Multifunctional REE Selective Hybrid Membranes Based on Ion-Imprinted Polymers and Modified Multiwalled Carbon Nanotubes: A Physicochemical Characterization
Abstract
1. Introduction
2. Results and Discussion
Characteristics of Synthesized REEIIPs and Hybrid Materials
3. Materials and Methods
3.1. Reagents
3.2. Ion-Imprinted Polymer Synthesis
3.3. Synthesis of MWCNTs
3.4. REEIIP Membrane Preparation
3.5. Membrane Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sager, M.; Wiche, O. Rare Earth Elements (REE): Origins, Dispersion, and Environmental Implications—A Comprehensive Review. Environments 2024, 11, 24. [Google Scholar] [CrossRef]
- Taggart, R.K.; Hower, J.C.; Hsu-Kim, H. Effects of Roasting Additives and Leaching Parameters on the Extraction of Rare Earth Elements from Coal Fly Ash. Int. J. Coal Geol. 2018, 196, 106–114. [Google Scholar] [CrossRef]
- Baba, A.; Howard, W.F.; Gunduz, O. Effects of Fly Ash from Coal-Burning Electrical Utilities on Ecosystem and Utilization of Fly Ash. In Groundwater Ecosystems; Springer: Dordrecht, The Netherlands, 2006; pp. 15–31. [Google Scholar]
- Muravyov, M.I.; Bulaev, A.G.; Melamud, V.S.; Kondrat’eva, T.F. Leaching of Rare Earth Elements from Coal Ashes Using Acidophilic Chemolithotrophic Microbial Communities. Microbiology 2015, 84, 194–201. [Google Scholar] [CrossRef]
- Van Loy, S.; Binnemans, K.; Van Gerven, T. Mechanochemical-Assisted Leaching of Lamp Phosphors: A Green Engineering Approach for Rare-Earth Recovery. Engineering 2018, 4, 398–405. [Google Scholar] [CrossRef]
- Nascimento, M.; Moreira, S.P.S.; de Souza, V.P. Adsorption of heavy metal cations using coal fly ash modified by hydrothermal method. Fuel 2009, 88, 1714–1719. [Google Scholar] [CrossRef]
- King, J.F.; Taggart, R.K.; Smith, R.C.; Hower, J.C.; Hsu-Kim, H. Aqueous acid and alkaline extraction of REE from coal ash. Int. J. Coal Geol. 2018, 195, 75–83. [Google Scholar] [CrossRef]
- Mutlu, B.K.; Cantoni, B.; Turolla, A.; Antonelli, M.; Hsu-Kim, H.; Wiesner, M.R. Application of nanofiltration for Rare Earth Elements recovery from coal fly ash leachate: Performance and cost evaluation. Chem. Eng. J. 2018, 349, 309–317. [Google Scholar] [CrossRef]
- Gasser, M.S.; Aly, M.I. Separation and recovery of rare earth elements from spent nickel-metal-hydride batteries using synthetic adsorbent. Int. J. Miner. Process 2013, 121, 31–38. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Huang, B.; Dong, Y.; Sun, X. The recovery of rare earth elements from coal combustion products by ionic liquids. Miner. Eng. 2019, 130, 142–147. [Google Scholar] [CrossRef]
- Hasegawa, H.; Rahman, I.M.M.; Egawa, Y.; Sawai, H.; Begum, Z.A.; Maki, T.; Mizutani, S. Recovery of the Rare Metals from Various Waste Ashes with the Aid of Temperature and Ultrasound Irradiation Using Chelants. Water Air Soil Pollut. 2014, 225, 2112. [Google Scholar] [CrossRef]
- Fang, H.; Cole, B.E.; Qiao, Y.; Bogart, J.A.; Cheisson, T.; Manor, B.C.; Carroll, P.J.; Schelter, E.J. Electro-kinetic separation of rare earth elements using a redox-active ligand. Angew. Chem. Int. Ed. 2017, 56, 13450–13454. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Ikeda, K.; Kamiyama, Y. Refining of a Rare Earth Including a Process for Separation by a Reverse Osmosis Membrane. U.S. Patent US5104544A, 14 April 1992. [Google Scholar]
- Wen, B.; Shan, X.Q.; Xu, S.G. Preconcentration of ultratrace REE in seawater with 8-hydroxyquinoline immobilized polyacrylonitrile hollow fiber membrane for determination by ICP-MS. Analyst 1999, 124, 621–626. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A. Characteristics of Some Selected Methods of Rare Earth Elements Recovery from Coal Fly Ashes. Metals 2021, 11, 142. [Google Scholar] [CrossRef]
- Ambare, D.N.; Ansari, S.A.; Anitha, M.; Kandwal, P.; Singh, D.K.; Singh, H.; Mohapatra, P.K. Non-dispersive solvent extraction of neodymium using a hollow fiber contactor: Mass transfer and modeling studies. J. Membr. Sci. 2013, 446, 106–112. [Google Scholar] [CrossRef]
- Fumes, B.H.; Ribeiro Silva, M.; Nascimento Andrade, F.; Domingues Nazario, C.E.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Moussa, M.; Pichon, V.; Mariet, C.; Vercouter, T.; Delaunay, N. Potential of IIP synthesized by trapping approach for selective SPE of lanthanides. Talanta 2016, 161, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Tajabadi, F. Carbon-based magnetic nanocomposites in solid phase dispersion for some of lanthanides, followed by their quantitation via ICP-OES. Microchim. Acta 2013, 180, 65–73. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, W.; Meng, M.; Lu, J.; Ma, F.; Gao, J.; Lin, X.; Yu, C. Multiple-functional molecularly imprinted nanocomposite membranes for high-efficiency selective separation applications: An imitated core-shell TiO2@PDA-based MIMs design. Compos. B Eng. 2020, 198, 108123. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Zhang, X.; Chen, H.; Lu, L.; Chai, R. Ionically Imprinting-Based Copper (II) Label-Free Detection for Preventing Hearing Loss. Engineering 2024, 33, 276–282. [Google Scholar] [CrossRef]
- Ortiz, S.D.; Pochat-Bohatier, C.; Cambedouzou, J.; Bechelany, M.; Miele, P. Current Trends in Pickering Emulsions: Particle Morphology and Applications. Engineering 2020, 6, 468–482. [Google Scholar] [CrossRef]
- Lazar, M.M.; Ghiorghita, C.A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution. Molecules 2023, 28, 2798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, B.; Wang, R. Insights into ion-imprinted materials for the recovery of metal ions: Preparation, evaluation and application. Sep. Purif. Technol. 2022, 298, 121469. [Google Scholar] [CrossRef]
- Rao, T.P.; Kala, R.; Daniel, S. Metal ion-imprinted polymers—Novel materials for selective recognition of inorganics. Anal. Chim. Acta 2006, 578, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Hande, P.E.; Samui, A.B.; Kulkarni, P.S. Highly selective monitoring of metals by using ion-imprinted polymers. Environ. Sci. Pollut. Res. 2015, 22, 7375–7404. [Google Scholar] [CrossRef] [PubMed]
- Erdem, O.; Saylan, Y.; Andaç, M.; Denizli, A. Molecularly Imprinted Polymers for Removal of Metal Ions: An Alternative Treatment Method. Biomimetics 2018, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, H. Construction of natural polymeric imprinted materials and their applications in water treatment: A review. J. Hazard. Mater. 2021, 403, 123643. [Google Scholar] [CrossRef] [PubMed]
- Karrat, A.; Lamaoui, A.; Amine, A.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Applications of Chitosan in Molecularly and Ion Imprinted Polymers. Chem. Afr. 2020, 3, 513–533. [Google Scholar] [CrossRef]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Dano, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [Google Scholar] [CrossRef] [PubMed]
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- El Ouardi, Y.; Giove, A.; Laatikainen, M.; Branger, C.; Laatikainen, K. Benefit of ion imprinting technique in solid-phase extraction of heavy metals, special focus on the last decade. J. Environ. Chem. Eng. 2021, 9, 106548. [Google Scholar] [CrossRef]
- Giakisikli, G. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Su, S. Determination of trace/ultratrace rare earth elements in environmental samples by ICP MS after MSPE with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 2014, 119, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Capillary microextraction combined with fluorinating assisted ETV ICP-OES for the determination of trace La, Eu, Dy and Y in human hair. Talanta 2013, 115, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Carbon nanostructures for separation, preconcentration and speciation of metal ions. TrAC Trends Anal. Chem. 2010, 29, 718–727. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Awietjan, S.; Jaroszewicz, J. The rheological and mechanical properties of magnetic hybrid membranes for gas mixtures separation. Mater. Lett. 2016, 183, 170–174. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Awietjan, S.; Molak, R.; Sysel, P. The magnetic inorganic-organic hybrid membranes based on polyimide matrices for gas separation. Compos. Part B Eng. 2017, 110, 161–170. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Awietjan, S.; Sysel, P. The studies on novel magnetic polyimide inorganic-organic hybrid membranes for air separation. Mater. Lett. 2017, 208, 14–18. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Kaszuwara, W.; Nyc, M.; Auguścik, M. Metal substituted sulfonated poly(2,6-dimethyl-1,4-phenylene oxide) hybrid membranes with magnetic fillers for gas separation. Sep. Purif. Technol. 2019, 210, 479–490. [Google Scholar] [CrossRef]
- Rybak, A.; Rybak, A.; Boncel, S.; Kolanowska, A.; Kaszuwara, W.; Kolev, S.D. Hybrid organic-inorganic membranes based on sulfonated poly (ether ether ketone) matrix and iron-encapsulated carbon nanotubes and their application in CO2 separation. RSC Adv. 2022, 12, 13367–13380. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Didamony, H.A.; El-Nour, K.M.A.; El-Zayat, L.; Adam, A.M.A. Charge-transfer interactions between piperidine as donor with different r- and p-acceptors: Synthesis and spectroscopic characterization. Arab. J. Chem. 2011, 4, 105–114. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan Schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Lewicka, K.; Smola-Dmochowska, A.; Śmigiel-Gac, N.; Kaczmarczyk, B.; Janeczek, H.; Barczyńska-Felusiak, R.; Szymanek, I.; Rychter, P.; Dobrzyński, P. Bactericidal Chitosan Derivatives and Their Superabsorbent Blends with ĸ-Carrageenan. Int. J. Mol. Sci. 2024, 25, 4534. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Li, J.; Sun, Y.; Wei, J.; Wei, M.; Wang, Y.; Yin, K.; Ahu, H.; Pan, H. Effects of transition metal substitution doping on the structure and magnetic properties of biphenylene. Phys. Chem. Chem. Phys. 2024, 26, 29948–29954. [Google Scholar] [CrossRef] [PubMed]
- Lebech, B.; Rainford, B.D. The magnetic structures of praseodymium and neodymium. J. Phys. Colloq. 1971, 32, C1-370–C1-371. [Google Scholar] [CrossRef]
- Ziębowicz, B.; Szewieczek, D.; Dobrzański, L.A.; Wysłocki, J.J.; Przybył, A. Structure and properties of the composite materials consisting of the nanocrystalline Fe73.5Cu1Nb3Si13.5B9 alloy powders and polyethylene. J. Mater. Process Technol. 2005, 162–163, 149–155. [Google Scholar] [CrossRef]
- Farkas, B.; de Leeuw, N.H. Effect of coverage on the magnetic properties of–COOH,–SH, and–NH2 ligand-protected cobalt nanoparticles. Nanoscale 2021, 13, 11844–11855. [Google Scholar] [CrossRef] [PubMed]
- Jamrozik, A.; Przewoznik, J.; Krysiak, S.; Korecki, J.; Trykowski, G.; Małolepszy, A.; Stobinski, L.; Burda, K. Effect of Grinding and the Mill Type on Magnetic Properties of Carboxylated Multiwall Carbon Nanotubes. Materials 2021, 14, 4057. [Google Scholar] [CrossRef] [PubMed]
- Rybak, A.; Rybak, A.; Boncel, S.; Kolanowska, A.; Jakobik-Kolon, A.; Bok-Badura, J.; Kaszuwara, W. Modern Rare Earth Imprinted Membranes for the Recovery of Rare Earth Metal Ions from Coal Fly Ash Extracts. Materials 2024, 17, 3087. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.W.; Zhao, H.Q.; Lai, Y.Q.; Li, J.; Liu, Y.X.; Deng, Z.G. Extraction of rare earth metals by liquid surfactant membranes containing Cyanex272 as a carrier. Miner. Metallurg. Eng. 2002, 22, 74–78. [Google Scholar]
- Davoodi-Nasab, P.; Rahbar-Kelishami, A.; Safdari, J.; Abolghasemi, H. Application of emulsion nanofluids membrane for the.extraction of gadolinium using response surface methodology. J. Mol. Liq. 2017, 244, 368–373. [Google Scholar] [CrossRef]
- Wannachod, P.; Chaturabul, S.; Pancharoen, U.; Lothongkum, A.W.; Patthaveekongka, W. The effective recovery of praseodymium from mixed rare earths via a hollow fiber supported liquid membrane and its mass transfer related. J. Alloy. Compd. 2011, 509, 354–361. [Google Scholar] [CrossRef]
- Murthy, Z.V.P.; Gaikwad, M.S. Separation of praseodymium (III) from aqueous solutions by nanofiltration. Can. Metall. Q. 2013, 52, 18–22. [Google Scholar] [CrossRef]
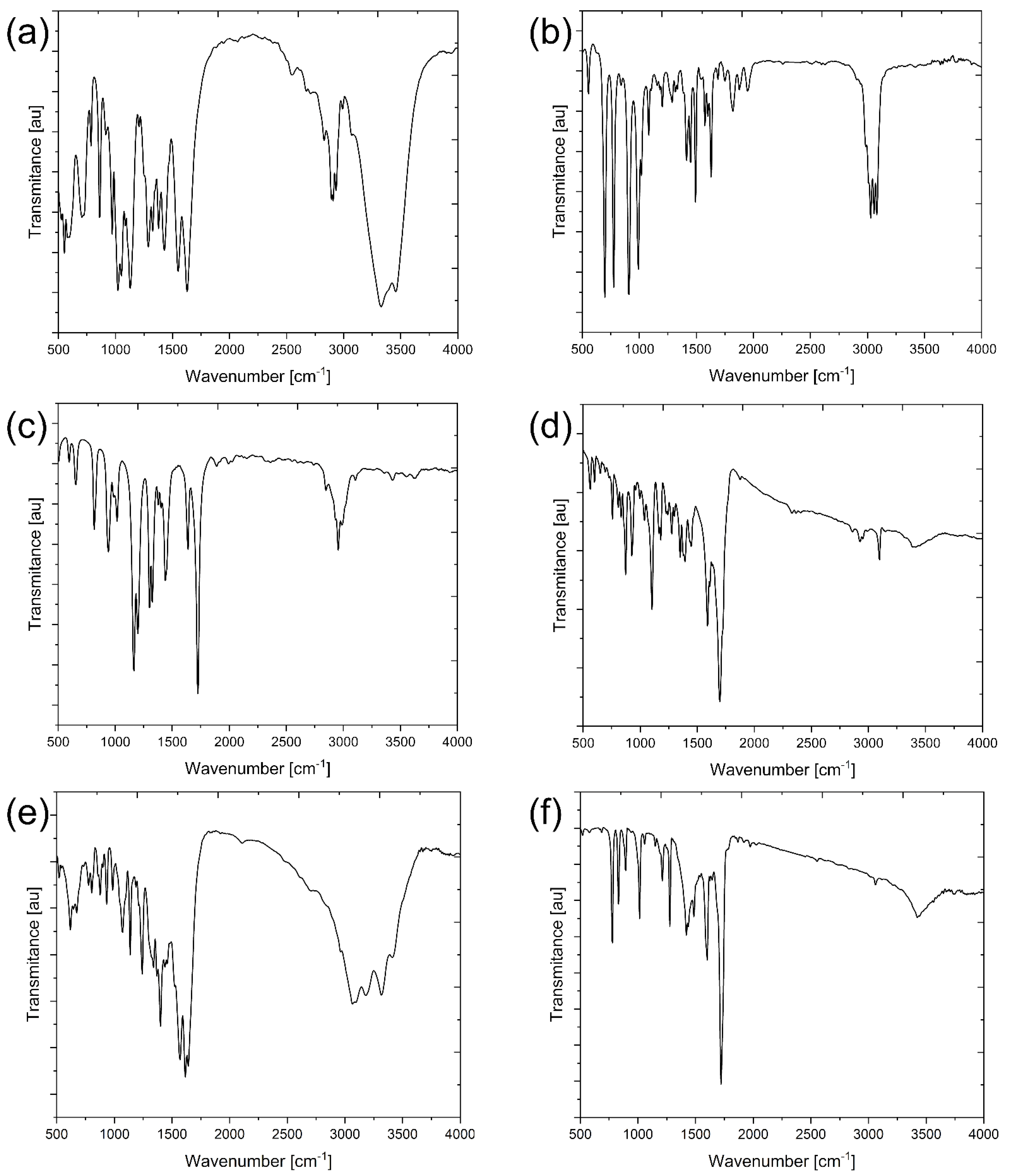
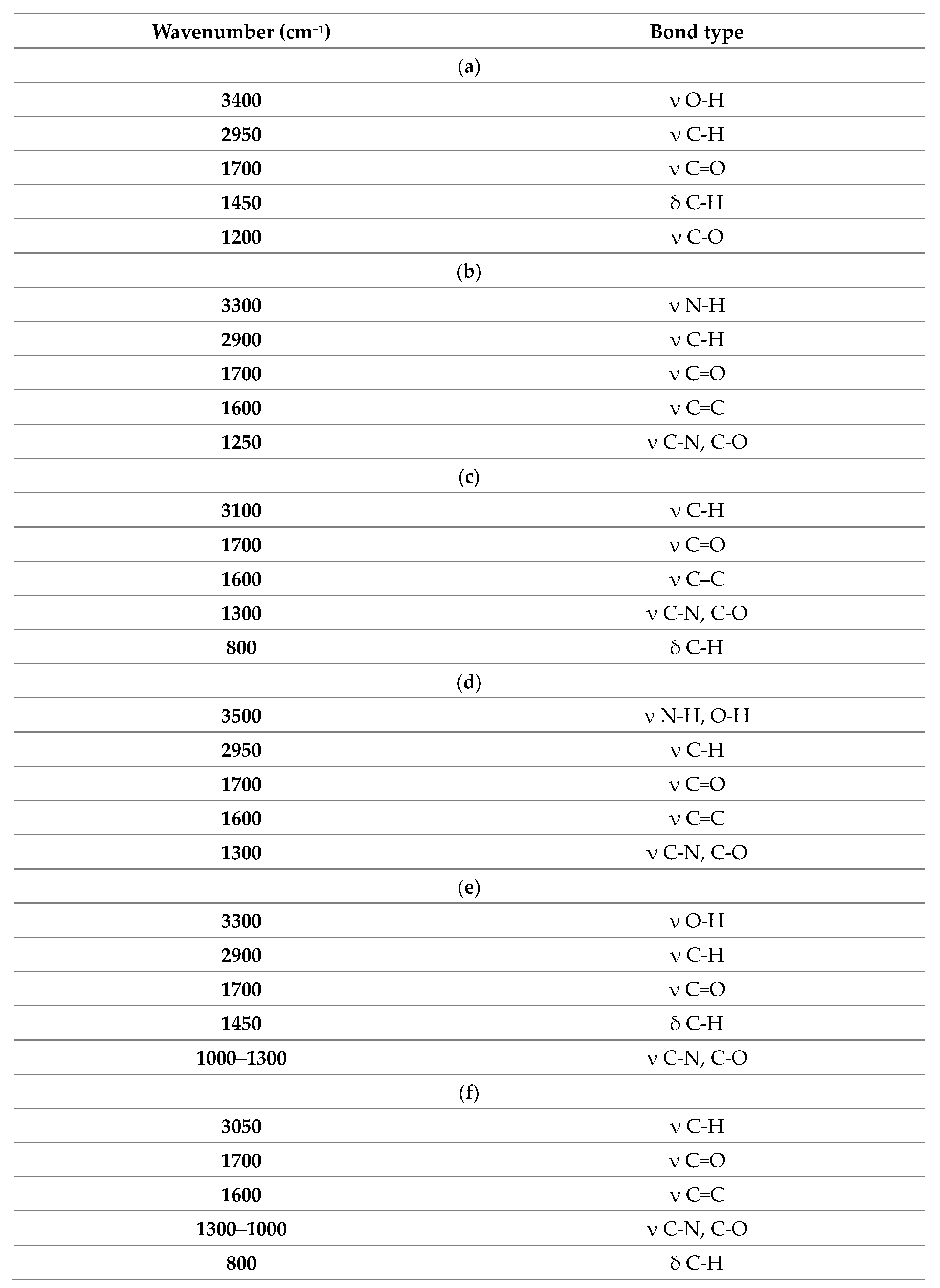
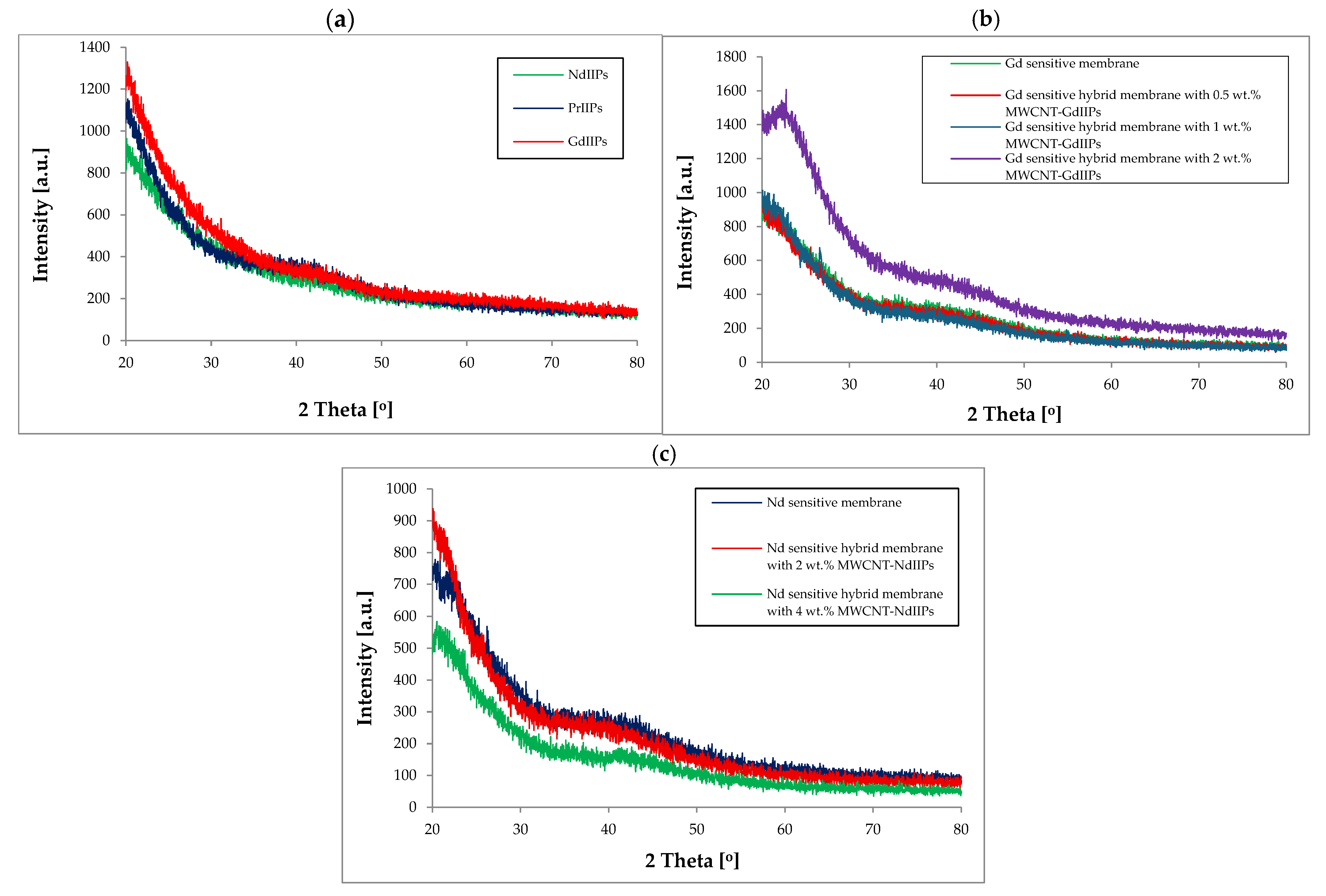
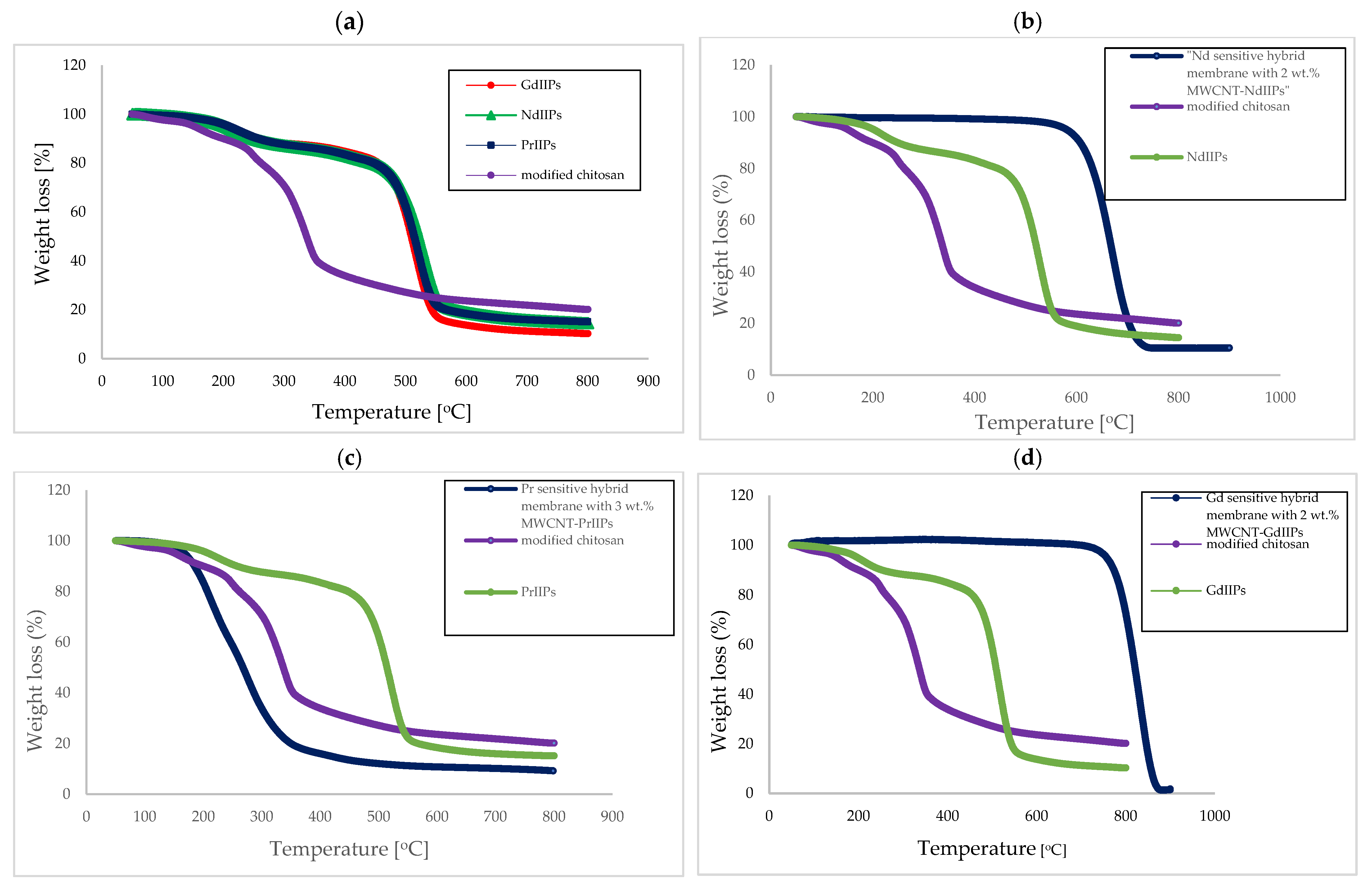
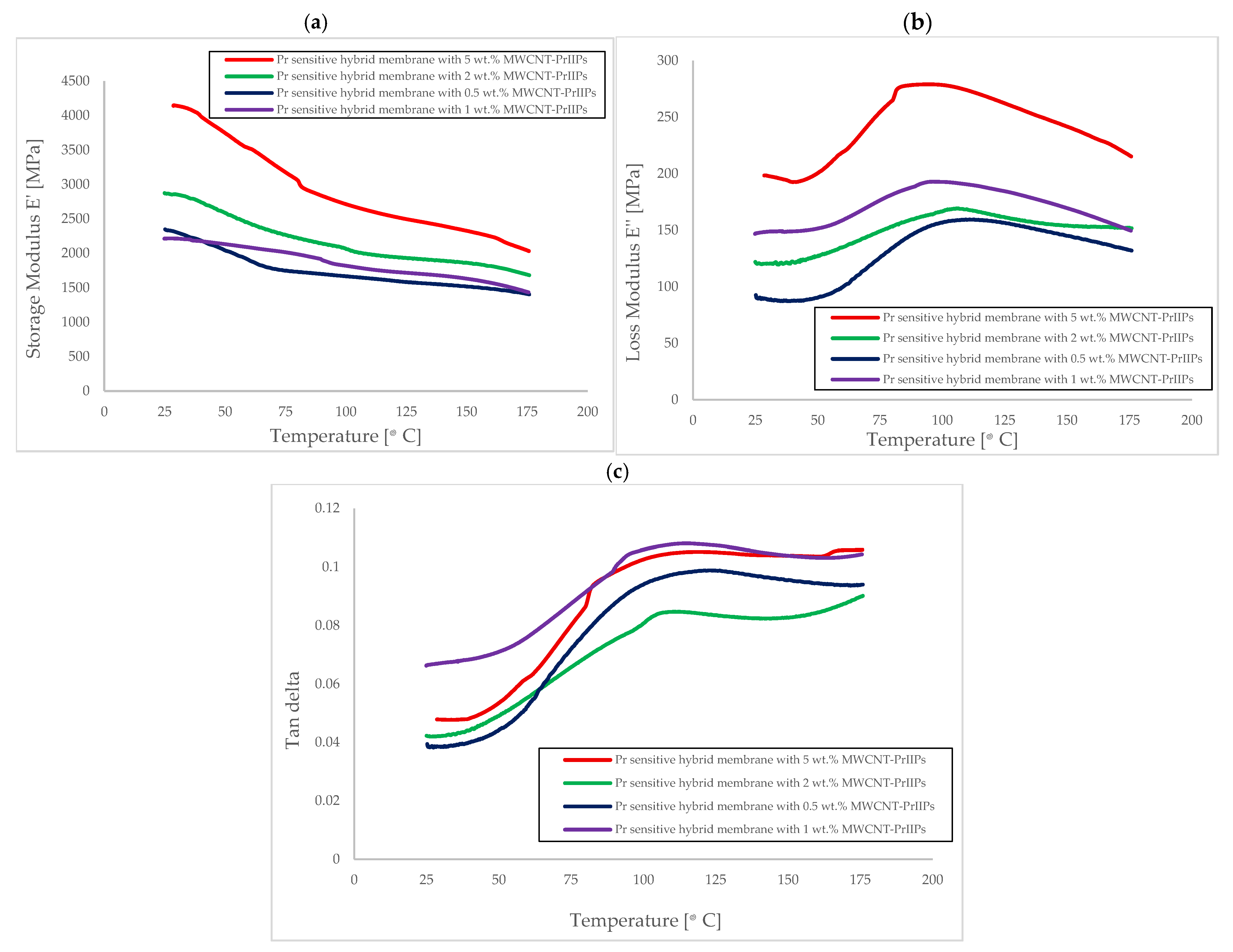
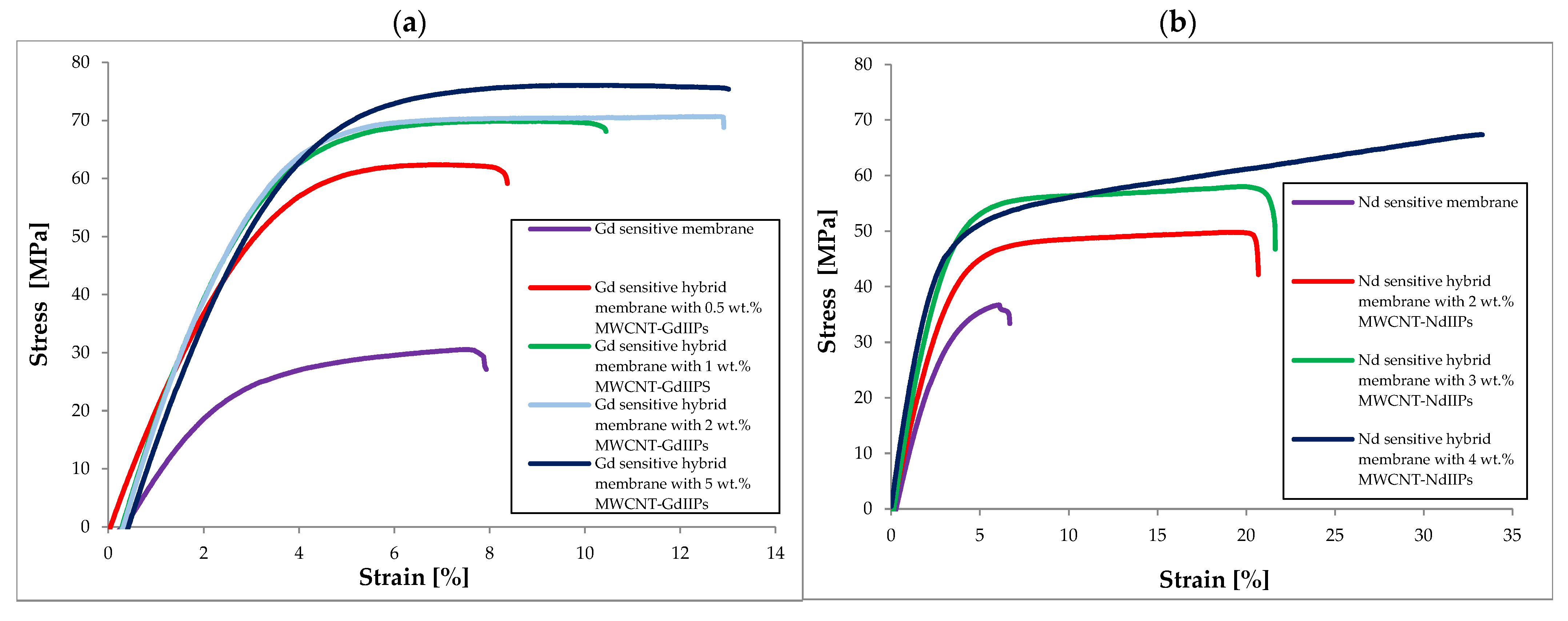
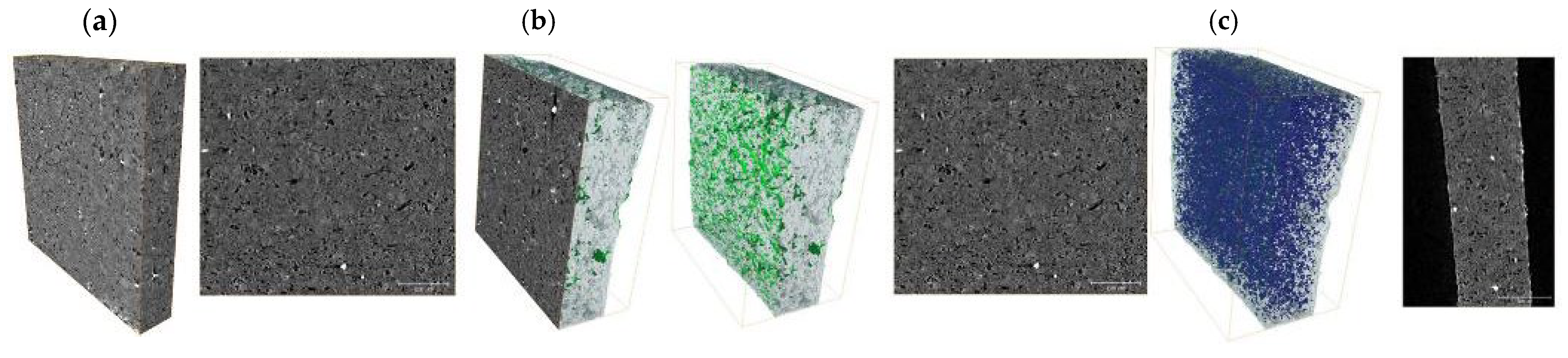


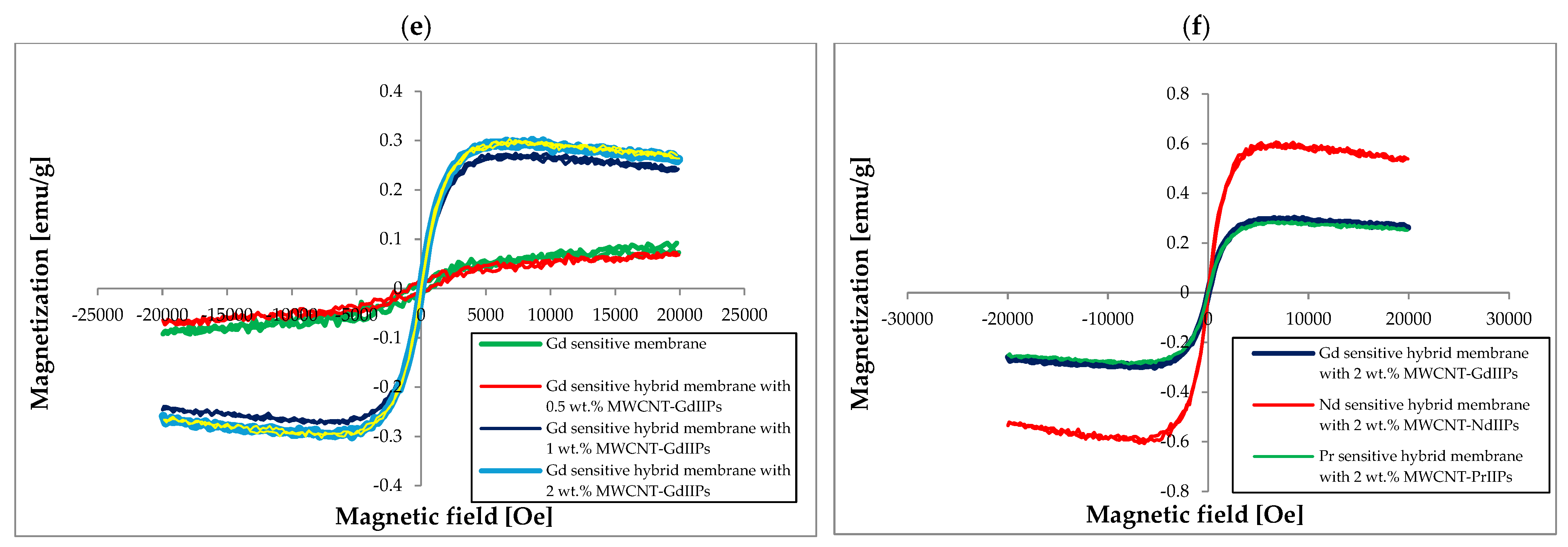

| Membrane | Rm [MPa] | Young’s Modulus E [MPa] | Elongation at Break [%] |
|---|---|---|---|
| Nd sensitive membrane | 36.72 ± 1.84 | 128.9 ± 6.3 | 6.68 ± 0.88 |
| Nd sensitive hybrid membrane with 2 wt.% MWCNT-NdIIPs | 49.80 ± 2.50 | 152.2 ± 7.4 | 20.68 ± 2.71 |
| Nd sensitive hybrid membrane with 3 wt.% MWCNT-NdIIPs | 58.03 ± 3.21 | 191.2 ± 9.3 | 21.63 ± 2.84 |
| Nd sensitive hybrid membrane with 4 wt.% MWCNT-NdIIPs | 67.37 ± 3.38 | 213.5 ± 10.4 | 33.30 ± 4.37 |
| Gd sensitive membrane | 30.55 ± 1.71 | 116.2 ± 7.4 | 7.93 ± 0.98 |
| Gd sensitive hybrid membrane with 0.5 wt.% MWCNT-GdIIPs | 62.37 ± 3.49 | 203.2 ± 9.9 | 8.37 ± 1.04 |
| Gd sensitive hybrid membrane with 1 wt.% MWCNT-GdIIPs | 69.88 ± 3.91 | 243.5 ± 15.6 | 10.44 ± 1.29 |
| Gd sensitive hybrid membrane with 2 wt.% MWCNT-GdIIPs | 70.68 ± 3.96 | 246.9 ± 15.8 | 12.91 ± 1.60 |
| Gd sensitive hybrid membrane with 5 wt.% MWCNT-GdIIPs | 76.06 ± 4.26 | 249.8 ± 16.0 | 13.01 ± 1.61 |
| Membrane | Coercivity Hc [Oe] | Saturation Magnetization, Ms [emu/g] | Remanence [emu/g] |
|---|---|---|---|
| Modified chitosan | 549.0 ± 27.9 | 0.085 ± 0.008 | 0.020 ± 0.001 |
| Modified chitosan imprinted with Nd | 736.4 ± 37.4 | 0.094 ± 0.009 | 0.010 ± 0.001 |
| Modified chitosan imprinted with Pr | 322.7 ± 16.4 | 0.157 ± 0.014 | 0.010 ± 0.001 |
| Modified chitosan imprinted with Gd | 804.5 ± 40.8 | 0.044 ± 0.004 | 0.010 ± 0.001 |
| NdIIPs | 781.8 ± 39.7 | 0.076 ± 0.007 | 0.020 ± 0.002 |
| PrIIPs | 895.4 ± 45.5 | 0.078 ± 0.007 | 0.020 ± 0.002 |
| GdIIPs | 818.2 ± 41.5 | 0.075 ± 0.007 | 0.020 ± 0.002 |
| Nd sensitive hybrid membrane with 1 wt.% MWCNT-NdIIPs | 133.09 ± 6.76 | 0.242 ± 0.022 | 0.003 ± 0.001 |
| Nd sensitive hybrid membrane with 2 wt.% MWCNT-NdIIPs | 126.62 ± 6.43 | 0.299 ± 0.028 | 0.003 ± 0.001 |
| Nd sensitive hybrid membrane with 3 wt.% MWCNT-NdIIPs | 65.75 ± 3.34 | 0.259 ± 0.024 | 0.002 ± 0.001 |
| Nd sensitive hybrid membrane with 4 wt.% MWCNT-NdIIPs | 48.87 ± 2.48 | 0.442 ± 0.041 | 0.009 ± 0.001 |
| Pr sensitive polymer membrane | 765.23 ± 38.85 | 0.079 ± 0.007 | 0.006 ± 0.001 |
| Pr sensitive hybrid membrane with 0.5 wt.% MWCNT-PrIIPs | 125.11 ± 6.35 | 0.236 ± 0.022 | 0.004 ± 0.001 |
| Pr sensitive hybrid membrane with 1 wt.% MWCNT-PrIIPs | 60.46 ± 3.07 | 0.286 ± 0.026 | 0.003 ± 0.001 |
| Pr sensitive hybrid membrane with 2 wt.% MWCNT-PrIIPs | 58.09 ± 2.95 | 0.288 ± 0.027 | 0.003 ± 0.001 |
| Pr sensitive hybrid membrane with 5 wt.% MWCNT-PrIIPs | 52.58 ± 2.67 | 0.329 ± 0.030 | 0.002 ± 0.001 |
| Gd sensitive polymer membrane | 811.61 ± 41.20 | 0.092 ± 0.008 | 0.008 ± 0.001 |
| Gd sensitive hybrid membrane with 0.5 wt.% MWCNT-GdIIPs | 808.92 ± 41.06 | 0.073 ± 0.007 | 0.010 ± 0.001 |
| Gd sensitive hybrid membrane with 1 wt.% MWCNT-GdIIPs | 155.10 ± 7.87 | 0.274 ± 0.025 | 0.007 ± 0.001 |
| Gd sensitive hybrid membrane with 2 wt.% MWCNT-GdIIPs | 40.27 ± 2.04 | 0.301 ± 0.028 | 0.005 ± 0.001 |
| Gd sensitive hybrid membrane with 5 wt.% MWCNT- GdIIPs | 22.86 ± 1.16 | 0.303 ± 0.028 | 0.005 ± 0.001 |
| Property | Nanocyl NC7000TM MWCNTs | In-House MWCNTs |
|---|---|---|
| Diameter [nm] | 9.5 | 60–70 |
| Length [mm] | 1.5 | >800 |
| Aspect ratio | 150 | >12,000 |
| Purity [wt.%] | 90 | 96 |
| Catalyst residue [wt.%] | <1 (+9 wt.% carrier Al2O3) | 4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybak, A.; Rybak, A.; Boncel, S.; Kolanowska, A.; Kaszuwara, W.; Nyc, M.; Molak, R.; Jaroszewicz, J.; Kolev, S.D. Multifunctional REE Selective Hybrid Membranes Based on Ion-Imprinted Polymers and Modified Multiwalled Carbon Nanotubes: A Physicochemical Characterization. Int. J. Mol. Sci. 2025, 26, 7136. https://doi.org/10.3390/ijms26157136
Rybak A, Rybak A, Boncel S, Kolanowska A, Kaszuwara W, Nyc M, Molak R, Jaroszewicz J, Kolev SD. Multifunctional REE Selective Hybrid Membranes Based on Ion-Imprinted Polymers and Modified Multiwalled Carbon Nanotubes: A Physicochemical Characterization. International Journal of Molecular Sciences. 2025; 26(15):7136. https://doi.org/10.3390/ijms26157136
Chicago/Turabian StyleRybak, Aleksandra, Aurelia Rybak, Sławomir Boncel, Anna Kolanowska, Waldemar Kaszuwara, Mariusz Nyc, Rafał Molak, Jakub Jaroszewicz, and Spas D. Kolev. 2025. "Multifunctional REE Selective Hybrid Membranes Based on Ion-Imprinted Polymers and Modified Multiwalled Carbon Nanotubes: A Physicochemical Characterization" International Journal of Molecular Sciences 26, no. 15: 7136. https://doi.org/10.3390/ijms26157136
APA StyleRybak, A., Rybak, A., Boncel, S., Kolanowska, A., Kaszuwara, W., Nyc, M., Molak, R., Jaroszewicz, J., & Kolev, S. D. (2025). Multifunctional REE Selective Hybrid Membranes Based on Ion-Imprinted Polymers and Modified Multiwalled Carbon Nanotubes: A Physicochemical Characterization. International Journal of Molecular Sciences, 26(15), 7136. https://doi.org/10.3390/ijms26157136










