Abstract
Cognitive aging trajectories differ widely across individuals, and genetic factors such as APOE and BDNF polymorphisms may contribute to this variability. While APOE ε4 has been widely studied, the influence of APOE ε2, particularly in interaction with sex, remains underexplored. This study aims to examine the longitudinal trajectory of APOE ε2 individuals on cognitive performance, and their interactions with sex, age, and BDNF Val66Met polymorphism, in a population-based cohort of older adults with vascular risk. We analyzed data from 386 participants (mean age: 71.8) from the Barcelona-AsIA Neuropsychology Study, followed over a 7-year period. Verbal memory, verbal fluency, and visuospatial domains were assessed. Linear regression models tested associations between cognitive change and genotypes, controlling for age, sex, education, depression, and vascular risk. Interaction terms and permutation testing were applied. Regression to the mean (RTM) effects were assessed. BDNF showed no significant associations with cognitive performance. RTM effects were evident across subgroups, particularly among ε2 carriers, suggesting this phenomenon partly explains the divergent results over time. APOE ε2 does not confer a consistent protective effect on cognition over time. Our results highlight that APOE ε2 may be detrimental to verbal memory in aging males.
1. Introduction
Most cognitive abilities decline with age, affecting the ability to perform basic daily functions and leading to a reduced sense of well-being [1,2]. Nevertheless, interindividual differences are considerable and not uniform throughout the lifespan. The identification of the factors involved in cognitive decline processes in late life is of utmost relevance for the development of effective and more personalized interventions [3].
Among the various genetic factors involved, Apolipoprotein E (APOE) plays a pivotal role in the risk of Alzheimer’s disease (AD) [4,5,6]. The functional impact of the APOE alleles—ε2, ε3, and ε4—has long been considered within a framework that ranges from beneficial to adverse across the life course, affecting cognition distinctly during different life stages [7,8]. The ε4 allele has been extensively examined due to its role as a genetic risk factor for cognitive decline in individuals with normal aging, mild cognitive impairment, and Alzheimer’s disease [9,10]. In contrast, the ε2 allele has been traditionally associated with enhanced cognitive performance across various domains in aging individuals, as well as a slower rate of age-related cognitive decline in both healthy and cognitively impaired populations [11,12,13,14]. However, emerging research suggests that its impact on cognition is inconsistent and appears to be limited [15]. The comprehensive review conducted by Kim et al. concluded that the evidence for the cognitive benefits of ε2 is mixed, and methodological limitations may have contributed to misleading conclusions in some prior studies [15]. In another large-scale observational study conducted by Wood et al., the authors revealed sex-specific effects, showing that among non-Hispanic White adults, ε2 carriers experienced slower cognitive decline only in men, but not in women [16]. Overall, ε2 is still linked to a lower risk of Alzheimer’s disease and longer lifespans, but its effects on cognition, especially in healthy aging, are not all equally positive. Recent research indicates that these effects vary by sex, ethnicity, and phenotype. Even so, it remains the case that studies focused on the role of ε2 in cognition have attracted less attention in comparison with studies based on the role of ε4 in cognition due mainly to the limited frequency of the ε2 variant (7% in the global community [17]) and the design of studies in which ε2 carriers are either excluded or combined with ε3 in a non-ε4 group. Although APOE is a key genetic factor for AD and cognition, its effects vary across individuals, and several factors have been proposed as modulators of this heterogeneity.
One of the primary variables that can influence how APOE affects cognition is sex. Its influence on the association between the APOE ε4 allele, cognitive function, and Alzheimer’s disease risk has been highlighted in several studies [4,18,19,20]. Women are at higher risk of developing late-onset AD (LOAD), probably due to an intensification of the effects of the loss of sex hormones during peri-/post-menopause [21,22]. The female brain may be more susceptible to cognitive decline and neurodegeneration as a result of fluctuating estrogen levels during perimenopause and the marked drop in estrogen following menopause [23,24,25]. In fact, brain glucose hypometabolism, Aβ deposition, and loss in brain volume commence at perimenopause [26,27,28,29]. Numerous studies have documented a more pronounced negative impact of the ε4 allele in women across a range of Alzheimer’s disease biomarkers [30,31,32,33,34,35]. Only two studies centered on the protective effects that APOE ε2 seems to have in females [36,37]. The first one, conducted by [36], was a 4 to 7-year longitudinal study in which the authors reported a more pronounced protective effect of APOE ε2 among women in predicting performance on a delayed recall task. The second one, conducted by [37], was a Genome-Wide Association study in which the authors reported a significant association between APOE ε2 and slower cognitive decline in women. Although those studies showed sex differences in the effects of APOE on cognition, few studies to date have explicitly examined the interactive effects of sex*APOE on cognitive performance. In our previous cross-sectional study [38], we reported for the first time a significant interaction between sex*APOE ε2 in females in the verbal memory and verbal fluency domains compared to men. To the best of our knowledge, only one longitudinal study explicitly examined APOE × sex interactions [39], and they found no significant effects of sex × APOE ε4 on cognitive performance. More recent longitudinal studies, however, have started to provide insight into this subject. For example, Burns et al. (2025) found that APOE ε4 status and sex have differential impacts on the brain’s longitudinal dynamics of excitation–inhibition balance. This differential impact is discussed as a preclinical indicator of cognitive decline in female APOE ε4 carriers. Further evidence for sex-modulated effects of APOE on cognitive outcomes comes from Dessy et al. (2024), who looked at the disentangled effects of gender and sex on APOE ε4–related neurocognitive impairment [40,41]
Longitudinal studies reported that ε4 carriers had an increased risk of developing mild cognitive impairment (MCI) [42], AD [43], and vascular dementia [44]. Less abundant literature has investigated the effects of ε2 on cognition, suggesting that ε2 carriers exhibit moderately preserved cognitive function over time. In young adults, it has been shown that APOE ε2 carriers had better spatial strategies compared to ε3 and ε4 carriers [45]. In older adults, APOE ε2 carriers have shown a protective effect on a measure of global cognitive function [46], and a reduced risk and delayed onset of AD [47,48,49]. Other studies question the presence of such benefits, even suggesting a cognitive disadvantage [50,51,52], or no effect of ε2 on cognitive change ratios [53,54].
Other potential factors that may interact with the effect of APOE on cognition include lipidic profile, inflammation, interactions with polymorphisms in other genes—such as Sponding-1 (SPON1) rs11023139 [55] or the Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism [56,57]—as well as dietary protein intake [58], having positive or negative age beliefs [59], physical activity [60,61], or education [62].
Special attention deserves the interaction between the APOE and the BDNF. BDNF is a key player in synaptic plasticity, long-term potentiation, and hippocampus-dependent memory, and it is necessary for normal cognitive function. According to new research, APOE genotype and BDNF interact, specifically affecting cognitive outcomes over time. The APOE-ε4 allele significantly altered the association between the BDNF Val66Met polymorphism and cognitive impairment, suggesting an interactive risk profile in nonclinical aging, according to Ji et al.’s (2024) study of older adults living in the community. Vilor-Tejedor et al. (2020) demonstrated that the BDNF Met variant compensates for APOE-ε4-related decreases in hippocampal subfield volumes in middle-aged, cognitively unimpaired individuals from the ALFA cohort, indicating structural resilience mechanisms associated with genotype interaction. Edmunds et al. (2025) demonstrated that circulating BDNF expression mediates verbal learning and memory in a cohort enriched for Alzheimer’s risk. [63,64,65]. All of these studies demonstrate the importance of BDNF in supporting cognitive health and reducing the risk of APOE, particularly in female subgroups and prior to the onset of clinical symptoms. To the best of our knowledge, there is no previous longitudinal research studying the interaction between sex and APOE ε2 on cognition, nor the interaction between BDNF and APOE. This type of research is crucial for understanding the mechanisms underlying the protective and deleterious effects of APOE on cognition over time. This study aims to analyze the effects of the APOE genotype on changes in cognitive performance over seven years, as well as its interaction with sex, age, and BDNF genotype. Based on our previous results [38], we anticipate that the ε2 allele will be associated with a lower rate of cognitive decline, particularly in verbal memory and fluency domains, and among female participants. Additionally, we anticipate that the combination of APOE ε4+ and the BDNF Met allele will be significantly associated with worse memory performance compared to other genotypes, and that APOE ε2, as a positive regulator of BDNF, may have a protective effect [63,66,67].
2. Results
2.1. Subjects
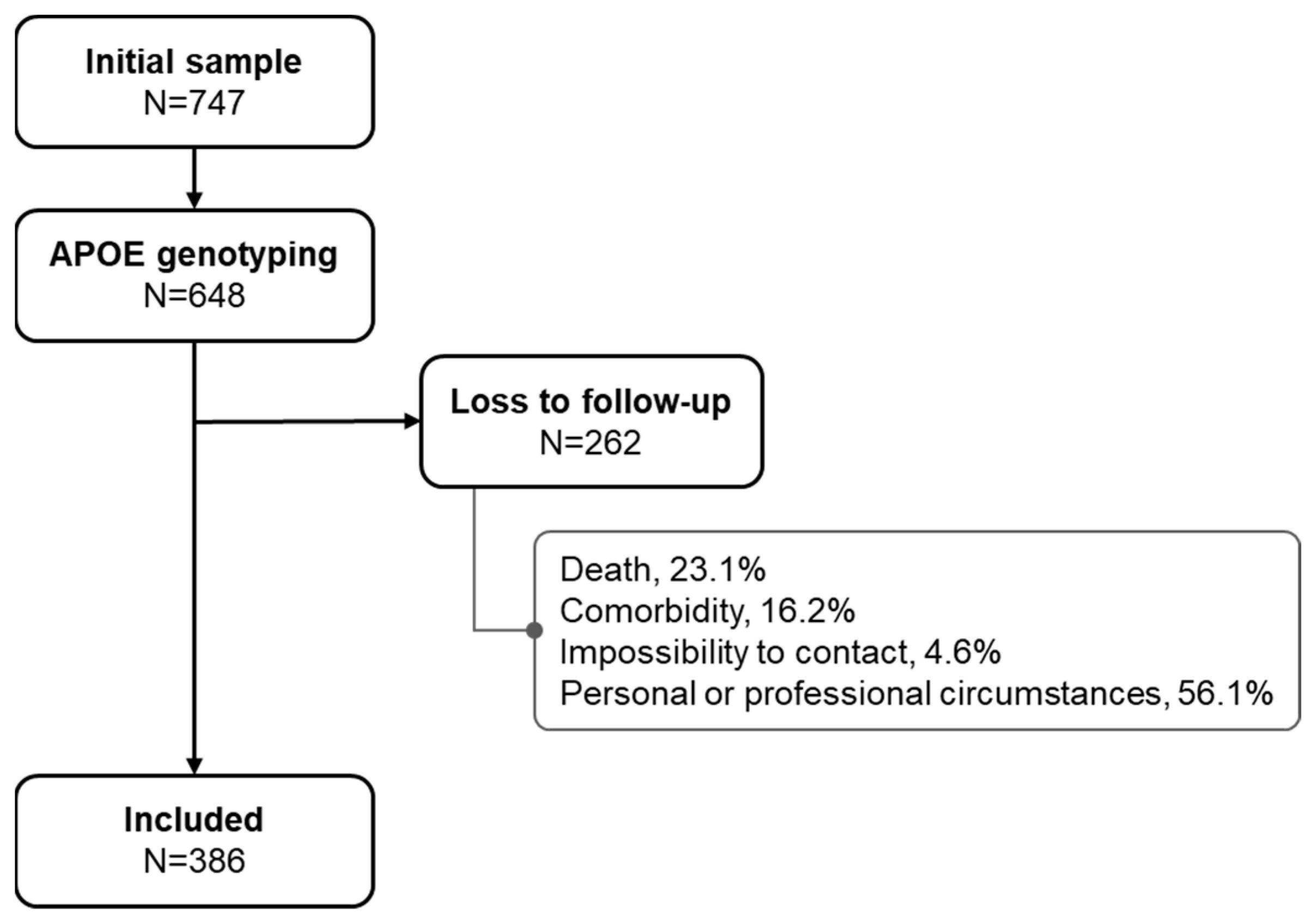
The initial sample, which underwent a complete neuropsychological (NPS) assessment between 2007 and 2010, included 747 subjects, of whom 648 had APOE genotyping. Of these, a total of 386 were accepted as being cognitively reassessed between April 2016 and May 2017. (Figure 1).
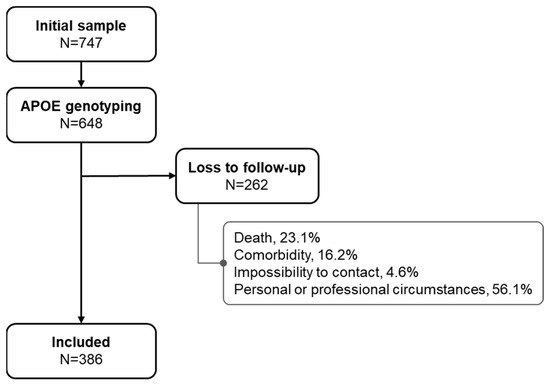
Figure 1.
Flowchart of the study population.
The mean age of the final sample was 71.82 years (SD = 6.58). Moreover, the 36.80% of the participants were females (n = 142) and reported 6.76 (SD = 4.22) years of education on average. Summary details of the sample are described in Table 1.

Table 1.
Sociodemographic, clinical, and neuropsychological sample characteristics.
Sociodemographic characteristics comparison between subjects who only completed the NPS baseline evaluation (n = 260) and subjects with baseline and follow-up assessments (n = 386) showed that subjects that did not undergo a new complete NPS assessment were older (t (648) = −5.07; p < 0.01), with less education years (t (648) = 2.70; p = 0.01) and lower cognitive performance (p < 0.01 for all three domains) and with higher levels of vascular risk factors based on the REGICOR score (t (648) = −3.23; p < 0.01) than subjects who were reassessed in the follow-up (Table S1). Conversely, there were no differences in allele frequency between these subgroups.
2.2. Genotype and Cognitive Domains
Our primary goal was to investigate the potential associations between the genetic variants analyzed and changes in cognitive performance. When we analyzed cognitive change (i.e., follow-up minus baseline difference), we observed a higher decline in verbal memory performance among ε2-carriers, especially among male participants (Table 2). After permutations, results remained significant only among male participants (β = −0.382; 95% CI: −0.670/−0.095; p = 0.01; p-perm = 0.027). Women showed no evidence of such an effect. Similar effects were observed when comparing participants carrying the APOE ε2 allele (ε2/ε2 and ε2/ε3) to those with the ε3/ε3 genotype, excluding ε4 carriers.

Table 2.
Linear regression results for the association between APOE alleles and changes in Z-scores from baseline to follow-up for cognitive domains.
Regarding the APOE ε4 allele, no significant associations were found between changes in performance across any of the cognitive domains studied and the APOE ε4 allele, after adjusting for covariates (Table 2).
The associations observed between the APOE ε2 allele and verbal memory performance were confirmed in the subgroup of participants without depressive symptoms (GDS > 5, N = 303), thus ruling out potential confounding effects due to depression.
2.3. Cross-Sectional Analyses
These initial longitudinal findings appeared to be discrepant with our hypotheses and previously published cross-sectional results from the same cohort. In our early study, we found that ε2-carriers performed significantly better in the verbal memory and fluency domains at baseline, and these results were particularly notable among women [38]. To assess whether differences between participants with only the baseline assessment and those with both baseline and follow-up assessments might account for these discrepancies, we ran association analyses of cognitive performance in the baseline assessment separately for each subgroup. Results matched our previously published findings in both subgroups separately: a significant protective association of the ε2 allele on the verbal memory and fluency domains among women at baseline (Tables S2 and S3).
At follow-up, ε2 carriers showed a similar cognitive performance to that of their non-carrier counterparts. The protective effects of ε2 found at baseline were not present in the follow-up assessment, and there were no significant cognitive differences between APOE genotypes (Table 3). We observed an adverse trend effect among males (p = 0.056) and a protective trend effect among females (p = 0.073) in the verbal memory domain, although these observations did not reach statistical significance. The other variants studied in the APOE gene did not show relevant results in the association analyses on cognitive performance at follow-up.

Table 3.
Linear regression results for the association between APOE alleles and Z-scores at follow-up for cognitive domains.
Regression to the Mean
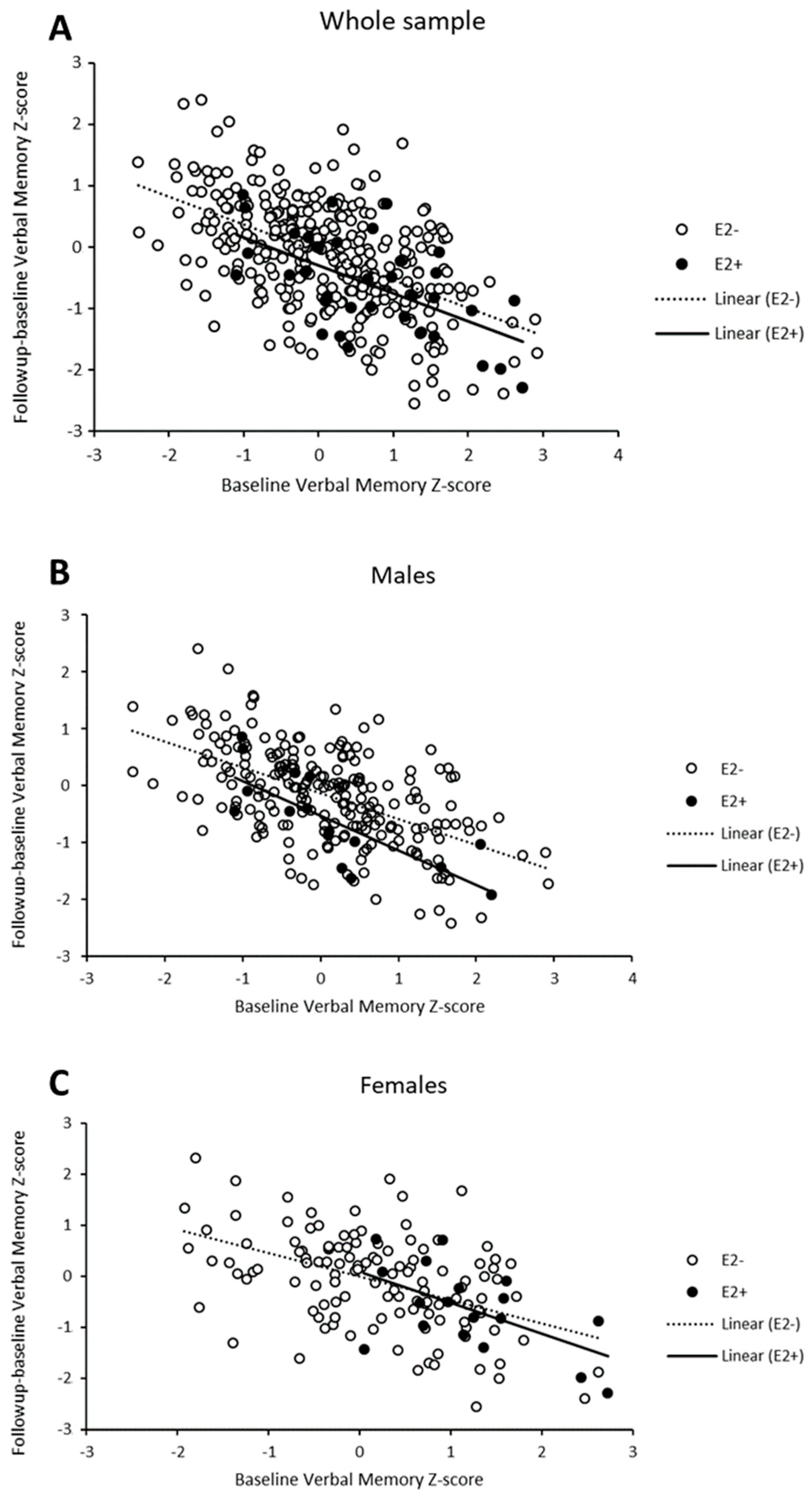
In order to reconcile the apparently conflicting findings of the effect of the ε2 allele on verbal memory (a protective role among women at baseline, no effect at follow-up, and a detrimental effect on longitudinal change among men), we explored whether a potential regression to the mean (RTM) effect could contribute to our observations of the longitudinal effects. Examination of the data revealed RTM effects in all subgroups (Figure 2). Overall, individuals whose baseline results in the verbal memory domain were higher than the average tended to perform worse in the follow-up measurement and cognitive changes were likely to retrieve negative values (lower right side of the plot). The inverse pattern was observed for individuals with low baseline scores. In addition, there were differences in RTM effects depending on ε2-carrier status and sex. In the whole sample, the intensity of RTM effects was seemingly similar (i.e., regression lines with the same slope) in ε2 carriers and non-carriers (Figure 2A), with a trend towards more negative values in cognitive change among ε2 carriers as revealed in the previous analyses. However, we observed that the effects were different among ε2 carriers and non-carriers when stratifying by sex (Figure 2B,C). Individuals carrying ε2 alleles showed more intense RTM effects compared to their non-carrier counterparts in both male and female subgroups, as illustrated by steeper slopes of the regression lines.
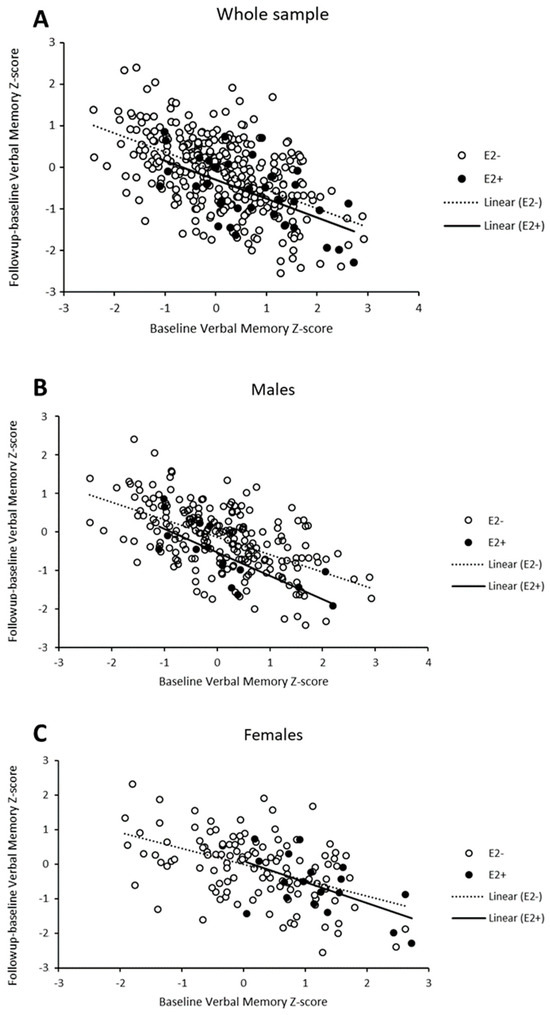
Figure 2.
Scatter-plot of verbal memory Z-scores across time points showing change (follow-up minus baseline) against baseline scores for the whole sample (A) and stratified for males (B) and females (C).
We did include cognitive performance in the baseline evaluation as a covariate in longitudinal analyses to account for potential RTM effects. This covariate was highly significant in most of the regression models, an indication that it successfully corrected (at least partially) biases induced by extreme baseline values. When we repeated analyses in the verbal memory domain, adjusting by the mean between the first and second questionnaires’ scores as an alternative method to capture RTM effects, we retrieved very similar results, with adverse effects of the ε2 allele from baseline to follow-up (i.e., higher memory decline) in the whole sample and among males (Table S4).
2.4. Gene*Age and Gene*Gene Interactions
We further explored the effect that gene-age and gene-gene interactions could play in the evolution of cognitive performance during the aging process. Regarding interactions with age, we did not identify significant interactive effects in the regression models (p > 0.3). However, a stratified analysis revealed that the detrimental effects of the ε2 allele on longitudinal change in verbal memory were only present among the younger males (i.e., younger than 71 years old, the median age in our cohort) (Table S5).
Since our previous analyses in the baseline assessment revealed significant APOE*BDNF interactions on cognitive performance, we also explored the presence of gene-gene interactive effects on cognitive decline in the longitudinal dataset. However, we did not find significant results (p > 0.05, Table S6). Moreover, similarly to our previous observations at baseline, BDNF did not show any significant association, as an independent factor, with the cognitive domains at either follow-up or the longitudinal level.
3. Discussion
APOE is widely recognized as a significant factor in cognitive success or decline during the aging process. Nevertheless, the complexity of its genotype interactions with age, sex, and other variables, such as vascular risk factors, hormones, and BDNF, remains a matter of controversy [68]; the mechanisms through which it exerts its effects also remain unclear. In this paper, we investigated the effects of genetic variants in the APOE gene on the longitudinal change in cognition in a large cohort of community-dwelling older adults. We focused on studying the interactive effects of sex, the BDNF Met allele, and APOE ε2 on cognition. Our results showed a detrimental association of APOE ε2 in males with changes in verbal memory, whereas no significant protective impacts were found for females in any cognitive domain. We did not find any influence of age or BDNF in our results.
Research centered on the role of APOE ε2 on cognitive decline is scarce, and the results are mixed. Some studies have reported a positive effect of APOE ε2 on reducing cognitive decline in both healthy and demented populations [13,46,69,70], as well as improved performance in short-term and long-term memory [71,72,73,74] and executive function and attention [13]. Other studies have questioned the presence of such benefits, even suggesting a cognitive disadvantage [50,51,52,75]. None of these studies, however, focused on the differential effect of sex on their results. Only a few studies have focused on the differential effect of APOE ε2 on cognition by sex. While one study reported a protective effect of APOE ε2 among women in predicting performance on a delayed recall task [36], another observed a protective effect of APOE ε2 against cognitive decline in men [16].
In our previously published cross-sectional study [38], we reported a significant interaction between sex and APOE ε2 on cognitive performance in females. Specifically, we found that female APOE ε2 carriers performed better in the domains of verbal memory and verbal fluency and that total cholesterol mediated these protective effects on cognition, providing a physiological mechanism for the observed genetic effects. In the present longitudinal study, and contrary to our expectations, the effect of the APOE ε2 genotype was deleterious for males in the verbal memory domain compared to non-ε2 carriers. That is, male ε2 carriers experienced more cognitive decline than non-ε2 carriers, finding evidence of an adverse effect of APOE ε2 on cognition in males. We did not, however, find the expected protective effect of APOE ε2 in females in the verbal memory and verbal fluency domains. This result can be explained by the differential characteristics found between subjects who received a second assessment and those who dropped out of the study. Subjects who dropped out of the study were older, less educated, had lower cognitive performance, and exhibited higher levels of vascular risk factors compared to the subjects who were followed up in the study. In fact, when we run separate cross-sectional analyses for subjects with both assessments and participants with only the baseline assessment, our main results match our previously published findings: a significant protective effect of the APOE ε2 allele on the verbal memory and fluency domains among women in both groups. It is possible that we retained subjects in our sample who performed better cognitively and had a better lifestyle, considering they had lower levels of vascular risk factors compared to the participants who dropped out. When we conducted a cross-sectional analysis at the follow-up visit (seven years after the initial assessment), we did not find significant effects of the ε2 allele; however, we observed trends towards a protective effect in women and a detrimental effect in men. Furthermore, although we controlled for the RTM effects, the fact that the RTM effects varied depending on ε2-carrier status and sex could have contributed to the apparent discrepancy between our previous study and this one. APOE ε2 carriers showed more intense RTM effects than non-carriers, separately in both the male and female subgroups.
Regarding age, one potential explanation for the lack of significant effects of age on the APOE genotype status could be that our age range is limited to 13 years. In contrast, studies focused on the effect of age on APOE ε2 carriers considered several decades for their analysis [76].
Several factors, beyond age and sex, may be interacting or mediating the effect of APOE on cognition and could serve as a putative explanation for the results found in our present study. Among them, the lipidic profile stands out as particularly relevant. APOE is involved in the transport of lipids in the body and brain [77]. It is known that APOE ε4 is associated with higher levels of low-density lipoprotein cholesterol (LDL-C) and total cholesterol (TC), as well as an increased risk of dementia [78]. In contrast, APOE ε2 is associated with elevated very low-density lipoprotein (VLDL) and a lower risk of dementia [79]. Women typically exhibit higher levels of high-density lipoprotein cholesterol (HDL-C) and lower levels of LDL-C and VLDL compared to age-matched men [80]. However, over time, the TC and LDL-C profiles become less favorable in women with lower age-specific anti-Müllerian hormone levels, a hormone related to ovarian reserve [81]. This would explain, for example, the higher risk of cerebrovascular disease factors in women at menopause [81]. It is known that cholesterol accumulation leads to subsequent neuroinflammation and neurodegeneration, leading to cognitive dysfunction. In our previous study, we found that TC mediated the protective effect of APOE ε2 on cognitive performance in females, suggesting that sex differences should be considered when analyzing the effect of specific genes on cognition. Unfortunately, we did not have available data on lipid levels at follow-up, and therefore, we cannot analyze their mediator effect as we did at baseline. This is a significant limitation in our study that prevents us from further developing a putative explanation regarding the change in the lipidic profile in women and how this change could interact with APOE ε2 and cognition.
Sexual dimorphism in brain function is driven by complex biological pathways involving sex hormones. Females generally exhibit higher cerebral blood flow (CBF) than males—a difference that persists with aging despite the overall age-related decline in CBF [82,83,84]. Most studies attribute this advantage to estrogens, which have well-established neuroprotective and vasoregulatory roles [85]. In contrast, the impact of androgens on neuroinflammation and cerebrovascular regulation is less understood. Evidence suggests that androgens can exert both protective and detrimental effects on the cerebral vasculature [86]. Furthermore, androgen levels decline from early adulthood due to reduced testicular function, contributing to oxidative stress, diminished synaptic plasticity, and cognitive decline [87,88].
Other factors that should be considered as potential modulators of the observed APOE*sex effects include BDNF [56,89], dietary protein intake [58], having positive or negative age beliefs [59], education [62], physical activity [60,61], and air pollution [90]. In particular, at baseline, we previously found a significant interaction between genotypes in the APOE and BDNF genes [38] that has not been replicated in our present longitudinal work. Regarding physical activity, for example, we have recently reported that higher cardiorespiratory fitness (CRF) mediated the cognitive benefits of physical activity on executive function and attention-speed domains only in men but not in women [91]. Moreover, although we did not collect information on environmental exposures, increasing evidence points to a relevant effect of air pollution on cognition, and some of its effects appear to be sex-specific [90].
Considering all the aforementioned potential limitations, the results from the present study were consistent with a protective effect of the APOE ε2 allele on verbal memory, especially among women, which diminished with age. That would explain the protective effect at baseline, the trend at follow-up, and the absence of a longitudinal effect. Among men, the protective effect was absent or substantially smaller, and a combination of biases in the cognitive profile of dropouts (i.e., participants in the longitudinal phase presented higher cognitive performances at baseline) and RTM effects could explain the apparently adverse longitudinal effects of ε2.
4. Materials and Methods
4.1. Sample/Participants
The Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) Neuropsychology Study is a longitudinal, prospective population-based study [47]. The study was conducted from March 2007 to May 2017 at the Hospital Universitari Germans Trias i Pujol in Badalona, Spain. Briefly, 933 participants over 50 years of age, with a moderate–to–high vascular risk in REGICOR [92], were recruited. REGICOR is a version of the Framingham risk score adapted and validated for the Spanish population, which includes age, sex, smoking, diabetes, TC, HDL-C, and blood pressure to estimate vascular risk. Exclusion criteria included dementia (Mini-Mental State Examination score < 25), history of stroke or transient ischemic attack, coronary heart disease, chronic neurological disease, psychiatric disorder, severe disability or institutionalization, and other medical conditions that could affect cognitive assessment and function. Recruitment protocol details have been previously reported [93,94]. Participants underwent a baseline assessment between 2007 and 2010, which included cognitive and behavioral assessments, as well as the extraction of blood samples. After different quality controls, 648 individuals had baseline data on genotype and neuropsychology (see [38] for further details). At follow-up, participants underwent cognitive reassessment between April 2016 and May 2017. The study was approved by the Germans Trias i Pujol University Hospital Ethics Committee (IRB00002131). All participants gave their written consent to participate in the study.
4.2. Neuropsychological and Behavioral Assessment
Participants were assessed using a comprehensive neuropsychological battery at the baseline and 7 years later in the follow-up visit. Performance on each test (direct scores) was standardized to Z-scores, separately for each visit. Factorial analysis from baseline data previously published [95] generated three cognitive domains: (a) Visuospatial skills/speed, (b) Verbal memory, and (c) Verbal fluency (Table S7). Cognitive change in the longitudinal study was calculated as the difference in the Z-scores between visits (follow-up minus baseline). Depression was assessed at both time points with the Short Geriatric Depression Scale (GDS-15), with scores >5 considered indicative of potential depression [96].
4.3. Genetic Analyses
Extensive details of the genetic analyses have already been published [20]. Briefly, blood samples were obtained following an overnight fast and stored in a biobank at −80 °C until DNA was extracted using an ISOLATE II Blood DNA Kit (BIOLINE, London, UK). APOE (rs429358 and rs7412) and BDNF (rs6265) SNPs were genotyped using KASPar assays by an external facility (Progenika Biopharma S.A., Derio, Spain). Negative controls and sample duplicates were included for quality control. APOE genotypes from the two SNPs were recoded into the classical ε2/ε3/ε4 allele classification following the standard nomenclature. We removed samples with more than one missing genotype, and all SNPs in our sample were in Hardy–Weinberg equilibrium.
4.4. Statistical Analyses
We used an independent sample t-test to analyze sociodemographic and cognitive differences between subjects who completed both the baseline and follow-up assessments and subjects who were lost to follow-up and did not complete the follow-up assessment. Demographic and clinical data were analyzed using the Statistical Package for Social Sciences version 24 (SPSS Inc., Chicago, IL, USA).
To examine the effects of APOE genotypes on longitudinal cognitive change, we performed linear regression analyses on the difference scores (follow-up minus baseline) for each cognitive domain. Covariates included age, sex, years of education, depressive symptoms, and cardiovascular risk (as measured by the REGICOR score). Baseline cognitive performance was included as a covariate to reduce potential bias and account for regression-to-the-mean effects. Significant results were further tested using permutation procedures (1000 permutations) to confirm robustness and reduce the risk of Type I error. To evaluate cross-sectional associations between APOE genotypes and cognition, we conducted separate linear regression analyses at baseline and follow-up. We also conducted stratified analyses based on depressive symptoms (GDS > 5) to identify potential confounding effects on the observed associations. These analyses also included the same covariates. All genetic association analyses (longitudinal and cross-sectional) were performed using PLINK 1.9 ([52]; www.cog-genomics.org/plink/1.9/ accessed on 1 February 2024) under additive genetic models. APOE genotype was modeled using comparisons of ε2 vs. non-ε2 and ε4 vs. non-ε4 alleles. Interaction effects between APOE genotypes, BDNF, sex, and age were also explored.
We examined the presence of ‘regression to the mean’ (or ‘regression towards the mean’, RTM) effects in our observations to control for potential biases that can affect analyses of longitudinal data. RTM is a phenomenon that may arise when the value of a random variable is extreme (either low or high) at a sampling point; a future measurement of the variable would be closer to the mean [97]. RTM effects become more noticeable when follow-up measurements are compared between sub-samples with differences in baseline values, as in our case, where ε2 carriers showed higher verbal memory scores at baseline. RTM effects should have been partially corrected in the association analyses, since longitudinal analyses were already adjusted for the baseline score. However, this crude adjustment might not be enough under specific scenarios of interindividual variability and measurement error [98]. We conduct post hoc analyses of the significant association results from the longitudinal study, adjusting for the mean difference between baseline and follow-up scores [99]. Moreover, examination for RTM effects included plotting cognitive score change (follow-up minus baseline values) over baseline scores, stratifying by ε2 carrier status and sex.
5. Conclusions
In summary, sex is a physiological factor that contributes to changes in various biological pathways, determining whether an individual will experience impaired cognition with aging, depending on their genetic background. However, studies specifically addressing these interactions remain scarce. We emphasize the importance of carefully examining the sex-specific effects of APOE ε2 on cognitive decline and brain metabolism, structure, and function throughout the lifespan. Overall, our results are consistent with a protective effect of the APOE ε2 allele, which is especially relevant among women. However, this effect appears to diminish with age, as previously noted in some studies [100]. This may explain the remarkable effect observed in our previous cross-sectional analysis at baseline in women, with effect sizes on verbal memory of 0.83 SD (95% CI: 0.49–1.18; p-perm = 10−4) per ε2 allele carried, and its attenuation at follow-up in the current dataset, where only a weak non-significant trend remained. In contrast, among men, the adverse longitudinal effect of ε2 on verbal memory may be partially explained by regression to the mean (RTM), especially given the absence of significant effects at baseline or follow-up. Importantly, this supposed detrimental effect was more negligible (0.38 SD; 95% CI: 0.09–0.67; p-perm = 0.03) than the protective effect observed in women at baseline. The differential effect of APOE ε2 on sex warrants further investigation before firm conclusions can be drawn.
Notable limitations of this study include (1) a low number of participants with frequency of APOE ε2 alleles in the sample; (2) potential RTM effects, even after controlling for them; (3) sample loss between baseline and follow-up, with dropouts showing poorer cognitive performance, lower education level, and more vascular risk factors, especially in males; and (4) the lack of follow-up data on key modulators, such as lipid profile and cognitive reserve.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262110591/s1.
Author Contributions
Conceptualization, I.C.C., E.L.-C., P.T., M.M. (Maria Mataró) and M.V.; methodology, L.P.-S., M.B., M.M. (Maria Mataró) and M.V.; formal analysis, N.L.-V., R.D.-A., L.P.-S., G.P. and M.V.; investigation, N.L.-V., R.D.-A., J.L.-O., J.J.S.-R., J.M., E.L.-C., C.C., M.M. (Mónica Millán), P.T., G.P., M.C.-C., C.H., P.M.-A., M.P.-A., M.H.-P., T.C., A.G.F., M.M. (Maria Mataró) and M.V.; data curation, N.L.-V., J.L.-O., J.J.S.-R., J.M., E.L.-C., G.P., M.M. (Maria Mataró) and M.V.; writing—original draft, N.L.-V., R.D.-A., M.V. and M.M. (Maria Mataró); writing—review and editing, all the authors, supervision, I.C.C., E.L.-C., C.C., P.T., M.M. (Maria Mataró) and M.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants FPU014/01460 to N.L.-V., 2009FI_B00285 to J.M. from the Generalitat of Catalonia, AP2006-00311 to J.J.S.-R. from the Ministerio de Ciencia y Educación, the Regional Government of Navarre to J.L.-O., and FIS PI 15/00605 IP to E.L.-C. from the Ministerio de Ciencia e Innovación and ISCIII, and from SEJ2006-15399/PSIC, FIS PI 15/00605 IP E.L.C. and the ICREA Academia program to M.M (Maria Mataró).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Germans Trias i Pujol University Hospital Ethics Committee (protocol code PI-16-063, 29 April 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy and ethical restrictions.
Acknowledgments
The authors thank all the participants from the Barcelona AsIA Study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APOE | Apolipoprotein E |
| BDNF | Brain-derived neurotrophic factor |
| RTM | Regression to the mean |
| AD | Alzheimer’s disease |
| LOAD | Late-onset Alzheimer’s disease |
| RCFT | Rey Complex Figure Test |
| MCI | Mild cognitive impairment |
| SPON1 | Sponding-1 |
| NPS | Neuropsychological |
| GDS-15 | Short Geriatric Depression Scale |
| CI | Confidence Interval |
| LDL-C | Low-density lipoprotein cholesterol |
| TC | Total cholesterol |
| VLDL | Very low-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| CBF | Cerebral blood flow |
| CRF | Cardiorespiratory fitness |
| SNPs | Single-nucleotide polymorphisms |
References
- Burton, R.L.; O’Connell, M.E.; Morgan, D.G. Cognitive and Neuropsychiatric Correlates of Functional Impairment Across the Continuum of No Cognitive Impairment to Dementia. Arch. Clin. Neuropsychol. 2018, 33, 795–807. Available online: https://academic.oup.com/acn/article/33/7/795/4668722 (accessed on 3 April 2025). [CrossRef]
- Dexter, M.; Ossmy, O. The effects of typical ageing on cognitive control: Recent advances and future directions. Front. Aging Neurosci. 2023, 15, 1231410. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1231410/full (accessed on 3 April 2025). [CrossRef]
- Suri, S.; Heise, V.; Trachtenberg, A.J.; Mackay, C.E. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci. Biobehav. Rev. 2013, 37, 2878–2886. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0149763413002340 (accessed on 3 April 2025). [CrossRef]
- Fan, J.; Tao, W.; Li, X.; Li, H.; Zhang, J.; Wei, D.; Chen, Y.; Zhang, Z.L. The Contribution of Genetic Factors to Cognitive Impairment and Dementia: Apolipoprotein E Gene, Gene Interactions, and Polygenic Risk. Int. J. Mol. Sci. 2019, 20, 1177. Available online: https://www.mdpi.com/1422-0067/20/5/1177 (accessed on 4 April 2025). [CrossRef] [PubMed]
- Belloy, M.E.; Andrews, S.J.; Le Guen, Y.; Cuccaro, M.; Farrer, L.A.; Napolioni, V.; Greicius, M.D. APOE Genotype and Alzheimer Disease Risk Across Age, Sex, and Population Ancestry. JAMA Neurol. 2023, 80, 1284. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2811630 (accessed on 4 April 2025). [CrossRef] [PubMed]
- de Rojas, I.; Moreno-Grau, S.; Tesi, N.; Grenier-Boley, B.; Andrade, V.; Jansen, I.E.; Pedersen, N.L.; Stringa, N.; Zettergren, A.; Hernández, I.; et al. Common variants in Alzheimer’s disease and risk stratification by polygenic risk scores. Nat. Commun. 2021, 12, 3417. Available online: https://www.nature.com/articles/s41467-021-22491-8 (accessed on 4 April 2025). [CrossRef] [PubMed]
- Tuminello, E.R.; Han, S. The apolipoprotein e antagonistic pleiotropy hypothesis: Review and recommendations. Int. J. Alzheimer’s Dis. 2011, 2011, 726197. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. Available online: https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-022-00566-4 (accessed on 4 April 2025). [CrossRef]
- O’Donoghue, M.C.; Murphy, S.E.; Zamboni, G.; Nobre, A.C.; Mackay, C.E. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 2018, 104, 103–123. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0010945218301060 (accessed on 4 April 2025). [CrossRef]
- Morrison, C.; Oliver, M.D.; Berry, V.; Kamal, F.; Dadar, M. The influence of APOE status on rate of cognitive decline. GeroScience 2024, 46, 3263–3274. Available online: https://link.springer.com/10.1007/s11357-024-01069-4 (accessed on 4 April 2025). [CrossRef]
- Chen, J.; Shu, H.; Wang, Z.; Liu, D.; Shi, Y.; Xu, L.; Zhang, Z. Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. Oncotarget 2016, 7, 58789. Available online: http://www.oncotarget.com/fulltext/11289 (accessed on 4 April 2025). [CrossRef][Green Version]
- Kim, Y.J.; Seo, S.W.; Park, S.B.; Yang, J.J.; Lee, J.S.; Lee, J.; Jang, Y.K.; Kim, S.T.; Lee, K.H.; Lee, J.M.; et al. Protective effects of APOE e2 against disease progression in subcortical vascular mild cognitive impairment patients: A three-year longitudinal study. Sci. Rep. 2017, 7, 1910. Available online: http://www.nature.com/articles/s41598-017-02046-y (accessed on 4 April 2025). [CrossRef]
- Sinclair, L.I.; Pleydell-Pearce, C.W.; Day, I.N.M. Possible positive effect of the APOE ε2 allele on cognition in early to mid-adult life. Neurobiol. Learn. Mem. 2017, 146, 37–46. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1074742717301582 (accessed on 5 April 2025). [CrossRef]
- Wang, S.-M.; Kang, D.W.; Um, Y.H.; Kim, S.; Kim, R.E.Y.; Kim, D.; Lee, C.U.; Lim, H.K. Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition. Int. J. Mol. Sci. 2023, 24, 11250. Available online: https://www.mdpi.com/1422-0067/24/14/11250 (accessed on 5 April 2025). [CrossRef]
- Kim, H.; Devanand, D.P.; Carlson, S.; Goldberg, T.E. Apolipoprotein E Genotype e2: Neuroprotection and Its Limits. Front. Aging Neurosci. 2022, 14, 919712. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2022.919712/full (accessed on 5 April 2025). [CrossRef]
- Wood, M.E.; Xiong, L.Y.; Wong, Y.Y.; Buckley, R.F.; Swardfager, W.; Masellis, M.; Lim, A.S.P.; Nichols, E.; Joie, R.; Casaletto, K.B.; et al. Sex differences in associations between APOE ε2 and longitudinal cognitive decline. Alzheimer’s Dement. 2023, 19, 4651–4661. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13036 (accessed on 6 April 2025). [CrossRef]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. Available online: https://www.nature.com/articles/ng1934 (accessed on 7 April 2025). [CrossRef] [PubMed]
- Hobel, Z.; Isenberg, A.L.; Raghupathy, D.; Mack, W.; Pa, J. APOE ε4 Gene Dose and Sex Effects on Alzheimer’s Disease MRI Biomarkers in Older Adults with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2019, 71, 647–658. Available online: https://journals.sagepub.com/doi/10.3233/JAD-180859?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 8 April 2025). [CrossRef] [PubMed]
- Walters, S.; Contreras, A.G.; Eissman, J.M.; Mukherjee, S.; Lee, M.L.; Choi, S.-E.; Scollard, P.; Trittschuh, E.H.; Mez, J.B.; Bush, W.S.; et al. Associations of Sex, Race, and Apolipoprotein E Alleles With Multiple Domains of Cognition Among Older Adults. JAMA Neurol. 2023, 80, 929. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2806771 (accessed on 8 April 2025). [CrossRef]
- Srisaikaew, P.; Chad, J.A.; Mahakkanukrauh, P.; Anderson, N.D.; Chen, J.J. Effect of sex on the APOE4-aging interaction in the white matter microstructure of cognitively normal older adults using diffusion-tensor MRI with orthogonal-tensor decomposition (DT-DOME). Front. Neurosci. 2023, 17, 1049609. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2023.1049609/full (accessed on 8 April 2025).
- Gilsanz, P.; Lee, C.; Corrada, M.M.; Kawas, C.H.; Quesenberry, C.P.; Whitmer, R.A. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology 2019, 92, e2005–e2014. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000007326 (accessed on 8 April 2025). [CrossRef] [PubMed]
- Moutinho, S. Women twice as likely to develop Alzheimer’s disease as men—But scientists do not know why. Nat. Med. 2025, 31, 704–707. Available online: https://www.nature.com/articles/s41591-025-03564-3 (accessed on 8 April 2025). [CrossRef]
- Bove, R.; Secor, E.; Chibnik, L.B.; Barnes, L.L.; Schneider, J.A.; Bennett, D.A.; De Jager, P.L. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014, 82, 222–229. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000000033 (accessed on 8 April 2025). [CrossRef] [PubMed]
- Mervosh, N.; Devi, G. Estrogen, menopause, and Alzheimer’s disease: Understanding the link to cognitive decline in women. Front. Mol. Biosci. 2025, 12, 1634302. Available online: https://www.frontiersin.org/articles/10.3389/fmolb.2025.1634302/full (accessed on 10 April 2025). [CrossRef]
- Mosconi, L.; Nerattini, M.; Matthews, D.C.; Jett, S.; Andy, C.; Williams, S.; Yepez, C.B.; Zarate, C.; Carlton, C.; Fauci, F.; et al. In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology. Sci. Rep. 2024, 14, 12680. Available online: https://www.nature.com/articles/s41598-024-62820-7 (accessed on 10 April 2025). [CrossRef]
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Varsavsky, I.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Sex differences in Alzheimer risk. Neurology 2017, 89, 1382–1390. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000004425 (accessed on 10 April 2025). [CrossRef]
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Osorio, R.S.; Connaughty, C.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS ONE 2017, 12, e0185926. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29016679 (accessed on 10 April 2025). [CrossRef]
- Mishra, A.; Wang, Y.; Yin, F.; Vitali, F.; Rodgers, K.E.; Soto, M.; Mosconi, L.; Wang, T.; Brinton, R.D. A tale of two systems: Lessons learned from female mid-life aging with implications for Alzheimer’s prevention & treatment. Ageing Res. Rev. 2022, 74, 101542. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1568163721002890 (accessed on 12 April 2025). [PubMed]
- Jett, S.; Dyke, J.P.; Andy, C.; Schelbaum, E.; Jang, G.; Boneu Yepez, C.; Pahlajani, S.; Diaz, I.; Brinton, R.D.; Mosconi, L. Sex and menopause impact 31P-Magnetic Resonance Spectroscopy brain mitochondrial function in association with 11C-PiB PET amyloid-beta load. Sci. Rep. 2022, 12, 22087. Available online: https://www.nature.com/articles/s41598-022-26573-5 (accessed on 12 April 2025). [CrossRef] [PubMed]
- Babapour Mofrad, R.; Tijms, B.M.; Scheltens, P.; Barkhof, F.; van der Flier, W.M.; Sikkes, S.A.M.; Teunissen, C.E. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 2020, 95, e2378–e2388. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000010629 (accessed on 14 April 2025). [CrossRef]
- Sundermann, E.E.; Tran, M.; Maki, P.M.; Bondi, M.W. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer’s disease markers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 438–447. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1016/j.dadm.2018.06.004 (accessed on 14 April 2025). [CrossRef]
- Arnold, M.; Nho, K.; Kueider-Paisley, A.; Massaro, T.; Huynh, K.; Brauner, B.; MahmoudianDehkordi, S.; Louie, G.; Moseley, M.A.; Thompson, J.W.; et al. Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 2020, 11, 1148. Available online: https://www.nature.com/articles/s41467-020-14959-w (accessed on 14 April 2025). [CrossRef]
- Xu, X.; Kwon, J.; Yan, R.; Apio, C.; Song, S.; Heo, G.; Yang, Q.; Timsina, J.; Liu, M.; Budde, J.; et al. Sex Differences in Apolipoprotein E and Alzheimer Disease Pathology Across Ancestries. JAMA Netw. Open 2025, 8, e250562. Available online: http://www.ncbi.nlm.nih.gov/pubmed/40067298 (accessed on 14 April 2025). [CrossRef]
- Boccalini, C.; Peretti, D.E.; Scheffler, M.; Mu, L.; Griffa, A.; Testart, N.; Allali, G.; Prior, J.O.; Ashton, N.J.; Zetterberg, H.; et al. Sex differences in the association of Alzheimer’s disease biomarkers and cognition in a multicenter memory clinic study. Alzheimer’s Res. Ther. 2025, 17, 46. Available online: http://www.ncbi.nlm.nih.gov/pubmed/39966925 (accessed on 15 April 2025). [CrossRef]
- Nemes, S.; Logan, P.E.; Manchella, M.K.; Mundada, N.S.; La Joie, R.; Polsinelli, A.J.; Hammers, D.B.; Koeppe, R.A.; Foroud, T.M.; Nudelman, K.N.; et al. Sex and APOE ε4 carrier effects on atrophy, amyloid PET, and tau PET burden in early-onset Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, S49–S63. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13403 (accessed on 15 April 2025). [CrossRef] [PubMed]
- Hyman, B.T.; Gomez-Isla, T.; Briggs, M.; Chung, H.; Nichols, S.; Kohout, F.; Wallace, R. Apolipoprotein E and cognitive change in an elderly population. Ann. Neurol. 1996, 40, 55–66. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ana.410400111 (accessed on 15 April 2025). [CrossRef] [PubMed]
- Davies, G.; Armstrong, N.; Bis, J.C.; Bressler, J.; Chouraki, V.; Giddaluru, S.; Hofer, E.; Ibrahim-Verbaas, C.A.; Kirin, M.; Lahti, J.; et al. Genetic contributions to variation in general cognitive function: A meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53,949). Mol. Psychiatry 2015, 20, 183–192. Available online: https://www.nature.com/articles/mp2014188 (accessed on 15 April 2025). [CrossRef]
- Lamonja-Vicente, N.; Dacosta-Aguayo, R.; López-Olóriz, J.; Prades-Senovilla, L.; Roig-Coll, F.; Castells-Sánchez, A.; Soriano-Raya, J.J.; Clemente, I.; Miralbell, J.; Barrios, M.; et al. Sex-Specific Protective Effects of APOE ε2 on Cognitive Performance. J. Gerontol. Ser. A 2021, 76, 41–49. Available online: https://academic.oup.com/biomedgerontology/article/76/1/41/5913014 (accessed on 17 April 2025). [CrossRef] [PubMed]
- Reas, E.T.; Laughlin, G.A.; Bergstrom, J.; Kritz-Silverstein, D.; Barrett-Connor, E.; McEvoy, L.K. Effects of APOE on cognitive aging in community-dwelling older adults. Neuropsychology 2019, 33, 406–416. Available online: http://doi.apa.org/getdoi.cfm?doi=10.1037/neu0000501 (accessed on 17 April 2025). [CrossRef]
- Dessy, T.; Barhdadi, A.; Cyr, M.-C.; Sandoval, J.; Bherer, L.; Rouleau, J.; Provost, S.; Lemieux Perreault, L.P.; Sylvestre, M.P.; Gagliano Taliun, S.A.; et al. Disentangling the effects of sex and gender on APOE ε4-related neurocognitive impairment. Alzheimer’s Dement. 2025, 17, e70111. Available online: http://www.ncbi.nlm.nih.gov/pubmed/40352685 (accessed on 17 April 2025). [CrossRef]
- Burns, A.P.; Fortel, I.; Zhan, L.; Lazarov, O.; Mackin, R.S.; Demos, A.P.; Bendlin, B.; Leow, A. Longitudinal excitation-inhibition balance altered by sex and APOE-ε4. Commun. Biol. 2025, 8, 488. Available online: https://www.nature.com/articles/s42003-025-07876-5 (accessed on 17 April 2025). [CrossRef]
- Barabash, A.; Marcos, A.; Ancín, I.; Vázquez-Alvarez, B.; de Ugarte, C.; Gil, P.; Fernández, C.; Encinas, M.; López-Ibor, J.J.; Cabranes, J.A. APOE, ACT and CHRNA7 genes in the conversion from amnestic mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1254–1264. Available online: https://linkinghub.elsevier.com/retrieve/pii/S019745800700423X (accessed on 18 April 2025). [CrossRef]
- Bretsky, P.; Guralnik, J.M.; Launer, L.; Albert, M.; Seeman, T.E. The role of APOE-ε4 in longitudinal cognitive decline. Neurology 2003, 60, 1077–1081. Available online: https://www.neurology.org/doi/10.1212/01.WNL.0000055875.26908.24 (accessed on 18 April 2025). [CrossRef]
- Chuang, Y.-F.; Hayden, K.M.; Norton, M.C.; Tschanz, J.; Breitner, J.C.S.; Welsh-Bohmer, K.A.; Zandi, P.P. Association between APOE ε4 Allele and Vascular Dementia: The Cache County Study. Dement. Geriatr. Cogn. Disord. 2010, 29, 248–253. Available online: https://karger.com/article/doi/10.1159/000285166 (accessed on 20 April 2025). [CrossRef]
- Konishi, K.; Bhat, V.; Banner, H.; Poirier, J.; Joober, R.; Bohbot, V.D. APOE2 Is Associated with Spatial Navigational Strategies and Increased Gray Matter in the Hippocampus. Front. Hum. Neurosci. 2016, 10, 349. Available online: http://journal.frontiersin.org/Article/10.3389/fnhum.2016.00349/abstract (accessed on 20 April 2025). [CrossRef]
- Sweigart, B.; Andersen, S.L.; Gurinovich, A.; Cosentino, S.; Schupf, N.; Perls, T.T.; Sebastiani, P. APOE E2/E2 Is Associated with Slower Rate of Cognitive Decline with Age. J. Alzheimer’s Dis. 2021, 83, 853–860. Available online: https://journals.sagepub.com/doi/full/10.3233/JAD-201205 (accessed on 20 April 2025). [CrossRef] [PubMed]
- Tiraboschi, P.; Hansen, L.A.; Masliah, E.; Alford, M.; Thal, L.J.; Corey-Bloom, J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 2004, 62, 1977–1983. Available online: https://www.neurology.org/doi/10.1212/01.WNL.0000128091.92139.0F (accessed on 21 April 2025). [CrossRef] [PubMed]
- Conejero-Goldberg, C.; Gomar, J.J.; Bobes-Bascaran, T.; Hyde, T.M.; Kleinman, J.E.; Herman, M.M.; Chen, S.; Davies, P.; Goldberg, T.E. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol. Psychiatry 2014, 19, 1243–1250. Available online: https://www.nature.com/articles/mp2013194 (accessed on 22 April 2025). [CrossRef] [PubMed]
- Serrano-Pozo, A.; Qian, J.; Monsell, S.E.; Betensky, R.A.; Hyman, B.T. APOE ε2 is associated with milder clinical and pathological Alzheimer’s disease. Ann. Neurol. 2015, 77, 917–929. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ana.24369 (accessed on 22 April 2025). [CrossRef]
- Lancaster, C.; Tabet, N.; Rusted, J. The APOE paradox: Do attentional control differences in mid-adulthood reflect risk of late-life cognitive decline. Neurobiol. Aging 2016, 48, 114–121. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0197458016301932 (accessed on 22 April 2025). [CrossRef]
- Marioni, R.E.; Campbell, A.; Scotland, G.; Hayward, C.; Porteous, D.J.; Deary, I.J. Differential effects of the APOE e4 allele on different domains of cognitive ability across the life-course. Eur. J. Hum. Genet. 2016, 24, 919–923. [Google Scholar] [CrossRef]
- Alexander, D.M.; Williams, L.M.; Gatt, J.M.; Dobson-Stone, C.; Kuan, S.A.; Todd, E.G.; Schofield, P.R.; Cooper, N.J.; Gordon, E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol. Psychol. 2007, 75, 229–238. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0301051107000476 (accessed on 22 April 2025). [CrossRef]
- Batterham, P.J.; Bunce, D.; Cherbuin, N.; Christensen, H. Apolipoprotein E ε4 and Later-Life Decline in Cognitive Function and Grip Strength. Am. J. Geriatr. Psychiatry 2013, 21, 1010–1019. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1064748113000407 (accessed on 24 April 2025). [CrossRef]
- Schiepers, O.J.G.; Harris, S.E.; Gow, A.J.; Pattie, A.; Brett, C.E.; Starr, J.M.; Deary, I.J. APOE E4 status predicts age-related cognitive decline in the ninth decade: Longitudinal follow-up of the Lothian Birth Cohort 1921. Mol. Psychiatry 2012, 17, 315–324. Available online: https://www.nature.com/articles/mp2010137 (accessed on 24 April 2025). [CrossRef]
- Fernandez, S.; Burnham, S.C.; Milicic, L.; Savage, G.; Maruff, P.; Peretti, M.; Sohrabi, H.R.; Lim, Y.Y.; Weinborn, M.; Ames, D.; et al. SPON1 Is Associated with Amyloid-β and APOE ε4-Related Cognitive Decline in Cognitively Normal Adults. J. Alzheimer’s Dis. Rep. 2021, 5, 111–120. Available online: https://journals.sagepub.com/doi/10.3233/ADR-200246 (accessed on 24 April 2025). [CrossRef]
- Lim, Y.Y.; Villemagne, V.L.; Laws, S.M.; Ames, D.; Pietrzak, R.H.; Ellis, K.A.; Harrington, K.D.; Bourgeat, P.; Salvado, O.; Darby, D.; et al. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2457–2464. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0197458013002042 (accessed on 24 April 2025). [CrossRef]
- Lim, Y.Y.; Hassenstab, J.; Cruchaga, C.; Goate, A.; Fagan, A.M.; Benzinger, T.L.S.; Maruff, P.; Snyder, P.J.; Masters, C.L.; Allegri, R.; et al. BDNF Val66Met moderates memory impairment, hippocampal function and tau in preclinical autosomal dominant Alzheimer’s disease. Brain 2016, 139, 2766–2777. Available online: https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/aww200 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Zhang, Y.; Jin, X.; Lutz, M.W.; Ju, S.-Y.; Liu, K.; Guo, G.; Zeng, Y.; Yao, Y. Interaction between APOE ε4 and dietary protein intake on cognitive decline: A longitudinal cohort study. Clin. Nutr. 2021, 40, 2716–2725. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0261561421001436 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Levy, B.R.; Slade, M.D.; Pietrzak, R.H.; Ferrucci, L. When Culture Influences Genes: Positive Age Beliefs Amplify the Cognitive-Aging Benefit of APOE ε2. J. Gerontol. Ser. B 2020, 75, e198–e203. Available online: https://academic.oup.com/psychsocgerontology/article/75/8/e198/5896592 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Stringa, N.; van Schoor, N.M.; Milaneschi, Y.; Ikram, M.A.; Del Panta, V.; Koolhaas, C.M.; Voortman, T.; Bandinelli, S.; Wolters, F.J.; Huisman, M. Physical Activity as Moderator of the Association Between APOE and Cognitive Decline in Older Adults: Results from Three Longitudinal Cohort Studies. J. Gerontol. Ser. A 2020, 75, 1880–1886. Available online: https://academic.oup.com/biomedgerontology/article/75/10/1880/5763509 (accessed on 26 April 2025). [CrossRef]
- Kim, R.; Park, S.; Yoo, D.; Jun, J.-S.; Jeon, B. Association of Physical Activity and APOE Genotype with Longitudinal Cognitive Change in Early Parkinson Disease. Neurology 2021, 96, e2429–e2437. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000011852 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Xiang, Q.; Andersen, S.L.; Perls, T.T.; Sebastiani, P. Studying the Interplay Between Apolipoprotein E and Education on Cognitive Decline in Centenarians Using Bayesian Beta Regression. Front. Genet. 2021, 11, 606831. Available online: https://www.frontiersin.org/articles/10.3389/fgene.2020.606831/full (accessed on 27 April 2025). [CrossRef]
- Ji, S.; Kang, J.; Han, C.; Xu, X.; Chen, M.; Chen, J.; Chhetri, J.K.; Pan, J.; Chan, P. Potential role of APOE ε4 allele as a modifier for the association of BDNF Val66Met polymorphisms and cognitive impairment in community-dwelling older adults. Front. Aging Neurosci. 2024, 16, 1330193. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1330193/full (accessed on 27 April 2025). [CrossRef]
- Vilor-Tejedor, N.; Operto, G.; Evans, T.E.; Falcon, C.; Crous-Bou, M.; Minguillón, C.; Cacciaglia, R.; Milà-Alomà, M.; Grau-Rivera, O.; Suárez-Calvet, M.; et al. Effect of BDNF Val66Met on hippocampal subfields volumes and compensatory interaction with APOE-ε4 in middle-age cognitively unimpaired individuals from the ALFA study. Brain Struct. Funct. 2020, 225, 2331–2345. Available online: https://link.springer.com/10.1007/s00429-020-02125-3 (accessed on 1 May 2025). [CrossRef] [PubMed]
- Edmunds, K.J.; Pandos, A.A.; Hoang, I.; Mamlouk, G.M.; Motovylyak, A.; Lose, S.R.; Asthana, S.; Stremlau, M.; Johnson, S.C.; van Praag, H.; et al. BDNF expression mediates verbal learning and memory in women in a cohort enriched with risk for Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2025, 17, e70062. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/dad2.70062 (accessed on 1 May 2025). [CrossRef]
- Cechova, K.; Andel, R.; Angelucci, F.; Chmatalova, Z.; Markova, H.; Laczó, J.; Vyhnalek, M.; Matoska, V.; Kaplan, V.; Nedelska, Z.; et al. Impact of APOE and BDNF Val66Met Gene Polymorphisms on Cognitive Functions in Patients with Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2020, 73, 247–257. Available online: https://journals.sagepub.com/doi/10.3233/JAD-190464?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 1 May 2025). [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. Available online: https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-022-00279-0 (accessed on 2 May 2025). [CrossRef]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0960076016300589 (accessed on 2 May 2025). [CrossRef] [PubMed]
- Gong, L.; Xu, R.; Liu, D.; Lan, L.; Zhang, B.; Zhang, C. The Specific Impact of Apolipoprotein E Epsilon 2 on Cognition and Brain Function in Cognitively Normal Elders and Mild Cognitive Impairment Patients. Front. Aging Neurosci. 2020, 11, 374. Available online: https://www.frontiersin.org/article/10.3389/fnagi.2019.00374/full (accessed on 2 May 2025). [CrossRef]
- Insel, P.S.; Hansson, O.; Mattsson-Carlgren, N. Association Between Apolipoprotein E ε2 vs ε4, Age, and β-Amyloid in Adults Without Cognitive Impairment. JAMA Neurol. 2021, 78, 229. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2771412 (accessed on 3 May 2025). [CrossRef] [PubMed]
- Wilson, R.S. The apolipoprotein E varepsilon2 allele and decline in episodic memory. J. Neurol. Neurosurg. Psychiatry 2002, 73, 672–677. Available online: https://jnnp.bmj.com/lookup/doi/10.1136/jnnp.73.6.672 (accessed on 3 May 2025). [CrossRef]
- Small, B.J.; Rosnick, C.B.; Fratiglioni, L.; Bäckman, L. Apolipoprotein E and Cognitive Performance: A Meta-Analysis. Psychol. Aging 2004, 19, 592–600. Available online: https://doi.apa.org/doi/10.1037/0882-7974.19.4.592 (accessed on 4 May 2025). [CrossRef] [PubMed]
- Wisdom, N.M.; Callahan, J.L.; Hawkins, K.A. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol. Aging 2011, 32, 63–74. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0197458009000384 (accessed on 4 May 2025). [CrossRef]
- Zokaei, N.; Board, A.G.; Slavkova, E.; EMackay, C.; Nobre, A.C.; Husain, M. Superior short-term memory in APOE ε2 carriers across the age range. Behav. Brain Res. 2021, 397, 112918. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0166432820306173 (accessed on 5 May 2025). [CrossRef]
- Meyer, M.R.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Steffens, D.C.; Wyse, B.W.; Breitner, J.C. APOE genotype predicts when—Not whether—One is predisposed to develop Alzheimer disease. Nat. Genet. 1998, 19, 321–322. Available online: https://www.nature.com/articles/ng0898_321 (accessed on 5 May 2025). [CrossRef]
- Shinohara, M.; Kanekiyo, T.; Tachibana, M.; Kurti, A.; Shinohara, M.; Fu, Y.; Zhao, J.; Han, X.; Sullivan, P.M.; Rebeck, G.W.; et al. APOE2 is associated with longevity independent of Alzheimer’s disease. eLife 2020, 9, e62199. Available online: https://elifesciences.org/articles/62199 (accessed on 6 May 2025). [CrossRef]
- Muros, M.; Rodríguez-Ferrer, C. Apolipoprotein E polymorphism influence on lipids, apolipoproteins and Lp(a) in a Spanish population underexpressing apo E4. Atherosclerosis 1996, 121, 13–21. Available online: https://linkinghub.elsevier.com/retrieve/pii/0021915095066438 (accessed on 6 May 2025). [CrossRef]
- Bennet, A.M.; Di Angelantonio, E.; Ye, Z.; Wensley, F.; Dahlin, A.; Ahlbom, A.; Keavney, B.; Collins, R.; Wiman, B.; de Faire, U.; et al. Association of Apolipoprotein E Genotypes With Lipid Levels and Coronary Risk. JAMA 2007, 298, 1300. Available online: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.298.11.1300 (accessed on 7 May 2025). [CrossRef]
- Reiman, E.M.; Arboleda-Velasquez, J.F.; Quiroz, Y.T.; Huentelman, M.J.; Beach, T.G.; Caselli, R.J.; Chen, Y.; Su, Y.; Myers, A.J.; Hardy, J.; et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5000-person neuropathological study. Nat. Commun. 2020, 11, 667. Available online: https://www.nature.com/articles/s41467-019-14279-8 (accessed on 8 May 2025). [CrossRef]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. Available online: https://academic.oup.com/jcem/article/96/4/885/2720832 (accessed on 8 May 2025). [CrossRef] [PubMed]
- Tehrani, F.R.; Erfani, H.; Cheraghi, L.; Tohidi, M.; Azizi, F. Lipid profiles and ovarian reserve status: A longitudinal study. Hum. Reprod. 2014, 29, 2522–2529. Available online: https://academic.oup.com/humrep/article-lookup/doi/10.1093/humrep/deu249 (accessed on 8 May 2025). [CrossRef] [PubMed]
- Tontisirin, N.; Muangman, S.L.; Suz, P.; Pihoker, C.; Fisk, D.; Moore, A.; Lam, A.M.; Vavilala, M.S. Early Childhood Gender Differences in Anterior and Posterior Cerebral Blood Flow Velocity and Autoregulation. Pediatrics 2007, 119, e610–e615. Available online: https://publications.aap.org/pediatrics/article/119/3/e610/70449/Early-Childhood-Gender-Differences-in-Anterior-and (accessed on 8 May 2025). [CrossRef]
- Satterthwaite, T.D.; Shinohara, R.T.; Wolf, D.H.; Hopson, R.D.; Elliott, M.A.; Vandekar, S.N.; Ruparel, K.; Calkins, M.E.; Roalf, D.R.; Gennatas, E.D.; et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc. Natl. Acad. Sci. USA 2014, 111, 8643–8648. Available online: https://pnas.org/doi/full/10.1073/pnas.1400178111 (accessed on 10 May 2025). [CrossRef] [PubMed]
- Rodriguez, G.; Warkentin, S.; Risberg, J.; Rosadini, G. Sex Differences in Regional Cerebral Blood Flow. J. Cereb. Blood Flow Metab. 1988, 8, 783–789. Available online: https://journals.sagepub.com/doi/10.1038/jcbfm.1988.133?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 10 May 2025). [CrossRef]
- Sunday, L.; Osuna, C.; Krause, D.N.; Duckles, S.P. Age alters cerebrovascular inflammation and effects of estrogen. Am. J. Physiol. Circ. Physiol. 2007, 292, H2333–H2340. Available online: https://www.physiology.org/doi/10.1152/ajpheart.01057.2006 (accessed on 10 May 2025). [CrossRef]
- Abi-Ghanem, C.; Robison, L.S.; Zuloaga, K.L. Androgens’ effects on cerebrovascular function in health and disease. Biol. Sex Differ. 2020, 11, 35. Available online: https://bsd.biomedcentral.com/articles/10.1186/s13293-020-00309-4 (accessed on 11 May 2025). [CrossRef]
- Banica, T.; Verroken, C.; Reyns, T.; Mahmoud, A.; T’Sjoen, G.; Fiers, T.; Kaufman, J.M.; Lapauw, B. Early Decline of Androgen Levels in Healthy Adult Men: An Effect of Aging Per Se? A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, e1074–e1083. Available online: https://academic.oup.com/jcem/article/106/4/e1074/6056486 (accessed on 11 May 2025). [CrossRef]
- Cai, Z.; Li, H. An Updated Review: Androgens and Cognitive Impairment in Older Men. Front. Endocrinol. 2020, 11, 586909. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33281745 (accessed on 12 May 2025). [CrossRef]
- Lim, Y.Y.; Villemagne, V.L.; Laws, S.M.; Pietrzak, R.H.; Snyder, P.J.; Ames, D.; Ellis, K.A.; Harrington, K.; Rembach, A.; Martins, R.N.; et al. APOE and BDNF polymorphisms moderate amyloid β-related cognitive decline in preclinical Alzheimer’s disease. Mol. Psychiatry 2015, 20, 1322–1328. Available online: http://www.nature.com/articles/mp2014123 (accessed on 12 May 2025). [CrossRef]
- Cativiela-Campos, B.; Ruiz-Sobremazas, D.; Rodulfo-Cárdenas, R.; Barrasa, A.; Sánchez-Santed, F.; Colomina, M.T.; Aschner, M.; López-Granero, C. What are the consequences of PM air pollution exposure on elderly behavior? A systematic review. Environ. Pollut. 2025, 375, 126279. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0269749125006529 (accessed on 12 May 2025). [CrossRef] [PubMed]
- Roig-Coll, F.; Castells-Sánchez, A.; Lamonja-Vicente, N.; Torán-Monserrat, P.; Pera, G.; García-Molina, A.; Tormos, J.M.; Montero-Alía, P.; Alzamora, M.T.; Dacosta-Aguayo, R.; et al. Effects of Aerobic Exercise, Cognitive and Combined Training on Cognition in Physically Inactive Healthy Late-Middle-Aged Adults: The Projecte Moviment Randomized Controlled Trial. Front. Aging Neurosci. 2020, 12, 590168. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2020.590168/full (accessed on 12 May 2025). [CrossRef] [PubMed]
- Marrugat, J.; Subirana, I.; Comin, E.; Cabezas, C.; Vila, J.; Elosua, R.; Nam, B.H.; Ramos, R.; Sala, J.; Solanas, P.; et al. Validity of an adaptation of the Framingham cardiovascular risk function: The VERIFICA study. J. Epidemiol. Community Health 2007, 61, 40–47. Available online: http://jech.bmj.com/cgi/doi/10.1136/jech.2005.038505 (accessed on 12 May 2025). [CrossRef]
- López-Cancio, E.; Dorado, L.; Millán, M.; Reverté, S.; Suñol, A.; Massuet, A.; Mataró, M.; Galán, A.; Alzamora, M.; Pera, G.; et al. The population-based Barcelona-Asymptomatic Intracranial Atherosclerosis Study (ASIA): Rationale and design. BMC Neurol. 2011, 11, 22. Available online: http://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-11-22 (accessed on 12 May 2025). [CrossRef] [PubMed]
- Crespo-Cuevas, A.M.; Canento, T.; Hernández-Perez, M.; Cáceres, C.; González, A.; Ispierto, L.; Mataró, M.; Vilas, D.; Planas-Ballvé, A.; Martin, L.; et al. The Barcelona-Asymptomatic Intracranial Atherosclerosis (AsIA) study: Subclinical cervico-cerebral stenosis and middle cerebral artery pulsatility index as predictors of long-term incident cognitive impairment. Atherosclerosis 2020, 312, 104–109. Available online: https://linkinghub.elsevier.com/retrieve/pii/S002191502030469X (accessed on 13 May 2025). [CrossRef]
- Miralbell, J.; López-Cancio, E.; López-Oloriz, J.; Arenillas, J.F.; Barrios, M.; Soriano-Raya, J.J.; Galán, A.; Cáceres, C.; Alzamora, M.; Pera, G.; et al. Cognitive Patterns in Relation to Biomarkers of Cerebrovascular Disease and Vascular Risk Factors. Cerebrovasc. Dis. 2013, 36, 98–105. Available online: https://karger.com/article/doi/10.1159/000352059 (accessed on 14 May 2025). [CrossRef]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. Available online: https://www.tandfonline.com/doi/full/10.1300/J018v05n01_09 (accessed on 14 May 2025). [CrossRef]
- Barnett, A.G. Regression to the mean: What it is and how to deal with it. Int. J. Epidemiol. 2004, 34, 215–220. Available online: https://academic.oup.com/ije/article-lookup/doi/10.1093/ije/dyh299 (accessed on 14 May 2025). [CrossRef] [PubMed]
- Chiolero, A.; Paradis, G.; Rich, B.; Hanley, J.A. Assessing the Relationship between the Baseline Value of a Continuous Variable and Subsequent Change Over Time. Front. Public Health 2013, 1, 29. Available online: http://journal.frontiersin.org/article/10.3389/fpubh.2013.00029/abstract (accessed on 15 May 2025). [CrossRef]
- Oldham, P.D. A note on the analysis of repeated measurements of the same subjects. J. Chronic. Dis. 1962, 15, 969–977. Available online: https://linkinghub.elsevier.com/retrieve/pii/0021968162901169 (accessed on 15 May 2025). [CrossRef]
- Corrada, M.M.; Paganini-Hill, A.; Berlau, D.J.; Kawas, C.H. Apolipoprotein E genotype, dementia, and mortality in the oldest old: The 90+ Study. Alzheimer’s Dement. 2013, 9, 12–18. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1016/j.jalz.2011.12.004 (accessed on 15 May 2025). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).