Maternal Genotype and Dietary Vitamin A Modify Aortic Arch Phenotypes in a Mouse Model of 22q11DS
Abstract
1. Introduction
2. Results
2.1. Experimental Setup and Groups
2.2. Litter Size Is Modulated by Maternal Genotype and Dietary Vitamin A Dosage
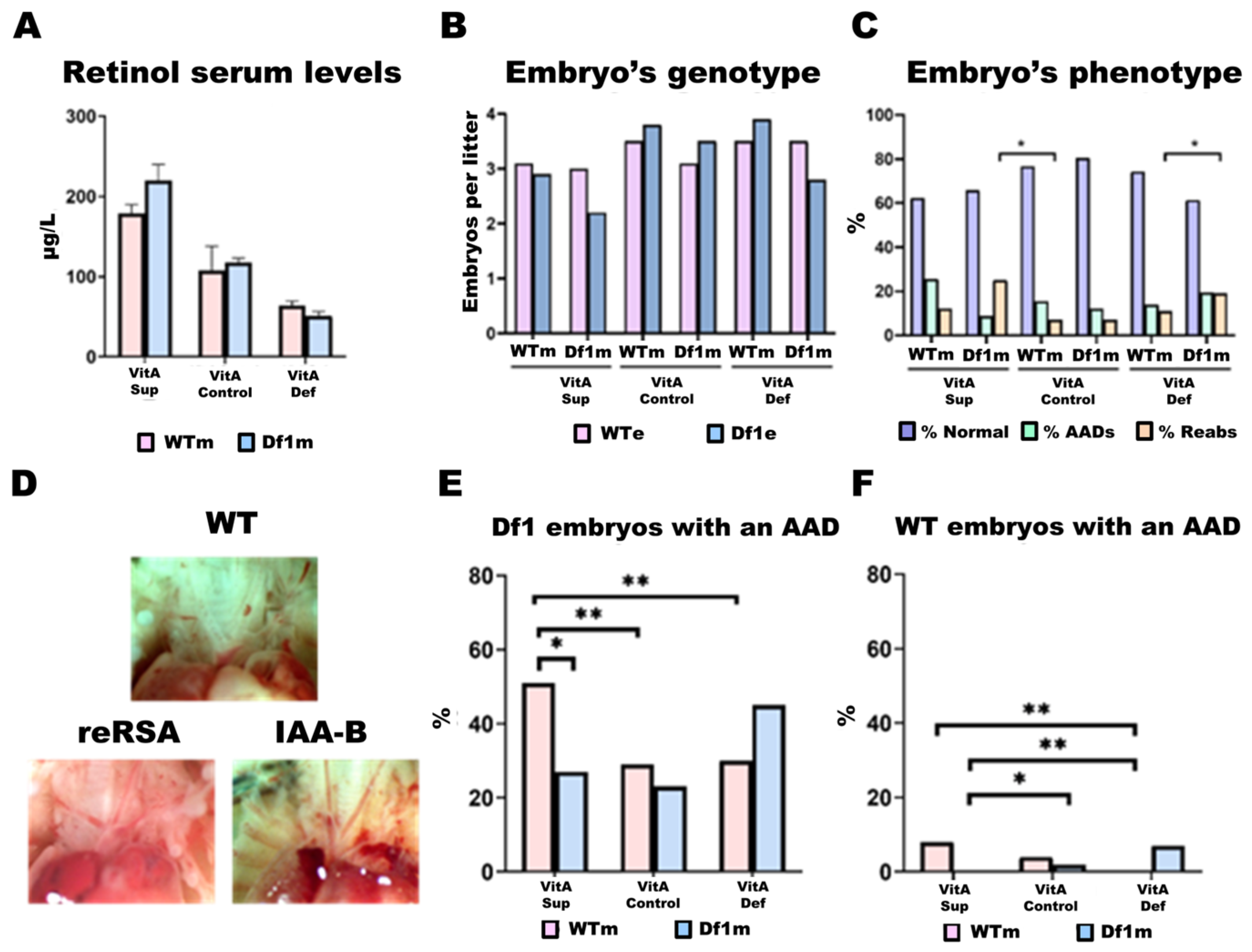
2.2.1. Vitamin A in the Diet Determines Retinol Levels in the Blood
2.2.2. Litter Size
2.2.3. Df1/+ Embryos vs. WT Embryos
2.3. Frequency of Aortic Arch Defects (AADs)
2.4. AAD Penetrance in Df1/+ Embryos Is Modulated by Maternal Genotype and Dietary VitA Dosage
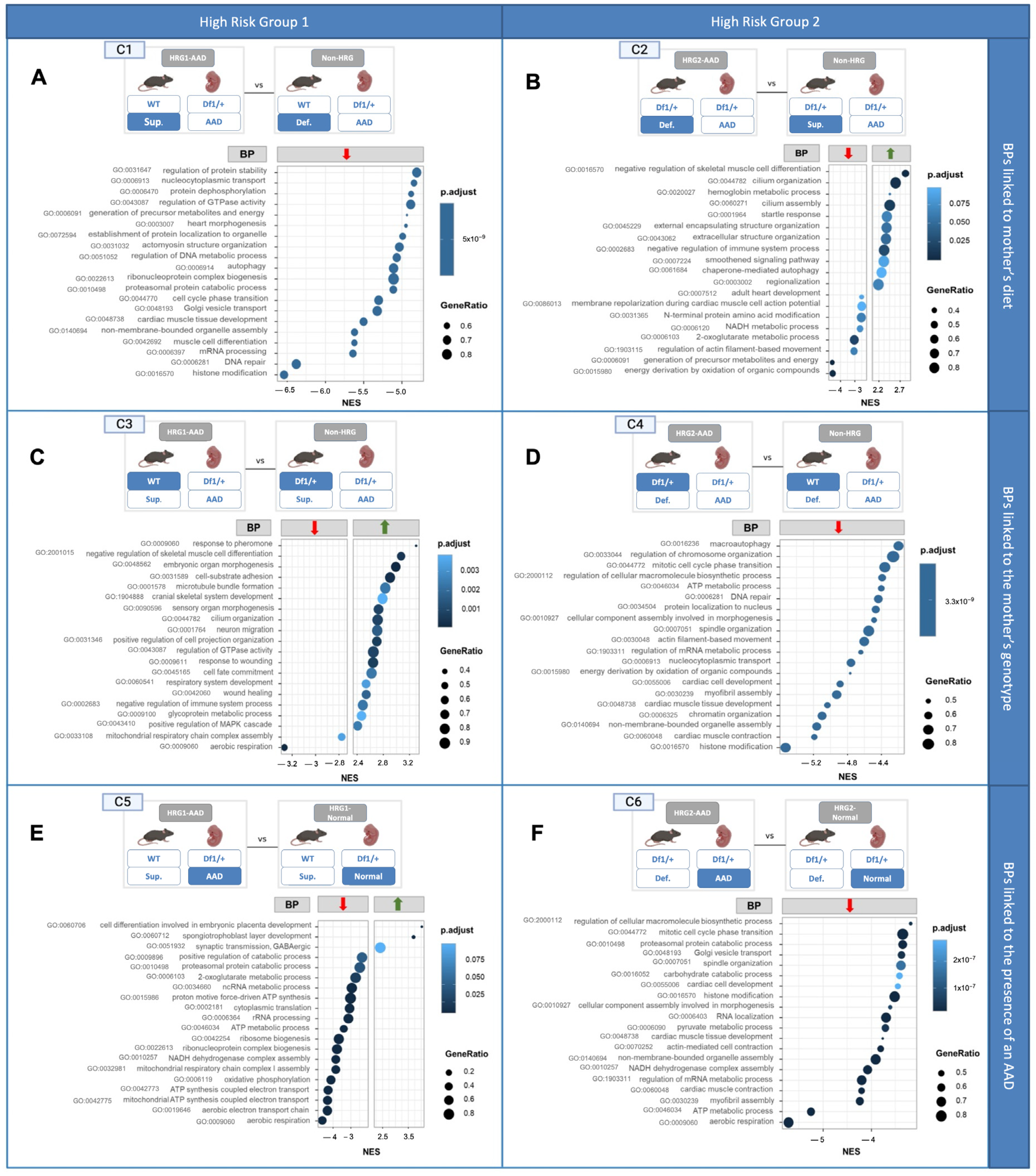
2.5. Differential Gene Expression Analysis
2.5.1. Gene Expresson and Pathway Analysis
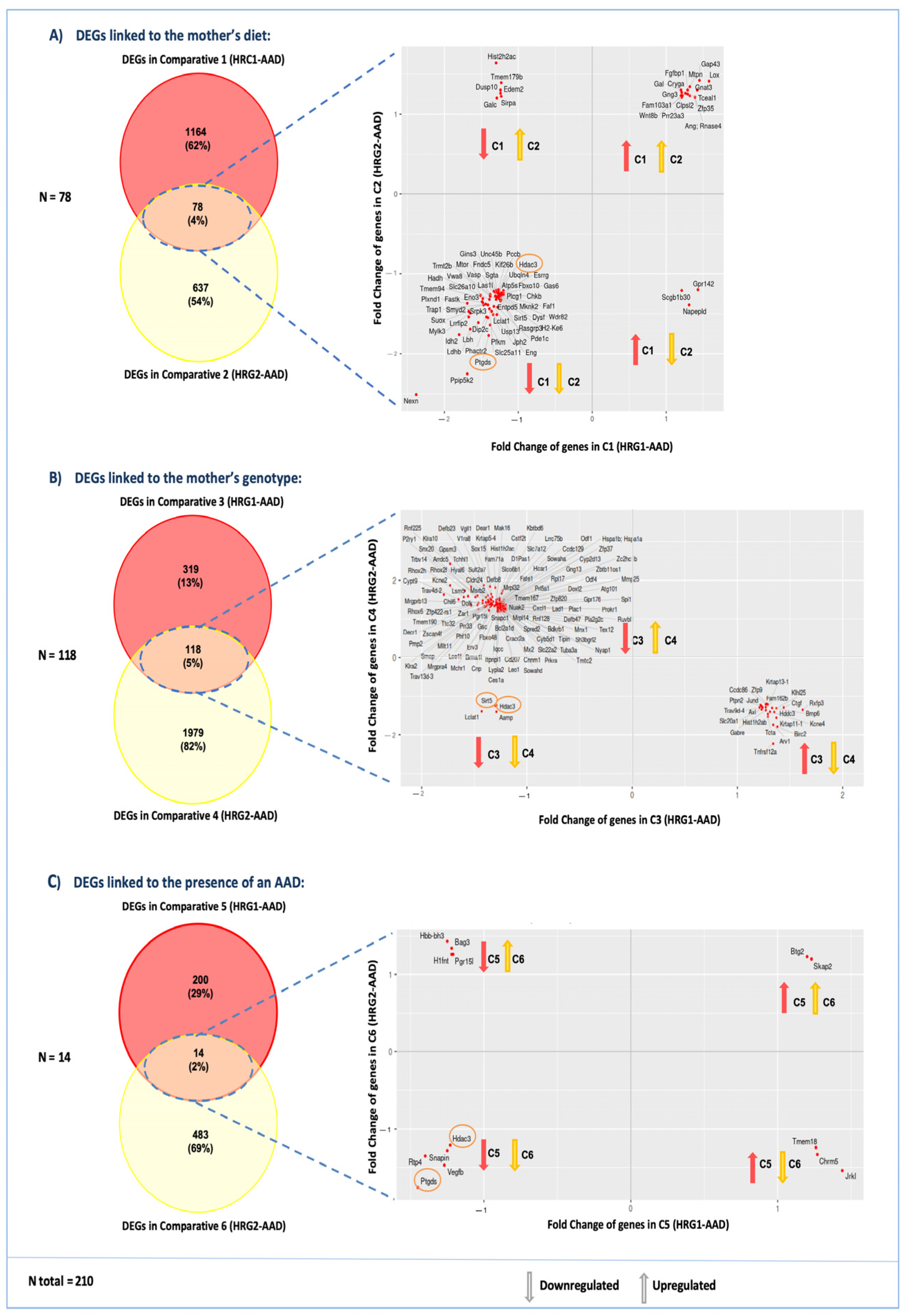
2.5.2. Changes in Embryonic Cardiac Gene Expression Linked to the Mother’s Diet
- Comparison 1 (C1): Cardiac gene expression from Df1/+ embryos with AADs from WT mothers fed a VitA-Sup diet (HRG1-AAD) versus Df1/+ embryos with AADs from WT mothers fed a VitA-Def diet (non-HRG1) (Figure 3(C1)).
- Comparison 2 (C2): Cardiac gene expression from Df1/+ embryos with an AAD from Df1/+ mothers fed a VitA-Def diet (HRG2-AAD) versus cardiac gene expression from Df1/+ embryos with an AAD from Df1/+ mothers fed a VitA-Sup diet (non-HRG2) (Figure 3(C2)).
2.5.3. Changes in Embryonic Cardiac Gene Expression Linked to the Mother’s Genotype
- Comparison 3 (C3): Cardiac gene expression from Df1/+ embryos with AADs from WT mothers fed a VitA-Sup diet (HRG1-AAD) versus cardiac gene expression from Df1/+ embryos with an AAD from Df1/+ mothers fed a VitA-Sup diet (non-HRG2) (Figure 3(C3)).
- Comparison 4 (C4): Cardiac gene expression of Df1/+ embryos with an AAD born from Df1/+ mothers fed a VitA-Def diet (HRG2-AAD) versus cardiac gene expression of Df1/+ embryos with an AAD born from WT mothers fed a VitA-Def diet (non-HRG1) (Figure 3(C4)).
2.5.4. Changes in Embryonic Cardiac Gene Expression Are Linked to the Presence of an AAD
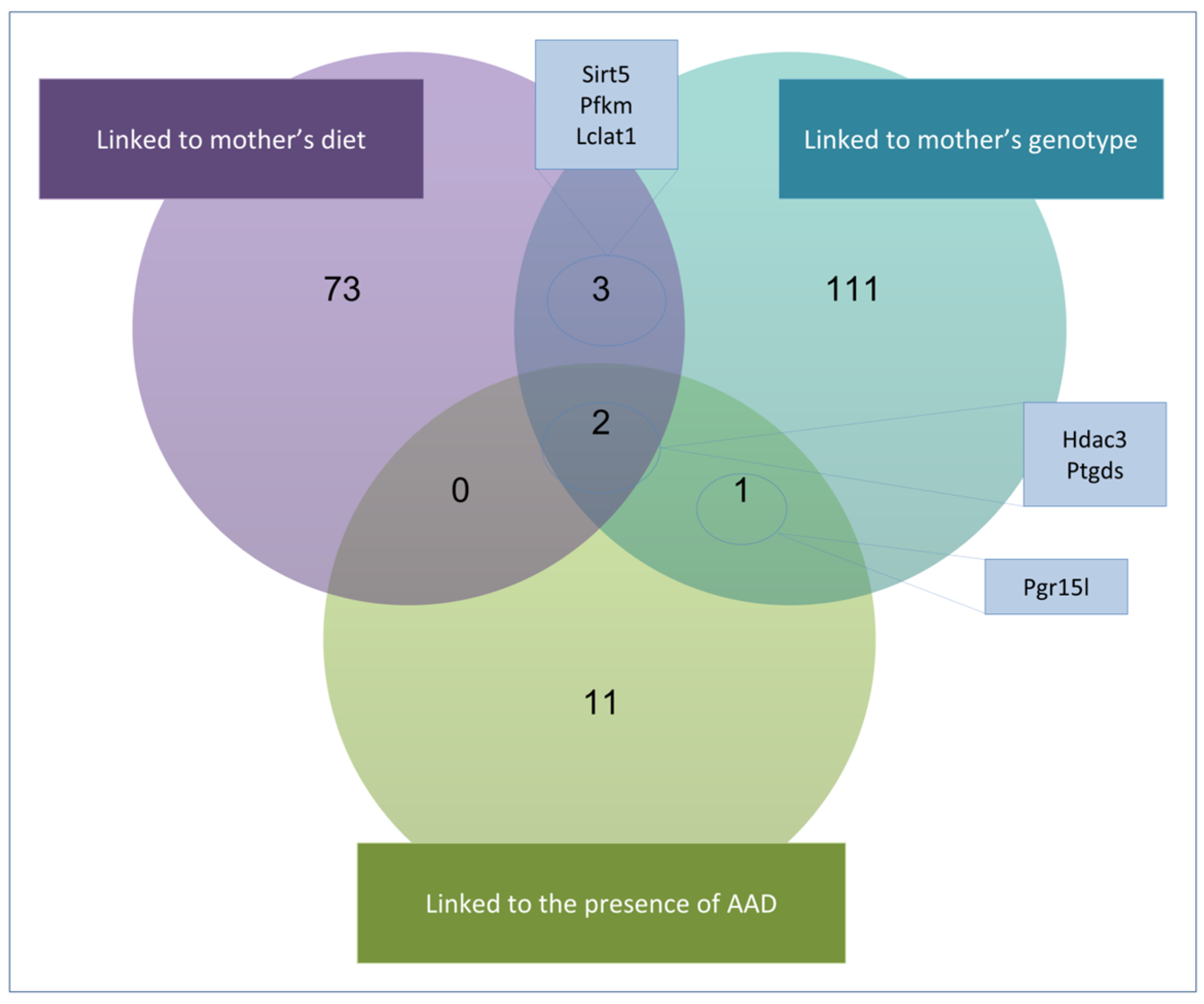
2.6. Gene Expression and Candidate Genes
3. Discussion
4. Conclusions
5. Limitations of the Research
6. Materials and Methods
6.1. Mice and Diets
6.1.1. Embryo Genotyping and Sex Identification via PCR
6.1.2. Phenotypic and Histological Analysis
6.2. RNA Isolation and Quantification
6.3. Transcriptome Analysis
6.3.1. Gene Set Enrichment Analysis
6.3.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Aortic Arch Defect |
| BPs | Biological Processes |
| CHDs | Congenital heart defects |
| DEF | Deficient |
| DEGs | Differentially expressed genes |
| Df1/+ | Mouse model of 22q11.2DS |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| IAA-B | Interrupted aortic arch type B |
| NCCs | Neural crest cells |
| reRSA/ARSA | Retroesophageal or aberrant right subclavian artery |
| SUPL | Supplemented |
| VitA | Vitamin A |
| WT | Wild Type |
References
- Hoffman, J.I.E.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef]
- Bruneau, B.G. The developmental genetics of congenital heart disease. Nature 2008, 451, 943–948. [Google Scholar] [CrossRef]
- Dolk, H.; Loane, M.; Garne, E. Congenital heart defects in Europe: Prevalence and perinatal mortality, 2000 to 2005. Circulation 2011, 123, 841–849. [Google Scholar] [CrossRef]
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2022, 19, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Jin, S.C.; Sierant, M.C.; Lu, Z.; Li, B.; Lu, Q.; Morton, S.U.; Zhang, J.; López-Giráldez, F.; Nelson-Williams, C.; et al. Recessive genetic contribution to congenital heart disease in 5,424 probands. Proc. Natl. Acad. Sci. USA 2025, 122, e2419992122. [Google Scholar] [CrossRef] [PubMed]
- Sierant, M.C.; Chih Jin, S.; Bilguvar, K.; Morton, S.U.; Dong, W.; Jiang, W.; Lu, Z.; Li, B.; López-Giráldez, F.; Tikhonova, I.; et al. Genomic analysis of 11,555 probands identifies 60 dominant congenital heart disease genes. Proc. Natl. Acad. Sci. USA 2025, 122, 2420343122. [Google Scholar] [CrossRef] [PubMed]
- Prendiville, T.; Jay, P.Y.; Pu, W.T. Insights into the genetic structure of congenital heart disease from human and murine studies on monogenic disorders. Cold Spring Harb. Perspect. Med. 2014, 4, a013946. [Google Scholar] [CrossRef]
- Yasuhara, J.; Garg, V. Genetics of congenital heart disease: A narrative review of recent advances and clinical implications. Transl. Pediatr. 2021, 10, 2366–2386. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.R.; Molina Gomes, D.; Ferreira, J.C.P.B.; Dupont, C.; Alesi, V.; Gouas, L.; Horelli-Kuitunen, N.; Choy, K.W.; García-Herrero, S.; de la Vega, A.G.; et al. Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenat. Diagn. 2015, 35, 801–809. [Google Scholar] [CrossRef]
- Edelmann, L.; Pandita, R.K.; Spiteri, E.; Funke, B.; Goldberg, R.; Palanisamy, N.; Chaganti, R.S.K.; Magenis, E.; Shprintzen, R.J.; Morrow, B.E. A Common Molecular Basis for Rearrangement Disorders on Chromosome 22q11. Hum. Mol. Genet. 1999, 8, 1157–1167. [Google Scholar] [CrossRef]
- Edelmann, L.; Pandita, R.K.; Morrow, B.E. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am. J. Hum. Genet. 1999, 64, 1076. [Google Scholar] [CrossRef]
- Shaikh, T.H.; Kurahashi, H.; Saitta, S.C.; O’Hare, A.M.; Hu, P.; Roe, B.A.; Driscoll, D.A.; McDonald-McGinn, D.M.; Zackai, E.H.; Budarf, M.L.; et al. Chromosome 22-specific low copy repeats and the 22q11.2 deletion syndrome: Genomic organization and deletion endpoint analysis. Hum. Mol. Genet. 2000, 9, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Merscher, S.; Funke, B.; Epstein, J.A.; Heyer, J.; Puech, A.; Lu, M.M.; Xavier, R.J.; Demay, M.B.; Russell, R.G.; Factor, S.; et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 2001, 104, 619–629. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Baldini, A. Recovery from arterial growth delay reduces penetrance of cardiovascular defects in mice deleted for the DiGeorge syndrome region. Hum. Mol. Genet. 2001, 10, 997–1002. [Google Scholar] [CrossRef]
- Jerome, L.A.; Papaioannou, V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001, 27, 286–291. [Google Scholar] [CrossRef]
- Baldini, A.; Fulcoli, F.G.; Illingworth, E. Tbx1: Transcriptional and Developmental Functions. Curr. Top. Dev. Biol. 2017, 122, 223–243. [Google Scholar] [CrossRef]
- Racedo, S.E.; McDonald-Mcginn, D.M.; Chung, J.H.; Goldmuntz, E.; Zackai, E.; Emanuel, B.S.; Zhou, B.; Funke, B.; Morrow, B.E. Mouse and human CRKL is dosage sensitive for cardiac outflow tract formation. Am. J. Hum. Genet. 2015, 96, 235–244. [Google Scholar] [CrossRef]
- Goldmuntz, E. 22Q11.2 Deletion Syndrome and Congenital Heart Disease. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 64–72. [Google Scholar] [CrossRef]
- Stalmans, I.; Lambrechts, D.; De Smet, F.; Jansen, S.; Wang, J.; Maity, S.; Kneer, P.; von der Ohe, M.; Swillen, A.; Maes, C.; et al. VEGF: A modifier of the del22q11 (DiGeorge) syndrome? Nat. Med. 2003, 9, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Stalmans, I. Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome. Verh. K. Acad. Geneeskd. Belg. 2005, 67, 229–276. [Google Scholar] [PubMed]
- Zhao, Y.; Diacou, A.; Johnston, H.R.; Musfee, F.I.; McDonald-McGinn, D.M.; McGinn, D.; Crowley, T.B.; Repetto, G.M.; Swillen, A.; Breckpot, J.; et al. Complete Sequence of the 22q11.2 Allele in 1,053 Subjects with 22q11.2 Deletion Syndrome Reveals Modifiers of Conotruncal Heart Defects. Am. J. Hum. Genet. 2020, 106, 26–40. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Shi, L.; McDonald-McGinn, D.M.; Crowley, T.B.; McGinn, D.E.; Tran, O.T.; Miller, D.; Lin, J.R.; Zackai, E.; et al. Chromatin regulators in the TBX1 network confer risk for conotruncal heart defects in 22q11.2DS. Npj Genom. Med. 2023, 8, 17. [Google Scholar] [CrossRef]
- Guo, T.; Chung, J.H.; Wang, T.; McDonald-Mcginn, D.M.; Kates, W.R.; Hawuła, W.; Coleman, K.; Zackai, E.; Emanuel, B.S.; Morrow, B.E. Histone Modifier Genes Alter Conotruncal Heart Phenotypes in 22q11.2 Deletion Syndrome. Am. J. Hum. Genet. 2015, 97, 869–877. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Botta, A.; Jurecic, V.; Carattini-Rivera, S.; Cheah, Y.C.; Rosenblatt, H.M.; Bradley, A.; Baldini, A. Congenital heart disease in mice deficient for the DiGeorge syndrome region. Nature 1999, 401, 379–383. [Google Scholar] [CrossRef]
- Guna, A.; Butcher, N.J.; Bassett, A.S. Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. J. Neurodev. Disord. 2015, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Motahari, Z.; Moody, S.A.; Maynard, T.M.; Lamantia, A.S. In the line-up: Deleted genes associated with DiGeorge/22q11.2 deletion syndrome: Are they all suspects? J. Neurodev. Disord. 2019, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, E.A.; Vitelli, F.; Su, H.; Morishima, M.; Huynh, T.; Pramparo, T.; Jurecic, V.; Ogunrinu, G.; Sutherland, H.F.; Scambler, P.J.; et al. Tbx1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 2001, 410, 97–101. [Google Scholar] [CrossRef]
- Sugrue, K.F.; Sarkar, A.A.; Leatherbury, L.; Zohn, I.E. The ubiquitin ligase HECTD1 promotes retinoic acid signaling required for development of the aortic arch. DMM Dis. Model. Mech. 2019, 12, dmm036491. [Google Scholar] [CrossRef]
- Sugrue, K.F.; Zohn, I.E. Reduced maternal vitamin A status increases the incidence of normal aortic arch variants. Genesis 2019, 57, e23326. [Google Scholar] [CrossRef] [PubMed]
- Vermot, J.; Niederreither, K.; Garnier, J.-M.; Chambon, P.; Dollé, P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc. Natl. Acad. Sci. USA 2003, 100, 1763–1768. [Google Scholar] [CrossRef]
- Ryckebusch, L.; Bertrand, N.; Mesbah, K.; Bajolle, F.; Niederreither, K.; Kelly, R.G.; Zaffran, S. Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of digeorge syndrome. Circ. Res. 2010, 106, 686–694. [Google Scholar] [CrossRef]
- Yitsege, G.; Stokes, B.A.; Sabatino, J.A.; Sugrue, K.F.; Banyai, G.; Paronett, E.M.; Karpinski, B.A.; Maynard, T.M.; LaMantia, A.S.; Zohn, I.E. Variations in maternal vitamin A intake modifies phenotypes in a mouse model of 22q11.2 deletion syndrome. Birth Defects Res. 2020, 112, 1194–1208. [Google Scholar] [CrossRef]
- Finnell, R.H.; Shaw, G.M.; Lammer, E.J.; Brandl, K.L.; Carmichael, S.L.; Rosenquist, T.H. Gene-nutrient interactions: Importance of folates and retinoids during early embryogenesis. Toxicol. Appl. Pharmacol. 2004, 198, 75–85. [Google Scholar] [CrossRef]
- Vecoli, C.; Pulignani, S.; Foffa, I.; Andreassi, M. Congenital Heart Disease: The Crossroads of Genetics, Epigenetics and Environment. Curr. Genom. 2014, 15, 390–399. [Google Scholar] [CrossRef]
- Kolmaga, A.; Trafalska, E.; Gaszyńska, E.; Gawron-Skarbek, A.; Witkowski, S.; Murlewska, J.; Respondek-Liberska, M.; Strzelecka, I. Vitamin D and LC-PUFA and the Presence of Fetal Heart Defects—A Further Part of a Case-Control Study. Nutrients 2025, 17, 18. [Google Scholar] [CrossRef]
- McMullan, A.; Zwierzynski, J.B.; Jain, N.; Haneline, L.S.; Shou, W.; Kua, K.L.; Hota, S.K.; Durbin, M.D. Role of Maternal Obesity in Offspring Cardiovascular Development and Congenital Heart Defects. J. Am. Heart Assoc. 2025, 14, e039684. [Google Scholar] [CrossRef]
- Graham, A.; Hikspoors, J.P.J.M.; Lamers, W.H.; Anderson, R.H.; Bamforth, S.D. Morphogenetic processes in the development and evolution of the arteries of the pharyngeal arches: Their relations to congenital cardiovascular malformations. Front. Cell Dev. Biol. 2023, 11, 1259175. [Google Scholar] [CrossRef]
- Hiruma, T.; Nakajima, Y. Development of pharyngeal arch arteries in early mouse embryo. J. Anat. 2002, 201, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Brand, T. Heart development: Molecular insights into cardiac specification and early morphogenesis. Dev. Biol. 2003, 258, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kodo, K.; Uchida, K.; Yamagishi, H. Genetic and Cellular Interaction During Cardiovascular Development Implicated in Congenital Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 653244. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, S.; Zheng, M.; Zhao, X.; Le, T.P.; Findley, T.O.; Wang, J. The Cardiac Neural Crest Cells in Heart Development and Congenital Heart Defects. J. Cardiovasc. Dev. Dis. 2021, 8, 89. [Google Scholar] [CrossRef]
- Reich, D.E.; Cargill, M.; Bolk, S.; Ireland, J.; Sabeti, P.C.; Richter, D.J.; Lavery, T.; Kouyoumjian, R.; Farhadian, S.F.; Ward, R.; et al. Linkage disequilibrium in the human genome. Nature 2001, 411, 199–204. [Google Scholar] [CrossRef]
- Vitelli, F.; Taddei, I.; Morishima, M.; Meyers, E.N.; Lindsay, E.A.; Baldini, A. A genetic link between Tbx1 and fibroblast growth factor signaling. Development 2002, 129, 4605–4611. [Google Scholar] [CrossRef]
- Guris, D.L.; Duester, G.; Papaioannou, V.E.; Imamoto, A. Dose-dependent interaction of Tbx1 and Crkl and locally aberrant RA signaling in a model of del22q11 syndrome. Dev. Cell 2006, 10, 81–92. [Google Scholar] [CrossRef]
- Zhang, Z.; Baldini, A. In vivo response to high-resolution variation of Tbx1 mRNA dosage. Hum. Mol. Genet. 2008, 17, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Randall, V.; Mccue, K.; Roberts, C.; Kyriakopoulou, V.; Beddow, S.; Barrett, A.N.; Vitelli, F.; Prescott, K.; Shaw-Smith, C.; Devriendt, K.; et al. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. J. Clin. Investig. 2009, 119, 3301–3310. [Google Scholar] [CrossRef]
- Calmont, A.; Ivins, S.; Van Bueren, K.L.; Papangeli, I.; Kyriakopoulou, V.; Andrews, W.D.; Martin, J.F.; Moon, A.M.; Illingworth, E.A.; Basson, M.A.; et al. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in thepharyngeal ectoderm. Development 2009, 136, 3173–3183. [Google Scholar] [CrossRef]
- Papangeli, I.; Scambler, P.J. Tbx1 genetically interacts with the transforming growth factor-β/bone morphogenetic protein inhibitor Smad7 during great vessel remodeling. Circ. Res. 2013, 112, 90–102. [Google Scholar] [CrossRef]
- Anderson, R.H.; Bamforth, S.D. Morphogenesis of the Mammalian Aortic Arch Arteries. Front. Cell Dev. Biol. 2022, 10, 892900. [Google Scholar] [CrossRef] [PubMed]
- Taddei, I.; Morishima, M.; Huynh, T.; Lindsay, E.A. Genetic factors are major determinants of phenotypic variability in a mouse model of the DiGeorge/del22q11 syndromes. Proc. Natl. Acad. Sci. USA 2001, 98, 11428–11431. [Google Scholar] [CrossRef] [PubMed]
- Yutzey, K.E. DiGeorge Syndrome, Tbx1, and Retinoic Acid Signaling Come Full Circle. Circ. Res. 2010, 106, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Ivins, S.M.; James, C.T.; Scambler, P.J. Retinoic acid down-regulates Tbx1 expression in vivo and in vitro. Dev. Dyn. 2005, 232, 928–938. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Deng, S.; Liu, B.; Jing, X.; Yan, Y.; Liu, Y.; Wang, J.; Zhou, X.; She, Q. The molecular mechanisms of cardiac development and related diseases. Signal Transduct. Target. Ther. 2024, 9, 368. [Google Scholar] [CrossRef]
- Fukuda, R.; Marín-Juez, R.; El-Sammak, H.; Beisaw, A.; Ramadass, R.; Kuenne, C.; Guenther, S.; Konzer, A.; Bhagwat, A.M.; Graumann, J.; et al. Stimulation of glycolysis promotes cardiomyocyte proliferation after injury in adult zebrafish. EMBO Rep. 2020, 21, e49752. [Google Scholar] [CrossRef]
- Chen, S.; Zou, Y.; Song, C.; Cao, K.; Cai, K.; Wu, Y.; Zhang, Z.; Geng, D.; Sun, W.; Ouyang, N.; et al. The role of glycolytic metabolic pathways in cardiovascular disease and potential therapeutic approaches. Basic Res. Cardiol. 2023, 118, 48. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, K.A.; Abraham, D.M.; Martin, A.S.; Mao, L.; Liu, J.; Gu, H.; Locasale, J.W.; Hirschey, M.D. Sirtuin 5 is required for mouse survival in response to cardiac pressure overload. J. Biol. Chem. 2017, 292, 19767. [Google Scholar] [CrossRef]
- Jia, D.; Zhang, J.; Nie, J.; Andersen, J.P.; Rendon, S.; Zheng, Y.; Liu, X.; Tian, Z.; Shi, Y. Cardiolipin remodeling by ALCAT1 links hypoxia to coronary artery disease by promoting mitochondrial dysfunction. Mol. Ther. 2021, 29, 3498–3511. [Google Scholar] [CrossRef]
- Lee, H.S.; Yun, S.J.; Ha, J.M.; Jin, S.Y.; Ha, H.K.; Song, S.H.; Kim, C.D.; Bae, S.S. Prostaglandin D2 stimulates phenotypic changes in vascular smooth muscle cells. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef]
- He, R.; He, Z.; Zhang, T.; Liu, B.; Gao, M.; Li, N.; Geng, Q. HDAC3 in action: Expanding roles in inflammation and inflammatory diseases. Cell Prolif. 2025, 58, e13731. [Google Scholar] [CrossRef]
- Urvalek, A.M.; Gudas, L.J. Retinoic Acid and Histone Deacetylases Regulate Epigenetic Changes in Embryonic Stem Cells. J. Biol. Chem. 2014, 289, 19519. [Google Scholar] [CrossRef]
- Singh, N.; Trivedi, C.M.; Lu, M.; Mullican, S.E.; Lazar, M.A.; Epstein, J.A. Histone deacetylase 3 regulates smooth muscle differentiation in neural crest cells and development of the cardiac outflow tract. Circ. Res. 2011, 109, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.L.; Potthoff, M.J.; Haberland, M.; Qi, X.; Matsuzaki, S.; Humphries, K.M.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. Maintenance of cardiac energy metabolism by histone deacetylase 3 in mice. J. Clin. Investig. 2008, 118, 3588–3597. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Singh, N.; Mullican, S.E.; Everett, L.J.; Li, L.; Yuan, L.; Liu, X.; Epstein, J.A.; Lazar, M.A. Diet-induced Lethality Due to Deletion of the Hdac3 Gene in Heart and Skeletal Muscle. J. Biol. Chem. 2011, 286, 33301. [Google Scholar] [CrossRef] [PubMed]
- Niederreither, K.; Vermot, J.; Schuhbaur, B.; Chambon, P.; Dollé, P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development 2002, 129, 3563–3574. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]

| Embryo’s Phenotype | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet | Mother’s Genotype | Embryo’s Genotype | Total n Embryos | N of AAD | Total n of AAD | N of Normal Phenotype | Total n of Normal | % AAD | % Normal | Litters (n) |
| VitA Sup. | WT | WT | 37 | 3 | 21 | 34 | 51 | 8.11 | 91.89 | 12 |
| Df1 | 35 | 18 | 17 | 51.43 | 48.57 | |||||
| Df1 | WT | 30 | 0 | 6 | 30 | 46 | 0.00 | 100.00 | 10 | |
| Df1 | 22 | 6 | 16 | 27.27 | 72.73 | |||||
| VitA Cont. | WT | WT | 46 | 2 | 16 | 44 | 79 | 4.35 | 95.65 | 13 |
| Df1 | 49 | 14 | 35 | 28.57 | 71.43 | |||||
| Df1 | WT | 47 | 1 | 13 | 46 | 86 | 2.13 | 97.87 | 15 | |
| Df1 | 52 | 12 | 40 | 23.08 | 76.92 | |||||
| VitA Def. | WT | WT | 42 | 0 | 14 | 42 | 75 | 0.00 | 100.00 | 12 |
| Df1 | 47 | 14 | 33 | 29.79 | 70.21 | |||||
| Df1 | WT | 14 | 1 | 6 | 13 | 19 | 7.14 | 92.86 | 4 | |
| Df1 | 11 | 5 | 6 | 45.45 | 54.55 | |||||
| Total | 432 | 76 | 76 | 356 | 356 | 17.59 | 82.41 | 66 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amengual-Cladera, E.; Llull-Alberti, M.V.; Ventayol-Guirado, M.; Jimenez-Barcelo, J.A.; Rocha, J.E.; Muncunill, J.; Hernandez-Rodriguez, J.; Medina-Chávez, D.; Lynton-Pons, E.; Sureda-Horrach, P.; et al. Maternal Genotype and Dietary Vitamin A Modify Aortic Arch Phenotypes in a Mouse Model of 22q11DS. Int. J. Mol. Sci. 2025, 26, 10595. https://doi.org/10.3390/ijms262110595
Amengual-Cladera E, Llull-Alberti MV, Ventayol-Guirado M, Jimenez-Barcelo JA, Rocha JE, Muncunill J, Hernandez-Rodriguez J, Medina-Chávez D, Lynton-Pons E, Sureda-Horrach P, et al. Maternal Genotype and Dietary Vitamin A Modify Aortic Arch Phenotypes in a Mouse Model of 22q11DS. International Journal of Molecular Sciences. 2025; 26(21):10595. https://doi.org/10.3390/ijms262110595
Chicago/Turabian StyleAmengual-Cladera, Emilia, Maria Victòria Llull-Alberti, Marc Ventayol-Guirado, Juan Antonio Jimenez-Barcelo, Jairo Enrique Rocha, Josep Muncunill, Jessica Hernandez-Rodriguez, Daniela Medina-Chávez, Elionor Lynton-Pons, Paula Sureda-Horrach, and et al. 2025. "Maternal Genotype and Dietary Vitamin A Modify Aortic Arch Phenotypes in a Mouse Model of 22q11DS" International Journal of Molecular Sciences 26, no. 21: 10595. https://doi.org/10.3390/ijms262110595
APA StyleAmengual-Cladera, E., Llull-Alberti, M. V., Ventayol-Guirado, M., Jimenez-Barcelo, J. A., Rocha, J. E., Muncunill, J., Hernandez-Rodriguez, J., Medina-Chávez, D., Lynton-Pons, E., Sureda-Horrach, P., Asensio, V. J., Ruiz-Guerra, L., Tubau, A., Juan-Clar, M., Bilio, M., Morrow, B., Vives-Bauzà, C., Lania, G., Illingworth, E., ... Heine-Suñer, A. D. (2025). Maternal Genotype and Dietary Vitamin A Modify Aortic Arch Phenotypes in a Mouse Model of 22q11DS. International Journal of Molecular Sciences, 26(21), 10595. https://doi.org/10.3390/ijms262110595









