Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages
Abstract
1. Introduction
2. Results
2.1. GroEL Accelerates AAA Formation in CaCl2 Immersion-Induced Mice
2.2. GroEL Induces Higher Cytokine Production in CaCl2 Immersion-Induced AAA Rats
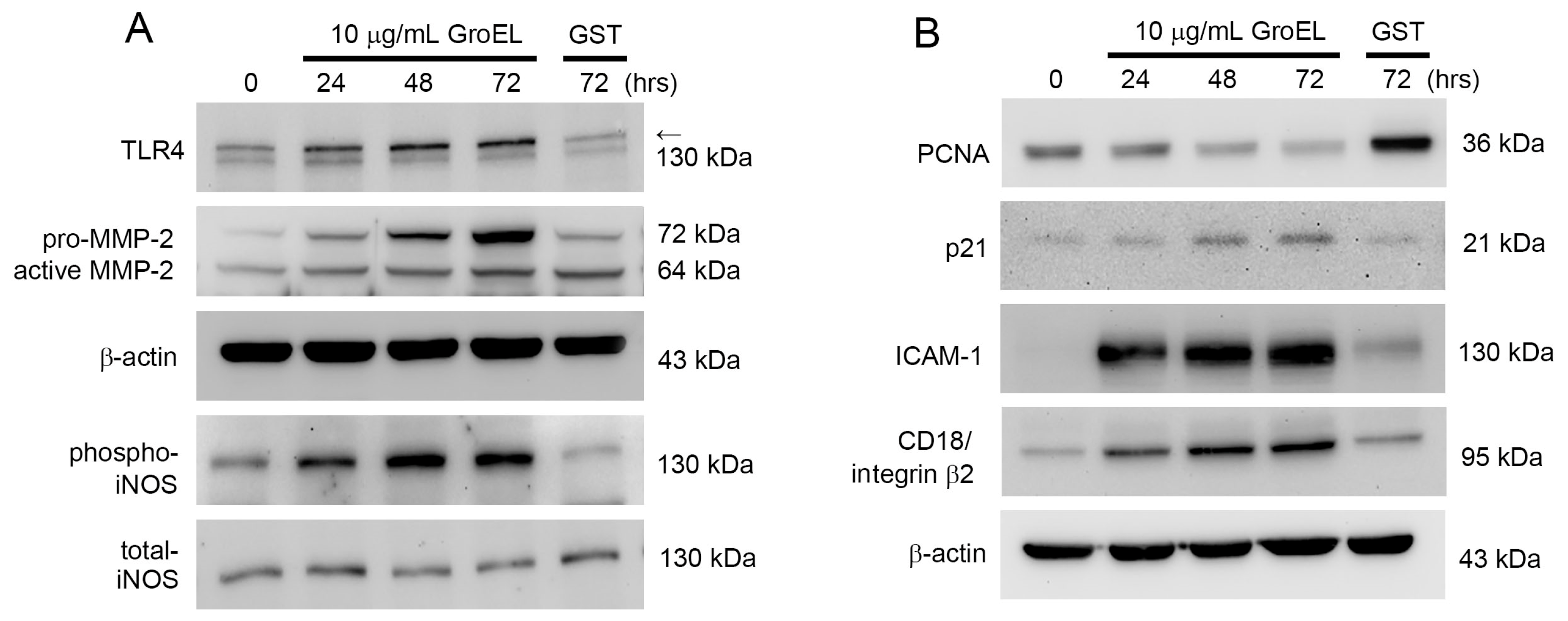
2.3. GroEL Induces Protein Expression Associated with Inflammation and AAA Formation in THP-1 Cells
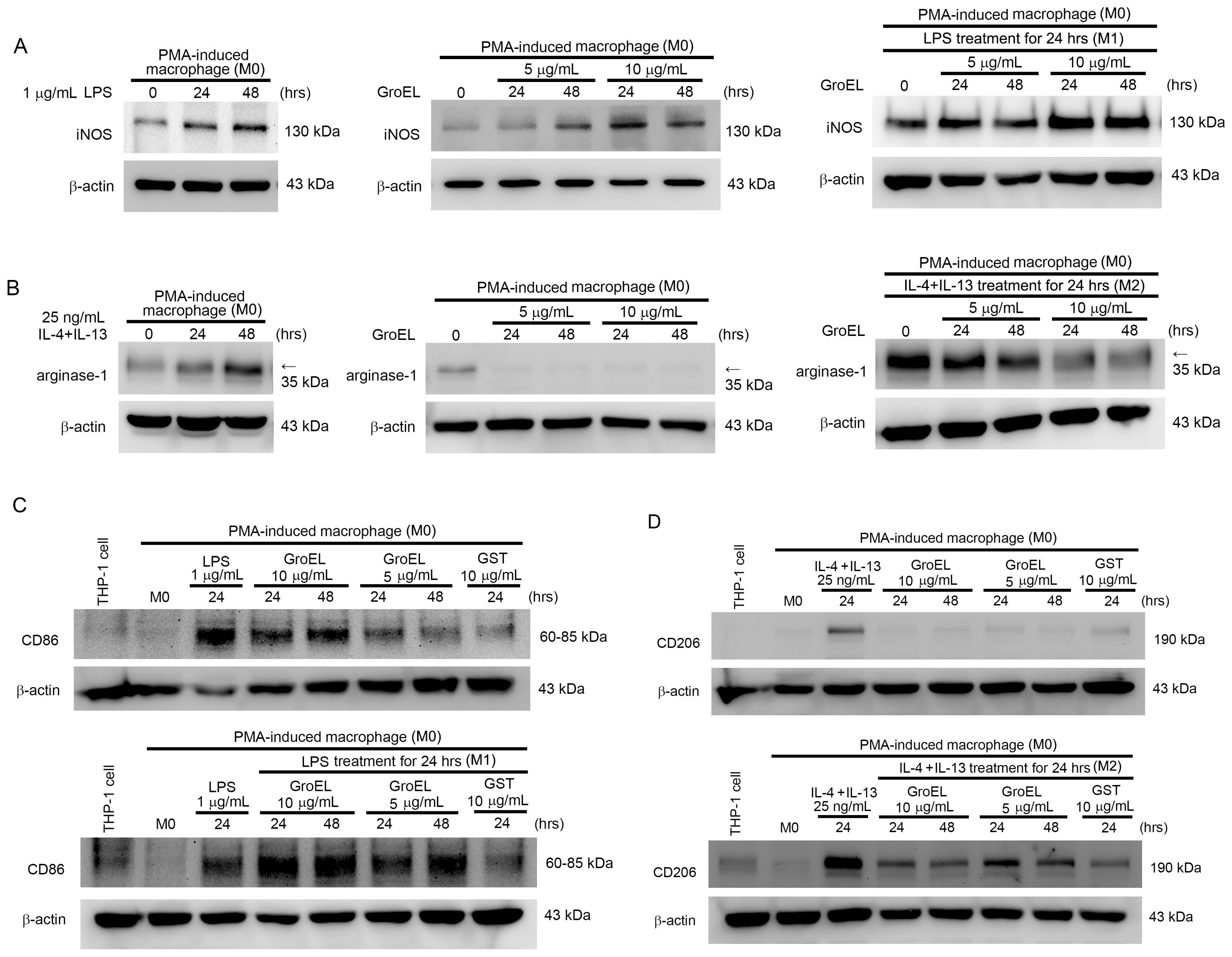
2.4. GroEL Induces M1 Macrophage Polarization and Inhibits IL-4/IL-13-Induced M2 Macrophage Polarization
2.5. GroEL Induces M1 Macrophage Polarization via TM and IRF5 Expression
2.6. GroEL Accelerates AAA Formation in Ang II-Induced Mice Through Induction of M1 Polarization of Macrophages
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.1.1. Induction of AAA in Rats Through CaCl2 Immersion
4.1.2. Induction of AAA in Mice Through Administration of Angiotensin II
4.1.3. Morphological Analysis Using Hematoxylin and Eosin Staining and Micro-Computed Tomography
4.1.4. Immunohistochemistry
4.1.5. Enzyme-Linked Immunosorbent Assay
4.1.6. Verhoeff–Van Gieson Staining and IHC
4.2. In Vitro Study
4.2.1. Cultivation of THP-1 Cells
4.2.2. Production and Purification of Recombinant P. gingivalis GroEL Protein
4.2.3. Knockdown of TM Gene Expression by siRNA Transfection
4.2.4. Flow Cytometry for Analysis of Cell Differentiation
4.2.5. Western Blot Analysis for Protein Production
4.3. Statistical Analyses
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | abdominal aortic aneurysm |
| Ang II | angiotensin II |
| CRP | C-reactive protein |
| eNOS | endothelial nitric oxide synthase |
| GST | glutathione S-transferase |
| HSP60 | heat shock protein 60 |
| H&E | hematoxylin and eosin |
| iNOS | inducible nitric oxide synthase |
| ICAM | intercellular adhesion molecule |
| IRF5 | interferon regulatory factor 5 |
| IFN-γ | interferon gamma |
| IL | interleukin |
| LPS | lipopolysaccharide |
| MMP | metalloproteinase |
| PMA | phorbol myristate acetate |
| P. gingivalis | Porphyromonas gingivalis |
| PCNA | proliferating cell nuclear antigen |
| SMCs | smooth muscle cells |
| TM | thrombomodulin |
| TLR4 | toll-like receptor 4 |
| VVG | Verhoeff–Van Gieson |
References
- Ding, F.; Wu, D.; Han, X.; Cheng, L.J.; Sun, Z.; Lv, Y.L. Oral hygiene and periodontal conditions in the Chinese patients with aortic aneurysm. BMC Oral Health 2018, 18, 136. [Google Scholar] [CrossRef]
- Suzuki, J.; Aoyama, N.; Aoki, M.; Tada, Y.; Wakayama, K.; Akazawa, H.; Shigematsu, K.; Hoshina, K.; Izumi, Y.; Komuro, I.; et al. High incidence of periodontitis in Japanese patients with abdominal aortic aneurysm. Int. Heart J. 2014, 55, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Aoyama, N.; Aoki, M.; Tada, Y.; Wakayama, K.; Akazawa, H.; Shigematsu, K.; Hoshina, K.; Izumi, Y.; Komuro, I.; et al. Incidence of periodontitis in Japanese patients with cardiovascular diseases: A comparison between abdominal aortic aneurysm and arrhythmia. Heart Vessel. 2015, 30, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Liu, B.; Gao, P.; Wang, C.; Fang, S.; Huo, Z.; Song, Q.; Dong, D.; Wu, X.; Li, G. RAGE deficiency ameliorates abdominal aortic aneurysm progression. Inflamm. Res. 2025, 74, 63. [Google Scholar] [CrossRef]
- Kurihara, N.; Inoue, Y.; Iwai, T.; Umeda, M.; Huang, Y.; Ishikawa, I. Detection and localization of periodontopathic bacteria in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2004, 28, 553–558. [Google Scholar] [CrossRef]
- Kregielczak, A.; Dorocka-Bobkowska, B.; Slomski, R.; Oszkinis, G.; Krasinski, Z. Periodontal status and the incidence of selected bacterial pathogens in periodontal pockets and vascular walls in patients with atherosclerosis and abdominal aortic aneurysms. PLoS ONE 2022, 17, e0270177. [Google Scholar] [CrossRef] [PubMed]
- Marques da Silva, R.; Caugant, D.A.; Lingaas, P.S.; Geiran, O.; Tronstad, L.; Olsen, I. Detection of Actinobacillus actinomycetemcomitans but not bacteria of the red complex in aortic aneurysms by multiplex polymerase chain reaction. J. Periodontol. 2005, 76, 590–594. [Google Scholar] [CrossRef]
- Okuda, K.; Ishihara, K.; Nakagawa, T.; Hirayama, A.; Inayama, Y.; Okuda, K. Detection of Treponema denticola in atherosclerotic lesions. J. Clin. Microbiol. 2001, 39, 1114–1117. [Google Scholar] [CrossRef]
- Deshpande, R.G.; Khan, M.B.; Genco, C.A. Invasion of aortic and heart endothelial cells by Porphyromonas gingivalis. Infect. Immun. 1998, 66, 5337–5343. [Google Scholar] [CrossRef]
- Deshpande, R.G.; Khan, M.; Genco, C.A. Invasion strategies of the oral pathogen Porphyromonas gingivalis: Implications for cardiovascular disease. Invasion Metastasis 1998, 18, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Isogai, E.; Ueda, I. Porphyromonas gingivalis fimbriae induce adhesion of monocytic cell line U937 to endothelial cells. Microbiol. Immunol. 2000, 44, 17–22. [Google Scholar] [CrossRef]
- Khlgatian, M.; Nassar, H.; Chou, H.H.; Gibson, F.C., 3rd; Genco, C.A. Fimbria-dependent activation of cell adhesion molecule expression in Porphyromonas gingivalis-infected endothelial cells. Infect. Immun. 2002, 70, 257–267. [Google Scholar] [CrossRef]
- Delbosc, S.; Alsac, J.M.; Journe, C.; Louedec, L.; Castier, Y.; Bonnaure-Mallet, M.; Ruimy, R.; Rossignol, P.; Bouchard, P.; Michel, J.B.; et al. Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats. PLoS ONE 2011, 6, e18679. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, M.; Silveira, A.; Eriksson, P.; Swedenborg, J. Protease activity in the multi-layered intra-luminal thrombus of abdominal aortic aneurysms. Atherosclerosis 2011, 218, 294–299. [Google Scholar] [CrossRef]
- Swedenborg, J.; Eriksson, P. The intraluminal thrombus as a source of proteolytic activity. Ann. N. Y. Acad. Sci. 2006, 1085, 133–138. [Google Scholar] [CrossRef]
- Kim, H.W.; Blomkalns, A.L.; Ogbi, M.; Thomas, M.; Gavrila, D.; Neltner, B.S.; Cassis, L.A.; Thompson, R.W.; Weiss, R.M.; Lindower, P.D.; et al. Role of myeloperoxidase in abdominal aortic aneurysm formation: Mitigation by taurine. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1168–H1179. [Google Scholar] [CrossRef]
- Ramos-Mozo, P.; Madrigal-Matute, J.; Martinez-Pinna, R.; Blanco-Colio, L.M.; Lopez, J.A.; Camafeita, E.; Meilhac, O.; Michel, J.B.; Aparicio, C.; Vega de Ceniga, M.; et al. Proteomic analysis of polymorphonuclear neutrophils identifies catalase as a novel biomarker of abdominal aortic aneurysm: Potential implication of oxidative stress in abdominal aortic aneurysm progression. Arter. Thromb. Vasc. Biol. 2011, 31, 3011–3019. [Google Scholar] [CrossRef]
- McCormick, M.L.; Gavrila, D.; Weintraub, N.L. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arter. Thromb. Vasc. Biol. 2007, 27, 461–469. [Google Scholar] [CrossRef]
- Lin, F.Y.; Huang, C.Y.; Lu, H.Y.; Shih, C.M.; Tsao, N.W.; Shyue, S.K.; Lin, C.Y.; Chang, Y.J.; Tsai, C.S.; Lin, Y.W.; et al. The GroEL protein of Porphyromonas gingivalis accelerates tumor growth by enhancing endothelial progenitor cell function and neovascularization. Mol. Oral Microbiol. 2015, 30, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Shih, C.M.; Tsao, N.W.; Lin, Y.W.; Shih, C.C.; Chiang, K.H.; Shyue, S.K.; Chang, Y.J.; Hsieh, C.K.; Lin, F.Y. The GroEL protein of Porphyromonas gingivalis regulates atherogenic phenomena in endothelial cells mediated by upregulating toll-like receptor 4 expression. Am. J. Transl. Res. 2016, 8, 384–404. [Google Scholar] [PubMed]
- Lin, F.Y.; Hsiao, F.P.; Huang, C.Y.; Shih, C.M.; Tsao, N.W.; Tsai, C.S.; Yang, S.F.; Chang, N.C.; Hung, S.L.; Lin, Y.W. Porphyromonas gingivalis GroEL induces osteoclastogenesis of periodontal ligament cells and enhances alveolar bone resorption in rats. PLoS ONE 2014, 9, e102450. [Google Scholar] [CrossRef]
- Wilmink, A.B.; Quick, C.R. Epidemiology and potential for prevention of abdominal aortic aneurysm. Br. J. Surg. 1998, 85, 155–162. [Google Scholar] [CrossRef]
- Lu, H.Y.; Huang, C.Y.; Shih, C.M.; Chang, W.H.; Tsai, C.S.; Lin, F.Y.; Shih, C.C. Dipeptidyl peptidase-4 inhibitor decreases abdominal aortic aneurysm formation through GLP-1-dependent monocytic activity in mice. PLoS ONE 2015, 10, e0121077. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Y.; Huang, C.Y.; Shih, C.M.; Lin, Y.W.; Tsai, C.S.; Lin, F.Y.; Shih, C.C. A potential contribution of dipeptidyl peptidase-4 by the mediation of monocyte differentiation in the development and progression of abdominal aortic aneurysms. J. Vasc. Surg. 2017, 66, 1217–1226.e1211. [Google Scholar] [CrossRef] [PubMed]
- Salhi, L.; Rijkschroeff, P.; Van Hede, D.; Laine, M.L.; Teughels, W.; Sakalihasan, N.; Lambert, F. Blood Biomarkers and Serologic Immunological Profiles Related to Periodontitis in Abdominal Aortic Aneurysm Patients. Front. Cell. Infect. Microbiol. 2021, 11, 766462. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Lin, F.Y.; Lai, Z.H.; Tsai, C.S.; Tsai, Y.T.; Huang, Y.S.; Liu, C.W. Porphyromonas gingivalis GroEL accelerates abdominal aortic aneurysm formation by matrix metalloproteinase-2 SUMOylation in vascular smooth muscle cells: A novel finding for the activation of MMP-2. Mol. Oral Microbiol. 2024; Online ahead of print. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhou, Y.Z.; Wu, Y.; Wu, Q.Y.; Liao, X.B.; Fu, X.M.; Zhou, X.M. Diverse roles of macrophage polarization in aortic aneurysm: Destruction and repair. J. Transl. Med. 2018, 16, 354. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, J.; Meng, X.; Lu, X.; Zhang, L.; Chen, R. Polarized Macrophages in Periodontitis: Characteristics, Function, and Molecular Signaling. Front. Immunol. 2021, 12, 763334. [Google Scholar] [CrossRef]
- Bianchi, S.; Torge, D.; Rinaldi, F.; Piattelli, M.; Bernardi, S.; Varvara, G. Platelets’ Role in Dentistry: From Oral Pathology to Regenerative Potential. Biomedicines 2022, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Hu, M.; Shen, M.; Kassiri, Z. Extracellular matrix, regional heterogeneity of the aorta, and aortic aneurysm. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Shi, G.P. Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2006, 31, 453–463. [Google Scholar] [CrossRef]
- Nakagomi, A. Effect of toll-like receptor in periodontal bacteria-accelerated abdominal aortic aneurysms. Circ. J. 2013, 77, 1414–1415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elmore, J.R.; Keister, B.F.; Franklin, D.P.; Youkey, J.R.; Carey, D.J. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann. Vasc. Surg. 1998, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Liapis, C.D. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr. Med. Res. Opin. 2004, 20, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, T.R.; Tarin, C.; Gomez, M.; Lavin, B.; Aracil, E.; Orte, L.M.; Zaragoza, C. Nitric oxide induces the progression of abdominal aortic aneurysms through the matrix metalloproteinase inducer EMMPRIN. Am. J. Pathol. 2009, 175, 1421–1430. [Google Scholar] [CrossRef]
- Lee, K.Y. M1 and M2 polarization of macrophages: A mini-review. Med. Biol. Sci. Eng. 2019, 2, 5. [Google Scholar] [CrossRef]
- Tsai, C.S.; Lin, Y.W.; Huang, C.Y.; Shih, C.M.; Tsai, Y.T.; Tsao, N.W.; Lin, C.S.; Shih, C.C.; Jeng, H.; Lin, F.Y. Thrombomodulin regulates monocye differentiation via PKCdelta and ERK1/2 pathway in vitro and in atherosclerotic artery. Sci. Rep. 2016, 6, 38421. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Mitchell, R.N.; Libby, P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arter. Thromb. Vasc. Biol. 2006, 26, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Wassef, M.; Baxter, B.T.; Chisholm, R.L.; Dalman, R.L.; Fillinger, M.F.; Heinecke, J.; Humphrey, J.D.; Kuivaniemi, H.; Parks, W.C.; Pearce, W.H.; et al. Pathogenesis of abdominal aortic aneurysms: A multidisciplinary research program supported by the National Heart, Lung, and Blood Institute. J. Vasc. Surg. 2001, 34, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.; Gineitis, A.; Wagberg, F.; Angquist, K.A. Activity of matrix metalloproteinase-2 and -9 in abdominal aortic aneurysms. Relation to size and rupture. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Bode, M.K.; Soini, Y.; Melkko, J.; Satta, J.; Risteli, L.; Risteli, J. Increased amount of type III pN-collagen in human abdominal aortic aneurysms: Evidence for impaired type III collagen fibrillogenesis. J. Vasc. Surg. 2000, 32, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Krettek, A.; Sukhova, G.K.; Libby, P. Elastogenesis in human arterial disease: A role for macrophages in disordered elastin synthesis. Arter. Thromb. Vasc. Biol. 2003, 23, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, L.; Bergqvist, D.; Lindback, J.; Parsson, H. Expansion of small-diameter abdominal aortic aneurysms is not reflected by the release of inflammatory mediators IL-6, MMP-9 and CRP in plasma. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Blomkalns, A.L.; Gavrila, D.; Thomas, M.; Neltner, B.S.; Blanco, V.M.; Benjamin, S.B.; McCormick, M.L.; Stoll, L.L.; Denning, G.M.; Collins, S.P.; et al. CD14 directs adventitial macrophage precursor recruitment: Role in early abdominal aortic aneurysm formation. J. Am. Heart Assoc. 2013, 2, e000065. [Google Scholar] [CrossRef]
- Li, F.; Downing, B.D.; Smiley, L.C.; Mund, J.A.; Distasi, M.R.; Bessler, W.K.; Sarchet, K.N.; Hinds, D.M.; Kamendulis, L.M.; Hingtgen, C.M.; et al. Neurofibromin-deficient myeloid cells are critical mediators of aneurysm formation in vivo. Circulation 2014, 129, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Tazume, H.; Miyata, K.; Tian, Z.; Endo, M.; Horiguchi, H.; Takahashi, O.; Horio, E.; Tsukano, H.; Kadomatsu, T.; Nakashima, Y.; et al. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arter. Thromb. Vasc. Biol. 2012, 32, 1400–1409. [Google Scholar] [CrossRef]
- Daugherty, A.; Powell, J.T. Recent highlights of ATVB: Aneurysms. Arter. Thromb. Vasc. Biol. 2014, 34, 691–694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lentz, S.R.; Chen, Y.; Sadler, J.E. Sequences required for thrombomodulin cofactor activity within the fourth epidermal growth factor-like domain of human thrombomodulin. J. Biol. Chem. 1993, 268, 15312–15317. [Google Scholar] [CrossRef] [PubMed]
- Bajzar, L. Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arter. Thromb. Vasc. Biol. 2000, 20, 2511–2518. [Google Scholar] [CrossRef] [PubMed]
- Senet, P.; Peyri, N.; Berard, M.; Dubertret, L.; Boffa, M.C. Thrombomodulin, a functional surface protein on human keratinocytes, is regulated by retinoic acid. Arch. Dermatol. Res. 1997, 289, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Nishioka, J.; Hayashi, T.; Kosaka, Y. Functionally active thrombomodulin is present in human platelets. J. Biochem. 1988, 104, 628–632. [Google Scholar] [CrossRef] [PubMed]
- McCachren, S.S.; Diggs, J.; Weinberg, J.B.; Dittman, W.A. Thrombomodulin expression by human blood monocytes and by human synovial tissue lining macrophages. Blood 1991, 78, 3128–3132. [Google Scholar] [CrossRef]
- Soff, G.A.; Jackman, R.W.; Rosenberg, R.D. Expression of thrombomodulin by smooth muscle cells in culture: Different effects of tumor necrosis factor and cyclic adenosine monophosphate on thrombomodulin expression by endothelial cells and smooth muscle cells in culture. Blood 1991, 77, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Chung, H.C.; Luo, C.Y.; Chao, T.H.; Shyu, K.G.; Shi, G.Y.; Wu, H.L. Thrombomodulin is upregulated in cardiomyocytes during cardiac hypertrophy and prevents the progression of contractile dysfunction. J. Card. Fail. 2010, 16, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, N.A.; Blevins, E.A.; Miller, W.M.; Perry, A.R.; Talmage, K.E.; Mullins, E.S.; Flick, M.J.; Queiroz, K.C.; Shi, K.; Spek, C.A.; et al. Thrombomodulin is a determinant of metastasis through a mechanism linked to the thrombin binding domain but not the lectin-like domain. Blood 2011, 118, 2889–2895. [Google Scholar] [CrossRef]
- Zhang, Y.; Weiler-Guettler, H.; Chen, J.; Wilhelm, O.; Deng, Y.; Qiu, F.; Nakagawa, K.; Klevesath, M.; Wilhelm, S.; Bohrer, H.; et al. Thrombomodulin modulates growth of tumor cells independent of its anticoagulant activity. J. Clin. Investig. 1998, 101, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Shi, G.Y.; Chang, Y.S.; Han, H.S.; Kuo, C.H.; Liu, C.; Huang, H.C.; Chang, Y.J.; Chen, P.S.; Wu, H.L. Evidence of human thrombomodulin domain as a novel angiogenic factor. Circulation 2005, 111, 1627–1636. [Google Scholar] [CrossRef]
- Huang, H.C.; Shi, G.Y.; Jiang, S.J.; Shi, C.S.; Wu, C.M.; Yang, H.Y.; Wu, H.L. Thrombomodulin-mediated cell adhesion: Involvement of its lectin-like domain. J. Biol. Chem. 2003, 278, 46750–46759. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouwer, M.; Collen, D.; Conway, E.M. Thrombomodulin-protein C-EPCR system: Integrated to regulate coagulation and inflammation. Arter. Thromb. Vasc. Biol. 2004, 24, 1374–1383. [Google Scholar] [CrossRef] [PubMed]
- Koutsi, A.; Papapanagiotou, A.; Papavassiliou, A.G. Thrombomodulin: From haemostasis to inflammation and tumourigenesis. Int. J. Biochem. Cell Biol. 2008, 40, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.S.; Tsai, Y.T.; Lin, C.Y.; Lin, T.C.; Huang, G.S.; Hong, G.J.; Lin, F.Y. Expression of thrombomodulin on monocytes is associated with early outcomes in patients with coronary artery bypass graft surgery. Shock 2010, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Huang, C.Y.; Shih, C.M.; Chang, W.L.; Shyue, S.K.; Tsai, Y.T.; Lin, C.Y.; Lee, C.Y.; Chang, Y.J.; Chang, N.C.; et al. The C-Terminal Domain of Thrombomodulin Regulates Monocyte Migration with Interleukin-6 Stimulation. Eur. J. Inflamm. 2014, 12, 27–39. [Google Scholar] [CrossRef]
- Wang, Y.; Krishna, S.; Golledge, J. The calcium chloride-induced rodent model of abdominal aortic aneurysm. Atherosclerosis 2013, 226, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Shih, C.M.; Huang, C.Y.; Tsai, Y.T.; Loh, S.H.; Li, C.Y.; Lin, C.Y.; Lin, Y.W.; Tsai, C.S. Dipeptidyl Peptidase-4 Inhibitor Decreases Allograft Vasculopathy Via Regulating the Functions of Endothelial Progenitor Cells in Normoglycemic Rats. Cardiovasc. Drugs Ther. 2021, 35, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, J.L.; Stubbe, J.; Lindholt, J.S. Animal models used to explore abdominal aortic aneurysms: A systematic review. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 487–499. [Google Scholar] [CrossRef] [PubMed]
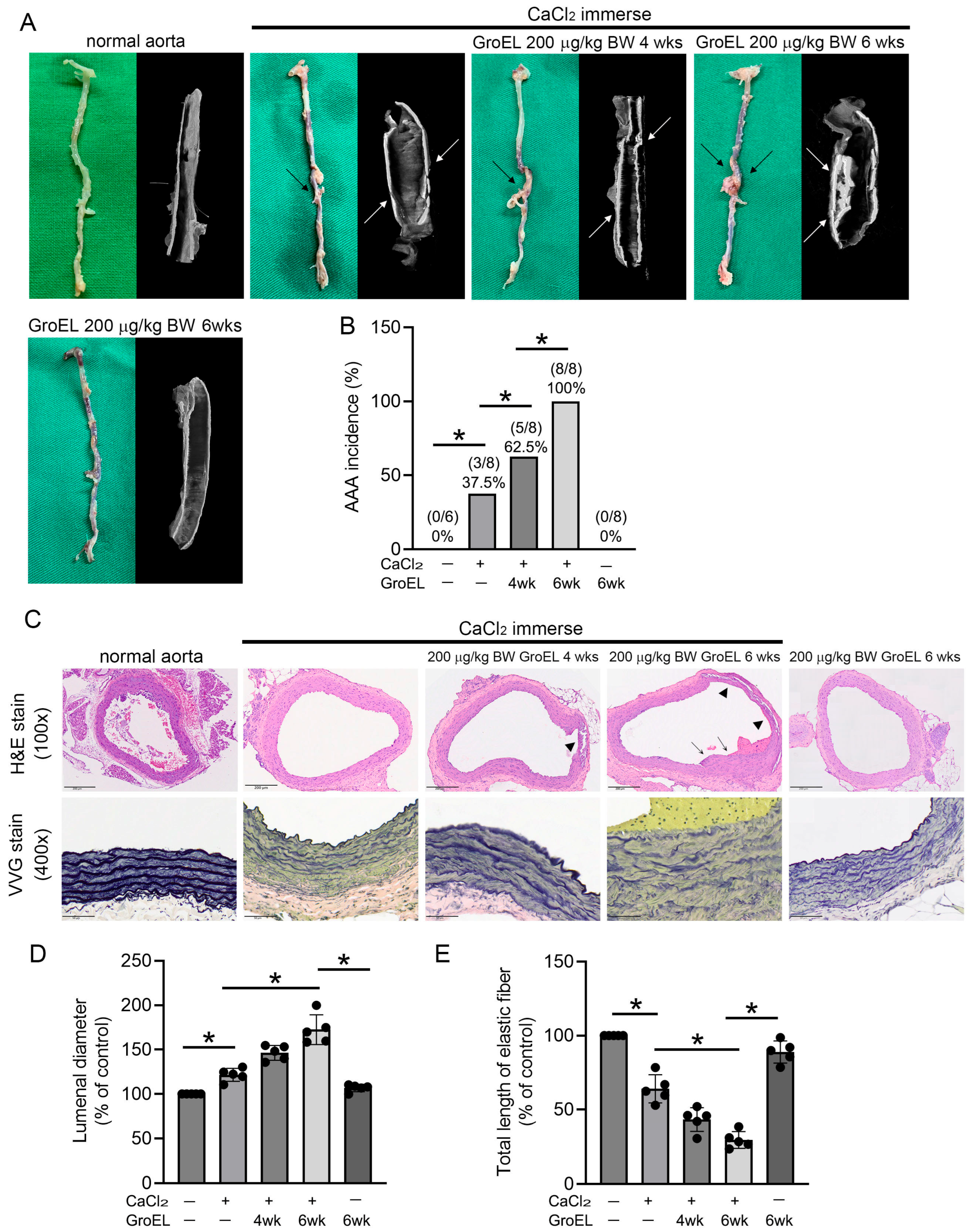


| Control (Naïve) | CaCl2 Immerse | CaCl2 Immerse Plus 200 μg/kg BW GroEL | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 Weeks | Baseline | 6 Weeks | Baseline | 6 Weeks | |
| Body weight (g) | 305.8 ± 9.8 | 340.3 ± 9.5 | 318.0 ± 10.9 | 344.8 ± 12.4 | 311.3 ± 8.0 | 334.8 ± 15.0 |
| CRP (mg/dL) | 31.3 ± 7.2 | 40.8 ± 9.1 | 37.3 ± 7.8 | 149.5 ± 29.3 ab | 32.8 ± 9.4 | 288.8 ± 19.8 abc |
| TNF-α (pg/mL) | 67.9 ± 14.3 | 55.3 ± 7.7 | 52.1 ± 24.9 | 940.0 ± 178.6 ab | 41.7 ± 22.2 | 1731.0 ± 119.5 abc |
| IL-1β (pg/mL) | 75.5 ± 5.8 | 73.8 ± 6.9 | 80.1 ± 6.7 | 105.2 ± 9.7 ab | 77.0 ± 7.3 | 331.8 ± 53.6 abc |
| IL-6 (pg/mL) | 28.8 ± 4.3 | 27.1 ± 1.5 | 27.1 ± 3.8 | 39.9 ± 10.7 ab | 29.7 ± 2.0 | 291.7 ± 76.1 abc |
| IL-2 (pg/mL) | 3.5 ± 0.8 | 3.7 ± 1.0 | 3.5 ± 0.7 | 3.7 ± 0.6 | 3.5 ± 0.8 | 9.7 ± 2.0 abc |
| INF-γ (pg/mL) | 3.5 ± 1.0 | 3.7 ± 1.1 | 3.2 ± 0.6 | 24.8 ± 4.3 ab | 3.2 ± 0.5 | 46.0 ± 13.9 abc |
| Control | PMA | 5 μg/mL GroEL | 10 μg/mL GroEL | |
|---|---|---|---|---|
| G0/G1 phase | 21.6 ± 5.5% | 56.9 ± 8.1% p < 0.05 | 64.1 ± 1.9% p < 0.05 | 68.4 ± 9.1% p < 0.05 |
| S phase | 55.7 ± 7.2% | 29.7 ± 6.4% p < 0.05 | 20.4 ± 5.6% p < 0.05 | 19.4 ± 2.3% p < 0.05 |
| G2/M phase | 16.5 ± 6.1% | 6.4 ± 3.1% p < 0.05 | 6.6 ± 2.3% p < 0.05 | 7.2 ± 3.1% p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-W.; Tsai, Y.-T.; Cheng, M.-J.; Shih, C.-M.; Huang, C.-Y.; Tsai, C.-S.; Sung, S.-Y.; Lai, Z.-H.; Liu, C.-W.; Lin, F.-Y. Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages. Int. J. Mol. Sci. 2025, 26, 7781. https://doi.org/10.3390/ijms26167781
Lin Y-W, Tsai Y-T, Cheng M-J, Shih C-M, Huang C-Y, Tsai C-S, Sung S-Y, Lai Z-H, Liu C-W, Lin F-Y. Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages. International Journal of Molecular Sciences. 2025; 26(16):7781. https://doi.org/10.3390/ijms26167781
Chicago/Turabian StyleLin, Yi-Wen, Yi-Ting Tsai, Ming-Jen Cheng, Chun-Ming Shih, Chun-Yao Huang, Chien-Sung Tsai, Shih-Ying Sung, Ze-Hao Lai, Chen-Wei Liu, and Feng-Yen Lin. 2025. "Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages" International Journal of Molecular Sciences 26, no. 16: 7781. https://doi.org/10.3390/ijms26167781
APA StyleLin, Y.-W., Tsai, Y.-T., Cheng, M.-J., Shih, C.-M., Huang, C.-Y., Tsai, C.-S., Sung, S.-Y., Lai, Z.-H., Liu, C.-W., & Lin, F.-Y. (2025). Porphyromonas gingivalis GroEL Accelerates Abdominal Aortic Aneurysm Formation by Induction of M1 Polarization in Macrophages. International Journal of Molecular Sciences, 26(16), 7781. https://doi.org/10.3390/ijms26167781







