Natural Products in Alzheimer’s Disease: A Systematic Review of Clinical Trials and Underlying Molecular Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Collection
2.3. Quality Assessment
2.4. Critical Appraisal Rationale
2.5. Systematic Review Registration
2.6. PRISMA Statement
2.7. Synthesis of Results
3. Results and Discussion
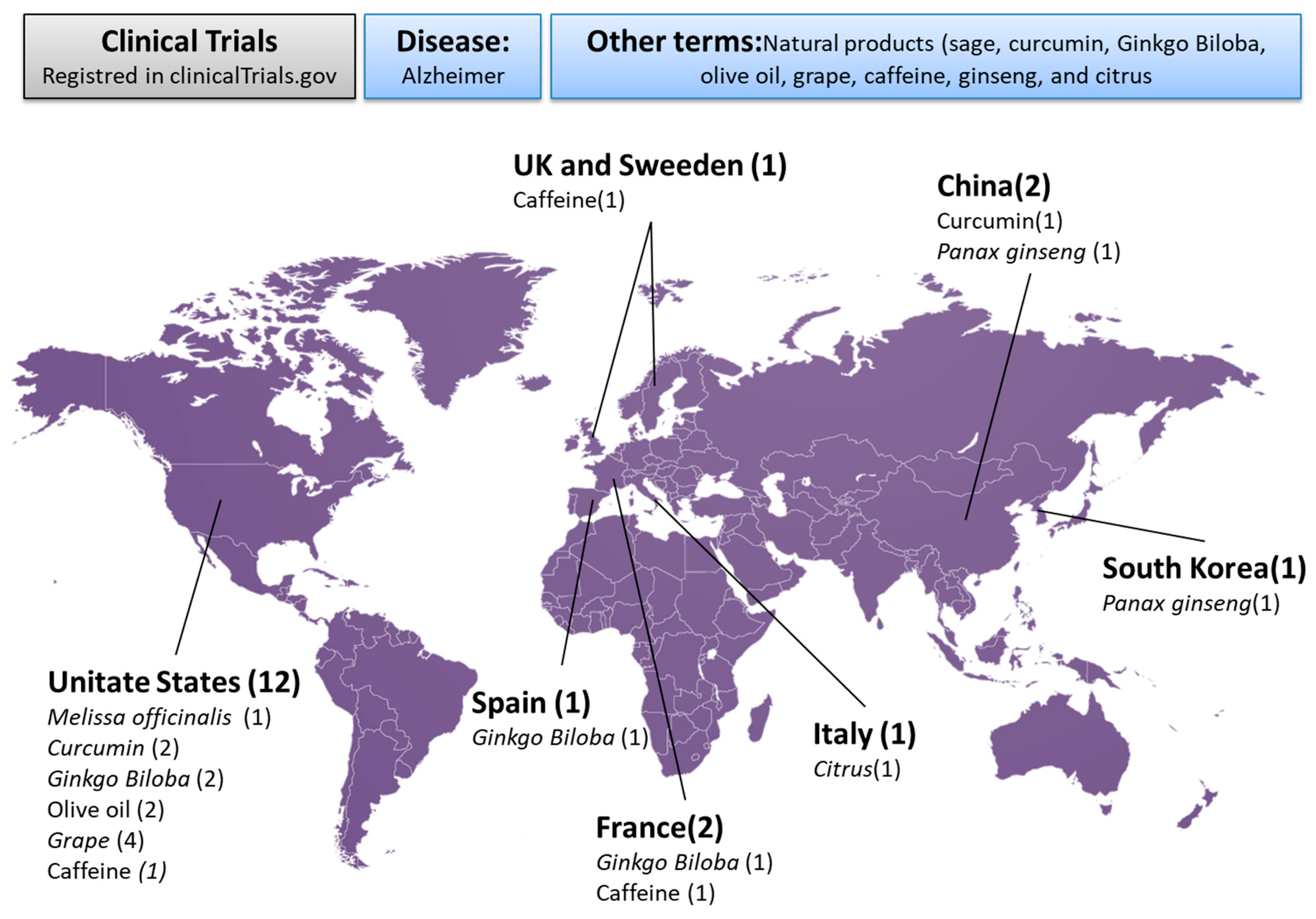
3.1. Study Selection
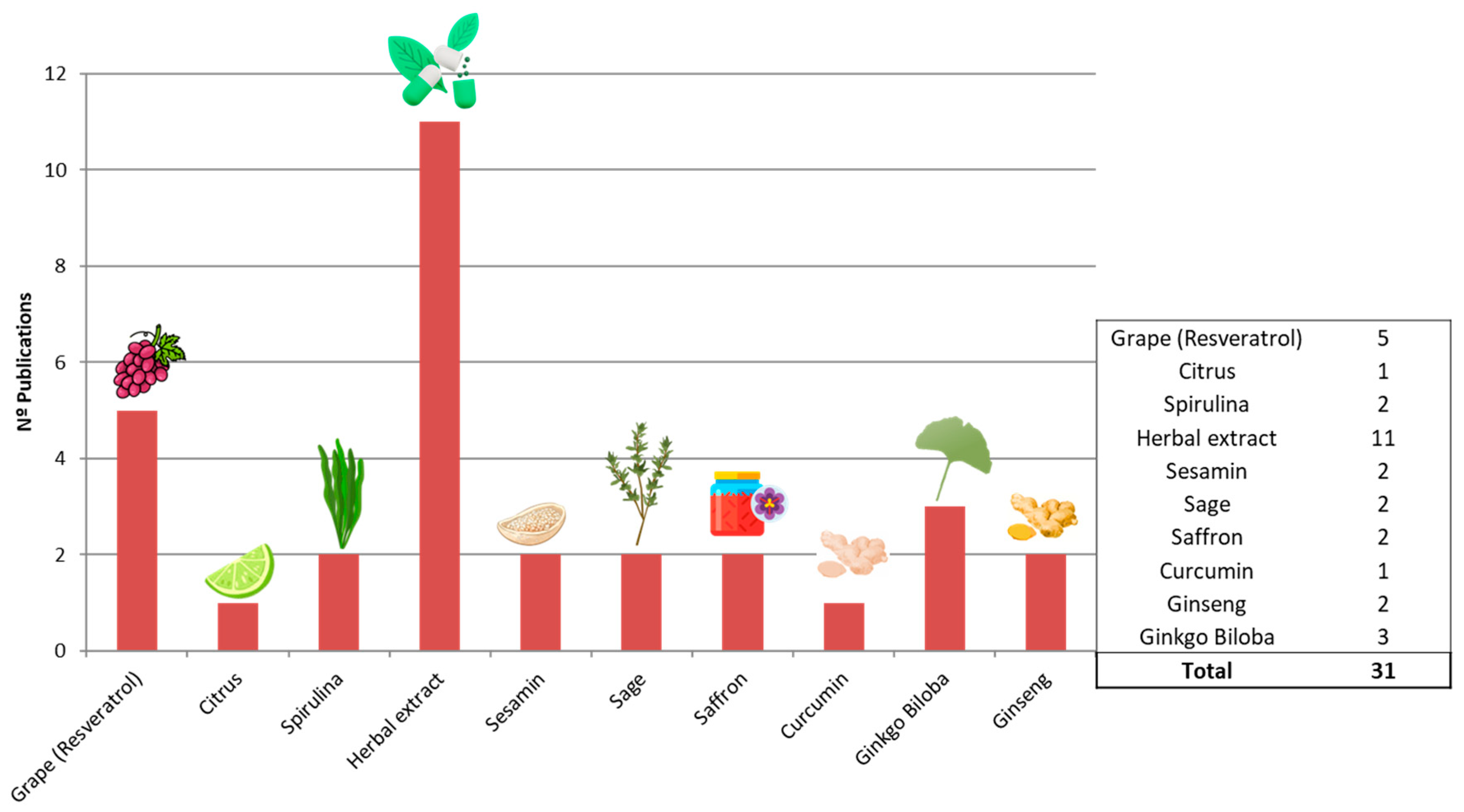
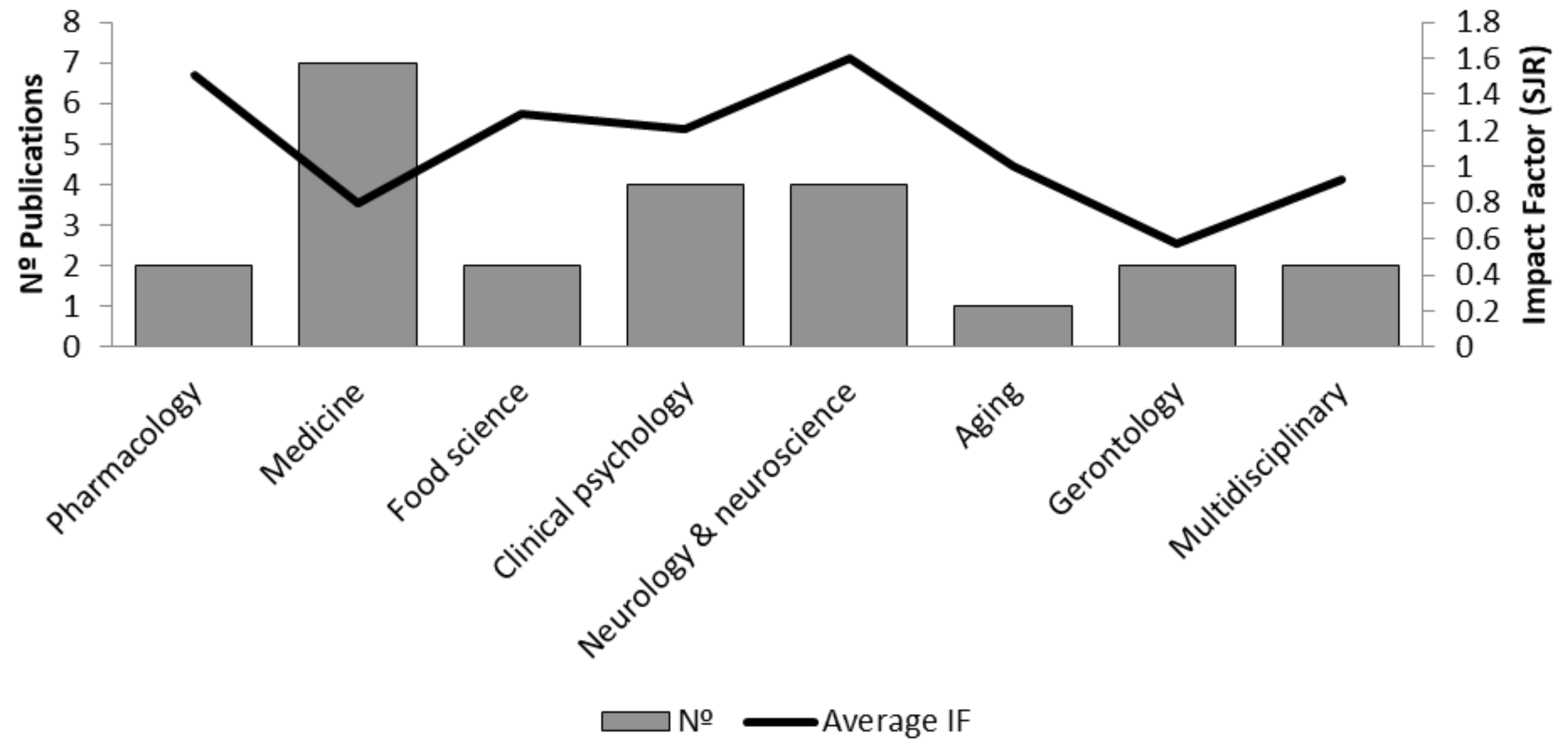
3.2. Analysis of Included Studies
3.3. Herbal Extracts
3.4. Resveratrol
3.5. Saffron
3.6. Sesame-Related Compounds
3.7. Ginseng and Ginkgo biloba
3.8. Curcumin
3.9. Melissa officinalis
3.10. Spirulina
3.11. Citrus
| First Author and Year | Treatment | Objectives | Subjects | Age | Inclusion Criteria (Cognitive Test Score) | Outcomes Assessment | Adverse Side Effects | Main Findings |
|---|---|---|---|---|---|---|---|---|
| Herbal extracts | ||||||||
| [67] Kudoh et al., 2016. | Ninjin’yoeito (NYT) formula (7.5 g/day). | Evaluate efficacy for AD treatment. | 30 AD patients. | 50–85 years. | MMSE, ADAS-cog, CDR. | MMSE, ADAS-Cog, CDR-SB, MoCA. | Mild gastrointestinal discomfort. | NYT improved ADAS-cog and MMSE scores significantly compared to donepezil. |
| [58] Wang et al., 2020. | Jiannao Yizhi Formula (JYF) (10 g/day). | Cognitive improvement in AD. | 40 AD patients. | 50–85 years. | ADAS-Cog, MMSE, MoCA. | ADAS-Cog, MMSE, MoCA. | Mild headache, dizziness. | JYF demonstrated similar efficacy to donepezil in improving cognitive function. |
| [50] Zhang et al., 2015. | Bushen capsules (BSC) (varied dose). | MCI cognitive improvement. | 30 MCI patients. | 50–85 years. | MMSE, AVLT, ROCF. | MMSE, AVLT, ROCF. | No adverse side effects noted. | BSC significantly improved memory and cognitive function after 3–12 months. |
| [53] Furukawa et al., 2017. | Yokukansan (YKS, TJ-54). | Evaluate efficacy in psychological symptoms of AD. | 35 AD patients. | 50–85 years. | NPI-Q, MMSE. | NPI-Q, MMSE. | No adverse effects. | No significant improvement in psychological symptoms of dementia. |
| [47] Sadhu et al., 2014. | Polyherbal formula (1 g/day). | Evaluate cognitive improvement in AD. | 50 AD patients. | 50–85 years. | DSS, FAQ, GDS. | DSS, FAQ, GDS. | No significant adverse effects. | Polyherbal formulation improved cognitive function better than donepezil. |
| [51] Zhang et al., 2015. | Yishen Huazhuo decoction (62 g/day). | Cognitive function in AD. | 60 AD patients. | 50–85 years. | ADAS-cog, MMSE. | ADAS-cog, MMSE. | No significant adverse effects. | YHD outperformed donepezil in improving cognitive scores. |
| [56] Yang et al., 2019. | Huannao Yicong Formula (HYF) (10 g/day). | Cognitive improvement in AD. | 40 AD patients. | 50–85 years. | MMSE, MoCA, CM-SS. | MMSE, MoCA, CM-SS. | No significant adverse effects. | HYF had similar efficacy to donepezil in improving cognitive scores. |
| [71] Shin et al., 2021. | Kami-guibi-tang (KGT). | Improve cognition and memory in AD. | 45 AD patients. | 50–85 years. | CDR-SB, SNSB-D. | CDR-SB, SNSB-D. | No adverse effects. | KGT significantly improved CDR-SB and SNSB-D scores. |
| [62] Zhang et al., 2019. | Bushen capsules (BSC). | Cognitive function in aMCI. | 35 MCI patients. | 50–85 years. | MMSE, AVLT, ROCF. | MMSE, AVLT, ROCF. | No significant adverse effects. | BSC significantly improved cognitive functions in aMCI patients after 12 months. |
| [70] Uchida, K. et al., 2024 | Matcha green tea (natural) | To assess the effect of matcha green tea on cognitive function and sleep quality in older adults with cognitive decline. | Older adults with cognitive decline. | 60–85 years | Cognitive decline (MMSE < 26). | Cognitive function tests, sleep quality assessment. | No major adverse effects reported. | Matcha green tea showed positive effects on cognitive functions and sleep quality. |
| Resveratrol | ||||||||
| [74] Moussa et al., 2017. | RES (500–2000 mg/day) for 52 weeks. | To evaluate the safety and efficacy of RES in AD patients. | 100 AD patients. | ≥49 years. | MMSE ≤ 24, ADAS-Cog ≥ 14. | MMSE, ADAS-Cog, CSF markers, plasma MMP-9, Aβ42 levels. | Weight loss (like placebo group). | RES slowed cognitive decline, improved MMSE, and reduced CSF MMP-9 and Aβ42 levels. Suggests neuroinflammation modulation and immune activation in AD patients. |
| [75] Li B. et al., 2023. | Grape seed procyanidins extract (GSPE). | To evaluate the effect of GSPE on cognitive function in elderly individuals with mild cognitive impairment (MCI). | 71 participants (35 GSPE, 36 placebo). | ≥60 years. | Diagnosis of MCI. | Montreal Cognitive Assessment (MoCA). | No significant adverse effects reported. | No significant improvement in cognitive function with GSPE supplementation over 6 months compared to placebo. |
| [61] Liu X et al., 2025 | Resveratrol (vs. placebo) | To determine whether resveratrol modulates CSF biomarkers of neurodegeneration, inflammation, microglial activation in Alzheimer’s disease. | Placebo (n = 21) vs. resveratrol (n = 30) from a prior multicenter trial | older adult age range | Participants from prior multicenter phase 2 trial of AD — mild-to-moderate Alzheimer’s disease | Biomarkers in CSF: neuron-specific enolase (NSE), phosphorylated neurofilaments (PNF), cathepsin D, MMP-9, TREM2, angiogenin, others. | None significantly different from placebo group. | Resveratrol reduced CSF levels of TREM2, MMP-9, reduced markers of neuronal damage (e.g. NSE, PNF), reduced cathepsin D, altered angiogenin. Suggests anti-inflammatory and neuroprotective effect in AD |
| [55] Zhu et al., 2018. | RES (10 mg/day) + glucose (5 g) + malate (5 g) for 12 months. | To evaluate the effects of RES in MCI patients. | 60 MCI patients. | ≥50 years. | MMSE 18–26, ADAS-Cog 12–30. | MMSE, ADAS-Cog, NPI, CSF biomarkers. | No significant adverse effects reported. | No significant changes in MMSE, ADAS-Cog, or NPI scores compared to placebo. Results indicate no cognitive improvement with RES treatment. |
| [52] Turner et al., 2015. | RES (500–2000 mg/day) for 52 weeks. | To evaluate the effect of RES on cognitive function and Aβ levels in AD patients. | 75 AD patients. | ≥50 years. | MMSE < 24, ADAS-Cog ≥ 14. | MMSE, ADAS-Cog, Aβ40, Aβ42, tau, brain volume. | Nausea, diarrhea (like placebo group). | RES did not improve cognitive scores. (MMSE, ADAS-Cog), and Aβ levels were lower in the placebo group. It slowed brain volume loss, but no greater cognitive or functional benefit. |
| Saffron | ||||||||
| [79] Tsolaki et al., 2020. | Saffron (30 mg/day). | Cognitive impairment management in MCI. | <35 MCI patients. | 50–85 years. | MMSE. | MMSE. | No adverse side effects. | Saffron improved the MMSE score in MCI patients compared to placebo. |
| [78] Farokhnia et al., 2014. | Saffron extract (30 mg/day). | Evaluating efficacy in AD treatment. | 30 AD patients. | 50–85 years. | SCIRS, FAST. | SCIRS, FAST. | No adverse effects. | Saffron had similar efficacy to memantine in reducing cognitive decline in AD. |
| Sesame | ||||||||
| [69] Jung et al., 2021. | Sesame oil cake extract (SOCE). | Cognitive improvement in MCI. | 45 MCI patients. | 50–85 years. | CNT, Aβ (1–40) levels. | CNT, Aβ levels. | No significant adverse effects. | SOCE improved cognitive function and reduced Aβ levels in MCI patients. |
| [84] Ito et al., 2018. | Sesamin (10 mg/day) and Astaxanthin (6 mg/day). | Cognitive function improvement in MCI. | 50 MCI patients. | 50–85 years. | Processing speed, task complexity. | Processing speed, task complexity. | Dizziness, cold, diarrhea. | The combination of sesamin and astaxanthin improved cognitive function in MCI patients. |
| Ginseng and Ginkgo biloba | ||||||||
| [45] Heo et al., 2012 | Ginseng (SG-135, 4.5 mg/day). | Evaluate cognitive effects in AD patients. | 40 AD patients. | 65–85 years. | MMSE, ADAS-Cog. | MMSE, ADAS-Cog at 12 and 24 weeks. | Mild GI issues in 21% of participants. | SG-135 improved MMSE, ADAS-Cog, showing positive cognitive effects in AD. |
| [85] Kim et al., 2013 | Ginkgo biloba (standardized extract). | Investigate neuroprotective effects in AD. | 50 AD patients. | 65–85 years. | ADAS-Cog, MMSE. | ADAS-Cog, MMSE, CDR-SB. | GI discomfort (minor). | Ginkgo biloba improved cognitive function, reduced amyloid deposition, neuroprotective. |
| [46] Yakoot et al., 2013 | Ginkgo biloba (standardized extract). | Examine neuroprotective effects in AD. | 30 AD patients. | 60–80 years. | MMSE, ADAS-Cog. | MMSE, ADAS-Cog, CDR-SB. | Headache (minor), GI discomfort. | Ginkgo biloba enhanced brain circulation, improving cognitive function. |
| [86] Herrschaft et al., 2012 | Ginkgo biloba extract EGb 761® (240 mg/day). | Evaluate the efficacy and safety of EGb 761® in dementia with neuropsychiatric features. | 410 patients with dementia (Alzheimer’s or vascular). | ≥50 years. | MMSE 10–24. | Neuropsychiatric Inventory (NPI), ADAS-Cog, CIBIC-Plus. | No serious adverse effects reported. | EGb 761® significantly improved neuropsychiatric symptoms and cognitive function compared to placebo. |
| [87] Ihl et al., 2012 | Ginkgo biloba extract EGb 761® (240 mg/day). | Evaluate the efficacy and tolerability of EGb 761® in AD and vascular dementia. | 404 patients with Alzheimer’s or vascular dementia. | ≥50 years. | MMSE 10–26. | ADAS-Cog, SKT, CGI. | No serious adverse effects reported. | EGb 761® improved cognition and daily activities compared to placebo. |
| [88] Vellas et al., 2012 | Ginkgo biloba extract EGb 761® (240 mg/day). | Assess long-term use of EGb 761® for preventing AD in elderly individuals with memory complaints. | 2854 subjects without dementia. | ≥70 years. | MMSE ≥26. | Incidence of AD, ADAS-Cog, CDR. | No significant differences in adverse events between groups. | No significant reduction in Alzheimer’s incidence with EGb 761® compared to placebo. |
| Curcumin | ||||||||
| [91] Ringman et al., 2012 | Curcumin (C3 Complex® 2–4 g/day). | Assess Curcumin’s effect on cognitive decline. | 60 AD patients. | 65–85 years. | MMSE, ADAS-Cog, NPI. | MMSE, ADAS-Cog, NPI, CSF Aβ, tau, p-tau. | Minor GI issues (21%). | Curcumin did not significantly improve cognitive scores or Aβ biomarkers in AD. |
| Melissa officinalis | ||||||||
| [60] Noguchi-Shinohara et al., 2020. | Melissa officinalis (500 mg rosmarinic acid). | Investigating cognitive effects in mild AD. | 23 AD patients. | 60–80 years. | MMSE, ADAS-Cog, DAD, CDR. | MMSE, ADAS-Cog, NPI-Q. | No serious adverse events. | No significant cognitive improvement; NPI-Q improved irritability. |
| [93] Noguchi-Shinohara et al., 2023. | Melissa officinalis (500 mg rosmarinic acid). | Investigating effect on cognitive decline. | 323 older adults. | 65–85 years. | Clinical Dementia Rating (CDR). | CDR, cognitive function tests. | No serious adverse events. | M. officinalis reduced cognitive decline in adults without hypertension. |
| Spirulina | ||||||||
| [96] Choi et al., 2022. | Spirulina maxima (70% ethanol extract). | Investigate cognitive and memory improvement. | 80 MCI patients. | 50–80 years. | CNT, MoCA, Aβ biomarkers. | CNT, MoCA, plasma antioxidant capacity. | No significant side effects. | Spirulina improved visual, verbal memory, and antioxidant levels in MCI patients. |
| [97] Tamtaji et al., 2023. | Spirulina (500 mg/day). | Assess cognitive and metabolic effects in AD. | 60 AD patients. | 65–85 years. | MMSE. | MMSE, metabolic markers. | No significant side effects. | Spirulina significantly improved MMSE and metabolic parameters in AD patients. |
| Citrus | ||||||||
| [99] Galluzzi et al., 2022 | Citrus peel extract (rich in AUR and NAR). | Investigate cognitive and biomarker effects. | 50 older adults. | 60–80 years. | Subjective cognitive decline (SCD). | Cognitive tests, biomarkers of oxidative stress. | No significant adverse events. | Citrus extract improved cognition and biomarkers in SCD patients. |
3.12. Overall Quality Assessment
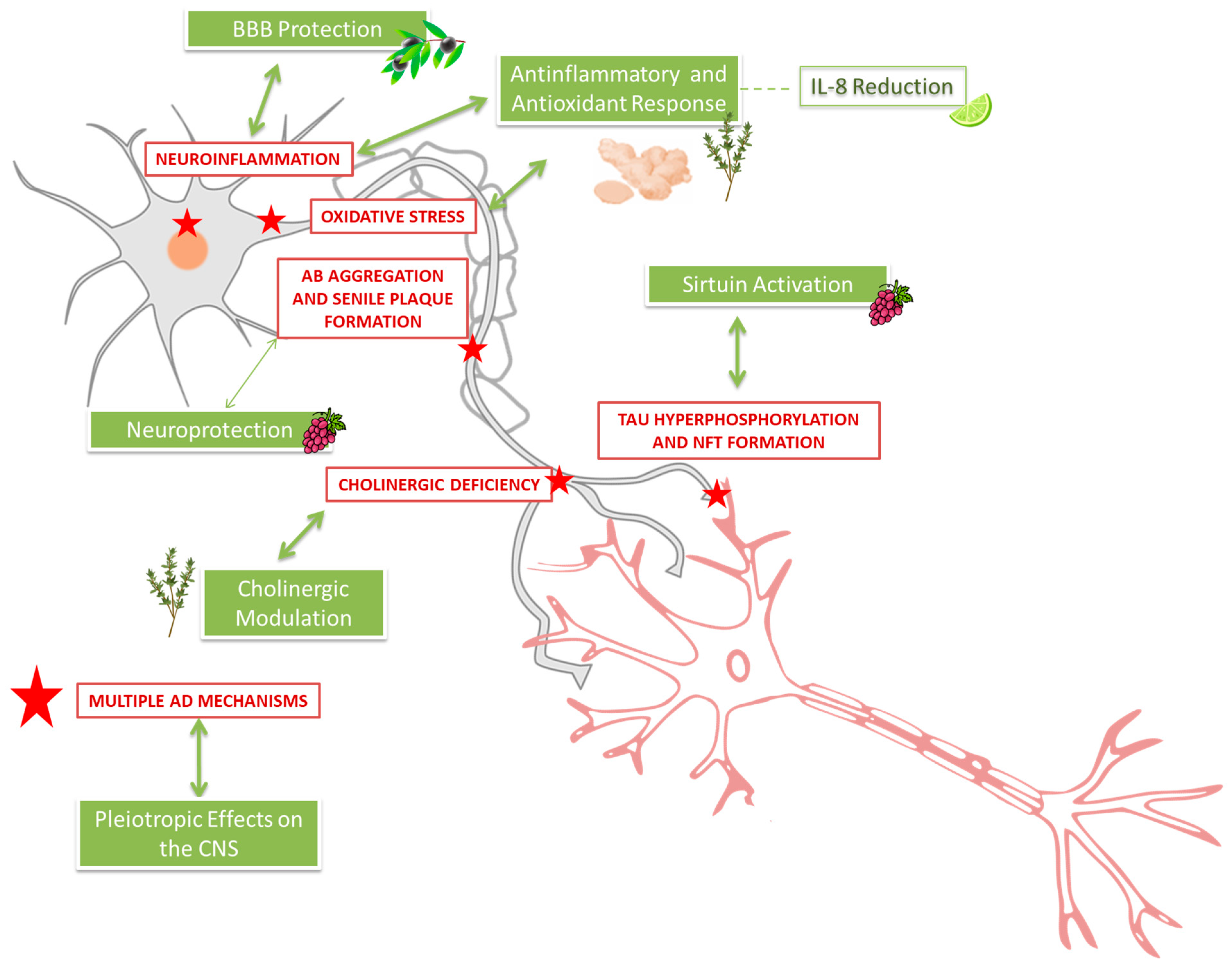
3.13. Molecular Mechanisms in Alzheimer’s Disease and Their Coverage in Clinical Trials
3.13.1. Key Molecular Mechanisms in AD Pathology
3.13.2. Coverage of Mechanisms by the Reviewed Clinical Trials
3.13.3. Underexplored or Uncovered Mechanisms in Clinical Trials
- -
- -
- -
- -
- Hippocampal neurogenesis: Humanin and related peptides under investigation, no trials included here [116].
- -
3.14. Synthesis of Results
4. Conclusions
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| Ach | Acetylcholine |
| AchE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| AD7C-NTP | Alzheimer-associated neuronal thread protein |
| ADAS-Cog | Cognitive subscale of the AD Assessment Scale |
| ADCS-ADL | AD Cooperative Study-Activities of Daily Living |
| ADL | Activities of Daily Living |
| aMCI | Amnestic mild cognitive impairment |
| APMC | Aloe polymannose multinutrient complex |
| AVLT | Auditory Verbal Learning Test |
| Aβ | Amyloid β-peptide |
| Aβ42 | Amyloid-β protein 42 |
| BHY | Bushenhuatanyizhi |
| BPSD | Behavioral and psychological symptoms of dementia |
| BSC | Bushen capsules |
| CDR | Clinical Dementia Rating |
| CDR-SOB | Clinical Dementia Rating Scale Sum of Boxes |
| CM-SS | ADAS-Cognitive Chinese Medicine Symptom Scale |
| CNSVS | Central Nervous System Vital Signs |
| CNT | Computerized neurocognitive function test |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| DAD | Disability Assessment for Dementia scale |
| DL | Davaie Loban |
| DSM-V | Diagnostic and Statistical Manual of Mental Disorders |
| DSS | Digital Symbol Substitution (subtest of the Wechsler Adult Intelligence Scale) |
| ECG | Electrocardiogram |
| EEG | Electroencephalogram |
| FAQ | Functional Activity Questionnaire |
| FAST | Functional Assessment Staging |
| FGF | Fibroblast growth factor |
| FIM | Functional Independence Measure |
| FRSSD | Functional Rating Scale of Symptoms of Dementia |
| GDS | Geriatric Depression Scale |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HAMD | Hamilton Depression Rating Scale |
| HYF | Huannao Yicong Formula |
| IL | Interleukin |
| JYF | Jiannao Yizhi Formula |
| KGT | Kami-guibi-tang |
| LDL | Low-density lipoprotein |
| MCI | Mild cognitive impairment |
| MDC | Macrophage-derived chemokine |
| MMP | Matrix metalloproteinase |
| MMPs | Metalloproteinases |
| MMSE | Modified Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment score |
| MRI | Magnetic resonance imaging |
| NCT | National Clinical Trial |
| NFTs | Neurofibrillary tangles |
| NMDA | N-methyl-D-aspartate |
| NPI | Neuropsychiatric Inventory |
| NPI-Q | Neuropsychiatric Inventory Brief Questionnaire Form |
| NTB | Neuropsychological Test Battery |
| NYT | Ninjin’yoeito formula |
| PET | Positron emission tomography |
| PHF-tau | Paired helical filament |
| PON1 | Paraoxonase 1 |
| RES | Resveratrol |
| ROCF | Rey Osterrieth Complex Figure test |
| SCIRS | Severe Cognitive Impairment Rating Scale |
| SM70EE | Spirulina maxima 70% ethanol extract |
| SNSB | Seoul Neuropsychological Screening Battery |
| SOCE | Sesame oil cake extract |
| sVOI | Standardized volumes of interest |
| Tau | Microtubule-associated protein tau |
| TBARS | Thiobarbituric acid reactive substances |
| TNF-α | Tumor necrosis factor alpha |
| YHD | Yishen Huazhuo decoction |
References
- Akhondzadeh, S.; Shafiee Sabet, M.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi, S.S.; Yousefi, M.H.; Alimardani, R.; Jamshidi, A.H. A 22-week, multi-center, randomized, double-blind controlled trial of Crocus sativus in the treatment of mild-to-moderate Alzheimer’s disease. Psychopharmacology 2010, 207, 637–643. [Google Scholar] [CrossRef]
- Tajadini, H.; Choopani, R.; Saifadini, R. Lifestyle Methods for Prevention of Alzheimer’s Disease From the Perspective of Traditional Persian Medicine. J. Evid. Based Complement. Altern. Med. 2016, 21, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, N.; He, L.; Wang, J.; Yang, Y.; Ping, F.; Xu, L.; Zhang, H.; Li, W.; Li, Y. Global Burden of Dementia Death from 1990 to 2019, with Projections to 2050: An Analysis of 2019 Global Burden of Disease Study. J. Prev. Alzheimers Dis. 2024, 11, 1013–1021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomino, C.; Ilari, S.; Solfrizzi, V.; Malafoglia, V.; Zilio, G.; Russo, P.; Proietti, S.; Marcolongo, F.; Scapagnini, G.; Muscoli, C.; et al. Mild Cognitive Impairment and Mild Dementia: The Role of Ginkgo biloba (EGb 761®). Pharmaceuticals 2021, 14, 305. [Google Scholar] [CrossRef]
- Alzheimer’s Disease International. Dementia Statistics. Available online: https://www.alzint.org/about/dementia-facts-figures/dementia-statistics/ (accessed on 14 June 2025).
- Geda, Y.E. Mild cognitive impairment in older adults. Curr. Psychiatry Rep. 2012, 14, 320–327. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Ohta, Y.; Shimizu, M.; Maruyama, J.; Mochizuki, M. Meta-analysis of the efficacy and safety of Ginkgo biloba extract for the treatment of dementia. J. Pharm. Health Care Sci. 2015, 1, 14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cardoso, B.R.; Busse, A.L.; Hare, D.J.; Cominetti, C.; Horst, M.A.; McColl, G.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M. Pro198Leu polymorphism affects the selenium status and GPx activity in response to Brazil nut intake. Food Funct. 2016, 7, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, C.; Zhao, A.; Wang, Z.; Zuo, S.; Lin, L. Effects of reminiscence therapy on cognitive function in older adults with cognitive impairment: A systematic review and meta-analysis of randomized controlled trials. Arch. Gerontol. Geriatr. 2025, 139, 106021. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Andrieu, S.; Sampaio, C.; Carrillo, M.C.; Khachaturian, Z.S.; Dubois, B.; Feldman, H.H.; Petersen, R.C.; Siemers, E.; Stern, Y. Report of the task force on designing clinical trials in early (predementia) AD. Neurology 2011, 76, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Cerejeira, J.; Lagarto, L.; Mukaetova-Ladinska, E.B. Behavioral and psychological symptoms of dementia. Front. Neurol. 2012, 3, 73. [Google Scholar] [CrossRef]
- Steinberg, M.; Shao, H.; Zandi, P.; Lyketsos, C.G.; Welsh-Bohmer, K.A.; Norton, M.C.; Tschanz, J.T. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: The Cache County Study. Int. J. Geriatr. Psychiatry 2008, 23, 170–177. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, E.; Rajmohan, V.; Raghunath, B. Neurobiology of Alzheimer’s disease. Indian J. Psychiatry 2009, 51, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Fratiglion, L.; Johansson, B.; Berg, S.; Mortimer, J.A.; Reynolds, C.A.; Fiske, A.; Pedersen, N.L. Complete ascertainment of dementia in the Swedish Twin Registry: The HARMONY study. Neurosci. Lett. 2006, 16, 687–693. [Google Scholar] [CrossRef]
- Ihara, R.; Iwata, A.; Suzuki, K.; Ikeuchi, T.; Kuwano, R.; Iwatsubo, T.; Japanese Alzheimer’s Disease Neuroimaging Initiative. Clinical and cognitive characteristics of preclinical Alzheimer’s disease in the Japanese Alzheimer’s Disease Neuroimaging Initiative cohort. Alzheimers Dement. 2018, 4, 645–651. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef]
- Mudher, A.; Lovestone, S. Alzheimer’s disease—Do tauists and baptists finally shake hands? Trends Neurosci. 2002, 25, 22–26. [Google Scholar] [CrossRef]
- Nordberg, A.; Winblad, B. Reduced number of [3H]nicotine and [3H]acetylcholine binding sites in the frontal cortex of Alzheimer brains. Neurosci. Lett. 1986, 72, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.; Snowden, J.S.; Mann, D.M.; Bowen, D.M.; Sims, N.R.; Northen, B.; Yates, P.O.; Davison, A.N. Alzheimer’s disease: A correlative study. J. Neurol. Neurosurg. Psychiatry 1986, 49, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Ruthirakuhan, M.; Guan, D.X.; Mortby, M.; Gatchel, J.; Babulal, G.M. Updates and future perspectives on neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2025, 21, e70079. [Google Scholar] [CrossRef] [PubMed]
- Parsons, C.G.; Stoffler, A.; Danysz, W. Memantine: A NMDA receptor antagonist that improves memory by restoration of homeostasis in the glutamatergic system--too little activation is bad, too much is even worse. Neuropsychopharmacology 2007, 32, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Ramassamy, C.; Doré, S.; Christen, Y.; Poirier, J.; Quirion, R. The Ginkgo biloba extract (EGb 761) protects hippocampal neurons against cell death induced by β-amyloid. Eur. J. Neurosci. 2000, 12, 1882–1890. [Google Scholar] [CrossRef]
- Smith, J.V.; Luo, Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J. Alzheimers Dis. 2003, 5, 287–300. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 1207, 1109–1122. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-β in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.B.; Hoda, M.N.; Bhatia, K.; Haque, R.; Fazili, I.S.; Jamal, A.; Khan, J.S.; Katare, D.P. Neuroprotective effect of sesame seed oil in 6-hydroxydopamine-induced neurotoxicity in mice model. Neurochem. Res. 2012, 37, 516–526. [Google Scholar] [CrossRef]
- Ahmed, H.H.; Salem, A.M.; Sabry, G.M.; Husein, A.A.; Kotob, S.E. Possible therapeutic uses of Salvia triloba and Piper nigrum in Alzheimer’s disease-induced rats. J. Med. Food 2013, 16, 437–446. [Google Scholar] [CrossRef]
- Talebi, M.; İlgün, S.; Ebrahimi, V.; Farkhondeh, T.; Ebrahimi, H.; Samarghandian, S. Zingiber officinale ameliorates Alzheimer’s disease and cognitive impairments: Lessons from preclinical studies. Biomed. Pharmacother. 2021, 133, 111088. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Farghaly, H.A.; Abdel-Wadood, Y.A.; Gomaa, G.A. Potential therapeutic effects of boswellic acids/Boswellia serrata extract in the prevention and therapy of type 2 diabetes and Alzheimer’s disease. Naunyn-Schmiedebergs Arch. Pharmacol. 2021, 394, 2167–2185. [Google Scholar] [CrossRef]
- Gregory, J.; Vengalasetti, Y.V.; Bredesen, D.E.; Rao, R.V. Neuroprotective herbs for the management of Alzheimer’s disease. Biomolecules 2021, 11, 468. [Google Scholar] [CrossRef]
- Caruso, G.; Torrisi, S.A.; Mogavero, M.P.; Currenti, W.; Castellano, S.; Godos, J.; Ferri, R.; Galvano, F.; Leggio, G.M.; Grosso, G.; et al. Polyphenols and neuroprotection: Therapeutic implications for cognitive decline. Pharmacol. Ther. 2022, 232, 108013. [Google Scholar] [CrossRef]
- De Giuseppe, R.; Colleoni, M.; Cremaschi, M.; Daconto, L.; Di Napoli, I.; Gallace, A.; Guzzetti, L.; Labra, M.; Maurino, A.; Tomasinelli, C.E.; et al. How to preserve healthy aging through nutritional strategies: The Food NET project. Mediterr. J. Nutr. Metab. 2022, 15, 91–101. [Google Scholar] [CrossRef]
- Godos, J.; Grosso, G. Dietary antioxidants and brain health: Focus on cognitive and affective disorders. Antioxidants 2021, 10, 1659. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Navarro -Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Vera-Ramírez, L.; Forbes-Hernández, T.J.; Esteban-Muñoz, A.; Giampieri, F.; Bullón, P.; Sánchez-González, C.; Quiles, J.L. An oleuropein-rich olive leaf extract reduces β-amyloid and tau proteotoxicity through regulation of oxidative- and heat shock-stress responses. Food Chem. Toxicol. 2022, 162, 112914. [Google Scholar]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Sánchez-González, C.; Rivas-García, L.; Llopis, J.; Cianciosi, D.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; et al. Strawberry methanolic extract attenuates Alzheimer’s β-amyloid production and oxidative stress by SKN-1/NRF and DAF-16/FOXO mediated mechanisms in C. elegans. Food Chem. 2022, 372, 131272. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Muñoz-Ollero, P.; Jiménez-Trigo, V.; Esteban-Muñoz, A.; Tutusaus, K.; Giampieri, F.; Battino, M.; Sánchez-González, C.; Rivas-García, L.; et al. Amyloid β-but not Tau-induced neurotoxicity is suppressed by Manuka honey via HSP-16.2 and SKN-1/Nrf2 pathways in an in vivo model of Alzheimer’s disease. Food Funct. 2022, 13, 11185–11199. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Natural compounds for Alzheimer’s disease therapy: A systematic review of preclinical and clinical studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Auti, S.T.; Kulkarni, Y.A. A systematic review on the role of natural products in modulating the pathways in Alzheimer’s disease. Int. J. Vitam. Nutr. Res. 2017, 87, 99–116. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective natural products for Alzheimer’s disease. Cells 2021, 10, 613. [Google Scholar] [CrossRef]
- Heo, J.-H.; Lee, S.-T.; Chu, K.; Oh, M.-J.; Park, H.-J.; Shim, J.-Y.; Kim, M. Heat-processed ginseng enhances the cognitive function in patients with moderately severe Alzheimer’s disease: A randomized controlled trial. Nutr. Neurosci. 2012, 15, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Yakoot, M.; Salem, A.; Helmy, S. Effect of Memo, a natural formula combination, on Mini-Mental State Examination scores in patients with mild cognitive impairment: A randomized double-blind placebo-controlled trial. Clin. Interv. Aging 2013, 8, 975–981. [Google Scholar] [CrossRef]
- Sadhu, A.; Upadhyay, P.; Agrawal, A.; Ilango, K.; Karmakar, D.; Singh, G.P.I.; Dubey, G.P. Management of cognitive determinants in senile dementia of Alzheimer’s type: Therapeutic potential of a novel polyherbal drug product. Clin. Drug Investig. 2014, 34, 1235–1243. [Google Scholar] [CrossRef]
- Lewis, J.E.; McDaniel, H.R.; Agronin, M.E.; Loewenstein, D.A.; Riveros, J.; Mestre, R.; Martinez, M.; Colina, N.; Abreu, D.; Konefal, J.; et al. The effect of an aloe polymannose multinutrient complex on cognitive and immune functioning in Alzheimer’s disease. J. Alzheimers Dis. 2013, 33, 359–365. [Google Scholar] [CrossRef]
- Liu, P.; Kong, M.; Liu, S.; Chen, G.; Wang, P. Effect of reinforcing kidney-essence, removing phlegm, and promoting mental therapy on treating Alzheimer’s disease. J. Tradit. Chin. Med. 2013, 33, 291–295. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, C.; Zhang, L.; Cui, Y.; Gu, Y.; Guo, J.; Wu, D.; Li, Q.; Song, W. Cognitive improvement during treatment for mild Alzheimer’s disease with a Chinese herbal formula: A randomized controlled trial. PLoS ONE 2015, 10, e0130353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, K.; Wei, D.; Guo, R.; Li, H.; Wang, Y.; Zhang, Z. The effects of Bushen capsule on episodic memory in amnestic mild cognitive impairment patients: A pilot placebo-controlled fMRI study. J. Alzheimers Dis. 2015, 46, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Furukawa, K.; Tomita, N.; Uematsu, D.; Okahara, K.; Shimada, H.; Ikeda, M.; Matsui, T.; Kozaki, K.; Fujii, M.; Ogawa, T.; et al. Randomized double-blind placebo-controlled multicenter trial of Yokukansan for neuropsychiatric symptoms in Alzheimer’s disease. Geriatr. Gerontol. Int. 2017, 17, 214–221. [Google Scholar] [CrossRef]
- Lee, J.; Torosyan, N.; Silverman, D.H. Examining the impact of grape consumption on brain metabolism and cognitive function in patients with mild decline in cognition: A double-blinded placebo-controlled pilot study. Exp. Gerontol. 2017, 87, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.W.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate to slow progression of Alzheimer’s disease. Alzheimers Dement. Transl. Res. Clin. Interv. 2018, 4, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.-P.; Fang, J.-Y.; Wang, H.-C.; Wei, Y.; Cao, Y.; Liu, J.-G.; Liu, L.-T.; Li, H. Effect and safety of Huannao Yicong formula in patients with mild-to-moderate Alzheimer’s disease: A randomized, double-blinded, donepezil-controlled trial. Chin. J. Integr. Med. 2019, 25, 1636–1644. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, C.; Wei, D.; Li, H.; Leung, E.L.-H.; Deng, Q.; Liu, Z.; Fan, X.-X.; Zhang, Z. Long-term efficacy of Chinese medicine Bushen Capsule on cognition and brain activity in patients with amnestic mild cognitive impairment. Pharmacol. Res. 2019, 146, 104319. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Liu, N.-Y.; Zhang, S.; Yang, Y.; Wang, Z.-Y.; Wei, Y.; Liu, J.-G.; Pei, H.; Li, H. Clinical experience in treatment of Alzheimer’s disease with Jiannao Yizhi formula and routine western medicine. Chin. J. Integr. Med. 2020, 26, 249–256. [Google Scholar] [CrossRef]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A randomized clinical trial of Greek high phenolic early harvest extra virgin olive oil in mild cognitive impairment: The MICOIL pilot study. J. Alzheimers Dis. 2020, 78, 589–603. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Hamaguchi, T.; Sakai, K.; Komatsu, J.; Iwasa, K.; Horimoto, M.; Nakamura, H.; Yamada, M.; Ono, K. Effects of Melissa officinalis Extract Containing Rosmarinic Acid on Cognition in Older Adults Without Dementia: A Randomized Controlled Trial. J. Alzheimers Dis. 2023, 91, 805–814. [Google Scholar] [CrossRef]
- Liu, X.; Baxley, S.; Hebron, M.; Turner, R.S.; Moussa, C. Resveratrol Attenuates CSF Markers of Neurodegeneration and Neuroinflammation in Individuals with Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 5044. [Google Scholar] [CrossRef]
- Zhang, Y.; Noh, K.; Song, W. Chinese herbal medicines on cognitive function and activity of daily living in senior adults with Alzheimer’s disease: A systematic review and meta-analysis. Integr. Med. Res. 2019, 8, 92–100. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Xu, Z.; Liu, S.; Liang, X.; Zhang, Y.; Jin, X. Clinical efficacy and safety of compound Congrong Yizhi Capsules on Alzheimer’s disease in mainland China: A systematic review with trial sequential analysis and GRADE assessment. J. Ethnopharmacol. 2023, 309, 116208. [Google Scholar] [CrossRef]
- Tajadini, H.; Saifadini, R.; Choopani, R.; Mehrabani, M.; Kamalinejad, M.; Haghdoost, A.A. Herbal medicine Davaie Loban in mild to moderate Alzheimer’s disease: A 12-week randomized double-blind placebo-controlled clinical trial. Complement. Ther. Med. 2015, 23, 767–772. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Revised Cochrane Risk-of-Bias Tool for Randomized Trials (RoB 2): Guidance Document. Version 6.0 (Updated July 2019). Available online: https://www.riskofbias.info (accessed on 14 June 2025).
- Friedli, M.J.; Inestrosa, N.C. Huperzine A and Its Neuroprotective Molecular Signaling in Alzheimer’s Disease. Molecules 2021, 26, 6531. [Google Scholar] [CrossRef]
- Kudoh, C.; Arita, R.; Honda, M.; Kishi, T.; Komatsu, Y.; Asou, H.; Mimura, M. Effect of ninjin’yoeito, a Kampo (traditional Japanese) medicine, on cognitive impairment and depression in patients with Alzheimer’s disease: 2 years of observation. Psychogeriatrics 2016, 16, 85–92. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.J.; Jung, E.S.; Ha, K.C.; Baek, H.I.; Park, Y.K.; Han, S.K.; Chae, S.W.; Lee, S.O.; Chung, Y.C. Efficacy and Safety of Sesame Oil Cake Extract on Memory Function Improvement: A 12-Week, Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2021, 13, 2606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchida, K.; Meno, K.; Korenaga, T.; Liu, S.; Suzuki, H.; Baba, Y.; Tagata, C.; Araki, Y.; Tsunemi, S.; Aso, K.; et al. Effect of matcha green tea on cognitive functions and sleep quality in older adults with cognitive decline: A randomized controlled study over 12 months. PLoS ONE 2024, 19, e0309287. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Kim, H.R.; Jahng, G.H.; Jin, C.; Kwon, S.; Cho, S.Y.; Park, S.U.; Jung, W.S.; Moon, S.K.; Ko, C.N.; et al. Efficacy and safety of Kami-guibi-tang for mild cognitive impairment: A pilot, randomized, double-blind, placebo-controlled trial. BMC Complement. Med. Ther. 2021, 21, 251. [Google Scholar] [CrossRef]
- Sun, A.Y.; Wang, Q.; Simonyi, A.; Sun, G.Y. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol. Neurobiol. 2010, 41, 375–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, M.M.; Chen, J.L.; Lee, T.H.; Liu, H.; Shanmugam, V.; Hsieh, H.L. Brain Protective Effect of Resveratrol via Ameliorating Interleukin-1β-Induced MMP-9-Mediated Disruption of ZO-1 Arranged Integrity. Biomedicines 2022, 10, 1270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflammation 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cheng, J.; Cheng, G.; Zhu, H.; Liu, B.; Yang, Y.; Dai, Q.; Li, W.; Bao, W.; Rong, S. The effect of grape seed procyanidins extract on cognitive function in elderly people with mild cognitive impairment: A randomized, double-blind, placebo-controlled clinical trial. Heliyon 2023, 9, e16994. [Google Scholar] [CrossRef]
- Azargoonjahromi, A.; Abutalebian, F. Unraveling the therapeutic efficacy of resveratrol in Alzheimer’s disease: An umbrella review of systematic evidence. Nutr. Metab. 2024, 21, 15. [Google Scholar] [CrossRef]
- Ayati, Z.; Yang, G.; Ayati, M.H.; Emami, S.A.; Chang, D. Saffron for mild cognitive impairment and dementia: A systematic review and meta-analysis of randomised clinical trials. BMC Complement. Med. Ther. 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Farokhnia, M.; Shafiee Sabet, M.; Iranpour, N.; Gougol, A.; Yekehtaz, H.; Alimardani, R.; Farsad, F.; Kamalipour, M.; Akhondzadeh, S. Comparing the efficacy and safety of Crocus sativus L. with memantine in patients with moderate to severe Alzheimer’s disease: A double-blind randomized clinical trial. Hum. Psychopharmacol. 2014, 29, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, M.; Karathanasi, E.; Lazarou, I.; Dovas, K.; Verykouki, E.; Karacostas, A.; Georgiadis, K.; Tsolaki, A.; Adam, K.; Kompatsiaris, I.; et al. Efficacy and Safety of Crocus sativus L. in Patients with Mild Cognitive Impairment: One Year Single-Blind Randomized, with Parallel Groups, Clinical Trial. J. Alzheimers Dis. 2020, 75, 613–623. [Google Scholar] [CrossRef]
- Kaji, H.; Matsui-Yuasa, I.; Matsumoto, K.; Omura, A.; Kiyomoto, K.; Kojima-Yuasa, A. Sesaminol prevents Parkinson’s disease by activating the Nrf2-ARE signaling pathway. Heliyon 2020, 6, e05342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, P.; Zhao, F.; Wang, Z.; Wang, Q.; Chai, X.; Hou, G.; Meng, Q. Sesame (Sesamum indicum L.): A Comprehensive Review of Nutritional Value, Phytochemical Composition, Health Benefits, Development of Food, and Industrial Applications. Nutrients 2022, 14, 4079. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akhondzadeh, S.; Sabet, M.S.; Harirchian, M.H.; Togha, M.; Cheraghmakani, H.; Razeghi, S.; Hejazi SSh Yousefi, M.H.; Alimardani, R.; Jamshidi, A.; Zare, F.; et al. Saffron in the treatment of patients with mild to moderate Alzheimer’s disease: A 16-week, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2010, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Z.; Zuo, Z.W.; Liu, Y. Recent status of sesaminol and its glucosides: Synthesis, metabolism, and biological activities. Crit. Rev. Food Sci. Nutr. 2023, 63, 12043–12056. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of Composite Supplement Containing Astaxanthin and Sesamin on Cognitive Functions in People with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimers Dis. 2018, 62, 1767–1775, Erratum in J. Alzheimers Dis. 2019, 68, 839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morató, X.; Marquié, M.; Tartari, J.P.; Lafuente, A.; Abdelnour, C.; Alegret, M.; Jofresa, S.; Buendía, M.; Pancho, A.; Aguilera, N.; et al. A randomized, open-label clinical trial in mild cognitive impairment with EGb 761 examining blood markers of inflammation and oxidative stress. Sci. Rep. 2023, 13, 5406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ihl, R.; Bachinskaya, N.; Korczyn, A.D.; Vakhapova, V.; Tribanek, M.; Hoerr, R.; Napryeyenko, O.; GOTADAY Study Group. Efficacy and safety of a once-daily formulation of Ginkgo biloba extract EGb 761 in dementia with neuropsychiatric features: A randomized controlled trial. J. Alzheimers Dis. 2012, 31, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Vellas, B.; Coley, N.; Ousset, P.J.; Berrut, G.; Dartigues, J.F.; Dubois, B.; Grandjean, H.; Pasquier, F.; Piette, F.; Robert, P.; et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): A randomised placebo-controlled trial. Lancet Neurol. 2012, 11, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Cole, G.M.; Masterman, D.L.; Cummings, J.L. A potential role of the curry spice curcumin in Alzheimer’s disease. Curr. Alzheimer Res. 2005, 2, 131–136. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and cognition: A randomised, placebo-controlled, double-blind study of community-dwelling older adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef] [PubMed]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Nagai, T.; Kobayashi, S.; Komatsu, J.; Samuraki-Yokohama, M.; Iwasa, K.; Yokoyama, K.; Nakamura, H.; et al. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 2020, 10, 18627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capelli, B.; Cysewski, G.R. Potential health benefits of spirulina microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, W.K.; Kim, T.H.; Ryu, Y.K.; Park, A.; Lee, Y.J.; Heo, S.J.; Oh, C.; Chung, Y.C.; Kang, D.H. The Effects of Spirulina maxima Extract on Memory Improvement in Those with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2022, 14, 3714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tamtaji, O.R.; Heidari-Soureshjani, R.; Asemi, Z.; Kouchaki, E. The effects of spirulina intake on clinical and metabolic parameters in Alzheimer’s disease: A randomized, double-blind, controlled trial. Phytother. Res. 2023, 37, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galluzzi, S.; Marizzoni, M.; Gatti, E.; Bonfiglio, N.S.; Cattaneo, A.; Epifano, F.; Frisoni, G.B.; Genovese, S.; Geviti, A.; Marchetti, L.; et al. Citrus supplementation in subjective cognitive decline: Results of a 36-week, randomized, placebo-controlled trial. Nutr. J. 2024, 23, 135. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Shao, S.; Ye, X.; Su, W.; Wang, Y. Curcumin alleviates Alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J. Chem. Neuroanat. 2023, 134, 102363. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Hu, Q.; Xiao, X.; Ou, L.; Chen, Y.; Luo, S.; Cheng, Y.; Jiang, Y.; Ma, X.; et al. The emerging possibility of the use of geniposide in the treatment of cerebral diseases: A review. Chin. Med. 2021, 16, 86. [Google Scholar] [CrossRef]
- Al Rihani, S.B.; Darakjian, L.I.; Kaddoumi, A. Oleocanthal-Rich Extra-Virgin Olive Oil Restores the Blood-Brain Barrier Function through NLRP3 Inflammasome Inhibition Simultaneously with Autophagy Induction in TgSwDI Mice. ACS Chem. Neurosci. 2019, 10, 3543–3554. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D. If amyloid drives Alzheimer disease, why have anti-amyloid therapies not yet slowed cognitive decline? PLoS Biol. 2022, 20, e3001694. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, D.; Liu, Y.; Li, L. Geniposide-mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer’s disease. Aging 2019, 11, 536–548. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zheng, W.; Wang, T.; Xie, J.W.; Wang, S.L.; Zhao, B.L.; Teng, W.P.; Wang, Z.Y. Huperzine A activates Wnt/β-catenin signaling and enhances the nonamyloidogenic pathway in an Alzheimer transgenic mouse model. Neuropsychopharmacology 2011, 36, 1073–1089. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Gasiorowska, A.; Wydrych, M.; Drapich, P.; Zadrozny, M.; Steczkowska, M.; Niewiadomski, W.; Niewiadomska, G. The Biology and Pathobiology of Glutamatergic, Cholinergic, and Dopaminergic Signaling in the Aging Brain. Front. Aging Neurosci. 2021, 13, 654931. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, F.; Ma, X.; Perry, G.; Zhu, X. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: Recent advances. Mol. Neurodegener. 2020, 15, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Dreses-Werringloer, U.; Vingtdeux, V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009, 4, 20. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Sulaimee, N.H.B.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.M.; Kesavapany, S. Curcumin Ameliorates Neuroinflammation, Neurodegeneration, and Memory Deficits in p25 Transgenic Mouse Model that Bears Hallmarks of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 6, 1101–1113. [Google Scholar] [CrossRef]
- AlHayani, D.A.; Kubaev, A.; Uthirapathy, S.; Mandaliya, V.; Ballal, S.; Kalia, R.; Arya, R.; Gabble, B.C.; Alasheqi, M.Q.; Kadhim, A.J. Insights Into the Therapeutic Potential of SIRT1-modifying Compounds for Alzheimer’s Disease: A Focus on Molecular Mechanisms. J. Mol. Neurosci. 2025, 75, 1–21. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Kurita, M.; Aiso, S.; Nishimoto, I.; Matsuoka, M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol. Biol. Cell. 2009, 20, 2864–2873. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.K.; Nigam, S.V.; Weitz, J.A.; Dave, J.R.; Doctor, B.P.; Ved, H.S. The NMDA receptor ion channel: A site for binding of Huperzine A. J. Appl. Toxicol. 2001, 21, S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Shanmugam, M.K.; Kumar, A.P.; Yap, C.T.; Sethi, G.; Bishayee, A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer 2019, 125, 1228–1246. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Wang, R.; Tang, X.C. Neuroprotective effects of huperzine A. A natural cholinesterase inhibitor for the treatment of Alzheimer’s disease. Neurosignals 2005, 14, 71–82. [Google Scholar] [CrossRef]
- Xu, S.S.; Gao, Z.X.; Weng, Z.; Du, Z.M.; Xu, W.A.; Yang, J.S.; Zhang, M.L.; Tong, Z.H.; Fang, Y.S.; Chai, X.S.; et al. Efficacy of tablet huperzine-A on memory, cognition, and behavior in Alzheimer’s disease. Zhongguo Yao Li Xue Bao 1995, 16, 391–395. [Google Scholar] [PubMed]
- Guo, L.X.; Xia, Z.N.; Gao, X.; Yin, F.; Liu, J.H. Glucagon-like peptide 1 receptor plays a critical role in geniposide-regulated insulin secretion in INS-1 cells. Acta Pharmacol. Sin. 2012, 33, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, F.; Liu, J.; Liu, Z.; Guo, L.; Xia, Z.; Zidichouski, J. Geniposide attenuates insulin-deficiency-induced acceleration of β-amyloidosis in an APP/PS1 transgenic model of Alzheimer’s disease. Neurochem. Int. 2015, 89, 7–16. [Google Scholar] [CrossRef] [PubMed]

| Inclusion Criteria |
|
| Exclusion criteria |
|
| NCT | Conditions | Intervention Design | Outcomes | Study Population | Locations | Phase | Related Publications |
|---|---|---|---|---|---|---|---|
| Sage | |||||||
| 1001637 | AD. | Sage or Salvia officinalis pills or no treatment during a 10-day period. Capsules taken by mouth. | -Tests to determine attention, memory, and visual cognition -EEG and ECG. | 111 subjects (50–90 years old, all genders, no healthy volunteers). | Oregon, USA. | 1 | PMID: 12605619. PMID:2895683. |
| Curcumin | |||||||
| 00099710 | AD. | Two different doses of Curcumin C3 Complex® (2 g per day and 4 g per day) or a placebo, for the initial 6 months of the trial. | -Inflammation, oxidative damage, and cholesterol levels on blood and cerebrospinal fluid. -Cognition, behavior, and daily function. | 33 subjects (>50 years old, all genders, no healthy volunteers). | California, USA. | 2 | PMID: 11571321. PMID: 23107780. |
| 1716637 | AD. | Etanercept (25 mg/week) for 6 weeks. Diet for 6/12 weeks: Curcumin, Omega-3, Quercetin, Resveratrol. | -Cognitive test. | 12 subjects (60–85 years old, all genders, healthy volunteers). | Florida, USA. | 1 | PMID: 18644112. |
| 164749 | AD. | AD patients: placebo (1 g/day) or Curcumin (4 g/day), for six months. All: Ginkgo biloba leaf extract (120 mg/day). | -Cognitive test -Blood samples analyzed for levels of isoprostane, amyloid beta protein, metals, and cholesterol. | 36 subjects (>50 years old, all genders, no healthy volunteers). | Hong Kong, China. | 2 | PMID: 18204357. PMID: 17951067. |
| Ginkgo biloba | |||||||
| 10803 | AD. Dementia. | Ginkgo biloba (240 mg/day) for 6/12 weeks. Or EGb761® (120 mg twice a day) for 6/12 weeks. | -MMSE. -ADAS-Cog. -Neuropsychological domains of memory, attention, visual-spatial construction, language, and executive functions, based on sums of z scores of individual tests. | >1500 subjects (>75 years old, all genders, healthy volunteers). | California, USA. | 3 | PMID: 20040554. PMID: 20123670. PMID: 19017911. |
| 814346 | AD. | EGb761® (120 mg/day) for 18 months. | -18-Fluorodeoxyglucose-PET. -Cognitive tests—verbal fluency. -CDR. -GDS. | 49 subjects (>65 years old, all genders, no healthy volunteers). | France. | 2 | Not provided. |
| 42172 | AD. Cognitive disorders. | Donepezil or placebo for the first 6 months. Donepezil + Ginkgo biloba. | -PET. -MRI. | 40 subjects (>65 years old, all genders, healthy volunteers). | Iowa, USA. | 4 | Not provided. |
| 1009476 | AD. Dementia. | Galantamine (8 mg,16 mg, 24 mg) for 12 months. Nootropics in accordance with the recommendations. | -Tolerability. -Vital functions. -Global GDS. -MMSE. -Dementia-associated behavioral symptoms. | 1134 subjects (>50 years old, all genders, no healthy volunteers). | Spain. | Not indicated. | Not provided. |
| Olive oil | |||||||
| 3824197 | AD. | Extra-virgin olive oil enriched with oleocanthal, and other phenolic compounds added to daily diet. OR Olive oil with low phenolic content added to daily diet. | -Functional MRI imaging. -Blood–brain barrier function by dynamic contrast-enhanced MRI. -Cognitive tests. | 25 subjects (55–75 years old, all genders, no healthy volunteers). | Alabama, USA. | Not applicable. | Not provided. |
| 2921672 | AD. Cognitive impairment. | Diet consisting of fruits, vegetables, grains, dairy, olive oils, seafood, and nuts for 9 weeks. | -Determine feasibility. | 30 subjects (>65 years old, all genders, healthy volunteers). | Kansas, USA. | Not applicable. | Not provided. |
| Grape | |||||||
| 2502253 | AD. Mild cognitive impairment. | Grape seed polyphenolic extract and resveratrol. | -Assessment of adverse events. -Levels of BDPP. -Neuropsychiatric Inventory and Cornell Scale for Depression in Dementia. -Memory, executive function, and attention measures. | 14 subjects (50–90 years old, all genders, no healthy volunteers). | Maryland, USA. | 1 | Not provided. |
| 1504854 | AD. | 500 mg RES by mouth once a day increasing at 13 weeks to a maximum of 1 g twice a day with or without food for one year. | -Volumetric MRI. -ADCS-ADL. | 119 subjects (50–90 years old, all genders, no healthy volunteers). | 26 different locations in USA. | 2 | PMID: 28086917. PMID: 33426901. |
| 0678431 | AD. | RES with glucose and malate. Dietary supplements are delivered in grape juice for one year. | -ADAS-Cog. | 27 subjects (50–90 years old, all genders, no healthy volunteers). | New York, USA. | 3 | PMID: 30480082. |
| 3361410 | AD. | 36 g of grape powder to be taken twice/day (total of 72 g/day) for 12 months or placebo. | -Regional cerebral metabolism, changes in neuropsychological performance measures. | >65 years old, all genders, no healthy volunteers). | LA, USA. | Not applicable. | Not provided. |
| Caffeine | |||||||
| 0726726 | AD | 5 mg Midazolam, 10 mg Warfarin, 10 mg vitamin K, 200 mg caffeine, 40 mg Omeprazole, 30 mg Dextromethorphan daily. | -Pharmacokinetic effects of BMS-708163 on interacting drugs. -Safety variables (adverse events, vital signs, safety labs). | 22 subjects (18–45 years old, male, healthy volunteers). | New Jersey, USA. | 1 | Not provided. |
| 0692510 | AD. | Cocktail mix: CYP1A2 (caffeine), CYP2B6 (Bupropion), CYP2C8 (Rosiglitazone), CYP2C19 (Omeprazole), CYP3A4 (Midazolam), UGT1A1 (Bilirubin). Single dose of mix for 7 days. | -PK variables. -Safety variables (adverse events, vital signs, safety labs). | 18 subjects (18–45 years old, male, healthy volunteers). | Sweden and UK. | 1 | Not provided. |
| 4570085 | AD. | Caffeine (100 mg/day) for 6 weeks, increasing after 3 weeks until 400 mg/day. | -Changes in NTB scores. -MMSE. -NTB subscores. | 248 subjects (>50 years old, all genders, no healthy volunteers). | France. | 3 | Not provided. |
| Ginseng | |||||||
| 0391833 | AD. Memory decline. | Panax ginseng powder (4.5 g/day) for 12 weeks. | -MMSE. -ADAS-Cog. -Biomarkers including hematopoietic progenitor cell count. | 97 subjects (40 years old, female, no healthy volunteers). | Seoul, South Korea. | 1 and 2. | PMID: 18580589. |
| 03221894 | AD. | One capsule/day in 150 mL warm water: 10 g ginseng, 30 g Rehmannia glutinosa, 10 g Acorus tatarinowii, 10 g Polygala tenuifolia, 10 g Epimedium brevicornu, 10 g Cornus officinalis, 10 g Cistanche deserticola, 10 g Curcuma aromatic, 10 g Salvia miltiorrhiza, 10 g Angelica sinensis, 10 g Gastrodia elata, and 10 g Berberine. | -MMSE. -ADCS-ADL. -CDR. | 120 subjects (50–85 years old, all genders, no healthy volunteers). | Beijing, China. | Not applicable. | Not provided. |
| Citrus | |||||||
| 4744922 | SCD. | One capsule extracts citrus a day for 9 months or placebo. | -Cognitive outcome (R-BANS). -Biological outcome (IL-8). | 60 to 75 Years. | Italy. | Not aplicable. | PMID: 36253765. PMID: 25205962. |
| Cognitive Test | Key Domains/Aspects Evaluated | Normal Score Range/Interpretation |
|---|---|---|
| MMSE (Modified Mini-Mental State Exam). | Orientation, short-term memory, attention, visuospatial, language skills, understanding instructions. | ≥25 = Normal; <24 = Cognitive impairment. |
| ADAS-Cog (Cognitive Subscale of ADAS). | Word recall, recognition, naming objects, following instructions, comprehension, ideational apraxia, attention, and orientation. | Higher scores (0–75) = Greater cognitive impairment. |
| NPI (Neuropsychiatric Inventory). | Neuropsychiatric symptoms (behavioral and psychological). | No standard score range, higher scores indicate greater severity of symptoms. |
| DSS (Digital Symbol Substitution). | Processing speed, cognitive flexibility. | No standard score range, lower scores indicate cognitive decline. |
| MoCA (Montreal Cognitive Assessment). | Attention, executive functions, memory, language, visuals, visuospatial abilities, orientation. | ≥26 = Normal; <26 = Cognitive impairment. |
| GDS (Geriatric Depression Scale). | Depression symptoms in the elderly. | ≥5 = Depressive symptoms likely. |
| ADAS-Cog Chinese Medicine Symptom Scale (CM-SS). | Cognitive function with a focus on a Traditional Chinese Medicine perspective. | No standard score range, higher scores indicate greater impairment. |
| CDR-SOB (Clinical Dementia Rating Scale Sum of Boxes). | Cognitive impairment, severity of dementia symptoms (e.g., memory, orientation, judgment). | Higher scores indicate more severe cognitive impairment. |
| AVLT (Auditory Verbal Learning Test). | Immediate and delayed word recall. | No standard score range, lower scores indicate greater memory impairment. |
| ROCF (Rey Osterrieth Complex Figure Test). | Visuospatial and memory functions. | No standard score range, lower scores indicate poorer visuospatial memory. |
| SNSB (Seoul Neuropsychological Screening Battery). | Various cognitive functions (attention, memory, language, visuals). | No standard score range, overall performance indicates cognitive ability. |
| FIM (Functional Independence Measure). | Functional ability (e.g., self-care, mobility, communication). | Higher scores indicate better functional independence. |
| SCIRS (Severe Cognitive Impairment Rating Scale). | Severity of cognitive impairment and related behaviors. | Higher scores indicate more severe impairment. |
| FAST (Functional Assessment Staging). | Stage of Alzheimer’s disease based on functional decline | Higher scores indicate later stages of AD. |
| CNSVS (Central Nervous System Vital Signs). | Cognitive functions (e.g., attention, memory, executive function). | No standard score range, lower scores indicate cognitive decline. |
| Low risk of bias | Bias arising from the randomization process | Bias due to deviations from the intended interventions | Bias due to missing outcome data | Bias in measurement of the outcome | Bias in selection of the reported result | Overall risk of bias | |
| Some concern | |||||||
| High risk of bias | |||||||
| Herbal extracts | [67] | ||||||
| [58] | |||||||
| [50] | |||||||
| [53] | |||||||
| [47] | |||||||
| [51] | |||||||
| [56] | |||||||
| [71] | |||||||
| [62] | |||||||
| [70] | |||||||
| [67] | |||||||
| RES and grape | [74] | ||||||
| [75] | |||||||
| [61] | |||||||
| [55] | |||||||
| [52] | |||||||
| Saffron | [79] | ||||||
| [78] | |||||||
| Sesame related compounds | [69] | ||||||
| [84] | |||||||
| Ginseng | [85] | ||||||
| Ginseng + Ginkgo biloba | [46] | ||||||
| Ginkgo biloba | [86] | ||||||
| [87] | |||||||
| [88] | |||||||
| Melissa officinalis | [60] | ||||||
| [93] | |||||||
| Curcumin | [91] | ||||||
| Spirulina | [96] | ||||||
| [97] | |||||||
| Citrus | [99] |
| Mechanism | Intervention(s) | Molecular Target | Clinical Trials |
|---|---|---|---|
| Cholinergic dysfunction | Sage extract | Cholinergic receptors (↑ ACh activity) | NCT1001637 |
| Oxidative stress and inflammation | Curcumin, Resveratrol, Grape polyphenols, Citrus extract | ROS, IL-8, inflammatory cytokines | NCT0099710, NCT00678431, NCT4744922 |
| BBB integrity | Extra virgin olive oil, Resveratrol | Cytokine-induced BBB disruption | NCT03824197, NCT1504854 |
| Sirtuin pathway/aging | Resveratrol | SIRT1 activation, tau regulation | NCT1504854, NCT00678431 |
| Amyloid and tau pathology | Grape polyphenols (indirect), possibly Curcumin | Aβ aggregation, tau phosphorylation | Indirect via biomarkers |
| Pleiotropic CNS effects | Caffeine, Citrus extract | Multiple neurotransmitter and cytokine pathways | NCT4570085, NCT4744922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayo Jimenez, M.T.; Rivas-García, L.; Sánchez-González, C.; Grosso, G.; Lipari, V.; Vera-Ramírez, L.; Battino, M.; Giampieri, F.; Quiles, J.L.; Forbes-Hernández, T.Y. Natural Products in Alzheimer’s Disease: A Systematic Review of Clinical Trials and Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2025, 26, 10631. https://doi.org/10.3390/ijms262110631
Bayo Jimenez MT, Rivas-García L, Sánchez-González C, Grosso G, Lipari V, Vera-Ramírez L, Battino M, Giampieri F, Quiles JL, Forbes-Hernández TY. Natural Products in Alzheimer’s Disease: A Systematic Review of Clinical Trials and Underlying Molecular Mechanisms. International Journal of Molecular Sciences. 2025; 26(21):10631. https://doi.org/10.3390/ijms262110631
Chicago/Turabian StyleBayo Jimenez, Maria T., Lorenzo Rivas-García, Cristina Sánchez-González, Giuseppe Grosso, Vivian Lipari, Laura Vera-Ramírez, Maurizio Battino, Francesca Giampieri, José L. Quiles, and Tamara Y. Forbes-Hernández. 2025. "Natural Products in Alzheimer’s Disease: A Systematic Review of Clinical Trials and Underlying Molecular Mechanisms" International Journal of Molecular Sciences 26, no. 21: 10631. https://doi.org/10.3390/ijms262110631
APA StyleBayo Jimenez, M. T., Rivas-García, L., Sánchez-González, C., Grosso, G., Lipari, V., Vera-Ramírez, L., Battino, M., Giampieri, F., Quiles, J. L., & Forbes-Hernández, T. Y. (2025). Natural Products in Alzheimer’s Disease: A Systematic Review of Clinical Trials and Underlying Molecular Mechanisms. International Journal of Molecular Sciences, 26(21), 10631. https://doi.org/10.3390/ijms262110631












