nadhman, A.; Chintamaneni, P.K.; Fonte, P. Solid Lipid Nanoparticles vs. Nanostructured Lipid Carriers: A Comparative Review. Pharmaceutics 2023, 15, 1593. [Google Scholar] [CrossRef]
- Westesen, K.; Bunjes, H.; Koch, M.H.J. Physicochemical characterization of lipid nanoparticles and evaluation of their drug loading capacity and sustained release potential. J. Control. Release 1997, 48, 223–236. [Google Scholar] [CrossRef]
- Tamjidi, F.; Shahedi, M.; Varshosaz, J.; Nasirpour, A. Nanostructured lipid carriers (NLC): A potential delivery system for bioactive food molecules. Innov. Food Sci. Emerg. Technol. 2013, 19, 29–43. [Google Scholar] [CrossRef]
- Song, A.; Zhang, X.; Li, Y.; Mao, X.; Han, F. Effect of Liquid-to-Solid Lipid Ratio on Characterizations of Flurbiprofen-Loaded Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) for Transdermal Administration. Drug Dev. Ind. Pharm. 2016, 42, 1308–1314. [Google Scholar] [CrossRef]
- Cirri, M.; Bragagni, M.; Mennini, N.; Mura, P. Development of a new delivery system consisting in “drug–in cyclodextrin–in nanostructured lipid carriers” for ketoprofen topical delivery. Eur. J. Pharm. Biopharm. 2012, 80, 46–53. [Google Scholar] [CrossRef]
- Renuka, M.T.; Ganesh, N.S.; Gopinath, E.; Ranjitha, K.S.; Chandy, V. Nanostructured Lipid Carriers, Novel Approach for Drug Delivery: A Comprehensive Review. Int. J. Pharm. Pharm. Res. 2021, 20, 319–338. [Google Scholar]
- Alloush, T.; Yurtdaş Kırımlıoğlu, G. Development of Vaginal In Situ Gel Containing ISN/HP-β-CD Inclusion Complex for Enhanced Solubility and Antifungal Efficacy. Polymers 2025, 17, 514. [Google Scholar] [CrossRef] [PubMed]
- Kommineni, N.; Butreddy, A.; Sainaga Jyothi, V.G.S.; Angsantikul, P. Freeze-drying for the preservation of immunoengineering products. iScience 2022, 25, 105127. [Google Scholar] [CrossRef] [PubMed]
- Păduraru, L.; Panainte, A.-D.; Peptu, C.-A.; Apostu, M.; Vieriu, M.; Bibire, T.; Sava, A.; Bibire, N. Smart Drug Delivery Systems Based on Cyclodextrins and Chitosan for Cancer Therapy. Pharmaceuticals 2025, 18, 564. [Google Scholar] [CrossRef]
- Pires, F.Q.; da Silva, J.K.R.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. Lipid nanoparticles as carriers of cyclodextrin inclusion complexes: A promising approach for cutaneous delivery of a volatile essential oil. Colloids Surf. B Biointerfaces 2019, 182, 110382. [Google Scholar] [CrossRef]
- Khan, M.I.; Hossain, M.I.; Hossain, M.K.; Rubel, M.H.K.; Hossain, K.M.; Mahfuz, A.M.U.B.; Anik, M.I. Recent Progress in Nanostructured Smart Drug Delivery Systems for Cancer Therapy: A Review. ACS Appl. Bio Mater. 2022, 5, 971–1012. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.H. Effective Delivery Routes and Strategies for Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC). Curr. Pharm. Des. 2017, 23, 6592–6601. [Google Scholar] [CrossRef]
- Battaglia, L.; Gallarate, M. Lipid nanoparticles: State of the art, new preparation methods and challenges in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 497–508. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Bochicchio, S.; Lamberti, G.; Barba, A.A. Polymer–Lipid Pharmaceutical Nanocarriers: Innovations by New Formulations and Production Technologies. Pharmaceutics 2021, 13, 198. [Google Scholar] [CrossRef]
- Parhi, R.; Suresh, P. Preparation and Characterization of Solid Lipid Nanoparticles—A Review. Curr. Drug Discov. Technol. 2012, 9, 2–16. [Google Scholar] [CrossRef]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Müller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Mishra, V.; Bansal, K.K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J.M. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.; Park, J.W.; Yoon, I.-S. Solid lipid nanoparticles (SLNs) and nanostructured lipid carriers (NLCs): Recent advances in drug delivery. J. Pharm. Investig. 2013, 43, 353–362. [Google Scholar] [CrossRef]
- Makoni, P.A.; Wa Kasongo, K.; Walker, R.B. Short Term Stability Testing of Efavirenz-Loaded Solid Lipid Nanoparticle (SLN) and Nanostructured Lipid Carrier (NLC) Dispersions. Pharmaceutics 2019, 11, 397. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Tang, C.-H.; Chen, H.-L.; Dong, J.-R. Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs) as Food-Grade Nanovehicles for Hydrophobic Nutraceuticals or Bioactives. Appl. Sci. 2023, 13, 1726. [Google Scholar] [CrossRef]
- Rajoriya, V.; Gupta, R.; Vengurlekar, S.; Singh, U.S. Nanostructured lipid carriers (NLCs): A promising candidate for lung cancer targeting. Int. J. Pharm. 2024, 655, 123986. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.d.C.V.; Muehlmann, L.A. Characteristics and Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. J. Nanotheranostics 2024, 5, 188–211. [Google Scholar] [CrossRef]
- Pandey, S.; Shaikh, F.; Gupta, A.; Tripathi, P.; Yadav, J.S. A Recent Update: Solid Lipid Nanoparticles for Effective Drug Delivery. Adv. Pharm. Bull. 2022, 12, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar] [CrossRef]
- Colaco, V.; Roy, A.A.; Naik, G.A.R.R.; Mondal, A.; Mutalik, S.; Dhas, N. Advancement in Lipid-Based Nanocomposites for Theranostic Applications in Lung Carcinoma Treatment. OpenNano 2024, 15, 100199. [Google Scholar] [CrossRef]
- Khan, S.; Sharma, A.; Jain, V. An Overview of Nanostructured Lipid Carriers and Its Application in Drug Delivery through Different Routes. Adv. Pharm. Bull. 2023, 13, 446–460. [Google Scholar] [CrossRef]
- Kimura, N.; Maeki, M.; Sato, Y.; Ishida, A.; Tani, H.; Harashima, H.; Tokeshi, M. Development of a Microfluidic-Based Post-Treatment Process for Size-Controlled Lipid Nanoparticles and Application to siRNA Delivery. ACS Appl. Mater. Interfaces 2020, 12, 34011–34020. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Xia, D.; Shrestha, N.; van de Streek, J.; Mu, H.; Yang, M. Spray Drying of Fenofibrate Loaded Nanostructured Lipid Carriers. Asian J. Pharm. Sci. 2016, 11, 507–515. [Google Scholar] [CrossRef]
- Izquierdo, P.; Esquena, J.; Tadros, T.F.; Dederen, C.; Garcia, M.J.; Azemar, N.; Solans, C. Formation and Stability of Nano-Emulsions Prepared Using the Phase Inversion Temperature Method. Langmuir 2002, 18, 26–30. [Google Scholar] [CrossRef]
- Roger, K.; Cabane, B.; Olsson, U. Formation of 10–100 nm Size-Controlled Emulsions through a Sub-PIT Cycle. Langmuir 2010, 26, 3860–3867. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Sarpietro, M.G.; Ottimo, S.; Puglisi, G.; Castelli, F. Differential Scanning Calorimetry Studies on Sunscreen Loaded Solid Lipid Nanoparticles Prepared by the Phase Inversion Temperature Method. Int. J. Pharm. 2011, 415, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, L.; Sinico, C.; Castangia, I.; Carbone, C.; Puglisi, G. Idebenone-Loaded Solid Lipid Nanoparticles for Drug Delivery to the Skin: In Vitro Evaluation. Int. J. Pharm. 2012, 434, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Singh, S.K. Preparation and Characterization of Solid Lipid Nanoparticles of Furosemide Using Quality by Design. Part. Sci. Technol. 2018, 36, 695–709. [Google Scholar] [CrossRef]
- Gao, S.; McClements, D.J. Formation and Stability of Solid Lipid Nanoparticles Fabricated Using Phase Inversion Temperature Method. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 79–87. [Google Scholar] [CrossRef]
- Shinde, U.A.; Parmar, S.J.; Easwaran, S. Metronidazole-Loaded Nanostructured Lipid Carriers to Improve Skin Deposition and Retention in the Treatment of Rosacea. Drug Dev. Ind. Pharm. 2019, 45, 1039–1051. [Google Scholar] [CrossRef]
- Gomes, G.V.L.; Sola, M.R.; Rochetti, A.L.; Fukumasu, H.; Vicente, A.A.; Pinho, S.C. β-Carotene and α-Tocopherol Coencapsulated in Nanostructured Lipid Carriers of Murumuru (Astrocaryum murumuru) Butter Produced by Phase Inversion Temperature Method: Characterisation, Dynamic In Vitro Digestion and Cell Viability Study. J. Microencapsul. 2019, 36, 43–52. [Google Scholar] [CrossRef]
- Carbone, C.; Tomasello, B.; Ruozi, B.; Renis, M.; Puglisi, G. Preparation and Optimization of PIT Solid Lipid Nanoparticles via Statistical Factorial Design. Eur. J. Med. Chem. 2012, 49, 110–117. [Google Scholar] [CrossRef]
- Izquierdo, P.; Feng, J.; Esquena, J.; Tadros, T.F.; Dederen, J.C.; Garcia, M.J.; Azemar, N.; Solans, C. The Influence of Surfactant Mixing Ratio on Nano-Emulsion Formation by the PIT Method. J. Colloid Interface Sci. 2005, 285, 388–394. [Google Scholar] [CrossRef]
- García-Fuentes, M.; Torres, D.; Alonso, M.J. Design of Lipid Nanoparticles for the Oral Delivery of Hydrophilic Macromolecules. Colloids Surf. B Biointerfaces 2003, 27, 159–168. [Google Scholar] [CrossRef]
- Singh, S.; Dobhal, A.K.; Jain, A.; Pandit, J.K.; Chakraborty, S. Formulation and Evaluation of Solid Lipid Nanoparticles of a Water Soluble Drug: Zidovudine. Chem. Pharm. Bull. 2010, 58, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Nogueira, T.; Gehm, C.; Ferreira, D.; Sarmento, B. Chitosan-Coated Solid Lipid Nanoparticles Enhance the Oral Absorption of Insulin. Drug Deliv. Transl. Res. 2011, 1, 299–308. [Google Scholar] [CrossRef]
- Mazur, K.L.; Feuser, P.E.; Valério, A.; Poester Cordeiro, A.; Indiani de Oliveira, C.; Assolini, J.P.; Pavanelli, W.R.; Sayer, C.; Araújo, P.H.H. Diethyldithiocarbamate Loaded in Beeswax-Copaiba Oil Nanoparticles Obtained by Solventless Double Emulsion Technique Promote Promastigote Death In Vitro. Colloids Surf. B Biointerfaces 2019, 176, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Nabi-Meibodi, M.; Vatanara, A.; Rouholamini Najafabadi, A.; Rouini, M.R.; Ramezani, V.; Gilani, K.; Etemadzadeh, S.M.H.; Azadmanesh, K. The Effective Encapsulation of a Hydrophobic Lipid-Insoluble Drug in Solid Lipid Nanoparticles Using a Modified Double Emulsion Solvent Evaporation Method. Colloids Surf. B Biointerfaces 2013, 112, 408–414. [Google Scholar] [CrossRef]
- Severino, P.; Silveira, E.F.; Loureiro, K.; Chaud, M.V.; Antonini, D.; Lancellotti, M.; Sarmento, V.H.; da Silva, C.F.; Santana, M.H.A.; Souto, E.B. Antimicrobial Activity of Polymyxin-Loaded Solid Lipid Nanoparticles (PLX-SLN): Characterization of Physicochemical Properties and In Vitro Efficacy. Eur. J. Pharm. Sci. 2017, 106, 177–184. [Google Scholar] [CrossRef]
- Patel, P.R.; Haemmerich, D. Review on Electrospray Nanoparticles for Drug Delivery: Exploring Applications. Polym. Adv. Technol. 2024, 35, e6507. [Google Scholar] [CrossRef]
- Trotta, M.; Cavalli, R.; Trotta, C.; Bussano, R.; Costa, L. Electrospray Technique for Solid Lipid-Based Particle Production. Drug Dev. Ind. Pharm. 2010, 36, 431–438. [Google Scholar] [CrossRef]
- Lüdtke, F.L.; Silva, T.J.; da Silva, M.G.; Hashimoto, J.C.; Ribeiro, A.P.B. Lipid Nanoparticles: Formulation, Production Methods and Characterization Protocols. Foods 2025, 14, 973. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, C.; Liu, X.; Mackie, A.; Zhang, M.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Co-Encapsulation of Curcumin and β-Carotene in Pickering Emulsions Stabilized by Complex Nanoparticles: Effects of Microfluidization and Thermal Treatment. Food Hydrocoll. 2022, 122, 107064. [Google Scholar] [CrossRef]
- Shirvani, A.; Goli, S.A.H.; Varshosaz, J.; Salvia-Trujillo, L.; Martín-Belloso, O. Fabrication of Edible Solid Lipid Nanoparticle from Beeswax/Propolis Wax by Spontaneous Emulsification: Optimization, Characterization and Stability. Food Chem. 2022, 387, 132934. [Google Scholar] [CrossRef]
- Frohlich, J.K.; Meyer, P.A.; Stein, T.; Tonussi, C.R.; Lemos-Senna, E. Development and In Vivo Evaluation of Lipid-Based Nanocarriers Containing Jatropha isabellei Dry Extract from the Dichloromethane Fraction Intended for Oral Treatment of Arthritic Diseases. Braz. J. Pharm. Sci. 2022, 58, e19178. [Google Scholar] [CrossRef]
- Shah, R.M.; Malherbe, F.; Eldridge, D.; Palombo, E.A.; Harding, I.H. Physicochemical Characterization of Solid Lipid Nanoparticles (SLNs) Prepared by a Novel Microemulsion Technique. J. Colloid Interface Sci. 2014, 428, 286–294. [Google Scholar] [CrossRef] [PubMed]
- López, K.L.; Ravasio, A.; González-Aramundiz, J.V.; Zacconi, F.C. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) Prepared by Microwave and Ultrasound-Assisted Synthesis: Promising Green Strategies for the Nanoworld. Pharmaceutics 2023, 15, 1333. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B. Recent Developments on Ultrasound-Assisted One-Pot Multicomponent Synthesis of Biologically Relevant Heterocycles. Ultrason. Sonochem. 2017, 35, 15–35. [Google Scholar] [CrossRef]
- Rivero-Barbarroja, G.; Benito, J.M.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-Based Functional Glyconanomaterials. Nanomaterials 2020, 10, 2517. [Google Scholar] [CrossRef]
- Li, X.; Jin, Z.; Bai, Y.; Svensson, B. Progress in cyclodextrins as important molecules regulating catalytic processes of glycoside hydrolases. Biotechnol. Adv. 2024, 72, 108326. [Google Scholar] [CrossRef]
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2022, 3, 1–31. [Google Scholar] [CrossRef]
- Abdellatif, A.A.H.; Ahmed, F.; Mohammed, A.M.; Alsharidah, M.; Al-Subaiyel, A.; Samman, W.A.; Alhaddad, A.A.; Al-Mijalli, S.H.; Amin, M.A.; Barakat, H.; et al. Recent Advances in the Pharmaceutical and Biomedical Applications of Cyclodextrin-Capped Gold Nanoparticles. Int. J. Nanomed. 2023, 18, 3247–3281. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; de Rossi, R.H. Complex systems that incorporate cyclodextrins to get materials for some specific applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. [Google Scholar] [CrossRef]
- D’Aria, F.; Pagano, B.; Giancola, C. Thermodynamic properties of hydroxypropyl-β-cyclodextrin/guest interaction: A survey of recent studies. J. Therm. Anal. Calorim. 2022, 147, 4889–4897. [Google Scholar] [CrossRef]
- Alloush, T.; Yurtdaş Kırımlıoğlu, G. Enhancing the Solubility of Isoconazole Nitrate Using Methyl-β-Cyclodextrin: Formulation and Characterization of Inclusion Complexes. Molecules 2025, 30, 1654. [Google Scholar] [CrossRef] [PubMed]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, formation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Boczar, D.; Michalska, K. Cyclodextrin Inclusion Complexes with Antibiotics and Antibacterial Agents as Drug-Delivery Systems—A Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1389. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M.V. Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, S.; Pan, Y.; Qu, W.; Tao, Y.; Chen, D.; Huang, L.; Liu, Z.; Wang, Y.; Yuan, Z. Preparation, characterization and pharmacokinetics of doxycycline hydrochloride and florfenicol polyvinylpyrrolidone microparticle entrapped with hydroxypropyl-β-cyclodextrin inclusion complexes suspension. Colloids Surf. B Biointerfaces 2016, 141, 634–642. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, S.; Li, Z.; Gu, Z.; Xu, S.; Ban, X.; Hong, Y.; Cheng, L.; Li, C. A review of controlled release from cyclodextrins: Release methods, release systems and application. Crit. Rev. Food Sci. Nutr. 2023, 63, 4744–4756. [Google Scholar] [CrossRef]
- Loftsson, T.; Vogensen, S.B.; Brewster, M.E.; Konráðsdóttir, F. Effects of cyclodextrins on drug delivery through biological membranes. J. Pharm. Sci. 2007, 96, 2532–2546. [Google Scholar] [CrossRef]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Sousa Lobo, J.M. Cyclodextrins as drug carriers in pharmaceutical technology: The state of the art. Curr. Pharm. Des. 2018, 24, 1405–1433. [Google Scholar] [CrossRef]
- Azman, M.; Sabri, A.H.; Anjani, Q.K.; Mustaffa, M.F.; Hamid, K.A. Intestinal Absorption Study: Challenges and Absorption Enhancement Strategies in Improving Oral Drug Delivery. Pharmaceuticals 2022, 15, 975. [Google Scholar] [CrossRef]
- Ferreira, L.; Campos, J.; Veiga, F.; Cardoso, C.; Paiva-Santos, A.C. Cyclodextrin-based delivery systems in parenteral formulations: A critical update review. Eur. J. Pharm. Biopharm. 2022, 178, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Fang, L. Biomaterials as novel penetration enhancers for transdermal and dermal drug delivery systems. Drug Deliv. 2013, 20, 199–209. [Google Scholar] [CrossRef]
- Santos, A.C.; Costa, D.; Ferreira, L.; Guerra, C.; Pereira-Silva, M.; Pereira, I.; Peixoto, D.; Ferreira, N.R.; Veiga, F. Cyclodextrin-Based Delivery Systems for In Vivo-Tested Anticancer Therapies. Drug Deliv. Transl. Res. 2021, 11, 49–71. [Google Scholar] [CrossRef]
- Cunha-Filho, M.S.S.; Dacunha-Marinho, B.; Torres-Labandeira, J.J.; Martínez-Pacheco, R.; Landin, M. Characterization of β-Lapachone and Methylated β-Cyclodextrin Solid-State Systems. AAPS PharmSciTech 2007, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y. Raloxifene/SBE-β-CD Inclusion Complexes Formulated into Nanoparticles with Chitosan to Overcome the Absorption Barrier for Bioavailability Enhancement. Pharmaceutics 2018, 10, 76. [Google Scholar] [CrossRef]
- Groeneboer, S.; Lambrecht, S.; Dhollander, A.; Jacques, P.; Vander Cruyssen, B.; Lories, R.J.; Devreese, K.; Chiers, K.; Elewaut, D.; Verbruggen, G. Optimized Alkylated Cyclodextrin Polysulphates with Reduced Risks on Thromboembolic Accidents Improve Osteoarthritic Chondrocyte Metabolism. Rheumatology 2011, 50, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Grecu, M.; Minea, B.; Foia, L.-G.; Bostanaru-Iliescu, A.-C.; Miron, L.; Nastasa, V.; Mares, M. Short Review on the Biological Activity of Cyclodextrin-Drug Inclusion Complexes Applicable in Veterinary Therapy. Molecules 2023, 28, 5565. [Google Scholar] [CrossRef]
- Mermelstein, F.; Hamilton, D.A.; Wright, C.; Lacouture, P.G.; Ramaiya, A.; Carr, D.B. Single-Dose and Multiple-Dose Pharmacokinetics and Dose Proportionality of Intravenous and Intramuscular HPβCD-Diclofenac (Dyloject) Compared with Other Diclofenac Formulations. Pharmacotherapy 2013, 33, 1012–1021. [Google Scholar] [CrossRef]
- Giri, B.R.; Lee, J.; Lim, D.Y.; Kim, D.W. Docetaxel/Dimethyl-β-Cyclodextrin Inclusion Complexes: Preparation, In Vitro Evaluation and Physicochemical Characterization. Drug Dev. Ind. Pharm. 2021, 47, 319–328. [Google Scholar] [CrossRef]
- Elder, D.P.; Kuentz, M.; Holm, R. Pharmaceutical Excipients—Quality, Regulatory and Biopharmaceutical Considerations. Eur. J. Pharm. Sci. 2016, 87, 88–99. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and Their Pharmaceutical Applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Wu, J.; Liu, X.; Wang, S.; Liu, Y.; Li, H.; Wang, H. Inclusion Complex of Quercetin with Sulfobutylether β-Cyclodextrin: Preparation, Characterization, Antioxidant and Antibacterial Activities and the Inclusion Mechanism. RSC Adv. 2024, 14, 9472–9481. [Google Scholar] [CrossRef]
- Saokham, P.; Loftsson, T. γ-Cyclodextrin. Int. J. Pharm. 2017, 516, 278–292. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. Biomed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S.; El-Saleh, F.; Lysenko, K.; Paz, F.A.A. Inclusion Compound of Efavirenz and γ-Cyclodextrin: Solid State Studies and Effect on Solubility. Molecules 2021, 26, 519. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.S. Molecular Mind Games: The Medicinal Action of Cyclodextrins in Neurodegenerative Diseases. Biomolecules 2023, 13, 666. [Google Scholar] [CrossRef]
- Ignaczak, A.; Orszański, Ł. In Search of the Most Stable Molecular Configuration of Heptakis(2,6-O-dimethyl)-β-Cyclodextrin and Its Complex with Mianserin: A Comparison of the B3LYP-GD2 and M062X-GD3 Results. J. Phys. Chem. B 2021, 125, 13077–13087. [Google Scholar] [CrossRef]
- Szente, L.; Tuza, K.; Herr, D.M.; Varga, E.; Szőcs, L.; Fenyvesi, É.; Puskás, I. Rapid Test for Identification of Heptakis(2,6-di-O-methyl) β-Cyclodextrin in Isomeric Mixtures. J. Incl. Phenom. Macrocycl. Chem. 2025, 105, 304. [Google Scholar] [CrossRef]
- Shelley, H.; Jayachandra Babu, R. Role of Cyclodextrins in Nanoparticle-Based Drug Delivery Systems. J. Pharm. Sci. 2018, 107, 1741–1753. [Google Scholar] [CrossRef]
- Dudhipala, N.; Ettireddy, S.; Youssef, A.A.A.; Puchchakayala, G. Cyclodextrin Complexed Lipid Nanoparticles of Irbesartan for Oral Applications: Design, Development, and In Vitro Characterization. Molecules 2021, 26, 7538. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Mennini, N.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Development and in vivo evaluation of an innovative “Hydrochlorothiazide-in Cyclodextrins-in Solid Lipid Nanoparticles” formulation with sustained release and enhanced oral bioavailability for potential hypertension treatment in pediatrics. Int. J. Pharm. 2017, 521, 73–83. [Google Scholar] [CrossRef]
- Amekyeh, H.; Sabra, R.; Billa, N. A Window for Enhanced Oral Delivery of Therapeutics via Lipid Nanoparticles. Drug Des. Dev. Ther. 2024, 18, 613–630. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin Based Nanoparticles for Drug Delivery and Theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Angi, R.; Kalóczkai, A.J.; Kovács, A.; Marton, A.; Bárdos, V.; Dormán, P.; Katona, G.; Agócs, A.; Csorba, A.; Nagy, Z.Z.; et al. Harnessing Cyclodextrins for Enhanced Ocular Delivery of Carotenoid Derivatives: From Development to ex vivo Characterization. Carbohydr. Polym. Technol. Appl. 2025, 9, 100718. [Google Scholar] [CrossRef]
- Cavalli, R.; Peira, E.; Caputo, O.; Gasco, M.R. Solid Lipid Nanoparticles as Carriers of Hydrocortisone and Progesterone Complexes with β-Cyclodextrins. Int. J. Pharm. 1999, 182, 59–69. [Google Scholar] [CrossRef]
- Parvez, S.; Yadagiri, G.; Gedda, M.R.; Singh, A.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Modified Solid Lipid Nanoparticles Encapsulated with Amphotericin B and Paromomycin: An Effective Oral Combination against Experimental Murine Visceral Leishmaniasis. Sci. Rep. 2020, 10, 12243. [Google Scholar] [CrossRef]
- Amasya, G.; Bakar-Ates, F.; Wintgens, V.; Amiel, C. Layer by Layer Assembly of Core-Corona Structured Solid Lipid Nanoparticles with β-Cyclodextrin Polymers. Int. J. Pharm. 2021, 592, 119994. [Google Scholar] [CrossRef]
- Mennini, N.; Cirri, M.; Maestrelli, F.; Mura, P. Comparison of Liposomal and NLC (Nanostructured Lipid Carrier) Formulations for Improving the Transdermal Delivery of Oxaprozin: Effect of Cyclodextrin Complexation. Int. J. Pharm. 2016, 515, 684–691. [Google Scholar] [CrossRef]
- Real, D.A.; Bolaños, K.; Priotti, J.; Yutronic, N.; Kogan, M.J.; Sierpe, R.; Donoso-González, O. Cyclodextrin-Modified Nanomaterials for Drug Delivery: Classification and Advances in Controlled Release and Bioavailability. Pharmaceutics 2021, 13, 2131. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Pyrak, B.; Rogacka-Pyrak, K.; Gubica, T.; Szeleszczuk, Ł. Exploring Cyclodextrin-Based Nanosponges as Drug Delivery Systems: Understanding the Physicochemical Factors Influencing Drug Loading and Release Kinetics. Int. J. Mol. Sci. 2024, 25, 3527. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Feng, H.; Zeng, R.; Li, S.; Zhang, Q. Macrocycle-Based Supramolecular Drug Delivery Systems: A Concise Review. Molecules 2024, 29, 3828. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Combined Approach of Cyclodextrin Complexationand Nanostructured Lipid Carriers for the Development of a Pediatric Liquid Oral Dosage Form of Hydrochlorothiazide. Pharmaceutics 2018, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, A.; Christman, R.; Kumari, D.; Tiwari, A.; North, E.J.; Chauhan, H. Preparation, Characterization, and In vitro Evaluation of Curcumin- and Resveratrol-Loaded Solid Lipid Nanoparticles. AAPS PharmSciTech 2019, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Permana, A.D.; Sam, A.; Marzaman, A.N.F.; Rahim, A.; Nainu, F.; Bahar, M.A.; Asri, R.M.; Chabib, L. Solid Lipid Nanoparticles Cyclodextrin-Decorated Incorporated into Gellan Gum-Based Dry Floating In Situ Delivery Systems for Controlled Release of Bioactive Compounds of Safflower (Carthamus tinctorius L): A Proof of Concept Study in Biorelevant Media. Int. J. Biol. Macromol. 2023, 237, 124084. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Öztürk, S.C.; Benito, J.M.; Esendağlı, G.; Bilensoy, E. Therapeutic Efficacy and Biodistribution of Paclitaxel-Bound Amphiphilic Cyclodextrin Nanoparticles: Analyses in 3D Tumor Culture and Tumor-Bearing Animals In Vivo. Nanomaterials 2021, 11, 515. [Google Scholar] [CrossRef]
- Grassiri, B.; Cesari, A.; Balzano, F.; Migone, C.; Kali, G.; Bernkop-Schnürch, A.; Uccello-Barretta, G.; Zambito, Y.; Piras, A.M. Thiolated 2-Methyl-β-Cyclodextrin as a Mucoadhesive Excipient for Poorly Soluble Drugs: Synthesis and Characterization. Polymers 2022, 14, 3170. [Google Scholar] [CrossRef]
- Asim, M.H.; Ijaz, M.; Rösch, A.C.; Bernkop-Schnürch, A. Thiolated Cyclodextrins: New Perspectives for Old Excipients. Coord. Chem. Rev. 2020, 420, 213433. [Google Scholar] [CrossRef]
- Baek, J.; So, J.; Shin, S.; Cho, C. Solid Lipid Nanoparticles of Paclitaxel Strengthened by Hydroxypropyl-β-Cyclodextrin as an Oral Delivery System. Int. J. Mol. Med. 2012, 30, 953–959. [Google Scholar] [CrossRef]
- Varan, G.; Varan, C.; Erdoğar, N.; Hıncal, A.A.; Bilensoy, E. Amphiphilic Cyclodextrin Nanoparticles. Int. J. Pharm. 2017, 531, 457–469. [Google Scholar] [CrossRef]
- Ajiboye, A.L.; Nandi, U.; Galli, M.; Trivedi, V. Olanzapine Loaded Nanostructured Lipid Carriers via High Shear Homogenization and Ultrasonication. Sci. Pharm. 2021, 89, 25. [Google Scholar] [CrossRef]
- Siddiqui, A.; Alayoubi, A.; El-Malah, Y.; Nazzal, S. Modeling the Effect of Sonication Parameters on Size and Dispersion Temperature of Solid Lipid Nanoparticles (SLNs) by Response Surface Methodology (RSM). Pharm. Dev. Technol. 2014, 19, 342–346. [Google Scholar] [CrossRef]
- Ji, X.-Y.; Zou, Y.-X.; Lei, H.-F.; Bi, Y.; Yang, R.; Tang, J.-H.; Jin, Q.-R. Advances in Cyclodextrins and Their Derivatives in Nano-Delivery Systems. Pharmaceutics 2024, 16, 1054. [Google Scholar] [CrossRef]
- Banasaz, S.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Encapsulation of Lipid-Soluble Bioactives by Nanoemulsions. Molecules 2020, 25, 3966. [Google Scholar] [CrossRef]
- Velhal, K.; Barage, S.; Roy, A.; Lakkakula, J.; Yamgar, R.; Alqahtani, M.S.; Yadav, K.K.; Ahn, Y.; Jeon, B.-H. A Promising Review on Cyclodextrin Conjugated Paclitaxel Nanoparticles for Cancer Treatment. Polymers 2022, 14, 3162. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Ferris, D.P.; Holcroft, J.M.; Kandela, I.; Stern, C.L.; Nassar, M.S.; Botros, Y.Y.; Stoddart, J.F. Encapsulation of Ibuprofen in CD-MOF and Related Bioavailability Studies. Mol. Pharm. 2017, 14, 1831–1839. [Google Scholar] [CrossRef]
- Loftsson, T.; Sigurdsson, H.H.; Másson, M.; Schipper, N. Preparation of Solid Drug/Cyclodextrin Complexes of Acidic and Basic Drugs. Die Pharm. 2004, 59, 25–29. Available online: https://pubmed.ncbi.nlm.nih.gov/14964417/ (accessed on 3 July 2025).
- Jansook, P.; Fülöp, Z.; Ritthidej, G.C. Amphotericin B Loaded Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carrier (NLCs): Physicochemical and Solid-Solution State Characterizations. Drug Dev. Ind. Pharm. 2019, 45, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Coleman, A.W.; Nicolis, I.; Keller, N.; Dalbiez, J.P. Aggregation of Cyclodextrins: An Explanation of the Abnormal Solubility of β-Cyclodextrin. J. Incl. Phenom. Mol. Recognit. Chem. 1992, 13, 139–143. [Google Scholar] [CrossRef]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Sousa Lobo, J.M.; Silva, A.C. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Dukhin, A.S.; Xu, R. Zeta-Potential Measurements. In Characterization of Nanoparticles; Hodoroaba, V.-D., Unger, W.E.S., Shard, A.G., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 213–224. [Google Scholar] [CrossRef]
- Lv, Z.; Wang, Z.; Wang, H.; Li, J.; Li, K. Adsorption of Cationic/Anionic Dyes and Endocrine Disruptors by Yeast/Cyclodextrin Polymer Composites. RSC Adv. 2024, 14, 6627–6641. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Huang, T.; Yang, Y.; Tu, J.; Zou, J.; Yang, H.; Yang, R. Application of Sodium Sulfobutylether-β-Cyclodextrin Based on Encapsulation. Carbohydr. Polym. 2024, 333, 121985. [Google Scholar] [CrossRef]
- Ghadiri, M.; Fatemi, S.; Vatanara, A.; Doroud, D.; Rouholamini Najafabadi, A.; Darabi, M.; Rahimi, A.A. Loading Hydrophilic Drug in Solid Lipid Media as Nanoparticles: Statistical Modeling of Entrapment Efficiency and Particle Size. Int. J. Pharm. 2012, 424, 128–137. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High Drug-Loading Nanomedicines: Progress, Current Status, and Prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Fornaguera, C.; Solans, C. Analytical Methods to Characterize and Purify Polymeric Nanoparticles. Int. J. Polym. Sci. 2018, 2018, 6387826. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Gonçalves, L.M.D.; Cirri, M.; Mennini, N.; Almeida, A.J. Cyclodextrin Complexation as a Fruitful Strategy for Improving the Performance of Nebivolol Delivery from Solid Lipid Nanoparticles. Int. J. Pharm. 2025, 668, 124972. [Google Scholar] [CrossRef]
- Lin, C.; Chen, F.; Ye, T.; Zhang, L.; Zhang, W.; Liu, D.; Xiong, W.; Yang, X.; Pan, W. A Novel Oral Delivery System Consisting in “Drug-in Cyclodextrin-in Nanostructured Lipid Carriers” for Poorly Water-Soluble Drug: Vinpocetine. Int. J. Pharm. 2014, 465, 90–96. [Google Scholar] [CrossRef]
- Morandeau, A.; Thiéry, M.; Dangla, P. Investigation of the Carbonation Mechanism of CH and C-S-H in Terms of Kinetics, Microstructure Changes and Moisture Properties. Cem. Concr. Res. 2014, 56, 153–170. [Google Scholar] [CrossRef]
- Attama, A.A.; Müller-Goymann, C.C. Effect of Beeswax Modification on the Lipid Matrix and Solid Lipid Nanoparticle Crystallinity. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 189–195. [Google Scholar] [CrossRef]
- Zlotnikov, I.D.; Streltsov, D.A.; Belogurova, N.G.; Kudryashova, E.V. Chitosan or Cyclodextrin Grafted with Oleic Acid Self-Assemble into Stabilized Polymeric Micelles with Potential of Drug Carriers. Life 2023, 13, 446. [Google Scholar] [CrossRef]
- Bednarek, E.; Bocian, W.; Michalska, K. Nuclear Magnetic Resonance Spectroscopic Study of the Inclusion Complex of (R)-Tedizolid with HDAS-β-CD, β-CD, and γ-Cyclodextrin in Aqueous Solution. J. Pharm. Biomed. Anal. 2019, 169, 170–180. [Google Scholar] [CrossRef]
- He, Y.; Fu, P.; Shen, X.; Gao, H. Cyclodextrin-Based Aggregates and Characterization by Microscopy. Micron 2008, 39, 495–516. [Google Scholar] [CrossRef]
- Gómez-Lázaro, L.; Martín-Sabroso, C.; Aparicio-Blanco, J.; Torres-Suárez, A.I. Assessment of In Vitro Release Testing Methods for Colloidal Drug Carriers: The Lack of Standardized Protocols. Pharmaceutics 2024, 16, 103. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. Pharmacokinetic study of solid-lipid-nanoparticles of altretamine complexed epichlorohydrin-β-cyclodextrin for enhanced solubility and oral bioavailability. Int. J. Biol. Macromol. 2017, 101, 24–31. [Google Scholar] [CrossRef]
- AlMajed, Z.; Salkho, N.M.; Sulieman, H.; Husseini, G.A. Modeling of the In Vitro Release Kinetics of Sonosensitive Targeted Liposomes. Biomedicines 2022, 10, 3139. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of Sustained-Action Medication. Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef]
- Liu, M.; Higashi, K.; Ueda, K.; Moribe, K. Supersaturation Maintenance of Carvedilol and Chlorthalidone by Cyclodextrin Derivatives: Pronounced Crystallization Inhibition Ability of Methylated Cyclodextrin. Int. J. Pharm. 2023, 637, 122876. [Google Scholar] [CrossRef]
- Liu, C.-H.; Chiu, H.-C.; Wu, W.-C.; Sahoo, S.L.; Hsu, C.-Y. Novel Lutein Loaded Lipid Nanoparticles on Porcine Corneal Distribution. J. Ophthalmol. 2014, 2014, 304694. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Are Nanostructured Lipid Carriers (NLCs) Better than Solid Lipid Nanoparticles (SLNs): Development, Characterizations and Comparative Evaluations of Clotrimazole-Loaded SLNs and NLCs? Eur. J. Pharm. Sci. 2012, 47, 139–151. [Google Scholar] [CrossRef]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Betamethasone-in-Cyclodextrin-in-Liposome: The Effect of Cyclodextrins on Encapsulation Efficiency and Release Kinetics. Int. J. Pharm. 2006, 312, 75–82. [Google Scholar] [CrossRef]
- Müller, R.H.; Shegokar, R.; Keck, C.M. 20 Years of Lipid Nanoparticles (SLN & NLC): Present State of Development & Industrial Applications. Curr. Drug Discov. Technol. 2011, 8, 207–227. [Google Scholar] [CrossRef]
- Upare, M.M.; Thorawade, K.M.; Mishra, A.P.; Nigam, M.; Waranuch, N. Lipid-Based Nanocarriers in Topical Applications for Skin Infections. Curr. Opin. Pharmacol. 2025, 83, 102541. [Google Scholar] [CrossRef]
- Aiassa, V.; Garnero, C.; Zoppi, A.; Longhi, M.R. Cyclodextrins and Their Derivatives as Drug Stability Modifiers. Pharmaceuticals 2023, 16, 1074. [Google Scholar] [CrossRef]
- Duchêne, D.; Bochot, A. Thirty Years with Cyclodextrins. Int. J. Pharm. 2016, 514, 58–72. [Google Scholar] [CrossRef]
- Hincal, A.A.; Eroğlu, H.; Bilensoy, E. Regulatory Status of Cyclodextrins in Pharmaceutical Products. In Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; Chapter 6; pp. 123–130. [Google Scholar] [CrossRef]
- Fernandes, F.; Dias-Teixeira, M.; Delerue-Matos, C.; Grosso, C. Critical Review of Lipid-Based Nanoparticles as Carriers of Neuroprotective Drugs and Extracts. Nanomaterials 2021, 11, 563. [Google Scholar] [CrossRef]
- Lu, Q. Bioresponsive and Multifunctional Cyclodextrin-Based Non-Viral Nanocomplexes in Cancer Therapy: Building Foundations for Gene and Drug Delivery, Immunotherapy and Bioimaging. Environ. Res. 2023, 234, 116507. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Heshmati Aghda, N.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D Printing Technologies for Drug Delivery Devices: Current Status and Future Perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]

| Cyclodextrin Type | Chemical Modification | Approx. Solubility in Water (mg/mL) | Approved Routes of Administration | Example Pharmaceutical Applications | References |
|---|---|---|---|---|---|
| HP-β-CD | 2-Hydroxypropyl substitution | >600 | Oral, IV, Intrathecal | Itraconazole oral/Intravenous (IV) solution (Sporanox®), Niemann–Pick Disease Type C, FSGS, vaccines | [91,92] |
| SBE-β-CD (Captisol®) | Sulfobutylether substitution of β-CD | ~500 | Oral, IV | Voriconazole IV formulation (VFend®), Solubilizing agent in marketed injectables (e.g., Voriconazole IV) | [91,93,94] |
| HP-γ-CD | 2-Hydroxypropyl substitution | >500 | Ophthalmic | Ophthalmic dexamethasone solutions | [95] |
| Randomly Methylated β-Cyclodextrin (RAMEB) | Random methylation (mixed methyl ethers) | ≥500 | Topical (nasal, ocular) | Nasal sprays, eye drops (as excipient) | [96,97,98] |
| 2,6-Di-O-methyl-β-Cyclodextrin (DIMEB) | 2,6-Di-O-methyl substitution | ~570 mg/mL | Investigational (pharmaceutical excipient) | Production of acellular pertussis vaccine (improving toxin secretion) | [91,99,100] |
| Sugammadex | γ-CD modified with carboxyl-thio ether side chains | ~100 mg/mL (in formulation) | IV | Reversal of neuromuscular blockade (Bridion®) | [91] |
| Application Area | CD Type | Drug/Compound | Carrier Type | Formulation and Benefits | References |
|---|---|---|---|---|---|
| Oral (Systemic) | HPβCD | Hydrochlorothiazide | NLC | Enhanced solubility, bioavailability, and sustained diuretic activity in pediatric use | [115] |
| Ocular (Topical) | HPβCD | Lutein | NLC | Improved corneal distribution and retention, enhanced transcorneal penetration, and formulation stability | [153] |
| Topical (Dermal) | βCD | Thyme oil (Essential oil) | NLC | Sustained release, enhanced skin permeation, and follicular targeting for topical antimicrobial therapy | [14] |
| Anticancer (Oral) | Epichlorohydrin-βCD (Poly-βCD) | Altretamine | SLN | Enhanced solubility, high entrapment efficiency, sustained release, and 2.75-fold increase in bioavailability | [103] |
| Anticancer (Oral) | HPβCD | Paclitaxel | SLN | Enhanced solubility, improved cellular uptake, increased lymphatic delivery, and greater oral bioavailability compared to unmodified solution | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alloush, T.; Demiralp, B. A Review of Formulation Strategies for Cyclodextrin-Enhanced Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs). Int. J. Mol. Sci. 2025, 26, 6509. https://doi.org/10.3390/ijms26136509
Alloush T, Demiralp B. A Review of Formulation Strategies for Cyclodextrin-Enhanced Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs). International Journal of Molecular Sciences. 2025; 26(13):6509. https://doi.org/10.3390/ijms26136509
Chicago/Turabian StyleAlloush, Tarek, and Burcu Demiralp. 2025. "A Review of Formulation Strategies for Cyclodextrin-Enhanced Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs)" International Journal of Molecular Sciences 26, no. 13: 6509. https://doi.org/10.3390/ijms26136509
APA StyleAlloush, T., & Demiralp, B. (2025). A Review of Formulation Strategies for Cyclodextrin-Enhanced Solid Lipid Nanoparticles (SLNs) and Nanostructured Lipid Carriers (NLCs). International Journal of Molecular Sciences, 26(13), 6509. https://doi.org/10.3390/ijms26136509








