Transient Receptor Potential Vanilloid 4 (TRPV4) in the Human Carotid Body
Abstract
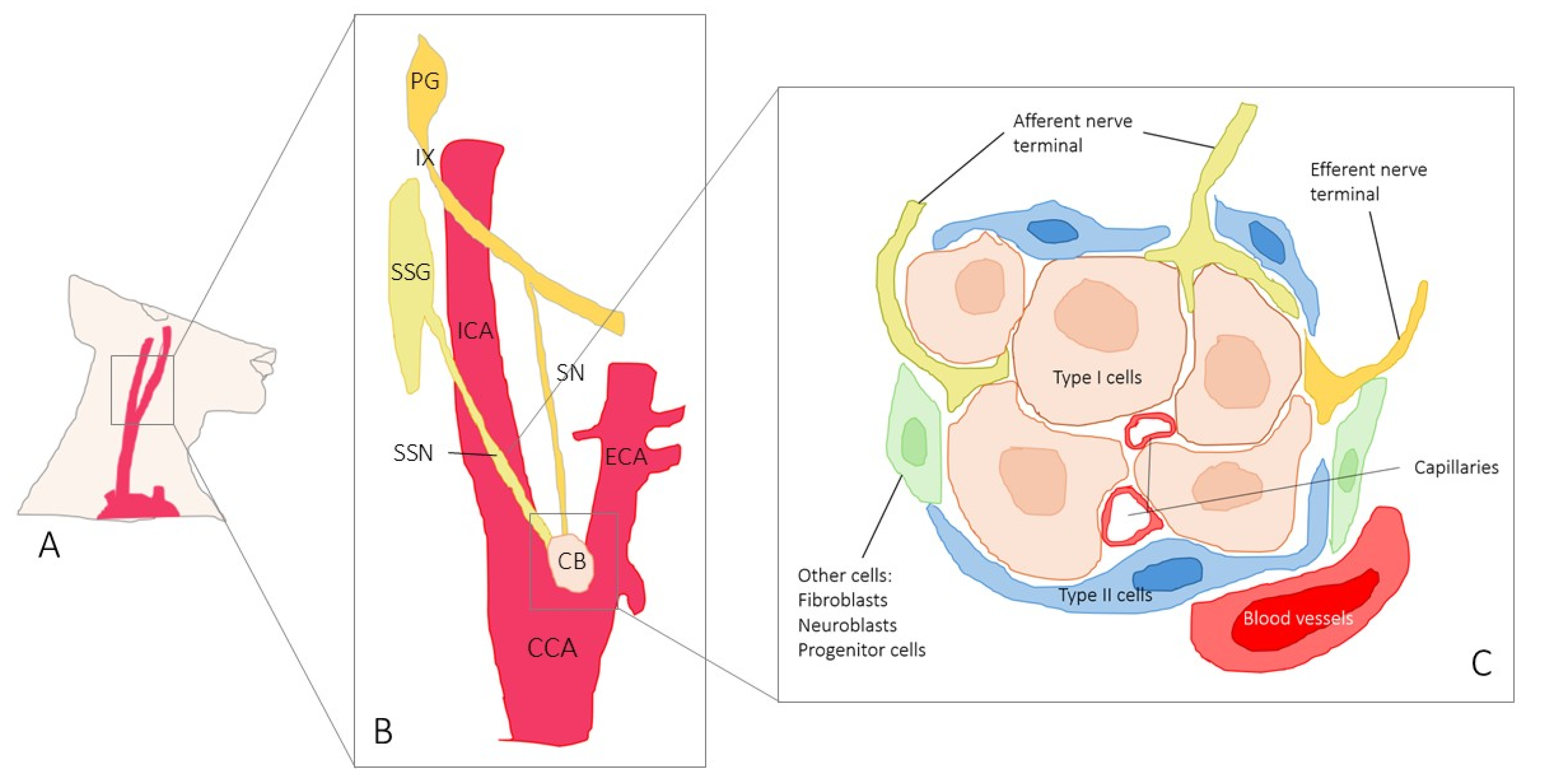
1. Introduction
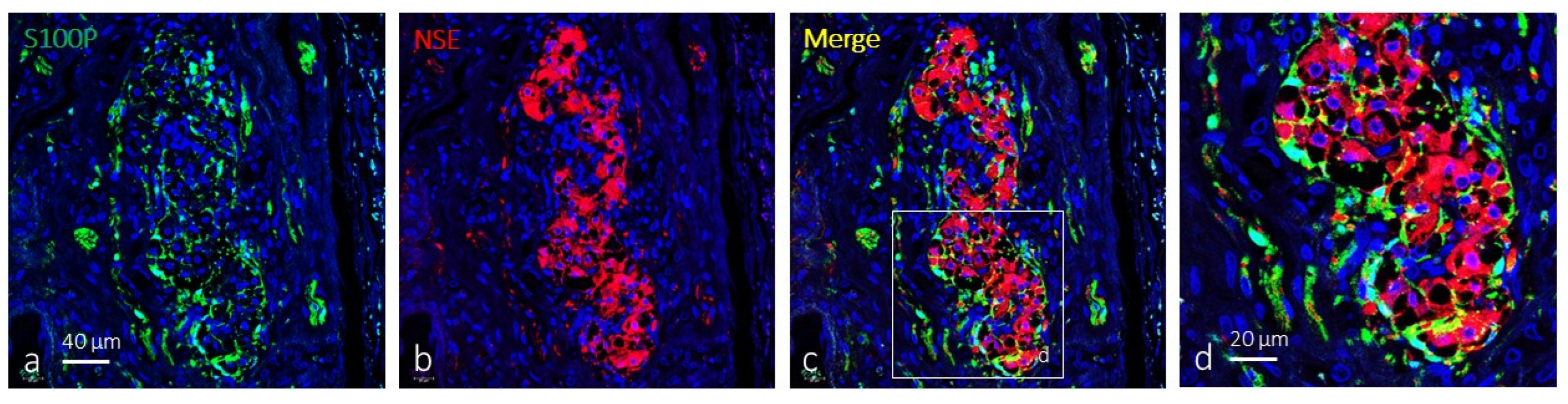
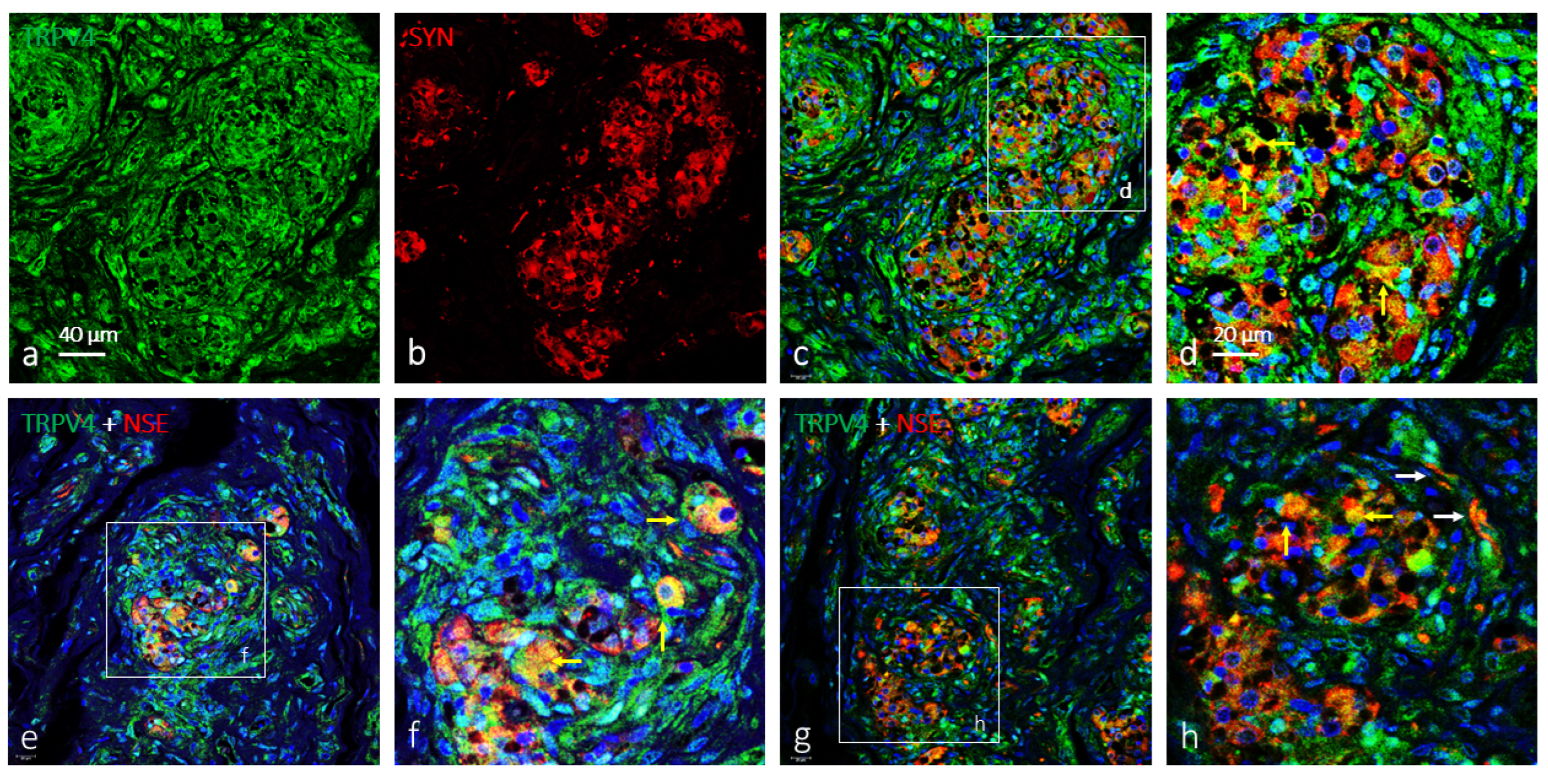
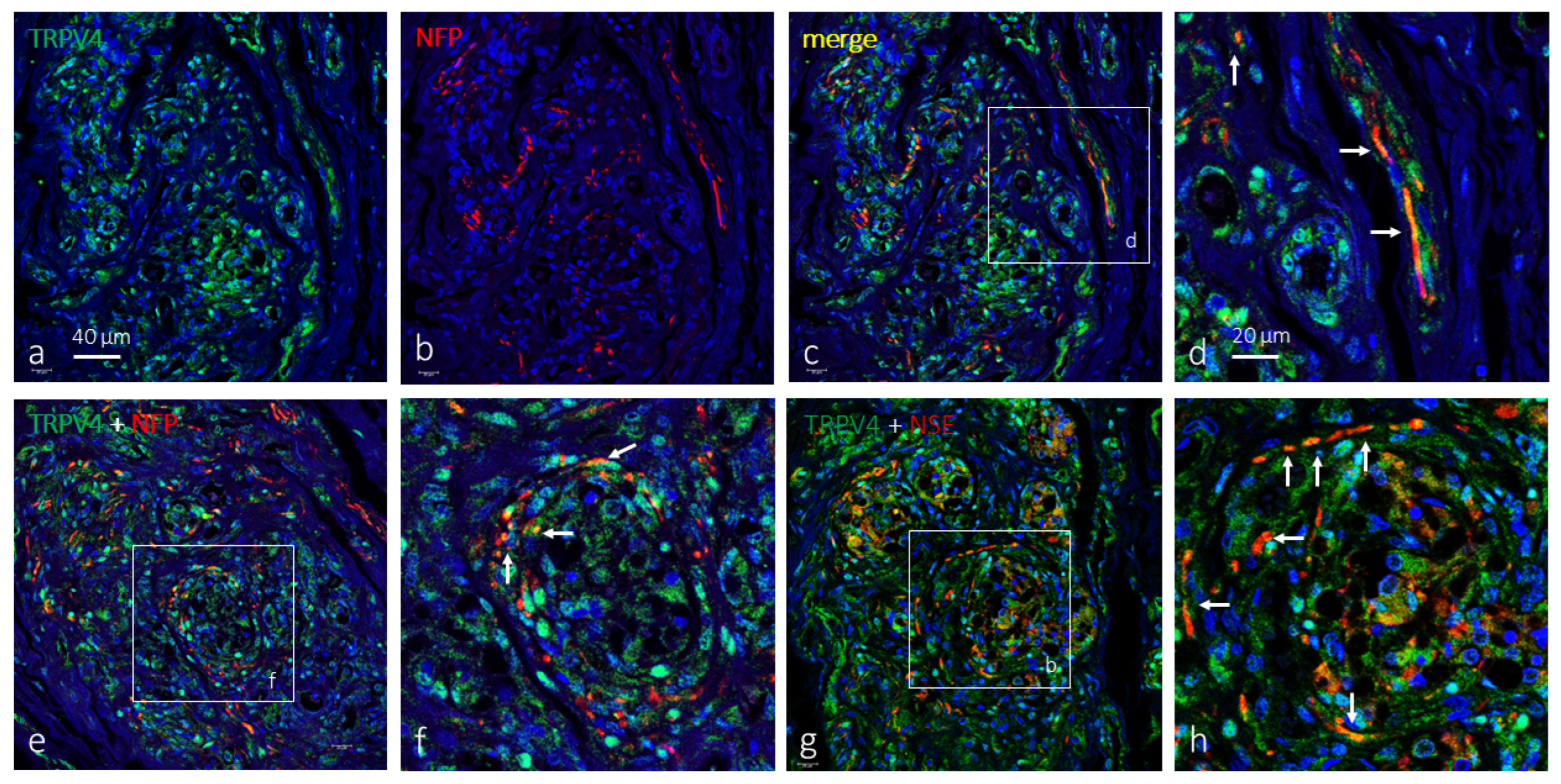
2. Results
Immunolocalization of TRPV4 in the Human Carotid Body
3. Discussion
4. Materials and Methods
4.1. Double Immunofluorescence
4.2. Quantitative Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega-Sáenz, P.; López-Barneo, J. Physiology of the Carotid Body: From Molecules to Disease. Annu. Rev. Physiol. 2020, 82, 127–149. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Alcayaga, J.; Chapleau, M.W.; Somers, V.K. Carotid body chemoreceptors: Physiology, pathology, and implications for health and disease. Physiol. Rev. 2021, 101, 1177–1235. [Google Scholar] [CrossRef]
- Shin, M.K.; Mitrut, R.; Gu, C.; Kim, L.J.; Yeung, B.H.Y.; Lee, R.; Pham, L.; Tang, W.Y.; Sham, J.S.K.; Cui, H.; et al. Pharmacological and Genetic Blockade of TRPM7 in the Carotid Body Treats Obesity-Induced Hypertension. Hypertension 2021, 78, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Carotid body hypersensitivity in intermittent hypoxia and obstructive sleep apnoea. J. Physiol. 2023, 601, 5481–5494. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Coker, R.H.; Stone, E.E.; Lacy, D.B.; Jabbour, K.; Williams, P.E.; Wasserman, D.H. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 2000, 49, 1434–1442. [Google Scholar] [CrossRef]
- Pardal, R.; López-Barneo, J. Low glucose-sensing cells in the carotid body. Nat. Neurosci. 2002, 5, 197–198. [Google Scholar] [CrossRef]
- Zhang, M.; Buttigieg, J.; Nurse, C.A. Neurotransmitter mechanisms mediating low-glucose signalling in cocultures and fresh tissue slices of rat carotid body. J. Physiol. 2007, 578 Pt 3, 735–750. [Google Scholar] [CrossRef]
- Shin, M.K.; Eraso, C.C.; Mu, Y.P.; Gu, C.; Yeung, B.H.Y.; Kim, L.J.; Liu, X.R.; Wu, Z.J.; Paudel, O.; Pichard, L.E.; et al. Leptin Induces Hypertension Acting on Transient Receptor Potential Melastatin 7 Channel in the Carotid Body. Circ. Res. 2019, 125, 989–1002. [Google Scholar] [CrossRef]
- Zheng, F.; Mu, S.; Rusch, N.J. Leptin Activates TRPM7 Channels in the Carotid Body As a Mechanism of Obesity-Related Hypertension. Circ. Res. 2019, 125, 1003–1005. [Google Scholar] [CrossRef]
- Thakkar, P.; Pauza, A.G.; Murphy, D.; Paton, J.F.R. Carotid body: An emerging target for cardiometabolic co-morbidities. Exp. Physiol. 2023, 108, 661–671. [Google Scholar] [CrossRef]
- Zera, T.; Moraes, D.J.A.; da Silva, M.P.; Fisher, J.P.; Paton, J.F.R. The Logic of Carotid Body Connectivity to the Brain. Physiology 2019, 34, 264–282. [Google Scholar] [CrossRef]
- Jendzjowsky, N.G.; Roy, A.; Barioni, N.O.; Kelly, M.M.; Green, F.H.Y.; Wyatt, C.N.; Pye, R.L.; Tenorio-Lopes, L.; Wilson, R.J.A. Preventing acute asthmatic symptoms by targeting a neuronal mechanism involving carotid body lysophosphatidic acid receptors. Nat. Commun. 2018, 9, 4030. [Google Scholar] [CrossRef]
- Jendzjowsky, N.G.; Roy, A.; Iftinca, M.; Barioni, N.O.; Kelly, M.M.; Herrington, B.A.; Visser, F.; Altier, C.; Wilson, R.J.A. PKCε stimulation of TRPV1 orchestrates carotid body responses to asthmakines. J. Physiol. 2021, 599, 1335–1354. [Google Scholar] [CrossRef]
- Katayama, P.L.; Leirão, I.P.; Kanashiro, A.; Menani, J.V.; Zoccal, D.B.; Colombari, D.S.A.; Colombari, E. The carotid body: A novel key player in neuroimmune interactions. Front. Immunol. 2022, 13, 1033774. [Google Scholar] [CrossRef]
- Del Rio, R.; Moya, E.A.; Parga, M.J.; Madrid, C.; Iturriaga, R. Carotid body inflammation and cardiorespiratory alterations in intermittent hypoxia. Eur. Respir. J. 2012, 39, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Iturriaga, R.; Del Rio, R.; Alcayaga, J. Carotid Body Inflammation: Role in Hypoxia and in the Anti-inflammatory Reflex. Physiology 2022, 37, 128–140. [Google Scholar] [CrossRef]
- Iturriaga, R. Carotid body contribution to the physio-pathological consequences of intermittent hypoxia: Role of nitro-oxidative stress and inflammation. J. Physiol. 2023, 601, 5495–5507. [Google Scholar] [CrossRef]
- Eyzaguirre, C.; Abudara, V. Carotid body glomus cells: Chemical secretion and transmission (modulation?) across cell-nerve ending junctions. Respir. Physiol. 1999, 115, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Eyzaguirre, C. Chemical and electric transmission in the carotid body chemoreceptor complex. Biol. Res. 2005, 38, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Eyzaguirre, C. Electric synapses in the carotid body-nerve complex. Respir. Physiol. Neurobiol. 2007, 157, 116–122. [Google Scholar] [CrossRef]
- Nurse, C.A.; Piskuric, N.A. Signal processing at mammalian carotid body chemoreceptors. Semin. Cell Dev. Biol. 2013, 24, 22–30. [Google Scholar] [CrossRef]
- Leonard, E.M.; Salman, S.; Nurse, C.A. Sensory Processing and Integration at the Carotid Body Tripartite Synapse: Neurotransmitter Functions and Effects of Chronic Hypoxia. Front. Physiol. 2018, 9, 225. [Google Scholar] [CrossRef]
- Leonard, E.M.; Nurse, C.A. The Carotid Body “Tripartite Synapse”: Role of Gliotransmission. Adv. Exp. Med. Biol. 2023, 1427, 185–194. [Google Scholar] [CrossRef]
- Prabhakar, N.R. Neurotransmitters in the carotid body. Adv. Exp. Med. Biol. 1994, 360, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Bairam, A.; Carroll, J.L. Neurotransmitters in carotid body development. Respir. Physiol. Neurobiol. 2005, 149, 217–232. [Google Scholar] [CrossRef]
- Buniel, M.C.; Schilling, W.P.; Kunze, D.L. Distribution of transient receptor potential channels in the rat carotid chemosensory pathway. J. Comp. Neurol. 2003, 464, 404–413. [Google Scholar] [CrossRef]
- Kim, I.; Fite, L.; Donnelly, D.F.; Kim, J.H.; Carroll, J.L. Possible Role of TRP Channels in Rat Glomus Cells. Adv. Exp. Med. Biol. 2015, 860, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Zubieta-DeUrioste, N.; Wilson, R.J.A. Beyond arterial blood gas sensing: The carotid body’s TRP to broad physiological function. J. Physiol. 2022, 600, 4965–4966. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Barbero, G.; García-Mesa, Y.; Cobo, R.; Cuendias, P.; Martín-Biedma, B.; García-Suárez, O.; Feito, J.; Cobo, T.; Vega, J.A. Acid-Sensing Ion Channels’ Immunoreactivity in Nerve Profiles and Glomus Cells of the Human Carotid Body. Int. J. Mol. Sci. 2023, 24, 17161. [Google Scholar] [CrossRef]
- Alba, E.; García-Mesa, Y.; Cobo, R.; Cuendias, P.; Martín-Cruces, J.; Suazo, I.; Martínez-Barbero, G.; Vega, J.A.; García-Suárez, O.; Cobo, T. Immunohistochemical Detection of PIEZO Ion Channels in the Human Carotid Sinus and Carotid Body. Biomolecules 2025, 15, 386. [Google Scholar] [CrossRef]
- Becker, D.; Bereiter-Hahn, J.; Jendrach, M. Functional interaction of the cation channel transient receptor potential vanilloid 4 (TRPV4) and actin in volume regulation. Eur. J. Cell Biol. 2009, 88, 141–152. [Google Scholar] [CrossRef]
- Toft-Bertelsen, T.; MacAulay, N. TRPing to the Point of Clarity: Understanding the Function of the Complex TRPV4 Ion Channel. Cells 2021, 10, 165. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Benítez-Angeles, M.; Sánchez-Hernández, R.; Morales-Lázaro, S.L.; Hiriart, M.; Morales-Buenrostro, L.E.; Torres-Quiroz, F. TRPV4: A Physio and Pathophysiologically Significant Ion Channel. Int. J. Mol. Sci. 2020, 21, 3837. [Google Scholar] [CrossRef]
- Liedtke, W.B. TRPV channels’ function in osmo- and mechanotransduction. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B., Heller, S., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Shibasaki, K. TRPV4 activation by thermal and mechanical stimuli in disease progression. Lab. Investig. 2020, 100, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/- mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [PubMed]
- Loukin, S.H.; Su, Z.; Kung, C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Lett. 2009, 583, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Thodeti, C.K.; Matthews, B.; Ravi, A.; Mammoto, A.; Ghosh, K.; Bracha, A.L.; Ingber, D.E. TRPV4 Channels Mediate Cyclic Strain–Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ. Res. 2009, 104, 1123–1130. [Google Scholar] [CrossRef]
- Matthews, B.D.; Thodeti, C.K.; Tytell, J.; Mammoto, A.; Overby, D.; Ingber, D.E. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface β1 integrins. Integr. Biol. 2010, 2, 435–442. [Google Scholar] [CrossRef]
- Adapala, R.K.; Katari, V.; Teegala, L.R.; Thodeti, S.; Paruchuri, S.; Thodeti, C.K. TRPV4 mechanotransduction in Fibrosis. Cells 2021, 10, 3053. [Google Scholar] [CrossRef]
- Plant, T.D.; Strotmann, R. TRPV4. Handb. Exp. Pharmacol. 2007, 179, 189–205. [Google Scholar] [CrossRef]
- Garcia-Elias, A.; Mrkonjić, S.; Jung, C.; Pardo-Pastor, C.; Vicente, R.; Valverde, M.A. The TRPV4 channel. Handb. Exp. Pharmacol. 2014, 222, 293–319. [Google Scholar] [CrossRef]
- Darby, W.G.; Grace, M.S.; Baratchi, S.; McIntyre, P. Modulation of TRPV4 by diverse mechanisms. Int. J. Biochem. Cell Biol. 2016, 78, 217–228. [Google Scholar] [CrossRef]
- Arniges, M.; Vázquez, E.; Fernández-Fernández, J.M.; Valverde, M.A. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J. Biol. Chem. 2004, 279, 54062–54068. [Google Scholar] [CrossRef]
- Hartmannsgruber, V.; Heyken, W.T.; Kacik, M.; Kaistha, A.; Grgic, I.; Harteneck, C.; Liedtke, W.; Hoyer, J.; Köhler, R. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE 2007, 2, e827. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Jaggi, A.S. TRPV4 channels: Physiological and pathological role in cardiovascular system. Basic Res. Cardiol. 2015, 110, 54. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, C.; Li, H.; Tang, C.; Kan, H.; Yang, Z.; Mao, A.; Ma, X. TRPV4 (Transient Receptor Potential Vanilloid 4) Mediates Endothelium-Dependent Contractions in the Aortas of Hypertensive Mice. Hypertension 2018, 71, 134–142. [Google Scholar] [CrossRef]
- Liu, L.; Guo, M.; Lv, X.; Wang, Z.; Yang, J.; Li, Y.; Yu, F.; Wen, X.; Feng, L.; Zhou, T. Role of Transient Receptor Potential Vanilloid 4 in Vascular Function. Front. Mol. Biosci. 2021, 8, 677661. [Google Scholar] [CrossRef] [PubMed]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef]
- Hu, W.; Ding, Y.; Li, Q.; Shi, R.; He, Y. Transient receptor potential vanilloid 4 channels as therapeutic targets in diabetes and diabetes-related complications. J. Diabetes Investig. 2020, 11, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Taguchi, K.; Kobayashi, T. Role of TRPV4 on vascular tone regulation in pathophysiological states. Eur. J. Pharmacol. 2023, 959, 176104. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Vega, A.M.; García-Villegas, R.; Rosenbaum, T. Roles for TRPV4 in disease: A discussion of possible mechanisms. Cell Calcium 2024, 124, 102972. [Google Scholar] [CrossRef]
- Jiang, D.; Guo, R.; Dai, R.; Knoedler, S.; Tao, J.; Machens, H.G.; Rinkevich, Y. The Multifaceted Functions of TRPV4 and Calcium Oscillations in Tissue Repair. Int. J. Mol. Sci. 2024, 25, 1179. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Ando, H.; Unno, S.; Roy, R.R.; Kitagawa, J. Pharmacological activation of transient receptor potential vanilloid 4 promotes triggering of the swallowing reflex in rats. Front. Cell. Neurosci. 2023, 17, 1149793. [Google Scholar] [CrossRef]
- Goldenberg, N.M.; Ravindran, K.; Kuebler, W.M. TRPV4: Physiological role and therapeutic potential in respiratory diseases. Naunyn Schmiedebergs Arch. Pharmacol. 2015, 388, 421–436. [Google Scholar] [CrossRef]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Negri, S.; Faris, P.; Berra-Romani, R.; Guerra, G.; Moccia, F. Endothelial transient receptor potential channels and vascular remodeling: Extracellular Ca2+ entry for angiogenesis, arteriogenesis and vasculogenesis. Front. Physiol. 2019, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Y.; Li, M.Y.; Li, Y.T.; Ning, J.Y.; Gou, X.C.; Shi, J.; Li, Y.Q. Expression and functional characterization of transient receptor potential vanilloid 4 in the dorsal root ganglion and spinal cord of diabetic rats with mechanical allodynia. Brain Res. Bull. 2020, 162, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tenorio Lopes, L.; Barioni, N.O.; Roeske, J.; Incognito, A.V.; Baker, J.; Raj, S.R.; Wilson, R.J.A. The molecular makeup of peripheral and central baroreceptors: Stretching a role for transient receptor potential (TRP), epithelial sodium channel (ENaC), acid sensing ion channel (ASIC), and Piezo channels. Cardiovasc. Res. 2022, 118, 3052–3070. [Google Scholar] [CrossRef]
- Rajasekhar, P.; Poole, D.P.; Liedtke, W.; Bunnett, N.W.; Veldhuis, N.A. P2Y1 receptor activation of the TRPV4 ion channel enhances purinergic signaling in satellite glial cells. J. Biol. Chem. 2015, 290, 29051–29062. [Google Scholar] [CrossRef]
- Fukazawa, A.; Hori, A.; Estrada, J.; Kim, H.K.; Hotta, N.; Iwamoto, G.A.; Smith, S.A.; Vongpatanasin, W.; Mizuno, M. Insulin potentiates mechanical responses in small dorsal root ganglion neurons by increasing the sensitization of TRPV4 channels. Am. J. Physiol. Cell Physiol. 2025, 328, C1982–C1994. [Google Scholar] [CrossRef]
- Kameda, Y. Molecular and cellular mechanisms of the organogenesis and development of the mammalian carotid body. Dev. Dyn. 2020, 249, 592–609. [Google Scholar] [CrossRef]
- Holzer, P. Acid-sensitive ion channels and receptors. Handb. Exp. Pharmacol. 2009, 194, 283–332. [Google Scholar] [CrossRef]
- Glitsch, M. Mechano- and pH-sensing convergence on Ca2+-mobilising proteins—A recipe for cancer? Cell Calcium. 2019, 80, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.; McNaughten, J.; McGahon, M.K.; Kelly, C.; Kyle, D.; Yong, P.H.; McGeown, J.G.; Curtis, T.M. Hyperglycemia and diabetes downregulate the functional expression of TRPV4 channels in retinal microvascular endothelium. PLoS ONE 2015, 10, e0128359. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, S.; Tanaka, T.; Ogawa, R.; Ito, S.; Ueno, S.; Koyama, H.; Tomotaka, O.; Sagawa, H.; Tanaka, T.; Iwakura, H.; et al. Potential role of TRPV4 in stretch-induced ghrelin secretion and obesity. Int. J. Endocrinol. 2022, 2022, 7241275. [Google Scholar] [CrossRef]
- Philip, N.; Yun, X.; Pi, H.; Murray, S.; Hill, Z.; Fonticella, J.; Perez, P.; Zhang, C.; Pathmasiri, W.; Sumner, S.; et al. Fatty acid metabolism promotes TRPV4 activity in lung microvascular endothelial cells in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2024, 326, L252–L265. [Google Scholar] [CrossRef]
- Holmes, A.P.; Hauton, D.; Kumar, P. The interaction between low glucose and hypoxia in the in vitro, rat carotid body. Adv. Exp. Med. Biol. 2012, 758, 123–127. [Google Scholar] [CrossRef]
- Shibasaki, K. TRPV4 ion channel as important cell sensors. J. Anesth. 2016, 30, 1014–1019. [Google Scholar] [CrossRef]
- Everaerts, W.; Nilius, B.; Owsianik, G. The vanilloid transient receptor potential channel TRPV4: From structure to disease. Prog. Biophys. Mol. Biol. 2010, 103, 2–17. [Google Scholar] [CrossRef]
- Cheng, Y.R.; Jiang, B.Y.; Chen, C.C. Acid-sensing ion channels: Dual function proteins for chemo-sensing and mechano-sensing. J. Biomed. Sci. 2018, 25, 46. [Google Scholar] [CrossRef]
- Wen, X.; Peng, Y.; Peng, Y.; Zhu, Y.; Yu, F.; Geng, L.; Zhou, T.; Wang, X.; Feng, L.; Meng, Q. Aortic smooth muscle TRPV4 channels regulate vasoconstriction in high salt-induced hypertension. Hypertens. Res. 2023, 46, 2356–2367. [Google Scholar] [CrossRef]
- Zhang, X.; Buckley, C.; Lee, M.D.; Salaun, C.; MacDonald, M.; Wilson, C.; McCarron, J.G. Increased TRPV4 channel expression enhances and impairs blood vessel function in hypertension. Hypertension 2025, 82, 57–68. [Google Scholar] [CrossRef]
- Iturriaga, R.; Oyarce, M.P.; Dias, A.C.R. Role of carotid body in intermittent hypoxia-related hypertension. Curr. Hypertens. Rep. 2017, 19, 38. [Google Scholar] [CrossRef]
- Iturriaga, R. Translating carotid body function into clinical medicine. J. Physiol. 2018, 596, 3067–3077. [Google Scholar] [CrossRef]
- Felippe, I.S.A.; Río, R.D.; Schultz, H.; Machado, B.H.; Paton, J.F.R. Commonalities and differences in carotid body dysfunction in hypertension and heart failure. J. Physiol. 2023, 601, 5527–5551. [Google Scholar] [CrossRef] [PubMed]
- Felippe, I.S.A.; Zera, T.; da Silva, M.P.; Moraes, D.J.A.; McBryde, F.; Paton, J.F.R. The sympathetic nervous system exacerbates carotid body sensitivity in hypertension. Cardiovasc. Res. 2023, 119, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Buffa, R.; Rindi, G.; Sessa, F.; Gini, A.; Capella, C.; Jahn, R.; Navone, F.; De Camilli, P.; Solcia, E. Synaptophysin immunoreactivity and small clear vesicles in neuroendocrine cells and related tumours. Mol. Cell. Probes. 1987, 1, 367–381. [Google Scholar] [CrossRef]
- Kondo, H.; Iwanaga, T.; Nakajima, T. Immunocytochemical study on the localization of neuron-specific enolase and S-100 protein in the carotid body of rats. Cell. Tissue Res. 1982, 227, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Otlyga, D.; Tsvetkova, E.; Junemann, O.; Saveliev, S. Immunohistochemical Characteristics of the Human Carotid Body in the Antenatal and Postnatal Periods of Development. Int. J. Mol. Sci. 2021, 225, 8222. [Google Scholar] [CrossRef]
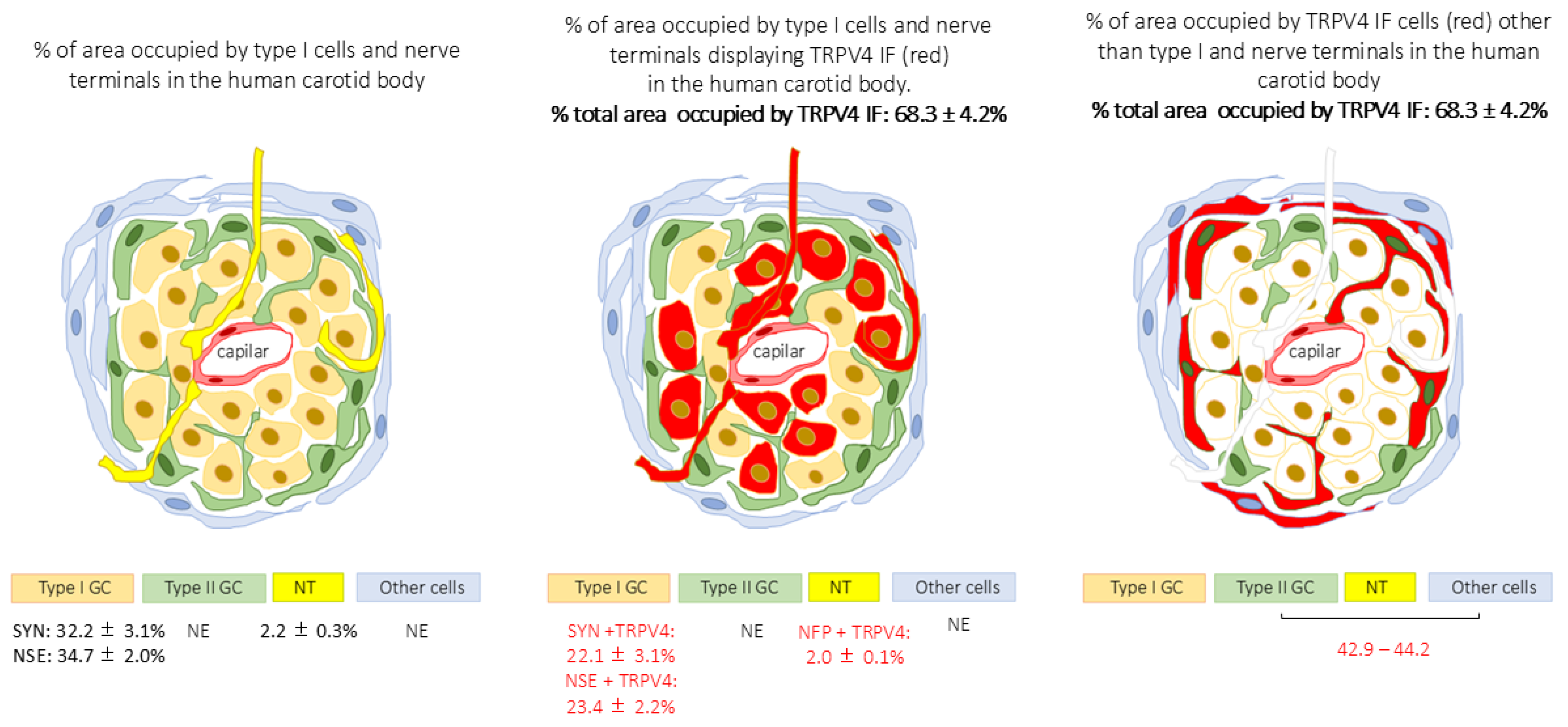
| % are occupied by TRPV4 IF cells | 68.3 ± 4.2% |
| % area occupied by SYN IF cells (type I cells) | 32.2 ± 3.1% |
| % area occupied by NSE IF cells (type I cells) | 34.7 ± 2.0% |
| % area occupied by NFP IF (nerve fibre terminals) | 2.2 ± 0.3% |
| % mergence of TRPV4 + SYN and % mergence TRPV4 + NSE (TRPV4 positive type I cells) | 22.1 ± 3.1% (68.6% of total type I cells) and 22.8 ± 2.6% (65.7% of total type I cells) |
| % mergence of TRPV4 + NFP (TRPV4 + nerve fibre terminals) | 2.0 ± 0.1% (90% of total nerve terminals) |
| Antigen | Origin | Dilution | Supplier |
|---|---|---|---|
| TRPV4 (LS-C401108) | Rabbit | 1:200 | LifeSpan BioSciences 1 |
| NSE (clone BBS/NC/VI-H14) | Mouse | 1:500 | Dako 2 |
| Synaptophysin (clone 27G12) | Mouse | Prediluted | Leica Biosystems 3 |
| NFP (clone 2F11) | Mouse | 1:200 | Roche 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Mesa, Y.; Alba, E.; Martínez-Barbero, G.; Suazo, I.; Cuendias, P.; Martín-Cruces, J.; Teulé-Trull, M.; Vega, J.A.; García-Suárez, O.; Cobo, T. Transient Receptor Potential Vanilloid 4 (TRPV4) in the Human Carotid Body. Int. J. Mol. Sci. 2025, 26, 10689. https://doi.org/10.3390/ijms262110689
García-Mesa Y, Alba E, Martínez-Barbero G, Suazo I, Cuendias P, Martín-Cruces J, Teulé-Trull M, Vega JA, García-Suárez O, Cobo T. Transient Receptor Potential Vanilloid 4 (TRPV4) in the Human Carotid Body. International Journal of Molecular Sciences. 2025; 26(21):10689. https://doi.org/10.3390/ijms262110689
Chicago/Turabian StyleGarcía-Mesa, Yolanda, Elda Alba, Graciela Martínez-Barbero, Iván Suazo, Patricia Cuendias, José Martín-Cruces, Mirian Teulé-Trull, José A. Vega, Olivia García-Suárez, and Teresa Cobo. 2025. "Transient Receptor Potential Vanilloid 4 (TRPV4) in the Human Carotid Body" International Journal of Molecular Sciences 26, no. 21: 10689. https://doi.org/10.3390/ijms262110689
APA StyleGarcía-Mesa, Y., Alba, E., Martínez-Barbero, G., Suazo, I., Cuendias, P., Martín-Cruces, J., Teulé-Trull, M., Vega, J. A., García-Suárez, O., & Cobo, T. (2025). Transient Receptor Potential Vanilloid 4 (TRPV4) in the Human Carotid Body. International Journal of Molecular Sciences, 26(21), 10689. https://doi.org/10.3390/ijms262110689






