Is PTEN rs397510595 an Unexpected Guardian in Canine Mammary Neoplasia?
Abstract
1. Introduction
2. Results
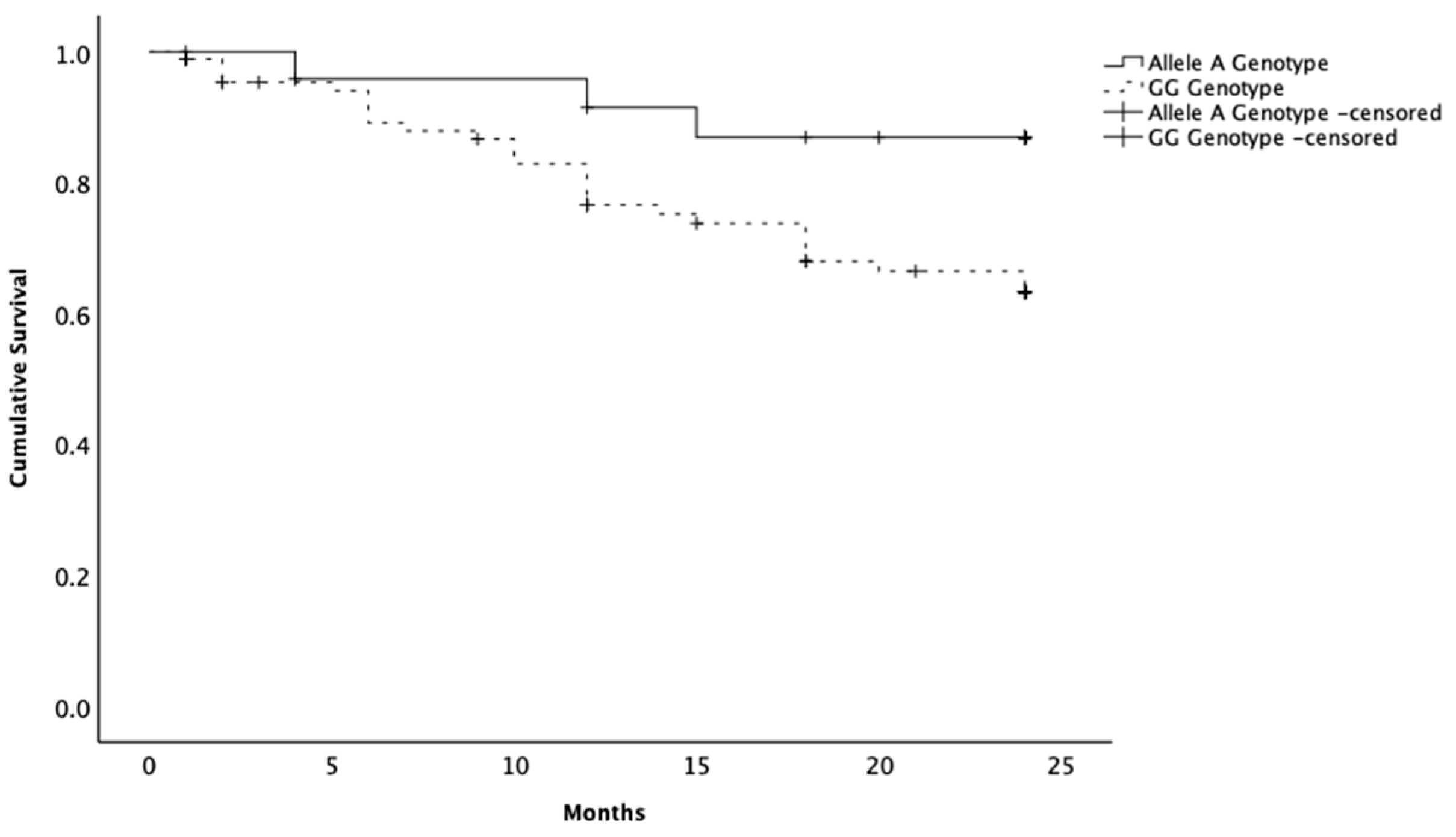
2.1. SNP rs397510595
2.2. SNP rs397513087
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Histopathology
4.3. Genotyping
4.4. Inclusion and Exclusion Criteria
4.5. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- Oliveira-Lopes, A.F.; Götze, M.M.; Lopes-Neto, B.E.; Guerreiro, D.D.; Bustamante-Filho, I.C.; Moura, A.A. Molecular and Pathobiology of Canine Mammary Tumour: Defining a Translational Model for Human Breast Cancer. Veter-Comp. Oncol. 2024, 22, 340–358. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Raposo, T.; Carvalho, M.I.; Prada, J.; Pires, I. Canine mammary tumours as a model to study human breast cancer: Most recent findings. Vivo 2011, 25, 455–465. [Google Scholar]
- Rivera, P.; von Euler, H. Molecular biological aspects on canine and human mammary tumors. Veter-Pathol. 2011, 48, 132–146. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002–2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef]
- Moe, L. Population-based incidence of mammary tumours in some dog breeds. J. Reprod. Fertil. Suppl. 2001, 57, 439–443. [Google Scholar]
- Schneider, R.; Dorn, C.R.; Taylor, D.O.N. Factors Influencing Canine Mammary Cancer Development and Postsurgical Survival2. JNCI J. Natl. Cancer Inst. 1969, 43, 1249–1261. [Google Scholar] [CrossRef]
- Borge, K.S.; Melin, M.; Rivera, P.; Thoresen, S.I.; Webster, M.T.; von Euler, H.; Lindblad-Toh, K.; Lingaas, F. The ESR1 gene is associated with risk for canine mammary tumours. BMC Veter-Res. 2013, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Canadas-Sousa, A.; Santos, M.; Dias-Pereira, P. Protective Effect of HER2 Gene Polymorphism rs24537331 in the Outcome of Canine Mammary Tumors. Animals 2023, 13, 1384. [Google Scholar] [CrossRef]
- Hunter, D.J. Gene–environment interactions in human diseases. Nat. Rev. Genet. 2005, 6, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Nowak, B.; Dzimira, S.; Liszka, B.; Zatoń-Dobrowolska, M. Identification of SNP markers for canine mammary gland tumours in females based on a genome-wide association study—Preliminary results. J. Veter-Res. 2023, 67, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Zhou, H.; Fan, H.; Yuan, Y. Single nucleotide polymorphisms and cancer susceptibility. Oncotarget 2017, 8, 110635–110649. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, H.C.; Chanock, S.J. SNPs in cancer research and treatment. Br. J. Cancer 2004, 90, 747–751. [Google Scholar] [CrossRef]
- Dahia, P.L. PTEN, a unique tumor suppressor gene. Endocr.-Relat. Cancer 2000, 7, 115–129. [Google Scholar] [CrossRef]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997, 275, 1943–1947. [Google Scholar] [CrossRef]
- Ma, F.; Laster, K.; Dong, Z. The comparison of cancer gene mutation frequencies in Chinese and U.S. patient populations. Nat. Commun. 2022, 13, 5651. [Google Scholar] [CrossRef]
- Serebriiskii, I.G.; Pavlov, V.; Tricarico, R.; Andrianov, G.; Nicolas, E.; Parker, M.I.; Newberg, J.; Frampton, G.; Meyer, J.E.; Golemis, E.A. Comprehensive characterization of PTEN mutational profile in a series of 34,129 colorectal cancers. Nat. Commun. 2022, 13, 1618. [Google Scholar] [CrossRef]
- Steck, P.A.; Pershouse, M.A.; Jasser, S.A.; Yung, W.A.; Lin, H.; Ligon, A.H.; Langford, L.A.; Baumgard, M.L.; Hattier, T.; Davis, T.; et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 1997, 15, 356–362. [Google Scholar] [CrossRef]
- Canadas, A.; Santos, M.; Nogueira, A.; Assis, J.; Gomes, M.; Lemos, C.; Medeiros, R.; Dias-Pereira, P. Canine mammary tumor risk is associated with polymorphisms in RAD51 and STK11 genes. J. Veter-Diagn. Investig. 2018, 30, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Enginler, S.O.; Akış, I.; Toydemir, T.S.F.; Oztabak, K.; Haktanir, D.; Gündüz, M.C.; Kırşan, I.; Fırat, I. Genetic variations of BRCA1 and BRCA2 genes in dogs with mammary tumours. Veter-Res. Commun. 2014, 38, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-L.; Huang, Y.-H.; Chang, T.-J.; Wong, M.-L.; Chang, S.-C. Single nucleotide variation in exon 11 of canine BRCA2 in healthy and cancerous mammary tissue. Veter-J. 2010, 184, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Melin, M.; Biagi, T.; Fall, T.; Häggström, J.; Lindblad-Toh, K.; von Euler, H. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009, 69, 8770–8774. [Google Scholar] [CrossRef] [PubMed]
- Thumser-Henner, P.; Nytko, K.J.; Bley, C.R. Mutations of BRCA2 in canine mammary tumors and their targeting potential in clinical therapy. BMC Veter-Res. 2020, 16, 30. [Google Scholar] [CrossRef]
- Cantley, L.C.; Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 4240–4245. [Google Scholar] [CrossRef]
- Maehama, T.; Dixon, J.E. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 1998, 273, 13375–13378. [Google Scholar] [CrossRef]
- Zhang, H.-P.; Jiang, R.-Y.; Zhu, J.-Y.; Sun, K.-N.; Huang, Y.; Zhou, H.-H.; Zheng, Y.-B.; Wang, X.-J. PI3K/AKT/mTOR signaling pathway: An important driver and therapeutic target in triple-negative breast cancer. Breast Cancer 2024, 31, 539–551. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Lebok, P.; Kopperschmidt, V.; Kluth, M.; Hube-Magg, C.; Özden, C.; Taskin, B.; Hussein, K.; Mittenzwei, A.; Lebeau, A.; Witzel, I.; et al. Partial PTEN deletion is linked to poor prognosis in breast cancer. BMC Cancer 2015, 15, 963. [Google Scholar] [CrossRef]
- Li, S.; Shen, Y.; Wang, M.; Yang, J.; Lv, M.; Li, P.; Chen, Z.; Yang, J. Loss of PTEN expression in breast cancer: Association with clinicopathological characteristics and prognosis. Oncotarget 2017, 8, 32043–32054. [Google Scholar] [CrossRef]
- Shoman, N.; Klassen, S.; McFadden, A.; Bickis, M.G.; Torlakovic, E.; Chibbar, R. Reduced PTEN expression predicts relapse in patients with breast carcinoma treated by tamoxifen. Mod. Pathol. 2005, 18, 250–259. [Google Scholar] [CrossRef]
- Koenig, A.; Bianco, S.R.; Fosmire, S.; Wojcieszyn, J.; Modiano, J.F. Expression and Significance of p53, Rb, p21/waf-1, p16/ink-4a, and PTEN Tumor Suppressors in Canine Melanoma. Veter-Pathol. 2002, 39, 458–472. [Google Scholar] [CrossRef]
- Molín, J.; José-López, R.; Ramírez, G.A.; Pumarola, M. Immunohistochemical Expression of PTEN in Canine Gliomas. Animals 2024, 14, 2115. [Google Scholar] [CrossRef]
- Asproni, P.; Ressel, L.; Millanta, F.; Vannozzi, I.; Poli, A. Co-localization of PTEN and E-cadherin in canine mammary hyperplasias and benign and malignant mammary tumors. Res. Veter-Sci. 2015, 103, 113–118. [Google Scholar] [CrossRef]
- Qiu, C.; Lin, D.; Wang, J.; Wang, L. Expression and significance of PTEN in canine mammary gland tumours. Res. Veter-Sci. 2008, 85, 383–388. [Google Scholar] [CrossRef]
- Ressel, L.; Millanta, F.; Caleri, E.; Innocenti, V.M.; Poli, A. Reduced PTEN protein expression and its prognostic implications in canine and feline mammary tumors. Veter-Pathol. 2009, 46, 860–868. [Google Scholar] [CrossRef]
- Diessler, M.E.; Castellano, M.C.; Portiansky, E.L.; Burns, S.; Idiart, J.R. Canine mammary carcinomas: Influence of histological grade, vascular invasion, proliferation, microvessel density andVEGFR2expression on lymph node status and survival time. Veter-Comp. Oncol. 2017, 15, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Pastor, N.; Ezquerra, L.J.; Santella, M.; Caballé, N.C.; Tarazona, R.; Durán, M.E. Prognostic significance of immunohistochemical markers and histological classification in malignant canine mammary tumours. Veter-Comp. Oncol. 2020, 18, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Li, X.; Qin, X.; Yu, C.; Jin, Y.; Qian, X. PTEN inhibitor improves vascular remodeling and cardiac function after myocardial infarction through PI3k/Akt/VEGF signaling pathway. Mol. Med. 2020, 26, 111. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Stolarov, J.; Myers, M.P.; Su, J.D.; Wigler, M.H.; Tonks, N.K.; Durden, D.L. PTEN controls tumor-induced angiogenesis. Proc. Natl. Acad. Sci. USA 2001, 98, 4622–4627. [Google Scholar] [CrossRef]
- Jiang, B.-H.; Liu, L.-Z. PI3K/PTEN Signaling in Angiogenesis and Tumorigenesis. Adv. Cancer Res. 2009, 102, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, S.O.; Cupitra, N.I.; Narvaez-Sanchez, R. Vascular adaptation to cancer beyond angiogenesis: The role of PTEN. Microvasc. Res. 2023, 147, 104492. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Kontos, C.D. PTEN Modulates Vascular Endothelial Growth Factor-Mediated Signaling and Angiogenic Effects. J. Biol. Chem. 2002, 277, 10760–10766. [Google Scholar] [CrossRef]
- Ma, J.; Sawai, H.; Ochi, N.; Matsuo, Y.; Xu, D.; Yasuda, A.; Takahashi, H.; Wakasugi, T.; Takeyama, H. PTEN regulate angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol. Cell. Biochem. 2009, 331, 161–171. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-D.; Zhang, Q.; Li, J.-H.; Hao, R.-M.; Ma, Y.; Wang, P.-Y.; Xie, S.-Y. Single nucleotide polymorphisms rs701848 and rs2735343 in PTEN increases cancer risks in an Asian population. Oncotarget 2017, 8, 96290–96300. [Google Scholar] [CrossRef]
- Zappulli, V.; Peña, L.; Rasotto, R.; Goldschmidt, M.H.; Gama, A.; Scruggs, J.L.; Kiupel, M. Surgical Pathology of Tumors of Domestic Animals Volume 2: Mammary Tumors; Kiupel, M., Ed.; Davis-Thompson DVM Foundation: Gurnee, IL, USA, 2019. [Google Scholar]
- Canadas, A.; França, M.; Pereira, C.; Vilaça, R.; Vilhena, H.; Tinoco, F.; Silva, M.J.; Ribeiro, J.; Medeiros, R.; Oliveira, P.; et al. Canine Mammary Tumors: Comparison of Classification and Grading Methods in a Survival Study. Veter-Pathol. 2019, 56, 208–219. [Google Scholar] [CrossRef]
- Haybittle, J.L.; Blamey, R.W.; Elston, C.W.; Johnson, J.; Doyle, P.J.; Campbell, F.C.; I Nicholson, R.; Griffiths, K. A prognostic index in primary breast cancer. Br. J. Cancer 1982, 45, 361–366. [Google Scholar] [CrossRef]
- Santos, M.; Gomes, C.; Marcos, R.; Santos, A.; De Matos, A.; Lopes, C.; Dias-Pereira, P. Value of the Nottingham Histological Grading Parameters and Nottingham Prognostic Index in Canine Mammary Carcinoma. Anticancer Res. 2015, 35, 4219–4227. [Google Scholar]
- Canadas-Sousa, A.; Santos, M.; Leal, B.; Medeiros, R.; Dias-Pereira, P. Estrogen receptors genotypes and canine mammary neoplasia. BMC Vet. Res. 2019, 15, 325. [Google Scholar] [CrossRef]
| Genotype | Frequency | Percentage |
|---|---|---|
| AA | 4 | 1.9 |
| GA | 32 | 15.5 |
| GG | 170 | 82.5 |
| Total | 206 | 100.0 |
| Genotype (n = Number of Cases) | |||
|---|---|---|---|
| Independent Variables | A Allele Carriers (n/%) | GG (n/%) | p |
| Age | |||
| <11 | 20 (14.0) | 123 (86.0) | |
| ≥11 | 16 (28.1) | 41 (71.9) | 0.019 |
| Number of neoplasia | |||
| Single | 12 (16.9) | 59 (83.1) | |
| Multiple | 24 (17.8) | 111 (82.2) | NS |
| Neoplasia size | |||
| ≤3 cm | 20 (17.5) | 94 (82.5) | |
| >3 cm | 14 (17.7) | 65 (82.3) | NS |
| Neoplasia size (only malignant) | |||
| ≤3 cm | 13 (20.3) | 51 (79.7) | |
| >3 cm | 13 (20.0) | 52 (80.0) | NS |
| Biological behavior | |||
| Benign | 8 (11.0) | 65 (89.0) | |
| Malignant | 28 (21.1) | 105 (78.9) | 0.068 |
| Mode of growth pattern | |||
| Expansive | 10 (19.6) | 41 (80.4) | |
| Infiltrative | 13 (27.7) | 34 (72.3) | |
| Invasive | 5 (14.3) | 30 (85.7) | NS |
| HG parameters scores | |||
| Tubule formation | |||
| Score 1 | 4 (16.0) | 21 (84.0) | |
| Score 2 | 13 (28.3) | 33 (71.7) | |
| Score 3 | 7 (20.0) | 28 (80.0) | NS |
| Nuclear pleomorphism | |||
| Score 1 | 1 (10.0) | 9 (90.0) | |
| Score 2 | 18 (26.5) | 50 (73.5) | |
| Score 3 | 5 (17.9) | 23 (82.1) | NS |
| Mitotic index | |||
| Score 1 | 9 (20.5) | 35 (79.5) | |
| Score 2 | 5 (19.2) | 21 (80.8) | |
| Score 3 | 10 (27.8) | 26 (72.2) | NS |
| HG | |||
| Grade I | 9 (24.3) | 28 (75.7) | |
| Grade II | 9 (19.6) | 37 (80.4) | |
| Grade III | 6 (26.1) | 17 (73.9) | NS |
| Vet-CPI | |||
| ≤4.0 | 15 (24.6) | 46 (75.4) | |
| >4.0 | 9 (20.0) | 36 (80.0) | NS |
| Vascular invasion | |||
| No | 27 (25.0) | 81 (75.0) | |
| Yes | 0 (0.0) | 22 (100.0) | 0.007 |
| Lymph node metastases | |||
| No | 19 (22.4) | 66 (77.6) | |
| Yes | 5 (16.1) | 26 (83.9) | NS |
| Genotype | Frequency | Percentage |
|---|---|---|
| CC | 186 | 90.7 |
| CT | 18 | 8.8 |
| TT | 1 | 0.5 |
| Total | 205 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canadas-Sousa, A.; Santos, M.; Dias-Pereira, P. Is PTEN rs397510595 an Unexpected Guardian in Canine Mammary Neoplasia? Int. J. Mol. Sci. 2025, 26, 10654. https://doi.org/10.3390/ijms262110654
Canadas-Sousa A, Santos M, Dias-Pereira P. Is PTEN rs397510595 an Unexpected Guardian in Canine Mammary Neoplasia? International Journal of Molecular Sciences. 2025; 26(21):10654. https://doi.org/10.3390/ijms262110654
Chicago/Turabian StyleCanadas-Sousa, Ana, Marta Santos, and Patrícia Dias-Pereira. 2025. "Is PTEN rs397510595 an Unexpected Guardian in Canine Mammary Neoplasia?" International Journal of Molecular Sciences 26, no. 21: 10654. https://doi.org/10.3390/ijms262110654
APA StyleCanadas-Sousa, A., Santos, M., & Dias-Pereira, P. (2025). Is PTEN rs397510595 an Unexpected Guardian in Canine Mammary Neoplasia? International Journal of Molecular Sciences, 26(21), 10654. https://doi.org/10.3390/ijms262110654






