Antiproliferative Evaluation of Dextran Polymer-Based Pomegranate Ethanolic Extract
Abstract
1. Introduction
2. Results
2.1. Extracts from Pomegranate Peels
2.2. ESI-MS/MS Characterization of SSE2-L and SSPD Matrices
2.3. Evaluation of the Phenolic Profile and Antioxidant Activity of the Extracts
2.4. Synthesis and Antioxidant Activity of Dextran Conjugate via Grafting Reaction
2.5. Safety Assessment
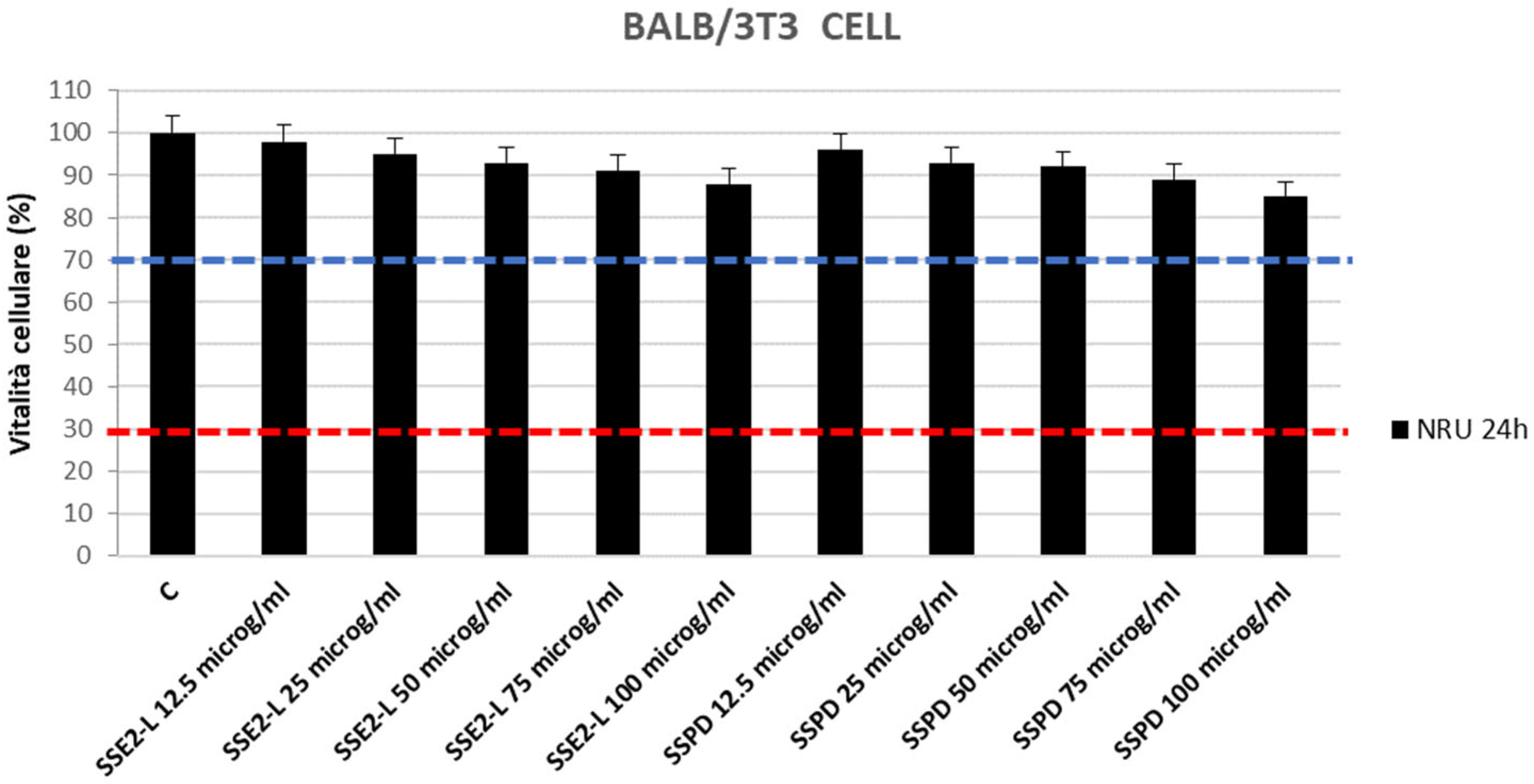
2.5.1. Cytotoxicity Evaluation: Neutral Red Uptake (NRU) Assay
2.5.2. In Vitro Analysis of Pro-Sensitizing Potential
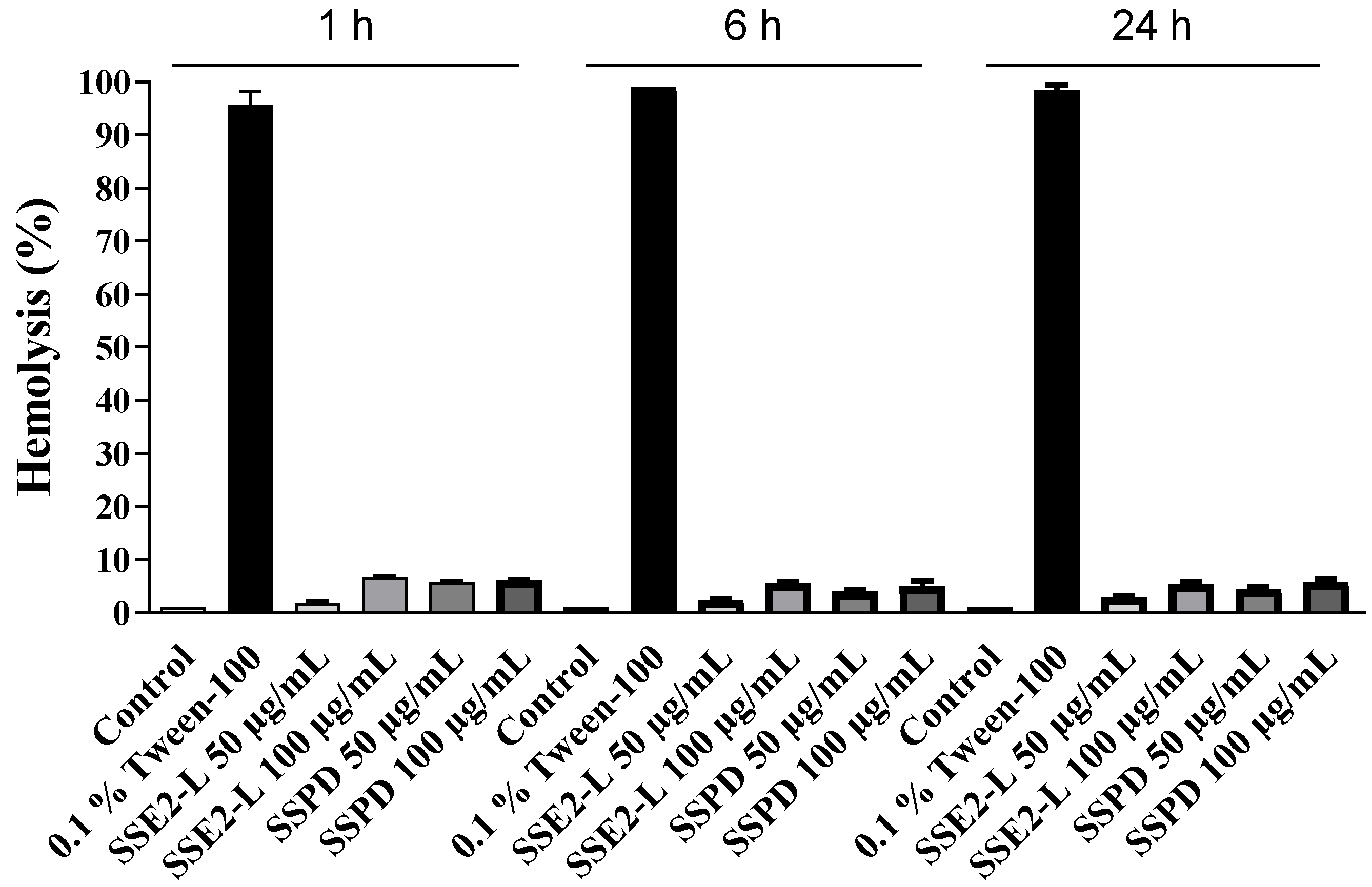
2.5.3. Hemolytic Activity of SSE2-L and SSPD on Peripheral Blood
2.6. Efficacy Assessment
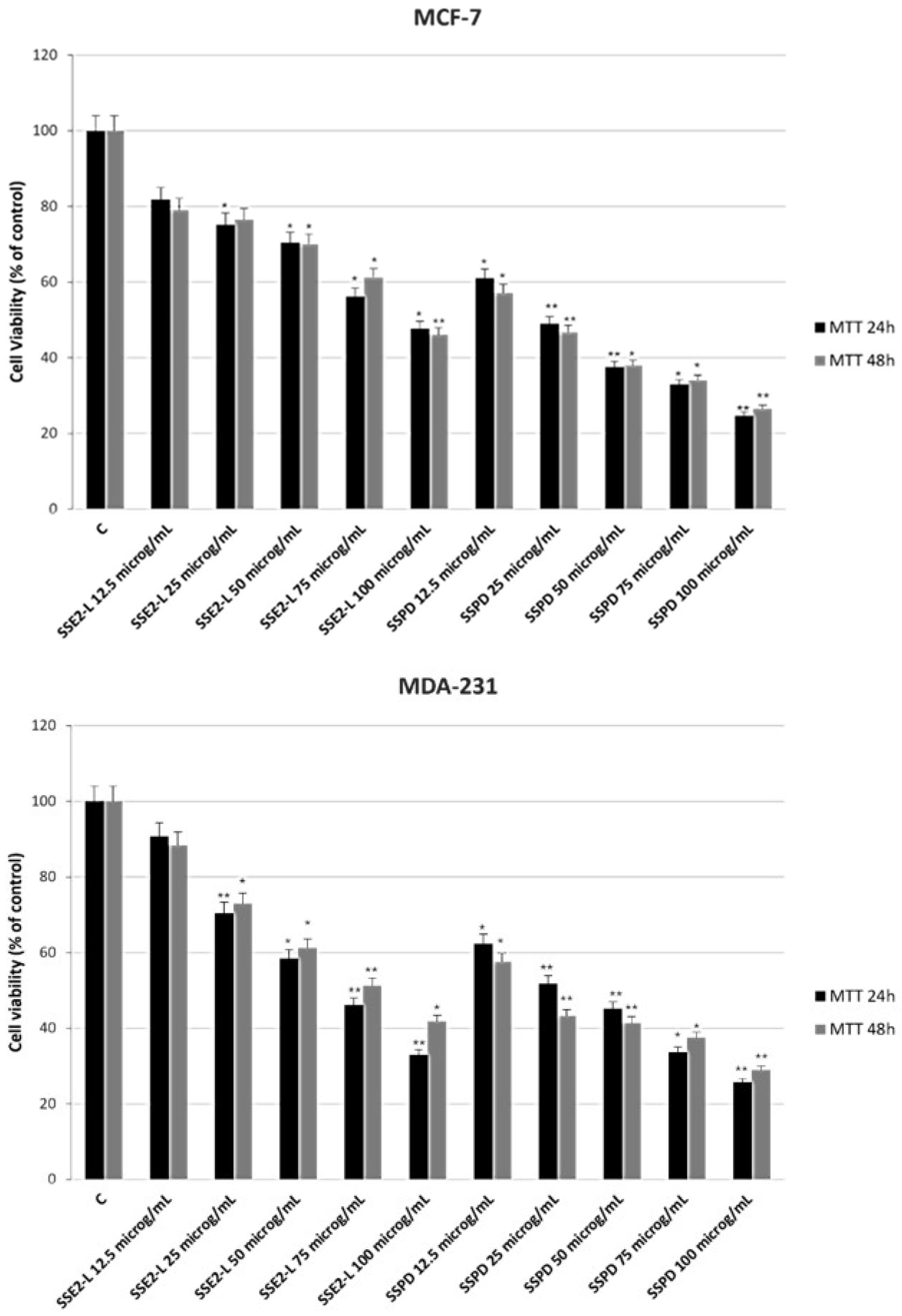
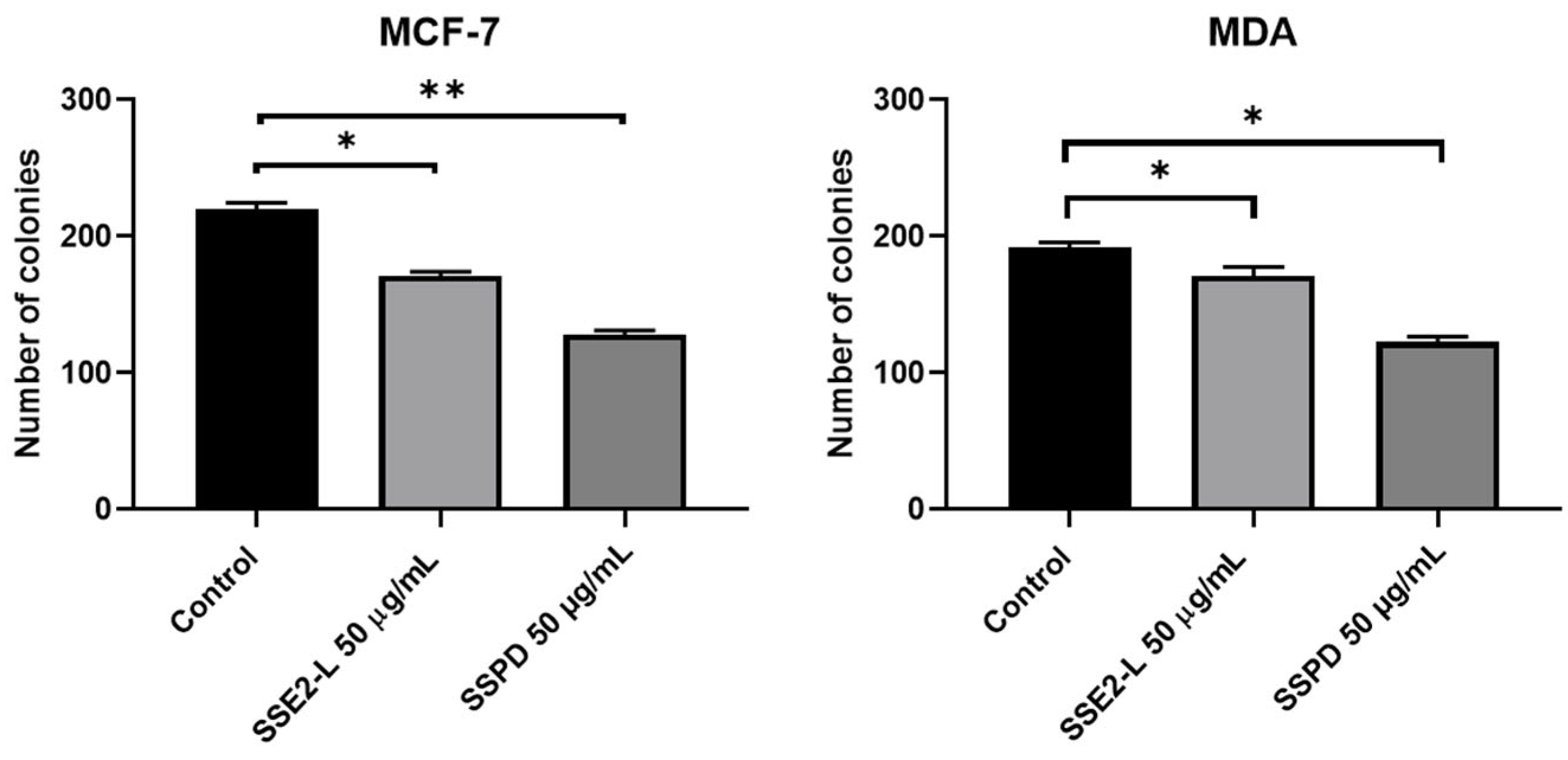
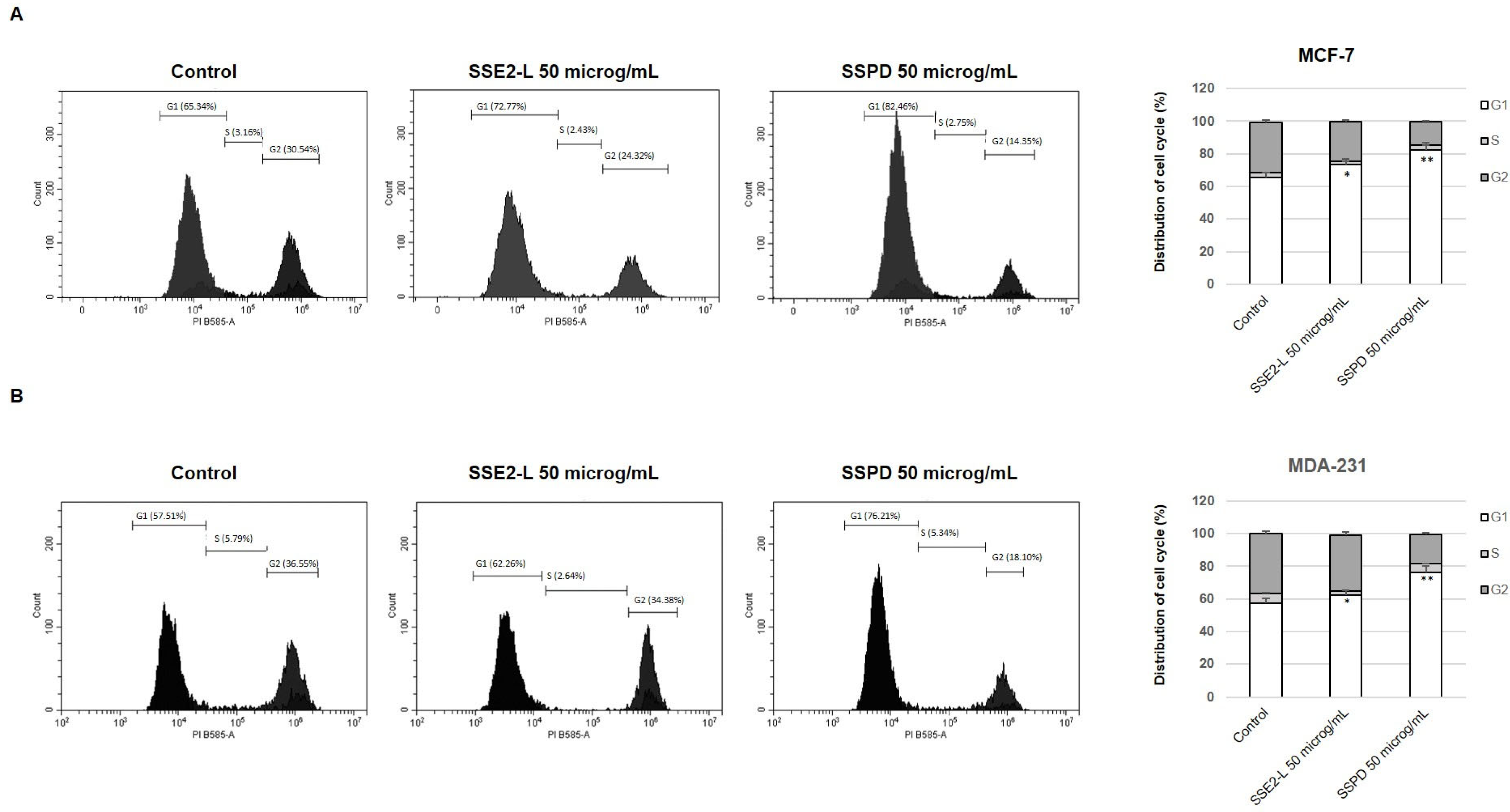
Anti-Proliferative Response of Breast Cancer Cells to SSE2-L and SSPD Treatments
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Extractive Method
4.3. Hydrolysable Tannins Evaluation
4.4. ESI-MS/MS Analysis
4.5. Evaluation of the Phenolic Profile and Antioxidant Activity of Extracts
4.5.1. Determination of Total Polyphenol Content
4.5.2. Determination of Flavonoid Content in Extracts
4.5.3. DPPH Assay
4.5.4. ABTS Assay
4.6. Synthesis of Dextran-Based Polymeric Conjugate via Grafting Reaction
4.7. Cell Cultures
4.8. Safety Testing
4.8.1. Neutral Red Uptake Assay
4.8.2. Human Cell Line Activation Test (h-CLAT)
4.8.3. Hemolysis Assay
4.9. Efficacy Testing
4.9.1. MTT Assay
4.9.2. Flow Cytometric Evaluation of DNA Content
4.9.3. Soft-Agar Colony Formation Assay
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J.A. Pomegranate and its health-promoting benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [PubMed]
- Jurenka, J.S. Therapeutic applications of pomegranate. Altern. Med. Rev. 2008, 13, 128–144. [Google Scholar]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F. Industrial Pomegranate Wastes and their Functional Benefits in Novel Food Formulations. In Mediterranean Fruits Bio-Wastes; Springer International Publishing: Cham, Switzerland, 2022; pp. 721–738. [Google Scholar]
- El-Shamy, S.; Farag, M.A. Novel trends in extraction and optimization methods of bioactives recovery from pomegranate fruit biowastes: Valorization purposes for industrial applications. Food Chem. 2021, 365, 130465. [Google Scholar] [CrossRef]
- Venkataramanamma, D.; Pamisetty, A.; Singh, R. Standardization of the conditions for extraction of polyphenols from pomegranate peel. J. Food Sci. Technol. 2016, 53, 2497–2503. [Google Scholar] [CrossRef]
- Abid, M.; Yaich, H.; Cheikh-Rouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC-MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef]
- Fahmy, H.A.; Farag, M.A. Ellagitannins and their metabolites as bioactive constituents of pomegranate: Recent advances and future directions. Food Chem. 2021, 367, 130682. [Google Scholar]
- Ain, H.B.U.; Tufail, T.; Bashir, S.; Ijaz, N.; Hussain, M.; Ikram, A.; Farooq, M.A.; Saewan, S.A. Nutritional importance and industrial uses of pomegranate peel: A critical review. Food Sci. Nutr. 2023, 11, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2009, 89, 1375–1387. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Selma, M.V.; Espin, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Hashmi, B.; Aja, P.M.; Atoki, A.V. Phytochemicals and pharmacology of pomegranate (Punica granatum L.): Nutraceutical benefits and industrial applications: A review. Front. Nutr. 2025, 12, 1528897. [Google Scholar] [CrossRef]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andres-Lacueva, C.; Alasalvar, C.; Orem, A.; Tomás-Barberán, F.A.; Espín, J.C. The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antiproliferative effects of pomegranate extract in MCF-7 breast cancer cells. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate ellagitannin-derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef]
- Esmaeli, N.; Dehabadi, M.D.; Ghanbari, A. Anticancer effects of pomegranate extract on breast cancer cell lines: A systematic review. Discov. Med. 2025, 5, 193. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Elmaliki, K.M.; Reliene, R. Pomegranate extract alters breast cancer stem cell properties and inhibits epithelial-to-mesenchymal transition. Nutr. Cancer 2017, 69, 1088–1098. [Google Scholar] [CrossRef]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vivo antioxidant ellagic acid derivatives from pomegranate juice. Clin. Nutr. 2005, 24, 982–995. [Google Scholar]
- Selma, M.V.; Espín, F.A.; Tomás-Barberán, J.C. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; García-Villalba, R.; Beltrán, D.; Frutos-Lisón, M.D.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Gut Bacteria Involved in Ellagic Acid Metabolism to Yield Human Urolithin Metabotypes Revealed. J. Agric. Food Chem. 2023, 71, 4029–4035. [Google Scholar] [CrossRef]
- Rudrapal, M.; Mishra, A.K.; Rani, L.; Sarwa, K.K.; Zothantluanga, J.H.; Khan, J.; Kamal, M.; Palai, S.; Bendale, A.R.; Talele, S.G.; et al. Nanodelivery of Dietary Polyphenols for Therapeutic Applications. Molecules 2022, 27, 8706. [Google Scholar] [CrossRef] [PubMed]
- Medana, C.; Spizzirri, U.G.; Schiavo, V.; Fusi, F.; Panti, A.; Saponara, S.; Marcolongo, P.; Affricano, A.; Asteggiano, A.; Aiello, F.; et al. Screening of the antioxidant and vasorelaxant activity of wine waste ultrasonic extracts, and HRMS targeted and semi-targeted profiling of glycosylated polyphenols vs free polyphenols. LWT Food Sci. Technol. 2024, 208, 116666. [Google Scholar] [CrossRef]
- Bataille, J.; Ricard, J. Dextrans: From biochemistry to biotechnology. Biotechnol. Adv. 1985, 3, 335–352. [Google Scholar]
- Varshosaz, J. Dextran conjugates in drug delivery. Expert Opin. Drug Deliv. 2012, 9, 509–523. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, K.W.; Wu, D.; Liu, H.; Li, H.; Zhang, J.; Gan, R. Pomegranate peel-derived punicalagin: Ultrasonic-assisted extraction, purification, and its α-glucosidase inhibitory mechanism. Food Chem. 2022, 374, 131635. [Google Scholar] [CrossRef]
- Jain, V.; Gopal, M.; Mundkinajeddu, D.; Viswanatha, G.L.; Manohar, D. Isolation and Standardization of Various Phytochemical Constituents from Methanolic Extracts of Fruit Rinds of Punica granatum. Chin. J. Nat. Med. 2011, 9, 414–420. [Google Scholar]
- Priya, H.; Singh, H. Formulation and evaluation of niosomes containing punicalagin from peels of punica granatum. J. Drug Deliv. Ther. 2012, 2, 56–67. [Google Scholar] [CrossRef]
- Sentandreu, E.; Cerdán-Calero, M.; Sendra, J.M. Phenolic profile characterization of pomegranate (Punica granatum) juice by high-performance liquid chromatography with diode array detection coupled to an electrospray ion trap mass analyzer. J. Food Compos. Anal. 2013, 30, 32–40. [Google Scholar] [CrossRef]
- Qu, W.; Breksa, A.P., III; Pan, Z.; Ma, H. Quantitative determination of major polyphenol constituents in pomegranate products. Food Chem. 2012, 132, 1585–1591. [Google Scholar] [CrossRef]
- Cao, H.; Saroglu, O.; Karadag, A.; Diaconeasa, Z.; Zoccatelli, G.; Conte-Junior, C.A.; Gonzalez-Aguilar, G.A.; Ou, J.; Bai, W.; Zamarioli, C.M.; et al. Available technologies on improving the stability of polyphenols in food processing. Food Front. 2021, 2, 109–139. [Google Scholar] [CrossRef]
- Lopes de Oliveira, I.; Domínguez-Rodríguez, G.; Montero, L.; Viganó, J.; Cifuentes, A.; Arial Rostagno, M.; Ibáñez, E. Advanced Extraction Techniques Combined with Natural Deep Eutectic Solvents for Extracting Phenolic Compounds from Pomegranate (Punica granatum L.) Peels. Int. J. Mol. Sci. 2024, 25, 9992. [Google Scholar] [CrossRef] [PubMed]
- Young, J.E.; Pan, Z.; The, H.E.; Menon, V.; Modereger, B.; Pesek, J.J.; Matyska, M.T.; Dao, L.; Takeoka, G. Phenolic composition of pomegranate peel extracts using a liquid chromatography-mass spectrometry approach with silica hydride columns. J. Sep. Sci. 2017, 40, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- Derakhshana, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Oliveri Conti, G.; Sadraba, E.K. Antioxidant activity and total phenolic content of ethanolic extract of pomegranate peels, juice and seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Elfalleh, W.; Hannachi, H.; Tlili, N.; Yahia, Y.; Nasri, N.; Ferchichi, A. Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med Plants Res. 2015, 6, 4724–4730. [Google Scholar] [CrossRef]
- Carullo, G.; Spizzirri, U.G.; Montopoli, M.; Cocetta, V.; Armentano, B.; Tinazzi, M.; Sciubba, F.; Giorgi, G.; Di Cocco, M.E.; Bohn, T.; et al. Milk Kefir Enriched with Inulin Grafted Seed Extract from White Wine Pomace: Chemical Characterization, Antioxidant Profile and in vitro Gastrointestinal Digestion. Int. J. Food Sci. Technol. 2022, 57, 4086–4095. [Google Scholar] [CrossRef]
- Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent advances in the conjugation approaches for enhancing the bioavailability of polyphenols. Food Hydrocoll. 2024, 146, 109221. [Google Scholar] [CrossRef]
- Blessy, J.; Saravanan, K.; Sagarika, V.K.; Avinash, R.P.; James, J.; Nandakumar, K.; Sabu, T. Green Chemistry Approach for Fabrication of Polymer Composites. Sustain. Chem. 2021, 2, 254–270. [Google Scholar] [CrossRef]
- Carullo, G.; Scarpelli, F.; Belsito, E.L.; Caputo, P.; Oliviero Rossi, C.; Mincione, A.; Leggio, A.; Crispini, A.; Restuccia, D.; Spizzirri, U.G.; et al. Formulation of New Baking (+)-Catechin Based Leavening Agents: Effects on Rheology, Sensory and Antioxidant Features during Muffin Preparation. Foods 2020, 9, 1569. [Google Scholar] [CrossRef] [PubMed]
- Restuccia, D.; Giorgi, G.; Spizzirri, U.G.; Sciubba, F.; Capuani, G.; Rago, V.; Carullo, G.; Aiello, F. Autochthonous white grape pomaces as bioactive source for functional jams. Int. J. Food Sci. Technol. 2019, 54, 1313–1320. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. ISO: Geneva, Switzerland, 2021.
- Aiello, F.; Malivindi, R.; Motta, M.F.; Crupi, P.; Nicoletti, R.; Benincasa, C.; Clodoveo, M.L.; Rago, V.; Spizzirri, U.G.; Restuccia, D. Synthesis and Characterization of a Biopolymer Pectin/Ethanolic Extract from Olive Mill Wastewater: In Vitro Safety and Efficacy Tests on Skin Wound Healing. Int. J. Mol. Sci. 2023, 24, 15075. [Google Scholar] [CrossRef]
- Manal, A.H.; Fatema, Y.S.; Grisilda, V.B.; Pooja, S.; Norhan, E.M.; Fariha, Y. Antiproliferative Potential of Pomegranate Peel Extract in MDA-MB Breast Cancer Cell Lines. Am. J. Biochem. Biotechnol. 2025, 21, 120–128. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Pinteala, M.; Simionescu, N. Dextran Formulations as Effective Delivery Systems of Therapeutic Agents. Molecules 2023, 28, 1086. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, N.; Wu, J.; Dong, P.; Lv, H.; Wang, Q.; Wang, S.; Yang, H.; Wang, S.; Li, X.; et al. A Novel Dextran-Based Dual Drug Conjugate Targeted Tumors with High Biodistribution Ratio of Tumors to Normal Tissues. Int. J. Nanomed. 2022, 17, 4895–4910. [Google Scholar] [CrossRef] [PubMed]
- Spizzirri, U.G.; Esposito, L.; Caputo, P.; Martuscelli, M.; Gaglianò, M.; Clodoveo, M.L.; De Luca, G.; Oliverio Rossi, C.; Savastano, M.; Scarcelli, E.; et al. Carob pulp flour as an innovative source of bioactive molecules for the preparation of high-value-added jellies. Helyion 2024, 10, e38354. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, R.; Spizzirri, U.G.; Aquino, G.; Basilicata, M.G.; Pepe, G.; Campiglia, P.; Celletti, S.; Tudino, V.; Dichiara, M.; Gemma, S.; et al. Biostimulants for Sustainable Food Production: Effects of Wood Distillate to Fortify Chickpea Flour for Development of Functional Bakery Products. ACS Food Sci. Technol. 2024, 4, 1640–1651. [Google Scholar] [CrossRef]
- Aiello, F.; Caputo, P.; Oliviero Rossi, C.; Restuccia, D.; Spizzirri, U.G. Formulation of Antioxidant Gummies Based on Gelatin Enriched with Citrus Fruit Peels Extract. Foods 2024, 13, 320. [Google Scholar] [CrossRef]
- Brindisi, M.; Bouzidi, C.; Frattaruolo, L.; Loizzo, M.R.; Tundis, R.; Dugay, A.; Deguin, B.; Cappello, A.R.; Cappello, M.S. Chemical Profile, Antioxidant, Anti-Inflammatory, and Anti-Cancer Effects of Italian Salvia rosmarinus Spenn. Methanol Leaves Extracts. Antioxidants 2020, 9, 826. [Google Scholar] [CrossRef]
- Università della Calabria. Unical: AOO1 Amministrazione Centrale; Doc. No. 234, 14 January 2021; Università della Calabria: Rende, Italy, 2021. [Google Scholar]
| Extract | TPC (mg GAE/g) | FC (mg CTE/g) | Scavenger Activity IC50 (mg mL−1) | |
|---|---|---|---|---|
| DPPH | ABTS | |||
| SSA1 | 19.53 ± 0.84 a | 65.21 ± 2.87 b | 0.1982 ± 0.0081 d | 0.0482 ± 0.0018 c |
| SSE2 | 19.43 ± 0.89 a | 31.32 ± 1.24 a | 0.1821 ± 0.0084 c | 0.0510 ± 0.0020 c |
| SSA1-L | 466.12 ± 16.24 c | 365.23 ± 15.41 d | 0.0186 ± 0.0008 b | 0.0093 ± 0.0004 a |
| SSE2-L | 318.08 ± 12.85 b | 177.33 ± 7.46 c | 0.0159 ± 0.0005 a | 0.0191 ± 0.0009 b |
| Positive control | ||||
| Ascorbic acid | 0.0017 ± 0.0004 | 0.0005 ± 0.0008 | ||
| Sample | CD86 * | CD54 * |
|---|---|---|
| SSE2-L 50 μg/mL | 57 | 71 |
| SSPD 50 μg/mL | 52 | 69 |
| Control | 35 | 59 |
| Positive Control (NISO4) | 191 | 236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spizzirri, U.G.; Motta, M.F.; Ferraro, S.; Strigaro, S.; Benincasa, C.; Nicoletti, R.; Astuto, F.; Comite, U.; Malivindi, R.; Aiello, F. Antiproliferative Evaluation of Dextran Polymer-Based Pomegranate Ethanolic Extract. Int. J. Mol. Sci. 2025, 26, 10618. https://doi.org/10.3390/ijms262110618
Spizzirri UG, Motta MF, Ferraro S, Strigaro S, Benincasa C, Nicoletti R, Astuto F, Comite U, Malivindi R, Aiello F. Antiproliferative Evaluation of Dextran Polymer-Based Pomegranate Ethanolic Extract. International Journal of Molecular Sciences. 2025; 26(21):10618. https://doi.org/10.3390/ijms262110618
Chicago/Turabian StyleSpizzirri, Umile Gianfranco, Marisa Francesca Motta, Sonia Ferraro, Silvia Strigaro, Cinzia Benincasa, Rosa Nicoletti, Francesco Astuto, Ubaldo Comite, Rocco Malivindi, and Francesca Aiello. 2025. "Antiproliferative Evaluation of Dextran Polymer-Based Pomegranate Ethanolic Extract" International Journal of Molecular Sciences 26, no. 21: 10618. https://doi.org/10.3390/ijms262110618
APA StyleSpizzirri, U. G., Motta, M. F., Ferraro, S., Strigaro, S., Benincasa, C., Nicoletti, R., Astuto, F., Comite, U., Malivindi, R., & Aiello, F. (2025). Antiproliferative Evaluation of Dextran Polymer-Based Pomegranate Ethanolic Extract. International Journal of Molecular Sciences, 26(21), 10618. https://doi.org/10.3390/ijms262110618








