Application of TiO2 in Photocatalytic Bacterial Inactivation: Review
Abstract
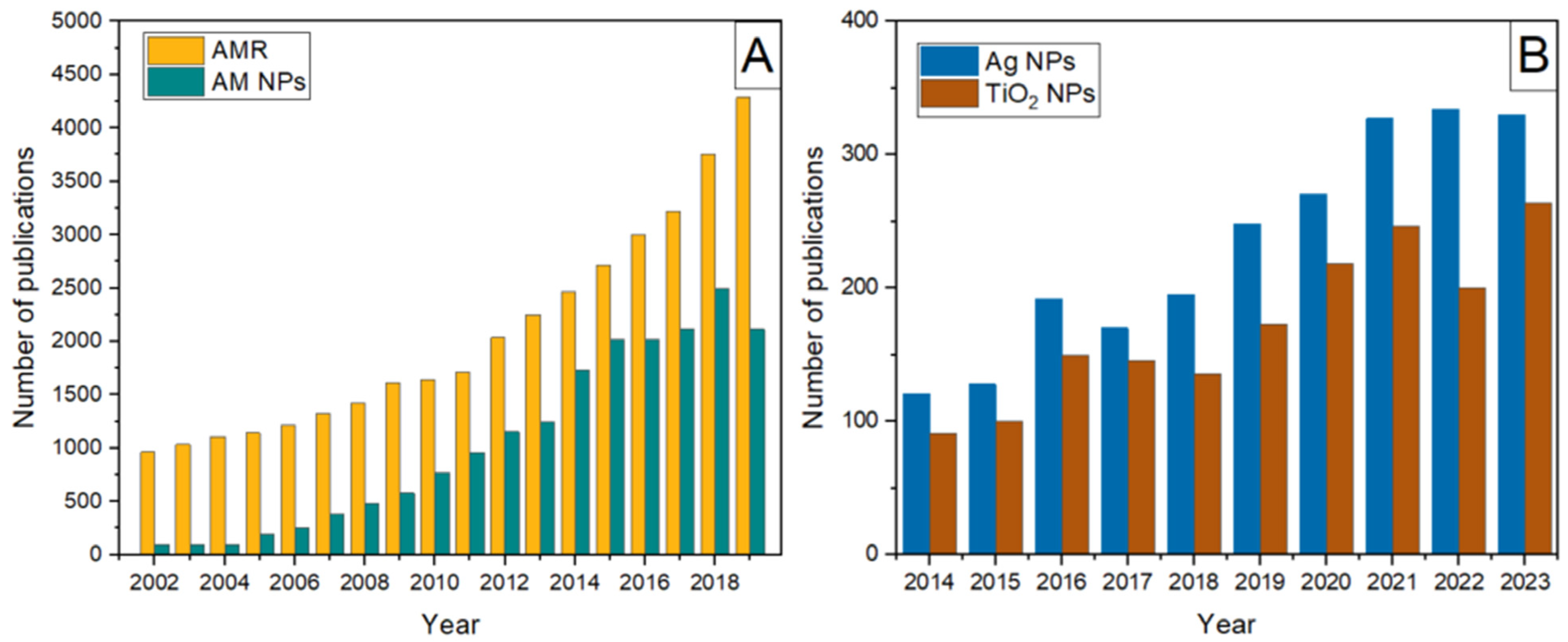
1. Introduction
2. TiO2—Basic Properties
2.1. Structure
2.2. Surface Charge
2.3. Energy Level Alignment—Optical Properties
3. Reactive Oxygen Species (ROS)
3.1. Generation of ROS
3.2. Redox Properties of ROS
4. Antimicrobial Activity of TiO2
4.1. Methods for In Vitro Determination of Antibacterial Activity
4.1.1. Disk-Diffusion Method
4.1.2. Dilution Tests
4.1.3. Time-Kill Method
4.2. TiO2 Suspensions
4.2.1. Antibacterial Activity of Commercial TiO2 (Degussa P25)
4.2.2. Antibacterial Activity of TiO2 Prepared by Chemical Methods
4.2.3. Antibacterial Activity of TiO2 Prepared by Biological Methods
4.3. Immobilized TiO2
4.3.1. Antibacterial Activity of Thin TiO2 Films
4.3.2. Antibacterial Activity of TiO2–Polymer Nanocomposites
4.4. TiO2—Noble Metal Heterostructures
4.5. Visible-Light-Responsive TiO2
4.5.1. Dopped TiO2
4.5.2. TiO2-Semiconductor Heterostructure
4.5.3. Surface-Modified TiO2 with Dyes and Interfacial Charge Transfer Complexes
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandler, C.I.R. Current accounts of antimicrobial resistance: Stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun. 2019, 5, 53. [Google Scholar] [CrossRef]
- Sweileh, W.M.; Mansour, A.M. Bibliometric analysis of global research output on antimicrobial resistance in the environment (2000–2019). Glob. Health Res. Policy 2020, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Chung, E.; Johnston, I.; Ren, G.; Cheong, Y.-K. Exploitation of Antimicrobial Nanoparticles and Their Applications in Biomedical Engineering. Appl. Sci. 2021, 11, 4520. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Prucek, R.; Kolář, M.; Večeřová, R.; Pizúrová, N.; Sharma, V.K.; Nevečná, T.; Zbořil, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Rai, M.; Yadev, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Laurentino, L.S.; Santos, L.M.G.D.; Silva, C.B.; de Barros, A.L.R.; Thomas, P.C.L.; Moreira, J.C. The Use of Silver Nanoparticles as Antimicrobial Agents between 2014 and 2023 in Brazil and Worldwide: A Bibliometric Review. J. Braz. Chem. Soc. 2025, 36, 20240167. [Google Scholar] [CrossRef]
- Hanim, S.A.M.; Malek, N.A.N.N.; Ibrahim, Z. Analyses of surface area, porosity, silver release and antibacterial activity of amine-functionalized, silver-exchanged zeolite NaY. Vacuum 2017, 143, 344–347. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, H.; Wang, Z.; Liu, S.; Hao, L.; Sang, Y.; Liu, D.; Wang, J.; Boughton, R.I. Silver nanoparticle-decorated porous ceramic composite for water treatment. J. Membr. Sci. 2009, 331, 50–56. [Google Scholar] [CrossRef]
- Lazić, V.; Smičiklas, I.; Marković, J.; Lončarević, D.; Dostanić, J.; Ahrenkiel, S.P.; Nedeljković, J.M. Antibacterial ability of supported silver nanoparticles by functionalized hydroxyapatite with 5-aminosalicylic acid. Vacuum 2018, 148, 62–68. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Gray, D.G. Bactericidal paper impregnated with silver nanoparticles for point-of-use water treatment. Environ. Sci. Technol. 2011, 45, 1992–1998. [Google Scholar] [CrossRef] [PubMed]
- Marini, M.; De Niederhausern, S.; Iseppi, R.; Bondi, M.; Sabia, C.; Toselli, M.; Pilati, F. Antibacterial activity of plastics coated with silver-doped organic-inorganic hybrid coatings prepared by sol—gel processes. Biomacromolecules 2007, 8, 1246–1254. [Google Scholar] [CrossRef]
- Radetić, M.; Ilić, V.; Vodnik, V.; Dimitrijević, S.; Jovančić, P.; Šaponjić, Z.; Nedeljković, J.M. Antibacterial effect of silver nanoparticles deposited on corona-treated polyester and polyamide fabrics. Polym. Adv. Technol. 2008, 19, 1816–1821. [Google Scholar] [CrossRef]
- Travan, A.; Pelillo, C.; Donati, I.; Marsich, E.; Benincasa, M.; Scarpa, T.; Semeraro, S.; Turco, G.; Gennero, R.; Paoletti, S. Non-cytotoxic silver nanoparticle-polysaccharide nanocomposites with antimicrobial activity. Biomacromolecules 2009, 10, 1429–1435. [Google Scholar] [CrossRef]
- Ilić, V.; Šaponjić, Z.; Vodnik, V.; Potkonjak, B.; Jovančić, P.; Nedeljković, J.; Radetić, M. The influence of silver content on antimicrobial activity and color of cotton fabrics functionalized with Ag nanoparticles. Carbohyd. Polym. 2009, 78, 564–569. [Google Scholar] [CrossRef]
- Ilić, V.; Šaponjić, Z.; Vodnik, V.; Lazarević, S.; Dimitrijević, S.; Jovančić, P.; Nedeljković, J.M.; Radetić, M. Bactericidal efficiency of silver nanoparticles deposited onto radio frequency plasma pretreated polyester fabrics. Ind. Eng. Chem. Res. 2010, 49, 7287–7293. [Google Scholar] [CrossRef]
- Mthombeni, N.H.; Mpenyana-Monyatsi, L.; Onyango, M.S.; Momba, M.N.B. Breakthrough analysis for water disinfection using silver nanoparticles coated resin beads in fixed-bed column. J. Hazard. Mater. 2012, 217–218, 133–140. [Google Scholar] [CrossRef]
- Vukoje, I.; Lazić, V.; Vodnik, V.; Mitrić, M.; Jokić, B.; Ahrenkiel, S.P.; Nedeljković, J.M.; Radetić, M. The influence of triangular silver nanoplates on antimicrobial activity and color of cotton fabrics pretreated with chitosan. J. Mater. Sci. 2014, 49, 4453–4460. [Google Scholar] [CrossRef]
- Vukoje, I.D.; Džunuzović, E.S.; Lončarević, D.R.; Dimitrijević, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Synthesis, characterization, and antimicrobial activity of silver nanoparticles on poly(GMA-co-EGDMA) polymer support. Polym. Compos. 2017, 38, 1206–1214. [Google Scholar] [CrossRef]
- Davidović, S.; Lazić, V.; Miljković, M.; Gordić, M.; Sekulić, M.; Marinović-Cincović, M.; Ratnayake, I.S.; Ahrenkiel, S.P.; Nedeljković, J.M. Antibacterial ability of immobilized silver nanoparticles in agar-agar films co-doped with magnesium ions. Carbohyd. Polym. 2019, 224, 115187. [Google Scholar] [CrossRef]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf 2020, 26, 100575. [Google Scholar] [CrossRef]
- Lok, C.-N.; Ho, C.-M.; Chen, R.; He, Q.-Y.; Yu, W.-Y.; Sun, H.; Tam, P.K.-H.; Chiu, J.-F.; Che, C.-M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Tończyk, A.; Niedziałkowska, K.; Lisowska, K. Ecotoxic effect of mycogenic silver nanoparticles in water and soil environment. Sci. Rep. 2025, 15, 10815. [Google Scholar] [CrossRef]
- Serov, D.A.; Gritsaeva, A.V.; Yanbaev, F.M.; Simakin, A.V.; Gudkov, S.V. Review of Antimicrobial Properties of Titanium Dioxide Nanoparticles. Int. J. Mol. Sci. 2024, 25, 10519. [Google Scholar] [CrossRef]
- Karthikeyan, K.T.; Nithya, A.; Jothivenkatachalam, K. Photocatalytic and antimicrobial activities of chitosan-TiO2 nanocomposite. Int. J. Biol. Macromol. 2017, 104, 1762–1773. [Google Scholar] [CrossRef]
- Rajput, P.; Deshpande, M.P.; Bhoi, H.R.; Suchak, N.M.; Desai, P.H.; Chaki, S.H.; Pandya, S.J.; Mishra, M.; Bhatt, S.V.; Tiwari, D.K.; et al. Photocatalytic and antibacterial activity of Yttrium doped TiO2 nanostructure. Chem. Phys. Impact 2022, 5, 100101. [Google Scholar] [CrossRef]
- Rosa, R.H.; Silva, R.S.; Nascimento, L.L.; Okura, M.H.; Patrocinio, A.O.T.; Rossignolo, J.A. Photocatalytic and Antimicrobial Activity of TiO2 Films Deposited on Fiber-Cement Surfaces. Catalysts 2023, 13, 861. [Google Scholar] [CrossRef]
- Soylu, N.Y.; Soylu, A.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Kucukbayrak, S.; Taygun, M.E. Photocatalytic and Antimicrobial Properties of Electrospun TiO2–SiO2–Al2O3–ZrO2–CaO–CeO2 Ceramic Membranes. ACS Omega 2023, 8, 10836–10850. [Google Scholar] [CrossRef] [PubMed]
- Atacan, K.; Güy, N.; Özacar, M. Recent advances in photocatalytic coatings for antimicrobial surfaces. Curr. Opin. Chem. Eng. 2022, 36, 100777. [Google Scholar] [CrossRef]
- van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Schraa, G.; Zehnder, A.J.B. Electrophoretic mobility and hydrophobicity as a measured to predict the initial steps of bacterial adhesion. Appl. Environ. Microbiol. 1987, 53, 1898–1901. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y. Photocatalytic effect on plasmid DNA damage under different UV irradiation time. Build. Environ. 2008, 43, 253–257. [Google Scholar] [CrossRef]
- Kim, S.; Ghafoor, K.; Lee, J.; Feng, M.; Hong, J.; Lee, D.U.; Park, J. Bacterial inactivation in water, DNA strand breaking, and membrane damage induced by ultraviolet-assisted titanium dioxide photocatalysis. Water Res. 2013, 47, 4403–4411. [Google Scholar] [CrossRef]
- Pigeot-Rémya, S.; Simoneta, F.; Errazuriz-Cerdad, E.; Lazzaronie, J.C.; Atlane, D.; Guillarda, C. Photocatalysis and disinfection of water: Identification of potential bacterial targets. Appl. Catal. B Environ. 2011, 104, 390–398. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, X.; Zhao, J.; Sun, T.; Zhu, Y. Mechanism of TiO2 nanotube UV-photocatalytic degradation of antibiotic resistance genes in wastewater sludge and blocking of their transfer. Front. Environ. Sci. 2025, 13, 1590101. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanism behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sust. Energ. Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Melián, E.P.; Díaz, O.G.; Méndez, A.O.; López, C.R.; Suárez, M.N.; Rodríguez, J.M.D.; Navío, J.A.; Hevia, D.F.; Peña, J.P. Efficient and affordable hydrogen production by water photo-splitting using TiO2-based Photocatalysts. Int. J. Hydrogen Energ. 2013, 38, 2144–2155. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A critical review in strategies to improve photocatalytic water splitting towards hydrogen production. Int. J. Hydrogen Energ. 2019, 44, 540–577. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photoch. Photobio C 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Kubacka, A.; Diez, M.S.; Rojo, D.; Bargiela, R.; Ciordia, S.; Zapico, I.; Albar, J.P.; Barbas, C.; Santos, V.A.P.M.D.; Fernández-Garcıá, M.; et al. Understanding the antimicrobial mechanism of TiO2-based nanocomposite films in a pathogenic bacterium. Sci. Rep. 2014, 4, 4134. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Kusior, A.; Banas, J.; Trenczek-Zajac, A.; Zubrzycka, P.; Micek-Ilnicka, A.; Radecka, M. Structural properties of TiO2 nanomaterials. J. Mol. Struct. 2018, 1157, 327–336. [Google Scholar] [CrossRef]
- Gatou, M.-A.; Syrrakou, A.; Lagopati, N.; Pavlatou, E.A. Photocatalytic TiO2-Based Nanostructures as a Promising Material for Diverse Environmental Applications: A Review. Reactions 2024, 5, 135–194. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- González-Reyes, L.; Hernández-Pérez, I.; Arceo, L.D.-B.; Dorantes-Rosales, H.; Arce-Estrada, E.; Suárez-Parra, R.; Cruz-Rivera, J.J. Temperature effects during Ostwald ripening on structural and bandgap properties of TiO2 nanoparticles prepared by sonochemical synthesis. Mater. Sci. Eng. B 2010, 175, 9–13. [Google Scholar] [CrossRef]
- Low, I.M.; Albetran, H.; Prida, V.M.; Vega, V.; Manurung, P.; Ionescu, M. A comparative study on crystallization behavior, phase stability, and binding energy in pure and Cr-doped TiO2 nanotubes. J. Mater. Res. 2013, 28, 304–312. [Google Scholar] [CrossRef]
- Kosmulski, M. The significance of the difference in the point of zero charge between rutile and anatase. Adv. Colloid Interface Sci. 2002, 99, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Pagnout, C.; Jomini, S.; Dadhwal, M.; Caillet, C.; Thomas, F.; Bauda, P. Role of electrostatic interactions in the toxicity of titanium dioxide nanoparticles toward Escherichia coli. Colloids Surf. B Biointerfaces 2012, 92, 315–321. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Vijaya, P. Evaluation of In-vitro Biocompatibility and Antimicrobial activities of Titanium Dioxide (TiO2) Nanoparticles by Hydrothermal Method. Nano Biomed. Eng. 2021, 13, 36–43. [Google Scholar] [CrossRef]
- Wasa, A.; Land, J.G.; Gorthy, R.; Krumdieck, S.; Bishop, C.; Godsoe, W.; Heinemann, J.A. Antimicrobial and biofilm-disrupting nanostructured TiO2 coating demonstrating photoactivity and dark activity. FEMS Microbiol. Lett. 2021, 368, fnab039. [Google Scholar] [CrossRef]
- Rathore, C.; Yadav, V.K.; Gacem, A.; AbdelRahim, S.K.; Verma, R.K.; Chundawat, R.S.; Gnanamoorthy, G.; Yadav, K.K.; Choudhary, N.; Sahoo, D.K.; et al. Microbial synthesis of titanium dioxide nanoparticles and their importance in wastewater treatment and antimicrobial activities: A review. Front. Microbiol. 2023, 14, 1270245. [Google Scholar] [CrossRef]
- Bak, T.; Nowotny, J.; Rekas, M.; Sorrell, C.C. Photo-electrochemical hydrogen generation from water using solar energy. Materials-related aspects. Int. J. Hydrogen Energ. 2002, 27, 991–1022. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide ion: Generation and chemical implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Wood, P.M. The potential diagram for oxygen at pH 7. Biochem. J. 1988, 253, 287–289. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Bio. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef]
- Schwarz, H.A.; Dodson, R.W. Equilibrium between hydroxyl radicals and thallium(II) and the oxidation potential of OH(aq). J. Phys. Chem. 1984, 88, 3643–3647. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (•OH/•O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Bajić, V.; Spremo-Potparević, B.; Živković, L.; Čabarkapa, A.; Kotur-Stevuljević, J.; Isenović, E.; Sredojević, D.; Vukoje, I.; Lazić, V.; Ahrenkiel, S.P.; et al. Surface-modified TiO2 nanoparticles with ascorbic acid: Antioxidant properties and efficiency against DNA damage in vitro. Colloid Surf. B 2017, 155, 323–331. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Younis, A.B.; Haddad, Y.; Kosaristanova, L.; Smerkova, K. Titanium dioxide nanoparticles: Recent progress in antimicrobial applications. WIREs Nanomed. Nanobi. 2023, 15, 1860. [Google Scholar] [CrossRef]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef]
- Markov, S.L.; Vidaković, A.M. Testing methods for antimicrobial activity of TiO2 photocatalyst. Acta Period. Technol. 2014, 45, 141–152. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Correa, M.G.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.N.; Stocker, S.A.; Culver, D.H.; Thornsberry, C. Comparison of the E test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J. Clin. Microbiol. 1991, 29, 533–538. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Janczura, A.; Nowicka, J.; Kamysz, W. Methods Used for the Eradication of Staphylococcal Biofilms. Antibiotics 2019, 8, 174. [Google Scholar] [CrossRef]
- Almquist, C.B.; Biswas, P. Role of Synthesis Method and Particle Size of Nanostructured TiO2 on Its Photoactivity. J. Catal. 2002, 212, 145–156. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Xiong, G.; Shao, R.; Droubay, T.C.; Joly, A.G.; Beck, K.M.; Chambers, S.A.; Hess, W.P. Photoemission electron microscopy of TiO2 anatase films embedded with rutile nanocrystals. Adv. Funct. Mater. 2007, 17, 2133–2138. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Foerster, S.; Unemo, M.; Hathaway, L.J.; Low, N.; Althaus, C.L. Time-kill curve analysis and pharmacodynamic modelling for in vitro evaluation of antimicrobials against Neisseria gonorrhoeae. BMC Microbiol. 2016, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.C.; Klostermann, P.; Rice, E.W.; Clark, R.M. Inactivation of Escherichia coli by Titanium Dioxide Photocatalytic Oxidation. Appl. Environ. Microbiol. 1993, 59, 1668–1670. [Google Scholar] [CrossRef]
- Bekbölet, M.; Araz, C.V. Inactivation of Escherichia coli by photocatalytic oxidation. Chemosphere 1996, 32, 959–965. [Google Scholar] [CrossRef]
- Bekbölet, M. Photocatalytic bactericidal activity of TiO2 in aqueous suspensions of E. coli. Water Sci. Technol. 1997, 35, 95–100. [Google Scholar] [CrossRef]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [CrossRef]
- Vidal, A.; Díaz, A.I.; El Hraiki, A.; Romero, M.; Muguruza, I.; Senhaji, F.; González, J. Solar photocatalysis for detoxification and disinfection of contaminated water: Pilot plant studies. Catal. Today 1999, 54, 283–290. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.-C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A 2000, 130, 163–170. [Google Scholar] [CrossRef]
- Ibáñez, J.A.; Litter, M.I.; Pizarro, R.A. Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae. Comparative study with other Gram (−) bacteria. J. Photochem. Photobiol. A 2003, 157, 81–85. [Google Scholar] [CrossRef]
- McLoughlin, O.A.; Ibáñez, P.F.; Gernjak, W.; Rodriguez, S.M.; Gill, L.W. Photocatalytic disinfection of water using low cost compound parabolic collectors. Sol. Energy 2004, 77, 625–633. [Google Scholar] [CrossRef]
- Duffy, E.F.; Al Touati, F.; Kehoe, S.C.; McLoughlin, O.A.; Gill, L.W.; Gernjak, W.; Oller, I.; Maldonado, M.I.; Malato, S.; Cassidy, J.; et al. A novel TiO2-assisted solar photocatalytic batch-process disinfection reactor for the treatment of biological and chemical contaminants in domestic drinking water in developing countries. Sol. Energy 2004, 77, 649–655. [Google Scholar] [CrossRef]
- McLoughlin, O.A.; Kehoe, S.C.; McGuigan, K.G.; Duffy, E.F.; Al Touati, F.; Gernjak, W.; Alberola, I.O.; Rodríguez, S.M.; Gill, L.W. Solar disinfection of contaminated water: A comparison of three small-scale reactors. Sol. Energy 2004, 77, 657–664. [Google Scholar] [CrossRef]
- Kim, S.Y.; Nishioka, M.; Taya, M. Promoted proliferation of an SOD-deficient mutant of Escherichia coli under oxidative stress induced by photoexcited TiO2. FEMS Microbiol. Lett. 2004, 236, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Seven, O.; Dindar, B.; Aydemir, S.; Metin, D.; Ozinel, M.A.; Icli, S. Solar photocalytic disinfection of a group of bacteria and fungi aqueous suspensions with TiO2, ZnO and sahara desert dust. J. Photochem. Photobiol. A 2004, 165, 103–107. [Google Scholar] [CrossRef]
- Fernández, P.; Blanco, J.; Sichel, C.; Malato, S. Water disinfection by solar photocatalysis using compound parabolic collectors. Catal. Today 2005, 101, 345–352. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO2 photocatalytic disinfection. Appl. Environ. Microbiol. 2005, 71, 270–275. [Google Scholar] [CrossRef]
- Robertson, J.M.C.; Robertson, P.K.J.; Lawton, L.A. A comparison of the effectiveness of TiO2 photocatalysis and UVA photolysis for the destruction of three pathogenic micro-organisms. J. Photochem. Photobiol. A 2005, 175, 51–56. [Google Scholar] [CrossRef]
- Lonnen, J.; Kilvington, S.; Kehoe, S.C.; Al-Touati, F.; McGuigan, K.G. Solar and photocatalytic disinfection of protozoan, fungal and bacterial microbes in drinking water. Wat. Res. 2005, 39, 877–883. [Google Scholar] [CrossRef]
- Gumy, D.; Morais, C.; Bowen, P.; Pulgarin, C.; Giraldo, S.; Hadju, R.; Kiwi, J. Catalytic activity of commercial of TiO2 powders for the abatement of the bacteria (E. coli) under solar simulated light: Influence of the isoelectric point. Appl. Catal. B 2006, 63, 76–84. [Google Scholar] [CrossRef]
- Gumy, D.; Rincon, A.G.; Hajdu, R.; Pulgarin, C. Solar photocatalysis for detoxification and disinfection of water: Different types of suspended and fixed TiO2 catalysts study. Sol. Energy 2006, 80, 1376–1381. [Google Scholar] [CrossRef]
- Gogniat, G.; Dukan, S. TiO2 photocatalysis causes DNA damage via Fenton reaction-generated hydroxyl radicals during the recovery period. Appl. Environ. Microbiol. 2007, 73, 7740–7743. [Google Scholar] [CrossRef] [PubMed]
- Benabbou, A.K.; Derriche, Z.; Felix, C.; Lejeune, P.; Guillard, C. Photocatalytic inactivation of Escherichia coli: Effect of concentration of TiO2 and microorganism, nature, and intensity of UV irradiation. Appl. Catal. B 2007, 76, 257–263. [Google Scholar] [CrossRef]
- Pal, A.; Pehkonen, S.O.; Yu, L.E.; Ray, M.B. Photocatalytic inactivation of Gram-positive and Gram-negative bacteria using fluorescent light. J. Photoch. Photobio. A 2007, 186, 335–341. [Google Scholar] [CrossRef]
- Block, S.S.; Seng, V.P.; Goswami, D.W. Chemically enhanced sunlight for killing bacteria. J. Sol. Energy Eng. Trans. ASME 1997, 119, 85–91. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Robertson, P.K.J.; Officer, S.; Pollard, P.M.; McCullagh, C.; Robertson, J.M.C. Variables to be considered when assessing the photocatalytic destruction of bacterial pathogens. Chemosphere 2009, 74, 1374–1378. [Google Scholar] [CrossRef]
- Fiorentino, A.; Rizzo, L.; Guilloteau, H.; Bellanger, X.; Merlin, C. Comparing TiO2 photocatalysis and UV-C radiation for inactivation and mutant formation of Salmonella typhimurium TA102. Environ. Sci. Pollut. Res. 2017, 24, 1871–1879. [Google Scholar] [CrossRef]
- Araña, J.; Melián, J.A.H.; Rodríguez, J.M.D.; Díaz, O.G.; Viera, A.; Peña, J.P.; Sosa, P.M.M.; Jiménez, V.E. TiO2-photocatalysis as a tertiary treatment of naturally treated wastewater. Catal. Today 2002, 76, 279–289. [Google Scholar] [CrossRef]
- Melián, J.A.H.; Rodríguez, J.M.D.; Suárez, A.V.; Rendón, E.T.; Campo, C.V.D.; Arana, J.; Peña, J.P. The photocatalytic disinfection of urban waste waters. Chemosphere 2000, 41, 323–327. [Google Scholar] [CrossRef]
- Muszkat, L.; Feigelson, L.; Bir, L.; Muszkat, K.A.; Teitel, M.; Dornay, I.; Kirchner, B.; Kritzman, G. Solar photo-inactivation of phytopathogens by trace level hydrogen peroxide and titanium dioxide photocatalysis. Phytoparasitica 2005, 33, 267–274. [Google Scholar] [CrossRef]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of Photocatalytic Bactericidal Action of Powdered Semiconductor TiO2 on Mutans Streptococci. J. Photochem. Photohiol. B Biol. 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Agulló-Barceló, M.; Polo-López, M.I.; Lucena, F.; Jofre, J.; Fernández-Ibáñez, P. Solar Advanced Oxidation Processes as disinfection tertiary treatments for real wastewater: Implications for water reclamation. Appl. Catal. B 2013, 136–137, 341–350. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2008, 43, 2085–2103. [Google Scholar] [CrossRef]
- Shandilya, M.; Rai, R.; Singh, J. Review: Hydrothermal technology for smart materials. Adv. Appl. Ceram. 2016, 115, 354–376. [Google Scholar] [CrossRef]
- Kim, B.; Kim, D.; Cho, D.; Cho, S. Bactericidal effect of TiO2 photocatalyst on selected food-borne pathogenic bacteria. Chemosphere 2003, 52, 277–281. [Google Scholar] [CrossRef]
- Swetha, S.; Santhosh, S.M.; Balakrishna, R.G. Synthesis and Comparative Study of Nano-TiO2 Over Degussa P-25 in Disinfection of Water. Photochem. Photobiol. 2010, 86, 628–632. [Google Scholar] [CrossRef]
- Salinas, J.L.A.; Aguilar, J.R.P.; Hernández, S.A.M.; Cruz, J.S. Bactericidal Activity of TiO2 on Cells of Pseudomonas aeruginosa ATCC 27853. Int. J. Photoenergy 2013, 2013, 954914. [Google Scholar]
- Hitkova, H.; Stoyanova, A.; Ivanova, N.; Sredkova, M.; Popova, V.; Iordanova, R.; Bachvarova-Nedelcheva, A. Study of Antibacterial Activity of Nonhydrolytic Synthesized TiO2 against E. coli, P. Aeruginosa and S. aureus. J. Optoelectron. Biome. 2012, 4, 9–17. [Google Scholar]
- Coleman, H.M.; Marquis, C.P.; Scott, J.A.; Chin, S.-S.; Amal, R. Bactericidal effects of titanium dioxide-based photocatalysts. Chem. Eng. J. 2005, 113, 55–63. [Google Scholar] [CrossRef]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef]
- Prasad, G.K.; Agarwal, G.S.; Singh, B.; Rai, G.P.; Vijayaraghavan, R. Photocatalytic inactivation of Bacillus anthracis by titania nanomaterials. J. Hazard. Mater. 2009, 165, 506–510. [Google Scholar] [CrossRef]
- Priyanka, K.P.; Sukirtha, T.H.; Balakrishna, K.M.; Varghese, T. Microbicidal activity of TiO2 nanoparticles synthesised by sol–gel method. IET Nanobiotechnol. 2016, 10, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Almashhori, K.; Ali, T.T.; Saeed, A.; Alwafi, R.; Aly, M.; Al-Hazmi, F.E. Antibacterial and photocatalytic activities of controllable (anatase/rutile) mixed phase TiO2 nanophotocatalysts synthesized via a microwave-assisted sol–gel method. New J. Chem. 2020, 44, 562–570. [Google Scholar] [CrossRef]
- Yaemsunthorn, K.; Kobielusz, M.; Macyk, W. TiO2 with tunable anatase-to-rutile nanoparticles ratios: How does the photoactivity depend on the phase composition and the nature of photocatalytic reaction? ACS Appl. Nano Mater. 2021, 4, 633–643. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumaghim, J.L. A Review of the Antioxidant Mechanisms of Polyphenol Compounds Related to Iron Binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Jameel, M.S.; Aziz, A.A.; Dheyab, M.A. Green synthesis: Proposed mechanism and factors influencing the synthesis of platinum nanoparticles. Green Process. Synth. 2020, 9, 386–398. [Google Scholar] [CrossRef]
- Santhosh, P.B.; Genova, J.; Chamati, H. Green Synthesis of Gold Nanoparticles: An Eco-Friendly Approach. Chemistry 2022, 4, 345–369. [Google Scholar] [CrossRef]
- Miljković, M.; Lazić, V.; Davidović, S.; Milivojević, A.; Papan, J.; Fernandes, M.M.; Lanceros, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Selective Antimicrobial Performance of Biosynthesized Silver Nanoparticles by Horsetail Extract Against E. coli. J. Inorg. Organomet. P. 2020, 30, 2598–2607. [Google Scholar] [CrossRef]
- Pirković, A.; Lazić, V.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Kuzman, S.; Antić-Stanković, J.; Božić, D.; Krivokuća, M.J.; Nedeljković, J.M. Comparative analysis of Ag NPs functionalized with olive leaf extract and oleuropein and toxicity in human trophoblast cells and peripheral blood lymphocytes. Mutagenesis 2023, 38, 169–181. [Google Scholar] [CrossRef]
- Irshad, S.; Iftikhar, S.; Riaz, M.; Mahmood, A.; Mushtaq, A.; Saleem, Y.; Shamim, R.; Akter, Q.S. Chemical fingerprinting, antimicrobial, antioxidant, anti-inflammatory, and anticancer potential of greenly synthesized silver nanoparticles from pistachio (Pistacia vera) nuts and senna (Cassia angustifolia Vahl.) leaves. Food Sci. Nutr. 2024, 12, 4989–5006. [Google Scholar] [CrossRef]
- Sethy, N.K.; Arif, Z.; Mishra, P.K.; Kumar, P. Green synthesis of TiO2 nanoparticles from Syzygium cumini extract for photo-catalytic removal of lead (Pb) in explosive industrial wastewater. Green Process. Synth. 2020, 9, 171–181. [Google Scholar] [CrossRef]
- Rodríguez-Jiménez, R.-A.; Panecatl-Bernal, Y.; Carrillo-López, J.; Méndez-Rojas, M.-Á.; Romero-López, A.; Pacio-Castillo, M.; Vivaldo, I.; Morales-Sánchez, A.; Arce, R.D.; Caram, J.; et al. Influence of Ethanolic Plant Extracts on Morphology and Size Distribution of Sol-Gel Prepared TiO2 Nanoparticles. ChemistrySelect 2021, 6, 3958–3968. [Google Scholar] [CrossRef]
- Rahmawati, D.; Permana, M.D.; Eddy, D.R.; Saito, N.; Takei, T.; Suryana; Noviyanti, A.R.; Rahayu, I.; Helal, M.H.; El-Bahy, Z.M. Synthesis of TiO2 nanoparticles using red spinach leaf extract (Amaranthus tricolor L.) for photocatalytic of methylene blue degradation. Green Chem. Lett. Rev. 2024, 17, 2352571. [Google Scholar] [CrossRef]
- Abduh, N.A.Y.; Algarni, T.S.; Al, B. Green synthesis of Zn-doped TiO2 nanomaterials for photocatalytic degradation of crystal violet and methylene blue dyes under sunlight. Biomass Convers. Bior. 2025, 15, 4849–4865. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Bama, K.; Bhavani, M.; Jegatheeswaran, S.; Ambika, S.; Sangili, A.; Nithya, P.; Sumathi, R. Obtaining titanium dioxide nanoparticles with spherical shape and antimicrobial properties using M. citrifolia leaves extract by hydrothermal method. J. Photoch. Photobio. B 2017, 171, 117–124. [Google Scholar]
- Jayaseelan, C.; Rahuman, A.A.; Roopan, S.M.; Kirthi, A.V.; Venkatesan, J.; Kim, S.-K.; Iyappan, M.; Siva, C. Biological approach to synthesize TiO2 nanoparticles using Aeromonas hydrophila and its antibacterial activity. Spectrochim. Acta A 2013, 107, 82–89. [Google Scholar] [CrossRef]
- Gebre, S.H.; Sendeku, M.G. New frontiers in the biosynthesis of metal oxide nanoparticles and their environmental applications: An overview. SN Appl. Sci. 2019, 1, 928. [Google Scholar] [CrossRef]
- Bahri, S.S.; Harun, Z.; Hubadillah, S.K.; Salleh, W.N.W.; Rosman, N.; Kamaruddin, N.H.; Azhar, F.H.; Sazali, N.; Ahmad, R.A.R.; Basri, H. Review on recent advance biosynthesis of TiO2 nanoparticles from plant-mediated materials: Characterization, mechanism and application. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1142, 012005. [Google Scholar] [CrossRef]
- Aslam, M.; Abdullah, A.Z.; Rafatullah, M. Recent development in the green synthesis of titanium dioxide nanoparticles using plant-based biomolecules for environmental and antimicrobial applications. J. Ind. Eng. Chem. 2021, 98, 1–16. [Google Scholar] [CrossRef]
- Almutairi, M.H.; Khan, S.; Fozia, F.; Aslam, M.; Ahmad, I.; Sillanpää, M.; Almutairi, B.O.; Ziaullah, Z. Green chemistry approach to synthesize titanium dioxide nanoparticles using Fagonia Cretica extract, novel strategy for developing antimicrobial and antidiabetic therapies. Green Process. Synth. 2024, 13, 20240134. [Google Scholar] [CrossRef]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Bavanilatha, M.; Yoshitha, L.; Nivedhitha, S.; Sahithya, S. Bioactive studies of TiO2 nanoparticles synthesized using Glycyrrhiza glabra. Biocatal. Agric. Biotechnol. 2019, 19, 101131. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Priyamvada, B.; Khanna, V.G.; Kumar, D.K.; Sujin, P.J. Eclipta prostrata leaf aqueous extract mediated synthesis of titanium dioxide nanoparticles. Mater. Lett. 2012, 68, 115–117. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A.A.; Jayaseelan, C.; Santhoshkumar, T.; Marimuthu, S.; Kamaraj, C.; Bagavan, A.; Zahir, A.A.; Kirthi, A.V.; Elango, G.; et al. Solanum trilobatum extract-mediated synthesis of titanium dioxide nanoparticles to control Pediculus humanus capitis, Hyalomma anatolicum anatolicum and Anopheles subpictus. Parasitol. Res. 2014, 113, 469–479. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Singh, V.; Yadav, A.K.; Bharagava, R.N.; Thapa, K.B. Green synthesis of TiO2 nanoparticles using leaf extract of Jatropha curcas L. for photocatalytic degradation of tannery wastewater. Chem. Eng. J. 2018, 336, 386–396. [Google Scholar] [CrossRef]
- Rathi, V.H.; Jeice, A.R. Green fabrication of titanium dioxide nanoparticles and their applications in photocatalytic dye degradation and microbial activities. Chem. Phys. Impact 2023, 6, 100197. [Google Scholar] [CrossRef]
- Rajh, T.; Nedeljković, J.M.; Chen, L.X.; Poluektov, O.; Thurnauer, M.C. Improving optical and charge separation properties of nanocrystalline TiO2 by surface modification with vitamin C. J. Phys. Chem. B 1999, 103, 3515–3519. [Google Scholar] [CrossRef]
- Janković, I.A.; Šaponjić, Z.V.; Čomor, M.I.; Nedeljković, J.M. Surface modification of colloidal TiO2 nanoparticles with bidentate benzene derivatives. J. Phys. Chem. C 2009, 113, 12645–12652. [Google Scholar] [CrossRef]
- Santhoshkumar, T.; Rahuman, A.A.; Jayaseelan, C.; Rajakumar, G.; Marimuthu, S.; Kirthi, A.V.; Velayutham, K.; Thomas, J.; Venkatesan, J.; Kim, S.-K. Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac. J. Trop. Med. 2014, 7, 968–976. [Google Scholar] [CrossRef]
- Abu-Dalo, M.; Jaradat, A.; Albiss, B.A.; Al-Rawashdeh, N.A.F. Green synthesis of TiO2 NPs/pristine pomegranate peel extract nanocomposite and its antimicrobial activity for water disinfection. J. Environ. Chem. Eng. 2019, 7, 103370. [Google Scholar] [CrossRef]
- Rajkumari, J.; Magdalane, C.M.; Siddhardha, B.; Madhavan, J.; Ramalingam, G.; Al-Dhabi, N.A.; Arasu, M.V.; Ghilan, A.K.M.; Duraipandiayan, V.; Kaviyarasu, K. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill and evaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photoch. Photobio. B 2019, 201, 111667. [Google Scholar] [CrossRef] [PubMed]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef] [PubMed]
- Eisa, N.E.; Almansour, S.; Alnaim, I.A.; Ali, A.M.; Algrafy, E.; Ortashi, K.M.; Awad, M.A.; Virk, P.; Hendi, A.A.; Eissa, F.Z. Eco-synthesis and characterization of titanium nanoparticles: Testing its cytotoxicity and antibacterial effects. Green Process. Synth. 2020, 9, 462–468. [Google Scholar] [CrossRef]
- Ahmad, W.; Jaiswal, K.K.; Soni, S. Green synthesis of titanium dioxide (TiO2) nanoparticles by using Mentha arvensis leaves extract and its antimicrobial properties. Inorg. Nano-Met. Chem. 2020, 50, 1032–1038. [Google Scholar] [CrossRef]
- Ansari, A.; Siddiqui, V.U.; Rehman, W.U.; Akram, M.K.; Siddiqi, W.A.; Alosaimi, A.M.; Hussein, M.A.; Rafatullah, M. Green Synthesis of TiO2 Nanoparticles Using Acorus calamus Leaf Extract and Evaluating Its Photocatalytic and In Vitro Antimicrobial Activity. Catalysts 2022, 12, 1451, Correction in Catalysts 2022, 12, 181. [Google Scholar] [CrossRef]
- Shimi, A.K.; Ahmed, H.M.; Wahab, M.; Katheria, S.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A.; Rane, K.P. Synthesis and Applications of Green Synthesized TiO2 Nanoparticles for Photocatalytic Dye Degradation and Antibacterial Activity. J. Nanomater. 2022, 2022, 7060388. [Google Scholar] [CrossRef]
- Anbumani, D.; Dhandapani, K.V.; Manoharan, J.; Babujanarthanam, R.; Bashir, A.K.H.; Muthusamy, K.; Alfarhan, A.; Kanimozhi, K. Green synthesis and antimicrobial efficacy of titanium dioxide nanoparticles using Luffa acutangula leaf extract. J. King Saud Univ. Sci. 2022, 34, 101896. [Google Scholar] [CrossRef]
- Ansari, F.S.; Daneshjou, S. Optimizing the green synthesis of antibacterial TiO2-anatase phase nanoparticles derived from spinach leaf extract. Sci. Rep. 2024, 14, 22440. [Google Scholar] [CrossRef]
- Sunny, N.E.; Mathew, S.S.; Chandel, N.; Saravanan, P.; Rajeshkannan, R.; Rajasimman, M.; Vasseghian, Y.; Rajamohan, N.; Kumar, S.V. Green synthesis of titanium dioxide nanoparticles using plant biomass and their applications—A review. Chemosphere 2022, 300, 134612. [Google Scholar] [CrossRef]
- Rajaram, P.; Jeice, A.R.; Jayakumar, K. Review of green synthesized TiO2 nanoparticles for diverse applications. Surf. Interfaces 2023, 39, 102912. [Google Scholar] [CrossRef]
- Shakeel, N.; Piwoński, I.; Iqbal, P.; Kisielewska, A. Green Synthesis of Titanium Dioxide Nanoparticles: Physicochemical Characterization and Applications: A Review. Int. J. Mol. Sci. 2025, 26, 5454. [Google Scholar] [CrossRef]
- Han, C.; Lalley, J.; Namboodiri, D.; Cromer, K.; Nadagouda, M.N. Titanium dioxide-based antibacterial surfaces for water treatment. Curr. Opin. Chem. Eng. 2016, 11, 46–51. [Google Scholar] [CrossRef]
- De Pasquale, I.; Porto, C.L.; Dell’Edera, M.; Petronella, F.; Agostiano, A.; Curri, M.L.; Comparelli, R. Photocatalytic TiO2-Based Nanostructured Materials for Microbial Inactivation. Catalysts 2020, 10, 1382. [Google Scholar] [CrossRef]
- Schutte-Smith, M.; Erasmus, E.; Mogale, R.; Marogoa, N.; Jayiya, A.; Visser, H.G. Using visible light to activate antiviral and antimicrobial properties of TiO2 nanoparticles in paints and coatings: Focus on new developments for frequent-touch surfaces in hospitals. J. Coat. Technol. Res. 2023, 20, 789–817. [Google Scholar] [CrossRef]
- Maryani, E.; Nurjanah, N.S.; Hadisantoso, E.P.; Wijayanti, R.B. The Effect of TiO2 additives on the antibacterial properties (Escherichia coli and Staphylococcus aureus) of glaze on ceramic tiles. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012011. [Google Scholar] [CrossRef]
- Tsuang, Y.-H.; Sun, J.-S.; Huang, Y.-C.; Lu, C.-H.; Chang, W.H.-S.; Wan, C.-C. Studies of Photokilling of Bacteria Using Titanium Dioxide Nanoparticles. Artif. Organs 2008, 32, 167–174. [Google Scholar] [CrossRef]
- Jia, L.; Qiu, J.; Du, L.; Li, Z.; Liu, H.; Ge, S. TiO2 nanorod arrays as a photocatalytic coating enhanced antifungal and antibacterial efficiency of Ti substrates. Nanomedicine 2017, 12, 761–776. [Google Scholar] [CrossRef]
- Alharbi, M.; Althubyani, H.; Alarfaj, E.; Dastan, D.; Timoumi, A.; Tao, L.; Albetran, H.; Ţălu, Ş. Photocatalytic Performances of Dip-Coated Ag-Doped TiO2 Thin Films on Glass Substrates. Arch. Metall. Mater. 2024, 69, 987–996. [Google Scholar] [CrossRef]
- Ruvarac-Bugarčić, I.; Janković, I.; Konstantinović, Z.; Šaponjić, Z.; Nedeljković, J. Photocatalytic deposition of gold on nanocrystalline TiO2 films. Acta Chim. Slov. 2008, 55, 268–272. [Google Scholar]
- Babu, S.V.; David, M.; Patel, R.C. Two-step regression procedure for the optical characterization of thin films. Appl. Opt. 1991, 30, 839–846. [Google Scholar] [CrossRef]
- Cheng, T.C.; Chang, C.Y.; Chang, C.I.; Hwang, C.J.; Hsu, H.C.; Wang, D.Y.; Yao, K.S. Photocatalytic bactericidal effect of TiO2 film on fish pathogens. Surf. Coat. Tech. 2008, 203, 925–927. [Google Scholar] [CrossRef]
- Zarubica, A.; Vasić, M.; Antonijević, M.D.; Ranđelović, M.; Momčilović, M.; Krstić, J.; Nedeljković, J. Design and photocatalytic ability of ordered mesoporous TiO2 thin films. Mater. Res. Bull. 2014, 57, 146–151. [Google Scholar] [CrossRef]
- Assefpour-Dezfuly, M.; Vlachos, C.; Andrews, E.H. Oxide morphology and adhesive bonding on titanium surfaces. J. Mater. Sci. 1984, 19, 3626–3639. [Google Scholar] [CrossRef]
- Fu, Y.; Mo, A. A Review on the Electrochemically Self-organized Titania Nanotube Arrays: Synthesis, Modifications, and Biomedical Applications. Nanoscale Res. Lett. 2018, 13, 187. [Google Scholar] [CrossRef]
- Chan, C.M.N.; Ng, A.M.C.; Fung, M.K.; Cheng, H.S.; Guo, M.Y.; Djurišić, A.B.; Leung, F.C.C.; Chan, W.K. Antibacterial and photocatalytic activities of TiO2 nanotubes. J. Exp. Nanosci. 2013, 8, 859–867, Erratum in J. Exp. Nanosci. 2015, 10, 323. [Google Scholar] [CrossRef]
- Podporska-Carroll, J.; Panaitescu, E.; Quilty, B.; Wang, L.; Menon, L.; Pillai, S.C. Antimicrobial properties of highly efficient photocatalytic TiO2 nanotubes. Appl. Catal. B-Environ. 2015, 176, 70–75. [Google Scholar] [CrossRef]
- Opolot, E.E.; Wang, H.; Capadona, J.R.; von Recum, H.A.; Hamedani, H.A. Synergistic antibacterial activity and inhibition of TiO2 nanotube arrays and loaded antibiotics against gram-positive and gram-negative bacteria. Front. Biomater. Sci. 2024, 3, 1360443. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent advances in TiO2-functionalized textile surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E. Functionality of nano titanium dioxide on textiles with future aspects: Focus on wool. J. Photoch. Photobio. C 2011, 12, 293–303. [Google Scholar] [CrossRef]
- Radetić, M. Functionalization of textile materials with TiO2 nanoparticles. J. Photoch. Photobio. C 2013, 16, 62–76. [Google Scholar] [CrossRef]
- Uğur, Ş.S.; Sarııšık, M.; Aktaş, A.H. Nano-TiO2 based multilayer film deposition on cotton fabrics for UV-protection. Fibers Polym. 2011, 12, 190–196. [Google Scholar] [CrossRef]
- Rabiei, H.; Dehghan, S.F.; Montazer, M.; Khaloo, S.S.; Koozekonan, A.G. UV protection properties of workwear fabrics coated with TiO2 nanoparticles. Front. Public Health 2022, 10, 929095. [Google Scholar] [CrossRef]
- Cot, M.; Mijas, G.; Prieto-Fuentes, R.; Riba-Moliner, M.; Cayuela, D. The Influence of Titanium Dioxide (TiO2) Particle Size and Crystalline Form on the Microstructure and UV Protection Factor of Polyester Substrates. Polymers 2024, 16, 475. [Google Scholar] [CrossRef]
- Kong, H.; Song, J.; Jang, J. Photocatalytic Antibacterial Capabilities of TiO2-Biocidal Polymer Nanocomposites Synthesized by a Surface-Initiated Photopolymerization. Environ. Sci. Technol. 2010, 44, 5672–5676. [Google Scholar] [CrossRef]
- Choudhary, U.; Dey, E.; Bhattacharyya, R.; Ghosh, S.K. A Brief Review on Plasma Treatment of Textile Materials. Adv. Res. Text. Eng. 2018, 3, 1019. [Google Scholar] [CrossRef]
- Bouazizi, N.; Abed, A.; Giraud, S.; El Achari, A.; Campagne, C.; Morshed, M.N.; Thoumire, O.; El Moznine, R.; Cherkaoui, O.; Vieillard, J.; et al. Development of new composite fibers with excellent UV radiation protection. Physica E 2020, 118, 113905. [Google Scholar] [CrossRef]
- Saleem, M.; Naz, M.Y.; Shoukat, B.; Shukrullah, S.; Hussain, Z. Functionality and applications of non-thermal plasma activated textiles: A review. Mater. Today Proc. 2021, 47, S74–S82. [Google Scholar] [CrossRef]
- Harifi, T.; Montazer, M. Application of sonochemical technique for sustainable surface modification of polyester fibers resulting in durable nano-sonofinishing. Ultrason. Sonochem. 2017, 37, 158–168. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Molina, R.; Puač, N.; Jovančić, P.; Nedeljković, J.; Radetić, M. Improved properties of oxygen and argon RF plasma-activated polyester fabrics loaded with TiO2 nanoparticles. ACS Appl. Mater. Inter. 2010, 2, 1700–1706. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Molina, R.; Radoičić, M.; Esquena, J.; Jovančić, P.; Nedeljković, J.; Radetić, M. Multifunctional properties of polyester fabrics modified by corona discharge/air RF plasma and colloidal TiO2 nanoparticles. Polym. Compos. 2011, 32, 390–397. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Molina, R.; Radetić, T.; Jovančić, P.; Nedeljković, J.; Radetić, M. Novel properties of PES fabrics modified by corona discharge and colloidal TiO2 nanoparticles. Polym. Advan. Technol. 2011, 22, 703–709. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Lazović, S.; Baily, C.J.; Jovančić, P.; Nedeljković, J.; Radetić, M. Functionalization of cotton fabrics with corona/air RF plasma and colloidal TiO2 nanoparticles. Cellulose 2011, 18, 811–825. [Google Scholar] [CrossRef]
- De Vietro, N.; Tursi, A.; Beneduci, A.; Chidichimo, F.; Milella, A.; Fracassi, F.; Chatzisymeon, E. Giuseppe Chidichimo, Photocatalytic inactivation of Escherichia coli bacteria in water using low pressure plasma deposited TiO2 cellulose fabric. Photochem. Photobiol. Sci. 2019, 18, 2248–2258. [Google Scholar] [CrossRef] [PubMed]
- Salama, K.F.; AlJindan, R.; Alfadhel, A.; Akhtar, S.; Al-Suhaimi, E.A. Enhanced antimicrobial performance of textiles coated with TiO2 nanoparticles. J. Ind. Text. 2024, 54, 15280837241233743. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Radetić, T.; Jovančić, P.; Nedeljković, J.; Radetić, M. Functionalization of polyester fabrics with alginates and TiO2 nanoparticles. Carbohyd. Polym. 2010, 79, 526–532. [Google Scholar] [CrossRef]
- Raeisi, M.; Kazerouni, Y.; Mohammadi, A.; Hashemi, M.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A.; Davachi, S.M. Superhydrophobic cotton fabrics coated by chitosan and titanium dioxide nanoparticles with enhanced antibacterial and UV-protecting properties. Int. J. Biol. Macromol. 2021, 171, 158–165. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, D.; Yan, J.; Xiao, Y.; Gu, W.; Zang, C. Study on the photocatalytic and antibacterial properties of TiO2 nanoparticles-coated cotton fabrics. Materials 2019, 12, 2010. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Lauruengtana, V.; Surassmo, S.; Ruktanonchai, U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomed. NBM 2009, 5, 240–249. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Zhao, H.; Gao, Y.; Wang, J.; Jiang, H.; Boughton, R.I. Chemical assembly of TiO2 and TiO2@Ag nanoparticles on silk fiber to produce multifunctional fabrics. J. Colloid Interface Sci. 2011, 358, 307–315. [Google Scholar] [CrossRef]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interf. Sci. 2011, 363, 1–24. [Google Scholar]
- Galstyan, V.; Bhandari, M.P.; Sberveglieri, V.; Sberveglieri, G.; Comini, E. Metal Oxide Nanostructures in Food Applications: Quality Control and Packaging. Chemosensors 2018, 6, 16. [Google Scholar] [CrossRef]
- Rehim, M.H.A.; Alhamidi, J. TiO2/Polymer Nanocomposites for Antibacterial Packaging Applications. J. Adv. Food Technol. 2018, 1, 101. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. Shelf 2022, 31, 100806. [Google Scholar] [CrossRef]
- Mao, X.; Hao, C. Recent advances in the use of composite titanium dioxide nanomaterials in the food industry. J. Food Sci. 2024, 89, 1310–1323. [Google Scholar] [CrossRef]
- Li, J.; Zhang, D.; Hou, C. Application of Nano-Titanium Dioxide in Food Antibacterial Packaging Materials. Bioengineering 2025, 12, 19. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Huang, N.; Sun, Y.; Huai, X.; Miao, J.; Wang, S.; Liu, W.; Liu, Y.; Chen, Z.; et al. Types of nanomaterials commonly used in food packaging, film formation techniques, and recent advances in their applications. Int. J. Food Sci. Technol. 2025, 60, 036. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Lee, B.-K. PVA/CNC/TiO2 nanocomposite for food-packaging: Improved mechanical, UV/water vapor barrier, and antimicrobial properties. Carbohyd. Polym. 2022, 298, 120064. [Google Scholar] [CrossRef]
- Cerrada, M.L.; Serrano, C.; Sánchez-Chaves, M.; Fernández-García, M.; Fernández-Martín, F.; de Andrés, A.; Riobóo, R.J.J.; Kubacka, A.; Ferrer, M.; Fernández-García, M. Self-Sterilized EVOH-TiO2 Nanocomposites: Interface Effects on Biocidal Properties. Adv. Funct. Mater. 2008, 18, 1949–1960. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Del Nobile, M.A. Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT-Food Sci. Technol. 2013, 50, 702–706. [Google Scholar] [CrossRef]
- Othman, S.H.; Salam, N.R.A.; Zainal, N.; Basha, R.K.; Talib, R.A. Antimicrobial Activity of TiO2 Nanoparticle-Coated Film for Potential Food Packaging Applications. Int. J. Photoenergy 2014, 2014, 945930. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Aziz, M.E.A.; Morsi, S.M.M. Development and evaluation of antimicrobial LDPE/TiO2 nanocomposites for food packaging applications. Polym. Bull. 2023, 80, 5417–5431. [Google Scholar] [CrossRef]
- Chawengkijwanich, C.; Hayata, Y. Development of TiO2 powder-coated food packaging film and its ability to inactivate Escherichia coli in vitro and in actual tests. Int. J. Food Microbiol. 2008, 123, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Ochoa, A.; Ulloa, M.T.; Alvarez, E.; Canales, D.; Zapata, P.A. Poly(lactic acid)/TiO2 nanocomposites as alternative biocidal and antifungal materials. Mat. Sci. Eng. C-Mater. 2015, 57, 314–320. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yu, Y.; Hu, X.; Larbot, A. Influence of silver doping on the photocatalytic activity of titania films. Appl. Surf. Sci. 2002, 200, 239–247. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2 core–shell composite clusters under UV-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef]
- Vodnik, V.V.; Nedeljković, J.M. Influence of negative charge on the optical properties of a silver sol. J. Serb. Chem. Soc. 2000, 65, 195–200. [Google Scholar] [CrossRef]
- Sökmen, M.; Candan, F.; Sümer, Z. Disinfection of E. coli by the Ag–TiO2/UV system: Lipidperoxidation. J. Photochem. Photobiol. A 2001, 143, 241–244. [Google Scholar] [CrossRef]
- Kubacka, A.; Ferrer, M.; Martínez-Arias, A.; Fernández-García, M. Ag promotion of TiO2-anatase disinfection capability: Study of Escherichia coli inactivation. Appl. Catal. B 2008, 84, 87–93. [Google Scholar] [CrossRef]
- Musil, J.; Louda, M.; Cerstvy, R.; Baroch, P.; Ditta, I.B.; Steele, A.; Foster, H.A. Two-functional direct current sputtered silver-containing titanium dioxide thin films. Nanoscale Res. Lett. 2009, 4, 313–320. [Google Scholar] [CrossRef]
- Kubacka, A.; Cerrada, M.L.; Serrano, C.; Fernández-García, M.; Ferrer, M.; Fernández-García, M. Plasmonic Nanoparticle/Polymer Nanocomposites with Enhanced Photocatalytic Antimicrobial Properties. J. Phys. Chem. C 2009, 113, 9182–9190. [Google Scholar] [CrossRef]
- Akhavan, O. Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef]
- Amin, S.A.; Pazouki, M.; Hosseinnia, A. Synthesis of TiO2–Ag nanocomposite with sol–gel method and investigation of its antibacterial activity against E. coli. Powder Technol. 2009, 196, 241–245. [Google Scholar] [CrossRef]
- Necula, B.S.; Fratila-Apachitei, L.E.; Zaat, S.A.J.; Apachitei, I.; Duszczyk, J. In vitro antibacterial activity of porous TiO2–Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009, 5, 3573–3580. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G. Potent Antibacterial Activities of Ag/TiO2 Nanocomposite Powders Synthesized by a One-Pot Sol-Gel Method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef]
- Rodríguez-González, V.; Alfaro, S.O.; Torres-Martínez, L.M.; Cho, S.-H.; Lee, S.-W. Silver–TiO2 nanocomposites: Synthesis and harmful algae bloom UV-photoelimination. Appl. Catal. B-Environ. 2010, 98, 229–234. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Vodnik, V.; Potkonjak, B.; Jovančić, P.; Nedeljković, J.M.; Radetić, M. Multifunctional PES fabrics modified with colloidal Ag and TiO2 nanoparticles. Polym. Advan. Technol. 2011, 22, 2244–2249. [Google Scholar] [CrossRef]
- Yu, B.; Leung, K.M.; Guo, Q.; Lau, W.M.; Yang, J. Synthesis of Ag–TiO2 composite nano thin film for antimicrobial application. Nanotechnology 2011, 22, 115603. [Google Scholar] [CrossRef]
- Milošević, M.; Radoičić, M.; Šaponjić, Z.; Nunney, T.; Marković, D.; Nedeljković, J.; Radetić, M. In situ generation of Ag nanoparticles on polyester fabrics by photoreduction using TiO2 nanoparticles. J. Mater. Sci. 2013, 48, 5447–5455. [Google Scholar] [CrossRef]
- Li, H.; Cui, Q.; Feng, B.; Wang, J.; Lu, X.; Weng, J. Antibacterial activity of TiO2 nanotubes: Influence of crystal phase, morphology and Ag deposition. Appl. Surf. Sci. 2013, 284, 179–183. [Google Scholar] [CrossRef]
- Barudin, N.H.A.; Sreekantan, S.; Thong, O.M.; Sahgal, G. Antibacterial Activity of Ag-TiO2 Nanoparticles with Various Silver Contents. Mater. Sci. Forum 2013, 756, 238–245. [Google Scholar] [CrossRef]
- Vukoje, I.D.; Tomašević-Ilić, T.D.; Zarubica, A.R.; Dimitrijević, S.; Budimir, M.D.; Vranješ, M.R.; Šaponjić, Z.V.; Nedeljković, J.M. Silver film on nanocrystalline TiO2 support: Photocatalytic and antimicrobial ability. Mater. Res. Bull. 2014, 60, 824–829. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Wang, X.C.; Yang, J.L.; Jiang, X.C.; Yu, A.B. Synthesis of silver-titanium dioxide nanocomposites for antimicrobial applications. J. Nanopart. Res. 2014, 16, 2526. [Google Scholar] [CrossRef]
- Milošević, M.; Krkobabić, A.; Radoičić, M.; Šaponjić, Z.; Lazić, V.; Stoiljković, M.; Radetić, M. Antibacterial and UV protective properties of polyamide fabric impregnated with TiO2/Ag nanoparticles. J. Serb. Chem. Soc. 2015, 80, 705–715. [Google Scholar] [CrossRef]
- André, R.S.; Zamperini, C.A.; Mima, E.G.; Longo, V.M.; Albuquerque, A.R.; Sambrano, J.R.; Machado, A.L.; Vergani, C.E.; Hernandes, A.C.; Varela, J.A.; et al. Antimicrobial activity of TiO2:Ag nanocrystalline heterostructures: Experimental and theoretical insights. Chem. Phys. 2015, 459, 87–95. [Google Scholar] [CrossRef]
- Ali, T.; Ahmed, A.; Alam, U.; Uddin, I.; Tripathi, P.; Muneer, M. Enhanced photocatalytic and antibacterial activities of Ag-doped TiO2 nanoparticles under visible light. Mater. Chem. Phys. 2018, 212, 325–335. [Google Scholar] [CrossRef]
- Muflikhun, M.A.; Frommelt, M.C.; Farman, M.; Chua, A.Y.; Santos, G.N.C. Structures, mechanical properties and antibacterial activity of Ag/TiO2 nanocomposite materials synthesized via HVPG technique for coating application. Heliyon 2019, 5, e01475. [Google Scholar] [CrossRef]
- Rahmani, R.; Rosenberg, M.; Ivask, A.; Kollo, L. Comparison of Mechanical and Antibacterial Properties of TiO2/Ag Ceramics and Ti6Al4V-TiO2/Ag Composite Materials Using Combined SLM-SPS Techniques. Metals 2019, 9, 874. [Google Scholar] [CrossRef]
- Saraswati, M.; Permadani, R.L. Slamet The innovation of antimicrobial and self-cleaning using Ag/TiO2 nanocomposite coated on cotton fabric for footwear application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012091. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Ryl, J.; Nowaczyk, G.; Zielińska-Jurek, A. Morphology, Photocatalytic and Antimicrobial Properties of TiO2 Modified with Mono- and Bimetallic Copper, Platinum and Silver Nanoparticles. Nanomaterials 2019, 9, 1129. [Google Scholar] [CrossRef]
- Pirsa, S.; Farshchi, E.; Roufegarinejad, L. Antioxidant/Antimicrobial Film Based on Carboxymethyl Cellulose/Gelatin/TiO2–Ag Nano-Composite. J. Polym. Environ. 2020, 28, 3154–3163. [Google Scholar] [CrossRef]
- Bilek, O.; Fialova, T.; Otahal, A.; Adam, V.; Smerkova, K.; Fohlerova, Z. Antibacterial activity of AgNPs–TiO2 nanotubes: Influence of different nanoparticle stabilizers. RSC Adv. 2020, 10, 44601–44610. [Google Scholar] [CrossRef]
- Ardhi, B.P.; Alfin, M.M.; Pramono, E.; Wahyuningsih, S. Properties of TiO2/PDMS-Ag Composites as Antibacterial Self Cleaning. J. Phys. Conf. Ser. 2022, 2190, 012009. [Google Scholar] [CrossRef]
- Jia, J.; Giannakis, S.; Li, D.; Yan, B.; Lin, T. Efficient and sustainable photocatalytic inactivation of E. coli by an innovative immobilized Ag/TiO2 photocatalyst with peroxymonosulfate (PMS) under visible light. Sci. Total Environ. 2023, 901, 166376. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Vary, P.S.; Lin, C.-T. Anatase TiO2 Nanocomposites for Antimicrobial Coatings. J. Phys. Chem. B 2005, 109, 8889–8898. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X.; Liu, J.; Tian, Z.; Dai, L.; He, B.; Han, C.; Wu, Y.; Zeng, Z.; Hu, Z. On the role of localized surface plasmon resonance in UV-Vis light irradiated Au/TiO2 photocatalysis systems: Pros and cons. Nanoscale 2015, 7, 4114–4123. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, Z.; Li, P.; Li, W.; Li, C.; Wang, Y.; Chu, P.K. Degradable and Photocatalytic Antibacterial Au-TiO2/Sodium Alginate Nanocomposite Films for Active Food Packaging. Nanomaterials 2018, 8, 930. [Google Scholar] [CrossRef]
- Moon, K.-S.; Choi, E.-J.; Bae, J.-M.; Park, Y.-B.; Oh, S. Visible Light-Enhanced Antibacterial and Osteogenic Functionality of Au and Pt Nanoparticles Deposited on TiO2 Nanotubes. Materials 2020, 13, 3721. [Google Scholar]
- Paszkiewicz, O.; Wang, K.; Rakoczy, R.; Kordas, M.; Leniec, G.; Kowalska, E.; Markowska-Szczupak, A. Antimicrobial properties of pristine and Pt-modified titania P25 in rotating magnetic field conditions. CEP PI 2022, 178, 109010. [Google Scholar] [CrossRef]
- Quisenberry, L.R.; Loetscher, L.H.; Boyd, J.E. Catalytic inactivation of bacteria using Pd-modified titania. Catal. Commun. 2009, 10, 1417–1422. [Google Scholar] [CrossRef]
- Rajh, T.; Nedeljković, J.M.; Chen, L.X.; Tiede, D.M.; Thurnauer, M.C. Photoreduction of copper on TiO2 nanoparticles modified with polydentante ligands. J. Adv. Oxid. Technol. 1998, 3, 292–298. [Google Scholar]
- Ruvarac-Bugarčić, I.A.; Šaponjić, Z.V.; Zec, S.; Rajh, T.; Nedeljković, J.M. Photocatalytic reduction of cadmium on TiO2 nanoparticles modified with amino acids. Chem. Phys. Lett. 2005, 407, 110–113. [Google Scholar] [CrossRef]
- Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 2014, 8, 95–103. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’Shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New Insights into the Mechanism of Visible Light Photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold nanorods: Synthesis, characterization and applications. Coordin. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Kumar, S.G.; Devi, L.G. Review on Modified TiO2 Photocatalysis under UV/Visible Light: Selected Results and Related Mechanisms on Interfacial Charge Carrier Transfer Dynamics. J. Phys. Chem. A 2011, 115, 13211–13241. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Chen, X.; Burda, C. The Electronic Origin of the Visible-Light Absorption Properties of C-, N- and S-Doped TiO2 Nanomaterials. J. Am. Chem. Soc. 2008, 130, 5018–5019. [Google Scholar] [CrossRef]
- Yu, J.C.; Ho, W.; Yu, J.; Yip, H.; Wong, P.K.; Zhao, J. Efficient Visible-Light-Induced Photocatalytic Disinfection on Sulfur-Doped Nanocrystalline Titania. Environ. Sci. Technol. 2005, 39, 1175–1179. [Google Scholar] [CrossRef]
- Wong, M.-S.; Chu, W.-C.; Sun, D.-S.; Huang, H.-S.; Chen, J.-H.; Tsai, P.-J.; Lin, N.-T.; Yu, M.-S.; Hsu, S.-F.; Wang, S.-L.; et al. Visible-Light-Induced Bactericidal Activity of a Nitrogen-Doped Titanium Photocatalyst against Human Pathogens. Appl. Environ. Microbiol. 2006, 72, 6111–6116. [Google Scholar] [CrossRef]
- Li, Q.; Xie, R.; Li, Y.W.; Mintz, E.A.; Shang, J.K. Enhanced Visible-Light-Induced Photocatalytic Disinfection of E. coli by Carbon-Sensitized Nitrogen-Doped Titanium Oxide. Environ. Sci. Technol. 2007, 41, 5050–5056. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Mielczarski, E.; Mielczarski, J.; Castillo, N.C.; Kiwi, J.; Pulgarin, C. Escherichia coli inactivation by N, S co-doped commercial TiO2 powders under UV and visible light. Appl. Catal. B 2008, 84, 448–456. [Google Scholar] [CrossRef]
- Emeline, A.V.; Kuznetsov, V.N.; Rybchuk, V.K.; Serpone, N. Visible-Light-Active Titania Photocatalysts: The Case of N-Doped TiO2s—Properties and Some Fundamental Issues. Int. J. Photoenergy 2008, 2008, 258394. [Google Scholar] [CrossRef]
- Etacheri, V.; Michlits, G.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. A Highly Efficient TiO2−xCx Nano-heterojunction Photocatalyst for Visible Light Induced Antibacterial Applications. ACS Appl. Mater. Interfaces 2013, 5, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Darzi, S.J.; Mahjoub, A.R.; Bayat, A. Synthesis and characterization of visible light active S-doped TiO2 nanophotocatalyst. Int. J. Nano Dimens. 2016, 7, 33–40. [Google Scholar]
- Yadav, H.M.; Otari, S.V.; Bohara, R.A.; Mali, S.S.; Pawar, S.H.; Delekar, S.D. Synthesis and visible light photocatalytic antibacterial activity of nickel-doped TiO2 nanoparticles against Gram-positive and Gram-negative bacteria. J. Photochem. Photobiol. A 2014, 294, 130–136. [Google Scholar] [CrossRef]
- AL-Jawad, S.M.H.; Taha, A.A.; Salim, M.M. Synthesis and characterization of pure and Fe doped TiO2 thin films for antimicrobial activity. Optik 2017, 142, 42–53. [Google Scholar] [CrossRef]
- Ilkhechi, N.N.; Akbarpour, M.R.; Yavari, R.; Azar, Z. Sn4+ and La3+ co doped TiO2 nanoparticles and their optical, photocatalytic and antibacterial properties under visible light. J. Mater. Sci. Mater. Electron. 2017, 28, 16658–16664. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, M.; Yang, H.; Shi, D.; Wang, Y. Preparation, characterization and the antimicrobial properties of metal ion doped TiO2 nano-powders. Ceram. Int. 2018, 44, 5145–5154. [Google Scholar] [CrossRef]
- Nithya, N.; Bhoopathi, G.; Magesh, G.; Kumar, C.D.N. Neodymium doped TiO2 nanoparticles by sol-gel method for antibacterial and photocatalytic activity. Mat. Sci. Semicon. Proc. 2018, 83, 70–82. [Google Scholar] [CrossRef]
- Meng, D.; Liu, X.; Xie, Y.; Du, Y.; Yang, Y.; Xiao, C. Antibacterial Activity of Visible Light-Activated TiO2 Thin Films with Low Level of Fe Doping. Adv. Mater. Sci. Eng. 2019, 2019, 5819805. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Xu, F. Generating oxygen vacancies in Cu2+-doped TiO2 hollow spheres for enhanced photocatalytic activity and antimicrobial activity. Micro Nano Lett. 2020, 15, 535–539. [Google Scholar] [CrossRef]
- Ali, M.; Hussain, R.; Tariq, F.; Noreen, Z.; Toufiq, A.M.; Bokhari, H.; Akhtar, N.; Rahman, S.U. Highly effective visible light-activated cobalt-doped TiO2 nanoparticles for antibacterial coatings against Campylobacter jejuni. Appl. Nanosci. 2020, 10, 1005–1012. [Google Scholar] [CrossRef]
- Gomez-Polo, C.; Larumbe, S.; Gil, A.; Muñoz, D.; Fernández, L.R.; Barquín, L.F.; García-Prieto, A.; Fdez-Gubieda, M.L.; Muela, A. Improved photocatalytic and antibacterial performance of Cr doped TiO2 nanoparticles. Surf. Interfaces 2021, 22, 100867. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; El-Sayyad, G.S.; Hegazy, A.H.; Hamza, M.A.; Fathy, R.M.; El Shenawy, E.T.; Allam, N.K. Superior visible light antimicrobial performance of facet engineered cobalt doped TiO2 mesocrystals in pathogenic bacterium and fungi. Sci. Rep. 2021, 11, 5609. [Google Scholar] [CrossRef]
- Yuan, Y.; Ding, J.; Xu, J.; Deng, J.; Guo, J. TiO2 Nanoparticles Co-Doped with Silver and Nitrogen for Antibacterial Application. J. Nanosci. Nanotechnol. 2010, 10, 4868–4874. [Google Scholar] [CrossRef]
- Hamal, D.B.; Haggstrom, J.A.; Marchin, G.L.; Ikenberry, M.A.; Hohn, K.; Klabunde, K.J. A Multifunctional Biocide/Sporocide and Photocatalyst Based on Titanium Dioxide (TiO2) Codoped with Silver, Carbon, and Sulfur. Langmuir 2010, 26, 2805–2810. [Google Scholar] [CrossRef]
- Fisher, M.B.; Keane, D.A.; Fernández-Ibáñez, P.; Colreavy, J.; Hinder, S.J.; McGuigan, K.G.; Pillai, S.C. Nitrogen and copper doped solar light active TiO2 photocatalysts for water decontamination. Appl. Catal. B 2013, 130–131, 8–13. [Google Scholar] [CrossRef]
- AAshkarran, A.; Hamidinezhad, H.; Haddadi, H.; Mahmoudi, M. Double-doped TiO2 nanoparticles as an efficient visible-light-active photocatalyst and antibacterial agent under solar simulated light. Appl. Surf. Sci. 2014, 301, 338–345. [Google Scholar] [CrossRef]
- Janpetch, N.; Vanichvattanadecha, C.; Rujiravanit, R. Photocatalytic disinfection of water by bacterial cellulose/N–F co-doped TiO2 under fluorescent light. Cellulose 2015, 22, 3321–3335. [Google Scholar] [CrossRef]
- Ananpattarachai, J.; Boonto, Y.; Kajitvichyanukul, P. Visible light photocatalytic antibacterial activity of Ni-doped and N-doped TiO2 on Staphylococcus aureus and Escherichia coli bacteria. Environ. Sci. Pollut. Res. 2016, 23, 4111–4119. [Google Scholar] [CrossRef]
- Lakshmi, K.V.D.; Rao, T.S.; Padmaja, J.S.; Raju, I.M.; Alim, S.K.A.; Kalyani, P. Visible light driven mesoporous Mn and S co-doped TiO2 nano material: Characterization and applications in photocatalytic degradation of indigocarmine dye and antibacterial activity. Environ. Nanotechnol. Monit. Manag. 2018, 10, 494–504, Erratum in Environ. Nanotechnol. Monit. Manag. 2020, 14, 100394. [Google Scholar] [CrossRef]
- Tahmasebizad, N.; Hamedani, M.T.; Ghazani, M.S.; Pazhuhanfar, Y. Photocatalytic activity and antibacterial behavior of TiO2 coatings co-doped with copper and nitrogen via sol–gel method. J. Sol-Gel Sci. Technol. 2020, 93, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.; Monteiro, O.C.; Rego, A.M.B.D.; Ferraria, A.M.; Batista, M.; Santos, R.; Monteiro, S.; Freire, M.; Silva, E.R. Visible light-driven photodegradation of triclosan and antimicrobial activity against Legionella pneumophila with cobalt and nitrogen co-doped TiO2 anatase nanoparticles. J. Environ. Chem. Eng. 2021, 9, 106735. [Google Scholar] [CrossRef]
- Kunnamareddy, M.; Ganesan, S.; Hatamleh, A.A.; Alnafisi, B.K.; Rajendran, R.; Chinnasamy, R.; Arumugam, P.; Diravidamani, B.; Lo, H.-M. Enhancement in the visible light induced photocatalytic and antibacterial properties of titanium dioxide codoped with cobalt and sulfur. Environ. Res. 2023, 216, 114705. [Google Scholar] [CrossRef]
- Sukhadeve, G.K.; Bandewar, H.; Janbandhu, S.Y.; Jayaramaiah, J.R.; Gedam, R.S. Photocatalytic hydrogen production, dye degradation, and antimicrobial activity by Ag-Fe co-doped TiO2 nanoparticles. J. Mol. Liq. 2023, 369, 120948. [Google Scholar] [CrossRef]
- Cates, S.L.; Cates, E.L.; Cho, M.; Kim, J.-H. Synthesis and Characterization of Visible-to-UVC Upconversion Antimicrobial Ceramics. Environ. Sci. Technol. 2014, 48, 2290–2297. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er Co-doped TiO2 for rapid bacteria-killing using visible light. Bioact. Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef]
- Xu, J.; Liu, N.; Wu, D.; Gao, Z.; Song, Y.-Y.; Schmuki, P. Upconversion Nanoparticle-Assisted Payload Delivery from TiO2 under Near-Infrared Light Irradiation for Bacterial Inactivation. ACS Nano 2020, 14, 337–346. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, W.; Choi, W.; Yu, J.; Deng, Y.; Xie, X.; Lin, Z. Ternary Biocidal-Photocatalytic-Upconverting Nanocomposites for Enhanced Antibacterial Activity. ACS Sustain. Chem. Eng. 2022, 10, 4741–4749. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lan, Y.; Qu, J.; Hu, X.; Wang, A. Ag/AgBr/TiO2 Visible Light Photocatalyst for Destruction of Azodyes and Bacteria. J. Phys. Chem. B 2006, 110, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Elahifard, M.R.; Rahimnejad, S.; Haghighi, S.; Gholami, M.R. Apatite-Coated Ag/AgBr/TiO2 Visible-Light Photocatalyst for Destruction of Bacteria. J. Am. Chem. Soc. 2007, 129, 9552–9553. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Hu, C.; Hu, X.; Qu, J. Efficient destruction of pathogenic bacteria with AgBr/TiO2 under visible light irradiation. Appl. Catal. B 2007, 73, 354–360. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar] [CrossRef]
- Petronella, F.; Rtimi, S.; Comparelli, R.; Sanjines, R.; Pulgarin, C.; Curri, M.L.; Kiwi, J. Uniform TiO2/In2O3 surface films effective in bacterial inactivation under visible light. J. Photochem. Photobio. A 2014, 279, 1–7. [Google Scholar] [CrossRef]
- Gunasekaran, A.; Rajamani, A.K.; Masilamani, C.; Chinnappan, I.; Ramamoorthy, U.; Kaviyarasu, K. Synthesis and Characterization of ZnO Doped TiO2 Nanocomposites for Their Potential Photocatalytic and Antimicrobial Applications. Catalysts 2023, 13, 215. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Magesan, P.; Ganesan, P.; Umapathy, M.J. Ultrasonic-assisted synthesis of doped TiO2 nanocomposites: Characterization and evaluation of photocatalytic and antimicrobial activity. Optik 2016, 127, 5171–5180. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Lee, H.; Jang, H.S.; Kim, N.Y.; Joo, J.B. Cu-doped TiO2 hollow nanostructures for the enhanced photocatalysis under visible light conditions. J. Ind. Eng. Chem. 2021, 99, 352–363. [Google Scholar] [CrossRef]
- Mertah, O.; El Hajjaji, K.; El Amrani, S.; Tanji, K.; Goncharova, I.; Kherbeche, A. Visible-light Cu/TiO2@Ag3PO4 heterostructure photocatalyst for selective nitrate reduction and antimicrobial activity. Opt. Mater. 2022, 129, 112549. [Google Scholar] [CrossRef]
- de Lima, M.S.; Schio, A.L.; Aguzzoli, C.; de Souza, W.V.; Roesch-Ely, M.; Leidens, L.M.; Boeira, C.D.; Alvarez, F.; Elois, M.A.; Fongaro, G.; et al. Visible Light-Driven Photocatalysis and Antibacterial Performance of a Cu-TiO2 Nanocomposite. ACS Omega 2024, 9, 47122–47134. [Google Scholar] [CrossRef] [PubMed]
- Paschoalino, M.; Guedes, N.C.; Jardim, W.; Mielczarski, E.; Mielczarski, J.A.; Bowen, P.; Kiwi, J. Inactivation of E. coli mediated by high surface area CuO accelerated by light irradiation >360 nm. J. Photochem. Photobio. A 2008, 199, 105–111. [Google Scholar] [CrossRef]
- Borgarello, E.; Kiwi, J.; Pelizzetti, E.; Visca, M.; Grätzel, M. Sustained Water Cleavage by Visible Light. J. Am. Chem. Soc. 1981, 103, 6324–6329. [Google Scholar] [CrossRef]
- Yao, K.S.; Wang, D.Y.; Chang, C.Y.; Weng, K.W.; Yang, L.Y.; Lee, S.J.; Cheng, T.C.; Hwang, C.C. Photocatalytic disinfection of phytopathogenic bacteria by dye-sensitized TiO2 thin film activated by visible light. Surf. Coat. Tech. 2007, 202, 1329–1332. [Google Scholar] [CrossRef]
- Perillo, P.M.; Getz, F.C. Dye Sensitized TiO2 Nanopore Thin Films with Antimicrobial Activity Against Methicillin Resistant Staphylococcus Aureus Under Visible Light. World J. Appl. Chem. 2016, 1, 9–15. [Google Scholar]
- Giridhar, M.; BhojyuNaik, H.S.; Vishwanath, R.; Sudhamani, C.N.; Prabakar, M.C.; Kenchappa, R. Preparation of azo-dye sensitized TiO2 photocatalyst for inhibition of E-Coli bacteria under visible light irradiation. Mater. Today Proc. 2017, 4, 11671–11678. [Google Scholar] [CrossRef]
- Sułek, A.; Pucelik, B.; Kobielusz, M.; Łabuz, P.; Dubin, G.; Dąbrowski, J.M. Surface Modification of Nanocrystalline TiO2 Materials with Sulfonated Porphyrins for Visible Light Antimicrobial Therapy. Catalysts 2019, 9, 821. [Google Scholar] [CrossRef]
- Vallejo, W.; Navarro, K.; Díaz-Uribe, C.; Schott, E.; Zarate, X.; Romero, E. Zn(II)-tetracarboxy-phthalocyanine-Sensitized TiO2 Thin Films as Antimicrobial Agents under Visible Irradiation: A Combined DFT and Experimental Study. ACS Omega 2021, 6, 13637–13646. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, J.; Lovely, B.; Li, Y.; Ponder, M.; Waterman, K.; Kim, Y.-T.; Shuai, D.; Yin, Y.; Huang, H. Visible light-activated dye-sensitized TiO2 antibacterial film: A novel strategy for enhancing food safety and quality. J. Hazard. Mater. 2024, 480, 136296. [Google Scholar] [CrossRef]
- Higashimoto, S.; Nishi, T.; Yasukawa, M.; Azuma, M.; Sakata, Y.; Kobayashi, H. Photocatalysis of titanium dioxide modified by catechol-type interfacial surface complexes (ISC) with different substituted groups. J. Catal. 2015, 329, 286–290. [Google Scholar] [CrossRef]
- Fujisawa, J.; Hanaya, M. Light harvesting and direct electron injection by interfacial charge-transfer transitions between TiO2 and carboxy-anchor dye LEG4 in dye-sensitized solar cells. J. Phys. Chem. C 2018, 122, 8–15. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Liz-Márzan, L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013, 25, 1465–1476. [Google Scholar] [CrossRef]
- Lazić, V.; Mihajlovski, K.; Mraković, A.; Illés, E.; Stoiljković, M.; Ahrenkiel, S.P.; Nedeljković, J.M. Antimicrobial activity of silver nanoparticles supported by magnetite. ChemistrySelect 2019, 4, 4018–4024. [Google Scholar] [CrossRef]
- Lazić, V.; Nedeljković, J.M. Photocatalytic Reactions over TiO2-Based Interfacial Charge Transfer Complexes. Catalysts 2024, 14, 810. [Google Scholar] [CrossRef]
- Dekanski, D.; Spremo-Potparević, B.; Bajić, V.; Živković, L.; Topalović, D.; Sredojević, D.N.; Lazić, V.; Nedeljković, J.M. Acute toxicity study in mice of orally administrated TiO2 nanoparticles functionalized with caffeic acid. Food Chem. Toxicol. 2018, 115, 42–48. [Google Scholar] [CrossRef]
- Todorović, A.; Bobić, K.; Veljković, F.; Pejić, S.; Glumac, S.; Stanković, S.; Milovanović, T.; Vukoje, I.; Nedeljković, J.M.; Škodrić, S.R.; et al. Comparable Toxicity of Surface-Modified TiO2 Nanoparticles: An In Vivo Experimental Study on Reproductive Toxicity in Rats. Antioxidants 2024, 13, 231. [Google Scholar] [CrossRef]
- Shahriari-Khalaji, M.; Zabihi, F.; Bahi, A.; Sredojević, D.; Nedeljković, J.M.; Macharia, D.K.; Ciprian, M.; Yang, S.; Ko, F. Photon-driven bactericidal performance of surface-modified TiO2 nanofibers. J. Mater. Chem. C 2023, 11, 5796–5805. [Google Scholar] [CrossRef]
- Nikšić, V.; Pirković, A.; Spremo-Potparević, B.; Živković, L.; Topalović, D.; Nedeljković, J.M.; Lazić, V. Bioactivity Assessment of Functionalized TiO2 Powder with Dihydroquercetin. Int. J. Mol. Sci. 2025, 26, 1475. [Google Scholar] [CrossRef]
- Queirós, J.M.; Zheng, F.; Brito-Pereira, R.; Fernandes, M.M.; Carvalho, E.O.; Martins, P.M.; Lazić, V.; Nedeljković, J.M.; Lanceros-Mendez, S. Photocatalytic and antimicrobial polymer-based hybrid membranes with surface-modified TiO2 nanoparticles with 5-aminosalicylic acid and silver nanoparticles. RSC Sustain. 2025, 3, 4568–4582. [Google Scholar] [CrossRef]
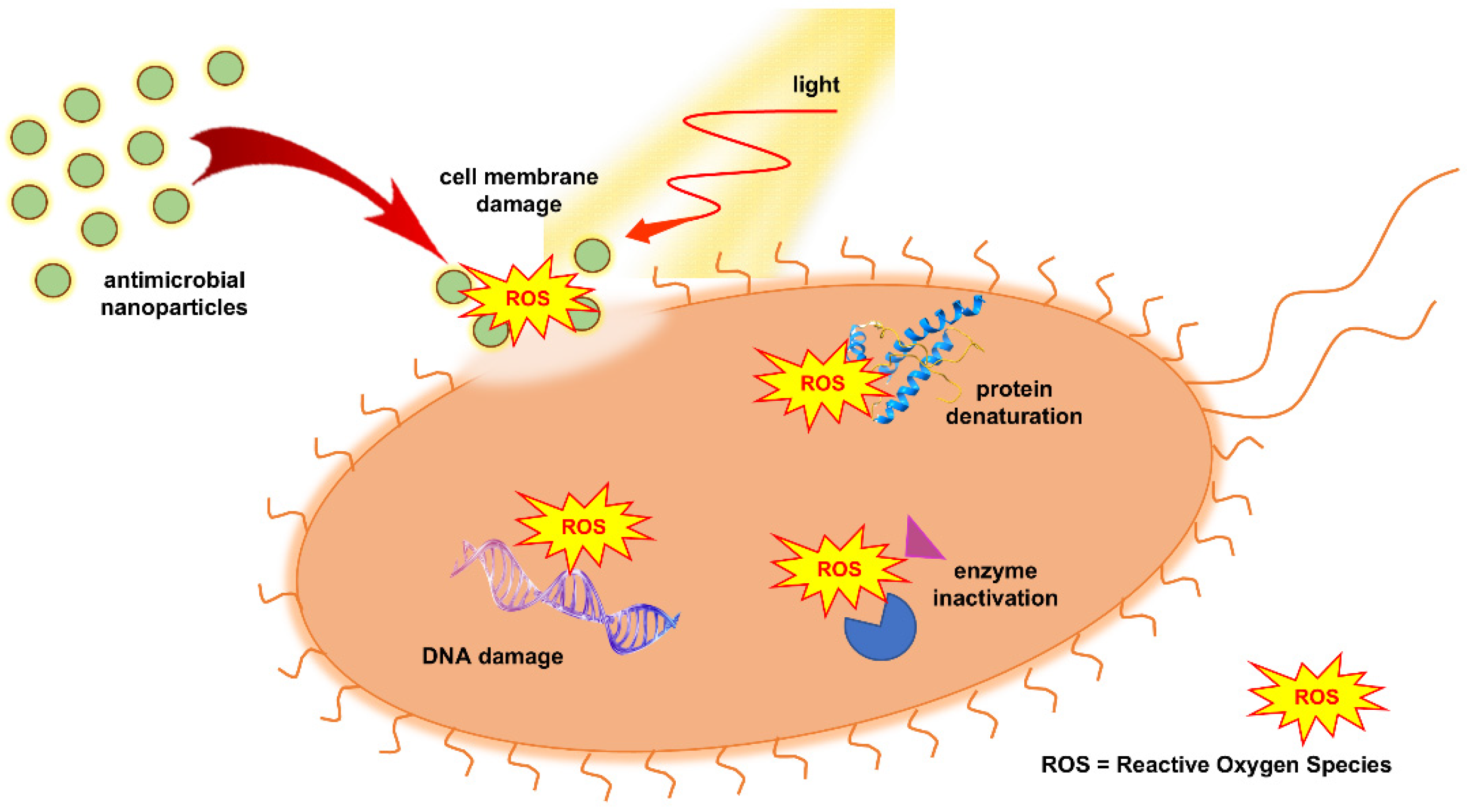
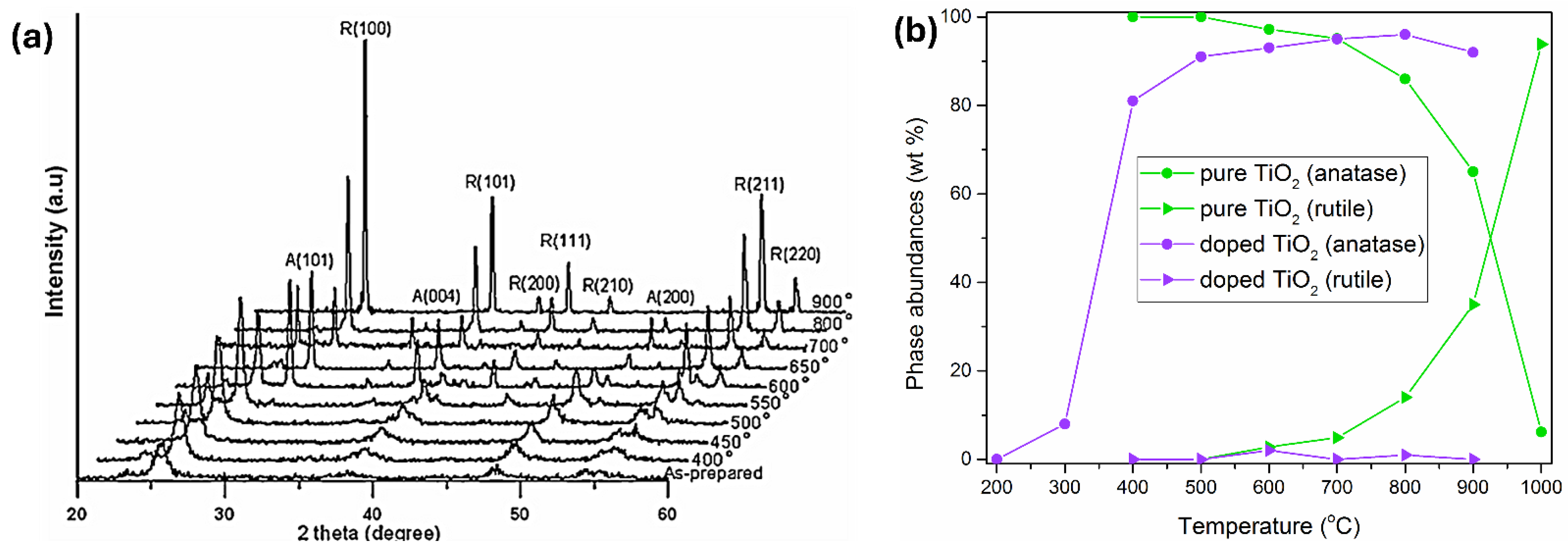
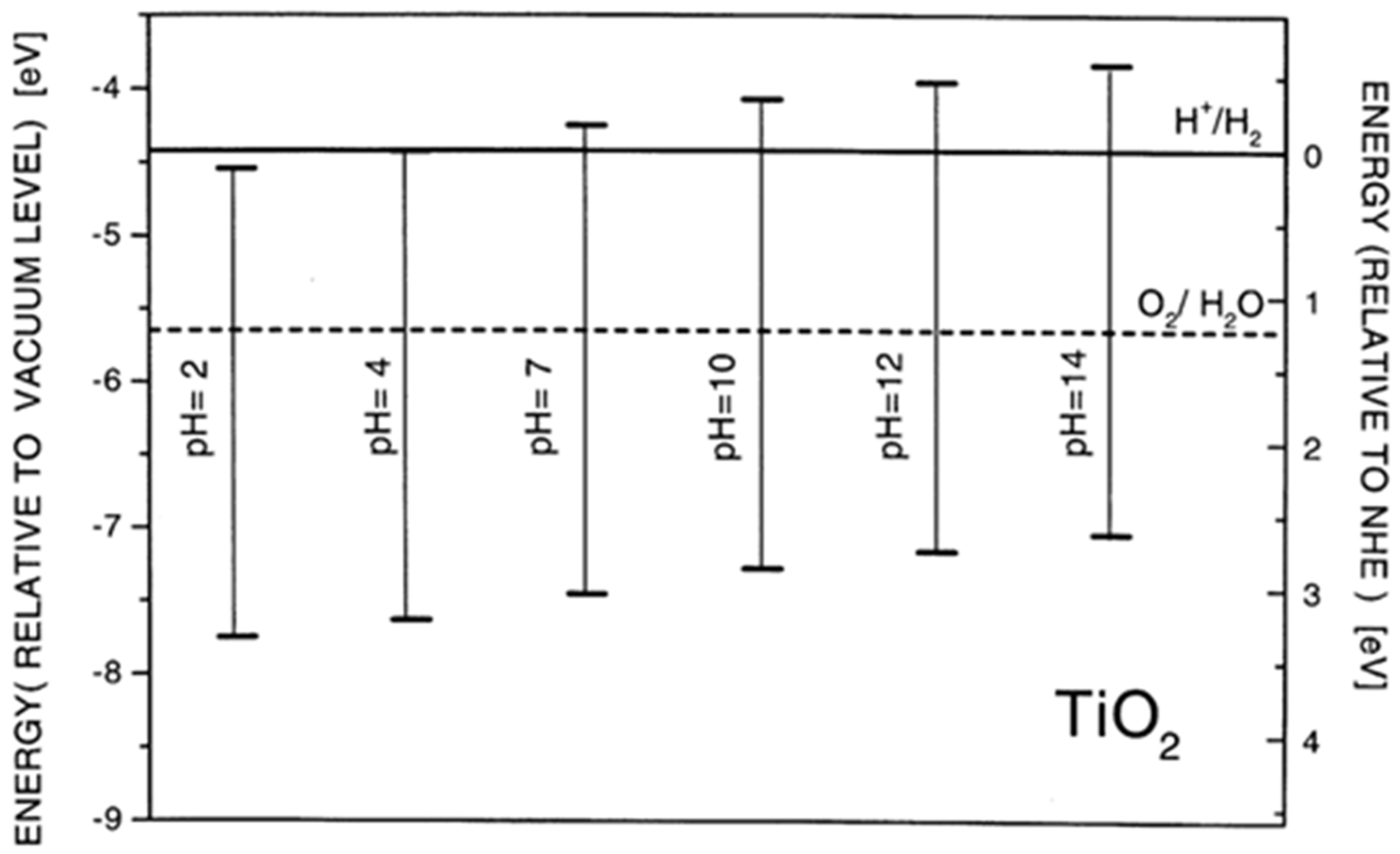


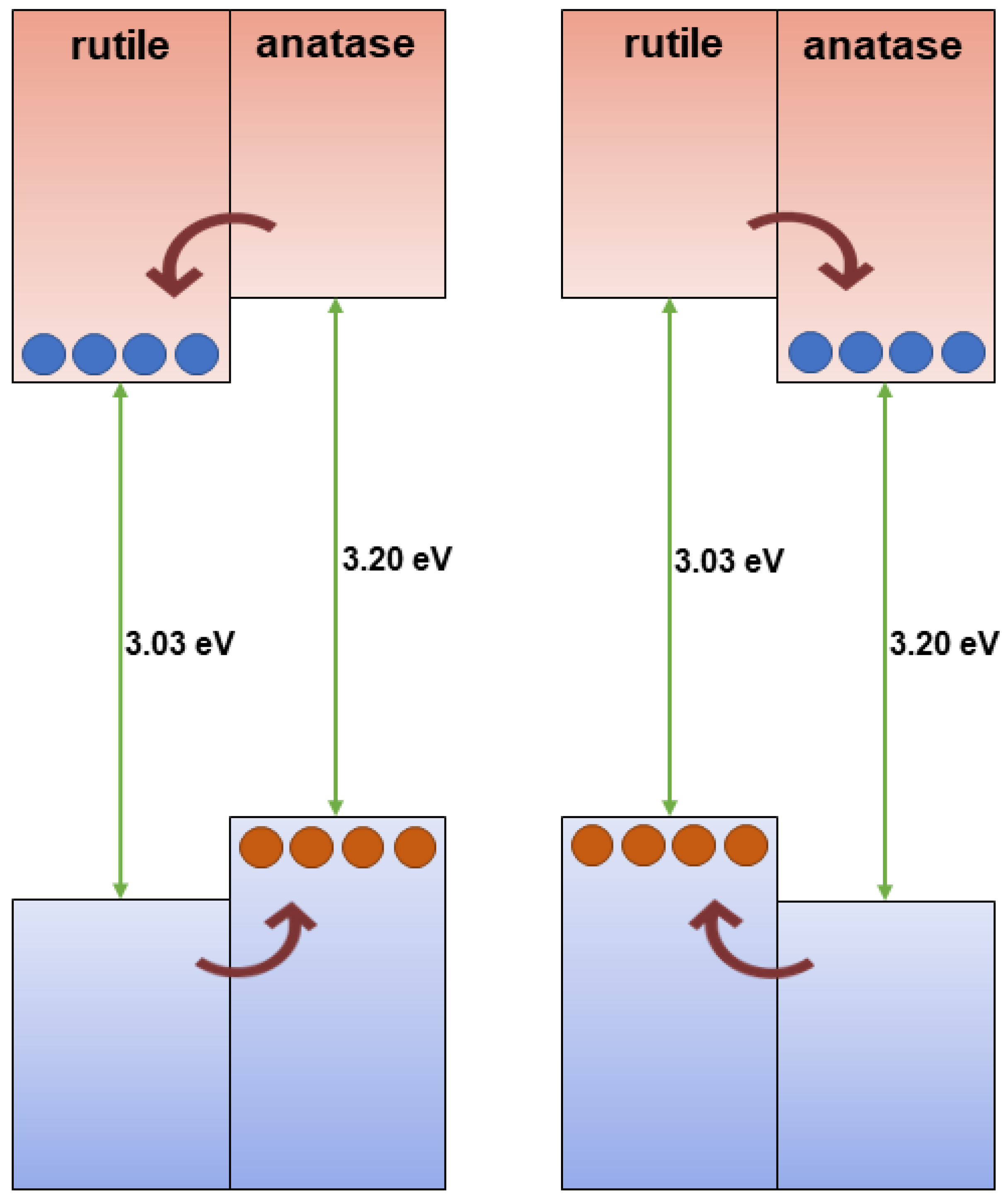
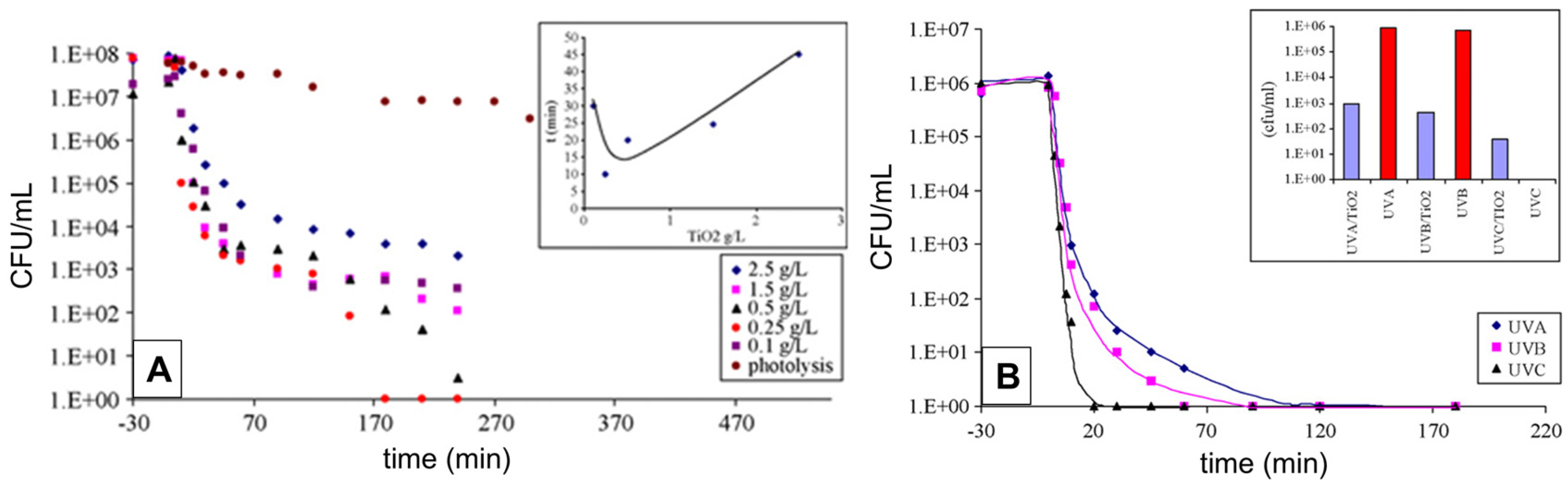
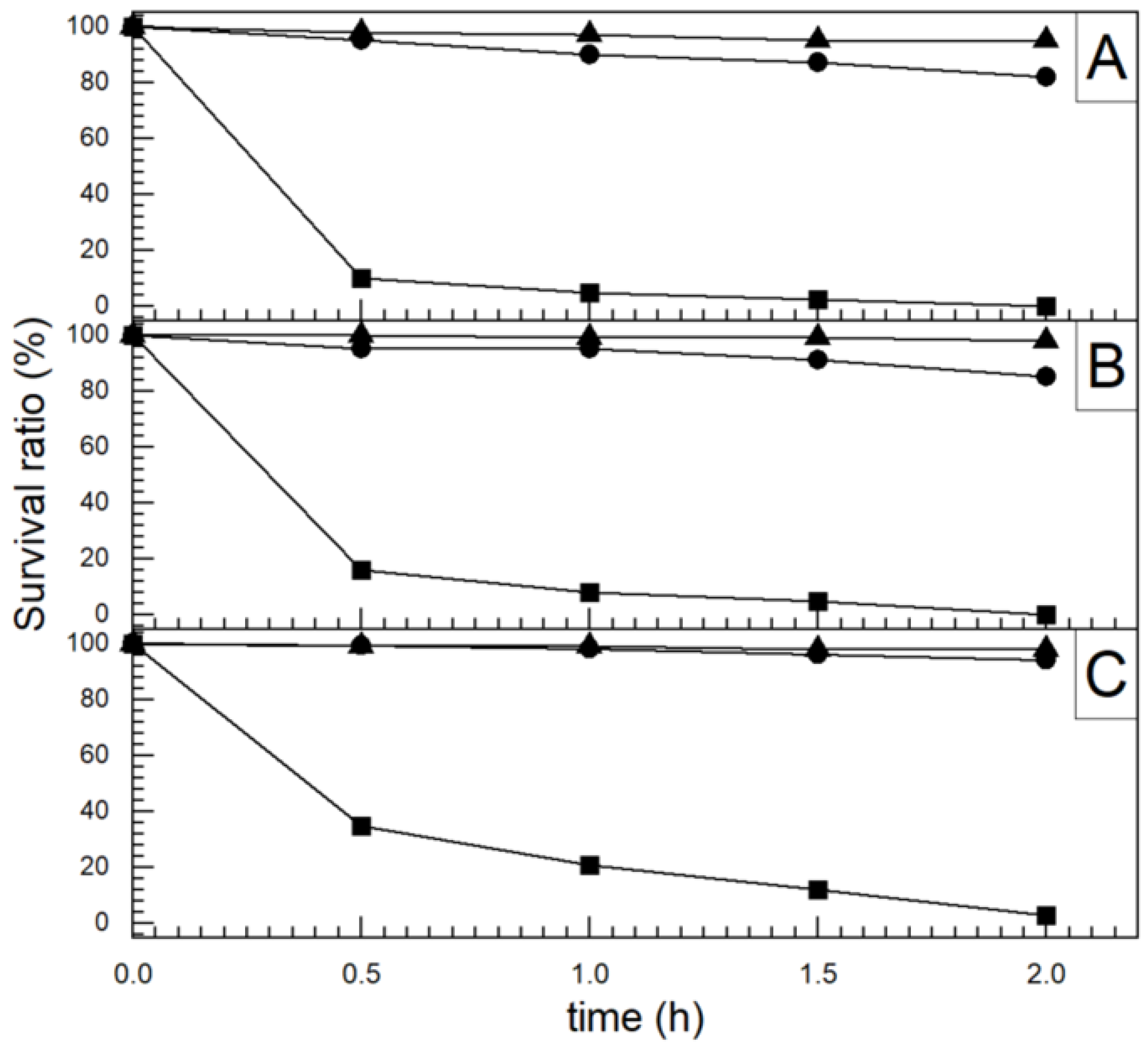


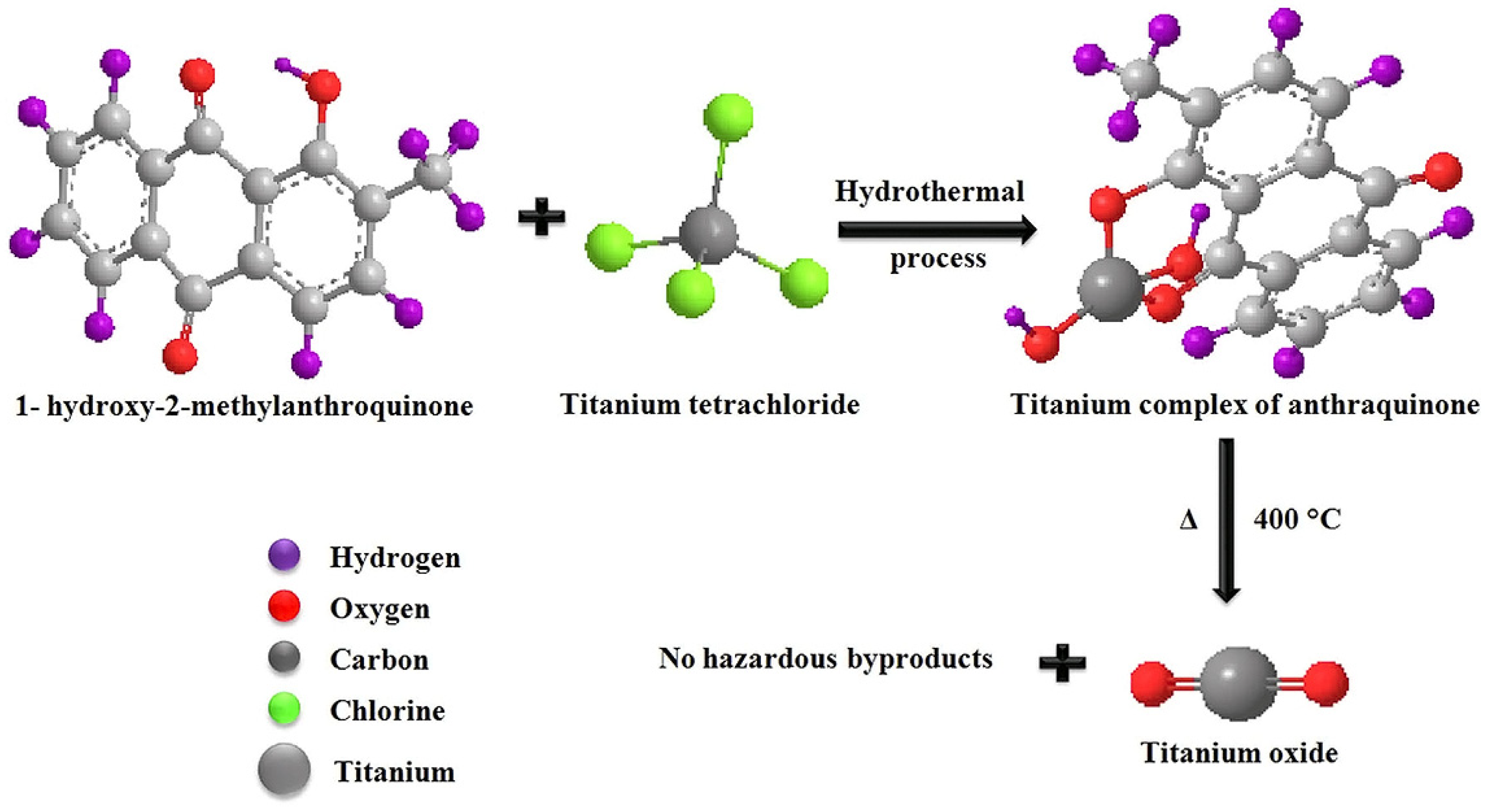
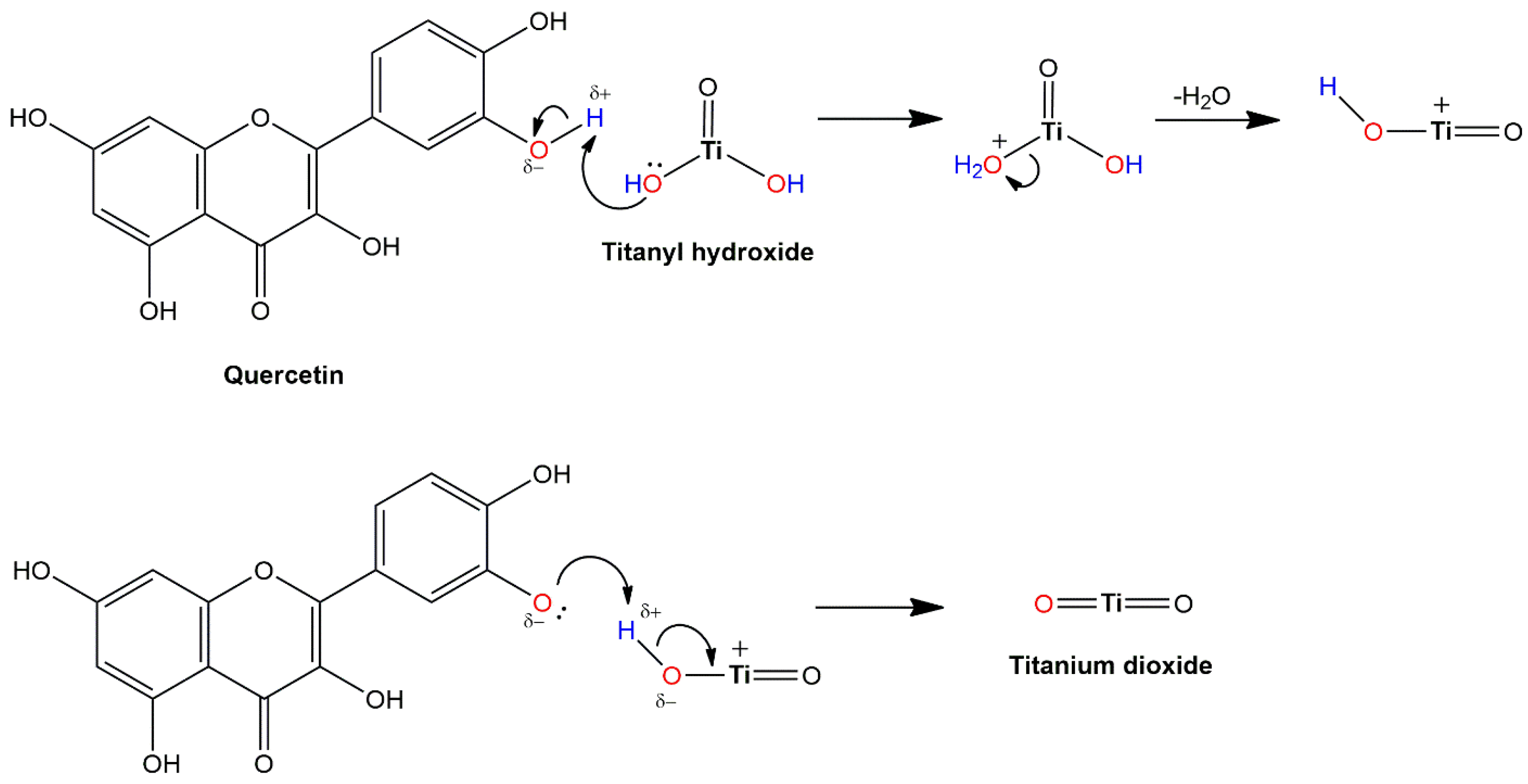

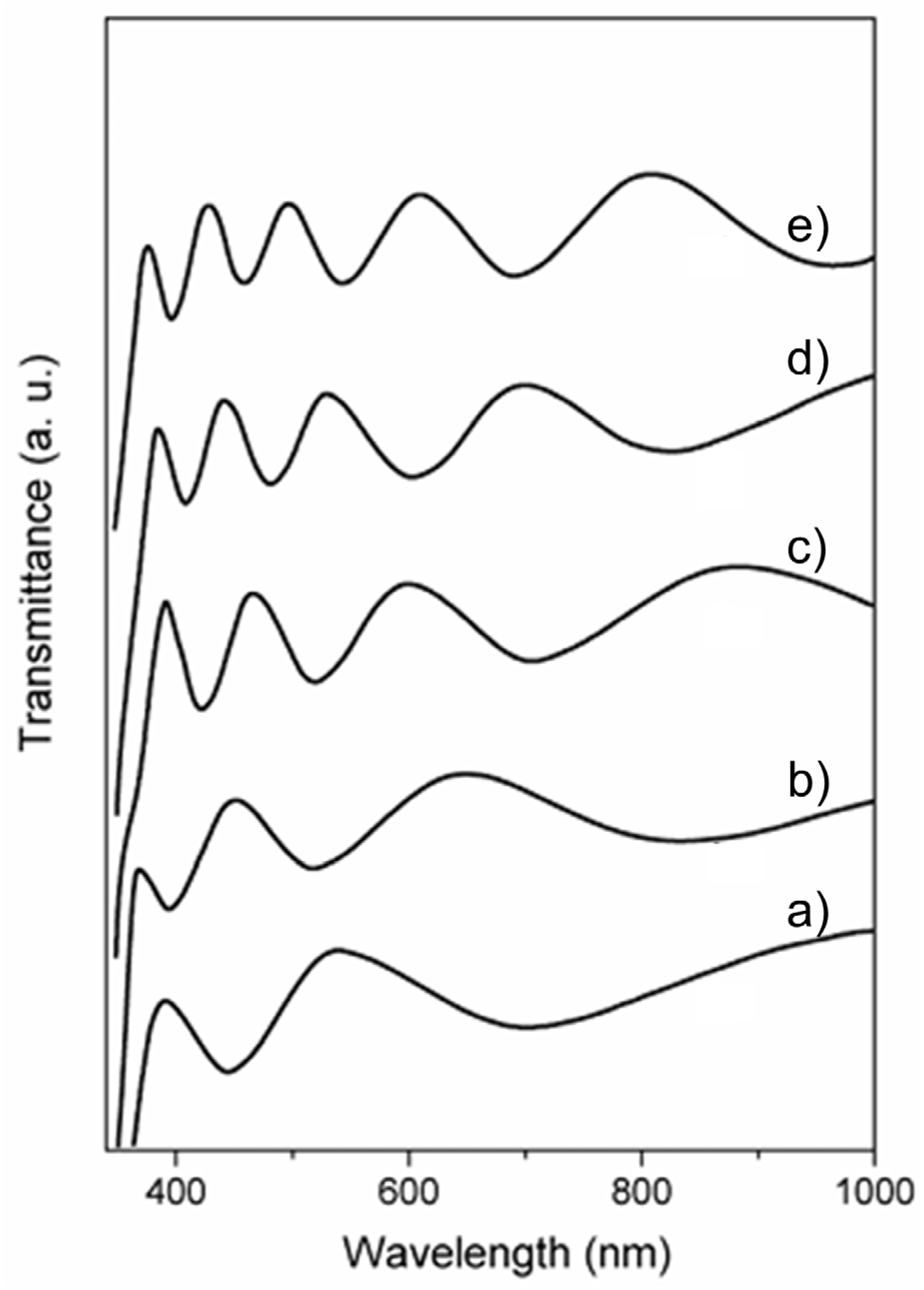
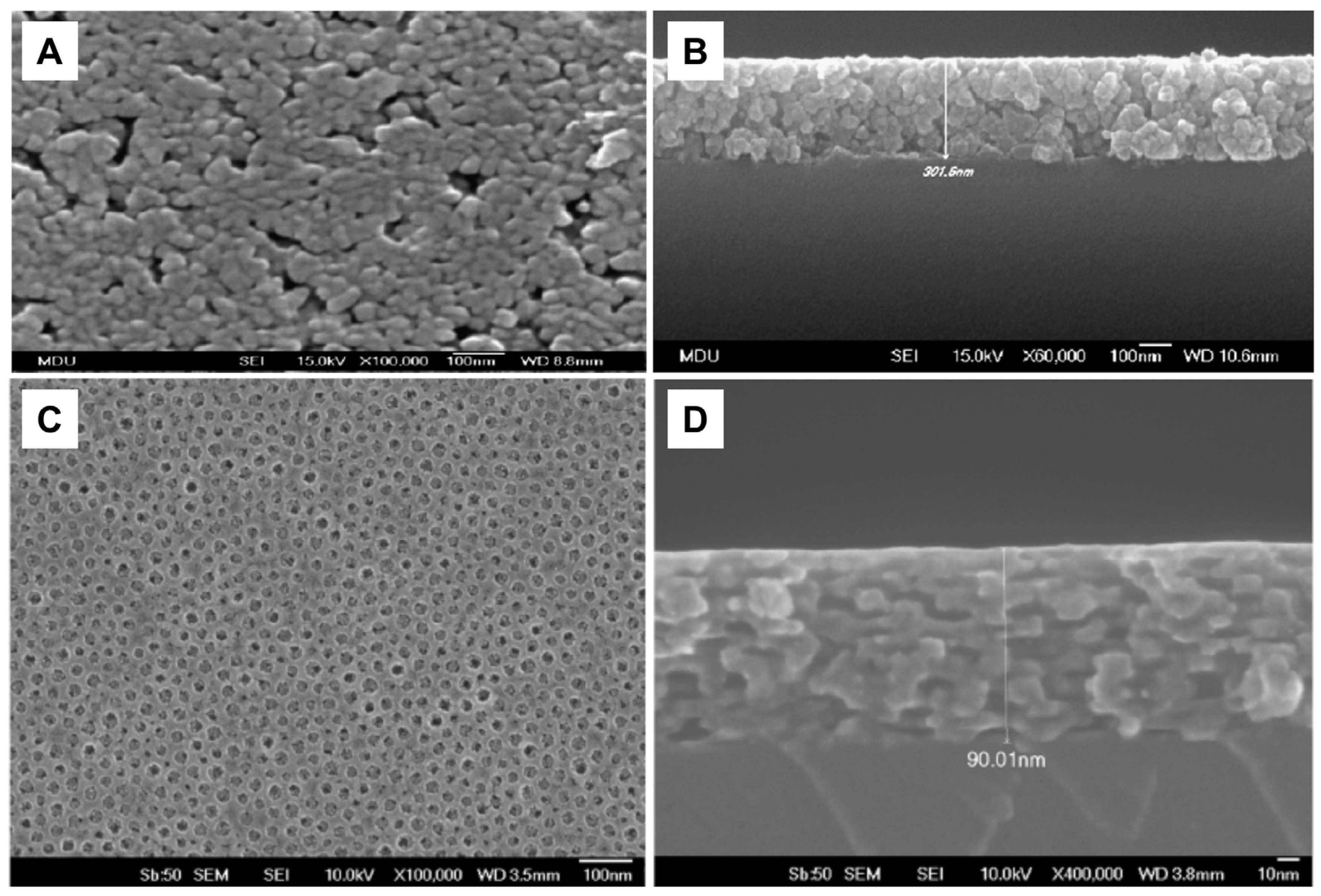

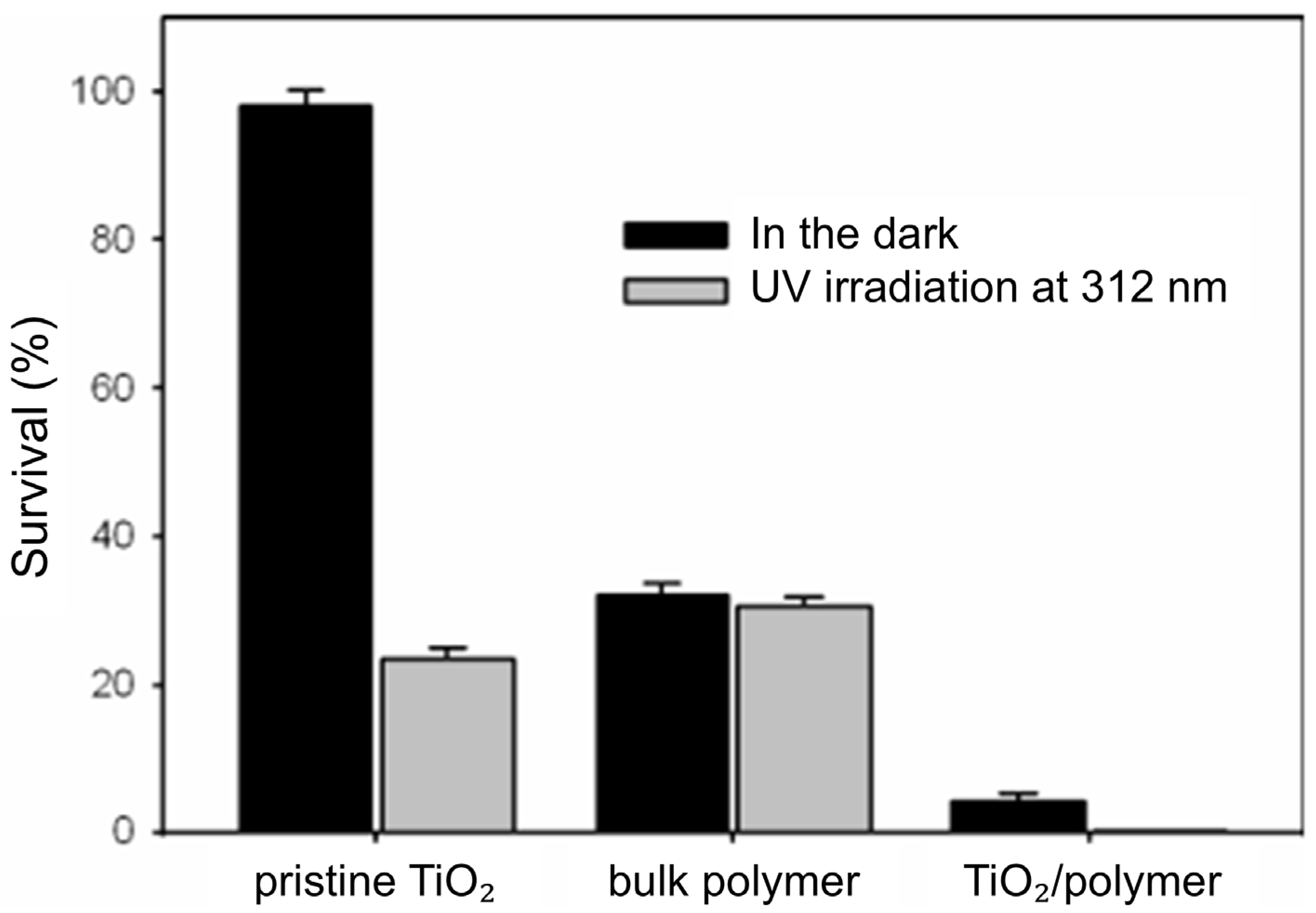
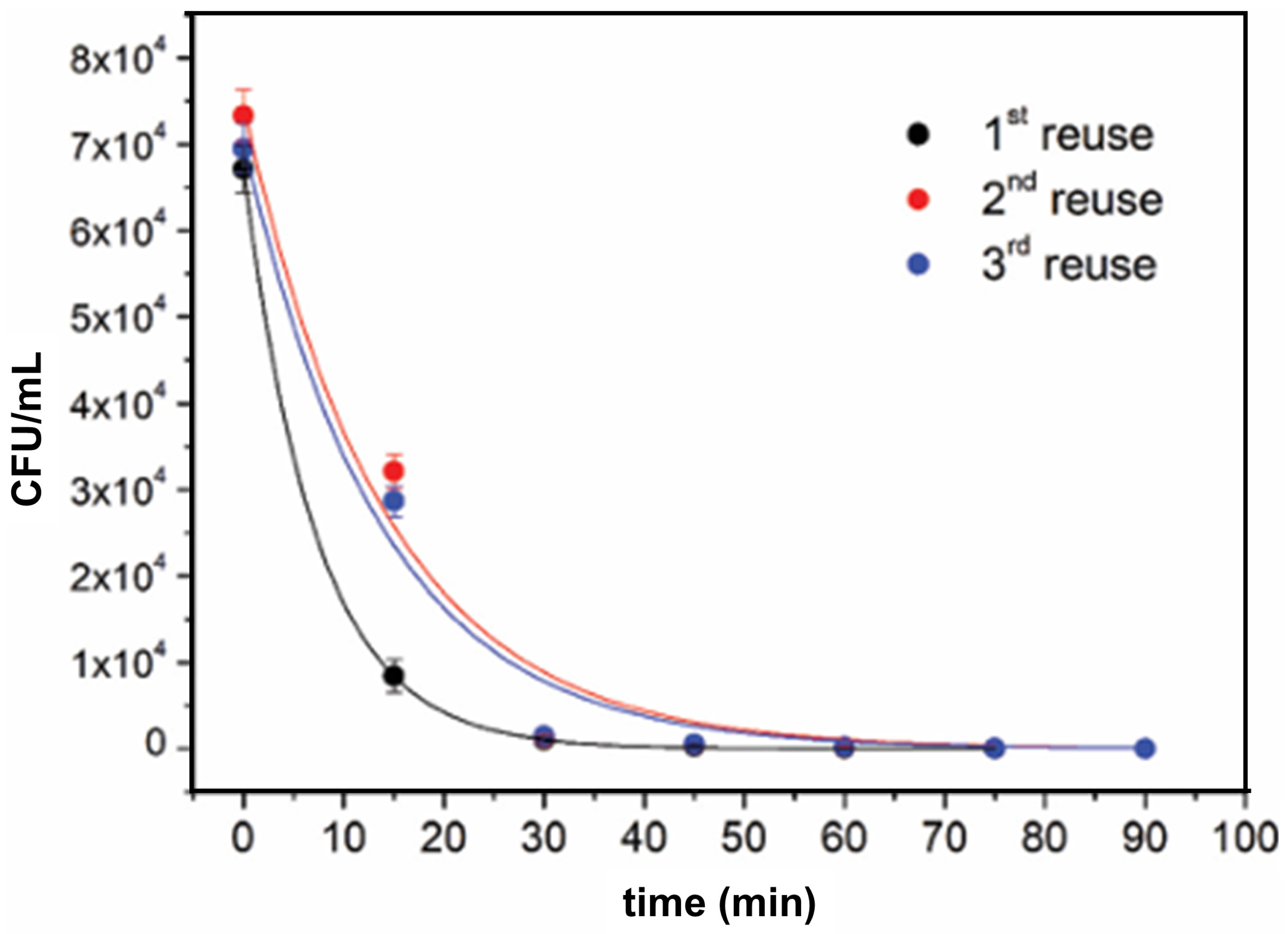

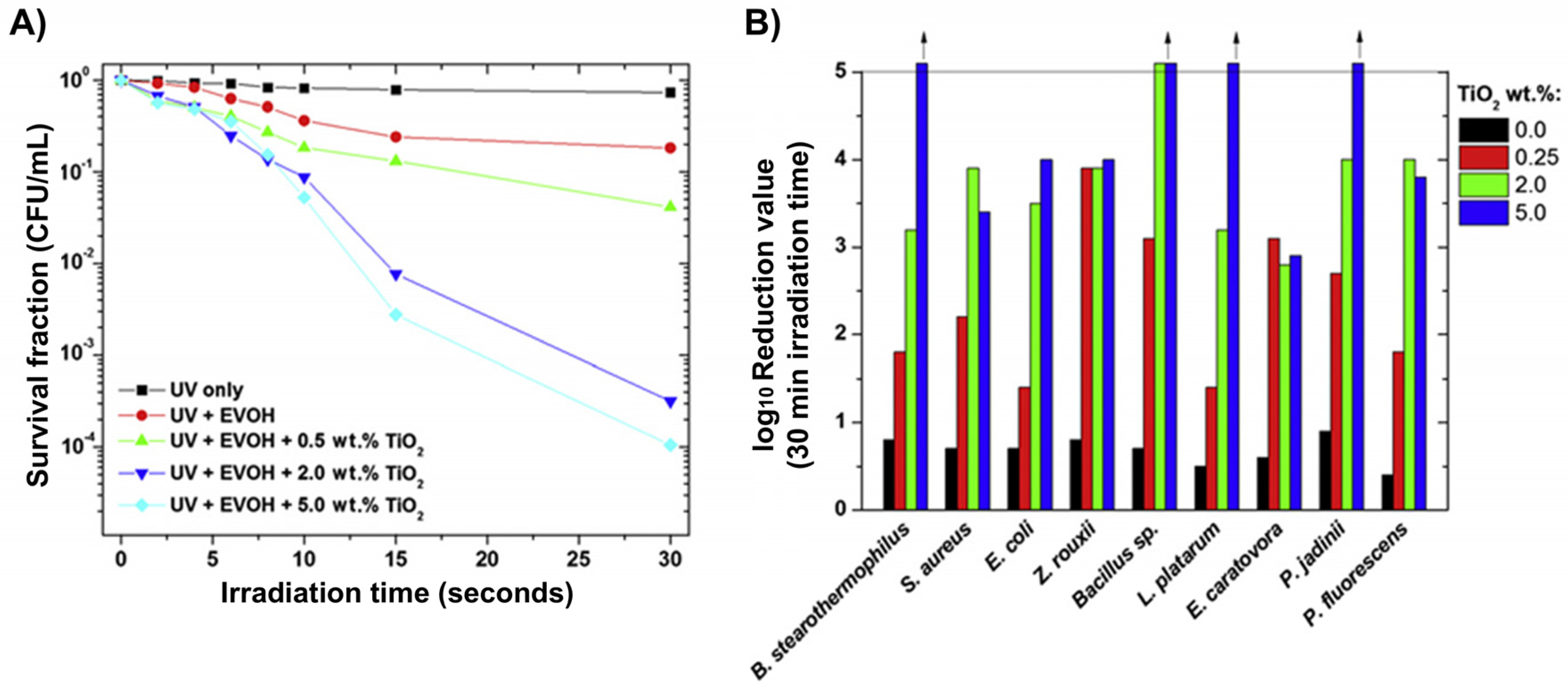
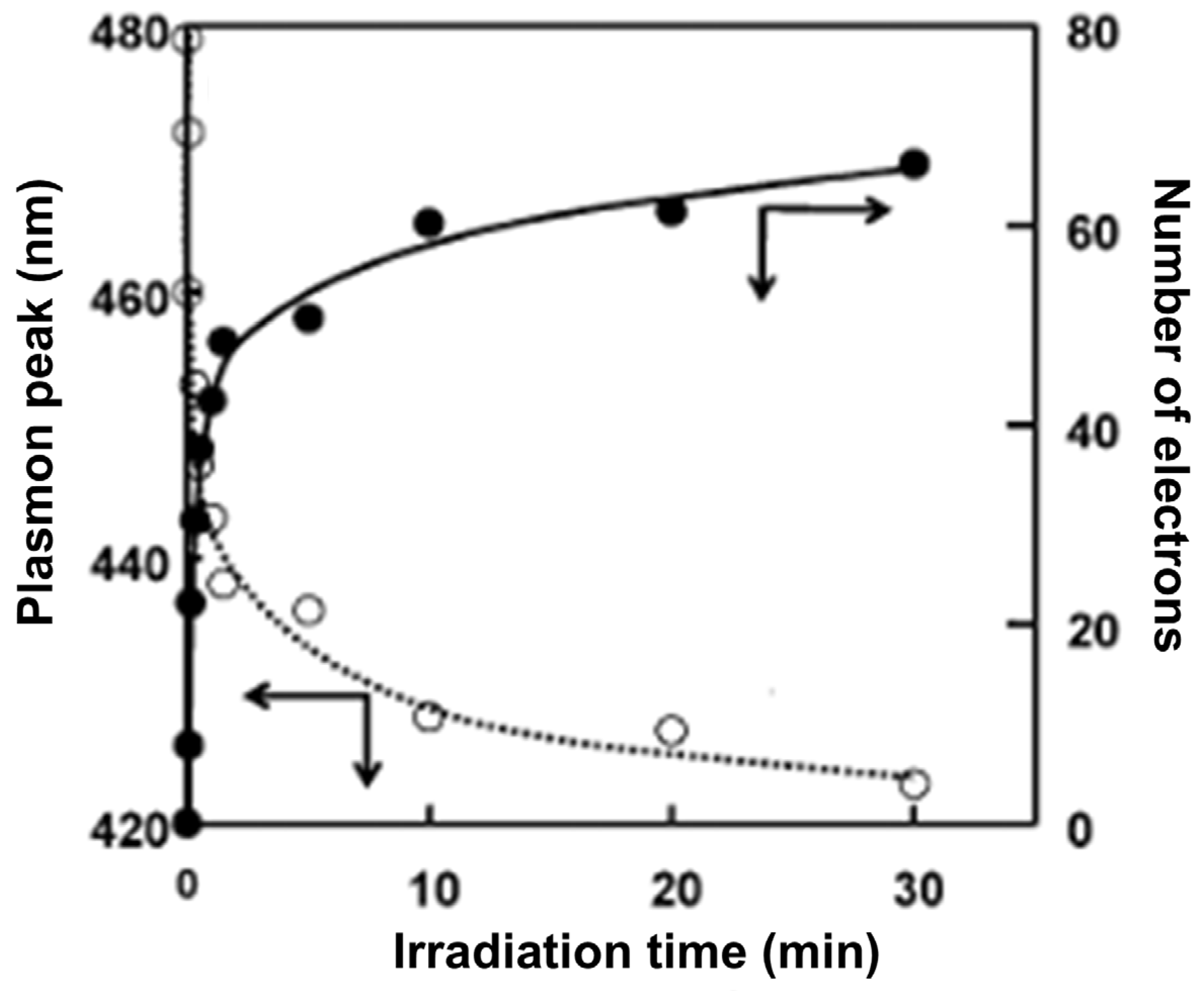




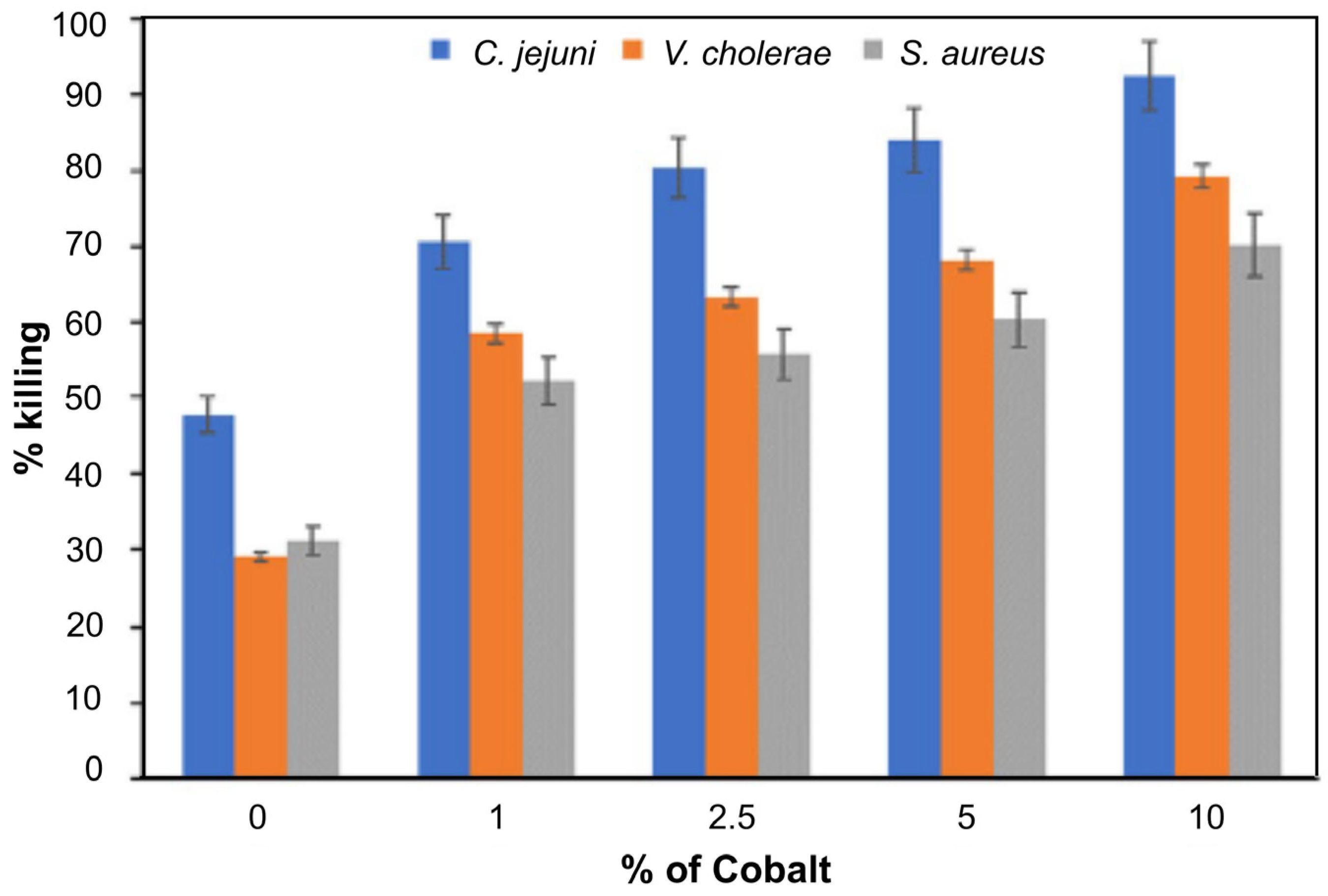
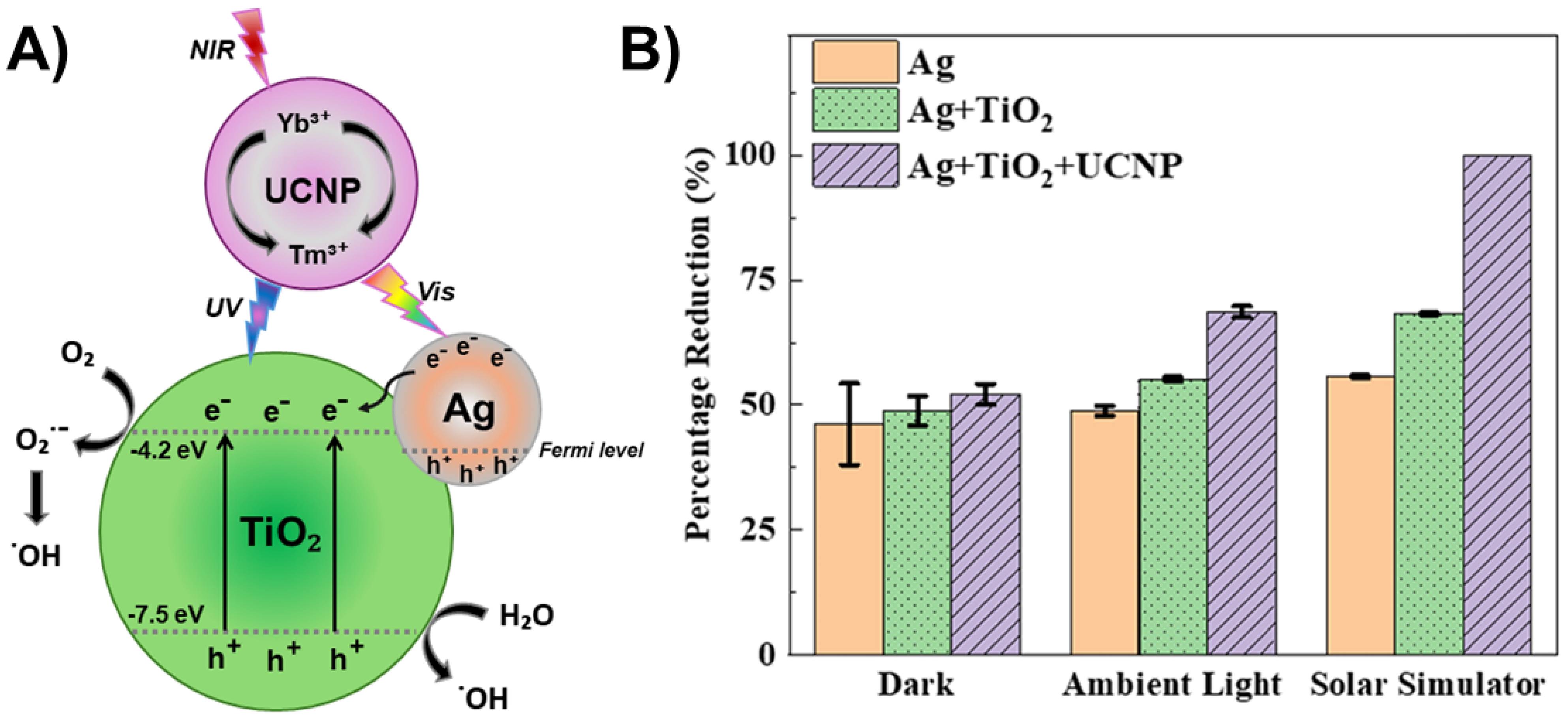
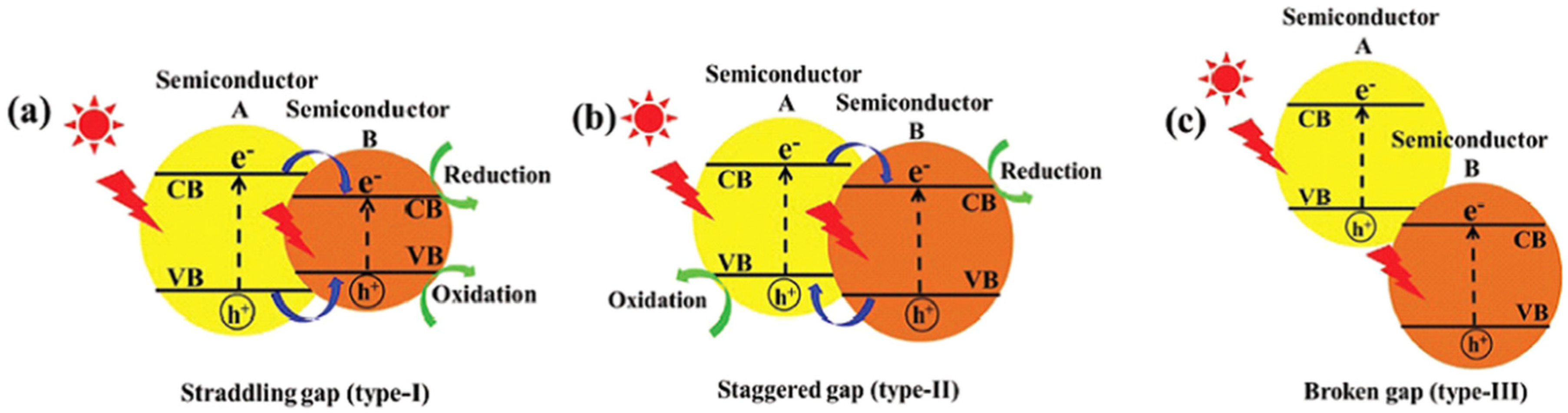



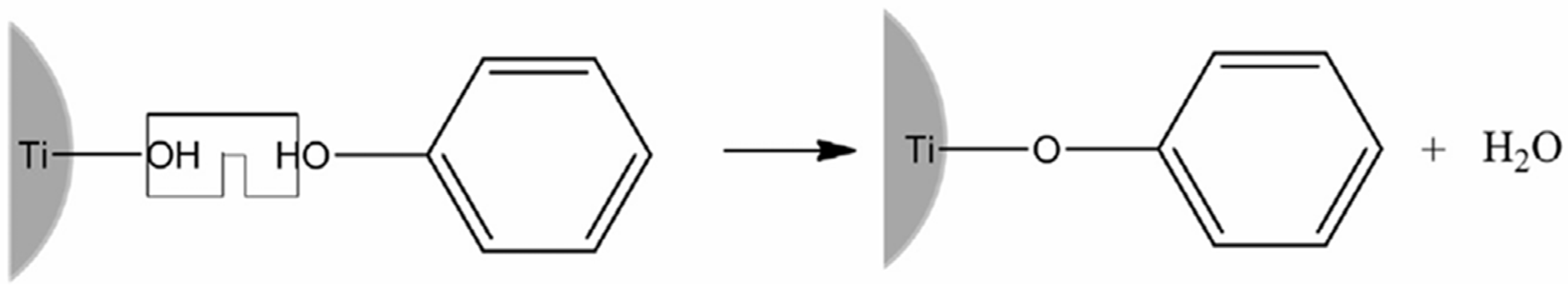
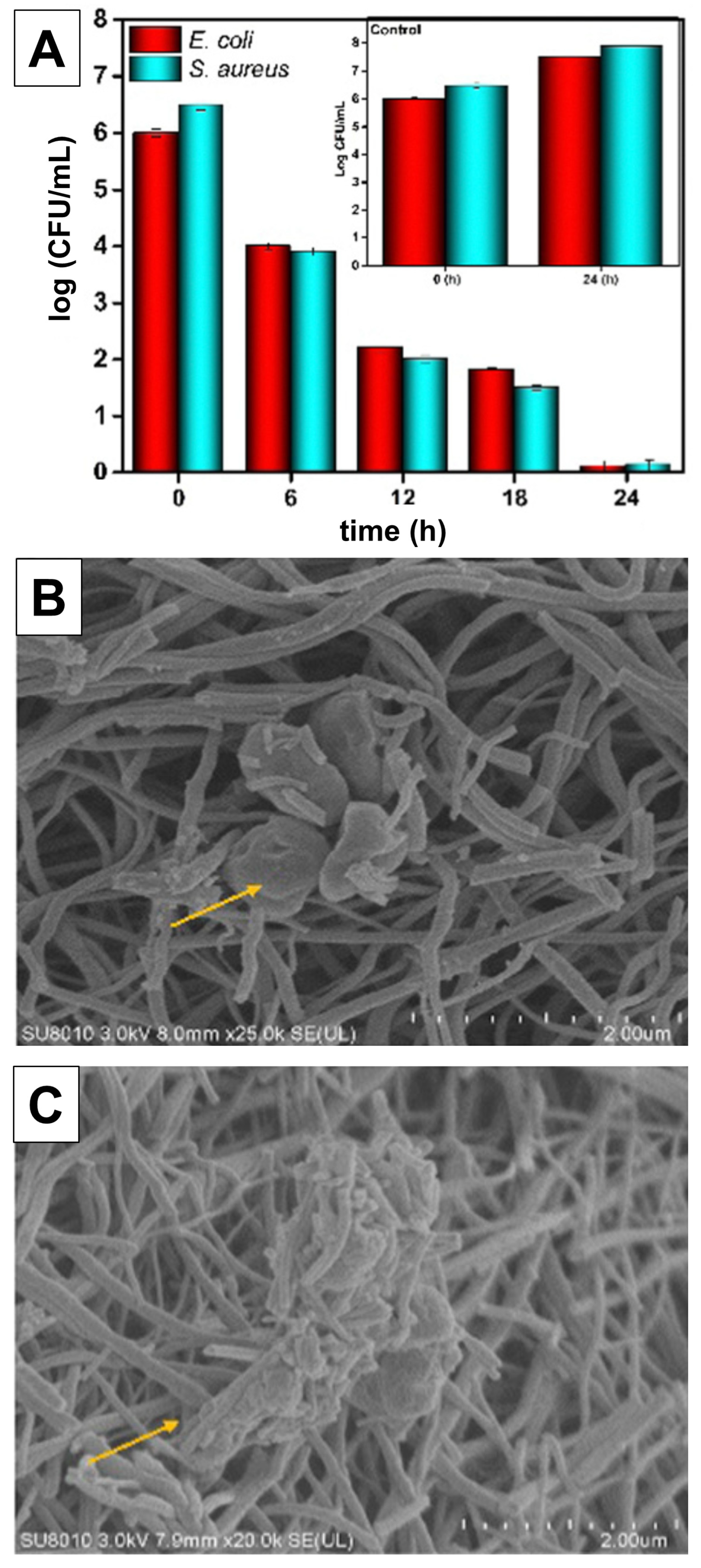
| Microorganism | Reference |
|---|---|
| Escherichia coli | [83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106] |
| Enterococcus faecalis | [88] |
| Enterobacter cloacae | [90] |
| Pseudomonas aeruginosa | [90,95,98,99] |
| Salmonella typhimurium | [90,106,107] |
| Staphylococcus aureus | [95,105,106] |
| Salmonella enteriditis | [98] |
| Bacillus subtilis | [99,104] |
| Pseudomonas fluorescens | [104] |
| Serratia marcescens | [105] |
| Coliforms | [108,109] |
| Streptoccocus faecalis | [109] |
| Erwinia carotovora | [110] |
| Pseudomonas syringae | [110] |
| Streptococcus sobrinus | [111] |
| Precursors | TiO2 Properties | Tested Pathogen | Reference | ||
|---|---|---|---|---|---|
| Plant Extract | Source of Ti | Crystal Phase | Morphology | ||
| Psidium guajava | TiO(OH)2 | A+R | spherical, 32.5 nm | A. hydrophila, P. mirabilis, E. coli, S. aureus, P. aeruginosa | [150] |
| Morinda citrifolia | TiCl4 | R | spherical, 10–20 nm | S. aureus, E. coli, B. subtilis, P. aeruginosa | [136] |
| Trigonella foenum-graecum | TiO(SO4) | A | spherical, 20–90 nm | E. faecalis, S. aureus, S. faecalis, B. subtilis, Y. enterocolitica, P. vulgaris, E. coli, P. aeruginosa, K. pneumonia | [142] |
| Glycyrrhiza glabra | TiO(SO4) | A | spherical, 60–140 nm | S. aureus, K. pneumonia. | [143] |
| Punica granatum | TTIP | A | various shapes, 1–5 μm | P. aeruginosa, E. coli, S. aureus | [151] |
| Aloe barbadensis | TiCl4 | A+B+R | spherical, ~20 nm | P. aeruginosa | [152] |
| Lupin bean | TTIP | A | spherical (9.2 nm) and nanorods | Enterococcus, E. coli | [154] |
| Mentha arvensis | TTIP | A | spherical, 20–70 nm | P. vulgaris, S. aureus, E. coli, A. cuboid | [155] |
| Acorus calamus | TTIP | A | Spherical, 11–30 nm | E. coli, P. aeruginosa, B. subtilis, S. aureus | [156] |
| Morus alba | TTIP | A | spherical, 24 nm | E. coli, S. aureus | [157] |
| Luffa acutangula | TiO(SO4) | R | hexagonal, 10–49 nm | B. subtilis, E. coli, E. faecalis, K. pneumoniae, S. aureus, P. aeruginosa | [158] |
| Nervila aragona, Ceaspina pulcherrima, Manihot esculante | TTIP | A | spherical, 15–28 nm | E. coli, S. aureus, P. aeruginosa | [147] |
| Spinacia oleracea | TiO(SO4) | A | spherical, 38 nm. | E. coli, S. aureus | [159] |
| Fagonia cretica | TiO(OH)2 | R | spherical, 20–80 nm | K. pneumoniae, S. aureus, P. aeruginosa, E. coli | [141] |
| Sample | Atomic Ratio (%) | |||
|---|---|---|---|---|
| C–C, C–H | C–O | C=O | O–C=O | |
| U-PES | 77.4 | 13.1 | 0.0 | 9.5 |
| O2-PES | 63.6 | 21.0 | 8.3 | 7.1 |
| Ar-PES | 70.4 | 14.2 | 10.3 | 5.1 |
| Time (min) | Viable Cell Concentration (CFU/mL) | |
|---|---|---|
| TiO2 | TiO2-Ag | |
| 0 | 1 × 107 | NVC |
| 10 | 1 × 106 | NVC |
| 15 | 0.5 × 106 | NVC |
| 20 | NVC | NVC |
| 30 | NVC | NVC |
| 40 | NVC | NVC |
| Co-Doped TiO2 | Fe-Doped TiO2 | ||
|---|---|---|---|
| Dopant Concentration (mol.-%) | Crystallite Size (nm) | Dopant Concentration (vol.-%) | Crystallite Size (nm) |
| 0.0 | 14.1 | 0.0 | 26.1 |
| 1.0 | 12.2 | 3.0 | 24.8 |
| 2.5 | 12.0 | 4.0 | 22.9 |
| 5.0 | 8,0 | 5.0 | 20.2 |
| 10.0 | 7.1 | 6.0 | 19.5 |
| Dopants | Heterostructure with Noble Metal | Pathogen | Reference | |
|---|---|---|---|---|
| Non-Metal | Metal Ion | |||
| N | Ag | E. coli, B. subtilis | [276] | |
| C, S | Ag | E. coli, B. subtilis | [277] | |
| N | Cu | E. coli, E. faecalis | [278] | |
| N | Ag | E. coli | [279] | |
| N, F | E. coli, S. aureus, | [280] | ||
| N | Ni | E. coli, S. aureus | [281] | |
| S | Mn | B. coagulans, K. pneumoniae | [282] | |
| N | Cu | E. coli, S. aureus | [283] | |
| N | Co | E. coli, S. aureus, L. pneumophila | [284] | |
| S | Co | E. coli, S. aureus | [285] | |
| Fe | Ag | E. coli, S. aureus | [286] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazić, V.; Nikšić, V.; Nedeljković, J.M. Application of TiO2 in Photocatalytic Bacterial Inactivation: Review. Int. J. Mol. Sci. 2025, 26, 10593. https://doi.org/10.3390/ijms262110593
Lazić V, Nikšić V, Nedeljković JM. Application of TiO2 in Photocatalytic Bacterial Inactivation: Review. International Journal of Molecular Sciences. 2025; 26(21):10593. https://doi.org/10.3390/ijms262110593
Chicago/Turabian StyleLazić, Vesna, Valentina Nikšić, and Jovan M. Nedeljković. 2025. "Application of TiO2 in Photocatalytic Bacterial Inactivation: Review" International Journal of Molecular Sciences 26, no. 21: 10593. https://doi.org/10.3390/ijms262110593
APA StyleLazić, V., Nikšić, V., & Nedeljković, J. M. (2025). Application of TiO2 in Photocatalytic Bacterial Inactivation: Review. International Journal of Molecular Sciences, 26(21), 10593. https://doi.org/10.3390/ijms262110593









