Aerogels Part 2. A Focus on the Less Patented and Marketed Airy Inorganic Networks Despite the Plethora of Possible Advanced Applications
Abstract
1. Introduction
2. Subclassification of Inorganic AGs
3. Conventional Synthetic Methods to Achieve AGs
3.1. Microstructure of AGs Prepared via the Synthetic Methods Previously Described
3.1.1. Sol–Gel Methods Starting from Molecular Precursors
3.1.2. Improved Sol–Gel Methods
Epoxide Addition (EA) Method
Dispersed Inorganic Method (DIS)
3.1.3. Nanoparticle-Based Methods
4. More in Deep in the Less Patented Classes of Inorganic Aerogels
4.1. Chalcogenides-Based Aerogels (CAGs)
4.1.1. Synthetic Methods and Possible Applications of Metal Chalcogenide Aerogels (MCAGs) and Mesoporous MCAGs (MMCAGs)
4.1.2. Case Studies Concerning Some Relevant Applications of CAGs
4.1.3. Author’s Summary and Considerations on CAGs
Current Research Status and Trends of CAGs with Key Issues in Future Research and Directions for Their Future Development
4.2. Metal-Based Aerogels (MAGs)
4.2.1. Sol–Gel Methods to Prepare Noble Metal and Metal Aerogels (NMAGs, MAGs)
4.2.2. Microstructure of MAGs by Sol–Gel Methods
4.2.3. Non-Sol–Gel Methods to Prepare Metallic Aerogels
Microstructure of MAGs by Non-Sol–Gel Methods
Dealloying Method
Combustion Method
Bio Templating Method
Salts Templating Methods
4.2.4. Case Studies on Synthesis and Applications of NMAGs and MAGs
Case Studies on Synthesis and Applications of Other MAGs
5. Interdisciplinarity of Aerogels
Current Research Hotspots and Future Development Trends: Author Consideration
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Wu, Y.; Liu, T.; Shi, Y.; Wang, H. Dramatically Enhancing Mechanical Properties of Hydrogels by Drying Reactive Polymers at Elevated Temperatures to Introduce Strong Physical and Chemical Crosslinks. Polymers 2022, 249, 124842. [Google Scholar] [CrossRef]
- Montes, S.; Maleki, H. Aerogels and Their Applications. In Colloidal Metal Oxide Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 337–399. [Google Scholar] [CrossRef]
- Hina Goyal Beyond Insulation: New Applications for Aerogels. Available online: https://www.cas.org/resources/cas-insights/aerogel-applications#:~:text=Inorganic%20aerogels%20not%20only%20encompass%20silica%20aerogels%20but,precursor%20materials%20like%20metal%20alkoxides%20or%20metal%20salts (accessed on 26 June 2025).
- Pajonk, G.M. Catalytic Aerogels. Catal. Today 1997, 35, 319–337. [Google Scholar] [CrossRef]
- Schneider, M.; Baiker, A. Aerogels in Catalysis. Catal. Rev. 1995, 37, 515–556. [Google Scholar] [CrossRef]
- Vallribera, A.; Molins, E. Aerogel Supported Nanoparticles in Catalysis. In Nanoparticles and Catalysis; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rechberger, F.; Niederberger, M. Synthesis of Aerogels: From Molecular Routes to 3-Dimensional Nanoparticle Assembly. Nanoscale Horiz. 2017, 2, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Hosticka, B.; Norris, P.M.; Brenizer, J.S.; Daitch, C.E. Gas Flow through Aerogels. J. Non Cryst. Solids 1998, 225, 293–297. [Google Scholar] [CrossRef]
- Aegerter, M.A.; Leventis, N.; Koebel, M.M. (Eds.) Aerogels Handbook; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7477-8. [Google Scholar]
- Burchell, M.J.; Graham, G.; Kearsley, A. Cosmic Dust Collection in Aerogel. Annu. Rev. Earth Planet Sci. 2006, 34, 385–418. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Hrubesh, L.W. Aerogel Applications. J. Non Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Thapliyal, P.C.; Singh, K. Aerogels as Promising Thermal Insulating Materials: An Overview. J. Mater. 2014, 2014, 127049. [Google Scholar] [CrossRef]
- Ayen, R.J.; Iacobucci, P.A. Metal Oxide Aerogel Preparation by Supercritical Extraction. Rev. Chem. Eng. 1988, 5, 157–198. [Google Scholar] [CrossRef]
- Gesser, H.D.; Goswami, P.C. Aerogels and Related Porous Materials. Chem. Rev. 1989, 89, 765–788. [Google Scholar] [CrossRef]
- Fricke, J.; Tillotson, T. Aerogels: Production, Characterization, and Applications. Thin Solid Film. 1997, 297, 212–223. [Google Scholar] [CrossRef]
- Akimov, Y.K. Fields of Application of Aerogels (Review). Instrum. Exp. Tech. 2003, 46, 287–299. [Google Scholar] [CrossRef]
- Rolison, D.R.; Dunn, B. Electrically Conductive Oxide Aerogels: New Materials in Electrochemistry. J. Mater. Chem. 2001, 11, 963–980. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Chemere, E.B.; Mhlabeni, T.L.; Mhike, W.; Mavhungu, M.L.; Shongwe, M.B. A Comprehensive Review of Types, Synthesis Strategies, Advanced Designing and Applications of Aerogels. R. Soc. Open Sci. 2025, 12, 241975. [Google Scholar] [CrossRef]
- Gaponik, N.; Herrmann, A.K.; Eychmüller, A. Colloidal Nanocrystal-Based Gels and Aerogels: Material Aspects and Application Perspectives. J. Phys. Chem. Lett. 2012, 3, 8–17. [Google Scholar] [CrossRef]
- Meti, P.; Wang, Q.; Mahadik, D.B.; Lee, K.Y.; Gong, Y.D.; Park, H.H. Evolutionary Progress of Silica Aerogels and Their Classification Based on Composition: An Overview. Nanomaterials 2023, 13, 1498. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Sheikh, J.N. Silica Centered Aerogels as Advanced Functional Material and Their Applications: A Review. J. Non Cryst. Solids 2023, 611, 122322. [Google Scholar] [CrossRef]
- Bag, S.; Arachchige, I.U.; Kanatzidis, M.G. Aerogels from Metal Chalcogenides and Their Emerging Unique Properties. J. Mater. Chem. 2008, 18, 3628–3632. [Google Scholar] [CrossRef]
- Bangi, U.K.H.; Lee, K.-Y.; Maldar, N.M.N.; Park, H.-H. Synthesis and Properties of Metal Oxide Aerogels via Ambient Pressure Drying. J. Nanosci. Nanotechnol. 2018, 19, 1217–1227. [Google Scholar] [CrossRef]
- Qiu, J.; Cao, H.; Liao, J.; Du, R.; Dou, K.; Tsidaeva, N.; Wang, W. 3D Porous Coral-like Co1.29Ni1.71O4 Microspheres Embedded into Reduced Graphene Oxide Aerogels with Lightweight and Broadband Microwave Absorption. J. Colloid. Interface Sci. 2022, 609, 12–22. [Google Scholar] [CrossRef]
- Xiong, T.; Li, Q.; Li, K.; Zhang, Y.; Zhu, W. Construction of Novel Magnesium Oxide Aerogel for Highly Efficient Separation of Uranium(VI) from Wastewater. Sep. Purif. Technol. 2022, 295, 121296. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Lee, K.J.; Kang, Y.; Kim, Y.H.; Baek, S.W.; Hwang, H. Synthesis of Silicon Carbide Powders from Methyl-Modified Silica Aerogels. Appl. Sci. 2020, 10, 6161. [Google Scholar] [CrossRef]
- Oschatz, M.; Boukhalfa, S.; Nickel, W.; Hofmann, J.P.; Fischer, C.; Yushin, G.; Kaskel, S. Carbide-Derived Carbon Aerogels with Tunable Pore Structure as Versatile Electrode Material in High Power Supercapacitors. Carbon 2017, 113, 283–291. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Kou, Z.; Li, W.; Mu, S. RuP2-Based Catalysts with Platinum-like Activity and Higher Durability for the Hydrogen Evolution Reaction at All PH Values. Angew. Chem.-Int. Ed. 2017, 56, 11559–11564. [Google Scholar] [CrossRef]
- Jiang, W.; Ruan, Q.; Xie, J.; Chen, X.; Zhu, Y.; Tang, J. Oxygen-Doped Carbon Nitride Aerogel: A Self-Supported Photocatalyst for Solar-to-Chemical Energy Conversion. Appl. Catal. B 2018, 236, 428–435. [Google Scholar] [CrossRef]
- Tang, J.; Feng, Y.; Feng, W. Photothermal Storage and Controllable Release of a Phase-Change Azobenzene/Aluminum Nitride Aerogel Composite. Compos. Commun. 2021, 23, 100575. [Google Scholar] [CrossRef]
- Wang, B.; Li, G.; Xu, L.; Liao, J.; Zhang, X. Nanoporous Boron Nitride Aerogel Film and Its Smart Composite with Phase Change Materials. ACS Nano 2020, 14, 16590–16599. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Warchol, J.; Matusik, J.; Tseng, W.L.; Rajesh, N.; Bajda, T. Heavy Metal and Organic Dye Removal via a Hybrid Porous Hexagonal Boron Nitride-Based Magnetic Aerogel. NPJ Clean Water 2022, 5, 24. [Google Scholar] [CrossRef]
- Jiang, X.; Du, R.; Hübner, R.; Hu, Y.; Eychmüller, A. A Roadmap for 3D Metal Aerogels: Materials Design and Application Attempts. Matter 2021, 4, 54–94. [Google Scholar] [CrossRef]
- Alfei, S. Aerogels Part 1: A Focus on the Most Patented Ultralight, Highly Porous Inorganic Networks and the Plethora of Their Advanced Applications. Gels 2025, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Trikalitis, P.N.; Chupas, P.J.; Armatas, G.S.; Kanatzidis, M.G. Porous Semiconducting Gels and Aerogels from Chalcogenide Clusters. Science 2007, 317, 490–493. [Google Scholar] [CrossRef]
- Bag, S.; Gaudette, A.F.; Bussell, M.E.; Kanatzidis, M.G. Spongy Chalcogels of Non-Platinum Metals Act as Effective Hydrodesulfurization Catalysts. Nat. Chem. 2009, 1, 217–224. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Malliakas, C.D.; He, J.; Kanatzidis, M.G. Selective Surfaces: Quaternary Co(Ni)MoS-Based Chalcogels with Divalent (Pb2+, Cd2+, Pd2+) and Trivalent (Cr3+, Bi3+) Metals for Gas Separation. Chem. Mater. 2012, 24, 3380–3392. [Google Scholar] [CrossRef]
- Oh, Y.; Bag, S.; Malliakas, C.D.; Kanatzidis, M.G. Selective Surfaces: High-Surface-Area Zinc Tin Sulfide Chalcogels. Chem. Mater. 2011, 23, 2447–2456. [Google Scholar] [CrossRef]
- Riley, B.J.; Chun, J.; Ryan, J.V.; Matyáš, J.; Li, X.S.; Matson, D.W.; Sundaram, S.K.; Strachan, D.M.; Vienna, J.D. Chalcogen-Based Aerogels as a Multifunctional Platform for Remediation of Radioactive Iodine. RSC Adv. 2011, 1, 1704–1715. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern Inorganic Aerogels. Angew. Chem.-Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef]
- Choi, H.; Parale, V.G.; Kim, T.; Choi, Y.S.; Tae, J.; Park, H.H. Structural and Mechanical Properties of Hybrid Silica Aerogel Formed Using Triethoxy(1-Phenylethenyl)Silane. Microporous Mesoporous Mater. 2020, 298, 110092. [Google Scholar] [CrossRef]
- Rashid, A.B.; Shishir, S.I.; Mahfuz, M.A.; Hossain, M.T.; Hoque, M.E. Silica Aerogel: Synthesis, Characterization, Applications, and Recent Advancements. Part. Part. Syst. Charact. 2023, 40, 2200186. [Google Scholar] [CrossRef]
- Niederberger, M.; Pinna, N. Metal Oxide Nanoparticles in Organic Solvents; Springer: London, UK, 2009; ISBN 978-1-84882-670-0. [Google Scholar]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2018, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H.; Poco, J.F.; Hrubesh, L.W.; Simpson, R.L. Use of Epoxides in the Sol-Gel Synthesis of Porous Iron(III) Oxide Monoliths from Fe(III) Salts. Chem. Mater. 2001, 13, 999–1007. [Google Scholar] [CrossRef]
- Gash, A.E.; Tillotson, T.M.; Satcher, J.H.; Hrubesh, L.W.; Simpson, R.L. New Sol–Gel Synthetic Route to Transition and Main-Group Metal Oxide Aerogels Using Inorganic Salt Precursors. J. Non Cryst. Solids 2001, 285, 22–28. [Google Scholar] [CrossRef]
- Gash, A.E.; Satcher, J.H.; Simpson, R.L. Strong Akaganeite Aerogel Monoliths Using Epoxides: Synthesis and Characterization. Chem. Mater. 2003, 15, 3268–3275. [Google Scholar] [CrossRef]
- Kido, Y.; Nakanishi, K.; Miyasaka, A.; Kanamori, K. Synthesis of Monolithic Hierarchically Porous Iron-Based Xerogels from Iron(III) Salts via an Epoxide-Mediated Sol-Gel Process. Chem. Mater. 2012, 24, 2071–2077. [Google Scholar] [CrossRef]
- Kucheyev, S.O.; Van Buuren, T.; Baumann, T.F.; Satcher, J.H.; Willey, T.M.; Meulenberg, R.W.; Felter, T.E.; Poco, J.F.; Gammon, S.A.; Terminello, L.J. Electronic Structure of Titania Aerogels from Soft X-Ray Absorption Spectroscopy. Phys. Rev. B 2004, 69, 245102. [Google Scholar] [CrossRef]
- Choi, J.; Shin, C.B.; Suh, D.J. Polyvanadate Dominant Vanadia-Alumina Composite Aerogels Prepared by a Non-Alkoxide Sol-Gel Method. J. Mater. Chem. 2009, 19, 7704–7709. [Google Scholar] [CrossRef]
- Peterson, G.R.; Hung-Low, F.; Gumeci, C.; Bassett, W.P.; Korzeniewski, C.; Hope-Weeks, L.J. Preparation-Morphology-Performance Relationships in Cobalt Aerogels as Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 1796–1803. [Google Scholar] [CrossRef]
- Reibold, R.A.; Poco, J.F.; Baumann, T.F.; Simpson, R.L.; Satcher, J.H. Synthesis and Characterization of a Low-Density Urania (UO3) Aerogel. J. Non Cryst. Solids 2003, 319, 241–246. [Google Scholar] [CrossRef]
- Zhang, H.D.; Li, B.; Zheng, Q.X.; Jiang, M.H.; Tao, X.T. Synthesis and Characterization of Monolithic Gd2O3 Aerogels. J. Non Cryst. Solids 2008, 354, 4089–4093. [Google Scholar] [CrossRef]
- Clapsaddle, B.J.; Neumann, B.; Wittstock, A.; Sprehn, D.W.; Gash, A.E.; Satcher, J.H.; Simpson, R.L.; Bäumer, M. A Sol-Gel Methodology for the Preparation of Lanthanide-Oxide Aerogels: Preparation and Characterization. J. Sol-Gel Sci. Technol. 2012, 64, 381–389. [Google Scholar] [CrossRef]
- Schäfer, H.; Milow, B.; Ratke, L. Synthesis of Inorganic Aerogels via Rapid Gelation Using Chloride Precursors. RSC Adv. 2013, 3, 15263–15272. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, L.; Shang, C.; Wang, X.; Bi, Y. Synthesis of a Low-Density Tantalum Oxide Tile-like Aerogel Monolithic. J. Sol-Gel Sci. Technol. 2010, 53, 307–311. [Google Scholar] [CrossRef]
- Davis, M.; Gümeci, C.; Kiel, C.; Hope-Weeks, L.J. Preparation of Porous Manganese Oxide Nanomaterials by One-Pot Synthetic Sol-Gel Method. J. Sol-Gel Sci. Technol. 2011, 58, 535–538. [Google Scholar] [CrossRef]
- Brown, P.D.; Gill, S.K.; Hope-Weeks, L.J. Influence of Solvent on Porosity and Microstructure of an Yttrium Based Aerogel. J. Mater. Chem. 2011, 21, 4204–4208. [Google Scholar] [CrossRef]
- Eid, J.; Pierre, A.C.; Baret, G. Preparation and Characterization of Transparent Eu Doped Y2O3 Aerogel Monoliths, for Application in Luminescence. J. Non Cryst. Solids 2005, 351, 218–227. [Google Scholar] [CrossRef]
- Reibold, R.A.; Poco, J.F.; Baumann, T.F.; Simpson, R.L.; Satcher, J.H. Synthesis and Characterization of a Nanocrystalline Thoria Aerogel. J. Non Cryst. Solids 2004, 341, 35–39. [Google Scholar] [CrossRef][Green Version]
- Yoo, J.; Bang, Y.; Han, S.J.; Park, S.; Song, J.H.; Song, I.K. Hydrogen Production by Tri-Reforming of Methane over Nickel–Alumina Aerogel Catalyst. J. Mol. Catal. A Chem. 2015, 410, 74–80. [Google Scholar] [CrossRef]
- Brown, P.; Cearnaigh, D.U.; Fung, E.K.; Hope-Weeks, L.J. Controlling the Morphology of a Zinc Ferrite-Based Aerogel by Choice of Solvent. J. Sol-Gel Sci. Technol. 2012, 61, 104–111. [Google Scholar] [CrossRef]
- Brown, P.; Hope-Weeks, L.J. The Synthesis and Characterization of Zinc Ferrite Aerogels Prepared by Epoxide Addition. J. Sol-Gel Sci. Technol. 2009, 51, 238–243. [Google Scholar] [CrossRef]
- Chervin, C.N.; Ko, J.S.; Miller, B.W.; Dudek, L.; Mansour, A.N.; Donakowski, M.D.; Brintlinger, T.; Gogotsi, P.; Chattopadhyay, S.; Shibata, T.; et al. Defective by Design: Vanadium-Substituted Iron Oxide Nanoarchitectures as Cation-Insertion Hosts for Electrochemical Charge Storage. J. Mater. Chem. A 2015, 3, 12059–12068. [Google Scholar] [CrossRef]
- Chervin, C.N.; Clapsaddle, B.J.; Chiu, H.W.; Gash, A.E.; Satcher, J.H.; Kauzlarich, S.M. A Non-Alkoxide Sol-Gel Method for the Preparation of Homogeneous Nanocrystalline Powders of La 0.85Sr 0.15MnO3. Chem. Mater. 2006, 18, 1928–1937. [Google Scholar] [CrossRef]
- Long, J.W.; Logan, M.S.; Carpenter, E.E.; Rolison, D.R. Synthesis and Characterization of Mn–FeOx Aerogels with Magnetic Properties. J. Non Cryst. Solids 2004, 350, 182–188. [Google Scholar] [CrossRef]
- Pettigrew, K.A.; Long, J.W.; Carpenter, E.E.; Baker, C.C.; Lytle, J.C.; Chervin, C.N.; Logan, M.S.; Stroud, R.M.; Rolison, D.R. Nickel Ferrite Aerogels with Monodisperse Nanoscale Building Blocks—The Importance of Processing Temperature and Atmosphere. ACS Nano 2008, 2, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Lan, Z.; Li, H.; Zhang, X.; Li, Q. Hydrogen Bonding Directed Assembly of Simonkolleite Aerogel by a Sol–Gel Approach. Mater. Des. 2016, 93, 503–508. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Shen, J.; Xiao, S.; Zhang, Z.; Liu, C.; Zhang, M. Monolithic Copper Oxide Aerogel via Dispersed Inorganic Sol–Gel Method. J. Non Cryst. Solids 2009, 355, 175–181. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Shen, J.; Gui, J.; Zhong, Y.; Liu, C.; Zhang, Z.; Wu, G. A Versatile Sol-Gel Route to Monolithic Oxidic Gels via Polyacrylic Acid Template. New J. Chem. 2011, 35, 1096–1102. [Google Scholar] [CrossRef]
- Talapin, D.V. Lego Materials. ACS Nano 2008, 2, 1097–1100. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Airaghi Leccardi, M.J.I.; Erdem, D.; Süess, M.J.; Niederberger, M. Anisotropically Structured Magnetic Aerogel Monoliths. Nanoscale 2014, 6, 13213–13221. [Google Scholar] [CrossRef]
- Rechberger, F.; Heiligtag, F.J.; Süess, M.J.; Niederberger, M. Assembly of BaTiO3 Nanocrystals into Macroscopic Aerogel Monoliths with High Surface Area. Angew. Chem.-Int. Ed. 2014, 53, 6823–6826. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, J.L.; Arachchige, I.U.; Brock, S.L. Porous Semiconductor Chalcogenide Aerogels. Science 2005, 307, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Heiligtag, F.J.; Rossell, M.D.; Süess, M.J.; Niederberger, M. Template-Free Co-Assembly of Preformed Au and TiO2 Nanoparticles into Multicomponent 3D Aerogels. J. Mater. Chem. 2011, 21, 16893–16899. [Google Scholar] [CrossRef]
- Rechberger, F.; Ilari, G.; Niederberger, M. Assembly of Antimony Doped Tin Oxide Nanocrystals into Conducting Macroscopic Aerogel Monoliths. Chem. Commun. 2014, 50, 13138–13141. [Google Scholar] [CrossRef]
- Zeng, G.; Shi, N.; Hess, M.; Chen, X.; Cheng, W.; Fan, T.; Niederberger, M. A General Method of Fabricating Flexible Spinel-Type Oxide/Reduced Graphene Oxide Nanocomposite Aerogels as Advanced Anodes for Lithium-Ion Batteries. ACS Nano 2015, 9, 4227–4235. [Google Scholar] [CrossRef]
- Niederberger, M. Nonaqueous Sol-Gel Routes to Metal Oxide Nanoparticles. Acc. Chem. Res. 2007, 40, 793–800. [Google Scholar] [CrossRef]
- Correa Baena, J.P.; Agrios, A.G. Antimony-Doped Tin Oxide Aerogels as Porous Electron Collectors for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 17028–17035. [Google Scholar] [CrossRef]
- Zhi, M.; Tang, H.; Wu, M.; Ouyang, C.; Hong, Z.; Wu, N. Synthesis and Photocatalysis of Metal Oxide Aerogels: A Review. Energy Fuels 2022, 36, 11359–11379. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The Evolution of “sol-Gel” Chemistry as a Technique for Materials Synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Y.C.; Feldmann, C. Polyol Synthesis of Nanoparticles: Status and Options Regarding Metals, Oxides, Chalcogenides, and Non-Metal Elements. Green Chem. 2015, 17, 4107–4132. [Google Scholar] [CrossRef]
- De Mello Donegá, C.; Liljeroth, P.; Vanmaekelbergh, D. Physicochemical Evaluation of the Hot-Injection Method, a Synthesis Route for Monodisperse Nanocrystals. Small 2005, 1, 1152–1162. [Google Scholar] [CrossRef]
- Van Embden, J.; Chesman, A.S.R.; Jasieniak, J.J. The Heat-up Synthesis of Colloidal Nanocrystals. Chem. Mater. 2015, 27, 2246–2285. [Google Scholar] [CrossRef]
- Rajamathi, M.; Seshadri, R. Oxide and Chalcogenide Nanoparticles from Hydrothermal/Solvothermal Reactions. Curr. Opin. Solid State Mater. Sci. 2002, 6, 337–345. [Google Scholar] [CrossRef]
- Hiemenz, P.C.; Rajagopalan, R. Principles of Colloid and Surface Chemistry: Third Edition, Revised and Expanded; Marcel Dekker Inc.: New York, NY, USA, 2016. [Google Scholar]
- Whitesides, G.M.; Boncheva, M. Beyond Molecules: Self-Assembly of Mesoscopic and Macroscopic Components. Proc. Natl. Acad. Sci. USA 2002, 99, 4769–4774. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S. Self-Assembly and Nanotechnology: A Force Balance Approach; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Gaponik, N.; Wolf, A.; Marx, R.; Lesnyak, V.; Schilling, K.; Eychmüller, A. Three-Dimensional Self-Assembly of Thiol-Capped CdTe Nanocrystals: Gels and Aerogels as Building Blocks for Nanotechnology. Adv. Mater. 2008, 20, 4257–4262. [Google Scholar] [CrossRef]
- Ranmohotti, K.G.S.; Gao, X.; Arachchige, I.U. Salt-Mediated Self-Assembly of Metal Nanoshells into Monolithic Aerogel Frameworks. Chem. Mater. 2013, 25, 3528–3534. [Google Scholar] [CrossRef]
- Hayase, G.; Nonomura, K.; Hasegawa, G.; Kanamori, K.; Nakanishi, K. Ultralow-Density, Transparent, Superamphiphobic Boehmite Nanofiber Aerogels and Their Alumina Derivatives. Chem. Mater. 2015, 27, 3–5, Correction in Chem. Mater. 2017, 29, 5413. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Colloidal Suspension Rheology; Cambridge University Press: Cambridge, UK, 2011; ISBN 9780521515993. [Google Scholar]
- Dawson, K.A. The Glass Paradigm for Colloidal Glasses, Gels, and Other Arrested States Driven by Attractive Interactions. Curr. Opin. Colloid Interface Sci. 2002, 7, 218–227. [Google Scholar] [CrossRef]
- Dickinson, E. On Gelation Kinetics in a System of Particles with Both Weak and Strong Interactions. J. Chem. Soc.-Faraday Trans. 1997, 93, 111–114. [Google Scholar] [CrossRef]
- Lu, P.J.; Zaccarelli, E.; Ciulla, F.; Schofield, A.B.; Sciortino, F.; Weitz, D.A. Gelation of Particles with Short-Range Attraction. Nature 2008, 453, 499–503. [Google Scholar] [CrossRef]
- Khan, M.M. Introduction and Fundamentals of Chalcogenides and Chalcogenides-Based Nanomaterials. In Chalcogenide-Based Nanomaterials as Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Brock, S.L.; Yu, H. Chalcogenide Aerogels. In Handbook of Aerogels; Springer: Berlin/Heidelberg, Germany, 2023; pp. 989–1010. [Google Scholar]
- Sukanya, R.; da Silva Alves, D.C.; Breslin, C.B. Review—Recent Developments in the Applications of 2D Transition Metal Dichalcogenides as Electrocatalysts in the Generation of Hydrogen for Renewable Energy Conversion. J. Electrochem. Soc. 2022, 169, 064504. [Google Scholar] [CrossRef]
- Wang, G.; Yu, M.; Feng, X. Carbon Materials for Ion-Intercalation Involved Rechargeable Battery Technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; Van Der Waal, J.C.; Luque, R. Biomass-Derived Porous Carbon Materials: Synthesis and Catalytic Applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Yao, Q.; Brock, S.L. Optical Sensing of Triethylamine Using CdSe Aerogels. Nanotechnology 2010, 21, 115502. [Google Scholar] [CrossRef] [PubMed]
- Gacoin, T.; Malier, L.; Boilot, J.P. New Transparent Chalcogenide Materials Using a Sol-Gel Process. Chem. Mater. 1997, 9, 1502–1504. [Google Scholar] [CrossRef]
- Ha, T.D.C.; Lee, H.; Vamvasakis, I.; Armatas, G.S.; Oh, Y.; Kim, M.G. Recent Developments in Porous Metal Chalcogenides for Environmental Remediation and Sustainable Energy. EcoMat 2023, 5, e12419. [Google Scholar] [CrossRef]
- Bai, J.; Yang, L.; Zhang, Y.; Sun, X.; Liu, J. Tin Sulfide Chalcogel Derived SnSx for CO2 Electroreduction. Mater. Lab 2022, 1, 220046. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.; He, X. Co(Ni)–Mo–Sx Chalcogels Films as PH-Universal Electrocatalysts for the H2 Evolution Reaction. Catal. Lett. 2020, 150, 623–630. [Google Scholar] [CrossRef]
- Herm, Z.R.; Krishna, R.; Long, J.R. CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2(dobdc). Microporous Mesoporous Mater. 2012, 151, 481–487. [Google Scholar] [CrossRef]
- Vamvasakis, I.; Subrahmanyam, K.S.; Kanatzidis, M.G.; Armatas, G.S. Template-Directed Assembly of Metal-Chalcogenide Nanocrystals into Ordered Mesoporous Networks. ACS Nano 2015, 9, 4419–4426. [Google Scholar] [CrossRef]
- Zou, Y.; Zhou, X.; Ma, J.; Yang, X.; Deng, Y. Recent Advances in Amphiphilic Block Copolymer Templated Mesoporous Metal-Based Materials: Assembly Engineering and Applications. Chem. Soc. Rev. 2020, 49, 1173–1208. [Google Scholar] [CrossRef]
- Kim, J.K.; Jeong, S.Y.; Lim, S.H.; Oh, J.H.; Park, S.K.; Cho, J.S.; Kang, Y.C. Recent Advances in Aerosol-Assisted Spray Processes for the Design and Fabrication of Nanostructured Metal Chalcogenides for Sodium-Ion Batteries. Chem. Asian J. 2019, 14, 3127–3140. [Google Scholar] [CrossRef]
- Patriarchea, C.; Vamvasakis, I.; Koutsouroubi, E.D.; Armatas, G.S. Enhancing Interfacial Charge Transfer in Mesoporous MoS2/CdS Nanojunction Architectures for Highly Efficient Visible-Light Photocatalytic Water Splitting. Inorg. Chem. Front. 2022, 9, 625–636. [Google Scholar] [CrossRef]
- Ashok, A.; Vasanth, A.; Nagaura, T.; Eguchi, M.; Motta, N.; Phan, H.P.; Nguyen, N.T.; Shapter, J.G.; Na, J.; Yamauchi, Y. Plasma-Induced Nanocrystalline Domain Engineering and Surface Passivation in Mesoporous Chalcogenide Semiconductor Thin Films. Angew. Chem.-Int. Ed. 2022, 61, e202114729. [Google Scholar] [CrossRef]
- Karakaya, C.; Solati, N.; Savacı, U.; Keleş, E.; Turan, S.; Çelebi, S.; Kaya, S. Mesoporous Thin-Film NiS2 as an Idealized Pre-Electrocatalyst for a Hydrogen Evolution Reaction. ACS Catal. 2020, 10, 15114–15122. [Google Scholar] [CrossRef]
- Riley, B.J.; Chong, S. Environmental Remediation with Functional Aerogels and Xerogels. Glob. Chall. 2020, 4, 2000013. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Rothenberger, A. KFeSbTe3: A Quaternary Chalcogenide Aerogel for Preferential Adsorption of Polarizable Hydrocarbons and Gases. J. Mater. Chem. A 2015, 3, 7786–7792. [Google Scholar] [CrossRef]
- Ha, T.D.C.; Lee, H.; Kang, Y.K.; Ahn, K.; Jin, H.M.; Chung, I.; Kang, B.; Oh, Y.; Kim, M.G. Multiscale Structural Control of Thiostannate Chalcogels with Two-Dimensional Crystalline Constituents. Nat. Commun. 2022, 13, 7876. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.D.C.; Do, H.H.; Lee, H.; Ha, N.N.; Ha, N.T.T.; Ahn, S.H.; Oh, Y.; Kim, S.Y.; Kim, M.G. A GO/CoMo3S13 Chalcogel Heterostructure with Rich Catalytic Mo-S-Co Bridge Sites for the Hydrogen Evolution Reaction. Nanoscale 2022, 14, 9331–9340. [Google Scholar] [CrossRef]
- Kang, Y.K.; Lee, H.; Ha, T.D.C.; Won, J.K.; Jo, H.; Ok, K.M.; Ahn, S.; Kang, B.; Ahn, K.; Oh, Y.; et al. Thiostannate Coordination Transformation-Induced Self-Crosslinking Chalcogenide Aerogel with Local Coordination Control and Effective Cs+ Remediation Functionality. J. Mater. Chem. A 2020, 8, 3468–3480, Correction in J. Mater. Chem. A 2022, 10, 1597–1597. [Google Scholar] [CrossRef]
- Stanić, V.; Etsell, T.H.; Pierre, A.C.; Mikula, R.J. Sol-Gel Processing of ZnS. Mater. Lett. 1997, 31, 35–38. [Google Scholar] [CrossRef]
- Stanić, V.; Pierre, A.C.; Etsell, T.H.; Mikula, R.J. Preparation of Tungsten Sulfides by Sol-Gel Processing. J. Non Cryst. Solids 1997, 220, 58–62. [Google Scholar] [CrossRef]
- Stanić, V.; Pierre, A.C.; Etsell, T.H.; Mikula, R.J. Preparation and Characterization of GeS2. J. Mater. Res. 1996, 11, 363–372. [Google Scholar] [CrossRef]
- Stanić, V.; Pierre, A.C.; Etsell, T.H.; Mikula, R.J. Influence of Reaction Parameters on the Microstructure of the Germanium Disulfide Gel. J. Am. Ceram. Soc. 2000, 83, 1790–1796. [Google Scholar] [CrossRef]
- Sanghera, J.S.; Scotto, C.; Bayya, S.; Aggarwal, I.D. Catalyzed Gelation of Amorphous Sulphides. J. Non Cryst. Solids 1999, 256–257, 31–35. [Google Scholar] [CrossRef]
- Shafaei-Fallah, M.; He, J.; Rothenberger, A.; Kanatzidis, M.G. Ion-Exchangeable Cobalt Polysulfide Chalcogel. J. Am. Chem. Soc. 2011, 133, 1200–1202. [Google Scholar] [CrossRef]
- Oh, Y.; Morris, C.D.; Kanatzidis, M.G. Polysulfide Chalcogels with Ion-Exchange Properties and Highly Efficient Mercury Vapor Sorption. J. Am. Chem. Soc. 2012, 134, 14604–14608. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Malliakas, C.D.; Sarma, D.; Armatas, G.S.; Wu, J.; Kanatzidis, M.G. Ion-Exchangeable Molybdenum Sulfide Porous Chalcogel: Gas Adsorption and Capture of Iodine and Mercury. J. Am. Chem. Soc. 2015, 137, 13943–13948. [Google Scholar] [CrossRef]
- Bag, S.; Kanatzidis, M.G. Chalcogels: Porous Metal-Chalcogenide Networks from Main-Group Metal Ions. Effect of Surface Polarizability on Selectivity in Gas Separation. J. Am. Chem. Soc. 2010, 132, 14951–14959. [Google Scholar] [CrossRef]
- Shafaei-Fallah, M.; Rothenberger, A.; Katsoulidis, A.P.; He, J.; Malliakas, C.D.; Kanatzidis, M.G. Extraordinary Selectivity of CoMo3S13 Chalcogel for C2H6 and CO2 Adsorption. Adv. Mater. 2011, 23, 4857–4860. [Google Scholar] [CrossRef]
- Coste, S.; Gautier, E.; Evain, M.; Bujoli-Doeuff, M.; Brec, R.; Jobic, S.; Kanatzidis, M.G. NaV1-XP2S6 (x = 0.16): A New Compound with Infinite Straight (1/∞)[V0.837P2 S6]- Chains That Exfoliate Forming Gels. Chem. Mater. 2003, 15, 2323–2327. [Google Scholar] [CrossRef]
- Islam, S.M.; Subrahmanyam, K.S.; Malliakas, C.D.; Kanatzidis, M.G. One-Dimensional Molybdenum Thiochlorides and Their Use in High Surface Area MoSx Chalcogels. Chem. Mater. 2014, 26, 5151–5160. [Google Scholar] [CrossRef]
- Banerjee, A.; Yuhas, B.D.; Margulies, E.A.; Zhang, Y.; Shim, Y.; Wasielewski, M.R.; Kanatzidis, M.G. Photochemical Nitrogen Conversion to Ammonia in Ambient Conditions with Femos-Chalcogels. J. Am. Chem. Soc. 2015, 137, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Yuhas, B.D.; Smeigh, A.L.; Samuel, A.P.S.; Shim, Y.; Bag, S.; Douvalis, A.P.; Wasielewski, M.R.; Kanatzidis, M.G. Biomimetic Multifunctional Porous Chalcogels as Solar Fuel Catalysts. J. Am. Chem. Soc. 2011, 133, 7252–7255. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Prasittichai, C.; Hupp, J.T.; Kanatzidis, M.G. Enhanced Electrocatalytic Reduction of CO2 with Ternary Ni-Fe 4S4 and Co-Fe 4S4-Based Biomimetic Chalcogels. J. Am. Chem. Soc. 2011, 133, 15854–15857. [Google Scholar] [CrossRef]
- Yuhas, B.D.; Smeigh, A.L.; Douvalis, A.P.; Wasielewski, M.R.; Kanatzidis, M.G. Photocatalytic Hydrogen Evolution from FeMoS-Based Biomimetic Chalcogels. J. Am. Chem. Soc. 2012, 134, 10353–10356. [Google Scholar] [CrossRef]
- Shim, Y.; Yuhas, B.D.; Dyar, S.M.; Smeigh, A.L.; Douvalis, A.P.; Wasielewski, M.R.; Kanatzidis, M.G. Tunable Biomimetic Chalcogels with Fe4S4 Cores and [SnnS2 n + 2]4-(n = 1, 2, 4) Building Blocks for Solar Fuel Catalysis. J. Am. Chem. Soc. 2013, 135, 2330–2337. [Google Scholar] [CrossRef]
- Shim, Y.; Young, R.M.; Douvalis, A.P.; Dyar, S.M.; Yuhas, B.D.; Bakas, T.; Wasielewski, M.R.; Kanatzidis, M.G. Enhanced Photochemical Hydrogen Evolution from Fe4S4-Based Biomimetic Chalcogels Containing M2+ (M = Pt, Zn, Co, Ni, Sn) Centers. J. Am. Chem. Soc. 2014, 136, 13371–13380. [Google Scholar] [CrossRef]
- Riley, B.J.; Chun, J.; Um, W.; Lepry, W.C.; Matyas, J.; Olszta, M.J.; Li, X.; Polychronopoulou, K.; Kanatzidis, M.G. Chalcogen-Based Aerogels as Sorbents for Radionuclide Remediation. Environ. Sci. Technol. 2013, 47, 7540–7547. [Google Scholar] [CrossRef]
- Riley, B.J.; Pierce, D.A.; Chun, J.; Matyáš, J.; Lepry, W.C.; Garn, T.G.; Law, J.D.; Kanatzidis, M.G. Polyacrylonitrile-Chalcogel Hybrid Sorbents for Radioiodine Capture. Environ. Sci. Technol. 2014, 48, 5832–5839. [Google Scholar] [CrossRef]
- Riley, B.J.; Pierce, D.A.; Lepry, W.C.; Kroll, J.O.; Chun, J.; Subrahmanyam, K.S.; Kanatzidis, M.G.; Alblouwy, F.K.; Bulbule, A.; Sabolsky, E.M. Consolidation of Tin Sulfide Chalcogels and Xerogels with and without Adsorbed Iodine. Ind. Eng. Chem. Res. 2015, 54, 11259–11267. [Google Scholar] [CrossRef]
- Ahmed, E.; Rothenberger, A. Adsorption of Volatile Hydrocarbons in Iron Polysulfide Chalcogels. Microporous Mesoporous Mater. 2014, 199, 74–82. [Google Scholar] [CrossRef]
- Ahmed, E.; Khanderi, J.; Anjum, D.H.; Rothenberger, A. Selective Adsorption of Volatile Hydrocarbons and Gases in High Surface Area Chalcogels Containing [ES3]3− Anions (E = As, Sb). Chem. Mater. 2014, 26, 6454–6460. [Google Scholar] [CrossRef]
- Ahmed, E.; Rothenberger, A. Enhancement in CO2 Adsorption Capacity and Selectivity in the Chalcogenide Aerogel CuSb2S4 by Post-Synthetic Modification with LiCl. Microporous Mesoporous Mater. 2016, 220, 247–252. [Google Scholar] [CrossRef]
- Brock, S.L.; Arachchige, I.U.; Kalebaila, K.K. Metal Chalcogenide Gels, Xerogels and Aerogels. Comments Inorg. Chem. 2006, 27, 103–126. [Google Scholar] [CrossRef]
- Arachchige, I.U.; Brock, S.L. Sol-Gel Methods for the Assembly of Metal Chalcogenide Quantum Dots. Acc. Chem. Res. 2007, 40, 801–809. [Google Scholar] [CrossRef]
- Wen, D.; Eychmüller, A. 3D Assembly of Preformed Colloidal Nanoparticles into Gels and Aerogels: Function-Led Design. Chem. Commun. 2017, 53, 12608–12621. [Google Scholar] [CrossRef]
- Nie, J.; Chandra Roy, S.; Dhami, S.; Islam, T.; Amin, R.; Zhu, X.; Taylor-Pashow, K.; Han, F.X.; Islam, S.M. K–Co–Mo–S x Chalcogel: High-Capacity Removal of Pb2+ and Ag+ and the Underlying Mechanisms. J. Mater. Chem. A. 2024, 12, 30063–30072. [Google Scholar] [CrossRef]
- Shao, P.; Chang, Z.; Li, M.; Lu, X.; Jiang, W.; Zhang, K.; Luo, X.; Yang, L. Mixed-Valence Molybdenum Oxide as a Recyclable Sorbent for Silver Removal and Recovery from Wastewater. Nat. Commun. 2023, 14, 1365. [Google Scholar] [CrossRef]
- Yang, L.; Xie, L.; Chu, M.; Wang, H.; Yuan, M.; Yu, Z.; Wang, C.; Yao, H.; Islam, S.M.; Shi, K.; et al. Mo3S132− Intercalated Layered Double Hydroxide: Highly Selective Removal of Heavy Metals and Simultaneous Reduction of Ag+ Ions to Metallic Ag0 Ribbons. Angew. Chem.-Int. Ed. 2022, 61, e202112511. [Google Scholar] [CrossRef]
- Celik, A.; Baker, D.R.; Arslan, Z.; Zhu, X.; Blanton, A.; Nie, J.; Yang, S.; Ma, S.; Han, F.X.; Islam, S.M. Highly Efficient, Rapid, and Concurrent Removal of Toxic Heavy Metals by the Novel 2D Hybrid LDH–[Sn2S6]. Chem. Eng. J. 2021, 426, 131696. [Google Scholar] [CrossRef]
- Rathee, G.; Kohli, S.; Awasthi, A.; Singh, N.; Chandra, R. MoS42− Intercalated NiFeTi LDH as an Efficient and Selective Adsorbent for Elimination of Heavy Metals. RSC Adv. 2020, 10, 19371–19381. [Google Scholar] [CrossRef]
- Ali, J.; Wang, H.; Ifthikar, J.; Khan, A.; Wang, T.; Zhan, K.; Shahzad, A.; Chen, Z.; Chen, Z. Efficient, Stable and Selective Adsorption of Heavy Metals by Thio-Functionalized Layered Double Hydroxide in Diverse Types of Water. Chem. Eng. J. 2018, 332, 387–397. [Google Scholar] [CrossRef]
- Xie, L.; Yu, Z.; Islam, S.M.; Shi, K.; Cheng, Y.; Yuan, M.; Zhao, J.; Sun, G.; Li, H.; Ma, S.; et al. Remarkable Acid Stability of Polypyrrole-MoS4: A Highly Selective and Efficient Scavenger of Heavy Metals Over a Wide PH Range. Adv. Funct. Mater. 2018, 28, 1800502. [Google Scholar] [CrossRef]
- Yuan, M.; Yao, H.; Xie, L.; Liu, X.; Wang, H.; Islam, S.M.; Shi, K.; Yu, Z.; Sun, G.; Li, H.; et al. Polypyrrole-Mo3S13: An Efficient Sorbent for the Capture of Hg2+ and Highly Selective Extraction of Ag+ over Cu2+. J. Am. Chem. Soc. 2020, 142, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, Q.; Islam, S.M.; Liu, Y.; Ma, S.; Kanatzidis, M.G. Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42− Ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh Fard, Z.; Malliakas, C.D.; Mertz, J.L.; Kanatzidis, M.G. Direct Extraction of Ag+ and Hg2+ from Cyanide Complexes and Mode of Binding by the Layered K2MgSn2S6 (KMS-2). Chem. Mater. 2015, 27, 1925–1928. [Google Scholar] [CrossRef]
- Chen, Z.; Jawad, A.; Liao, Z.; Zhou, Z.; Khan, A.; Wang, T.; Ifthikar, J.; Shahzad, A.; Chen, Z. Fe-MoS4: An Effective and Stable LDH-Based Adsorbent for Selective Removal of Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 28451–28463. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A Lignosulfonate-Modified Graphene Hydrogel with Ultrahigh Adsorption Capacity for Pb(II) Removal. J. Mater. Chem. A 2016, 4, 11888–11896. [Google Scholar] [CrossRef]
- Ogawa, M.; Saito, F. Easily Oxidizable Polysulfide Anion Occluded in the Interlayer Space of Mg/Al Layered Double Hydroxide. Chem. Lett. 2004, 33, 1030–1031. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, G.; Chu, L.; Liu, Y.; Liu, C.; Luo, S.; Wei, Y. Efficient Removal of Heavy Metal Ions with An EDTA Functionalized Chitosan/Polyacrylamide Double Network Hydrogel. ACS Sustain. Chem. Eng. 2017, 5, 843–851. [Google Scholar] [CrossRef]
- Huang, G.; Wang, D.; Ma, S.; Chen, J.; Jiang, L.; Wang, P. A New, Low-Cost Adsorbent: Preparation, Characterization, and Adsorption Behavior of Pb(II) and Cu(II). J. Colloid Interface Sci. 2015, 445, 294–302. [Google Scholar] [CrossRef]
- Alatalo, S.M.; Pileidis, F.; Mäkilä, E.; Sevilla, M.; Repo, E.; Salonen, J.; Sillanpää, M.; Titirici, M.M. Versatile Cellulose-Based Carbon Aerogel for the Removal of Both Cationic and Anionic Metal Contaminants from Water. ACS Appl. Mater. Interfaces 2015, 7, 25875–25883. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, Q.; Ji, X.; Wang, F.; Gao, X.; Zhao, M. High and Fast Adsorption of Cd(II) and Pb(II) Ions from Aqueous Solutions by a Waste Biomass Based Hydrogel. Sci. Rep. 2020, 10, 3285. [Google Scholar] [CrossRef] [PubMed]
- Manos, M.J.; Petkov, V.G.; Kanatzidis, M.G. H2xMnxSn3-XS6 (x = 0.11–0.25): A Novel Reusable Sorbent for Highly Specific Mercury Capture under Extreme PH Conditions. Adv. Funct. Mater. 2009, 19, 1087–1092. [Google Scholar] [CrossRef]
- Bao, J.; Fu, Y.; Bao, Z. Thiol-Functionalized Magnetite/Graphene Oxide Hybrid as a Reusable Adsorbent for Hg2+ Removal. Nanoscale Res. Lett. 2013, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Raju, M.M.; Kota, S.S. Highly Efficient Chalcogel-Based Molecular Filters. J. Chem. Eng. Data 2018, 63, 3449–3458. [Google Scholar] [CrossRef]
- Han, S.; Liu, K.; Hu, L.; Teng, F.; Yu, P.; Zhu, Y. Correction: Corrigendum: Superior Adsorption and Regenerable Dye Adsorbent Based on Flower-Like Molybdenum Disulfide Nanostructure. Sci. Rep. 2017, 7, 46887. [Google Scholar] [CrossRef]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-Inspired Synthesis of Polydopamine-Functionalized Graphene Hydrogel as Reusable Adsorbents for Water Purification. ACS Appl Mater. Interfaces 2013, 5, 425–432. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Qu, F. Synthesis of Ferromagnetic Ordered Mesoporous Carbons for Bulky Dye Molecules Adsorption. Chem. Eng. J. 2012, 193–194, 169–177. [Google Scholar] [CrossRef]
- Deka, J.R.; Lin, Y.H.; Kao, H.M. Ordered Cubic Mesoporous Silica KIT-5 Functionalized with Carboxylic Acid Groups for Dye Removal. RSC Adv. 2014, 4, 49061–49069. [Google Scholar] [CrossRef]
- Yu, J.X.; Li, B.H.; Sun, X.M.; Yuan, J.; Chi, R.A. Polymer Modified Biomass of Baker’s Yeast for Enhancement Adsorption of Methylene Blue, Rhodamine B and Basic Magenta. J. Hazard. Mater. 2009, 168, 1147–1154. [Google Scholar] [CrossRef]
- Anandkumar, J.; Mandal, B. Adsorption of Chromium(VI) and Rhodamine B by Surface Modified Tannery Waste: Kinetic, Mechanistic and Thermodynamic Studies. J. Hazard. Mater. 2011, 186, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Hou, M.F.; Ma, C.X.; Zhang, W.D.; Tang, X.Y.; Fan, Y.N.; Wan, H.F. Removal of Rhodamine B Using Iron-Pillared Bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef]
- Duan, S.; Li, J.; Liu, X.; Wang, Y.; Zeng, S.; Shao, D.; Hayat, T. HF-Free Synthesis of Nanoscale Metal-Organic Framework NMIL-100(Fe) as an Efficient Dye Adsorbent. ACS Sustain. Chem. Eng. 2016, 4, 3368–3378. [Google Scholar] [CrossRef]
- Saghanejhad Tehrani, M.; Zare-Dorabei, R. Highly Efficient Simultaneous Ultrasonic-Assisted Adsorption of Methylene Blue and Rhodamine B onto Metal Organic Framework MIL-68(Al): Central Composite Design Optimization. RSC Adv. 2016, 6, 27416–27425. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H.T. Adsorption and Desorption of Methylene Blue on Porous Carbon Monoliths and Nanocrystalline Cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. [Google Scholar] [CrossRef]
- Pal, S.; Ghorai, S.; Das, C.; Samrat, S.; Ghosh, A.; Panda, A.B. Carboxymethyl Tamarind-g-Poly(Acrylamide)/Silica: A High Performance Hybrid Nanocomposite for Adsorption of Methylene Blue Dye. Ind. Eng. Chem. Res. 2012, 51, 15546–15556. [Google Scholar] [CrossRef]
- Ai, L.; Li, M.; Li, L. Adsorption of Methylene Blue from Aqueous Solution with Activated Carbon/Cobalt Ferrite/Alginate Composite Beads: Kinetics, Isotherms, and Thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Lu, H.; Yang, L.; Wu, Y. Methylene Blue Adsorption onto Swede Rape Straw (Brassica Napus L.) Modified by Tartaric Acid: Equilibrium, Kinetic and Adsorption Mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Li, P.; Zhang, D.; Feng, C.L. C2-Symmetric Benzene-Based Hydrogels with Unique Layered Structures for Controllable Organic Dye Adsorption. Soft Matter 2012, 8, 3231–3238. [Google Scholar] [CrossRef]
- Paulino, A.T.; Guilherme, M.R.; Reis, A.V.; Campese, G.M.; Muniz, E.C.; Nozaki, J. Removal of Methylene Blue Dye from an Aqueous Media Using Superabsorbent Hydrogel Supported on Modified Polysaccharide. J. Colloid Interface Sci. 2006, 301, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Khoshnood, N. Removal of Cationic Dyes by Poly(AA-Co-AMPS)/Montmorillonite Nanocomposite Hydrogel. Desalination Water Treat. 2016, 57, 6372–6383. [Google Scholar] [CrossRef]
- Tian, Y.; Ji, C.; Zhao, M.; Xu, M.; Zhang, Y.; Wang, R. Preparation and Characterization of Baker’s Yeast Modified by Nano-Fe3O4: Application of Biosorption of Methyl Violet in Aqueous Solution. Chem. Eng. J. 2010, 165, 474–481. [Google Scholar] [CrossRef]
- Tehrani, M.S.; Zare-Dorabei, R. Competitive Removal of Hazardous Dyes from Aqueous Solution by MIL-68(Al): Derivative Spectrophotometric Method and Response Surface Methodology Approach. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 160, 8–18. [Google Scholar] [CrossRef]
- Hameed, B.H. Equilibrium and Kinetic Studies of Methyl Violet Sorption by Agricultural Waste. J. Hazard. Mater. 2008, 154, 204–212. [Google Scholar] [CrossRef]
- Azizian, S.; Haerifar, M.; Bashiri, H. Adsorption of Methyl Violet onto Granular Activated Carbon: Equilibrium, Kinetics and Modeling. Chem. Eng. J. 2009, 146, 36–41. [Google Scholar] [CrossRef]
- Xu, R.; Xiao, S.; Yuan, J.; Zhao, A. Adsorption of Methyl Violet from Aqueous Solutions by the Biochars Derived from Crop Residues. Bioresour. Technol. 2011, 102, 10293–10298. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, B.; Mei, D.; Zhang, H.; Liu, J. Adsorption of Methyl Violet from Aqueous Solution by Halloysite Nanotubes. Desalination 2011, 268, 111–116. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Wu, R.; Wang, J.; Li, H. Adsorption Behaviors of Acid and Basic Dyes on Crosslinked Amphoteric Starch. Chem. Eng. J. 2006, 117, 161–167. [Google Scholar] [CrossRef]
- Ho, Y.S.; Chiu, W.T.; Wang, C.C. Regression Analysis for the Sorption Isotherms of Basic Dyes on Sugarcane Dust. Bioresour. Technol. 2005, 96, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Feng, M.; Huang, X. Metal Chalcogenides as Ion-Exchange Materials for the Efficient Removal of Key Radionuclides: A Review. Fundam. Res. 2025, 5, 1969–1987. [Google Scholar] [CrossRef]
- Manos, M.J.; Ding, N.; Kanatzidis, M.G. Layered Metal Sulfides: Exceptionally Selective Agents for Radioactive Strontium Removal. Proc. Natl. Acad. Sci. USA 2008, 105, 3696–3699. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Highly Efficient and Rapid Cs+ Uptake by the Layered Metal Sulfide K2xMnxSn3−xS6 (KMS-1). J. Am. Chem. Soc. 2009, 131, 6599–6607. [Google Scholar] [CrossRef]
- Manos, M.J.; Kanatzidis, M.G. Layered Metal Sulfides Capture Uranium from Seawater. J. Am. Chem. Soc. 2012, 134, 16441–16446. [Google Scholar] [CrossRef]
- Mertz, J.L.; Fard, Z.H.; Malliakas, C.D.; Manos, M.J.; Kanatzidis, M.G. Selective Removal of Cs+, Sr2+, and Ni2+ by K2xMgxSn3-XS6 (x = 0.5–1) (KMS-2) Relevant to Nuclear Waste Remediation. Chem. Mater. 2013, 25, 2116–2127. [Google Scholar] [CrossRef]
- Xiao, C.; Hassanzadeh Fard, Z.; Sarma, D.; Song, T.B.; Xu, C.; Kanatzidis, M.G. Highly Efficient Separation of Trivalent Minor Actinides by a Layered Metal Sulfide (KInSn2S6) from Acidic Radioactive Waste. J. Am. Chem. Soc. 2017, 139, 16494–16497. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Bao, H.; Spanopoulos, I.; Ke, W.; Dong, X.; Xiao, C.; Kanatzidis, M.G. Selective Capture Mechanism of Radioactive Thorium from Highly Acidic Solution by a Layered Metal Sulfide. ACS Appl. Mater. Interfaces 2021, 13, 37308–37315. [Google Scholar] [CrossRef]
- Tang, J.H.; Jin, J.C.; Li, W.A.; Zeng, X.; Ma, W.; Li, J.L.; Lv, T.T.; Peng, Y.C.; Feng, M.L.; Huang, X.Y. Highly Selective Cesium(I) Capture under Acidic Conditions by a Layered Sulfide. Nat. Commun. 2022, 13, 658. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, P.; Zhang, Z.; Liu, J.; Dong, L.; Zhang, G. Effective, Rapid and Selective Adsorption of Radioactive Sr2+ from Aqueous Solution by a Novel Metal Sulfide Adsorbent. Chem. Eng. J. 2018, 351, 668–677. [Google Scholar] [CrossRef]
- Zhang, M.; Gu, P.; Yan, S.; Dong, L.; Zhang, G. Na/Zn/Sn/S (NaZTS): Quaternary Metal Sulfide Nanosheets for Efficient Adsorption of Radioactive Strontium Ions. Chem. Eng. J. 2020, 379, 122227. [Google Scholar] [CrossRef]
- Wang, K.Y.; Ding, D.; Sun, M.; Cheng, L.; Wang, C. Effective and Rapid Adsorption of Sr2+ Ions by a Hydrated Pentasodium Cluster Templated Zinc Thiostannate. Inorg. Chem. 2019, 58, 10184–10193. [Google Scholar] [CrossRef] [PubMed]
- Sarma, D.; Malliakas, C.D.; Subrahmanyam, K.S.; Islam, S.M.; Kanatzidis, M.G. K2xSn4-xS8-x (x = 0.65–1): A New Metal Sulfide for Rapid and Selective Removal of Cs+, Sr2+ and UO22+ Ions. Chem. Sci. 2016, 7, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.M.; Luo, D.; Ma, Z.J.; Zheng, B.; Cheng, F.F.; Xiong, W.W. Effective Enrichment of Low-Concentration Rare-Earth Ions by Three-Dimensional Thiostannate K2Sn2S5. ACS Appl Mater. Interfaces 2021, 13, 55188–55197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gu, P.; Zhang, M.; Yan, S.; Dong, L.; Zhang, G. Synthesis of a Robust Layered Metal Sulfide for Rapid and Effective Removal of Sr2+ from Aqueous Solutions. Chem. Eng. J. 2019, 372, 1205–1215. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Zhang, G.; Liu, S.; Dong, L.; Gu, P.; Hou, L. Rapid and Effective Removal of Strontium Ions from Aqueous Solutions by a Novel Layered Metal Sulfide NaTS-2. J. Radioanal. Nucl. Chem. 2023, 332, 2367–2378. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Liu, S.; Zhang, G.; Dong, L.; Gu, P.; Hou, L. Layered Metal Sulfide NMTS for Rapid Removal of Radioactive Strontium Ions from Aqueous Solution. Sep. Purif. Technol. 2023, 310, 122887. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G.; Ma, C.; Guo, Y.; Duo, J.; Li, M.; Deng, T. Cesium Removal from Wastewater: High-Efficient and Reusable Adsorbent K1.93Ti0.22Sn3S6.43. Chemosphere 2022, 305, 135406. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, C.; He, Y.; Li, M.; Liu, G.; Guo, Y.; Ji, D.; Deng, T. Novel Layered Iron Antimony Thiostannate Adsorbent of K1.61Fe0.04Sb0.03Sn3.1S7 for Cesium Green Recovery from Geothermal Water. J. Clean. Prod. 2022, 347, 131332. [Google Scholar] [CrossRef]
- Yang, C.; Cho, K. Rapid and Selective Removal of Cs+ from Water by Layered Potassium Antimony Thiostannate. J. Hazard. Mater. 2021, 403, 124105. [Google Scholar] [CrossRef]
- Yang, C.; Suh, Y.J.; Cho, K. Highly Selective Cesium Removal under Acidic and Alkaline Conditions Using a Novel Potassium Aluminum Thiostannate. Chemosphere 2022, 301, 134610. [Google Scholar] [CrossRef] [PubMed]
- Yohannan, J.P.; Vidyasagar, K. Syntheses, Structural Variants and Characterization of AInM′S4 (A = alkali Metals, Tl; M′ = Ge, Sn) Compounds; Facile Ion-Exchange Reactions of Layered NaInSnS4 and KInSnS4 Compounds. J. Solid State Chem. 2016, 238, 291–302. [Google Scholar] [CrossRef]
- Pogu, A.; Jaschin, P.W.; Varma, K.B.R.; Vidyasagar, K. Syntheses, Structural Variants and Characterization of A2CdSn2S6 (A = Cs, Rb and K) Compounds. J. Solid State Chem. 2019, 277, 713–720. [Google Scholar] [CrossRef]
- Liang, C.; Jia, M.; Wang, X.; Du, Z.; Men, J.; Ding, H. Preparation of Potassium Niobium Sulfide and Its Selective Adsorption Properties for Sr2+ and Co2+. J. Radioanal. Nucl. Chem. 2019, 322, 377–387. [Google Scholar] [CrossRef]
- Ding, N.; Chung, D.Y.; Kanatzidis, M.G. K6Cd4Sn3Se13: A Polar Open-Framework Compound Based on the Partially Destroyed Supertetrahedral [Cd4Sn4Se17]10− Cluster. Chem. Commun. 2004, 10, 1170–1171. [Google Scholar] [CrossRef]
- Wu, M.; Su, W.; Jasutkar, N.; Huang, X.; Li, J. An Open-Framework Bimetallic Chalcogenide Structure K3Rb3Zn4Sn3Se13 Built on a Unique [Zn4Sn3Se16]12− Cluster: Synthesis, Crystal Structure, Ion Exchange and Optical Properties. Mater. Res. Bull. 2005, 40, 21–27. [Google Scholar] [CrossRef]
- Yang, H.; Luo, M.; Luo, L.; Wang, H.; Hu, D.; Lin, J.; Wang, X.; Wang, Y.; Wang, S.; Bu, X.; et al. Highly Selective and Rapid Uptake of Radionuclide Cesium Based on Robust Zeolitic Chalcogenide via Stepwise Ion-Exchange Strategy. Chem. Mater. 2016, 28, 8774–8780. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, H.Y.; Li, J.; Li, L.Z.; Deng, Y.L.; Liu, S.H.; Feng, M.L.; Huang, X.Y. Fast and Selective Removal of Aqueous Uranium by a K+-Activated Robust Zeolitic Sulfide with Wide PH Resistance. Inorg. Chem. 2019, 58, 11622–11629. [Google Scholar] [CrossRef]
- Zeng, X.; Zeng, M.; Cai, P.W.; Tang, J.H.; Ma, W.; Feng, M.L.; Huang, X.Y. Ultra-Fast 137Cs Sequestration via a Layered Inorganic Indium Thioantimonate†. Environ. Sci. Adv. 2022, 1, 331–341. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Cheng, L.; Wang, K.Y.; Hao, X.; Wang, J.; Zhu, J.Y.; Sun, M.; Wang, C. PH-Controlled Switch over Coadsorption and Separation for Mixed Cs+ and Sr2+ by an Acid-Resistant Potassium Thioantimonate. Adv. Funct. Mater. 2022, 32, 2112717. [Google Scholar] [CrossRef]
- Rathore, E.; Pal, P.; Biswas, K. Reversible and Efficient Sequestration of Cesium from Water by the Layered Metal Thiophosphate K0.48Mn0.76PS3⋅H2O. Chem. Eur. J. 2017, 23, 11085–11092. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zeng, M.; Zhang, T.; Cai, P.W.; Feng, M.L.; Huang, X.Y. Efficient Uptake of Uranium(VI) by a Layered Manganese Thiophosphite Intercalated with NH4+. Chem. Eng. J. 2022, 429, 132474. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Sarma, D.; Malliakas, C.D.; Polychronopoulou, K.; Riley, B.J.; Pierce, D.A.; Chun, J.; Kanatzidis, M.G. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Qi, X.H.; Du, K.Z.; Feng, M.L.; Li, J.R.; Du, C.F.; Zhang, B.; Huang, X.Y. A Two-Dimensionally Microporous Thiostannate with Superior Cs+ and Sr2+ Ion-Exchange Property. J. Mater. Chem. A 2015, 3, 5665–5673. [Google Scholar] [CrossRef]
- Feng, M.L.; Sarma, D.; Qi, X.H.; Du, K.Z.; Huang, X.Y.; Kanatzidis, M.G. Efficient Removal and Recovery of Uranium by a Layered Organic-Inorganic Hybrid Thiostannate. J. Am. Chem. Soc. 2016, 138, 12578–12585. [Google Scholar] [CrossRef]
- Qi, X.H.; Du, K.Z.; Feng, M.L.; Gao, Y.J.; Huang, X.Y.; Kanatzidis, M.G. Layered A2Sn3S7·1.25H2O (A = Organic Cation) as Efficient Ion-Exchanger for Rare Earth Element Recovery. J. Am. Chem. Soc. 2017, 139, 4314–4317. [Google Scholar] [CrossRef]
- Gao, Y.J.; Sun, H.Y.; Li, J.L.; Qi, X.H.; Du, K.Z.; Liao, Y.Y.; Huang, X.Y.; Feng, M.L.; Kanatzidis, M.G. Selective Capture of Ba2+, Ni2+, and Co2+ by a Robust Layered Metal Sulfide. Chem. Mater. 2020, 32, 1957–1963. [Google Scholar] [CrossRef]
- Li, W.A.; Li, J.R.; Zhang, B.; Sun, H.Y.; Jin, J.C.; Huang, X.Y.; Feng, M.L. Layered Thiostannates with Distinct Arrangements of Mixed Cations for the Selective Capture of Cs+, Sr2+, and Eu3+ Ions. ACS Appl. Mater. Interfaces 2021, 13, 10191–10201. [Google Scholar] [CrossRef]
- Feng, M.; Li, J.; Jin, J.; Zhang, T.; Ma, W.; Zeng, X.; Sun, H.; Cheng, M.; Huang, X. Rapid and Selective Uptake of Cs+ and Sr2+ Ions by a Layered Thiostannate with Acid-Base and Irradiation Resistances. ACS ES T Water J. 2021, 1, 2440–2449. [Google Scholar] [CrossRef]
- Lan, Y.; Su, Z.; Li, X.; Jiang, Z.; Jin, J.; Xie, J.; Li, S. Synthesis of a New Microporous Indium Sulphide and Its Capabilities to the Separation of Strontium. J. Radioanal. Nucl. Chem. 2007, 273, 99–102. [Google Scholar] [CrossRef]
- Wang, K.Y.; Sun, M.; Ding, D.; Liu, H.W.; Cheng, L.; Wang, C. Di-Lacunary [In6S15]12− Cluster: The Building Block of a Highly Negatively Charged Framework for Superior Sr2+ Adsorption Capacities. Chem. Commun. 2020, 56, 3409–3412. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wang, K.Y.; Ding, D.; Zhu, J.Y.; Zhao, Y.M.; Cheng, L.; Wang, C. Removal of Sr2+ Ions by a High-Capacity Indium Sulfide Exchanger Containing Permeable Layers with Large Pores. Inorg. Chem. 2020, 59, 13822–13826. [Google Scholar] [CrossRef]
- Zhao, Y.M.; Sun, M.; Cheng, L.; Wang, K.Y.; Liu, Y.; Zhu, J.Y.; Zhang, S.; Wang, C. Efficient Removal of Ba2+, Co2+ and Ni2+ by an Ethylammonium-Templated Indium Sulfide Ion Exchanger. J. Hazard. Mater. 2022, 425, 128007. [Google Scholar] [CrossRef]
- Manos, M.J.; Malliakas, C.D.; Kanatzidis, M.G. Heavy-Metal-Ion Capture, Ion-Exchange, and Exceptional Acid Stability of the Open-Framework Chalcogenide (NH4)4In12Se20. Chem. Eur. J. 2007, 13, 51–58. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Li, J.R.; Zhang, B.; Sun, H.Y.; Ma, W.; Jin, J.C.; Feng, M.L.; Huang, X.Y. Robust and Flexible Thioantimonate Materials for Cs+ Remediation with Distinctive Structural Transformation: A Clear Insight into the Ion-Exchange Mechanism. ACS Appl. Mater. Interfaces 2021, 13, 5275–5283. [Google Scholar] [CrossRef]
- Feng, M.L.; Kong, D.N.; Xie, Z.L.; Huang, X.Y. Three-Dimensional Chiral Microporous Germanium Antimony Sulfide with Ion-Exchange Properties. Angew. Chem.-Int. Ed. 2008, 47, 8623–8626. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, M.L.; Cui, H.H.; Du, C.F.; Qi, X.H.; Shen, N.N.; Huang, X.Y. Syntheses, Crystal Structures, Ion-Exchange, and Photocatalytic Properties of Two Amine-Directed Ge-Sb-S Compounds. Inorg. Chem. 2015, 54, 8474–8481. [Google Scholar] [CrossRef]
- Ding, N.; Kanatzidis, M.G. Permeable Layers with Large Windows in [(CH3CH2CH2)2NH2]5In5Sb6S191.45H2O: High Ion-Exchange Capacity, Size Discrimination, and Selectivity for Cs Ions. Chem. Mater. 2007, 19, 3867–3869. [Google Scholar] [CrossRef]
- Wang, K.Y.; Feng, M.L.; Li, J.R.; Huang, X.Y. 4[In4SbS9SH]: A Novel Methylamine-Directed Indium Thioantimonate with Rb+ Ion-Exchange Property. J. Mater. Chem. A 2013, 1, 1709–1715. [Google Scholar] [CrossRef]
- Ding, N.; Kanatzidis, M.G. Selective Incarceration of Caesium Ions by Venus Flytrap Action of a Flexible Framework Sulfide. Nat. Chem. 2010, 2, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.L.; Sarma, D.; Gao, Y.J.; Qi, X.H.; Li, W.A.; Huang, X.Y.; Kanatzidis, M.G. Efficient Removal of [UO2]2+, Cs+, and Sr2+ Ions by Radiation-Resistant Gallium Thioantimonates. J. Am. Chem. Soc. 2018, 140, 11133–11140. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Zhang, T.; Jin, J.C.; Li, J.L.; Sun, Q.; Ai, Y.J.; Feng, M.L.; Huang, X.Y. Ultrafast and Selective Uptake of Eu3+ from Aqueous Solutions by Two Layered Sulfides. Chem. Eng. J. 2021, 420, 127613. [Google Scholar] [CrossRef]
- Wang, W.; Yang, H.; Luo, M.; Zhong, Y.; Xu, D.; Wu, T.; Lin, Z. A 36-Membered Ring Metal Chalcogenide with a Very Low Framework Density. Inorg. Chem. 2017, 56, 14730–14733. [Google Scholar] [CrossRef]
- Wang, L.; Pei, H.; Sarma, D.; Zhang, X.M.; MacRenaris, K.; Malliakas, C.D.; Kanatzidis, M.G. Highly Selective Radioactive 137 Cs+ Capture in an Open-Framework Oxysulfide Based on Supertetrahedral Cluster. Chem. Mater. 2019, 31, 1628–1634. [Google Scholar] [CrossRef]
- Li, J.R.; Huang, X.Y. 0.75[Ag1.25SnSe3]: A Three-Dimensionally Microporous Chalcogenide Exhibiting Framework Flexibility upon Ion-Exchange. Dalton Trans. 2011, 40, 4387–4390. [Google Scholar] [CrossRef]
- Liu, H.W.; Wang, K.Y.; Ding, D.; Sun, M.; Cheng, L.; Wang, C. Deep Eutectic Solvothermal Synthesis of an Open Framework Copper Selenidogermanate with PH-Resistant Cs+ Ion Exchange Properties. Chem. Commun. 2019, 55, 13884–13887. [Google Scholar] [CrossRef]
- Ding, D.; Cheng, L.; Wang, K.Y.; Liu, H.W.; Sun, M.; Wang, C. Efficient Cs+-Sr2+ Separation over a Microporous Silver Selenidostannate Synthesized in Deep Eutectic Solvent. Inorg. Chem. 2020, 59, 9638–9647. [Google Scholar] [CrossRef]
- Wang, K.Y.; Liu, Y.; Zhu, J.Y.; Cheng, L.; Wang, C. M-Sn-Q (M = Zn, Cd; Q = S, Se) Compounds Templated by (Alkyl)Ammonium Species: Synthesis, Crystal Structure, and Sr2+Adsorption Property. Inorg. Chem. 2022, 61, 19106–19118. [Google Scholar] [CrossRef]
- Zhang, R.C.; Yao, H.G.; Ji, S.H.; Liu, M.C.; Ji, M.; An, Y.L. (H2en)2Cu8Sn3S12: A Trigonal CuS3-Based Open-Framework Sulfide with Interesting Ion-Exchange Properties. Chem. Commun. 2010, 46, 4550–4552. [Google Scholar] [CrossRef]
- Zhang, R.C.; Zhang, J.C.; Cao, Z.; Wang, J.J.; Liang, S.S.; Cong, H.J.; Wang, H.J.; Zhang, D.J.; An, Y.L. Unusual Flexibility of Microporous Sulfides during Ion Exchange. Inorg. Chem. 2018, 57, 13128–13136. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Hu, D.; Luo, M.; Xue, C.; Li, D.; Wu, T. Hybrid Assembly of Different-Sized Supertetrahedral Clusters into a Unique Non-Interpenetrated Mn-In-S Open Framework with Large Cavity. Inorg. Chem. 2018, 57, 6710–6715. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Cheng, L.; Zhao, Y.M.; Li, M.Y.; Wang, Z.Z.; Wang, J.; Wang, C.; Wang, K.Y. Structural Investigation of the Efficient Capture of Cs+ and Sr2+ by a Microporous Cd-Sn-Se Ion Exchanger Constructed from Mono-Lacunary Supertetrahedral Clusters. Inorg. Chem. Front. 2022, 9, 2880–2894. [Google Scholar] [CrossRef]
- Feng, M.L.; Qi, X.H.; Zhang, B.; Huang, X.Y. [BiGeS4]: The First Organically Directed Bismuth Thiogermanate with Rb+ Ion Exchange Property. Dalton Trans. 2014, 43, 8184–8187. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, B.; Jian, Y.; Zhong, W.; Mu, W.; He, J.; Ma, Z.; Liu, G.; Luo, S. Ion-Exchange Characteristics of a Layered Metal Sulfide for Removal of Sr2+ from Aqueous Solutions. Sep. Sci. Technol. 2012, 47, 896–902. [Google Scholar] [CrossRef]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed]
- Pekala, R.W. Organic Aerogels from the Polycondensation of Resorcinol with Formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Lu, Y.; He, W.; Cao, T.; Guo, H.; Zhang, Y.; Li, Q.; Shao, Z.; Cui, Y.; Zhang, X. Elastic, Conductive, Polymeric Hydrogels and Sponges. Sci. Rep. 2014, 4, srep05792. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef]
- Bryning, M.B.; Milkie, D.E.; Islam, M.F.; Hough, L.A.; Kikkawa, J.M.; Yodh, A.G. Carbon Nanotube Aerogels. Adv. Mater. 2007, 19, 661–664. [Google Scholar] [CrossRef]
- Wang, J.; Ellsworth, M. Graphene Aerogels. ECS Trans. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Guo, J.; Fu, S.; Deng, Y.; Xu, X.; Laima, S.; Liu, D.; Zhang, P.; Zhou, J.; Zhao, H.; Yu, H.; et al. Hypocrystalline Ceramic Aerogels for Thermal Insulation at Extreme Conditions. Nature 2022, 606, 909–916. [Google Scholar] [CrossRef]
- Guo, P.; Su, L.; Peng, K.; Lu, D.; Xu, L.; Li, M.; Wang, H. Additive Manufacturing of Resilient SiC Nanowire Aerogels. ACS Nano 2022, 16, 6625–6633. [Google Scholar] [CrossRef]
- Lohe, M.R.; Rose, M.; Kaskel, S. Metal-Organic Framework (MOF) Aerogels with High Micro- and Macroporosity. Chem. Commun. 2009, 40, 6056–6058. [Google Scholar] [CrossRef] [PubMed]
- Mohanan, J.L.; Brock, S.L. A New Addition to the Aerogel Community: Unsupported CdS Aerogels with Tunable Optical Properties. J. Non-Cryst. Solids 2004, 350, 1–8. [Google Scholar] [CrossRef]
- Ning, W.; Yi, L.; Qian, C.; Xiaoyue, S.; Yue, H.; Yunjun, L.; Ran, D. Metal Aerogels: Controlled Synthesis and Applications. Chin. J. Inorg. Chem. 2023, 39, 2212014. [Google Scholar]
- Leventis, N.; Chandrasekaran, N.; Sotiriou-Leventis, C.; Mumtaz, A. Smelting in the Age of Nano: Iron Aerogels. J. Mater. Chem. 2009, 19, 63–65. [Google Scholar] [CrossRef]
- Jiang, B.; Wan, Z.; Kang, Y.; Guo, Y.; Henzie, J.; Na, J.; Li, H.; Wang, S.; Bando, Y.; Sakka, Y.; et al. Auto-Programmed Synthesis of Metallic Aerogels: Core-Shell Cu@Fe@Ni Aerogels for Efficient Oxygen Evolution Reaction. Nano Energy 2021, 81, 105644. [Google Scholar] [CrossRef]
- Bigall, N.C.; Herrmann, A.K.; Vogel, M.; Rose, M.; Simon, P.; Carrillo-Cabrera, W.; Dorfs, D.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Hydrogels and Aerogels from Noble Metal Nanoparticles. Angew. Chem.-Int. Ed. 2009, 48, 9731–9734. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmüller, A. Noble Metal Aerogels-Synthesis, Characterization, and Application as Electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef]
- Du, R.; Fan, X.; Jin, X.; Hübner, R.; Hu, Y.; Eychmüller, A. Emerging Noble Metal Aerogels: State of the Art and a Look Forward. Matter 2019, 1, 39–56. [Google Scholar] [CrossRef]
- Naskar, S.; Freytag, A.; Deutsch, J.; Wendt, N.; Behrens, P.; Köckritz, A.; Bigall, N.C. Porous Aerogels from Shape-Controlled Metal Nanoparticles Directly from Nonpolar Colloidal Solution. Chem. Mater. 2017, 29, 9208–9217. [Google Scholar] [CrossRef]
- Liu, W.; Rodriguez, P.; Borchardt, L.; Foelske, A.; Yuan, J.; Herrmann, A.K.; Geiger, D.; Zheng, Z.; Kaskel, S.; Gaponik, N.; et al. Bimetallic Aerogels: High-Performance Electrocatalysts for the Oxygen Reduction Reaction. Angew. Chem.-Int. Ed. 2013, 52, 9849–9852. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Jin, W.; Hübner, R.; Zhou, L.; Hu, Y.; Eychmüller, A. Engineering Multimetallic Aerogels for PH-Universal HER and ORR Electrocatalysis. Adv. Energy Mater. 2020, 10, 1903857. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Chen, K.; Clark, A.H.; Hübner, R.; Zhan, J.; Zhang, L.; Eychmüller, A.; Cai, B. Optimizing the Pd Sites in Pure Metallic Aerogels for Efficient Electrocatalytic H2O2 Production. Adv. Mater. 2023, 35, e2211512. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huang, H.; Chen, Y.; Lai, F.; Fu, H.; Zhang, L.; Zhang, N.; Bai, S.; Liu, T. High-Entropy Alloy Aerogels: A New Platform for Carbon Dioxide Reduction. Adv. Mater. 2023, 35, e2209242. [Google Scholar] [CrossRef]
- Jiao, L.; Xu, W.; Yan, H.; Wu, Y.; Gu, W.; Li, H.; Du, D.; Lin, Y.; Zhu, C. A Dopamine-Induced Au Hydrogel Nanozyme for Enhanced Biomimetic Catalysis. Chem. Commun. 2019, 55, 9865–9868. [Google Scholar] [CrossRef]
- Xu, J.; Sun, F.; Li, Q.; Yuan, H.; Ma, F.; Wen, D.; Shang, L. Ultrasmall Gold Nanoclusters-Enabled Fabrication of Ultrafine Gold Aerogels as Novel Self-Supported Nanozymes. Small 2022, 18, e2200525. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Xu, W.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. Polydopamine-Capped Bimetallic AuPt Hydrogels Enable Robust Biosensor for Organophosphorus Pesticide Detection. Small 2019, 15, e1900632. [Google Scholar] [CrossRef]
- Fang, Q.; Qin, Y.; Wang, H.; Xu, W.; Yan, H.; Jiao, L.; Wei, X.; Li, J.; Luo, X.; Liu, M.; et al. Ultra-Low Content Bismuth-Anchored Gold Aerogels with Plasmon Property for Enhanced Nonenzymatic Electrochemical Glucose Sensing. Anal. Chem. 2022, 94, 11030–11037. [Google Scholar] [CrossRef]
- Guan, S.; Xu, B.; Yang, Y.; Zhu, X.; Chen, R.; Ye, D.; Liao, Q. Gold Nanowire Aerogel-Based Biosensor for Highly Sensitive Ethanol Detection in Simulated Sweat. ACS Appl. Nano Mater. 2022, 5, 11091–11099. [Google Scholar] [CrossRef]
- Pan, W.; Liang, C.; Sui, Y.; Wang, J.; Liu, P.; Zou, P.; Guo, Z.; Wang, F.; Ren, X.; Yang, C. A Highly Compressible, Elastic, and Air-Dryable Metallic Aerogels via Magnetic Field-Assisted Synthesis. Adv. Funct. Mater. 2022, 32, 2204166. [Google Scholar] [CrossRef]
- Courthéoux, L.; Popa, F.; Gautron, E.; Rossignol, S.; Kappenstein, C. Platinum Supported on Doped Alumina Catalysts for Propulsion Applications. Xerogels versus Aerogels. J. Non-Cryst. Solids 2004, 350, 113–119. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Li, Y.; Long, C.; Zhou, S.; Hübner, R.; Li, Y.; Xue, G.; Lin, D.; Xu, W.; et al. Design of Metal Aerogels-Based 3D SERS Substrates by Gentle Compression. Adv. Funct. Mater. 2025, 35, 2412006. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, C.; Liu, K.; Wei, L.; Luo, Z.; Zeng, M.; Yi, Y. Promising Pure Gold Aerogel: In Situ Preparation by Composite Sol–Gel and Application in Catalytic Removal of Pollutants and SERS. J. Sol-Gel Sci. Technol. 2021, 99, 614–626. [Google Scholar] [CrossRef]
- Gao, X.; Esteves, R.J.A.; Nahar, L.; Nowaczyk, J.; Arachchige, I.U. Direct Cross-Linking of Au/Ag Alloy Nanoparticles into Monolithic Aerogels for Application in Surface-Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2016, 8, 13076–13085. [Google Scholar] [CrossRef]
- Wang, H.; Fang, Q.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Noble Metal Aerogels. ACS Appl. Mater. Interfaces 2020, 12, 52234–52250. [Google Scholar] [CrossRef]
- Burpo, F.J. Noble Metal Aerogels. In Springer Handbook of Aerogels; Aegerter, M.A., Leventis, N., Koebel, M., Steiner, S.A., III, Eds.; Springer Handbooks; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Herrmann, A.K.; Formanek, P.; Borchardt, L.; Klose, M.; Giebeler, L.; Eckert, J.; Kaskel, S.; Gaponik, N.; Eychmüller, A. Multimetallic Aerogels by Template-Free Self-Assembly of Au, Ag, Pt, and Pd Nanoparticles. Chem. Mater. 2014, 26, 1074–1083. [Google Scholar] [CrossRef]
- Gao, X.; Esteves, R.J.; Luong, T.T.H.; Jaini, R.; Arachchige, I.U. Oxidation-Induced Self-Assembly of Ag Nanoshells into Transparent and Opaque Ag Hydrogels and Aerogels. J. Am. Chem. Soc. 2014, 136, 7993–8002. [Google Scholar] [CrossRef]
- Kühn, L.; Herrmann, A.K.; Rutkowski, B.; Oezaslan, M.; Nachtegaal, M.; Klose, M.; Giebeler, L.; Gaponik, N.; Eckert, J.; Schmidt, T.J.; et al. Alloying Behavior of Self-Assembled Noble Metal Nanoparticles. Chem. Eur. J. 2016, 22, 13446–13450. [Google Scholar] [CrossRef] [PubMed]
- Oezaslan, M.; Herrmann, A.K.; Werheid, M.; Frenkel, A.I.; Nachtegaal, M.; Dosche, C.; Laugier Bonnaud, C.; Yilmaz, H.C.; Kühn, L.; Rhiel, E.; et al. Structural Analysis and Electrochemical Properties of Bimetallic Palladium–Platinum Aerogels Prepared by a Two-Step Gelation Process. ChemCatChem 2017, 9, 798–808. [Google Scholar] [CrossRef]
- Cai, B.; Dianat, A.; Hübner, R.; Liu, W.; Wen, D.; Benad, A.; Sonntag, L.; Gemming, T.; Cuniberti, G.; Eychmüller, A. Multimetallic Hierarchical Aerogels: Shape Engineering of the Building Blocks for Efficient Electrocatalysis. Adv. Mater. 2017, 29, 1605254. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Wen, D.; Liu, W.; Herrmann, A.K.; Benad, A.; Eychmüller, A. Function-Led Design of Aerogels: Self-Assembly of Alloyed PdNi Hollow Nanospheres for Efficient Electrocatalysis. Angew. Chem.-Int. Ed. 2015, 54, 13101–13105. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Geiger, D.; Borchardt, L.; Simon, F.; Kaskel, S.; Gaponik, N.; Eychmüller, A. High-Performance Electrocatalysis on Palladium Aerogels. Angew. Chem.-Int. Ed. 2012, 51, 5743–5747. [Google Scholar] [CrossRef]
- Yuan, H.; Gao, W.; Wan, X.; Ye, J.; Ma, F.; Wen, D. Surface Engineering of Pt Aerogels by Metal Phthalocyanine to Enhance the Electrocatalytic Property for Oxygen Reduction Reaction. Mater. Today Energy 2023, 37, 101379. [Google Scholar] [CrossRef]
- Lu, L.; Sun, X.; Ma, J.; Yang, D.; Wu, H.; Zhang, B.; Zhang, J.; Han, B. Highly Efficient Electroreduction of CO2 to Methanol on Palladium-Copper Bimetallic Aerogels. Angew. Chem. Int. Ed. 2018, 57, 14149. [Google Scholar] [CrossRef]
- Nyce, G.W.; Hayes, J.R.; Hamza, A.V.; Satcher, J.H. Synthesis and Characterization of Hierarchical Porous Gold Materials. Chem. Mater. 2007, 19, 344–346. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of Nanoporosity in Dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef]
- Zielasek, V.; Jürgens, B.; Schulz, C.; Biener, J.; Biener, M.M.; Hamza, A.V.; Bäumer, M. Gold Catalysts: Nanoporous Gold Foams. Angew. Chem.-Int. Ed. 2006, 45, 8241–8244. [Google Scholar] [CrossRef]
- Hodge, A.M.; Hayes, J.R.; Caro, J.A.; Biener, J.; Hamza, A.V. Characterization and Mechanical Behavior of Nanoporous Gold. Adv. Eng. Mater. 2006, 8, 853–857. [Google Scholar] [CrossRef]
- Biener, J.; Wittstock, A.; Zepeda-Ruiz, L.A.; Biener, M.M.; Zielasek, V.; Kramer, D.; Viswanath, R.N.; Weissmüller, J.; Bäumer, M.; Hamza, A.V. Surface-Chemistry-Driven Actuation in Nanoporous Gold. Nat. Mater. 2009, 8, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Nahar, L.; Farghaly, A.A.; Esteves, R.J.A.; Arachchige, I.U. Shape Controlled Synthesis of Au/Ag/Pd Nanoalloys and Their Oxidation-Induced Self-Assembly into Electrocatalytically Active Aerogel Monoliths. Chem. Mater. 2017, 29, 7704–7715. [Google Scholar] [CrossRef]
- Bryce, C.T.; Stephen, A.S.; Luther, E.P. Nanoporous Metal Foams. Angew. Chem.-Int. Ed. 2010, 49, 4544–4565. [Google Scholar]
- Tappan, B.C.; Huynh, M.H.; Hiskey, M.A.; Chavez, D.E.; Luther, E.P.; Mang, J.T.; Son, S.F. Ultralow-Density Nanostructured Metal Foams: Combustion Synthesis, Morphology, and Composition. J. Am. Chem. Soc. 2006, 128, 6589–6594. [Google Scholar] [CrossRef]
- Sotiropoulou, S.; Sierra-Sastre, Y.; Mark, S.S.; Batt, C.A. Biotemplated Nanostructured Materials. Chem. Mater. 2008, 20, 821–834. [Google Scholar] [CrossRef]
- Fan, T.X.; Chow, S.K.; Zhang, D. Biomorphic Mineralization: From Biology to Materials. Prog. Mater. Sci. 2009, 54, 542–659. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Losch, A.R.; Bui, J.K.; Forcherio, G.T.; Baker, D.R.; McClure, J.P.; Bartolucci, S.F.; Chu, D.D. Salt-Templated Platinum-Copper Porous Macrobeams for Ethanol Oxidation. Catalysts 2019, 9, 662. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Mitropoulos, A.N.; Bartolucci, S.F.; McClure, J.P.; Baker, D.R.; Losch, A.R.; Chu, D.D. Saltlated Platinum-Palladium Porous Macrobeam Synthesis. MRS Commun. 2019, 9, 662. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; Woronowicz, K.; Mitropoulos, A.N. Salt-Mediated Au-Cu Nanofoam and Au-Cu-Pd Porous Macrobeam Synthesis. Molecules 2018, 23, 1701. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Winter, S.J.; McClure, J.P.; Bartolucci, S.F.; Burns, A.R.; O’Brien, S.F.; Chu, D.D. Salt-Templated Hierarchically Porous Platinum Macrotube Synthesis. ChemistrySelect 2018, 3, 4542–4546. [Google Scholar] [CrossRef]
- Wen, D.; Herrmann, A.K.; Borchardt, L.; Simon, F.; Liu, W.; Kaskel, S.; Eychmüller, A. Controlling the Growth of Palladium Aerogels with High-Performance toward Bioelectrocatalytic Oxidation of Glucose. J. Am. Chem. Soc. 2014, 136, 2727–2730. [Google Scholar] [CrossRef]
- Du, R.; Hu, Y.; Hübner, R.; Joswig, J.-O.; Fan, X.; Schneider, K.; Eychmüller, A. Specific ion effects directed noble metal aerogels: Versatile manipulation for electrocatalysis and beyond. Sci. Adv. 2019, 5, eaaw4590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Du, R.; Wang, J.; Wang, Y.; Hübner, R.; Fan, X.; Senkovska, I.; Hu, Y.; Kaskel, S.; Eychmüller, A. Unveiling Reductant Chemistry in Fabricating Noble Metal Aerogels for Superior Oxygen Evolution and Ethanol Oxidation. Nat. Commun. 2020, 11, 1590. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Nallathambi, V.; Liu, Y.; Kresse, J.; Hübner, R.; Reichenberger, S.; Gault, B.; Zhan, J.; Eychmüller, A.; et al. Structural Regulation of Au-Pt Bimetallic Aerogels for Catalysing the Glucose Cascade Reaction. Adv. Mater. 2024, 36, e2405200. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhu, C.; Du, D.; Bi, C.; Xia, H.; Feng, S.; Engelhard, M.H.; Lin, Y. Kinetically controlled synthesis of AuPt bi-metallic aerogels and their enhanced electrocatalytic performances. J. Mater. Chem. A 2017, 5, 19626–19631. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Xia, H.; Du, D.; Lin, Y. Efficient Synthesis of MCu (M = Pd, Pt, and Au) Aerogels with Accelerated Gelation Kinetics and their High Electrocatalytic Activity. Adv. Mater. 2016, 28, 8779–8783. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, S.; Cai, W.; Xu, B.Z.; Cai, Z.; Wu, Y.; Luo, X.; Wei, X.; Liu, Z.; Gu, W.; et al. Largely Boosted Methanol Electrooxidation Using Ionic Liquid/PdCu Aerogels: Via Interface Engineering. Mater. Horiz. 2020, 7, 2407–2413. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Zhong, H.; Su, D.; Li, N.; Engelhard, M.H.; Xia, H.; Zhang, Q.; Feng, S.; Beckman, S.P.; et al. Nanovoid Incorporated IrxCu Metallic Aerogels for Oxygen Evolution Reaction Catalysis. ACS Energy Lett. 2018, 3, 2038–2044. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Luo, X.; Jiao, L.; Wei, X.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Ternary PtRuCu Aerogels for Enhanced Methanol Electrooxidation. Nanoscale 2019, 11, 10575–10580. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, C.; Tian, M.; Su, D.; Fu, M.; Engelhard, M.H.; Chowdhury, I.; Feng, S.; Du, D.; Lin, Y. Ultrafine Pd Ensembles Anchored-Au2Cu Aerogels Boost Ethanol Electrooxidation. Nano Energy 2018, 53, 206–212. [Google Scholar] [CrossRef]
- Henning, S.; Ishikawa, H.; Kühn, L.; Herranz, J.; Müller, E.; Eychmüller, A.; Schmidt, T.J. Unsupported Pt-Ni Aerogels with Enhanced High Current Performance and Durability in Fuel Cell Cathodes. Angew. Chem. Int. Ed. 2017, 56, 10707. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Joswig, J.O.; Fan, X.; Hübner, R.; Spittel, D.; Hu, Y.; Eychmüller, A. Disturbance-Promoted Unconventional and Rapid Fabrication of Self-Healable Noble Metal Gels for (Photo-)Electrocatalysis. Matter 2020, 2, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zerebecki, S.; Du, R.; Hübner, R.; Marzum, G.; Jiang, G.; Hu, Y.; Barcikowki, S.; Reichenberger, S.; Eychmüller, A. Promoting the Electrocatalytic Performance of Noble Metal Aerogels by Ligand-Directed Modulation. Angew. Chem.-Int. Ed. 2020, 59, 5706–5711. [Google Scholar] [CrossRef]
- Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Ryu, M.Y.; Palmer, J.L. Gelatin Biotemplated Platinum Aerogels. MRS Adv. 2018, 3, 2875–2880. [Google Scholar] [CrossRef]
- Burpo, F.J.; Mitropoulos, A.N.; Nagelli, E.A.; Palmer, J.L.; Morris, L.A.; Ryu, M.Y.; Kenneth Wickiser, J. Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. Molecules 2018, 23, 1405. [Google Scholar] [CrossRef]
- Burpo, F.J.; Palmer, J.L.; Mitropoulos, A.N.; Nagelli, E.A.; Morris, L.A.; Ryu, M.Y.; Wickiser, J.K. Synthesis Method for Cellulose Nanofiber Biotemplated Palladium Composite Aerogels. J. Vis. Exp. 2019, 147, e59176. [Google Scholar] [CrossRef]
- Cai, B.; Hübner, R.; Sasaki, K.; Zhang, Y.; Su, D.; Ziegler, C.; Vukmirovic, M.B.; Rellinghaus, B.; Adzic, R.R.; Eychmüller, A. Core–Shell Structuring of Pure Metallic Aerogels towards Highly Efficient Platinum Utilization for the Oxygen Reduction Reaction. Angew. Chem.-Int. Ed. 2018, 57, 2963–2966. [Google Scholar] [CrossRef]
- Du, R.; Joswig, J.O.; Hübner, R.; Zhou, L.; Wei, W.; Hu, Y.; Eychmüller, A. Freeze–Thaw-Promoted Fabrication of Clean and Hierarchically Structured Noble-Metal Aerogels for Electrocatalysis and Photoelectrocatalysis. Angew. Chem.-Int. Ed. 2020, 59, 8293–8300. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, P.; Xiahou, Y.; Song, Y.; Bi, C.; Zhan, J.; Du, W.; Huang, L.; Möhwald, H.; Xia, H. Regulating Surface Facets of Metallic Aerogel Electrocatalysts by Size-Dependent Localized Ostwald Ripening. ACS Appl. Mater. Interfaces 2018, 10, 23081–23093. [Google Scholar] [CrossRef]
- Wang, C.; Duan, W.; Xing, L.; Xiahou, Y.; Du, W.; Xia, H. Fabrication of Au Aerogels with {110}-Rich Facets by Size-Dependent Surface Reconstruction for Enzyme-Free Glucose Detection. J. Mater. Chem. B 2019, 7, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Cai, B.; Du, R.; Hübner, R.; Georgi, M.; Jiang, G.; Li, L.; Samadi Khoshkhoo, M.; Sun, H.; Eychmüller, A. Ligand-Exchange-Mediated Fabrication of Gold Aerogels Containing Different Au(I) Content with Peroxidase-like Behavior. Chem. Mater. 2019, 31, 10094–10099. [Google Scholar] [CrossRef]
- Hiekel, K.; Jungblut, S.; Georgi, M.; Eychmüller, A. Tailoring the Morphology and Fractal Dimension of 2D Mesh-like Gold Gels. Angew. Chem.-Int. Ed. 2020, 59, 12048–12054. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhu, C.; Li, Y.; Xia, H.; Engelhard, M.H.; Fu, S.; Du, D.; Lin, Y. A Facile Method for Synthesizing Dendritic Core-Shell Structured Ternary Metallic Aerogels and Their Enhanced Electrochemical Performances. Chem. Mater. 2016, 28, 7928–7934. [Google Scholar] [CrossRef]
- Shi, Q.; Zhu, W.; Zhong, H.; Zhu, C.; Tian, H.; Li, J.; Xu, M.; Su, D.; Li, X.; Liu, D.; et al. Highly Dispersed Platinum Atoms on the Surface of AuCu Metallic Aerogels for Enabling H2O2 Production. ACS Appl. Energy Mater. 2019, 2, 7722–7727. [Google Scholar] [CrossRef]
- Wen, D.; Liu, W.; Haubold, D.; Zhu, C.; Oschatz, M.; Holzschuh, M.; Wolf, A.; Simon, F.; Kaskel, S.; Eychmüller, A. Gold Aerogels: Three-Dimensional Assembly of Nanoparticles and Their Use as Electrocatalytic Interfaces. ACS Nano 2016, 10, 2559–2567. [Google Scholar] [CrossRef]
- Liu, W.; Haubold, D.; Rutkowski, B.; Oschatz, M.; Hübner, R.; Werheid, M.; Ziegler, C.; Sonntag, L.; Liu, S.; Zheng, Z.; et al. Self-Supporting Hierarchical Porous PtAg Alloy Nanotubular Aerogels as Highly Active and Durable Electrocatalysts. Chem. Mater. 2016, 28, 6477–6483. [Google Scholar] [CrossRef]
- Burpo, F.J.; Nagelli, E.A.; Morris, L.A.; McClure, J.P.; Ryu, M.Y.; Palmer, J.L. Direct Solution-Based Reduction Synthesis of Au, Pd, and Pt Aerogels. J. Mater. Res. 2017, 32, 4153–4165. [Google Scholar] [CrossRef]
- Shafaei Douk, A.; Saravani, H.; Noroozifar, M. Three-Dimensional Assembly of Building Blocks for the Fabrication of Pd Aerogel as a High Performance Electrocatalyst toward Ethanol Oxidation. Electrochim. Acta 2018, 275, 182–191. [Google Scholar] [CrossRef]
- Wang, J.; Chen, F.; Jin, Y.; Guo, L.; Gong, X.; Wang, X.; Johnston, R.L. In Situ High-Potential-Driven Surface Restructuring of Ternary AgPd-Ptdilute Aerogels with Record-High Performance Improvement for Formate Oxidation Electrocatalysis. Nanoscale 2019, 11, 14174–14185. [Google Scholar] [CrossRef]
- Zareie Yazdan-Abad, M.; Noroozifar, M.; Douk, A.S.; Modarresi-Alam, A.R.; Saravani, H. Shape Engineering of Palladium Aerogels Assembled by Nanosheets to Achieve a High Performance Electrocatalyst. Appl. Catal. B 2019, 250, 242–249. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Du, D.; Su, D.; Engelhard, M.H.; Lin, Y. Core-Shell PdPb@Pd Aerogels with Multiply-Twinned Intermetallic Nanostructures: Facile Synthesis with Accelerated Gelation Kinetics and Their Enhanced Electrocatalytic Properties. J. Mater. Chem. A 2018, 6, 7517–7521. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Zhu, C.; Du, D.; Eychmüller, A.; Lin, Y. Engineering Ordered and Nonordered Porous Noble Metal Nanostructures: Synthesis, Assembly, and Their Applications in Electrochemistry. Chem. Rev. 2015, 115, 8896–8943. [Google Scholar] [CrossRef]
- Sisk, C.N.; Hope-Weeks, L.J. Copper(Ii) Aerogels via 1, 2-Epoxide Gelation. J. Mater. Chem. 2008, 18, 2607–2610. [Google Scholar] [CrossRef]
- Shobe, A.M.; Gill, S.K.; Hope-Weeks, L.J. Monolithic CuO–NiO Aerogels via an Epoxide Addition Route. J. Non Cryst. Solids 2010, 356, 1337–1343. [Google Scholar] [CrossRef]
- Allaf, R.M.; Hope-Weeks, L.J. Synthesis of ZnO-CuO Nanocomposite Aerogels by the Sol-Gel Route. J. Nanomater. 2014, 2014, 491817. [Google Scholar] [CrossRef]
- Baghi, R.; Peterson, G.R.; Hope-Weeks, L.J. Thermal Tuning of Advanced Cu Sol-Gels for Mixed Oxidation State Cu/Cu XOy Materials. J. Mater. Chem. A 2013, 1, 10898–10902. [Google Scholar] [CrossRef]
- Bi, Y.; Ren, H.; Chen, B.; Chen, G.; Mei, Y.; Zhang, L. Synthesis Monolithic Copper-Based Aerogel with Polyacrylic Acid as Template. J. Sol-Gel Sci. Technol. 2012, 63, 140–145. [Google Scholar] [CrossRef]
- Bi, Y.; He, L.; Zhang, Y.; He, S.; Ren, H.; Zhang, L. Characterization of the Microstructures of Copper-Based Aerogels on the Sol–Gel Process. J. Sol-Gel Sci. Technol. 2014, 72, 415–420. [Google Scholar] [CrossRef]
- Bi, Y.; Ren, H.; He, L.; Zhang, Y.; He, S.; Zhang, L. Preparation and Characterization of Metallic Copper-Based Aerogel with the Building Block of Nano-Crystals. Mater. Lett. 2015, 139, 205–207. [Google Scholar] [CrossRef]
- Mahadik-Khanolkar, S.; Donthula, S.; Bang, A.; Wisner, C.; Sotiriou-Leventis, C.; Leventis, N. Polybenzoxazine Aerogels. 2. Interpenetrating Networks with Iron Oxide and the Carbothermal Synthesis of Highly Porous Monolithic Pure Iron(0) Aerogels as Energetic Materials. Chem. Mater. 2014, 26, 1318–1331. [Google Scholar] [CrossRef]
- Mahadik-Khanolkar, S. Polybenzoxazine Aerogels: Synthesis, Characterization, Conversion to Porous Carbons, and Energetic Composites. PhD Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2013. [Google Scholar]
- Rewatkar, P.M.; Soni, R.U.; Sotiriou-Leventis, C.; Leventis, N. A Cobalt Sunrise: Thermites Based on LiClO4-Filled Co(0) Aerogels Prepared from Polymer-Cross-Linked Cobaltia Xerogel Powders. ACS Appl. Mater. Interfaces 2019, 11, 22668–22676. [Google Scholar] [CrossRef] [PubMed]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Sotiriou-Leventis, C.; Lu, H. One-Pot Synthesis of Interpenetrating Inorganic/Organic Networks of CuO/Resorcinol-Formaldehyde Aerogels: Nanostructured Energetic Materials. J. Am. Chem. Soc. 2009, 131, 4576–4577. [Google Scholar] [CrossRef]
- Leventis, N.; Donthula, S.; Mandal, C.; Ding, M.S.; Sotiriou-Leventis, C. Explosive versus Thermite Behavior in Iron(0) Aerogels Infiltrated with Perchlorates. Chem. Mater. 2015, 27, 8126–8137. [Google Scholar] [CrossRef]
- Xu, W.; Du, A.; Xiong, J.; Zhang, Z.; Shen, J.; Zhou, B. Freestanding Titanium Metallic Aerogel. Mater. Des. 2016, 97, 93–97. [Google Scholar] [CrossRef]
- Jung, S.M.; Qi, J.; Oh, D.; Belcher, A.; Kong, J. M13 Virus Aerogels as a Scaffold for Functional Inorganic Materials. Adv. Funct. Mater. 2017, 27, 1603203. [Google Scholar] [CrossRef]
- Saeed, A.M.; Wisner, C.A.; Donthula, S.; Majedi Far, H.; Sotiriou-Leventis, C.; Leventis, N. Reuseable Monolithic Nanoporous Graphite-Supported Nanocatalysts (Fe, Au, Pt, Pd, Ni, and Rh) from Pyrolysis and Galvanic Transmetalation of Ferrocene-Based Polyamide Aerogels. Chem. Mater. 2016, 28, 4867–4877. [Google Scholar] [CrossRef]
- Lei, Q.; Guo, J.; Noureddine, A.; Wang, A.; Wuttke, S.; Brinker, C.J.; Zhu, W. Sol–Gel-Based Advanced Porous Silica Materials for Biomedical Applications. Adv. Funct. Mater. 2020, 30, 1909539. [Google Scholar] [CrossRef]
- Liu, H.; Du, H.; Zheng, T.; Liu, K.; Ji, X.; Xu, T.; Zhang, X.; Si, C. Cellulose Based Composite Foams and Aerogels for Advanced Energy Storage Devices. Chem. Eng. J. 2021, 426, 130817. [Google Scholar] [CrossRef]
- Idumah, C.I.; Ezika, A.C.; Okpechi, V.U. Emerging Trends in Polymer Aerogel Nanoarchitectures, Surfaces, Interfaces and Applications. Surf. Interfaces 2021, 25, 101258. [Google Scholar] [CrossRef]
- Nie, L.; Li, S.; Cao, M.; Han, N.; Chen, Y. A Brief Review of Preparation and Applications of Monolithic Aerogels in Atmospheric Environmental Purification. J. Environ. Sci. 2025, 149, 209–220. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Wright, S.; Sandberg, A.; Nguyen, B.N.; Van Keuls, F.W.; Mueller, C.H.; Rodríguez-Solís, R.; Miranda, F.A. Low dielectric polyimide aerogels as substrates for lightweight patch antennas. ACS Appl. Mater. Interfaces 2012, 4, 6346–6353. [Google Scholar] [CrossRef]
- Hebalkar, N.; Kollipara, K.S.; Ananthan, Y.; Sudha, M.K. Nanoporous Aerogels for Defense and Aerospace Applications. In Handbook of Advanced Ceramics and Composites; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Aiev, L.E.; Oh, J.; Kozlov, M.E.; Kuznetsov, A.A.; Fang, S.; Fonseca, A.F.; Ovalle, R.; Lima, M.D.; Haque, M.H.; Gartstein, Y.N. Giant-stroke, superelastic carbon nanotube aerogel muscles. Science 2009, 323, 1575–1578. [Google Scholar] [CrossRef]
- Zhou, L.; Zhai, S.; Chen, Y.; Xu, Z. Anisotropic cellulose nanofibers/polyvinyl alcohol/graphene aerogels fabricated by directional freeze-drying as effective oil adsorbents. Polymers 2019, 11, 712. [Google Scholar] [CrossRef]
- Sonu, S.S.; Rai, N.; Chauhan, I. Multifunctional Aerogels: A Comprehensive Review on Types, Synthesis and Applications of Aerogels. J. Sol-Gel Sci. Technol. 2023, 105, 324–336. [Google Scholar] [CrossRef]
- Yin, M.; Fu, Z.; Yu, X.; Wang, X.; Lu, Y. The Effect of Drying Methods on the Pore Structure of Balsa Wood Aerogels. Polymers 2025, 17, 1686. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, P.; Xiao, R.; Fu, R.; Liu, Y.; Ji, C.; Song, Q.; Miao, C.; Yu, H.; Gu, J.; et al. Robust Silica–Agarose Composite Aerogels with Interpenetrating Network Structure by In Situ Sol–Gel Process. Gels 2022, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shao, G.; Wu, X.; Cui, S.; Shen, X. Ag-Incorporated Cr-Doped BaTiO3 Aerogel toward Enhanced Photocatalytic Degradation of Methyl Orange. Nanomaterials 2024, 14, 848. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Lai, Y.-R.; Sung, H.-L.; Chung, T.-W.; Lin, K.-Y.A. Design of Amine-Modified Zr–Mg Mixed Oxide Aerogel Nanoarchitectonics with Dual Lewis Acidic and Basic Sites for CO2/Propylene Oxide Cycloaddition Reactions. Nanomaterials 2022, 12, 3442. [Google Scholar] [CrossRef]
- Liu, F.; Feng, D.; Yang, H.; Guo, X. Preparation of Macroporous Transition Metal Hydroxide Monoliths via a Sol-Gel Process Accompanied by Phase Separation. Sci. Rep. 2020, 10, 4331. [Google Scholar] [CrossRef]
- Granados-Carrera, C.M.; Castro-Criado, D.; Abdullah, J.A.A.; Jiménez-Rosado, M.; Perez-Puyana, V.M. Aerogels Based on Chitosan and Collagen Modified with Fe2O3 and Fe3O4 Nanoparticles: Fabrication and Characterization. Polymers 2025, 17, 133. [Google Scholar] [CrossRef]
- Menshutina, N.; Fedotova, O.; Abramov, A.; Golubev, E.; Sulkhanov, Y.; Tsygankov, P. Processes of Obtaining Nanostructured Materialswith a Hierarchical Porous Structure on the Example of Alginate Aerogels. Gels 2024, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, R.; Dhakal, G.; Shim, J.-J.; Kim, W.K. NiO/Ni Nanowafer Aerogel Electrodes for High Performance Supercapacitors. Nanomaterials 2022, 12, 3813. [Google Scholar] [CrossRef] [PubMed]
- Wo, X.; Yan, R.; Yu, X.; Xie, G.; Ma, J.; Cao, Y.; Li, A.; Huang, J.; Huo, C.; Li, F.; et al. One-Step Synthesis of 3D Graphene Aerogel Supported Pt Nanoparticles as Highly Active Electrocatalysts for Methanol Oxidation Reaction. Nanomaterials 2024, 14, 547. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.; Scaglione, F.; Celegato, F.; Coïsson, M.; Tiberto, P.; Rizzi, P. Electroless Cobalt Deposition on Dealloyed Nanoporous Gold Substrate: A Versatile Technique to Control Morphological and Magnetic Properties. Nanomaterials 2023, 13, 494. [Google Scholar] [CrossRef]
- Liu, J.; Gu, T.; Li, L.; Li, L. Synthesis of MnO/C/NiO-Doped Porous Multiphasic Composites for Lithium-Ion Batteries by Biomineralized Mn Oxides from Engineered Pseudomonas Putida Cells. Nanomaterials 2021, 11, 361. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhao, H.; Li, L.; Ju, D.; Wang, C.; An, B. Biomass-Derived Porous Carbon with a Good Balance between High Specific Surface Area and Mesopore Volume for Supercapacitors. Nanomaterials 2022, 12, 3804. [Google Scholar] [CrossRef]
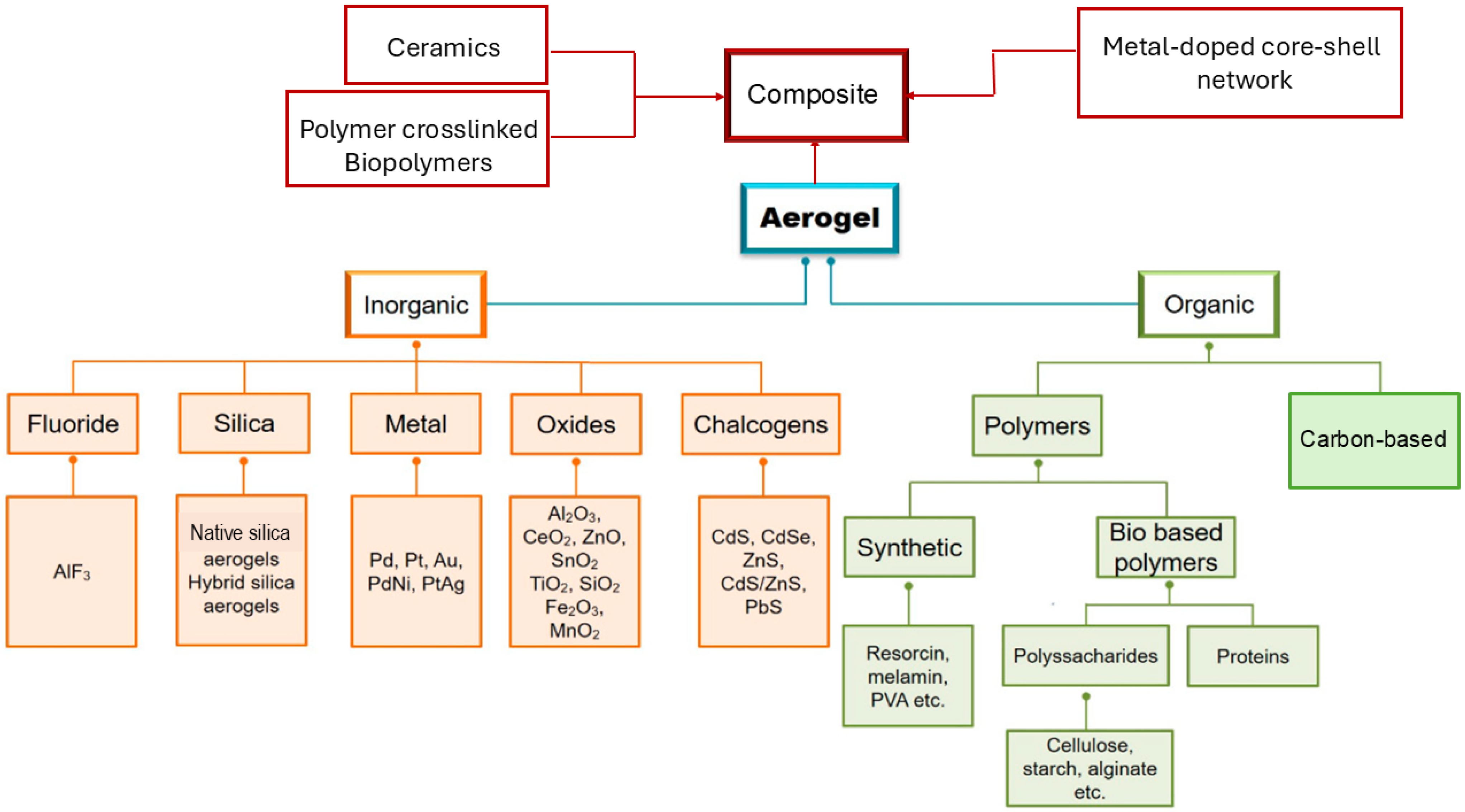
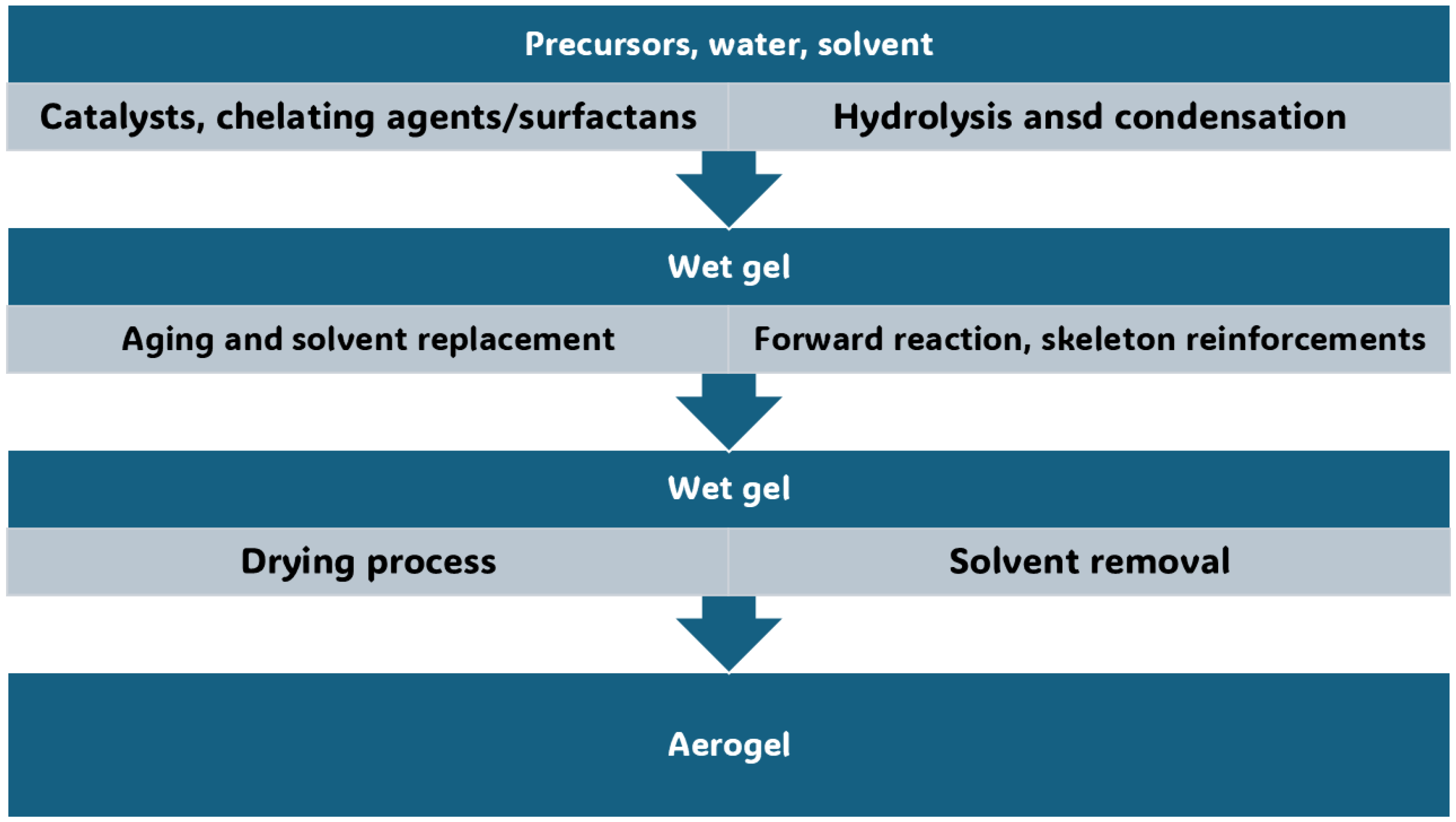

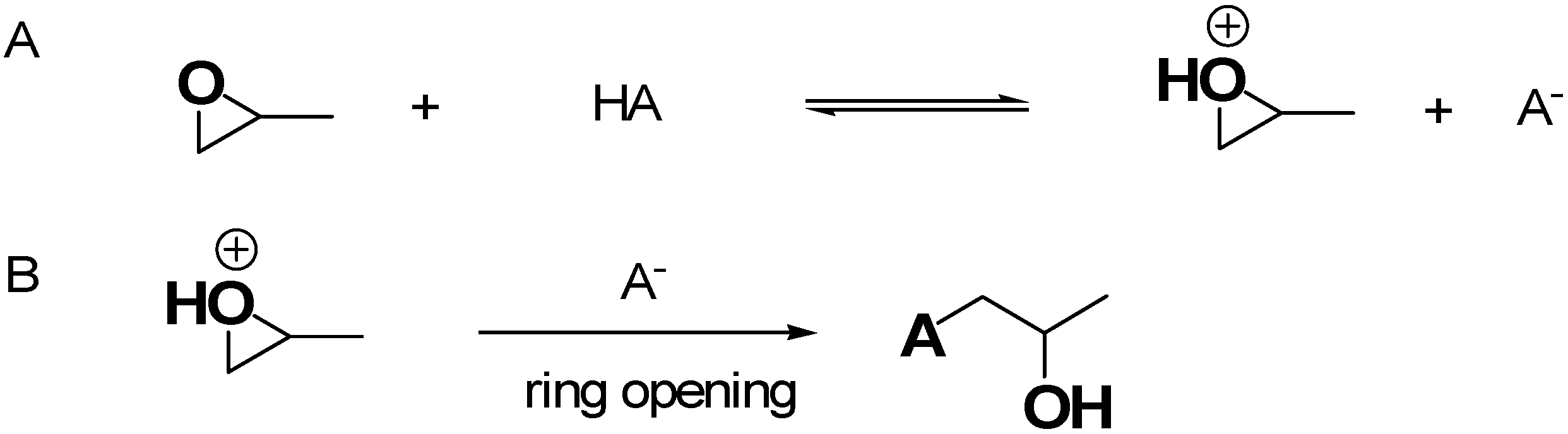
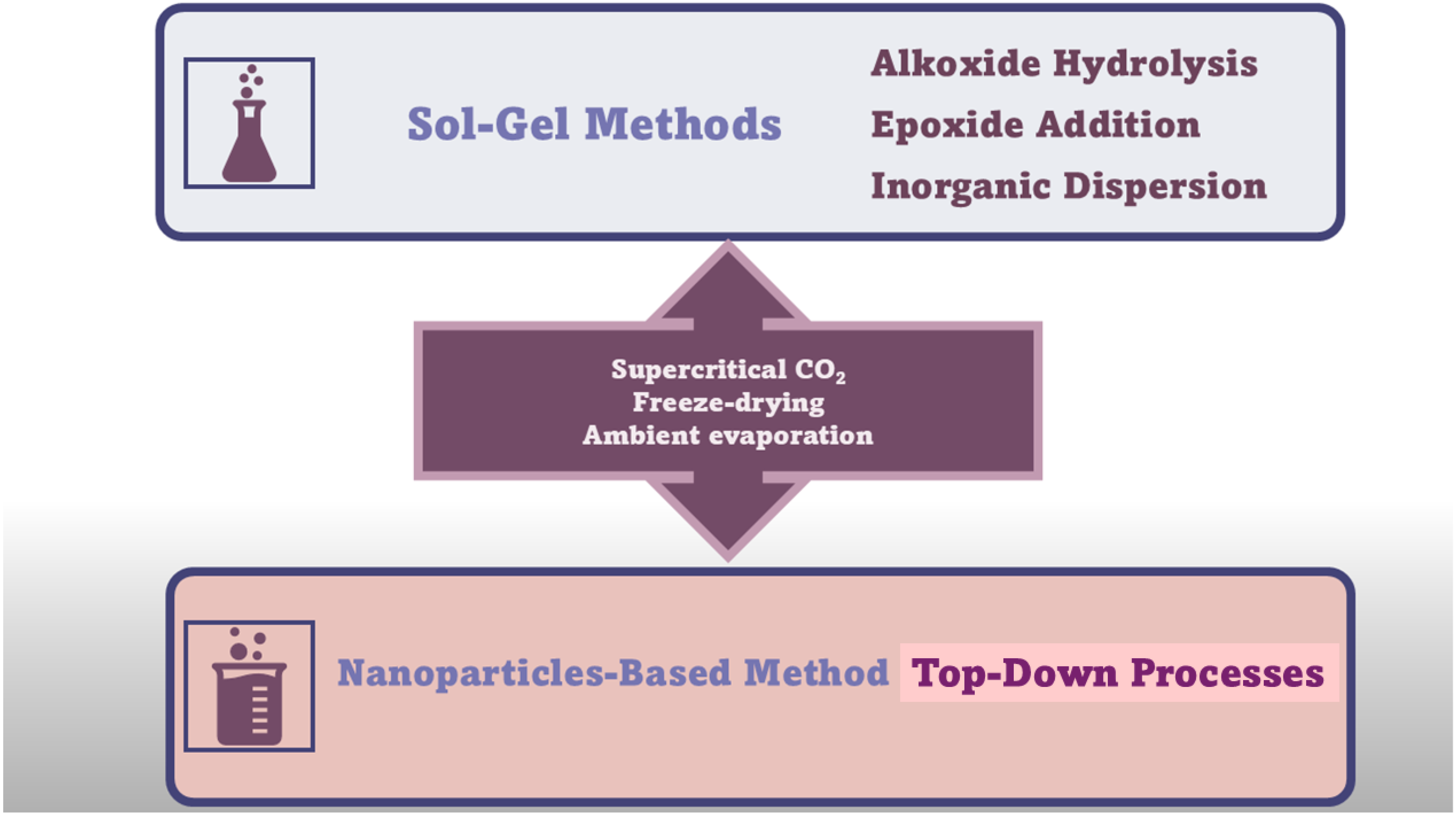
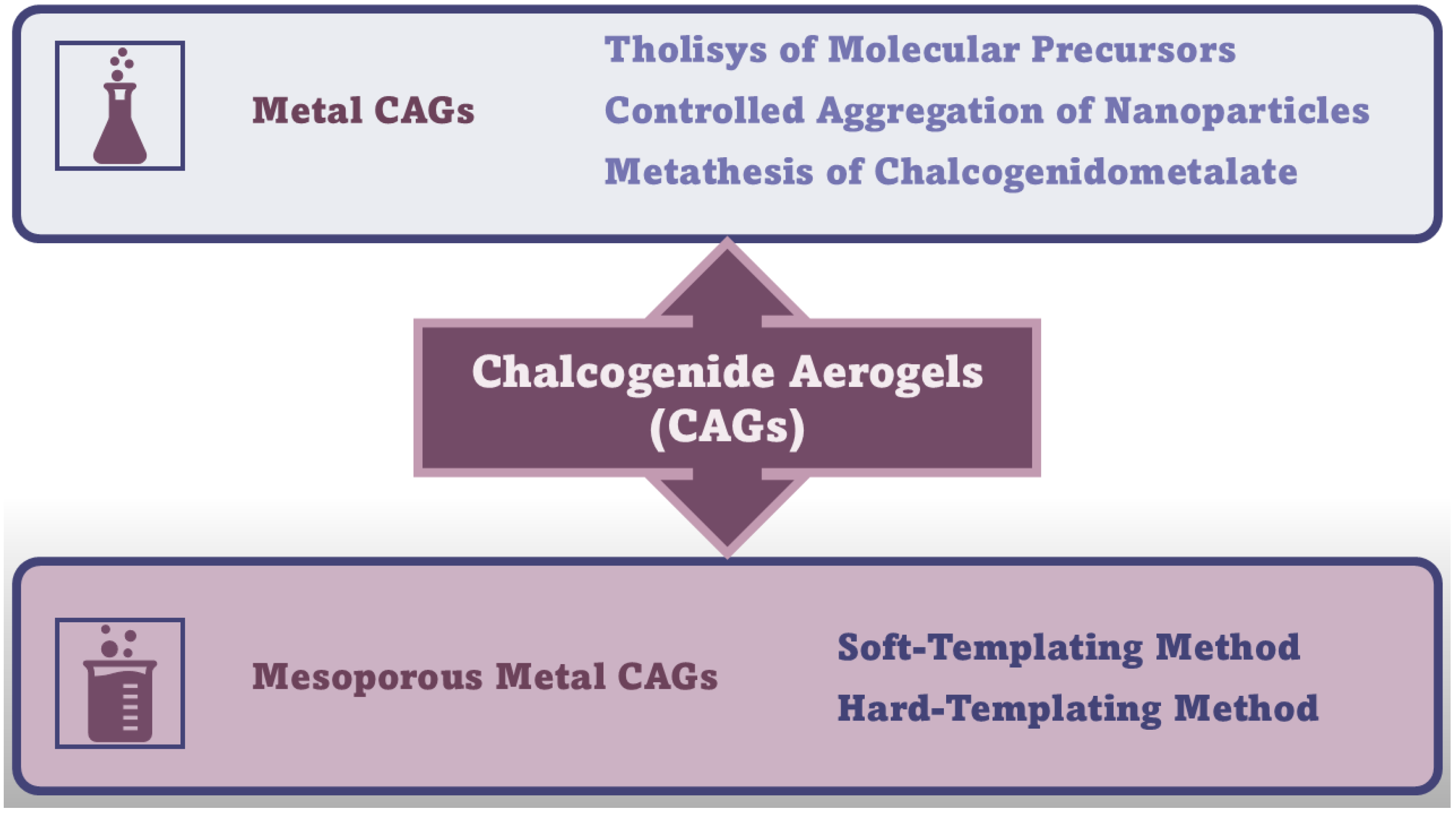

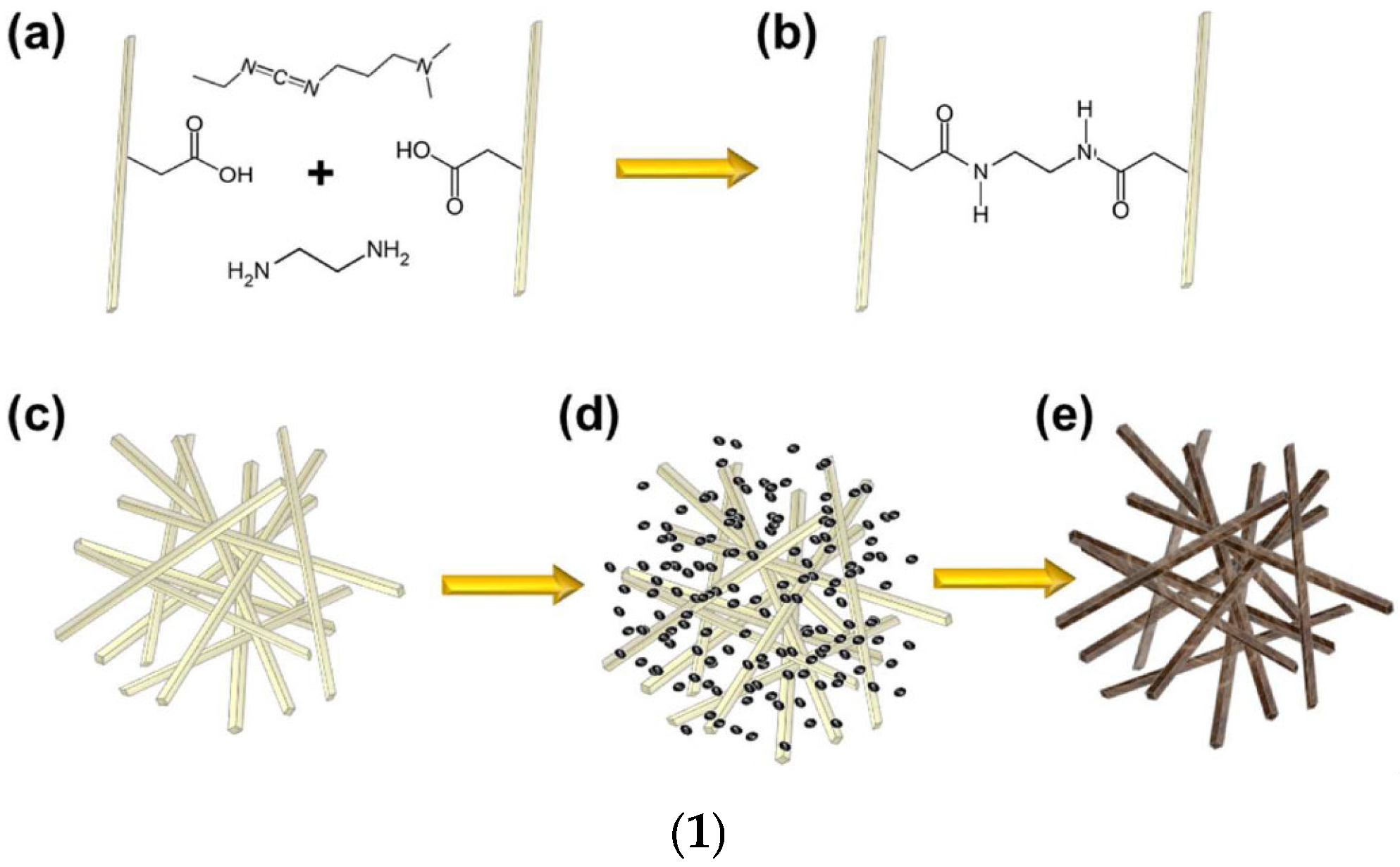
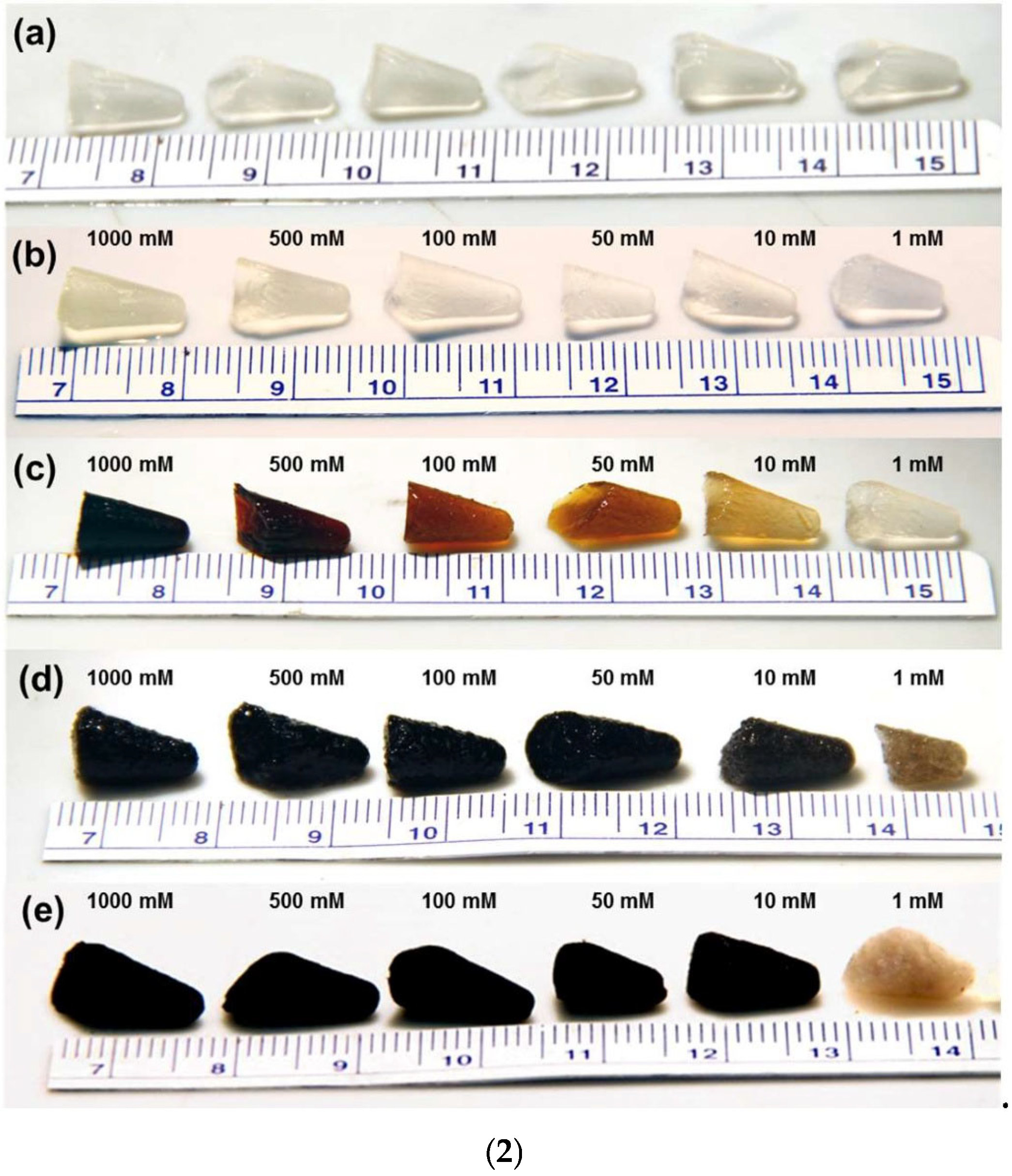
| Aerogels (AGs) | Sub Classes | Description/Subtypes | Refs |
|---|---|---|---|
| IAGs | SAGs | N.R. | [9,23] |
| CAGs | SCAGs | [9,24] | |
| OAGs | SnO2, W2O5, ZrO2, TiO2, Al2O3, MgO, Co3O4, NiO | [25,26,27,28] | |
| CBAGs | SC/AGs, CDC/AGs, MC/AGs, CHC/AGs, HAN/AGs, TIC/AGs, TUC/AGs | [29,30,31] | |
| NAGs | CN/AGs, AN/AGs, BN/AGs, SIN/AGs | [32,33,34,35] | |
| MAGs/NMAGs | INs, GNs, SNs | [36] |
| Formula | Chemical Name | Applications | Refs |
|---|---|---|---|
| TiO2 | Titanium oxide | N.R. | [52] |
| V2O5 | Vanadium oxide | N.R. | [53] |
| Co3O4 | Cobalt oxide | Supercapacitors | [54] |
| UO3 | Uranium trioxide | N.R. | [55] |
| Gd2O3 | Gadolinium oxide | N.R. | [56] |
| La2O3 | Lanthanide oxides | N.R. | [57] |
| ZnO2/TiO2, SiO2/TiO2 | Zinc/titanium oxide, silica/titanium oxide | N.R. | [58] |
| Ta2O5 | Tantalum oxide | N.R. | [59] |
| Mn3O4 | Manganese oxide | N.R. | [60] |
| Y2O3 | Yttrium oxide | N.R. | [61] |
| Eu-doped Y2O3 | Europium-doped yttrium oxide | Luminescence | [62] |
| Eu-doped ThO2 | Europium-doped oxide | N.R. | [63] |
| NiO/Al2O3 | Nickel oxide/alumina oxides | Hydrogen production | [64] |
| ZnFe2O4 | Zinc-ferrite oxides | N.R. | [65,66] |
| VFe2Ox | Vanadium-ferrite oxides | Electrochemical charge storage | [67] |
| La0.85Sr0.15MnO3 | Lanthanum-strontium-manganese oxides | Electronic conductivity | [68] |
| MnFe2O4 | Manganese-ferrite-oxide | Magnetism | [69] |
| NiFe2O4 | Nikel-ferrite oxide | Magnetic sector | [70] |
| Zn5(OH)8Cl2·H2O | Simonkolleite | Photoluminescence | [71] |
| Characteristics | MMCAGs | MCAGs |
|---|---|---|
| SF | ⬆ Polarizable surface, ⬇ Lewis’s basicity | ⬆ PS, ⬇ Lewis’s basicity |
| Synthesis | Soft-templating, hard-templating | Thiolytic hydrolysis, NPC, metathesis |
| Crystallinity | Crystalline to amorphous * | Nanocrystalline or amorphous |
| Porosity | Mesopores, narrow PSD, ⬆ SSA (516 m2/g) | Broad PSD, ⬆ SSA (755 m2/g) |
| AEE | Catalyst (HER/OER), PTC H2 production | Catalyst (HER, OER, CP), WR, AR |
| References | [111,112,113,114,115,116] | [108,109,117,118,119,120,121] |
| Mn+ | Ci (ppm) | Cf (ppb) | Mn+ Removal (%) | Kd (mL g−1) |
|---|---|---|---|---|
| Cu2+ | 10 × 103 | 872.0 | 91.30 | 1.05 × 104 |
| Hg2+ | 10 × 103 | 236.8 | 97.63 | 4.12 × 104 |
| Ag+ | 10 × 103 | 3.25 | 99.97 | 3.08 × 106 |
| Pb2+ | 10 × 103 | 0.10 | ∼100.0 | ∼108 |
| Cd2+ | 10 × 103 | 9.91 × 103 | 0.86 | 8.65 |
| Ni2+ | 10 × 103 | 9.12 × 103 | 8.8 | 96.49 |
| Zn2+ | 10 × 103 | 10 × 103 | 0.0 | 0.0 |
| Cations | Adsorbents | qm (mg g−1) | Refs |
|---|---|---|---|
| Ag+ | KCMS | 1377 | [149] |
| Amorphous MoOx | 2605 | [150] | |
| LDH-Mo3S13 | 1074 | [151] | |
| LDH-Sn2S6 | 978 | [152] | |
| Ni/Fe/Ti-MoS4-LDH | 856 | [153] | |
| Mn-LDH-MoS4 | 564 | [154] | |
| MoS4-ppy | 480 (pH~5) 725 (pH∼1) | [155] | |
| Mo3S13-ppy | 408 | [156] | |
| MoS4-LDH | 450 | [157] | |
| KMS-2 | 408 | [158] | |
| Fe-MoS4 | 565 | [159] | |
| Pb2+ | KCMS | 1146 | [149] |
| Lignosulfonate-modified graphene hydrogel | 1210 | [160] | |
| LDH-Sn2S6 | 579 | [152] | |
| MoS4-LDH | 290 | [157] | |
| Mn-MoS4 | 357 | [154] | |
| Fe-MoS4 | 345 | [159] | |
| EDTA-LDH | 180 | [161] | |
| CTS/PAM gel | 138 | [162] | |
| Mg2Al-LS-LDH | 123 | [163] | |
| Cellulose-based CAGs | 240 | [164] | |
| Biomass-based hydrogel | 422.7 | [165] | |
| Hg2+ | KCMS | 460 | [149] |
| LDH-Sn2S6 | 666 | [152] | |
| MoS4-LDH | 500 | [157] | |
| Mn-MoS4 | 594 | [154] | |
| Fe-MoS4 | 582 | [159] | |
| KMS-2 | 297 | [158] | |
| MoS4-ppy | 210 | [155] | |
| KMS-1 | 377 | [166] | |
| Thio-functionalized magnetic graphene oxide | 289 | [167] |
| Tap Water | MRW | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MIs | Ci (ppm) | Cf (ppm) | Removal ** | Kd (mL/g) | qm (mg/g) | Cf (ppm) | Removal ** | Kd (mL/g) | qm (mg/g) |
| Cu2+ | 10.0 | 3.6184 | 63.81 | 1782.4 | 6.381 | 3.1254 | 68.74 | 2.22 × 103 | 6.874 |
| Hg2+ | 10.0 | 0.2132 | 97.86 | 4.60 × 104 | 9.786 | 0.1749 | 98.25 | 5.68 × 104 | 9.825 |
| Ag+ | 10.0 | 0.0336 | 99.66 | 2.96 × 105 | 9.966 | 0.0524 | 99.48 | 2.08 × 105 | 9.948 |
| Pb2+ | 10.0 | 0.0686 | 99.31 | 1.51 × 105 | 9.931 | 0.0279 | 99.72 | 3.4 × 107 | 9.972 |
| Cd2+ | 10.0 | 8.0149 | 19.85 | 258.65 | 1.985 | 8.1857 | 18.14 | 2.30 × 102 | 1.814 |
| Ni2+ | 10.0 | 9.5000 | 4.64 | 48.63 | 0.463 | 9.3766 | 6.23 | 6.63 × 101 | 0.623 |
| Zn2+ | 10.0 | 10 | 0.0 | 0.0 | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 |
| Dyes | Co (mg/100 mL) | Qe,exp (mg/g) | K2 g mg−1 min−1 | Qe,cal (mg/g) | R2 |
|---|---|---|---|---|---|
| RhB | 100 | 416.33 | 0.0028 | 418.41 | 0.9997 |
| MB | 100 | 260.00 | 0.0072 | 263.15 | 0.9992 |
| MV | 100 | 280.89 | 0.0066 | 280.11 | 0.9983 |
| Adsorbent | Dyes | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|---|
| AC qm (mg/g) | KL (L/mg) | R2 | RL | n | Kf (L/mg) | R2 | ||
| SbS | RhB | 442.47 | 0.02181 | 0.9840 | 0.0353 | 3.09 | 11.03 | 0.7223 |
| MB | 303.95 | 0.08756 | 0.9956 | 0.0090 | 8.74 | 12.83 | 0.7174 | |
| MV | 210.52 | 0.06157 | 0.9751 | 0.0128 | 9.11 | 12.96 | 0.7149 | |
| Dyes | Adsorbent | qmax (mg/g) | Ref |
|---|---|---|---|
| RhB | Molybdenum sulfide (MoS2) | 291 | [169] |
| PDA-modified graphene hydrogel | 207.06 | [170] | |
| Activated carbon | 88.0 | [171] | |
| CK-30 (COOH functionalized KIT-5) | 270 | [172] | |
| Hydrothermal graphene hydrogel | 148.41 | [170] | |
| Unmodified biomass | 25.2 | [173] | |
| A-TRB | 212.77 | [174] | |
| Fe–Ben | 98.62 | [175] | |
| NMIL-100 (Fe) | 76.69 | [176] | |
| MIL-68 (Al) | 1111.11 | [177] | |
| Antimony sulfide (SbS) | 442.47 | [168] | |
| MB | MoS2 | 208 | [169] |
| Carbon monolith | 127.06 | [178] | |
| Cobalt ferrite/alginate | 33.6 | [179] | |
| CMT-g-PAM/silica nanocomposite | 43.86 | [180] | |
| Magnetic graphene oxide | 270.94 | [181] | |
| Swede rape straw C2-symmetric benzene-based | 246.4 | [182] | |
| Hydrogels with unique layered structures | 54.4 | [183] | |
| Superabsorbent hydrogel supported on modified polysaccharide | 48 | [184] | |
| Poly(AA-co-AMPS)MMT | 215 | [185] | |
| MIL-68 (Al) | 1666.67 | [177] | |
| MIL-68 (Al) | 666.67 | [186] | |
| SbS | 303.95 | [168] | |
| MV | Baker’s yeast modified by nano Fe3O4 | 60.8 | [187] |
| Sunflower seed hull | 92.6 | [188] | |
| Magnetic baker’s yeast biomass | 60.80 | [187] | |
| Granular activated carbon | 95 | [189] | |
| Bagasse fly ash | 26.2 | [190] | |
| Peanut straw char | 101 | [190] | |
| Halloysite nanotubes | 113.6 | [191] | |
| Cross-linked amphoteric starch | 333.3 | [192] | |
| Sugar cane dust | 50.4 | [193] | |
| SbS | 210.52 | [168] |
| Material | T | Target Ion | Kd (mL/g) | qm (mg/g) | T (min) | pH Range | Ref. |
|---|---|---|---|---|---|---|---|
| K2xMnxSn3−xS6 (x = 0.5–0.95) (KMS-1) | RT | Sr2+ | ≥105 | 77 | —— | 1.0–14 | [195] |
| Cs+ | ≥104 | 226 | 5 | 0.8–12 | [196] | ||
| UO22+ | ≥105 | 380 | 120 | 2.5–10 | [197] | ||
| K2xMgxSn3−xS6 (x = 0.5–1) (KMS-2) | RT | Cs+ | ≥103 | 531 | —— | 3.0–10 | [198] |
| Sr2+ | ≥104 | 87 | |||||
| KInSn2S6 (KMS-5) | RT | Eu3+ | ≥5.91 × 104 | 87 | 10 | 1.0–5 | [199] |
| 241Am | —— | —— | |||||
| Th4+ | 1.5 × 106 | 90.9 | 10 | —— | [200] | ||
| KInSnS4 (InSnS-1) | RT | Cs+ | >104 | 316.0 | 5 | 0.02–11.14 2 mol/L HNO3 | [201] |
| K1.87ZnSn1.68S5.30 (KZTS) | 298 K | Sr2+ | 1.28 × 106 | 19.3 | 10 | 3.0–11 | [202] |
| Na5Zn3.5Sn3.5S13·6H2O (NaZTS) | 298 K | Sr2+ | 2.67 × 105 | 32.3 | 5 | 3.0–12 | [203] |
| Na5Zn3.5Sn3.5S13·6H2O (ZnSnS-1) | 343 K | Sr2+ | 1.6 × 104 | 124.2 | 1440 | 2.5–13 | [204] |
| K2xSn4−xS8−x (x = 0.65–1) (KTS-3) | RT | Cs+ | 5.5 × 104 | 280 | 5 | 2.0–12 | [205] |
| Sr2+ | 3.9 × 105 | 102 | 2.0–12 | ||||
| UO22+ | 2.7 × 104 | 287 | 4.0–10 | ||||
| K2Sn2S5 (KTS-2) | 343 K | Yb3+ | >105 | 232.7 | 10 | —— | [206] |
| Na2Sn3S7 (NaTS) | 298 K | Sr2+ | 3.43 × 107 | 80 | 5 | 3.0–13 | [207] |
| Na1.94Sn2.87S7 (NaTS-2) | 298 K | Sr2+ | 2 × 106 | 88.9 | 60 | 3–11 | [208] |
| Na1.60Mg0.33Sn3.15S7 (NMTS) | 298 K | Sr2+ | 1.1 × 106 | 52.6 | 3 | 4–11 | [209] |
| K1.93Ti0.22Sn3S6.43 (KTSS) | RT | Cs+ | >104 | 450.12 | 1 | 3–12 | [210] |
| K1.61Fe0.04Sb0.03Sn3.1S7 (PIATS) | RT | Cs+ | 1.96 × 104 | 401.23 | 3 | 4–12 | [211] |
| K1.29Sb0.15Sn3S6.87 (KATS-2) | RT | Cs+ | 1.59 × 105 | 358 | 5 | 1–12 | [212] |
| K1.83Al0.48Sn3S7.64 (KAlSnS-3) | 298 K | Cs+ | 2.09 × 105 | 259.31 | 15 | 1–13 | [213] |
| KInSnS4 | RT | NH4+, Rb+, Cs+,Tl+, Sr2+, Ca2+, Ln3+ | [214] | ||||
| NaInSnS4 | RT | NH4+, Rb+, Cs+,Tl+, Sr2+, Ca2+, Ce3+ | |||||
| K2CdSn2S6 | RT | Rb+, Cs+,Tl+, Sr2+, Ce3+ | [215] | ||||
| K3.2Nb1.6S4.8 (KNbS) | 303 K | Sr2+ | —— | 80 | 25 | 4–10 | [216] |
| Co2+ | —— | 55 | 35 | 3.5–12 | |||
| K6Cd4Sn3Se13 | —— | Li+, Na+, Rb+ | [217] | ||||
| K3Rb3Zn4Sn3Se13 | 323 K | Cs+ | [218] | ||||
| Compounds | T | Target Ion | Kd (mL/g) | qm (mg/g)/RR (%) | T (min) | pH |
|---|---|---|---|---|---|---|
| FJSM-SnS | 338 K | Cs+ | 2.36 × 103 | 408.91 | 5 | 0.7–12.7 |
| Sr2+ | 8.89 × 104 | 65.19 RR | 5 | |||
| RT | UO22+ | 2.64 × 104 | 338.43 | 1200 | 2.1–11 | |
| 338 K | Eu3+ | >104 | 139.82 | <5 | 1.9–8.5 | |
| Tb3+ | 147.05 | <5 | ||||
| Nd3+ | 126.70 | —— | ||||
| 298 K | Ba2+ | 7.49 × 104 | 289.0 | <5 | 3.3–10.8 | |
| Ni2+ | 8.92 × 104 | 83.27 RR | 2.3–10.8 | |||
| Co2+ | 3.75 × 105 | 51.98 RR | ||||
| FJSM-SnS-2 | RT | Cs+ | 1.81 × 103 | 266.54 | 60 | 2.5–11.9 |
| Sr2+ | 4.47 × 103 | 59.41 RR | 60 | |||
| Eu3+ | >105 | 58.39 RR | 5 | |||
| FJSM-SnS-3 | RT | Cs+ | 2.62 × 103 | 109.68 | 60 | |
| Sr2+ | 3.59 × 103 | 57.81 RR | 60 | |||
| Eu3+ | >105 | 61.52 RR | 5 | |||
| FJSM-SnS-4 | RT | Cs+ | 5.83 × 103 | 388.94 | 30 | 0.0–11.1 |
| Sr2+ | 9.38 × 105 | 141.22 | 2 | |||
| InS-0 | —— | Sr2+ | —— | ∼59.6 RR | 60 | —— |
| Cs+ | —— | ∼41.2 RR | —— | —— | ||
| InS-1 | RT | Sr2+ | 1.57 × 105 | 105.35 | 1440 | 3.0–12 |
| InS-2 | RT | Sr2+ | >104 | 143 | 480 | 3.0–14 |
| Ba2+ | 2.3 × 105 | 211.73 | 20 | 3–13 | ||
| Ni2+ | 2.0 × 105 | 103.57 | 20 | 3–11 | ||
| Co2+ | 1.6 × 105 | 111.78 | 10 | 3–11 | ||
| (NH4)4In12Se20 | RT | Cs+Sr2+ | —— | —— | —— | —— |
| FJSM-SbS | 353K | Cs+ | 5.62 × 103 | 143.47 | 2 | 3.4–11.4 |
| [(Me)2NH2]2[Sb2GeS6] | RT | Cs+ | —— | ∼93 RR | —— | —— |
| Rb+ | —— | ∼85 RR | —— | —— | ||
| [CH3NH3]20Ge10Sb28S72·7H2O | 338 K | Cs+ | 5.46 × 103 | 230.91 | 2 | 2.8–11 |
| [(CH3CH2CH2)2NH2]5In5Sb6S19·1.45H2O | 348 K | Cs+ | —— | 228.61 | —— | 4.3–10 |
| [NH3CH3]4[In4SbS9SH] | 343 K | Rb+ | —— | ∼82.5 RR | —— | —— |
| [(CH3)2NH2]2[Ga2Sb2S7]·H2O | 348 K | Cs+ | —— | 100 RR | —— | 1.7–11.8 |
| FJSM-GAS-1 | RT | Cs+ | —— | 164 | —— | —— |
| Sr2+ | —— | 80 RR | —— | —— | ||
| UO22+ | 2.47 × 104 | 196 | 5 | 2.9–10 | ||
| Eu3+ | 6.39 × 105 | 127.7 | <2 | 2.5–8.2 | ||
| FJSM-GAS-2 | RT | UO22+ | 5.12 × 104 | 144 | 15 | 2.9–10 |
| Eu3+ | 6.00 × 105 | 115.8 | <2 | 2.5–8.2 | ||
| SCU-36 | RT | Cs+ | —— | ∼70 RR | —— | —— |
| InSnOS | RT | Cs+ | 105 | 537.7 | 5 | 4.0–10 |
| [(Me)2NH2]0.75 [Ag1.25SnSe3] | RT | Cs+ | —— | 93 RR | —— | —— |
| Rb+ | —— | 87 RR | —— | —— | ||
| CuGeSe-1 | 343 K | Cs+ | 3.5 × 103 | 225.3 | —— | 1.0–12 |
| AgSnSe-1 | RT | Cs+ | 3.17 × 104 | 174.4 | —— | 1.0–12 |
| [CH3CH2NH3]22Zn16Sn12Se51(H2O)4·16H2O | RT | Sr2+ | >104 | 104.17 | 20 | —— |
| (H2en)2Cu8Sn3S12 | 333 K | Cs+ | —— | ∼35 RR | —— | —— |
| 333 K | Rb+ | —— | ∼36 RR | —— | —— | |
| (dap)2(Hdap)4Cu8Ge3S18 | 318 K | Cs+ | —— | 90 RR | —— | —— |
| OCF-45-MnInS | RT | Cs+ | —— | 90 RR | —— | —— |
| 333 K | Cs+ | —— | 100 RR | —— | —— | |
| CdSnSe-1 | RT | Cs+ | 1.42 × 104 | 371.4 | 1440 | 4–13 |
| Sr2+ | 1.50 × 104 | 128.4 | 1440 | 3–13 | ||
| [(Me)2NH2] [BiGeS4] | RT | Rb+ | —— | 25 RR | —— | —— |
| UCR-28 | RT | Sr2+ | —— | 90 | 90–120 | —— |
| Material | T | Target Ion | Kd (mL/g) | qm (mg/g) or RR (%) | T (min) | pH Range | Ref. |
|---|---|---|---|---|---|---|---|
| K@RWY | RT | Cs+ | ≥105 | 310 | 5 | 2.9–11.8 | [219] |
| K@GaSnS-1 | RT | UO22+ | 1.03 × 104 | 147.6 | 15 | 2.75–10.87 | [220] |
| KIAS | RT | Cs+ | >104 | 309.6 | 1 | 1–13 | [221] |
| SbS-1 | RT | Cs+ | N.R. | 70.96 RR | 1440 | N.R. | [222] |
| Sr2+ | 49.48 RR | >2880 | |||||
| SbS-1K | RT | Cs+ | 1.06 × 105 | 318.77 | 40 | 0–12 | [222] |
| Sr2+ | 1.95 × 105 | 61.12 RR | 40 | 4–11 | |||
| K-MPS-1 | RT | Cs+ | >104 | 337.5 | 15 | 2–12 | [223] |
| N-MPS | RT | UO22+ | 2.23 × 104 | 854.36 | 360 | 2.8–12.2 | [224] |
| MT | Synthetic Methods | Morphologies | Compositions | Refs |
|---|---|---|---|---|
| TSM | C2H5OH or H2O2 | NWs | Au, Pt, Ag, PtAg, AuAg | [270] |
| 348 K, GRR | HNWs | Ni–PdxPty | [294] | |
| Cu-UPD | CSNWs | PdxAu–Pt | [329] | |
| 348 K, GRR | HNWs | PdNi | [295] | |
| Salting out | NWs | Au, Ag, Pd, Pt | [314] | |
| Freeze–thaw | Au, Pd, Rh, AuAg, AuPd, AuPt, AuRh | [330] | ||
| Excessive-reductant-directed | Au, Ag, Pd, Pt, Ru, Rh, Os, Ir | [315] | ||
| NaCl | Aum−nPd | [331] | ||
| Au | [332] | |||
| Ligand-exchange | Au | [333] | ||
| NaBH4 | Au, Pd, AuPd | [326] | ||
| C(NO2)4 | HNWs | Ag | [291] | |
| AuAg alloy | [287] | |||
| 348 K | NWs | Au, Ag, Pt, Pd | [290] | |
| Phase boundary gelation | NMs | Au | [334] | |
| C(NO2)4 | NWs | Au/Ag/Pd | [304] | |
| N2H4·H2O | NCs | Pt | [273] | |
| NaCl | NSs | Au/Ag, Pd/Ag, Pt/Ag | [94] | |
| AA, 35 °C | DCSs | Au@Pt3Pd | [335] | |
| NaBH4, 60 °C, GRR | NWs | AD-Pt@AuCu | [336] | |
| Au2Cu@Pd | [322] | |||
| Ca2+ | Pd | [313] | ||
| DA | Au | [337] | ||
| NaBH4, 25 °C, GRR | NTs | PtAg | [338] | |
| OSM | NaBH4, 25 °C | NWs | PdCD | [296] |
| DMAB, NaHPO2·H2O, 25 °C | Au, Pd, Pt | [339] | ||
| NH4F | AuPt, AuRh | [275] | ||
| Stirring | Au, Ag, Pd, Rh, Au–Ir, Au–Pd–Pt | [324] | ||
| NaBH4, 25 °C | PtNi | [323] | ||
| PtxPdy | [274] | |||
| CHOCOOH, 45 °C | NWs | Pd | [340] | |
| NaBH4, 60 °C | NVNWs | IrxCu | [320] | |
| NWs | IL/PdCu | [319] | ||
| AgPd-Ptdilute | [341] | |||
| AuPt | [298] | |||
| NaBH4, DA, 60 °C | NWs | AuPt-PDA | [280] | |
| CO, 50 °C | Pd | [342] | ||
| NaBH4, 60 °C | PdCu | [297] | ||
| NaH2PO2, 60 °C | CSINs | PdPb@Pd | [343] | |
| NaBH4, 60 °C | NWs | PtRuCu | [321] |
| Sector | Possible Application | Motivations | Examples/Advantages | Refs |
|---|---|---|---|---|
| Biomedicine | Controlled DDSs | Tuneable PS, ⬆ SSA, CRR of PP | SAGs for targeted CT * | [361] |
| Energy | Batteries, supercapacitors | ⬆ SSA | ⬆ Charge storage capacities ** | [81,362] |
| AE | Insulation | IP and resistance to ET | Spacecraft/satellites protection ** | [363] |
| EE | FS for AP and WP | ⬆ Trap particulates/contaminants | HPFS for EP ** | [364]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfei, S. Aerogels Part 2. A Focus on the Less Patented and Marketed Airy Inorganic Networks Despite the Plethora of Possible Advanced Applications. Int. J. Mol. Sci. 2025, 26, 10696. https://doi.org/10.3390/ijms262110696
Alfei S. Aerogels Part 2. A Focus on the Less Patented and Marketed Airy Inorganic Networks Despite the Plethora of Possible Advanced Applications. International Journal of Molecular Sciences. 2025; 26(21):10696. https://doi.org/10.3390/ijms262110696
Chicago/Turabian StyleAlfei, Silvana. 2025. "Aerogels Part 2. A Focus on the Less Patented and Marketed Airy Inorganic Networks Despite the Plethora of Possible Advanced Applications" International Journal of Molecular Sciences 26, no. 21: 10696. https://doi.org/10.3390/ijms262110696
APA StyleAlfei, S. (2025). Aerogels Part 2. A Focus on the Less Patented and Marketed Airy Inorganic Networks Despite the Plethora of Possible Advanced Applications. International Journal of Molecular Sciences, 26(21), 10696. https://doi.org/10.3390/ijms262110696






