A Stable RNA Vaccine Against the Regulatory Peptide Adrenomedullin Reduces Angiogenesis and Tumor Burden in a Subcutaneous Melanoma Model Without Inducing an Immunosuppressive Tumor Microenvironment
Abstract
1. Introduction
2. Results
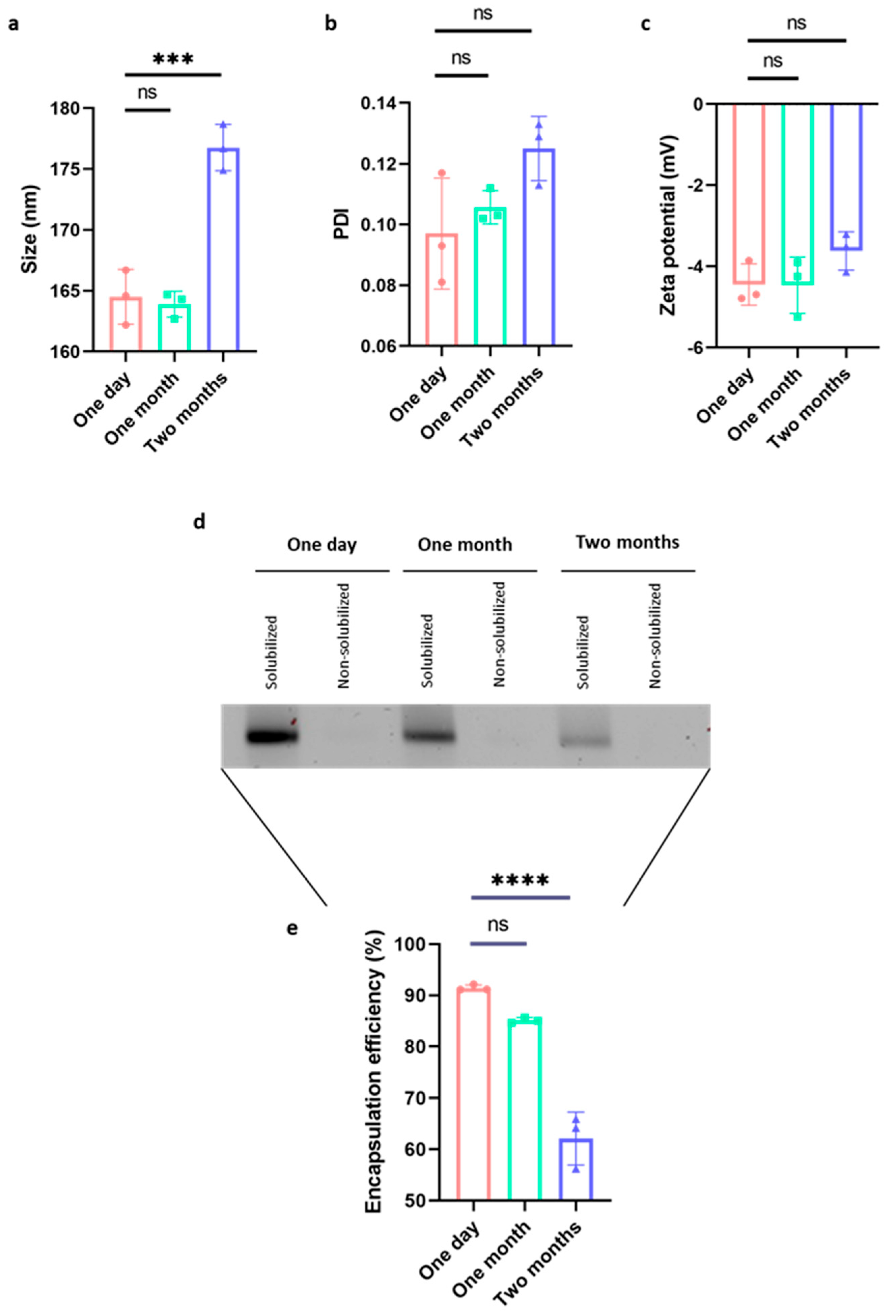
2.1. The Vaccine Is Highly Stable Even in the Absence of Cryoprotectants
2.2. Mouse Immunization Induced a Humoral and Cellular Immune Response in the Absence of Measurable Toxicity
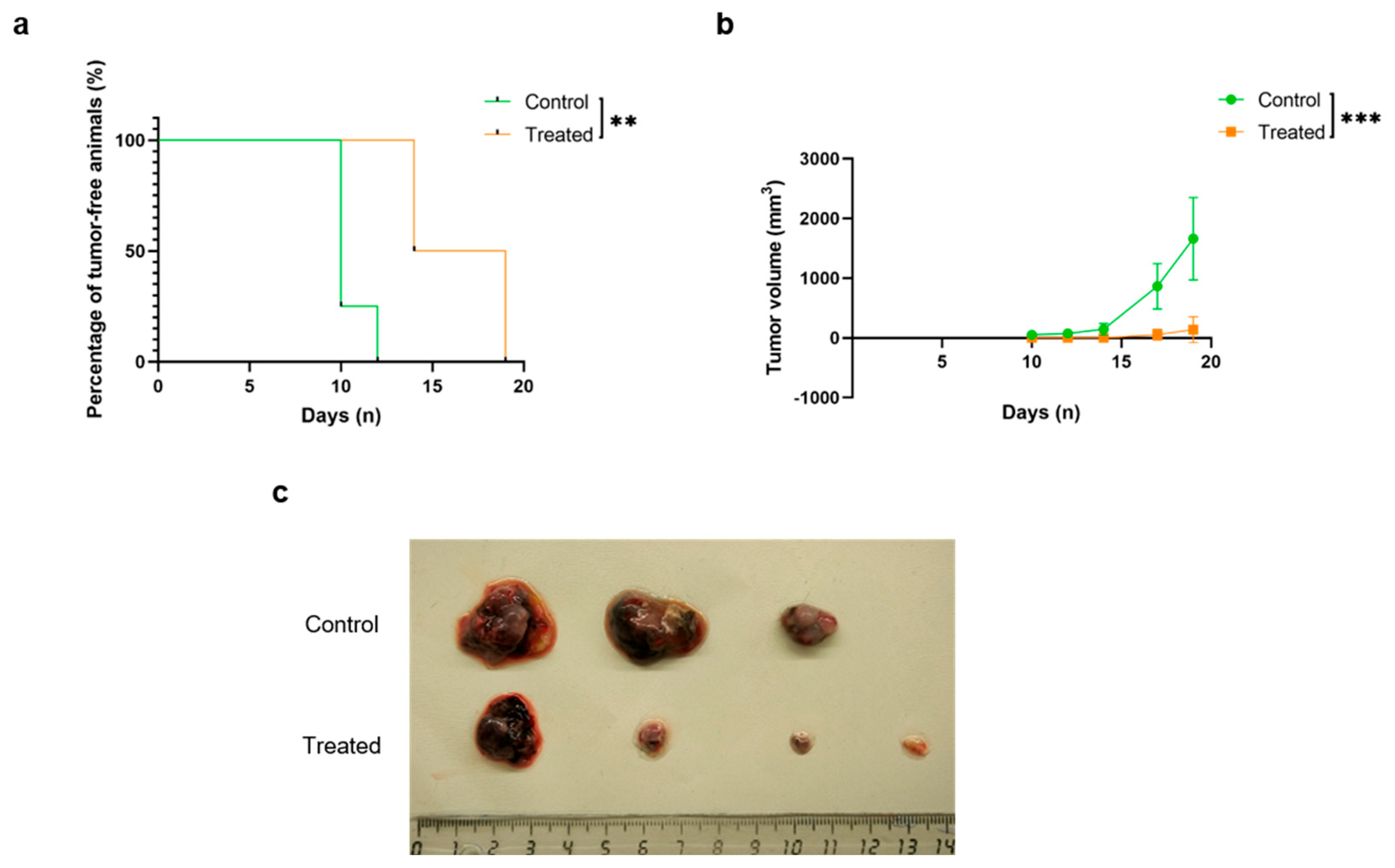
2.3. Mouse Immunization Resulted in a Delay in Tumor Initiation and Progression
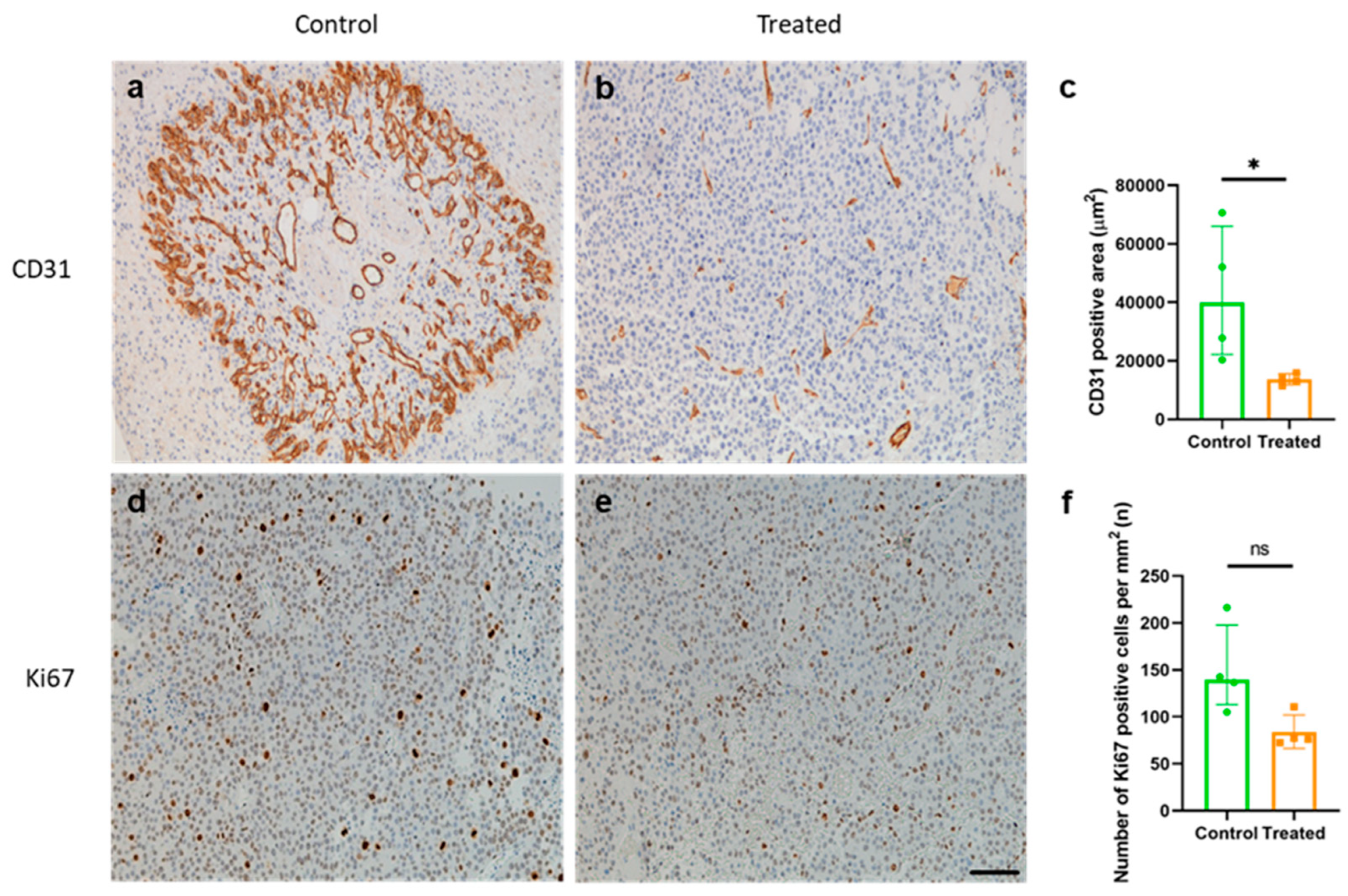
2.4. Mouse Immunization Resulted in a Reduction in Tumor Angiogenesis but Not in Tumor Cell Proliferation
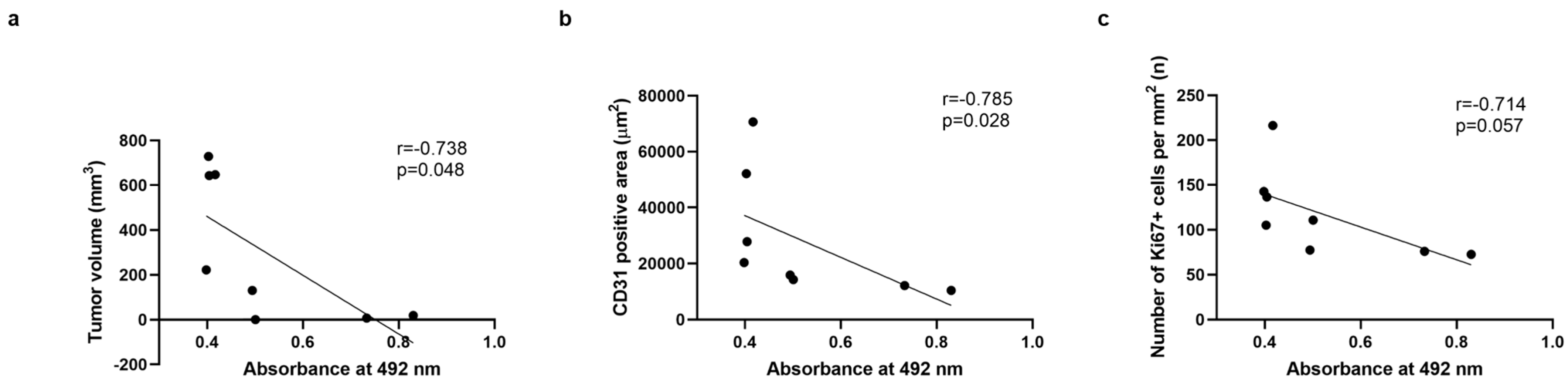
2.5. Correlation Studies Indicate a Significant Correlation Between Antibody Titers and a Decrease in Tumor Volume and Blood Vessel Density, but Not with Tumor Proliferation
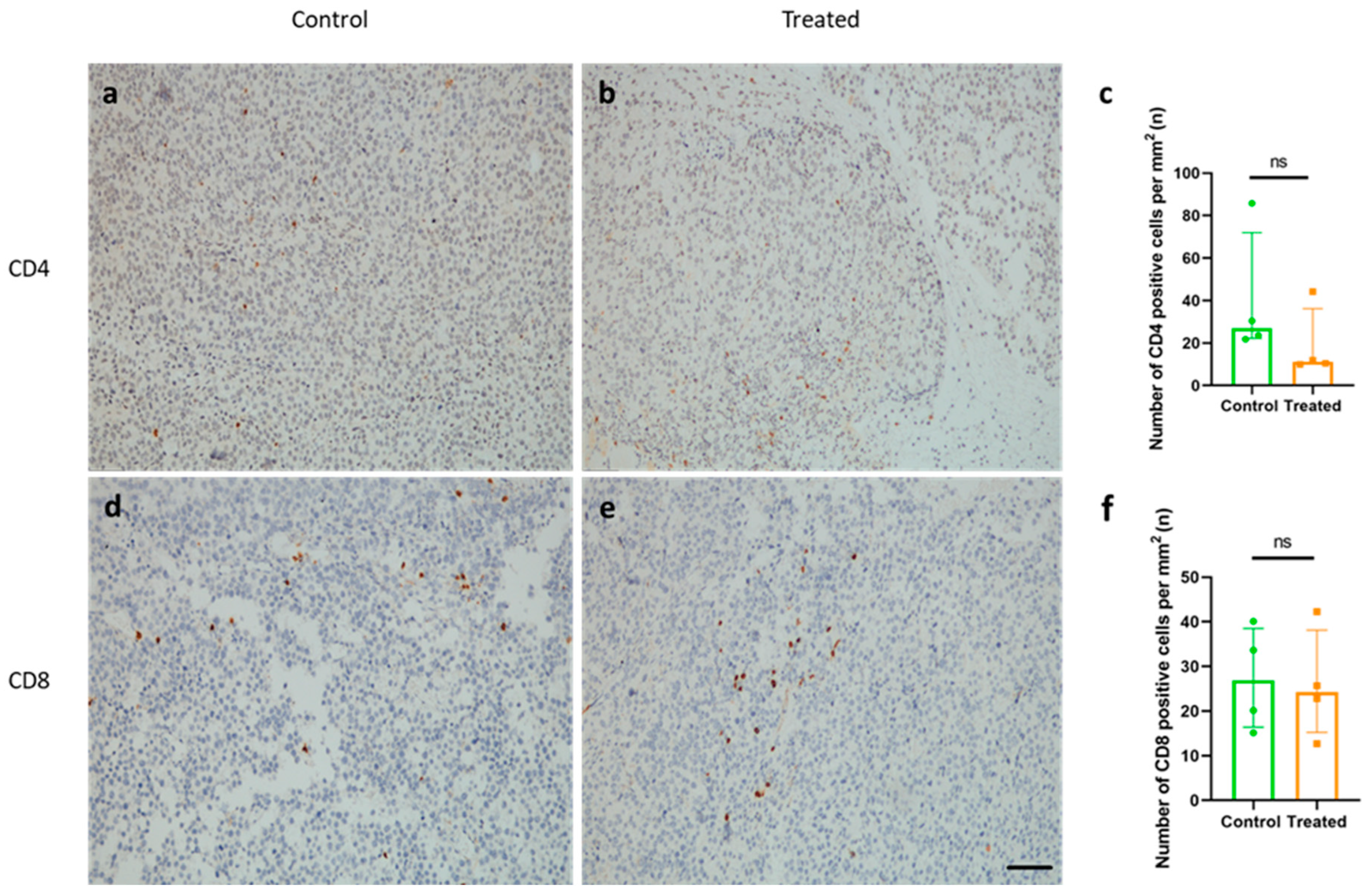
2.6. Immunization with KLH-AM Did Not Induce Changes in the Number of Tumor-Infiltrating CD4+ or CD8+ T Cells
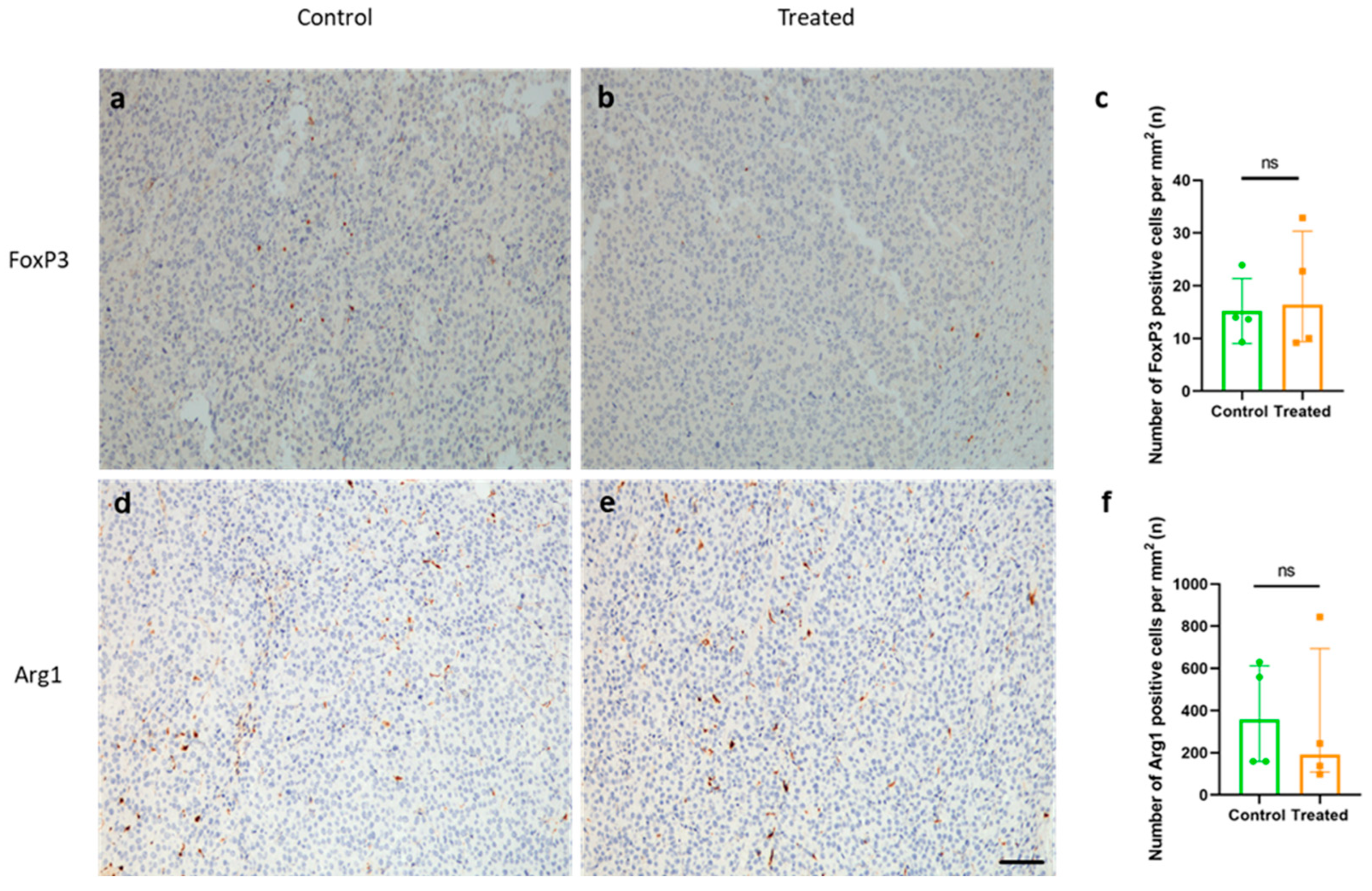
2.7. Immunization with KLH-AM Did Not Change the Features of the Immunosuppressive TME
3. Discussion
4. Materials and Methods
4.1. Transfection of E. coli, Amplification and Linearization of the DNA Template
4.2. In Vitro Transcription, Capping and Characterization
4.3. In Vitro Transcription Encapsulation of the mRNA in Lipid Nanoparticles
4.4. Characterization of Lipid Nanoparticles Following Long-Term Storage
4.5. Cell Lines and Culture Conditions
4.6. Animals and Immunization Protocol
4.7. Tumor Challenge
4.8. Serum Characterization (ELISA)
4.9. Isolation of Splenocytes
4.10. Flow Cytometry
4.11. Morphological and Microscopical Assessment of Resulting Tumors
4.12. Immunohistochemistry
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Adrenomedullin |
| ANOVA | Analysis of variance |
| CLR | Calcitonin receptor-like receptor |
| HIF | Hypoxia inducible factor |
| IVT | In vitro transcription |
| KLH | Keyhole limpet hemocyanin |
| LNP | Lipid nanoparticle |
| MHCI | Major histocompatibility complex I |
| PAMP | Proadrenomedullin N-terminal 20 peptide |
| PDAC | Pancreatic ductal adenocarcinoma |
| PDI | Polydispersity index |
| RAMP | Receptor activity-modifying protein |
| TAA | Tumor-associated antigen |
| TAM | Tumor-associated macrophage |
| TIL | Tumor infiltrating lymphocyte |
| TME | Tumor microenvironment |
| TSA | Tumor-specific antigen |
References
- World Health Organisation (WHO). Cancer Statistics in 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 1 July 2024).
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Joshi, D.C.; Sharma, A.; Prasad, S.; Singh, K.; Kumar, M.; Sherawat, K.; Tuli, H.S.; Gupta, M. Novel therapeutic agents in clinical trials: Emerging approaches in cancer therapy. Discov. Oncol. 2024, 15, 342. [Google Scholar] [CrossRef]
- Katt, M.E.; Wong, A.D.; Searson, P.C. Dissemination from a solid tumor: Examining the multiple parallel pathways. Trends Cancer 2018, 4, 20–37. [Google Scholar] [CrossRef]
- Folkman, J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–30. [Google Scholar] [CrossRef]
- Bowler, E.; Ladomery, M.R. Working with Hypoxia. In Redox-Mediated Signal Transduction: Methods and Protocols; Hancock, J.T., Conway, M., Eds.; Springer: New York, NY, USA, 2019; pp. 109–133. [Google Scholar] [CrossRef]
- Stephenson, J.A.; Goddard, J.C.; Al-Taan, O.; Dennison, A.R.; Morgan, B. Tumour Angiogenesis: A Growth Area—From John Hunter to Judah Folkman and Beyond. J. Cancer Res. 2013, 2013, 895019. [Google Scholar] [CrossRef]
- Adami, H.-O.; Csermely, P.; Veres, D.V.; Emilsson, L.; Løberg, M.; Bretthauer, M.; Kalager, M. Are rapidly growing cancers more lethal? Eur. J. Cancer 2017, 72, 210–214. [Google Scholar] [CrossRef]
- Garayoa, M.; Martínez, A.; Lee, S.; Pío, R.; An, W.G.; Neckers, L.; Trepel, J.; Montuenga, L.M.; Ryan, H.; Johnson, R.; et al. Hypoxia-inducible factor-1 (HIF-1) up-regulates adrenomedullin expression in human tumor cell lines during oxygen deprivation: A possible promotion mechanism of carcinogenesis. Mol. Endocrinol. 2000, 14, 848–862. [Google Scholar] [CrossRef]
- Din, S.M.U.; Streit, S.G.; Huynh, B.T.; Hana, C.; Abraham, A.-N.; Hussein, A. Therapeutic Targeting of Hypoxia-Inducible Factors in Cancer. Int. J. Mol. Sci. 2024, 25, 2060. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, K.; Kangawa, K.; Eto, T. Adrenomedullin and PAMP: Discovery, structures, and cardiovascular functions. Microsc. Res. Tech. 2002, 57, 3–13. [Google Scholar] [CrossRef] [PubMed]
- López, J.; Martínez, A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int. Rev. Cytol. 2002, 221, 1–92. [Google Scholar]
- Nikitenko, L.L.; Fox, S.B.; Kehoe, S.; Rees, M.C.P.; Bicknell, R. Adrenomedullin and tumour angiogenesis. Br. J. Cancer 2006, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Larráyoz, I.M.; Martínez-Herrero, S.; García-Sanmartín, J.; Ochoa-Callejero, L.; Martínez, A. Adrenomedullin and tumour microenvironment. J. Transl. Med. 2014, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Benyahia, Z.; Gaudy-Marqueste, C.; Berenguer-Daizé, C.; Chabane, N.; Dussault, N.; Cayol, M.; Vellutini, C.; Djemli, A.; Nanni, I.; Beaufils, N.; et al. Adrenomedullin Secreted by Melanoma Cells Promotes Melanoma Tumor Growth through Angiogenesis and Lymphangiogenesis. Cancers 2022, 14, 5909. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Reynolds, A.R. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis 2014, 17, 471–494, Erratum in Angiogenesis 2014, 17, 495–497. [Google Scholar] [CrossRef]
- Finn, O.J. Human Tumor Antigens Yesterday, Today, and Tomorrow. Cancer Immunol. Res. 2017, 5, 347–354. [Google Scholar] [CrossRef]
- Wagner, S.C.; Ichim, T.E.; Ma, H.; Szymanski, J.; Perez, J.A.; Lopez, J.; Bogin, V.; Patel, A.N.; Marincola, F.M.; Kesari, S. Cancer anti-angiogenesis vaccines: Is the tumor vasculature antigenically unique? J. Transl. Med. 2015, 13, 340. [Google Scholar] [CrossRef] [PubMed]
- Ugel, S.; Facciponte, J.G.; De Sanctis, F.; Facciabene, A. Targeting tumor vasculature: Expanding the potential of DNA cancer vaccines. Cancer Immunol. Immunother. 2015, 64, 1339–1348. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Peng, X.; Yang, Y.; Chen, Q.; Liu, J.; She, Q.; Tan, J.; Lou, C.; Liao, Z.; et al. mRNA vaccine in cancer therapy: Current advance and future outlook. Clin. Transl. Med. 2023, 13, e1384. [Google Scholar] [CrossRef]
- Caraviello, C.; Nazzaro, G.; Tavoletti, G.; Boggio, F.; Denaro, N.; Murgia, G.; Passoni, E.; Mancin, V.B.; Marzano, A.V. Melanoma Skin Cancer: A Comprehensive Review of Current Knowledge. Cancers 2025, 17, 2920. [Google Scholar] [CrossRef]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef]
- Tadic, S.; Ochoa-Callejero, L.; Narro-Íñiguez, J.; García-Sanmartín, J.; Martínez, A. An RNA vaccine against adrenomedullin reduces angiogenesis and tumor burden in a syngeneic metastatic melanoma mouse model. Front. Immunol. 2025, 16, 1604156. [Google Scholar] [CrossRef]
- Mandalà, M.; Ugolini, F.; Caldirola, L.; Rulli, E.; Manca, A.; Sini, M.C.; Simi, S.; Baroni, G.; Costabile, S.; De Giorgi, V.; et al. Tumour microenvironment heterogeneity in primary and metastatic paired melanoma samples and its correlation with genetic features and prognosis. Eur. J. Cancer 2025, 229, 115755. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Chen, N.; Ge, C.; Li, R.; Li, Z.; Zeng, B.; Li, C.; Wang, Y.; Xue, Y.; Song, X.; et al. Prognostic value of tumor-infiltrating lymphocytes in melanoma: A systematic review and meta-analysis. Oncoimmunology 2019, 8, 1593806. [Google Scholar] [CrossRef]
- Fujimura, T.; Kambayashi, Y.; Fujisawa, Y.; Hidaka, T.; Aiba, S. Tumor-Associated Macrophages: Therapeutic Targets for Skin Cancer. Front. Oncol. 2018, 8, 3. [Google Scholar] [CrossRef]
- Pan, Y.; Zhou, H.; Sun, Z.; Zhu, Y.; Zhang, Z.; Han, J.; Liu, Y.; Wang, Q. Regulatory T cells in solid tumor immunotherapy: Effect, mechanism and clinical application. Cell Death Dis. 2025, 16, 277. [Google Scholar] [CrossRef]
- Miller, M.J.; Martínez, A.; Unsworth, E.J.; Thiele, C.J.; Moody, T.W.; Elsasser, T.; Cuttitta, F. Adrenomedullin Expression in Human Tumor Cell Lines Its Potential Role as An Autocrine Growth Factor. J. Biol. Chem. 1996, 271, 23345–23351. [Google Scholar] [CrossRef]
- Pío, R.; Martínez, A.; Unsworth, E.J.; Kowalak, J.A.; Bengoechea, J.A.; Zipfel, P.F.; Elsasser, T.H.; Cuttitta, F. Complement Factor H Is a Serum-binding Protein for Adrenomedullin, and the Resulting Complex Modulates the Bioactivities of Both Partners. J. Biol. Chem. 2001, 276, 12292–12300. [Google Scholar] [CrossRef] [PubMed]
- Ouafik, L.; Sauze, S.; Boudouresque, F.; Chinot, O.; Delfino, C.; Fina, F.; Vuaroqueaux, V.; Dussert, C.; Palmari, J.; Dufour, H.; et al. Neutralization of adrenomedullin inhibits the growth of human glioblastoma cell lines in vitro and suppresses tumor xenograft growth in vivo. Am. J. Pathol. 2002, 160, 1279–1292. [Google Scholar] [CrossRef]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Bokov, D.; Markov, A.; Jalil, A.T.; Shalaby, M.N.; Suksatan, W.; Chupradit, S.; Al-Ghamdi, H.S.; Shomali, N.; Zamani, A.; et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun. Signal. 2022, 20, 49. [Google Scholar] [CrossRef]
- Folkman, J. Tumor Angiogenesis: Therapeutic Implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Wu, B.; Zhang, B.; Li, B.; Wu, H.; Jiang, M. Cold and hot tumors: From molecular mechanisms to targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 274. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Nguyen, F.D.; Noory, M.A.; Sharma, A. Current State of Animal (Mouse) Modeling in Melanoma Research. Cancer Growth Metastasis 2015, 8 (Suppl. S1), 81–94. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Gu, Y.; Zhang, P.; Fang, H.; Cao, Y.; Wang, J.; Lin, C.; Liu, H.; Zhang, H.; He, H.; et al. Intratumoral PD-1+CD8+ T cells associate poor clinical outcomes and adjuvant chemotherapeutic benefit in gastric cancer. Br. J. Cancer 2022, 127, 1709–1717. [Google Scholar] [CrossRef]
- Raju, T.K.; Tadic, S.; Garrido, P.; Ochoa-Callejero, L.; Narro-Íñiguez, J.; García-Sanmartín, J.; Martínez, A. A DNA Vaccine Against Proadrenomedullin N-Terminal 20 Peptide (PAMP) Reduces Angiogenesis and Increases Lymphocyte and Macrophage Infiltration but Has No Effect on Tumor Burden in a Mouse Model of Lung Metastasis. Vaccines 2025, 13, 586. [Google Scholar] [CrossRef] [PubMed]
- Matias, M.; Pinho, J.O.; Penetra, M.J.; Campos, G.; Reis, C.P.; Gaspar, M.M. The Challenging Melanoma Landscape: From Early Drug Discovery to Clinical Approval. Cells 2021, 10, 3088. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef]
- Sethna, Z.; Guasp, P.; Reiche, C.; Milighetti, M.; Ceglia, N.; Patterson, E.; Lihm, J.; Payne, G.; Lyudovyk, O.; Rojas, L.A.; et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature 2025, 639, 1042–1051. [Google Scholar] [CrossRef]
- Kamba, T.; McDonald, D.M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br. J. Cancer 2007, 96, 1788–1795. [Google Scholar] [CrossRef]
- Meadows, K.L.; Hurwitz, H.I. Anti-VEGF Therapies in the Clinic. Cold Spring Harb. Perspect. Med. 2012, 2, a006577. [Google Scholar] [CrossRef] [PubMed]
- Tadic, S.; Martínez, A. Nucleic acid cancer vaccines targeting tumor related angiogenesis. Could mRNA vaccines constitute a game changer? Front. Immunol. 2024, 15, 1433185. [Google Scholar] [CrossRef]
- Caron, K.M.; Smithies, O. Extreme hydrops fetalis and cardiovascular abnormalities in mice lacking a functional Adrenomedullin gene. Proc. Natl. Acad. Sci. USA 2001, 98, 615–619. [Google Scholar] [CrossRef]
- Martínez-Herrero, S.; Larrayoz, I.M.; Ochoa-Callejero, L.; Fernández, L.J.; Allueva, A.; Ochoa, I.; Martínez, A. Prevention of Bone Loss in a Model of Postmenopausal Osteoporosis through Adrenomedullin Inhibition. Front. Physiol. 2016, 7, 280. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Qiao, N.; Meng, Q.; Zhang, M.; Wang, X.; Jia, J.; Yang, S.; Qu, C.; Li, W.; et al. Adrenomedullin blockade suppresses sunitinib-resistant renal cell carcinoma growth by targeting the ERK/MAPK pathway. Oncotarget 2016, 7, 63374–63387. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, P.; Pang, X.; Hu, Y.; Zhang, Y. Adrenomedullin Up-regulates the Expression of Vascular Endothelial Growth Factor in Epithelial Ovarian Carcinoma Cells via JNK/AP-1 Pathway. Int. J. Gynecol. Cancer 2015, 25, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Ma, J.; Pang, X.; Dong, M. Adrenomedullin promotes angiogenesis in epithelial ovarian cancer through upregulating hypoxia-inducible factor-1α and vascular endothelial growth factor. Sci. Rep. 2017, 7, 40524. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Vázquez, R.; Riveiro, M.E.; Berenguer-Daizé, C.; O’kAne, A.; Gormley, J.; Touzelet, O.; Rezai, K.; Bekradda, M.; Ouafik, L. Targeting Adrenomedullin in Oncology: A Feasible Strategy With Potential as Much More Than an Alternative Anti-Angiogenic Therapy. Front. Oncol. 2021, 10, 589218. [Google Scholar] [CrossRef]
- Eto, T.; Kato, J.; Kitamura, K. Regulation of production and secretion of adrenomedullin in the cardiovascular system. Regul. Pept. 2003, 112, 61–69. [Google Scholar] [CrossRef]
- Crommelin, D.J.; Anchordoquy, T.J.; Volkin, D.B.; Jiskoot, W.; Mastrobattista, E. Addressing the Cold Reality of mRNA Vaccine Stability. J. Pharm. Sci. 2021, 110, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Talbot, A.; de Koning-Ward, T.F.; Layton, D. Left out in the cold-inequity in infectious disease control due to cold chain disparity. Vaccine 2025, 45, 126648. [Google Scholar] [CrossRef] [PubMed]
- Mata, E.; Broset, E.; Matute, C.; Stoian, A.; Adame, S.; Alejo, T.; López, A.; Andrés, B.; Heredero, J.; de Miguel, D.; et al. Preserved efficacy of lyophilized SARS-CoV-2 mRNA vaccine incorporating novel ionizable lipids after one year at 25 °C. npj Vaccines 2025, 10, 135. [Google Scholar] [CrossRef]
- Kim, H.-I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Overwijk, W.W.; Restifo, N.P. B16 as a Mouse Model for Human Melanoma. Curr. Protoc. Immunol. 2000, 39, 20-1. [Google Scholar] [CrossRef]
- Becker, J.C.; Houben, R.; Schrama, D.; Voigt, H.; Ugurel, S.; Reisfeld, R.A. Mouse models for melanoma: A personal perspective. Exp. Dermatol. 2010, 19, 157–164. [Google Scholar] [CrossRef]
- Ray, A.L.; Nofchissey, R.A.; Khan, M.A.; Reidy, M.A.; Lerner, M.R.; Wu, X.; Guo, S.; Hill, S.L.; Weygant, N.; Adams, S.F.; et al. The role of sex in the innate and adaptive immune environment of metastatic colorectal cancer. Br. J. Cancer 2020, 123, 624–632. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Tian, X.; Wei, X. Cancer vaccines: Current status and future directions. J. Hematol. Oncol. 2025, 18, 18. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, Y.; Yu, X.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.; Robinson, J.; Zhang, D.; et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, S.; Bao, S.; Wu, L.; Qu, H.; Wang, Z.; Dong, H.; Wu, J.; Jin, Y. A Universal Strategy of Anti-Tumor mRNA Vaccine by Harnessing “Off-the-Shelf” Immunity. Adv. Sci. 2025, 12, 2401287. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Callejero, L.; Pozo-Rodrigálvarez, A.; Martínez-Murillo, R.; Martínez, A. Lack of adrenomedullin in mouse endothelial cells results in defective angiogenesis, enhanced vascular permeability, less metastasis, and more brain damage. Sci. Rep. 2016, 6, 33495, Erratum in Sci. Rep. 2020, 10, 6283. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]

| Type of Antibody | Antibody | Manufacturer | Cat. No. | RRID | Dilution |
|---|---|---|---|---|---|
| Primary | Anti-mouse CD45 PerCP-Cyanine5.5 | BioLegend, San Diego, CA, USA | 103132 | AB_893340 | 1/100 |
| Anti-mouse CD4 APC-Cyanine7 | BioLegend | 100526 | AB_312727 | 1/200 | |
| Anti-mouse CD8b.2 FITC | BioLegend | 140404 | AB_10643587 | 1/100 | |
| Anti-mouse CD31 | Abcam, Cambridge, UK | ab281583 | AB_3096925 | 1/5000 | |
| Anti-mouse Ki67 (clone SP6) | Vitro Master Diagnostica, New York, NY, USA | MAD-000310QD-3 | AB_3677420 | 1/5 | |
| Anti-mouse CD4 | Abcam | ab288724 | AB_2941893 | 1:4000 | |
| Anti-mouse CD8 | Abcam | ab217344 | AB_2890649 | 1:2000 | |
| Anti-mouse FoxP3 | Thermo Fisher Scientific | 14-5773-82 | AB_467576 | 1:100 | |
| Anti-mouse Arg1 | Cell Signaling, Danvers, MA, USA | 93668 | AB_2800207 | 1:2000 | |
| Secondary | Novolink rabbit detection system | Leica, Wetzlar, Germany | RE7200CE | AB_3674357 | Prediluted |
| Peroxidase-AffiniPure Goat Anti-Rat IgG (H+L) | Jackson Immunoresearch, West Grove, PA, USA | 112-035-003 | AB_2338128 | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tadic, S.; García-Sanmartín, J.; Narro-Íñiguez, J.; Martínez, A. A Stable RNA Vaccine Against the Regulatory Peptide Adrenomedullin Reduces Angiogenesis and Tumor Burden in a Subcutaneous Melanoma Model Without Inducing an Immunosuppressive Tumor Microenvironment. Int. J. Mol. Sci. 2025, 26, 10745. https://doi.org/10.3390/ijms262110745
Tadic S, García-Sanmartín J, Narro-Íñiguez J, Martínez A. A Stable RNA Vaccine Against the Regulatory Peptide Adrenomedullin Reduces Angiogenesis and Tumor Burden in a Subcutaneous Melanoma Model Without Inducing an Immunosuppressive Tumor Microenvironment. International Journal of Molecular Sciences. 2025; 26(21):10745. https://doi.org/10.3390/ijms262110745
Chicago/Turabian StyleTadic, Srdan, Josune García-Sanmartín, Judit Narro-Íñiguez, and Alfredo Martínez. 2025. "A Stable RNA Vaccine Against the Regulatory Peptide Adrenomedullin Reduces Angiogenesis and Tumor Burden in a Subcutaneous Melanoma Model Without Inducing an Immunosuppressive Tumor Microenvironment" International Journal of Molecular Sciences 26, no. 21: 10745. https://doi.org/10.3390/ijms262110745
APA StyleTadic, S., García-Sanmartín, J., Narro-Íñiguez, J., & Martínez, A. (2025). A Stable RNA Vaccine Against the Regulatory Peptide Adrenomedullin Reduces Angiogenesis and Tumor Burden in a Subcutaneous Melanoma Model Without Inducing an Immunosuppressive Tumor Microenvironment. International Journal of Molecular Sciences, 26(21), 10745. https://doi.org/10.3390/ijms262110745








