Antibodies to Periodontal Bacteria Are Associated with Systemic Lupus Erythematosus and Autoantibody Positivity
Abstract
1. Introduction
2. Results
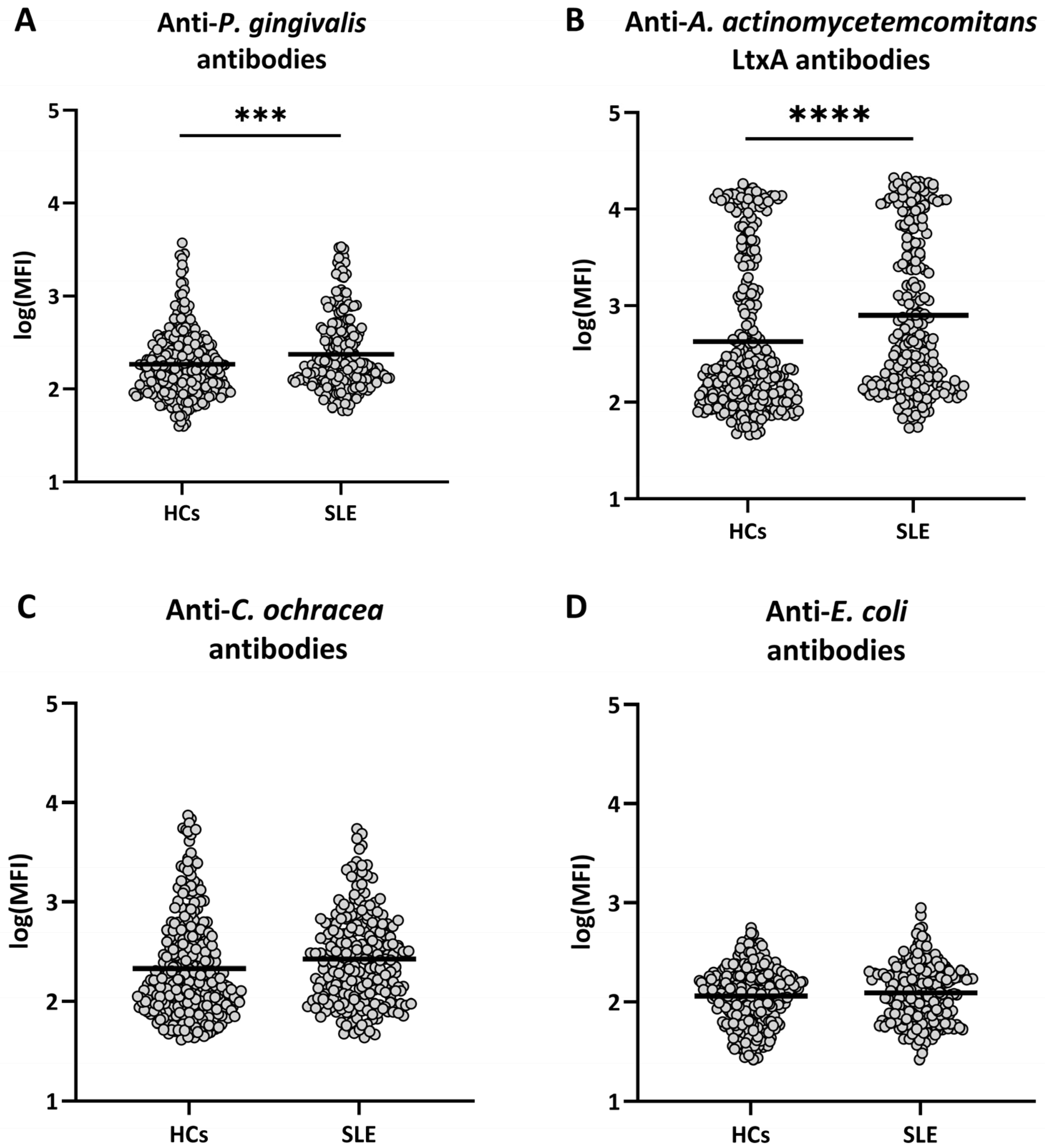
2.1. Antibodies Against Periodontal Bacteria in SLE and in Healthy Individuals
2.2. Antibodies Against Periodontal Bacteria and Smoking Status in SLE Etiology
2.3. Antibodies Against Periodontal Bacteria in Relation to SLE Autoantibodies
3. Discussion
4. Materials and Methods
4.1. Patients and Healthy Controls
4.2. Luminex Multiplex Assay for Anti-Bacterial Antibodies
4.3. Autoantibody Measurement
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | Attributable proportion due to interaction |
| dsDNA | Double-stranded DNA |
| IgG | Immunoglobulin G |
| LtxA | Leukotoxin A |
| OR | Odds ratio |
| SLE | Systemic lupus erythematosus |
| U1RNP | U1 ribonucleoprotein |
References
- Kivity, S.; Agmon-Levin, N.; Blank, M.; Shoenfeld, Y. Infections and autoimmunity—Friends or foes? Trends Immunol. 2009, 30, 409–414. [Google Scholar] [CrossRef]
- Bogdanos, D.P.; Smyk, D.S.; Rigopoulou, E.I.; Sakkas, L.I.; Shoenfeld, Y. Infectomics and autoinfectomics: A tool to study infectious-induced autoimmunity. Lupus 2015, 24, 364–373. [Google Scholar] [CrossRef]
- Esposito, S.; Bosis, S.; Semino, M.; Rigante, D. Infections and systemic lupus erythematosus. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1467–1475. [Google Scholar] [CrossRef]
- Zandman-Goddard, G.; Berkun, Y.; Barzilai, O.; Boaz, M.; Ram, M.; Anaya, J.; Shoenfeld, Y. Neuropsychiatric lupus and infectious triggers. Lupus 2008, 17, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Zandman-Goddard, G.; Berkun, Y.; Barzilai, O.; Boaz, M.; Blank, M.; Ram, M.; Sherer, Y.; Anaya, J.M.; Shoenfeld, Y. Exposure to Epstein-Barr virus infection is associated with mild systemic lupus erythematosus disease. Ann. N. Y. Acad. Sci. 2009, 1173, 658–663. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Ceccarelli, F.; Iaiani, G.; Perricone, C.; Giordano, A.; Amori, L.; Miranda, F.; Massaro, L.; Pacucci, V.A.; Truglia, S.; et al. Association between Staphylococcus aureus nasal carriage and disease phenotype in patients affected by systemic lupus erythematosus. Arthritis Res. Ther. 2016, 18, 177. [Google Scholar] [CrossRef]
- Jung, J.Y.; Suh, C.H. Infection in systemic lupus erythematosus, similarities, and differences with lupus flare. Korean J. Intern. Med. 2017, 32, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, L.; Aleti, G.; Choudhury, S.; Nguyen, D.; Yaskell, T.; Zhang, Y.; Li, W.; Nelson, K.E.; Neto, L.L.S.; Sant’ANa, A.C.P.; et al. Host-Microbial Interactions in Systemic Lupus Erythematosus and Periodontitis. Front. Immunol. 2019, 10, 2602. [Google Scholar] [CrossRef]
- Guo, J.; Cui, G.; Huang, W.; Zheng, Z.; Li, T.; Gao, G.; Huang, Z.; Zhan, Y.; Ding, S.; Liu, S.; et al. Alterations in the human oral microbiota in systemic lupus erythematosus. J. Transl. Med. 2023, 21, 95. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Johnson, D.K. The prevalence of oral manifestations of systemic lupus erythematosus. Quintessence Int. 1990, 21, 461–465. [Google Scholar]
- Novo, E.; Garcia-MacGregor, E.; Viera, N.; Chaparro, N.; Crozzoli, Y. Periodontitis and anti-neutrophil cytoplasmic antibodies in systemic lupus erythematosus and rheumatoid arthritis: A comparative study. J. Periodontol. 1999, 70, 185–188. [Google Scholar] [CrossRef]
- Mutlu, S.; Richards, A.; Maddison, P.; Scully, C. Gingival and periodontal health in systemic lupus erythematosus. Community Dent. Oral Epidemiol. 1993, 21, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, K.D.; Al-Zahrani, M.S.; Bahlas, S.M.; Kayal, R.A.; Zawawi, K.H. Periodontal findings in systemic lupus erythematosus patients and healthy controls. Saudi Med. J. 2015, 36, 463–468. [Google Scholar] [CrossRef]
- Corrêa, J.D.; Calderaro, D.C.; Ferreira, G.A.; Mendonça, S.M.S.; Fernandes, G.R.; Xiao, E.; Teixeira, A.L.; Leys, E.J.; Graves, D.T.; Silva, T.A. Subgingival microbiota dysbiosis in systemic lupus erythematosus: Association with periodontal status. Microbiome 2017, 5, 34. [Google Scholar] [CrossRef]
- Martínez, R.E.M.; Herrera, J.L.A.; Pérez, R.A.D.; Mendoza, C.A.; Manrique, S.I.R. Frequency of Porphyromonas gingivalis and fimA genotypes in patients with periodontitis and systemic lupus erythematosus. Lupus 2021, 30, 80–85. [Google Scholar] [CrossRef]
- Griffen, A.N.N.L.; Becker, M.R.; Lyons, S.R.; Moeschberger, M.L.; Leys, E.J. Prevalence of Porphyromonas gingivalis and Periodontal Health Status. J. Clin. Microbiol. 1998, 36, 3239–3242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chyuan, I.; Wang, Y.; Kuo, M.Y.; Chang, C.; Wu, K.; Hsu, P.; Nagasawa, T.; Wara-Aswapati, N.; Chen, Y. β2-Glycoprotein I-Dependent Anti-Cardiolipin Antibodies Associated With Periodontitis in Patients With Systemic Lupus Erythematosus. J. Periodontol. 2015, 86, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- de Vries, C.; Ruacho, G.; Kindstedt, E.; Potempa, B.A.; Potempa, J.; Klinge, B.; Lundberg, P.; Svenungsson, E.; Lundberg, K. Antibodies to Porphyromonas gingivalis Are Increased in Patients with Severe Periodontitis, and Associate with Presence of Specific Autoantibodies and Myocardial Infarction. J. Clin. Med. 2022, 11, 1008. [Google Scholar] [CrossRef]
- Bagavant, H.; Dunkleberger, M.L.; Wolska, N.; Sroka, M.; Rasmussen, A.; Adrianto, I.; Montgomery, C.; Sivils, K.; Guthridge, J.M.; James, J.A.; et al. Antibodies to periodontogenic bacteria are associated with higher disease activity in lupus patients. Clin. Exp. Rheumatol. 2019, 37, 106–111. [Google Scholar]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A * periodontopathogen? Periodontol. 2000 2010, 54, 78–105. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Könönen, E.; Paju, S.; Hyvärinen, K.; Gursoy, U.K.; Huumonen, S.; Knuuttila, M.; Suominen, A.L. Periodontal pathogen carriage, rather than periodontitis, determines the serum antibody levels. J. Clin. Periodontol. 2011, 38, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Massarenti, L.; Nielsen, C.H.; Danielsen, A.K.; Jensen, P.Ø.; Enevold, C.; Damgaard, C. Evaluation of circulating IgG antibodies against Porphyromonas gingivalis or its gingipains as serological markers of periodontitis and carriage of the bacterium. J. Periodontol. 2024, 96, 119–128. [Google Scholar] [CrossRef]
- Damgaard, C.; Danielsen, A.K.; Enevold, C.; Reinholdt, J.; Holmstrup, P.; Nielsen, C.H.; Massarenti, L. Circulating antibodies against leukotoxin A as marker of periodontitis Grades B and C and oral infection with Aggregatibacter actinomycetemcomitans. J. Periodontol. 2021, 92, 1795–1804. [Google Scholar] [CrossRef] [PubMed]
- Lakio, L.; Antinheimo, J.; Paju, S.; Buhlin, K.; Pussinen, P.J.; Alfthan, G. Tracking of plasma antibodies against Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis during 15 years. J. Oral Microbiol. 2009, 1, 1979. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Alfthan, G.; Mattila, K.; Asikainen, S. Multiserotype Enzyme-Linked Immunosorbent Assay as a Diagnostic Aid for Periodontitis in Large-Scale Studies. J. Clin. Microbiol. 2002, 40, 512–518. [Google Scholar] [CrossRef]
- Dahlén, G.; Luan, W.M.; Dahlgren, U.; Papapanou, P.P.; Baelum, V.; Fejerskov, O. Subgingival bacterial clusters and serum antibody response as markers of extent and severity of periodontitis in adult Chinese. Eur. J. Oral Sci. 2016, 124, 179–187. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Neiderud, A.M.; Disick, E.; Lalla, E.; Miller, G.C.; Dahlén, G. Longitudinal stability of serum immunoglobulin G responses to periodontal bacteria. J. Clin. Periodontol. 2004, 31, 985–990. [Google Scholar] [CrossRef]
- Maekawa, T.; Krauss, J.L.; Abe, T.; Jotwani, R.; Triantafilou, M.; Triantafilou, K.; Hashim, A.; Hoch, S.; Curtis, M.A.; Nussbaum, G.; et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe 2014, 15, 768–778. [Google Scholar] [CrossRef]
- Stathopoulou, P.G.; Benakanakere, M.R.; Galicia, J.C.; Kinane, D.F. The host cytokine response to Porphyromonas gingivalis is modified by gingipains. Oral Microbiol. Immunol. 2009, 24, 11–17. [Google Scholar] [CrossRef]
- Krueger, E.; Brown, A.C. Aggregatibacter actinomycetemcomitans leukotoxin: From mechanism to targeted anti-toxin therapeutics. Mol. Oral Microbiol. 2020, 35, 85–105. [Google Scholar] [CrossRef]
- Kharlamova, N.; Jiang, X.; Sherina, N.; Potempa, B.; Israelsson, L.; Quirke, A.-M.; Eriksson, K.; Yucel-Lindberg, T.; Venables, P.J.; Potempa, J.; et al. Antibodies to Porphyromonas gingivalis indicate interaction between oral infection, smoking and risk genes in rheumatoid arthritis etiology. Arthritis Rheumatol. 2016, 68, 604–613. [Google Scholar] [CrossRef]
- Costenbader, K.H.; Kim, D.J.; Peerzada, J.; Lockman, S.; Nobles-Knight, D.; Petri, M.; Karlson, E.W. Cigarette smoking and the risk of systemic lupus erythematosus: A meta-analysis. Arthritis Rheum. 2004, 50, 849–857. [Google Scholar] [CrossRef]
- Ghaussy, N.O.; Sibbitt, W.; Bankhurst, A.D.; Qualls, C.R. Cigarette smoking and disease activity in systemic lupus erythematosus. J. Rheumatol. 2003, 30, 1215–1221. [Google Scholar]
- Hussain, S.B.; Leira, Y.; Zehra, S.A.; Botelho, J.; Machado, V.; Ciurtin, C.; D’aIuto, F.; Orlandi, M. Periodontitis and Systemic Lupus Erythematosus: A systematic review and meta-analysis. J. Periodontal Res. 2022, 57, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2014, 15, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Belibasakis, G.N. Porphyromonas gingivalis: An invasive and evasive opportunistic oral pathogen. FEMS Microbiol. Lett. 2012, 333, 1–9. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Schenkein, H.; Berry, C.; Burmeister, J.; Brooks, C.; Barbour, S.; Best, A.; Tew, J. Anti-cardiolipin Antibodies in Sera from Patients with Periodontitis. J. Dent. Res. 2003, 82, 919–922. [Google Scholar] [CrossRef]
- Schenkein, H.A.; Best, A.M.; Brooks, C.N.; Burmeister, J.A.; Arrowood, J.A.; Kontos, M.C.; Tew, J.G. Anti-Cardiolipin and Increased Serum Adhesion Molecule Levels in Patients With Aggressive Periodontitis. J. Periodontol. 2007, 78, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Blank, M.; Krause, I.; Fridkin, M.; Keller, N.; Kopolovic, J.; Goldberg, I.; Tobar, A.; Shoenfeld, Y. Bacterial induction of autoantibodies to β2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J. Clin. Investig. 2002, 109, 797–804. [Google Scholar] [CrossRef]
- Wang, D.; Nagasawa, T.; Chen, Y.; Ushida, Y.; Kobayashi, H.; Takeuchi, Y.; Umeda, M.; Izumi, Y. Molecular mimicry of Aggregatibacter actinomycetemcomitans with β2 glycoprotein I. Oral Microbiol. Immunol. 2008, 23, 401–405. [Google Scholar] [CrossRef]
- Massarenti, L.; Enevold, C.; Damgaard, D.; Ødum, N.; Nielsen, C.H.; Jacobsen, S. Peptidylarginine deiminase-4 gene polymorphisms are associated with systemic lupus erythematosus and lupus nephritis. Scand. J. Rheumatol. 2018, 48, 133–140. [Google Scholar] [CrossRef]
- Tan, E.M.; Cohen, A.S.; Fries, J.F.; Masi, A.T.; Mcshane, D.J.; Rothfield, N.F.; Schaller, J.G.; Talal, N.; Winchester, R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982, 25, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Hocberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Erikstrup, C.; Sørensen, E.; Nielsen, K.R.; Bruun, M.T.; Petersen, M.S.; Rostgaard, K.; Thørner, L.W.; Larsen, M.; Mikkelsen, S.; Dinh, K.M.; et al. Cohort Profile: The Danish Blood Donor Study. Leuk. Res. 2022, 52, e162–e171. [Google Scholar] [CrossRef] [PubMed]
- Andersson, T.; Alfredsson, L.; Källberg, H.; Zdravkovic, S.; Ahlbom, A. Calculating measures of biological interaction. Eur. J. Epidemiol. 2005, 20, 575–579. [Google Scholar] [CrossRef] [PubMed]
| SLE (n = 223) | Healthy Controls (n = 301) | p-Value | |
|---|---|---|---|
| Age, median years (range) | 40 (17–72) | 48 (20–71) | <10−4 |
| Women, n (%) | 201 (90%) | 198 (66%) | <10−4 |
| Ever smokers, n (%) | 122 (55%) | 42 (14%) | <10−4 |
| Anti-dsDNA antibodies +, n (%) | 176 (79%) | n.a. | |
| Anti-Smith antibodies +, n (%) | 26 (12%) | n.a. | |
| Anti-U1RNP antibodies +, n (%) | 36 (16%) | n.a. | |
| Anti-cardiolipin antibodies +, n (%) | 109 (49%) | n.a. |
| Risk Factors | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Oral Carriage | Ever Smoker | |||
| P. gingivalis | ||||
| - | - | 1 (ref) | ||
| + | - | 2.65 | 1.33–5.29 | <0.01 |
| - | + | 10.34 | 5.91–18.08 | <10−15 |
| + | + | 42.48 | 14.5–124.58 | <10−11 |
| AP | 0.72 (0.41–1.02) | |||
| A. actinomycetemcomitans | ||||
| - | - | 1 (ref) | ||
| + | - | 2.91 | 1.57–5.40 | <0.001 |
| - | + | 13.57 | 7.74–23.77 | <10−19 |
| + | + | 23.29 | 7.43–72.97 | <10−7 |
| AP | 0.34 (−0.42–1.09) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massarenti, L.; Leffers, H.C.B.; Brodersen, T.; Hansen, P.R.; Damgaard, C.; Pedersen, O.B.; Jacobsen, S.; Nielsen, C.H. Antibodies to Periodontal Bacteria Are Associated with Systemic Lupus Erythematosus and Autoantibody Positivity. Int. J. Mol. Sci. 2025, 26, 10719. https://doi.org/10.3390/ijms262110719
Massarenti L, Leffers HCB, Brodersen T, Hansen PR, Damgaard C, Pedersen OB, Jacobsen S, Nielsen CH. Antibodies to Periodontal Bacteria Are Associated with Systemic Lupus Erythematosus and Autoantibody Positivity. International Journal of Molecular Sciences. 2025; 26(21):10719. https://doi.org/10.3390/ijms262110719
Chicago/Turabian StyleMassarenti, Laura, Henrik Christian Bidstrup Leffers, Thorsten Brodersen, Peter Riis Hansen, Christian Damgaard, Ole Birger Pedersen, Søren Jacobsen, and Claus Henrik Nielsen. 2025. "Antibodies to Periodontal Bacteria Are Associated with Systemic Lupus Erythematosus and Autoantibody Positivity" International Journal of Molecular Sciences 26, no. 21: 10719. https://doi.org/10.3390/ijms262110719
APA StyleMassarenti, L., Leffers, H. C. B., Brodersen, T., Hansen, P. R., Damgaard, C., Pedersen, O. B., Jacobsen, S., & Nielsen, C. H. (2025). Antibodies to Periodontal Bacteria Are Associated with Systemic Lupus Erythematosus and Autoantibody Positivity. International Journal of Molecular Sciences, 26(21), 10719. https://doi.org/10.3390/ijms262110719






