The Role of Omics Technology in Evaluating Plastic Pollution’s Effects on Plants: A Comprehensive Review
Abstract
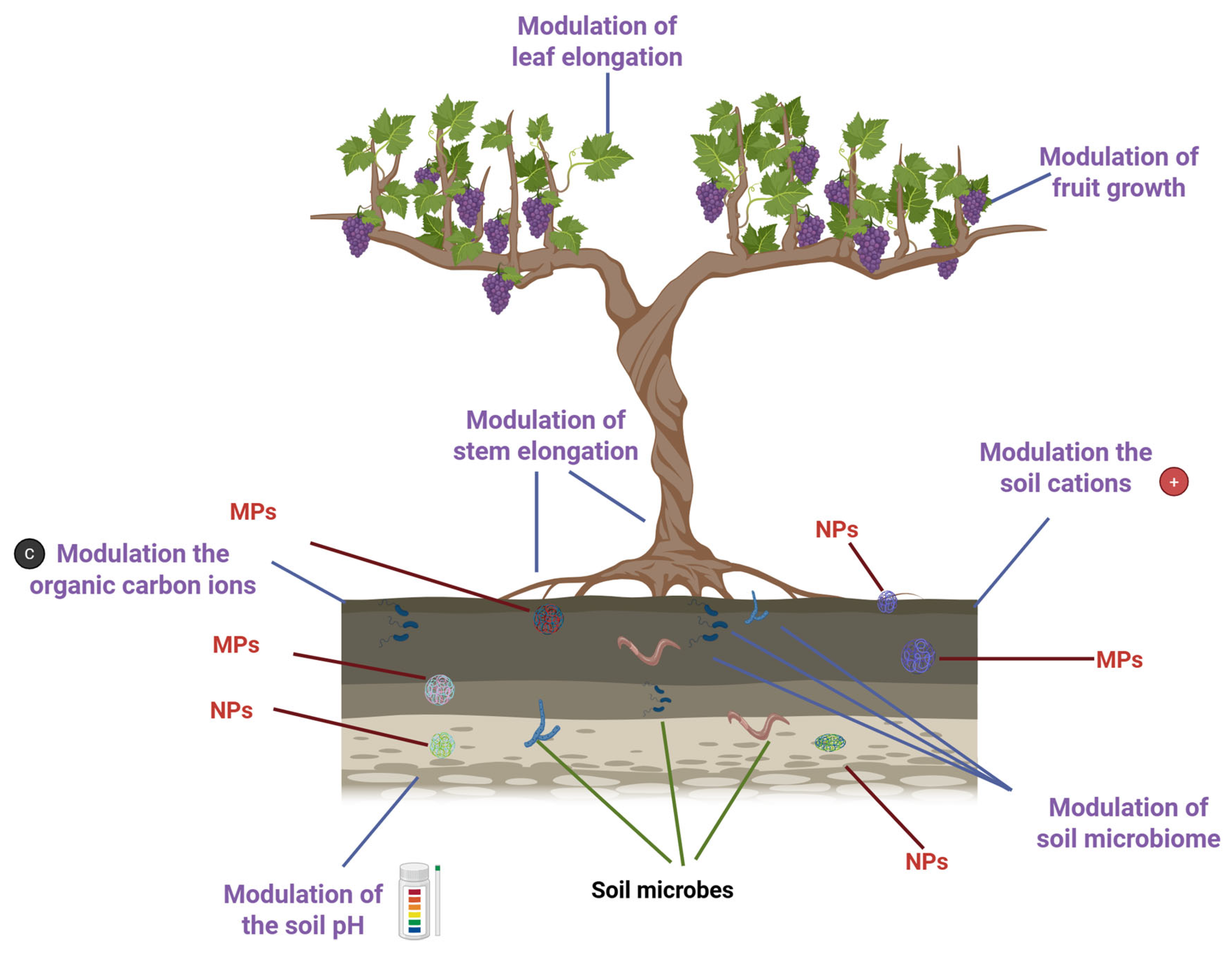
1. Introduction
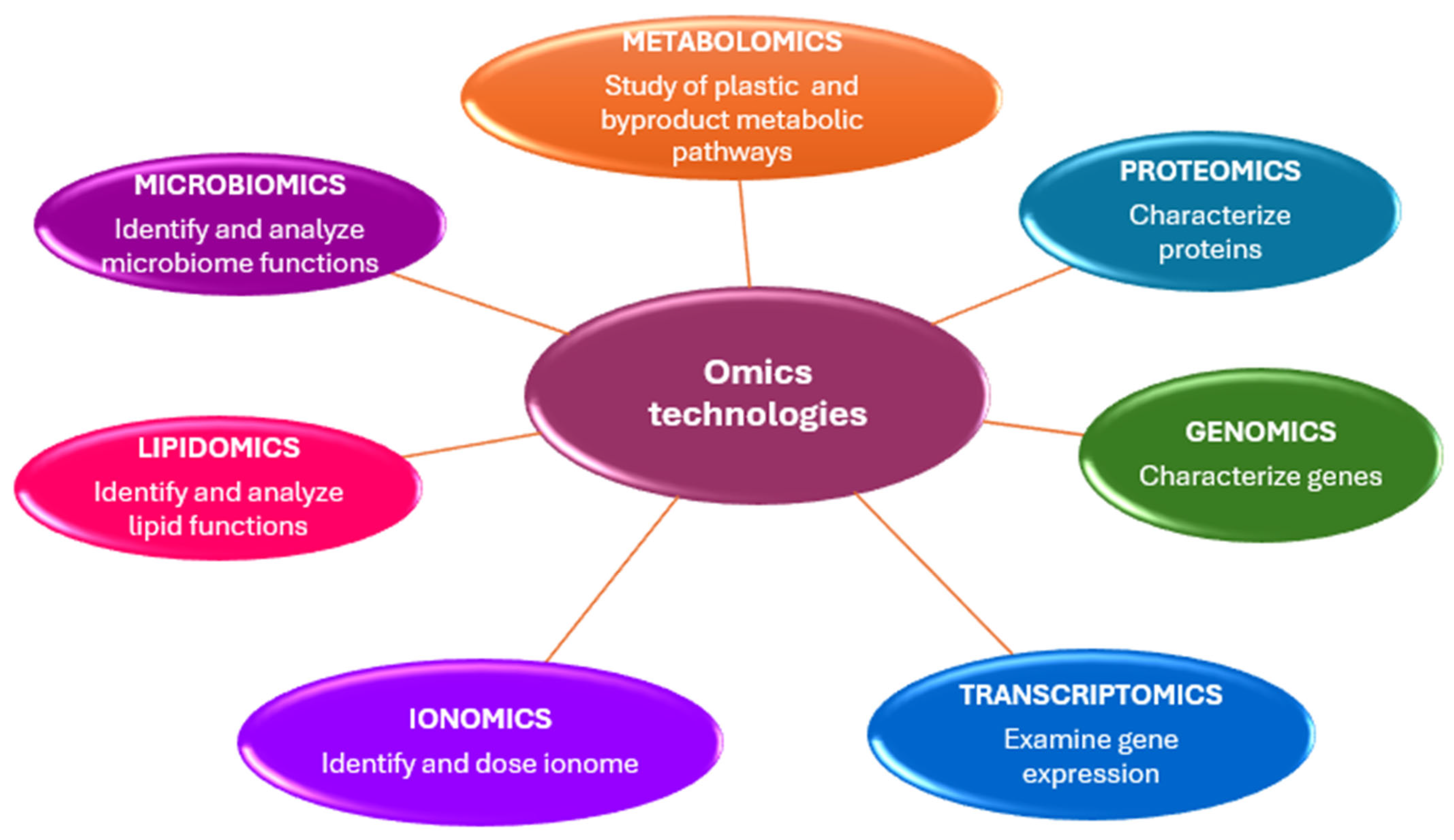
2. Omics Technologies
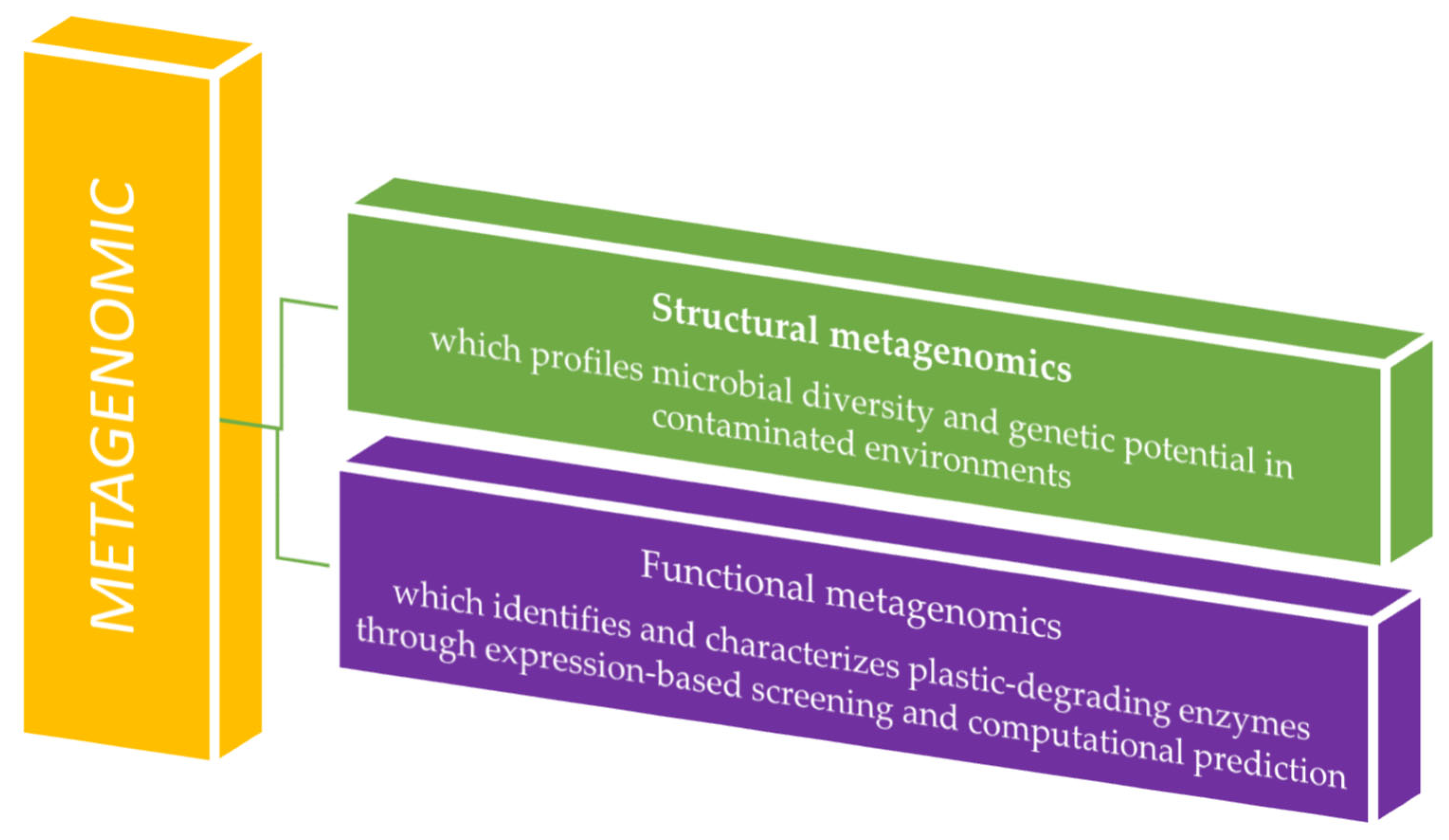
2.1. Metagenomics
2.1.1. The Technique in Metagenomic Analyses
2.1.2. Metagenomic Studies on Soil Microbial Responses to Plastic Derivatives
2.2. Epigenomics
2.3. Transcriptomics
2.3.1. The Technique in Transcriptomic Analyses
2.3.2. Transcriptomic Studies on Plant Responses to Plastic Derivatives
2.4. Metabolomics
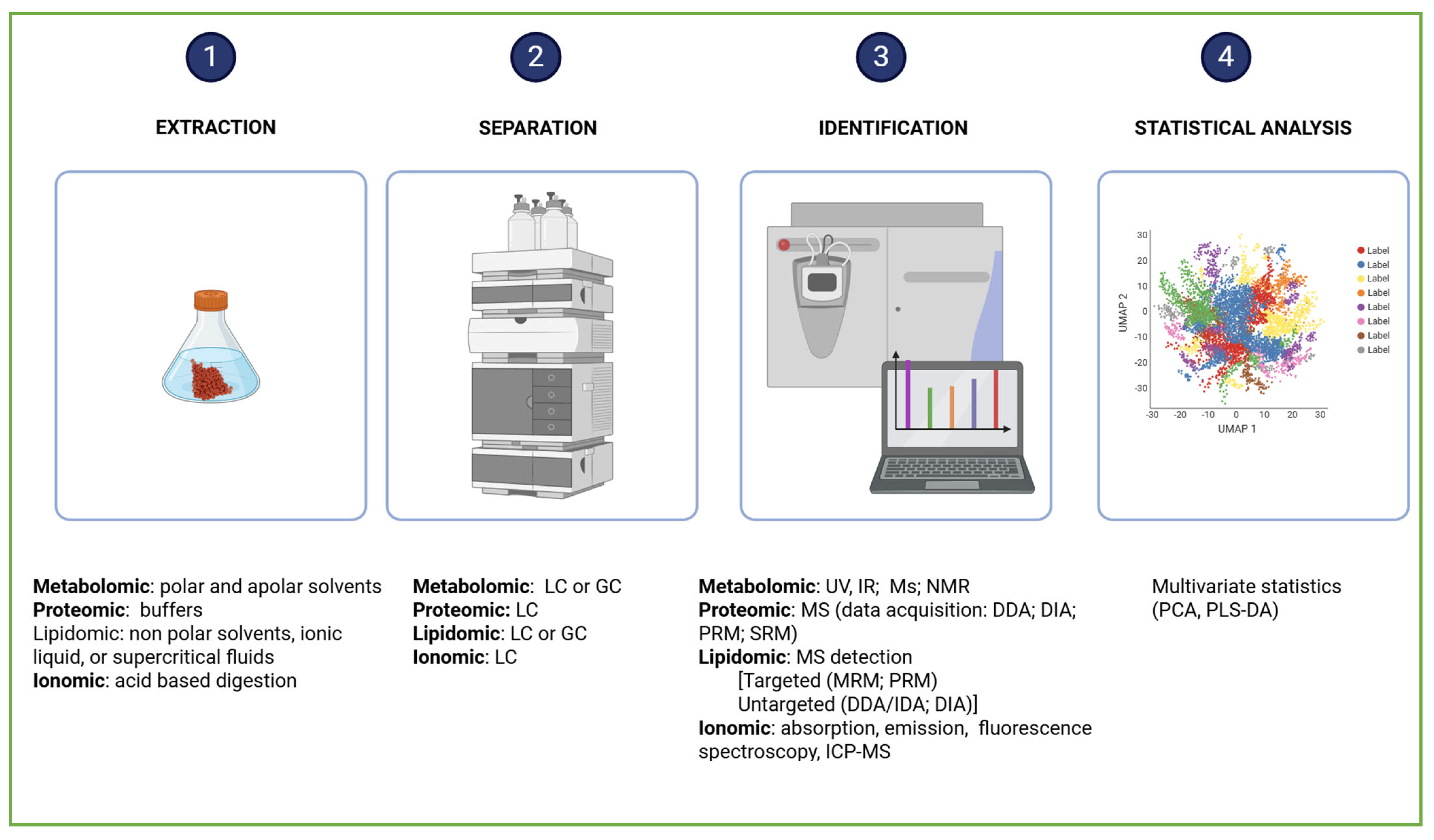
2.4.1. Techniques Used in Metabolomic Analyses
2.4.2. Plastic Derivatives’ Impact on the Modulation of Amino Acids
2.4.3. Plastic Derivatives’ Impact on the Saccharide Modulation
2.4.4. Plastic Derivatives’ Impact on the Organic Acid Modulation
2.4.5. Plastic Derivatives’ Impact on the Secondary Metabolites and Hormone Modulation
2.4.6. Plastic Derivatives’ Impact on Heavy Metal Tolerance in Plants
| Plant | Plastic Derivatives | Plastic Derivatives Levels | Effect | Reference |
|---|---|---|---|---|
| Capsicum annuum | polyethylene terephthalate (150 µm) | 20 mg · kg−1 and 200 mg · kg−1 | down-regulation levels of amino acids | [90] |
| Zea mays | polystyrene (100 nm) and polypropylene (10 µm) | 2% of soil mass | up-regulation of amino acids | [91] |
| Solidago canadensis | mixture of polyethylene pellets, fragments, and fibers (0.60 mm–1.00 mm) | 0.5% of the soil weight | down-regulation of carbohydrates | [92] |
| Cucumber | polystyrene (50–100 nm) | 50 and 100 mg/L | down-regulation of carbohydrates | [93] |
| Stevia rebaudiana | polystyrene (20 nm) | 10 mg/L | up-regulation of steviol glycosides | [94] |
| Triticum aestivum | polystyrene (120–254.4 nm) | 0.1 mg/L | Upregulation of threonic acid, boric acid, butanedioic acid, glycolic acid, aconitic acid, malic acid | [97] |
| Spinacia oleracea | CeO2 NPs (194.8–215.3 nm) | 0.3, and 3 mg/plant. | downregulation of 3-hydroxybenzoic acid, 4-hydroxybenzoic acid, and nicotinic acid | [98] |
| Cucumis sativus | nano-Cu (2590 nm) | 10 and 20 mg/L | downregulation of citric acid | [99] |
| Oryza sativa | polybrominated diphenyl ethers | 500 μg/L | down-regulation of amino acids | [89] |
| Oryza sativa | Di-(2-ethylhexyl) phthalate | 80, 160, and 320 ng/mL | downregulation of picolinic acid | [100] |
| Oryza sativa | polystyrene (<50 µm) | 50 mg L−1 and 250 mg L−1 | downregulation of organic acid | [101] |
| Oryza sativa | polystyrene (~20 nm) | 10, 50, and 100 mg L−1 | downregulation of jasmonate and lignin | [108] |
| Lactuca sativa | polystyrene (0.2 µm) | 10, 20, 30, 40, and 50 mg L−1 | upregulation of flavonoids, ascorbic acid, and terpenoids | [103] |
| Broccoli | low-density polyethylene (<2000 μm) | 0.01 mg L−1–10,000 mg L−1 | downregulation of the glucosinolates; upregulation of anthocyanins | [104] |
| Barley | polystyrene (<5 mm) | 2 g mL−1 | downregulation of auxins | [105] |
| Barley and cucumber | polystyrene (790 nm–4999 nm) | 0, 100, and 1000 mg L−1 | up-regulation of hormones in barley and down-regulation in cucumber | [106] |
| Barley | polystyrene (<5 mm) | 2 g mL−1 | Downregulation of indole-3-acetic acid, indole-3-butyric acid and dihydrozeatin | [110] |
| Glycine max | polystyrene (~73 nm) | 75 mg L−1 | downregulation of salicylic acid 2-O-β-glucoside and l-tryptophan | [109] |
2.5. Proteomics
2.5.1. The Technique in Proteomic Analyses
2.5.2. Proteomic Insights into Plant Responses to Plastic Exposure
MPs and NPs’ Effects on the Protein Levels in Plants
MPs and NPs’ Effects on the Cellular Signaling Networks
MPs and NPs’ Effects on the Redox Homeostasis
2.6. Lipidomics
2.6.1. The Technique in Lipidomic Analyses
2.6.2. Lipidomic Studies on Plant Responses to Plastic Derivatives
2.7. Ionomic
2.7.1. The Technique in Ionomic Analyses
2.7.2. Ionomic Insights into the Effects of Plastic-Derived Compounds on Plants
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pathak, P.; Sharma, S.; Ramakrishna, S. Circular transformation in plastic management lessens the carbon footprint of the plastic industry. Mater. Today Sustain. 2023, 22, 100365. [Google Scholar] [CrossRef]
- Schiavi, S.; Parmigiani, M.; Galinetto, P.; Albini, B.; Taglietti, A.; Dacarro, G. Plasmonic Nanomaterials for Micro- and Nanoplastics Detection. Appl. Sci. 2023, 13, 9291. [Google Scholar] [CrossRef]
- OECD Global Plastics Outlook—Plastics Waste by Region and End-of-Life Fate. 2023. Available online: https://ourworldindata.org/grapher/plastic-fate (accessed on 9 August 2024).
- Zeb, A.; Liu, W.; Ali, N.; Shi, R.; Wang, Q.; Wang, J.; Li, J.; Yin, C.; Liu, J.; Yu, M.; et al. Microplastic pollution in terrestrial ecosystems: Global implications and sustainable solutions. J. Hazard. Mater. 2024, 461, 132636. [Google Scholar] [CrossRef]
- Idris, S.N.; Amelia, T.S.M.; Bhubalan, K.; Lazim, A.M.M.; Zakwan, N.A.M.A.; Jamaluddin, M.I.; Santhanam, R.; Amirul, A.-A.A.; Vigneswari, S.; Ramakrishn, S. The degradation of single-use plastics and commercially viable bioplastics in the environment: A review. Environ. Res. 2023, 231, 115988. [Google Scholar] [CrossRef]
- He, S.; Wei, Y.; Yang, C.; He, Z. Interactions of Microplastics and Soil Pollutants in Soil-Plant Systems. Environ. Pollut. 2022, 315, 120357. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; De, J.; Sur, S.; Banerjee, P. Interaction of Microbes with Microplastics and Nanoplastics in the Agroecosystems—Impact on Antimicrobial Resistance. Pathogens 2023, 12, 888. [Google Scholar] [CrossRef] [PubMed]
- Liwarska-Bizukojc, E. Effect of (bio)plastics on soil environment: A review. Sci. Total Environ. 2021, 795, 148889. [Google Scholar] [CrossRef] [PubMed]
- Schiano, M.E.; D’Auria, L.J.; D’Auria, R.; Seccia, S.; Rofrano, G.; Signorelli, D.; Sansone, D.; Caprio, E.; Albrizio, S.; Cocca, M. Microplastic contamination in the agri-food chain: The case of honeybees and beehive products. Sci. Total Environ. 2024, 948, 174698. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC Trends Anal. Chem. 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Bello, F.A.; Folorunsho, A.B.; Chia, R.W.; Lee, J.Y.; Fasusi, S.A. Microplastics in agricultural soils: Sources, impacts on soil organisms, plants, and humans. Environ. Monit. Assess. 2025, 197, 448. [Google Scholar] [CrossRef]
- Seccia, S.; Dini, I. Development and Validation of an HPLC-DAD Method to Determine Alkylphenols in Milk. Beverages 2025, 11, 59. [Google Scholar] [CrossRef]
- Russo, G.; Laneri, S.; Di Lorenzo, R.; Neri, I.; Dini, I.; Ciampaglia, R.; Grumetto, L. Monitoring of Pollutants Content in Bottled and Tap Drinking Water in Italy. Molecules 2022, 27, 3990. [Google Scholar] [CrossRef] [PubMed]
- Tumwesigye, E.; Nnadozie, C.F.; Akamagwuna, F.C.; Noundou, X.S.; Nyakairu, G.W.; Odume, O.N. Microplastics as vectors of chemical contaminants and biological agents in freshwater ecosystems: Current knowledge status and future perspectives. Environ. Pollut. 2023, 330, 121829. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.W.; Lee, J.Y.; Cha, J.; Rodríguez-Seijo, A. Methods of soil sampling for microplastic analysis: A review. Environ. Chem. Lett. 2024, 22, 227–238. [Google Scholar] [CrossRef]
- Krekelbergh, N.; Li, J.; Kusumawardani, P.N.; Liu, Y.; Hu, J.; Sleutel, S.; Parakhonskiy, B.; Hoogenboom, R.; De Neve, S.; Skirtach, A. Comparison of Raman and fluorescence microscopy for identification of small (<2 μm) microplastics in soil. Environ. Pollut. 2025, 374, 126204. [Google Scholar] [CrossRef]
- Constant, M.; Billon, G.; Breton, N.; Alary, C. Extraction of Microplastics from Sediment Matrices: Experimental Comparative Analysis. J. Hazard. Mater. 2021, 420, 126571. [Google Scholar] [CrossRef]
- Dini, I.; Mancusi, A.; Seccia, S. From Harm to Hope: Tackling Microplastics’ Perils with Recycling Innovation. Molecules 2025, 30, 2535. [Google Scholar] [CrossRef]
- Ivanisevic, T.; Sewduth, R.N. Multi-Omics Integration for the Design of Novel Therapies and the Identification of Novel Biomarkers. Proteomes 2023, 11, 34. [Google Scholar] [CrossRef]
- Vailati-Riboni, M.; Palombo, V.; Loor, J.J. What are omics sciences? In Periparturient Diseases of Dairy Cows; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–7. [Google Scholar]
- Kumar, R.; Pandit, P.; Kumar, D.; Patel, Z.; Pandya, L.; Kumar, M.; Joshi, C.; Joshi, M. Landfill microbiome harbour plastic degrading genes: A metagenomic study of solid waste dumping site of Gujarat, India. Sci. Total Environ. 2021, 779, 146184. [Google Scholar] [CrossRef]
- Purohit, J.; Chattopadhyay, A.; Teli, B. Metagenomic exploration of plastic degrading microbes for biotechnological application. Curr. Genom. 2020, 21, 253–270. [Google Scholar] [CrossRef]
- Bhargava, P.; Khan, M.; Verma, A.; Singh, A.; Singh, S.; Vats, S.; Goel, R. Metagenomics as a tool to explore new insights from plant-microbe interface. In Plant Microbe Interface, 1st ed.; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 271–289. [Google Scholar]
- Dini, I.; d’Errico, G.; Troiano, E.; Gigliotti, C.; Vassetti, A.; Lotito, D.; Staropoli, A.; Parrella, G.; d’Errico, F.P.; Lorito, M.; et al. Combined Metagenomic and Metabolomic Analysis to Evaluate the Comprehensive Effects of Trichoderma and 6PP on Vineyard Ecosystems. Agriculture 2025, 15, 1441. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Yang, S.; Zhu, M.; Wang, X. Technology prospecting on enzymes: Application, marketing and engineering. Comput. Struct. Biotechnol. J. 2012, 2, e201209017. [Google Scholar] [CrossRef] [PubMed]
- Gaudêncio, S.P.; Bayram, E.; Lukić Bilela, L.; Cueto, M.; Díaz-Marrero, A.R.; Haznedaroglu, B.Z.; Jimenez, C.; Mandalakis, M.; Pereira, F.; Reyes, F.; et al. Advanced Methods for Natural Products Discovery: Bioactivity Screening, Dereplication, Metabolomics Profiling, Genomic Sequencing, Databases and Informatic Tools, and Structure Elucidation. Mar. Drugs 2023, 21, 308. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.G.Z.; Green, K.T.; Dutilh, B.E.; Edwards, R.A. SUPER-FOCUS: A Tool for Agile Functional Analysis of Shotgun Metagenomic Data. Bioinformatics 2016, 32, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Cui, W.Z.; Li, W.G.; Xu, S.; Sun, Y.H.; Xu, G.J.; Wang, F.Y. Effects of microplastics on cadmium accumulation by rice and arbuscular mycorrhizal fungal communities in Cd-contaminated soil. J. Hazard. Mater. 2023, 442, 130102. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Gong, X.; Sun, Y.; Zhang, S.; Wang, F. Metagenomic analysis reveals gene taxonomic and functional diversity response to microplastics and cadmium in an agricultural soil. Environ. Res. 2024, 251, 118673. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Wang, X.; Cheng, T.; Fu, K.; Qin, Z.; Feng, K. Biofilm Structural and Functional Features on Microplastic Surfaces in Greenhouse Agricultural Soil. Sustainability 2022, 14, 7024. [Google Scholar] [CrossRef]
- Niu, L.; Zhao, S.; Chen, Y.; Li, Y.; Zou, G.; Tao, Y.; Zhang, W.; Wang, L.; Zhang, H. Diversity and potential functional characteristics of phage communities colonizing microplastic biofilms. Environ. Res. 2023, 219, 115103. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Gullans, E.; Krohne, G.; Gerdts, G. The Plastisphere—Uncovering tightly attached plastic “specific” microorganisms. PLoS ONE 2019, 14, e0215859. [Google Scholar] [CrossRef]
- Seo, J.-S.; Keum, Y.-S.; Li, Q.X. Bacterial degradation of aromatic compounds. Int. J. Environ. Res. Public Health 2009, 6, 278–309. [Google Scholar] [CrossRef]
- Lenfant, N.; Hotelier, T.; Velluet, E.; Bourne, Y.; Marchot, P.; Chatonnet, A. ESTHER, the database of the α/β-hydrolase fold superfamily of proteins: Tools to explore diversity of functions. Nucleic Acids Res. 2012, 41, D423–D429. [Google Scholar] [CrossRef]
- Bollinger, A.; Thies, S.; Knieps-Grünhagen, E.; Gertzen, C.; Kobus, S.; Höppner, A.; Ferrer, M.; Gohlke, H.; Smits, S.H.; Jaeger, K.-E. A novel polyester hydrolase from the marine bacterium Pseudomonas aestusnigri–structural and functional insights. Front. Microbiol. 2020, 11, 114. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Taniguchi, I.; Oda, K. Ideonella sakaiensis, PETase, and MHETase: From identification of microbial PET degradation to enzyme characterization. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 648, pp. 187–205. [Google Scholar]
- Yang, Y.; Liu, W.; Zhang, Z.; Grossart, H.-P.; Gadd, G.M. Microplastics provide new microbial niches in aquatic environments. Appl. Microbiol. Biotechnol. 2020, 104, 6501–6511. [Google Scholar] [CrossRef]
- Sun, J.; Yang, W.; Li, M.; Zhang, S.; Sun, Y.; Wang, F. Metagenomic analysis reveals soil microbiome responses to microplastics and ZnO nanoparticles in an agricultural soil. J. Hazard. Mater. 2025, 492, 138164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.J.; Gao, S.S.; Han, H.; Chen, Z.J. Ecotoxicological effects of microplastic types and concentrations in combination with Cd on the bioenergy plant sweet sorghum: Growth and rhizosphere microbiome analysis. Ind. Crops Prod. 2024, 221, 119329. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.Z.; Long, B.B.; Gao, X.H. Effects of biodegradable and polyethylene film mulches and their residues on soil bacterial communities. Environ. Sci. Pollut. Res. 2022, 29, 89698–89711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Qin, X.; Jia, W.; Chai, L.; Huang, M.; Huang, Y. Microplastics from mulching film is a distinct habitat for bacteria in farmland soil. Sci. Total Environ. 2019, 688, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Xiao, Y. Microplastics increase soil microbial network complexity and trigger diversity-driven community assembly. Environ. Pollut. 2023, 333, 122095. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, S.; Li, H.; Jian, S.; Liu, F.; Li, X. Reprogramming of microbial community in barley root endosphere and rhizosphere soil by polystyrene plastics with different particle sizes. Sci. Total Environ. 2023, 866, 161420. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Zhao, Z.; He, J.; Li, W.; Li, J.; Xu, W.A.; Ma, Y.; Niu, Z. Colonization characteristics of bacterial communities on microplastics compared with ambient environments (water and sediment) in Haihe Estuary. Sci. Total Environ. 2020, 708, 134876. [Google Scholar] [CrossRef]
- Ranauda, M.A.; Zuzolo, D.; Maisto, M.; Tartaglia, M.; Scarano, P.; Prigioniero, A.; Sciarrillo, R.; Guarino, C. Microplastics affect soil-plant system: Implications for rhizosphere biology and fitness of sage (Salvia officinalis L.). Environ. Pollut. 2024, 346, 123656. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, P.; Wang, Y.; Zhang, Y.; Xu, T.; Zhang, Y.; Xi, J.; Hou, L.; Li, L.; Zhang, Z.; et al. Negative Effects of Poly(Butylene Adipate-Co-Terephthalate) Microplastics on Arabidopsis and Its Root-Associated Microbiome. J. Hazard. Mater. 2022, 437, 129294. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Tu, Y.; Chen, C.; Wang, F.; Yang, Y.; Hu, Y. Biofilms on microplastic surfaces and their effect on pollutant adsorption in the aquatic environment. J. Mater. Cycles Waste Manag. 2024, 26, 3303–3323. [Google Scholar] [CrossRef]
- Stenger, K.S.; Wikmark, O.G.; Bezuidenhout, C.C.; Molale-Tom, L.G. Microplastics pollution in the ocean: Potential carrier of resistant bacteria and resistance genes. Environ. Pollut. 2021, 291, 118130. [Google Scholar] [CrossRef] [PubMed]
- Zettler, E.R.; Mincer, T.J.; Amaral-Zettler, L.A. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 2013, 47, 7137–7146. [Google Scholar] [CrossRef]
- Fossi, M.C.; Coppola, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsili, L.; Panti, C.; De Sabata, E.; Clò, S. Large filter feeding marine organisms as indicators of microplastic in the pelagic environment: The case studies of the Mediterranean basking shark (Cetorhinus maximus) and fin whale (Balaenoptera physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef]
- Sun, M.; Ye, M.; Jiao, W.; Feng, Y.; Yu, P.; Liu, M.; Jiao, J.; He, X.; Liu, K.; Zhao, Y. Changes in tetracycline partitioning and bacteria/phage-comediated ARGs in microplastic-contaminated greenhouse soil facilitated by sophorolipid. J. Hazard. Mater. 2018, 345, 131–139. [Google Scholar] [CrossRef]
- Wolffe, A.P.; Guschin, D. Review: Chromatin Structural Features and Targets That Regulate Transcription. J. Struct. Biol. 2000, 129, 102–122. [Google Scholar] [CrossRef]
- Liu, C.; Xin, Y.; Xu, L.; Cai, Z.; Xue, Y.; Liu, Y.; Xie, D.; Liu, Y.; Qi, Y. Arabidopsis ARGONAUTE 1 Binds Chromatin to Promote Gene Transcription in Response to Hormones and Stresses. Dev. Cell 2018, 44, 348–361. [Google Scholar] [CrossRef]
- da Costa, G.S.; Cerqueira, A.F.; de Brito, C.R.; Mielke, M.S.; Gaiotto, F.A. Epigenetics Regulation in Responses to Abiotic Factors in Plant Species: A Systematic Review. Plants 2024, 13, 2082. [Google Scholar] [CrossRef] [PubMed]
- Miryeganeh, M. Plants’ Epigenetic Mechanisms and Abiotic Stress. Genes 2021, 12, 1106. [Google Scholar] [CrossRef] [PubMed]
- Kawakatsu, T.; Nery, J.R.; Castanon, R.; Ecker, J.R. Dynamic DNA methylation reconfiguration during seed development and germination. Genome Biol. 2017, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic Mechanisms of Plant Adaptation to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef]
- Duarte-Aké, F.; Us-Camas, R.; De-la-Peña, C. Epigenetic Regulation in Heterosis and Environmental Stress: The Challenge of Producing Hybrid Epigenomes to Face Climate Change. Epigenomes 2023, 7, 14. [Google Scholar] [CrossRef]
- Tang, K.H.D. Genotoxicity of Microplastics on Living Organisms: Effects on Chromosomes, DNA and Gene Expression. Environments 2025, 12, 10. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Zhu, Z.; Bai, X.; Ma, J. Adsorption behavior of organic pollutants and metals on micro/nanoplastics in the aquatic environment. Sci. Total Environ. 2019, 694, 133643. [Google Scholar] [CrossRef]
- Dainelli, M.; Castellani, M.B.; Pignattelli, S.; Falsini, S.; Ristori, S.; Papini, A.; Colzi, I.; Coppi, A.; Gonnelli, C. Growth, physiological parameters and DNA methylation in Spirodela polyrhiza (L.) Schleid exposed to PET micro-nanoplastic contaminated waters. Plant Physiol. Biochem. 2024, 20, 108403. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A.; Guchhait, R.; De, S.; Pramanick, K. Cytogenotoxic potential of a hazardous material, polystyrene microparticles on Allium cepa L. J. Hazard. Mater. 2020, 385, 121560. [Google Scholar] [CrossRef]
- Zhuang, H.; Qin, M.; Liu, B.; Li, R.; Li, Z. Combination of transcriptomics, metabolomics and physiological traits reveals the effects of polystyrene microplastics on photosynthesis, carbon and nitrogen metabolism in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2023, 205, 108201. [Google Scholar] [CrossRef]
- Poliseno, L.; Lanza, M.; Pandolfi, P.P. Coding, or non-coding, that is the question. Cell Res. 2024, 34, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.-H.; Pilla, R.; Chen, C.-C.; Ishii, P.E.; Toresson, L.; Allenspach-Jorn, K.; Jergens, A.E.; Summers, S.; Swanson, K.S.; Volk, H.; et al. Correlation between Targeted qPCR Assays and Untargeted DNA Shotgun Metagenomic Sequencing for Assessing the Fecal Microbiota in Dogs. Animals 2023, 13, 2597. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.W.; Williams, A.; Uthe, H.; van Dam, N.M.; Mur, L.A.J.; Grant, M.R.; Pétriacq, P. Unravelling Plant Responses to Stress—The Importance of Targeted and Untargeted Metabolomics. Metabolites 2021, 11, 558. [Google Scholar] [CrossRef] [PubMed]
- Satrio, R.D.; Fendiyanto, M.H.; Miftahudin, M. Tools and Techniques Used at Global Scale Through Genomics, Transcriptomics, Proteomics, and Metabolomics to Investigate Plant Stress Responses at the Molecular Level. In Molecular Dynamics of Plant Stress and Its Management; Springer: Singapore, 2024; pp. 555–607. [Google Scholar]
- Lv, L.; Zhang, W.; Sun, L.; Zhao, A.; Zhang, Y.; Wang, L.; Liu, Y.; Li, Z.; Li, H.; Chen, X. Gene co-expression network analysis to identify critical modules and candidate genes of drought-resistance in wheat. PLoS ONE 2020, 15, e0236186. [Google Scholar] [CrossRef]
- Fu, F.; Long, B.B.; Huang, Q.; Li, J.J.; Zhou, W.J.; Yang, C. Integrated effects of residual plastic films on soil-rhizosphere microbe-plant ecosystem. J. Hazard. Mater. 2023, 445, 130420. [Google Scholar] [CrossRef]
- Cui, J.; Tian, H.; Qi, Y.; Hu, X.; Li, S.; Zhang, W.; Wei, Z.; Zhang, M.; Liu, Z.; Abolfathi, S. Impact of microplastic residues from polyurethane films on crop growth: Unraveling insights through transcriptomics and metabolomics analysis. Ecotoxicol. Environ. Saf. 2024, 283, 116826. [Google Scholar] [CrossRef]
- Wu, X.; Hou, H.; Liu, Y.; Yin, S.; Bian, S.; Liang, S.; Wan, C.; Yuan, S.; Xiao, K.; Liu, B.; et al. Microplastics affect rice (Oryza sativa L.) quality by interfering metabolite accumulation and energy expenditure pathways: A field study. J. Hazard. Mater. 2022, 422, 126834. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Guo, L.; Wang, P.; He, C.; Liu, D.; Bian, H.; Sheng, L. Effects of polystyrene nanoplastics with different functional groups on rice (Oryza sativa L.) seedlings: Combined transcriptome, enzymology, and physiology. Sci. Total Environ. 2022, 834, 155092. [Google Scholar] [CrossRef]
- Yu, C.; Zeng, H.; Wang, Q.; Chen, W.; Chen, W.; Yu, W.; Lou, H.; Wu, J. Multi-omics analysis reveals the molecular responses of Torreya grandis shoots to nanoplastic pollutant. J. Hazard. Mater. 2022, 436, 129181. [Google Scholar] [CrossRef]
- Teng, L.; Zhu, Y.; Li, H.; Song, X.; Shi, L. The Phytotoxicity of Microplastics to the Photosynthetic Performance and Transcriptome Profiling of Nicotiana Tabacum Seedlings. Ecotoxicol. Environ. Saf. 2022, 231, 113155. [Google Scholar] [CrossRef]
- Wang, Y.; Xiang, L.; Wang, F.; Wang, Z.; Bian, Y.; Gu, C.; Wen, X.; Kengara, F.O.; Schaffer, A.; Jiang, X.; et al. Positively charged microplastics induce strong lettuce stress responses from physiological, transcriptomic, and metabolomic perspectives. Environ. Sci. Technol. 2022, 56, 16907–16918. [Google Scholar] [CrossRef]
- Anzano, A.; Bonanomi, G.; Mazzoleni, S.; Lanzotti, V. Plant Metabolomics in Biotic and Abiotic Stress: A Critical Overview. Phytochem. Rev. 2021, 21, 503–524. [Google Scholar] [CrossRef]
- Dini, I. Flavonoid glycosides from Pouteria obovata (R. Br.) fruit flour. Food Chem. 2011, 124, 884–888. [Google Scholar]
- Xu, Y.; Fu, X. Reprogramming of Plant Central Metabolism in Response to Abiotic Stresses: A Metabolomics View. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Tenore, G.C.; Dini, A. Oleanane Saponins in “Kancolla,” a Sweet Variety of Chenopodium quinoa. J. Nat. Prod. 2002, 65, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Graziani, G.; Gaspari, A.; Fedele, F.L.; Sicari, A.; Vinale, F.; Cavallo, P.; Lorito, M.; Ritieni, A. New Strategies in the Cultivation of Olive Trees and Repercussions on the Nutritional Value of the Extra Virgin Olive Oil. Molecules 2020, 25, 2345. [Google Scholar] [CrossRef]
- Dini, I.; Marra, R.; Cavallo, P.; Pironti, A.; Sepe, I.; Troisi, J.; Scala, G.; Lombardi, P.; Vinale, F. Trichoderma Strains and Metabolites Selectively Increase the Production of Volatile Organic Compounds (VOCs) in Olive Trees. Metabolites 2021, 11, 213. [Google Scholar] [CrossRef]
- Dini, I.; Pascale, M.; Staropoli, A.; Marra, R.; Vinale, F. Effect of Selected Trichoderma Strains and Metabolites on Olive Drupes. Appl. Sci. 2021, 11, 8710. [Google Scholar] [CrossRef]
- Dini, I.; Graziani, G.; Fedele, F.L.; Sicari, A.; Vinale, F.; Castaldo, L.; Ritieni, A. An Environmentally Friendly Practice Used in Olive Cultivation Capable of Increasing Commercial Interest in Waste Products from Oil Processing. Antioxidants 2020, 9, 466. [Google Scholar] [CrossRef]
- Anwardeen, N.R.; Diboun, I.; Mokrab, Y.; Althani, A.A.; Elrayess, M.A. Statistical methods and resources for biomarker discovery using metabolomics. BMC Bioinform. 2023, 24, 250. [Google Scholar] [CrossRef]
- Sorochan Armstrong, M.D.; de la Mata, A.P.; Harynuk, J.J. Review of variable selection methods for discriminant-type problems in chemometrics. Front. Anal. Sci. 2022, 2, 867938. [Google Scholar] [CrossRef]
- Petrovsky, D.V.; Malsagova, K.A.; Rudnev, V.R.; Kulikova, L.I.; Pustovoyt, V.I.; Balakin, E.I.; Yurku, K.A.; Kaysheva, A.L. Bioinformatics Methods for Constructing Metabolic Networks. Processes 2023, 11, 3430. [Google Scholar] [CrossRef]
- Tao, H.; Zhou, L.; Yu, D.; Chen, Y.; Luo, Y.; Lin, T. Effects of polystyrene microplastics on the metabolic level of Pseudomonas aeruginosa. Sci. Total Environ. 2024, 922, 171335. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Le, X.C.; Zhu, L. Metabolomic analysis of two rice (Oryza sativa) varieties exposed to 2, 2′, 4, 4′-tetrabromodiphenyl ether. Environ. Pollut. 2018, 237, 308–317. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Y.; Guan, M.; Fu, Y.; Yang, X.; Hu, M.; Yang, R. The effect of Polyethylene Terephthalate (PET) Microplastic stress on the composition and gene regulatory network of amino acid in Capsicum annuum. Environ. Exp. Bot. 2024, 228, 106029. [Google Scholar] [CrossRef]
- Zhao, M.; Xu, L.; Wang, X.; Li, C.; Zhao, Y.; Cao, B.; Zhang, C.; Zhang, J.; Wang, J.; Chen, Y.; et al. Microplastics Promoted Cadmium Accumulation in Maize Plants by Improving Active Cadmium and Amino Acid Synthesis. J. Hazard. Mater. 2023, 447, 130788. [Google Scholar] [CrossRef]
- Iqbal, B.; Zhao, X.; Khan, K.Y.; Javed, Q.; Nazar, M.; Khan, I.; Zhao, X.; Li, G.; Du, D. Microplastics meet invasive plants: Unraveling the ecological hazards to agroecosystems. Sci. Total Environ. 2024, 906, 167756. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Shi, Z.; Shan, X.; Yang, S.; Zhang, Y.; Guo, X. Insights into growth-affecting effect of nanomaterials: Using metabolomics and transcriptomics to reveal the molecular mechanisms of cucumber leaves upon exposure to polystyrene nanoplastics (PSNPs). Sci. Total Environ. 2023, 866, 161247. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Scurtu, V.-F.; Coman, C.; Clapa, D.; Iancu, Ș.D.; Leopold, N.; Leopold, L.-F. Effects of Polystyrene Nanoplastics Exposure on in Vitro-Grown Stevia Rebaudiana Plants. Plant Physiol. Biochem. 2023, 197, 107634. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Bykova, N.V. Role of organic acids in the integration of cellular redox metabolism and mediation of redox signaling in photosynthetic tissues of higher plants. Free Radic. Biol. Med. 2018, 122, 74–85. [Google Scholar] [CrossRef]
- Jian, S.; Li, S.; Liu, F.; Liu, S.; Gong, L.; Jiang, Y.; Li, X. Elevated Atmospheric CO2 Concentration Changes the Eco-Physiological Response of Barley to Polystyrene Nanoplastics. Chem. Eng. J. 2023, 457, 141135. [Google Scholar] [CrossRef]
- Lian, J.; Wu, J.; Xiong, H.; Zeb, A.; Yang, T.; Su, X.; Su, L.; Liu, W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.). J. Hazard. Mater. 2020, 385, 121620. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Zhao, X.; Zhao, S.; Gu, X.; Du, W.; Wei, H.; Ji, R.; Zhao, L. Metabolomics Reveals the “Invisible” Responses of Spinach Plants Exposed to CeO2 Nanoparticles. Environ. Sci. Technol. 2019, 53, 6007. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Hu, J.; Zhou, H.; Adeleye, A.S.; Keller, A.A. 1H NMR and GC-MS Based Metabolomics Reveal Defense and Detoxification Mechanism of Cucumber Plant under Nano-Cu Stress. Environ. Sci. Technol. 2016, 50, 2000–2010. [Google Scholar] [CrossRef]
- Yu, X.; Xing, H.; Sun, J.; Du, X.; Lu, G.; Zhu, L. New insight into phytometabolism and phytotoxicity mechanism of widespread plasticizer di (2-ethylhexyl) phthalate in rice plants. Sci. Total Environ. 2023, 880, 163254. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Yin, S.; Xiao, K.; Xiong, Q.; Bian, S.; Liang, S.; Hou, H.; Hu, J.; Yang, J. Metabolomics Revealing the Response of Rice (Oryza sativa L.) Exposed to Polystyrene Microplastics. Environ. Pollut. 2020, 266, 115159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. An overview of compounds derived from the shikimate and phenylpropanoid pathways and their medicinal importance. Mini Rev. Med. Chem. 2017, 17, 1013–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiang, L.; Wang, F.; Redmile-Gordon, M.; Bian, Y.; Wang, Z.; Gu, C.; Jiang, X.; Schäffer, A.; Xing, B. Transcriptomic and metabolomic changes in lettuce triggered by microplastics-stress. Environ. Pollut. 2023, 320, 121081. [Google Scholar] [CrossRef]
- López-Belchí, M.D.; Toro, M.T.; Riveros, G.; Illanes, M.; Noriega, F.; Shoebitz, M.; García-Viguera, C.; Moreno, D.A. Brassica Sprouts Exposed to Microplastics: Effects on Phytochemical Constituents. Sci. Total Environ. 2022, 823, 153796. [Google Scholar] [CrossRef]
- Xiao, W.; Xiang, P.; Liao, W.; Xiong, Z.; Peng, L.; Zou, L.; Liu, B.; Li, Q. Effects of polystyrene microplastics on the growth and metabolism of highland barley seedlings based on LC-MS. Front. Plant Sci. 2024, 15, 1477605. [Google Scholar] [CrossRef] [PubMed]
- Bouaicha, O.; Tiziani, R.; Maver, M.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Trevisan, M.; Cesco, S.; Borruso, L.; Mimmo, T. Plant species-specific impact of polyethylene microspheres on seedling growth and the metabolome. Sci. Total Environ. 2022, 840, 156678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.-Q.; Lu, C.-H.; Mai, L.; Bao, L.-J.; Liu, L.-Y.; Zeng, E.Y. Response of rice (Oryza sativa L.) roots to nanoplastic treatment at seedling stage. J. Hazard. Mater. 2021, 401, 123412. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.K.; Han, Z.M.; Wang, Q.Y.; Wang, T.Y.; Sun, Z.H.; Yu, Y.; Han, X.R.; Yu, H.W. Toxicity effects of nanoplastics on soybean (Glycine max L.): Mechanisms and transcriptomic analysis. Chemosphere 2022, 313, 137571. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Guo, J.; Dong, Y.; Wang, Z.; Gong, L.; Li, X. Polystyrene microplastics disturb the redox homeostasis, carbohydrate metabolism and phytohormone regulatory network in barley. J. Hazard. Mater. 2021, 415, 125614. [Google Scholar] [CrossRef]
- Yibo, L.; Genshen, Y.; Yu, C.; Lei, X.; Ma, X.; Xing, X. Microplastics in the agricultural soils: Pollution behavior and subsequent effects. Land Degrad. Dev. 2024, 35, 4455–4471. [Google Scholar] [CrossRef]
- Feng, Z.; Ji, S.; Ping, J.; Cui, D. Recent advances in metabolomics for studying heavy metal stress in plants. TrAC Trends Anal. Chem. 2021, 143, 116402. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Wang, X.; Zeb, A.; Wang, Q.; Mo, F.; Shi, R.; Liu, J.; Yu, M.; Li, J.; et al. Assessing stress responses in potherb mustard (Brassica juncea var. multiceps) exposed to a synergy of microplastics and cadmium: Insights from physiology, oxidative damage, and metabolomics. Sci. Total Environ. 2024, 907, 167920. [Google Scholar]
- Liu, X.; Wang, Z.; Shi, G.; Gao, Y.; Zhang, H.; Liu, K. Effects of microplastics and salt single or combined stresses on growth and physiological responses of maize seedlings. Physiol. Plant. 2025, 177, e70106. [Google Scholar] [CrossRef]
- Al-Amrani, S.; Al-Jabri, Z.; Al-Zaabi, A.; Alshekaili, J.; Al-Khabori, M. Proteomics: Concepts and applications in human medicine. World J. Biol. Chem. 2021, 12, 57–69. [Google Scholar] [CrossRef]
- Danko, K.; Lukasheva, E.; Zhukov, V.A.; Zgoda, V.; Frolov, A. Detergent-Assisted Protein Digestion—On the Way to Avoid the Key Bottleneck of Shotgun Bottom-Up Proteomics. Int. J. Mol. Sci. 2022, 23, 13903. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Plant proteomic research for improvement of food crops under stresses: A review. Mol. Omics 2021, 17, 860–880. [Google Scholar] [CrossRef] [PubMed]
- Schlotterbeck, J.; Chatterjee, M.; Gawaz, M.; Lammerhofer, M. Comprehensive MS/MS profiling by UHPLC-ESI-QTOF-MS/MS using SWATH data-independent acquisition for the study of platelet lipidomes in coronary artery disease. Anal. Chim. Acta 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, T.; Boom, I.; Maliepaard, J.; Dubbelman, A.-C.; Harms, A.C.; Hankemeier, T. Data-Independent Acquisition for the Quantification and Identification of Metabolites in Plasma. Metabolites 2020, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-X.; Zhang, Y.; Fernie, A.R.; Liu, Y.-G.; Zhu, F.-Y. SWATH-MS-based proteomics: Strategies and applications in plants. Trends Biotechnol. 2021, 39, 433–437. [Google Scholar] [CrossRef]
- Aydoğan, C. Critical review of new advances in food and plant proteomics analyses by nano-LC/MS towards advanced foodomics. TrAC Trends Anal. Chem. 2024, 176, 117759. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Cukura, A.; Denny, P.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2022, 51, D523–D531. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Duarte, J.M.; Feng, Z.K.; Flatt, J.W.; Hudson, B.P.; Lowe, R.; Peisach, E.; Piehl, D.W.; Rose, Y.; et al. Protein Data Bank: A Comprehensive Review of 3D Structure Holdings and Worldwide Utilization by Researchers, Educators, and Students. Biomolecules 2022, 12, 1425. [Google Scholar] [CrossRef]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Multi’ omic data integration: A review of concepts, considerations, and approaches. Semin. Perinatol. 2021, 45, 151456. [Google Scholar] [CrossRef]
- Ansari, F.A.; Ratha, S.K.; Renuka, N.; Ramanna, L.; Gupta, S.K.; Rawat, I.; Bux, F. Effect of microplastics on growth and biochemical composition of microalga Acutodesmus obliquus. Algal Res. 2021, 56, 102296. [Google Scholar] [CrossRef]
- Lian, J.; Liu, W.; Sun, Y.; Men, S.; Wu, J.; Zeb, A.; Yang, T.; Ma, L.Q.; Zhou, W. Nanotoxicological effects and transcriptome mechanisms of wheat (Triticum aestivum L.) under stress of polystyrene nanoplastics. J. Hazard. Mater. 2022, 423, 127241. [Google Scholar] [CrossRef]
- Kim, D.; An, S.; Kim, L.; Byeon, Y.M.; Lee, J.; Choi, M.J.; An, Y.J. Translocation and chronic effects of microplastics on pea plants (Pisum sativum) in copper-contaminated Soil. J. Hazard. Mater. 2022, 436, 129194. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.Y.; Ali, B.; Ghani, H.U.; Cui, X.; Luo, X.; Ali, Z.; Ahmed, W.; Tan, J.; Lysenko, V.; Guo, Y. Polyvinyl chloride microplastics and drought co-exposure alter rice growth by affecting metabolomics and proteomics. Sci. Total Environ. 2024, 955, 177002. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.F.; Vazquez, C.I.; Chiang, C.Y.; Chiu, M.H.; Chen, C.S.; Ni, C.W.; Gong, G.C.; Quigg, A.; Santschi, P.H.; Chin, W.C. Nano-and microplastics trigger secretion of protein-rich extracellular polymeric substances from phytoplankton. Sci. Total Environ. 2020, 748, 141469. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, S.; Brestic, M.; Li, N.; Zhang, P.; Liu, L.; Li, X. Modulations in protein phosphorylation explain the physiological responses of barley (Hordeum vulgare) to nanoplastics and ZnO nanoparticles. J. Hazard. Mater. 2023, 443, 130196. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, H.; Li, T.; Yu, L.; Qi, Y.; Tian, G.; He, F.; Li, X.; Sun, N.; Liu, R. Size-dependent effects of nanoplastics on structure and function of superoxide dismutase. Chemosphere 2022, 309, 136768. [Google Scholar] [CrossRef]
- Yao, J.; Li, H.; Lan, J.; Bao, Y.; Du, X.; Zhao, Z.; Hu, G. Spectroscopic investigations on the interaction between nano plastic and catalase on molecular level. Sci. Total Environ. 2023, 863, 160903. [Google Scholar] [CrossRef]
- Kuźniak, E.; Gajewska, E. Lipids and Lipid-Mediated Signaling in Plant–Pathogen Interactions. Int. J. Mol. Sci. 2024, 25, 7255. [Google Scholar] [CrossRef]
- Kehelpannala, C.; Rupasinghe, T.; Hennessy, T.; Bradley, D.; Ebert, B.; Roessner, U. The State of the Art in Plant Lipidomics. Mol. Omics 2021, 17, 894–910. [Google Scholar] [CrossRef]
- Probst, K.V.; Wales, M.D.; Rezac, M.E.; Vadlani, P.V. Evaluation of green solvents: Oil extraction from oleaginous yeast Lipomyces starkeyi using cyclopentyl methyl ether (CPME). Biotechnol. Prog. 2017, 33, 1096–1103. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Han, X. Lipidomics for studying metabolism. Nat. Rev. Endocrinol. 2016, 12, 668–679. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.; Trengove, R.D.; Mullin, L.G.; Rainville, P.D.; Isaac, G.; Plumb, R.S.; Gethings, L.A.; Wilson, I.D. Rapid profiling method for the analysis of lipids in human plasma using ion mobility enabled-reversed phase-ultra high performance liquid chromatography/mass spectrometry. J. Chromatogr. A 2020, 1611, 460597. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wei, J.H.; Wei, B.K.; Chen, Z.Q.; Liu, H.L.; Zhang, W.Y.; Zhou, D.M. Metabolic response of lettuce (Lactuca sativa L.) to polystyrene nanoplastics and microplastics after foliar exposure. Environ. Sci. Nano 2024, 11, 4847–4861. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, Y.; Li, Y.; Liu, J. Optimistic effects of galaxolide and polystyrene microplastic stress on the physio-biochemical characteristics and metabolic profiles of an ornamental plant. Plant Physiol. Biochem. 2023, 196, 350–360. [Google Scholar] [CrossRef]
- Guschina, I.A.; Hayes, A.J.; Ormerod, S.J. Polystyrene Microplastics Decrease Accumulation of Essential Fatty Acids in Common Freshwater Algae. Environ. Pollut. 2020, 263, 114425. [Google Scholar] [CrossRef]
- Zhang, L.; Hoagland, L.; Yang, Y.; Becchi, P.P.; Sobolev, A.P.; Scioli, G.; La Nasa, J.; Biale, G.; Modugno, F.; Lucini, L. The combination of hyperspectral imaging, untargeted metabolomics and lipidomics highlights a coordinated stress-related biochemical reprogramming triggered by polyethylene nanoparticles in lettuce. Sci. Total Environ. 2025, 964, 178604. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Das, S.P.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Kumar, A.; Ramrao, D.P.; Sudhakar, C. Multi-Omics Pipeline and Omics-Integration Approach to Decipher Plant’s Abiotic Stress Tolerance Responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. Ionomic Approaches for Discovery of Novel Stress-Resilient Genes in Plants. Int. J. Mol. Sci. 2021, 22, 7182. [Google Scholar] [CrossRef]
- Paliwal, S.; Tripathi, M.K.; Tiwari, S.; Tripathi, N.; Payasi, D.K.; Tiwari, P.N.; Singh, K.; Yadav, R.K.; Asati, R.; Chauhan, S. Molecular Advances to Combat Different Biotic and Abiotic Stresses in Linseed (Linum usitatissimum L.): A Comprehensive Review. Genes 2023, 14, 1461. [Google Scholar] [CrossRef]
- Satismruti, K.; Senthil, N.; Vellaikumar, S.; Ranjani, R.V.; Raveendran, M. Plant Ionomics: A Platform for Identifying Novel Gene Regulating Plant Mineral Nutrition. Am. J. Plant Sci. 2013, 4, 1309–1315. [Google Scholar] [CrossRef]
- Tang, M.; Huang, Y.; Zhang, W.; Fu, T.; Zeng, T.; Huang, Y.; Yang, X. Effects of Microplastics on the Mineral Elements Absorption and Accumulation in Hydroponic Rice Seedlings (Oryza sativa L.). Bull. Environ. Contam. Toxicol. 2022, 108, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Dainelli, M.; Pignattelli, S.; Bazihizina, N.; Falsini, S.; Papini, A.; Baccelli, I.; Mancuso, S.; Coppi, A.; Castellani, M.B.; Colzi, I.; et al. Can Microplastics Threaten Plant Productivity and Fruit Quality? Insights from Micro-Tom and Micro-PET/PVC. Sci. Total Environ. 2023, 895, 165119. [Google Scholar] [CrossRef]
- Fu, Q.; Lai, J.L.; Ji, X.H.; Luo, Z.X.; Wu, G.; Luo, X.G. Alterations of the Rhizosphere Soil Microbial Community Composition and Metabolite Profiles of Zea Mays by Polyethylene-Particles of Different Molecular Weights. J. Hazard. Mater. 2022, 423, 127062. [Google Scholar] [CrossRef]
- Yang, R.C.; Cheng, L.; Li, Z.Q.; Cui, Y.L.; Liu, J.W.; Xu, D.; Liu, S.J.; Lin, Z.; Chen, J.G.; Zhang, Y.Q. Mechanism of microplastics in the reduction of cadmium toxicity in tomato. Ecotoxicol. Environ. Saf. 2025, 289, 117621. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dini, I.; Mancusi, R.; De Biasi, M.-G. The Role of Omics Technology in Evaluating Plastic Pollution’s Effects on Plants: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 10646. https://doi.org/10.3390/ijms262110646
Dini I, Mancusi R, De Biasi M-G. The Role of Omics Technology in Evaluating Plastic Pollution’s Effects on Plants: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(21):10646. https://doi.org/10.3390/ijms262110646
Chicago/Turabian StyleDini, Irene, Roberto Mancusi, and Margherita-Gabriella De Biasi. 2025. "The Role of Omics Technology in Evaluating Plastic Pollution’s Effects on Plants: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 21: 10646. https://doi.org/10.3390/ijms262110646
APA StyleDini, I., Mancusi, R., & De Biasi, M.-G. (2025). The Role of Omics Technology in Evaluating Plastic Pollution’s Effects on Plants: A Comprehensive Review. International Journal of Molecular Sciences, 26(21), 10646. https://doi.org/10.3390/ijms262110646








