Neural Correlates of Restless Legs Syndrome (RLS) Based on Electroencephalogram (EEG)—A Mechanistic Review
Abstract
1. Introduction
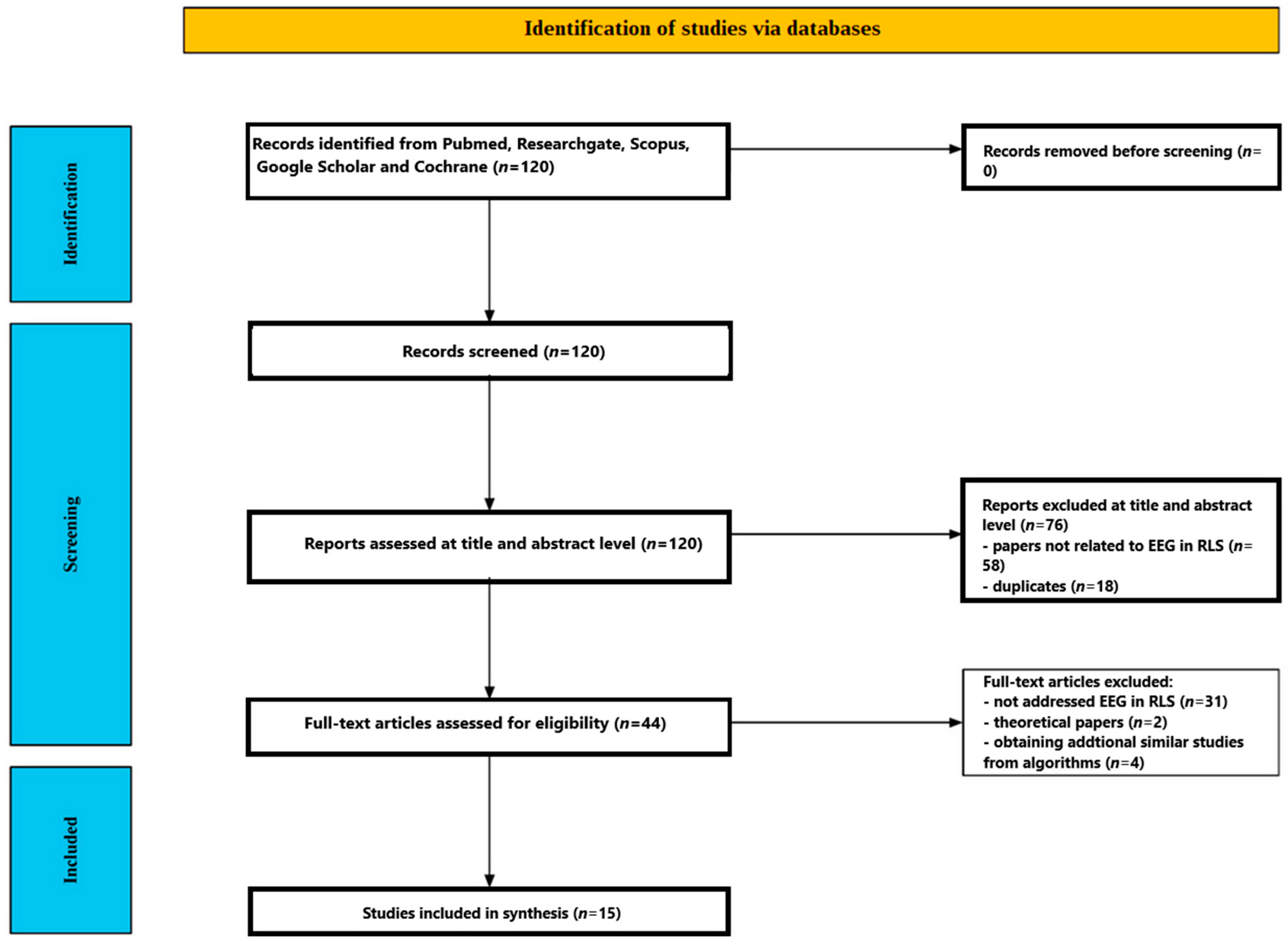
2. Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection Criteria
2.3. Screening Process
2.3.1. Title and Abstract Screening
2.3.2. Full-Text Assessment
3. Results
3.1. Participants
3.1.1. Sample Size and Sex Distribution
3.1.2. Age
3.1.3. Diagnostic Confirmation and Disease Severity
3.1.4. Medication Status and Comorbidity
3.1.5. Control and Comparison Groups
3.1.6. Screening Instruments
3.2. EEG Paradigms
3.2.1. Working Memory (Sternberg) Paradigms
- (a)
- Primary ERP focus: P300 (parietal and frontal amplitudes; latency secondary) in [51] and treatment response extension [53]; theta-band power dynamics and interregional phase synchrony during retrieval in [55]; and single-trial ERP topographies (150–250 ms) entered into explainable deep-learning classifiers in [57].
- (b)
- (c)
3.2.2. Visual Oddball Attention Paradigms
- (a)
- ERP components: Classic P300 (amplitude/latency) in [52]; extended component set including P2 (150–250 ms) and P3 (300–450 ms) with source localisation (LORETA) to medial prefrontal/anterior cingulate and precuneus generators in [54]; combined ERP + induced/evoked gamma-band activity and gamma phase synchrony (GBPS) with network metrics in [56].
- (b)
3.2.3. Circadian Cognitive-Control (Flanker) Paradigm
3.2.4. Vigilance-Controlled and Resting-State QEEG Mapping
- (a)
- Vigilance-controlled morning EEG (V-EEG): 3 min eyes-closed recordings after two lab nights in drug-free RLS vs. controls [60]; 21-channel montage; 36 derived spectral variables spanning 1.3–35 Hz bands (delta → beta-5) plus centroids/dominant frequency indices, extensive omnibus statistics, and topographic probability mapping.
- (b)
- Daytime mapping with multimodal cohort (RLS, PLMD, controls): Parallel 1.3–35 Hz spectral quantification and psychometric correlation in [61]; subset with overnight PSG.
- (c)
- Eyes-closed resting QEEG connectivity (RLS vs. primary insomnia): Five-minute recordings; source-space ciPLV connectivity across canonical bands (delta → gamma) controlling demographic and affective covariates in a large retrospective sample [58].
- (d)
- Alertness-check resting blocks embedded in oddball design: Alternating 20 s eyes-closed/open epochs repeated six times in [52] to ensure wakefulness and to compute relative band powers.
- (e)
- Resting-state EEG with standard activation procedures such as photic stimulation and hyperventilation [64].
3.2.5. Motor-State/Movement-Provocation Paradigms
- (a)
- Suggested Immobilization Test (SIT): One-hour quiet-wake limb-rest period before sleep in 53 drug-free patients; continuous EEG + heart rate capture around spontaneous leg movements categorized as periodic (PLM), isolated (ILM), or short-interval (SILM); 40 s peri-movement windows (−20/+20 s) analyzed for delta → beta absolute power trajectories and autonomic coupling [62].
- (b)
- Simple Paced Motor Response: Right-index button presses to auditory clicks (~6 s ISI) to elicit sensorimotor beta event-related desynchronization/synchronization (ERD/ERS) over C3/Cz; two beta sub-bands (14–20; 20–32 Hz) quantified for movement-related reactivity and post-movement rebound [63].
- (c)
- SIT: EEG data were processed offline to analyze movement-related changes in mu and beta rhythms. Specifically, the study examined ERD and post-movement beta synchronization (PMBS) [65].
3.2.6. Derived Network/Oscillatory Metrics Across Paradigms
3.3. EEG Measures and Outcomes
3.3.1. Resting-State Spectral Signatures
3.3.2. Vigilance Markers
3.3.3. Spectral Signatures During a Task
3.3.4. P300/P3 Amplitude
3.3.5. Global Field Power (GFP)
3.3.6. P300 Latency
3.3.7. P2 Amplitude
3.3.8. Treatment and Circadian Effects
3.3.9. Connectivity and Network Topology
3.3.10. Motor-Related Oscillations
4. Discussion
4.1. Cortical Hyperarousal and Abnormal Resting-State Spectra
4.2. Reduced P300 Amplitude—Typical Biomarker in RLS
4.3. Disrupted Oscillatory Synchrony and Small-World Topology
4.4. Circadian Modulation of Early Visual Attention in Restless Legs Syndrome
4.5. Motor–Cortex Excitability and Exaggerated Beta Rebound
4.6. Sensory-Integration Circuitry and Somatosensory Connectivity
4.7. Autonomic–Cortical Coupling with Leg Movements
4.8. Knowledge Gaps Identified from EEG Studies of RLS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mogavero, M.P.; Salemi, M.; Lanza, G.; Rinaldi, A.; Marchese, G.; Ravo, M.; Salluzzo, M.G.; Antoci, A.; DelRosso, L.M.; Bruni, O.; et al. Unveiling the pathophysiology of restless legs syndrome through transcriptome analysis. iScience 2024, 27, 109568. [Google Scholar] [CrossRef]
- Tang, M.; Sun, Q.; Zhang, Y.; Li, H.; Wang, D.; Wang, Y.; Wang, Z. Circadian rhythm in restless legs syndrome. Front. Neurol. 2023, 14, 1105463. [Google Scholar] [CrossRef]
- Antelmi, E.; Rocchi, L.; Latorre, A.; Belvisi, D.; Magrinelli, F.; Bhatia, K.P.; Tinazzi, M. Restless Legs Syndrome: Known Knowns and Known Unknowns. Brain Sci. 2022, 12, 118. [Google Scholar] [CrossRef]
- Ruppert, E. Restless arms syndrome: Prevalence, impact, and management strategies. Neuropsychiatr. Dis. Treat. 2019, 15, 1737–1750. [Google Scholar] [CrossRef]
- Riccardi, S.; Ferri, R.; Garbazza, C.; Miano, S.; Manconi, M. Pharmacological responsiveness of periodic limb movements in patients with restless legs syndrome: A systematic review and meta-analysis. J. Clin. Sleep. Med. 2023, 19, 811–822. [Google Scholar] [CrossRef]
- Gossard, T.R.; Trotti, L.M.; Videnovic, A.; St Louis, E.K. Restless Legs Syndrome: Contemporary Diagnosis and Treatment. Neurotherapeutics 2021, 18, 140–155. [Google Scholar] [CrossRef]
- Sharma, A.; Rai, N.K.; Singh, R. Clinical profile of restless leg syndrome and its effect on sleep and quality of life. J. Family Med. Prim. Care 2025, 14, 1359–1367. [Google Scholar] [CrossRef]
- Song, P.; Wu, J.; Cao, J.; Sun, W.; Li, X.; Zhou, T.; Shen, Y.; Tan, X.; Ye, X.; Yuan, C.; et al. Global Health Epidemiology Research Group (GHERG). The global and regional prevalence of restless legs syndrome among adults: A systematic review and modelling analysis. J. Glob. Health 2024, 14, 04113. [Google Scholar] [CrossRef] [PubMed]
- Gjevre, J.A.; Taylor Gjevre, R.M. Restless legs syndrome as a comorbidity in rheumatoid arthritis. Autoimmune Dis. 2013, 2013, 352782. [Google Scholar] [CrossRef]
- Milligan, S.A.; Chesson, A.L. Restless legs syndrome in the older adult: Diagnosis and management. Drugs Aging 2002, 19, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.M.; O’Hara, R.; Vitiello, M.V. Epidemiology of restless legs syndrome: A synthesis of the literature. Sleep Med. Rev. 2012, 16, 283–295. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.R.; Park, H.R.; Joo, E.Y. Sex-specific patterns of discomfort in patients with restless legs syndrome. J. Clin. Sleep Med. 2024, 20, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lepuzanovic, M.; Sinanovic, O.; Aziraj-Smajic, V.; Kapic, D.; Basagic, E.; Muftic, M. Prevalence of restless legs syndrome during pregnancy and postpartum period. J. Perinat. Med. 2024, 52, 852–857. [Google Scholar] [CrossRef]
- Gupta, R.; Dhyani, M.; Kendzerska, T.; Pandi-Perumal, S.R.; BaHammam, A.S.; Srivanitchapoom, P.; Pandey, S.; Hallett, M. Restless legs syndrome and pregnancy: Prevalence, possible pathophysiological mechanisms and treatment. Acta Neurol. Scand. 2016, 133, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Alzaabi, F.M.; Al Tarawneh, D.J.; Al Tarawneh, Y.J.; Khan, A.; Khan, M.A.M.; Siddiqui, T.W.; Siddiqui, R.W.; Nishat, S.M.H.; Alzaabi, A.A.; Siddiqui, S.W. Restless Legs and Iron Deficiency: Unraveling the Hidden Link and Unlocking Relief. Cureus 2025, 17, e82413. [Google Scholar] [CrossRef]
- Khan, A.; Kumar, H.; Rai, K.D.; Saeed, A.; Ishtiaq, J.; Tanveer Alam, M.; Chawla, S.; Haque, M.A. Efficacy and safety of intravenous ferric carboxymaltose in the treatment of Restless Legs Syndrome: A systematic review and meta-analysis. Front. Neurol. 2025, 15, 1503342. [Google Scholar] [CrossRef]
- Beliveau, V.; Stefani, A.; Birkl, C.; Kremser, C.; Gizewski, E.R.; Högl, B.; Scherfler, C. Revisiting brain iron deficiency in restless legs syndrome using magnetic resonance imaging. Neuroimage Clin. 2022, 34, 103024. [Google Scholar] [CrossRef]
- Frantom, P.A.; Seravalli, J.; Ragsdale, S.W.; Fitzpatrick, P.F. Reduction and oxidation of the active site iron in tyrosine hydroxylase: Kinetics and specificity. Biochemistry 2006, 45, 2372–2379. [Google Scholar] [CrossRef]
- Connor, J.R.; Patton, S.M.; Oexle, K.; Allen, R.P. Iron and restless legs syndrome: Treatment, genetics and pathophysiology. Sleep Med. 2017, 31, 61–70. [Google Scholar] [CrossRef]
- Hornyak, M.; Trenkwalder, C.; Kohnen, R.; Scholz, H. Efficacy and safety of dopamine agonists in restless legs syndrome. Sleep Med. 2012, 13, 228–236. [Google Scholar] [CrossRef]
- Allen, R.P.; Barker, P.B.; Horská, A.; Earley, C.J. Thalamic glutamate/glutamine in restless legs syndrome: Increased and related to disturbed sleep. Neurology 2013, 80, 2028–2034. [Google Scholar] [CrossRef]
- Griffin, E.; Brown, J.N. Pregabalin for the Treatment of Restless Legs Syndrome. Ann. Pharmacother. 2016, 50, 586–591. [Google Scholar] [CrossRef]
- Ondo, W.G. Methadone for refractory restless legs syndrome. Mov. Disord. 2005, 20, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.S.; Li, Y.; Koo, B.B.; Ondo, W.G.; Weinstock, L.B.; Champion, D.; Afrin, L.B.; Karroum, E.G.; Bagai, K.; Spruyt, K. Review of the role of the endogenous opioid and melanocortin systems in the restless legs syndrome. Brain 2024, 147, 26–38. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, C.O.; Carvalho, L.B.; Carlos, K.; Conti, C.; de Oliveira, M.M.; Prado, L.B.; Prado, G.F. Opioids for restless legs syndrome. Cochrane Database Syst. Rev. 2016, 2016, CD006941. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Quiroz, C.; Guitart, X.; Rea, W.; Seyedian, A.; Moreno, E.; Casadó-Anguera, V.; Díaz-Ríos, M.; Casadó, V.; Clemens, S.; et al. Pivotal Role of Adenosine Neurotransmission in Restless Legs Syndrome. Front. Neurosci. 2018, 11, 722. [Google Scholar] [CrossRef]
- Sönmez, A.T.; Demirci, H. Restless legs syndrome and hashimoto’s thyroiditis. Acta Neurol. Belg. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Suwała, S.; Rzeszuto, J.; Glonek, R.; Krintus, M.; Junik, R. Is Restless Legs Syndrome De Facto Thyroid Disease? Biomedicines 2022, 10, 2502. [Google Scholar] [CrossRef]
- Miyaue, K.; Isono, H. A Case Report of Grave’s Disease Presenting with an Episode of Restless Legs Syndrome. Cureus 2024, 16, e57354. [Google Scholar] [CrossRef]
- Geng, C.; Yang, Z.; Kong, X.; Xu, P.; Zhang, H. Association between thyroid function and disease severity in restless legs syndrome. Front. Neurol. 2022, 13, 974229. [Google Scholar] [CrossRef]
- Harrer, P.; Mirza-Schreiber, N.; Mandel, V.; Roeber, S.; Stefani, A.; Naher, S.; Wagner, M.; Gieger, C.; Waldenberger, M.; Peters, A.; et al. Epigenetic Association Analyses and Risk Prediction of RLS. Mov. Disord. 2023, 38, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Schormair, B.; Zhao, C.; Bell, S.; Didriksen, M.; Nawaz, M.S.; Schandra, N.; Stefani, A.; Högl, B.; Dauvilliers, Y.; Bachmann, C.G.; et al. Genome-wide meta-analyses of restless legs syndrome yield insights into genetic architecture, disease biology and risk prediction. Nat. Genet. 2024, 56, 1090–1099. [Google Scholar] [CrossRef]
- Sun, B.; Tan, B.; Zhang, P.; Zhu, L.; Wei, H.; Huang, T.; Li, C.; Yang, W. Iron deficiency anemia: A critical review on iron absorption, supplementation and its influence on gut microbiota. Food Funct. 2024, 15, 1144–1157. [Google Scholar] [CrossRef]
- Veres-Székely, A.; Szász, C.; Pap, D.; Szebeni, B.; Bokrossy, P.; Vannay, Á. Zonulin as a Potential Therapeutic Target in Microbiota-Gut-Brain Axis Disorders: Encouraging Results and Emerging Questions. Int. J. Mol. Sci. 2023, 24, 7548. [Google Scholar] [CrossRef]
- Darol, E.S.; Sahin, E.O.; Sayan, S.; Alemdar, M. Blood zonulin levels in restless legs syndrome: Insights into intestinal permeability. Rev. Assoc. Med. Bras. 2025, 71, e20241870. [Google Scholar] [CrossRef]
- Cheng, H.; Yang, W.; Xu, H.; Zhu, W.; Gong, A.; Yang, X.; Li, S.; Xu, H. Microbiota metabolites affect sleep as drivers of brain-gut communication (Review). Int. J. Mol. Med. 2025, 56, 130. [Google Scholar] [CrossRef]
- Nayak, C.S.; Anilkumar, A.C. EEG Normal Waveforms. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539805/ (accessed on 30 October 2025).
- Edgar, J.C.; Franzen, R.E.; McNamee, M.; Green, H.L.; Shen, G.; DiPiero, M.; Liu, S.; Airey, M.; Goldin, S.; Blaskey, L.; et al. A comparison of resting-state eyes-closed and dark-room alpha-band activity in children. Psychophysiology 2023, 60, e14285. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; De Blasio, F.M. EEG differences between eyes-closed and eyes-open resting remain in healthy ageing. Biol. Psychol. 2017, 129, 293–304. [Google Scholar] [CrossRef]
- Freschl, J.; Azizi, L.A.; Balboa, L.; Kaldy, Z.; Blaser, E. The development of peak alpha frequency from infancy to adolescence and its role in visual temporal processing: A meta-analysis. Dev. Cogn. Neurosci. 2022, 57, 101146. [Google Scholar] [CrossRef]
- Turner, C.; Baylan, S.; Bracco, M.; Cruz, G.; Hanzal, S.; Keime, M.; Kuye, I.; McNeill, D.; Ng, Z.; van der Plas, M.; et al. Developmental changes in individual alpha frequency: Recording EEG data during public engagement events. Imaging Neurosci. 2023, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Reddy, V.; Shumway, K.R.; Araujo, J.F. Physiology, Sleep Stages. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK526132/ (accessed on 30 October 2025).
- Snipes, S.; Krugliakova, E.; Meier, E.; Huber, R. The Theta Paradox: 4–8 Hz EEG Oscillations Reflect Both Sleep Pressure and Cognitive Control. J. Neurosci. 2022, 42, 8569–8586. [Google Scholar] [CrossRef]
- Sabate, M.; Llanos, C.; Enriquez, E.; Gonzalez, B.; Rodriguez, M. Fast modulation of alpha activity during visual processing and motor control. Neuroscience 2011, 189, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Barone, J.; Rossiter, H.E. Understanding the Role of Sensorimotor Beta Oscillations. Front. Syst. Neurosci. 2021, 15, 655886. [Google Scholar] [CrossRef] [PubMed]
- Doesburg, S.M.; Kitajo, K.; Ward, L.M. Increased gamma-band synchrony precedes switching of conscious perceptual objects in binocular rivalry. NeuroReport 2005, 16, 1139–1142. [Google Scholar] [CrossRef]
- Yamamoto, J.; Suh, J.; Takeuchi, D.; Tonegawa, S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell 2014, 157, 845–857. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y. The role of quantitative EEG biomarkers in Alzheimer’s disease and mild cognitive impairment: Applications and insights. Front. Aging Neurosci. 2025, 17, 1522552. [Google Scholar] [CrossRef]
- Woodman, G.F. A brief introduction to the use of event-related potentials in studies of perception and attention. Atten. Percept. Psychophys. 2010, 72, 2031–2046. [Google Scholar] [CrossRef]
- Patel, S.H.; Azzam, P.N. Characterization of N200 and P300: Selected studies of the Event-Related Potential. Int. J. Med. Sci. 2005, 2, 147–154. [Google Scholar] [CrossRef]
- Kim, S.M.; Choi, J.W.; Lee, C.; Lee, B.U.; Koo, Y.S.; Kim, K.H.; Jung, K.Y. Working memory deficit in patients with restless legs syndrome: An event-related potential study. Sleep Med. 2014, 15, 808–815. [Google Scholar] [CrossRef]
- Jung, K.Y.; Koo, Y.S.; Kim, B.J.; Ko, D.; Lee, G.T.; Kim, K.H.; Im, C.H. Electrophysiologic disturbances during daytime in patients with restless legs syndrome: Further evidence of cognitive dysfunction? Sleep Med. 2011, 12, 416–421. [Google Scholar] [CrossRef]
- Jung, K.Y.; Kim, S.M.; Song, J.Y.; Lee, B.U.; Lee, C.; Lee, S.K.; Koo, Y.S.; Cho, Y.W.; Choi, J.W.; Kim, K.H. Sternberg working memory performance following treatment with pramipexole in patients with moderate-to-severe restless legs syndrome. Sleep Med. 2015, 16, 703–708. [Google Scholar] [CrossRef]
- Cha, K.S.; Choi, J.W.; Jung, K.Y.; Kim, K.H. Frontal dysfunction in patients with restless legs syndrome performing a visual oddball task: An event-related potential source imaging study. Sleep Med. 2017, 36, 48–54. [Google Scholar] [CrossRef]
- Cha, K.S.; Sunwoo, J.S.; Byun, J.I.; Kim, T.J.; Shin, J.W.; Kim, K.H.; Jung, K.Y. Working memory deficits in patients with idiopathic restless legs syndrome are associated with abnormal theta-band neural synchrony. J. Sleep. Res. 2021, 30, e13287. [Google Scholar] [CrossRef]
- Choi, J.W.; Ko, D.; Lee, G.T.; Jung, K.Y.; Kim, K.H. Reduced neural synchrony in patients with restless legs syndrome during a visual oddball task. PLoS ONE 2012, 7, e42312. [Google Scholar] [CrossRef]
- Kim, M.; Kim, H.; Seo, P.; Jung, K.Y.; Kim, K.H. Explainable Machine-Learning-Based Characterization of Abnormal Cortical Activities for Working Memory of Restless Legs Syndrome Patients. Sensors 2022, 22, 7792. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, Y.M.; Kim, Y.R. A Comparative Investigation of Functional Connectivity Utilizing Electroencephalography in Insomnia Patients with and without Restless Leg Syndrome. Clin. Psychopharmacol. Neurosci. 2024, 22, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Brandt, M.D.; Schrempf, W.; Beste, C.; Stock, A.K. Neurophysiological mechanisms of circadian cognitive control in RLS patients—An EEG source localization study. Neuroimage Clin. 2017, 15, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Saletu, M.; Anderer, P.; Saletu, B.; Lindeck-Pozza, L.; Hauer, C.; Saletu-Zyhlarz, G. EEG mapping in patients with restless legs syndrome as compared with normal controls. Psychiatry Res. 2002, 115, 49–61. [Google Scholar] [CrossRef]
- Saletu, B.; Anderer, P.; Saletu, M.; Hauer, C.; Lindeck-Pozza, L.; Saletu-Zyhlarz, G. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. Sleep Med. 2002, 3, S35–S42. [Google Scholar] [CrossRef]
- Benbir Senel, G.; Tunali, A.; Demirel, O.; Köse, S.; Cakir, V.; Resadiyeli, B.; Karadeniz, D.; Ferri, R. Spectral EEG and heart rate changes associated with leg movements during the suggested immobilization test in patients with restless legs syndrome. J. Sleep Res. 2025, 34, e14394. [Google Scholar] [CrossRef] [PubMed]
- Schober, T.; Wenzel, K.; Feichtinger, M.; Schwingenschuh, P.; Strebel, A.; Krausz, G.; Pfurtscheller, G. Restless legs syndrome: Changes of induced electroencephalographic beta oscillations-an ERD/ERS study. Sleep 2004, 27, 147–150. [Google Scholar] [CrossRef]
- Titiz, A.P.; Mungan, S.; Mutlu, N.M.; Özcan, K.M.; Bilen, S.; Eruyar, E.; Fikri, A. Daytime cerebral electrical activity in patients with restless legs syndrome. Sleep Biol. Rhythms. 2016, 14, 193–198. [Google Scholar] [CrossRef]
- Tyvaert, L.; Houdayer, E.; Devanne, H.; Bourriez, J.L.; Derambure, P.; Monaca, C. Cortical involvement in the sensory and motor symptoms of primary restless legs syndrome. Sleep Med. 2009, 10, 1090–1096. [Google Scholar] [CrossRef]
- Lanza, G.; Ferri, R. The neurophysiology of hyperarousal in restless legs syndrome: Hints for a role of glutamate/GABA. Adv. Pharmacol. 2019, 84, 101–119. [Google Scholar]
- Liu, Z.; Guan, R.; Pan, L. Exploration of restless legs syndrome under the new concept: A review. Medicine 2022, 101, e32324. [Google Scholar] [CrossRef]
- Allen, R.P.; Earley, C.J. Restless legs syndrome: A review of clinical and pathophysiologic features. J. Clin. Neurophysiol. 2001, 18, 128–147. [Google Scholar] [CrossRef]
- Gamaldo, C.; Benbrook, A.R.; Allen, R.P.; Oguntimein, O.; Earley, C.J. Evaluating daytime alertness in individuals with Restless Legs Syndrome (RLS) compared to sleep restricted controls. Sleep Med. 2009, 10, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.M.; Sharon, D. Mood disorders in restless legs syndrome (Willis-Ekbom disease). J. Clin. Psychiatry 2014, 75, e679–e694. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Cosentino, F.I.; Manconi, M.; Rundo, F.; Bruni, O.; Zucconi, M. Increased electroencephalographic high frequencies during the sleep onset period in patients with restless legs syndrome. Sleep 2014, 37, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Cha, K.S.; Lee, S.; Yang, T.W.; Kim, K.T.; Park, B.S.; Jun, J.S.; Lim, J.A.; Byun, J.I.; Sunwoo, J.S.; et al. Brain regions associated with periodic leg movements during sleep in restless legs syndrome. Sci. Rep. 2020, 10, 1615. [Google Scholar] [CrossRef]
- Livint Popa, L.; Dragos, H.; Pantelemon, C.; Verisezan Rosu, O.; Strilciuc, S. The Role of Quantitative EEG in the Diagnosis of Neuropsychiatric Disorders. J. Med. Life 2020, 13, 8–15. [Google Scholar] [CrossRef]
- Oh, D.Y.; Park, S.M.; Choi, S.W. Daytime Neurophysiological Hyperarousal in Chronic Insomnia: A Study of qEEG. J. Clin. Med. 2020, 9, 3425. [Google Scholar] [CrossRef]
- Dressle, R.J.; Riemann, D. Hyperarousal in insomnia disorder: Current evidence and potential mechanisms. J. Sleep Res. 2023, 32, e13928. [Google Scholar] [CrossRef]
- Hedges, D.; Janis, R.; Mickelson, S.; Keith, C.; Bennett, D.; Brown, B.L. P300 Amplitude in Alzheimer’s Disease: A Meta-Analysis and Meta-Regression. Clin. EEG Neurosci. 2016, 47, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. The neurophysiology of P 300—An integrated review. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1480–1488. [Google Scholar]
- Linden, D.E. The p300: Where in the brain is it produced and what does it tell us? Neuroscientist 2005, 11, 563–576. [Google Scholar] [CrossRef]
- Soltani, M.; Knight, R.T. Neural origins of the P300. Crit. Rev. Neurobiol. 2000, 14, 199–224. [Google Scholar] [CrossRef] [PubMed]
- Özmüş, G.; Yerlikaya, D.; Gökçeoğlu, A.; Emek Savaş, D.D.; Çakmur, R.; Dönmez Çolakoğlu, B.; Yener, G.G. Demonstration of Early Cognitive Impairment in Parkinson’s Disease with Visual P300 Responses. Noro Psikiyatr. Ars. 2017, 54, 21–27. [Google Scholar] [CrossRef]
- Stanzione, P.; Fattapposta, F.; Giunti, P.; D’Alessio, C.; Tagliati, M.; Affricano, C.; Amabile, G. P300 variations in parkinsonian patients before and during dopaminergic monotherapy: A suggested dopamine component in P300. Electroencephalogr. Clin. Neurophysiol. 1991, 80, 446–453. [Google Scholar] [CrossRef]
- Sohn, Y.H.; Kim, G.W.; Huh, K.; Kim, J.S. Dopaminergic influences on the P300 abnormality in Parkinson’s disease. J. Neurol. Sci. 1998, 158, 83–87. [Google Scholar] [CrossRef]
- Prabhakar, S.; Syal, P.; Srivastava, T. P300 in newly diagnosed non-dementing Parkinson’s disease: Effect of dopaminergic drugs. Neurol. India 2000, 48, 239–242. [Google Scholar]
- Pineda, J.A.; Foote, S.L.; Neville, H.J. Effects of locus coeruleus lesions on auditory, long-latency, event-related potentials in monkey. J. Neurosci. 1989, 9, 81–93. [Google Scholar] [CrossRef]
- Castro, A.; Díaz, F. Effect of the relevance and position of the target stimuli on P300 and reaction time. Int. J. Psychophysiol. 2001, 41, 43–52. [Google Scholar] [CrossRef]
- Gray, H.M.; Ambady, N.; Lowenthal, W.T.; Deldin, P. P300 as an index of attention to self-relevant stimuli. J. Exp. Social. Psychol. 2004, 40, 216–224. [Google Scholar] [CrossRef]
- Moon, Y.J.; Song, J.Y.; Lee, B.U.; Koo, Y.S.; Lee, S.K.; Jun, K.Y. Comparison of Cognitive Function between Patients with Restless Legs Syndrome and Healthy Controls. Sleep Med. Res. 2014, 5, 20–24. [Google Scholar] [CrossRef]
- Celle, S.; Roche, F.; Kerleroux, J.; Thomas-Anterion, C.; Laurent, B.; Rouch, I.; Pichot, V.; Barthélémy, J.C.; Sforza, E. Prevalence and clinical correlates of restless legs syndrome in an elderly French population: The synapse study. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 167–173. [Google Scholar] [CrossRef]
- Fulda, S.; Beitinger, M.E.; Reppermund, S.; Winkelmann, J.; Wetter, T.C. Short-term attention and verbal fluency is decreased in restless legs syndrome patients. Mov. Disord. 2010, 25, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Arıkan, M.K.; İlhan, R.; Orhan, Ö.; Esmeray, M.T.; Turan, Ş.; Gica, Ş.; Bakay, H.; Pogarell, O.; Tarhan, K.N.; Metin, B. P300 parameters in major depressive disorder: A systematic review and meta-analysis. World J. Biol. Psychiatry 2024, 25, 255–266. [Google Scholar] [CrossRef]
- Hasan, L.A.; Hamdan, F.B.; Al-Mahdawi, A. P300 event-related potentials in people with epilepsy: Clinico-neurophysiologic study. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 93. [Google Scholar] [CrossRef]
- Ferri, R.; Rundo, F.; Bruni, O.; Terzano, M.G.; Stam, C.J. Small-world network organization of functional connectivity of EEG slow-wave activity during sleep. Clin. Neurophysiol. 2007, 118, 449–456. [Google Scholar] [CrossRef]
- Ansari Nasab, S.; Panahi, S.; Ghassemi, F.; Jafari, S.; Rajagopal, K.; Ghosh, D.; Perc, M. Functional neuronal networks reveal emotional processing differences in children with ADHD. Cogn. Neurodyn. 2022, 16, 91–100. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, K.T.; Kang, K.W.; Park, J.A.; Seo, J.G.; Kim, J.; Chang, H.; Kim, E.Y.; Cho, Y.W. RLS Subcommittee of the Korean Sleep Research Society. Alterations of Functional Connectivity in Patients with Restless Legs Syndrome. J. Clin. Neurol. 2022, 18, 290–297. [Google Scholar] [CrossRef]
- Ku, J.; Cho, Y.W.; Lee, Y.S.; Moon, H.J.; Chang, H.; Earley, C.J.; Allen, R.P. Functional connectivity alternation of the thalamus in restless legs syndrome patients during the asymptomatic period: A resting-state connectivity study using functional magnetic resonance imaging. Sleep Med. 2014, 15, 289–294. [Google Scholar] [CrossRef]
- Tuovinen, N.; Stefani, A.; Mitterling, T.; Heidbreder, A.; Frauscher, B.; Gizewski, E.R.; Poewe, W.; Högl, B.; Scherfler, C. Functional connectivity and topology in patients with restless legs syndrome: A case-control resting-state functional magnetic resonance imaging study. Eur. J. Neurol. 2021, 28, 448–458. [Google Scholar] [CrossRef]
- Lozoff, B. Early iron deficiency has brain and behavior effects consistent with dopaminergic dysfunction. J. Nutr. 2011, 141, 740S–746S. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Ren, Q.; Meng, J.; Gao, W.J.; Chang, Y.Z. Brain Iron Homeostasis and Mental Disorders. Antioxidants 2023, 12, 1997. [Google Scholar] [CrossRef] [PubMed]
- Llinás, R.R.; Ribary, U.; Jeanmonod, D.; Kronberg, E.; Mitra, P.P. Thalamocortical dysrhythmia: A neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl. Acad. Sci. USA 1999, 96, 15222–15227. [Google Scholar] [CrossRef]
- Choi, J.W.; Jeong, M.H.; Her, S.J.; Lee, B.U.; Cha, K.S.; Jung, K.Y.; Kim, K.H. Abnormal Sleep Delta Rhythm and Interregional Phase Synchrony in Patients with Restless Legs Syndrome and Their Reversal by Dopamine Agonist Treatment. J. Clin. Neurol. 2017, 13, 340–350. [Google Scholar] [CrossRef]
- Freichel, R.; Zink, N.; Chang, F.Y.; Vera, J.D.; Truong, H.; Michelini, G.; Loo, S.K.; Lenartowicz, A. Alpha event-related decreases during encoding in adults with ADHD—An investigation of sustained attention and working memory processes. Behav. Brain Res. 2024, 469, 115003. [Google Scholar] [CrossRef]
- Nagano-Saito, A.; Bellec, P.; Hanganu, A.; Jobert, S.; Mejia-Constain, B.; Degroot, C.; Lafontaine, A.L.; Lissemore, J.I.; Smart, K.; Benkelfat, C.; et al. Why Is Aging a Risk Factor for Cognitive Impairment in Parkinson’s Disease?-A Resting State fMRI Study. Front. Neurol. 2019, 10, 267. [Google Scholar] [CrossRef]
- Walters, A.S.; Zee, P.C. Why the worsening at rest and worsening at night criteria for Restless Legs Syndrome are listed separately: Review of the circadian literature on RLS and suggestions for future directions. Front. Neurol. 2023, 14, 1153273. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, K.S.; Blakemore, L.J.; Trombley, P.Q. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Front. Cell Neurosci. 2017, 11, 91. [Google Scholar] [CrossRef]
- Youdim, M.B.; Yehuda, S.; Ben-Uriah, Y. Iron deficiency-induced circadian rhythm reversal of dopaminergic-mediated behaviours and thermoregulation in rats. Eur. J. Pharmacol. 1981, 74, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bianco, L.E.; Wiesinger, J.; Earley, C.J.; Jones, B.C.; Beard, J.L. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J. Neurochem. 2008, 106, 205–215. [Google Scholar] [CrossRef]
- Vanegas, M.I.; Blangero, A.; Galvin, J.E.; Di Rocco, A.; Quartarone, A.; Ghilardi, M.F.; Kelly, S.P. Altered dynamics of visual contextual interactions in Parkinson’s disease. npj Parkinsons Dis. 2019, 5, 13. [Google Scholar] [CrossRef]
- Duret, L.C.; Nagoshi, E. The intertwined relationship between circadian dysfunction and Parkinson’s disease. Trends Neurosci. 2025, 48, 62–76. [Google Scholar] [CrossRef]
- Martínez-Pérez, V.; Palmero, L.B.; Campoy, G.; Fuentes, L.J. The role of chronotype in the interaction between the alerting and the executive control networks. Sci. Rep. 2020, 10, 11901. [Google Scholar] [CrossRef]
- Gumenyuk, V.; Howard, R.; Roth, T.; Korzyukov, O.; Drake, C.L. Sleep loss, circadian mismatch, and abnormalities in reorienting of attention in night workers with shift work disorder. Sleep 2014, 37, 545–556. [Google Scholar] [CrossRef]
- Barclay, N.L.; Myachykov, A. Sustained wakefulness and visual attention: Moderation by chronotype. Exp. Brain Res. 2017, 235, 57–68. [Google Scholar] [CrossRef]
- Song, T.; Xu, L.; Peng, Z.; Wang, L.; Dai, C.; Xu, M.; Shao, Y.; Wang, Y.; Li, S. Total sleep deprivation impairs visual selective attention and triggers a compensatory effect: Evidence from event-related potentials. Cogn. Neurodyn. 2023, 17, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.P.; Earley, C.J.; Jones, B.C.; Unger, E.L. Iron-deficiency and dopaminergic treatment effects on RLS-Like behaviors of an animal model with the brain iron deficiency pattern of the restless legs syndrome. Sleep Med. 2020, 71, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Winlow, W. Pramipexole in restless legs syndrome: An evidence-based review of its effectiveness on clinical outcomes. Core Evid. 2005, 1, 35–42. [Google Scholar]
- Silber, M.H.; Girish, M.; Izurieta, R. Pramipexole in the management of restless legs syndrome: An extended study. Sleep 2003, 26, 819–821. [Google Scholar] [CrossRef]
- Serafini, A.; Lorenzut, S.; Gigli, G.L.; Merlino, G.; Valente, M. The use of rotigotine in the treatment of restless legs syndrome. Ther. Adv. Neurol. Disord. 2010, 3, 241–248. [Google Scholar] [CrossRef]
- Vallderiola, F.; Compta, Y.; Aparicio, J.; Tarradellas, J.; Salazar, G.; Oliver, J.M.; Callén, A.; Delgado, T.; Nobbe, F. Effects of Night-Time Use of Rotigotine on Nocturnal Symptoms in Parkinson’s Disease. Parkinsons Dis. 2015, 2015, 475630. [Google Scholar] [CrossRef]
- Troutt, J.S.; Rudling, M.; Persson, L.; Ståhle, L.; Angelin, B.; Butterfield, A.M.; Schade, A.E.; Cao, G.; Konrad, R.J. Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 2012, 58, 1225–1232. [Google Scholar] [CrossRef]
- Schaap, C.C.; Hendriks, J.C.; Kortman, G.A.; Klaver, S.M.; Kroot, J.J.; Laarakkers, C.M.; Wiegerinck, E.T.; Tjalsma, H.; Janssen, M.C.; Swinkels, D.W. Diurnal rhythm rather than dietary iron mediates daily hepcidin variations. Clin. Chem. 2013, 59, 527–535. [Google Scholar] [CrossRef]
- Zaehle, T.; Rach, S.; Herrmann, C.S. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS ONE 2010, 5, e13766. [Google Scholar] [CrossRef]
- Gholamali Nezhad, F.; Martin, J.; Tassone, V.K.; Swiderski, A.; Demchenko, I.; Khan, S.; Chaudhry, H.E.; Palmisano, A.; Santarnecchi, E.; Bhat, V. Transcranial alternating current stimulation for neuropsychiatric disorders: A systematic review of treatment parameters and outcomes. Front. Psychiatry 2024, 15, 1419243. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Guo, Y.; Long, J. Beta rebound reduces subsequent movement preparation time by modulating of GABAA inhibition. Cereb. Cortex 2024, 34, bhae037. [Google Scholar] [CrossRef]
- Tergau, F.; Wischer, S.; Paulus, W. Motor system excitability in patients with restless legs syndrome. Neurology 1999, 52, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, S.C.; Queiroz de Paiva, J.P.; Kaelin-Lang, A.; Sterr, A.; Eckeli, A.L.; Winkler, A.M.; Fernandes do Prado, G.; Amaro, E., Jr.; Conforto, A.B. Short-interval intracortical inhibition is decreased in restless legs syndrome across a range of severity. Sleep Med. 2019, 62, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.; Mogavero, M.P.; Ferri, R.; Pani, T. Motor cortex excitability in restless legs syndrome: A systematic review and insights into pathophysiology via transcranial magnetic stimulation. Sleep Med. Rev. 2025, 79, 102027. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Madadi Asl, M.; Vahabie, A.H.; Valizadeh, A. The Origin of Abnormal Beta Oscillations in the Parkinsonian Corticobasal Ganglia Circuits. Parkinsons Dis. 2022, 2022, 7524066. [Google Scholar] [CrossRef]
- Heideman, S.G.; Quinn, A.J.; Woolrich, M.W.; van Ede, F.; Nobre, A.C. Dissecting beta-state changes during timed movement preparation in Parkinson’s disease. Prog. Neurobiol. 2020, 184, 101731. [Google Scholar] [CrossRef]
- Rossiter, H.E.; Davis, E.M.; Clark, E.V.; Boudrias, M.H.; Ward, N.S. Beta oscillations reflect changes in motor cortex inhibition in healthy ageing. NeuroImage 2014, 91, 360–365. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, K.T.; Lee, D.A.; Motamedi, G.K.; Cho, Y.W. Structural and functional multilayer network analysis in restless legs syndrome patients. J. Sleep Res. 2024, 33, e14104. [Google Scholar] [CrossRef]
- Kocar, T.D.; Müller, H.P.; Kassubek, J. Differential functional connectivity in thalamic and dopaminergic pathways in restless legs syndrome: A meta-analysis. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420941670. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, K.T.; Lee, D.A.; Cho, Y.W. Alterations of the thalamic nuclei volumes and intrinsic thalamic network in patients with restless legs syndrome. Sci. Rep. 2023, 13, 4415. [Google Scholar] [CrossRef]
- Kim, J.; Loggia, M.L.; Cahalan, C.M.; Harris, R.E.; Beissner, F.; Nat, P.; Garcia, R.G.; Kim, H.; Wasan, A.D.; Edwards, R.R.; et al. The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015, 67, 1395–1405. [Google Scholar] [CrossRef]
- Suñol, M.; Dudley, J.; Payne, M.F.; Tong, H.; Ting, T.V.; Kashikar-Zuck, S.; Coghill, R.C.; López-Solà, M. Reduced Cortico-Cortical Resting-State Connectivity in Sensory Systems Related to Bodily Pain in Juvenile Fibromyalgia. Arthritis Rheumatol. 2024, 76, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Hosomi, K.; Shimizu, T.; Okada, K.; Kadono, Y.; Mori, N.; Hori, Y.; Yahata, N.; Hirabayashi, T.; Kishima, H.; et al. Cross-Species Convergence of Functional Connectivity Changes in Thalamic Pain Across Human Patients and Model Macaques. J. Pain 2024, 25, 104661. [Google Scholar] [CrossRef]
- Kowalski, J.L.; Morse, L.R.; Troy, K.; Nguyen, N.; Battaglino, R.A.; Falci, S.P.; Linnman, C. Resting state functional connectivity differentiation of neuropathic and nociceptive pain in individuals with chronic spinal cord injury. NeuroImage Clin. 2023, 38, 103414. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Langguth, B.; Llinas, R. Thalamocortical Dysrhythmia: A Theoretical Update in Tinnitus. Front. Neurol. 2015, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Chouchou, F.; Mauguière, F.; Vallayer, O.; Catenoix, H.; Isnard, J.; Montavont, A.; Jung, J.; Pichot, V.; Rheims, S.; Mazzola, L. How the insula speaks to the heart: Cardiac responses to insular stimulation in humans. Hum. Brain Mapp. 2019, 40, 2611–2622. [Google Scholar] [CrossRef]
- Oppenheimer, S.M.; Gelb, A.; Girvin, J.P.; Hachinski, V.C. Cardiovascular effects of human insular cortex stimulation. Neurology 1992, 42, 1727–1732. [Google Scholar] [CrossRef]
- Benarroch, E.E. The central autonomic network: Functional organization, dysfunction, and perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Weavil, J.C.; Rossman, M.J.; Jessop, J.E.; Bledsoe, A.D.; Buys, M.J.; Supiano, M.S.; Richardson, R.S.; Amann, M. Exercise Pressor Reflex Contributes to the Cardiovascular Abnormalities Characterizing: Hypertensive Humans During Exercise. Hypertension. 2019, 74, 1468–1475. [Google Scholar] [CrossRef]
- Grotle, A.K.; Macefield, V.G.; Farquhar, W.B.; O’Leary, D.S.; Stone, A.J. Recent advances in exercise pressor reflex function in health and disease. Auton. Neurosci. 2020, 228, 102698. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.L.; Kaufman, M.P. Reflex Autonomic Responses Evoked by Group III and IV Muscle Afferents. In Translational Pain Research: From Mouse to Man; Kruger, L., Light, A.R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Boca Raton, FL, USA, 2010. Available online: https://www.ncbi.nlm.nih.gov/books/NBK57268/ (accessed on 8 October 2025).
- Grassi, G.; Bombelli, M.; Seravalle, G.; Dell’Oro, R.; Quarti-Trevano, F. Diurnal blood pressure variation and sympathetic activity. Hypertens. Res. 2010, 33, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Thosar, S.S.; Butler, M.P.; Shea, S.A. Role of the circadian system in cardiovascular disease. J. Clin. Investig. 2018, 128, 2157–2167. [Google Scholar] [CrossRef]
- Dauphinot, V.; Gosse, P.; Kossovsky, M.; Schott, A.M.; Rouch, I.; Pichot, V.; Gaspoz, J.M.; Roche, F.; Barthelemy, J.C. Autonomic nervous system activity is independently associated with the risk of shift in the non-dipper blood pressure pattern. Hypertens. Res. 2010, 33, 1032–1037. [Google Scholar] [CrossRef]
- Batool-Anwar, S.; Malhotra, A.; Forman, J.; Winkelman, J.; Li, Y.; Gao, X. Restless legs syndrome and hypertension in middle-aged women. Hypertension 2011, 58, 791–796. [Google Scholar] [CrossRef]
- Hwang, I.C.; Na, K.S.; Lee, Y.J.; Kang, S.G. Higher Prevalence of Hypertension among Individuals with Restless Legs Syndrome: A Meta-Analysis. Psychiatry Investig. 2018, 15, 701–709. [Google Scholar] [CrossRef]
- Sforza, E.; Roche, F.; Pichot, V. Determinants of Nocturnal Cardiovascular Variability and Heart Rate Arousal Response in Restless Legs Syndrome (RLS)/Periodic Limb Movements (PLMS). J. Clin. Med. 2019, 8, 1619. [Google Scholar] [CrossRef]
- Shoemaker, J.K.; Goswami, R. Forebrain neurocircuitry associated with human reflex cardiovascular control. Front. Physiol. 2015, 6, 240. [Google Scholar] [CrossRef]
- Beard, J.; Tobin, B.; Smith, S.M. Norepinephrine turnover in iron deficiency at three environmental temperatures. Am. J. Physiol. 1988, 255 Pt 2, R90–R96. [Google Scholar] [CrossRef] [PubMed]
- Swann, J.W.; Contrera, J.F. Depletion of cardiac norepinephrine during two forms of hemolytic anemia in the rat. Circ. Res. 1976, 38, 179–184. [Google Scholar] [CrossRef]
- Button, K.S.; Ioannidis, J.P.; Mokrysz, C.; Nosek, B.A.; Flint, J.; Robinson, E.S.; Munafò, M.R. Power failure: Why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013, 14, 365–376, Erratum in: Nat. Rev. Neurosci. 2013, 14, 451. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods 2007, 14, 365–376. [Google Scholar] [CrossRef]
- Aeschbach, D.; Matthews, J.R.; Postolache, T.T.; Jackson, M.A.; Giesen, H.A.; Wehr, T.A. Two circadian rhythms in the human electroencephalogram during wakefulness. Am. J. Physiol. 1999, 277, R1771–R1779. [Google Scholar]
- Gundel, A.; Hilbig, A. Circadian acrophases of powers and frequencies in the waking EEG. Int. J. Neurosci. 1983, 22, 125–133. [Google Scholar] [CrossRef]
- Bae, H.; Cho, Y.W.; Kim, K.T.; Allen, R.P.; Earley, C.J. Randomized, placebo-controlled trial of ferric carboxymaltose in restless legs syndrome patients with iron deficiency anemia. Sleep Med. 2021, 84, 179–186. [Google Scholar] [CrossRef]
- Lee, D.O.; Ziman, R.B.; Perkins, A.T.; Poceta, J.S.; Walters, A.S.; Barrett, R.W. XP053 Study Group. A randomized, double-blind, placebo-controlled study to assess the efficacy and tolerability of gabapentin enacarbil in subjects with restless legs syndrome. J. Clin. Sleep Med. 2011, 7, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Dauvilliers, Y.; Chenini, S.; Barateau, L.; Somers, V.K. Restless Legs Syndrome, Periodic Leg Movements, Hypertension and Cardiovascular Diseases. Circ. Res. 2025, 137, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Siersbaek-Nielsen, K.; Hansen, J.M.; Schioler, M.; Kristensen, M.; Stoier, M.; Olsen, P.Z. Electroencephalographic changes during and after treatment of hyperthyroidism. Acta Endocrinol. 1972, 70, 308–314. [Google Scholar] [CrossRef]
- Li, J.; Li, F. Hashimoto’s Encephalopathy and Seizure Disorders. Front. Neurol. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Khedr, E.M.; El Toony, L.F.; Tarkhan, M.N.; Abdella, G. Peripheral and central nervous system alterations in hypothyroidism: Electrophysiological findings. Neuropsychobiology 2000, 41, 88–94. [Google Scholar] [CrossRef]
- Jakhar, D.; Sarin, S.K.; Kaur, S. Gut microbiota and dynamics of ammonia metabolism in liver disease. npj Gut Liver. 2024, 1, 11. [Google Scholar] [CrossRef]
- Morris, H.; Kaplan, P.W.; Kane, N. Electroencephalography in encephalopathy and encephalitis. Pract. Neurol. 2024, 24, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.L.; Li, Y.Q.; Huang, K.M.; Kuang, R.G.; Lv, G.P.; Lu, X.F.; Li, J.M.; Desmond, P.V. Alterations in cerebral potentials evoked by rectal distention and drinking ice water in patients with irritable bowel syndrome. J. Gastroenterol. Hepatol. 2006, 21, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Blomhoff, S.; Jacobsen, M.B.; Spetalen, S.; Dahm, A.; Malt, U.F. Perceptual hyperreactivity to auditory stimuli in patients with irritable bowel syndrome. Scand. J. Gastroenterol. 2000, 35, 583–589. [Google Scholar] [CrossRef]
- Berman, S.M.; Naliboff, B.D.; Chang, L.; Fitzgerald, L.; Antolin, T.; Camplone, A.; Mayer, E.A. Enhanced preattentive central nervous system reactivity in irritable bowel syndrome. Am. J. Gastroenterol. 2002, 97, 2791–2797. [Google Scholar] [CrossRef]
- Billeci, L.; Callara, A.L.; Guiducci, L.; Prosperi, M.; Morales, M.A.; Calderoni, S.; Muratori, F.; Santocchi, E. A randomized controlled trial into the effects of probiotics on electroencephalography in preschoolers with autism. Autism 2023, 27, 117–132. [Google Scholar] [CrossRef]
| Correlations/Clinical Links | EEG/ERP Findings | Behavioral | Paradigm/Measure | Sample | Focus/Aim | Study |
|---|---|---|---|---|---|---|
| Parietal P300 ↓ correlated with longer illness duration; no links with PSQI/ISI/BDI | Parietal P300 amplitude ↓ in RLS across loads; load ↑ → P300 ↓, RT ↑; no group × load interaction | RLS slower RT; similar hit rate | Sternberg WM (2–4 digits); ERPs (P300) at F/C/P | 13 drug-naïve severe RLS (52 y); 13 HC (52 y) | RLS vs. HC working memory (WM) via P300 | [51] |
| P300 latency ↔ bothersomeness (VAS); no correlation with IRLS | Resting beta (26–30 Hz) ↑ frontal/central; P300 latency ↑ and amplitude ↓ esp. frontal/central (300–350 ms) | Accuracy comparable; RLS slower RT; ↑ bothersomeness (VAS); SSS similar | Resting EEG (27 ch); visual oddball; P300 | 17 female drug-naïve RLS (53.7 y); 13 female HC (54.6 y) | Daytime EEG/ERP abnormalities and P300 in RLS | [52] |
| IRLS 30.1→14.0; PSQI/ISI/BDI improved; sleep duration ↑ (ns); ESS ↔ | Parietal P300 amplitude ↑ across loads; frontal P300 changes track clinical improvement | RT ↓ across loads; hit rate ↑ (88→91%); omission errors ↓; commission ↔ | Modified Sternberg WM; ERPs (P300 at F/C/P) | 13 drug-naïve RLS, pre/post 12–16 wks pramipexole | Effect of pramipexole on WM and P300 | [53] |
| Supports dopaminergic frontal dysfunction | P2 amplitude ↓ frontally (Fz); source ↓ in ACC/mPFC; P3 amplitude ↓ and latency ↑; sources ↓ in ACC and precuneus; GFP ↓ | Accuracy intact; faster RT in RLS (possible compensatory strategy) | Visual oddball; ERPs (P2 150–250 ms; P3 300–450 ms); LORETA | 17 female RLS (53.7 y); 13 female HC (54.6 y) | Frontal involvement (P2/P3) with source localization | [54] |
| SWP negatively correlated with IRLS (r = −0.65) | Frontal TBA ↑ delayed and smaller; interregional TBPS ↓ (frontal-centered); graph: ↓ clustering, ↑ path length, ↓ small-world propensity | RLS slower RT; accuracy similar | Sternberg WM; EEG theta-band activity (4–6 Hz) and phase synchrony (wPLI) | 12 female RLS (53.4 y); 12 female HC (49.3 y) | Frontal theta dynamics and WM retrieval | [55] |
| Effects independent of sleepiness; path length modestly ↔ ferritin | P300 delayed and reduced; induced gamma ↓ and delayed (frontal/central); GBPS ↓ esp. 300–500 ms; longer path length, lower clustering | RLS slower RT; ↑ bothersomeness; accuracy high | Visual oddball; ERPs (P300); induced/evoked gamma; GBPS; graph metrics | 17 female RLS; 13 female HC | Gamma synchrony and network metrics during oddball | [56] |
| Relevance scores correlate with PSQI, ISI, IRLS | CNN accuracy ≈ 94% (AUC 0.93); relevance in L sup. frontal/parietal, temporal, insular, occipital regions | RLS slower RT; hit rate similar | Modified Sternberg WM; sLORETA features → CNN (LOOCV) | 13 RLS; 13 HC → final 9 RLS after exclusions | Explainable deep learning on single-trial ERPs in WM | [57] |
| RLS severity negatively ↔ connectivity in sensory cortices | ↑ connectivity in L primary somatosensory (BA1L); ↓ in R anterior PFC (BA10R) and R V1 (BA17R) vs. PI | No behavioral measures (resting) | Resting eyes-closed; ciPLV across bands; source-space | 107 RLS (46.2 y); 17 PI (48.4 y) | QEEG connectivity: RLS vs. Primary Insomnia | [58] |
| Not explained by severity, sleep quality, fatigue | N1 amplitude ↓ in RLS in evening (incompatible trials); source BA18; N2/P3 ↔ | RLS: larger PM interference (performance decline); HC stable | Flanker task AM (8–9) vs. PM (17–18); ERPs; sLORETA | 33 RLS (65.2 y); 29 HC (64.4 y) | Circadian effects on attention (flanker) in RLS | [59] |
| Profiles resemble depression; PSG: not in this study | ABS delta and alpha-2 ↑; alpha-1 ↓ (parietal); REL alpha-2 ↑ (occipital/left OT/right frontal); REL beta-3 ↑ (R parietal); ABS beta-5 ↓ (R frontal); centroid shifts → ‘dissociated vigilance’ | Higher depression/anxiety; poorer sleep; QoL ↓; ESS ↔ | Vigilance-controlled EEG (21 ch, 3 min); spectral metrics | 33 RLS (59.0 y); 33 HC (57.0 y) | Daytime EEG mapping and psychometrics in RLS | [60] |
| Both: ↑ PLM indices/arousals; RLS QoL ↓; PLMD ↑ sleepiness | RLS EEG: delta ↑, fast alpha ↑, slow alpha ↓; alpha centroid ↑; dom. freq ↑ but power ↓; total power ↔ | RLS: worse sleep/awakening quality; AM fine motor and RT impaired; PLMD: deficits in memory/attention/motor | EEG mapping; 2-night PSG (subset); morning tests | 33 RLS; 26 PLMD; 33 HC | Day/night EEG, PSG, and performance: RLS and PLMD | [61] |
| Suggests common cortical and sympathetic activation mechanisms | EEG power ↑ across all bands ~10 s pre-movement and post; HR ↑ from ~15 s pre-onset; strongest HR after SILM | (resting with movements) | 1-h SIT; EEG spectral (δ/θ/α/β) and heart rate around PLM/ILM/SILM | 53 drug-free RLS (51.9 y) | EEG/HR around leg movements during SIT | [62] |
| Interpreted as stronger post-movement inhibition/feedback | ERD during movement ↔; post-movement ERS markedly ↑ in RLS (C3 lower/upper beta; Cz upper beta) | (simple motor) | Right-hand button press to clicks; EEG beta ERD/ERS at C3/Cz | 10 primary RLS (45.5 y); 10 HC (46.3 y) | Motor cortical ERD/ERS during simple movement | [63] |
| Insomnia strongly associated with beta EEG patterns | Beta activity prevalent in RLS (56.5% vs. 20% HC); mild paroxysmal slowing in 34.8% RLS | High insomnia prevalence; many on pramipexole; daytime sleepiness in 60.9% | Awake resting EEG (18 ch); activation procedures | 23 RLS (52.3 y); 20 HC (47.1 y) | Awake morning EEG patterns in treated RLS | [64] |
| Cortical initiation for PLMW; feedback processing after PLMS | PLMS: no pre-movement ERD (subcortical/spinal) + strong PMBS; PLMW: clear pre-movement mu/beta ERD (cortical involvement) | Evening voluntary movement: RLS ERD ↑ & PMBS longer; HC ERD ↓ evening | EEG (38 ch) + EMG; PSG and SIT; voluntary ankle dorsiflexion AM vs. PM | 12 idiopathic RLS (49.7 y); 10 HC (52.7 y) | Cortical role in PLMS/PLMW and voluntary movement | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chmiel, J.; Kurpas, D. Neural Correlates of Restless Legs Syndrome (RLS) Based on Electroencephalogram (EEG)—A Mechanistic Review. Int. J. Mol. Sci. 2025, 26, 10675. https://doi.org/10.3390/ijms262110675
Chmiel J, Kurpas D. Neural Correlates of Restless Legs Syndrome (RLS) Based on Electroencephalogram (EEG)—A Mechanistic Review. International Journal of Molecular Sciences. 2025; 26(21):10675. https://doi.org/10.3390/ijms262110675
Chicago/Turabian StyleChmiel, James, and Donata Kurpas. 2025. "Neural Correlates of Restless Legs Syndrome (RLS) Based on Electroencephalogram (EEG)—A Mechanistic Review" International Journal of Molecular Sciences 26, no. 21: 10675. https://doi.org/10.3390/ijms262110675
APA StyleChmiel, J., & Kurpas, D. (2025). Neural Correlates of Restless Legs Syndrome (RLS) Based on Electroencephalogram (EEG)—A Mechanistic Review. International Journal of Molecular Sciences, 26(21), 10675. https://doi.org/10.3390/ijms262110675






