Sex and APOE ε2 Interactive Effects on the Longitudinal Change in Cognition in a Population-Based Cohort of Older Adults with Vascular Risk Factors
Abstract
1. Introduction
2. Results
2.1. Subjects
2.2. Genotype and Cognitive Domains
2.3. Cross-Sectional Analyses
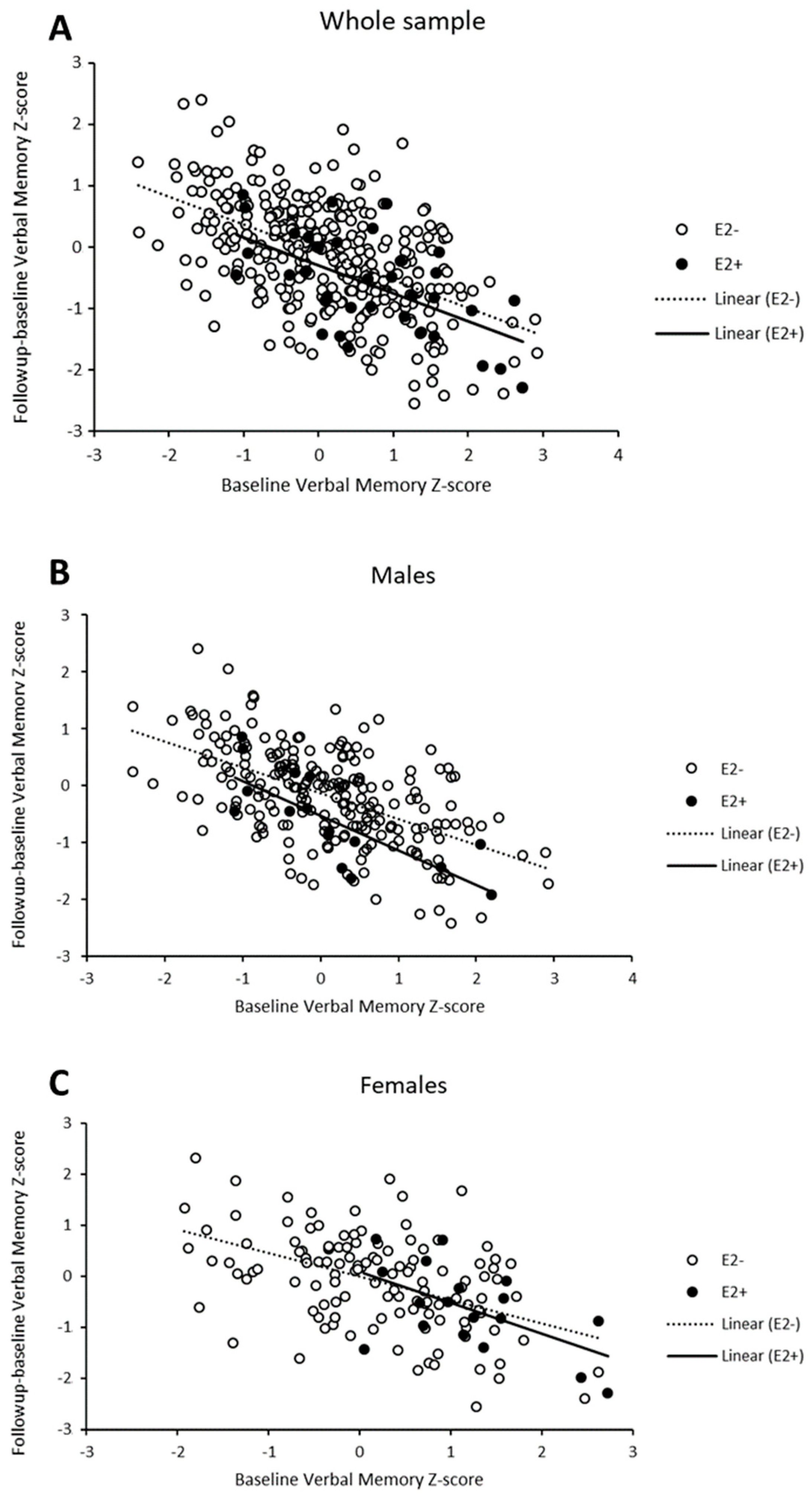
Regression to the Mean
2.4. Gene*Age and Gene*Gene Interactions
3. Discussion
4. Materials and Methods
4.1. Sample/Participants
4.2. Neuropsychological and Behavioral Assessment
4.3. Genetic Analyses
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APOE | Apolipoprotein E |
| BDNF | Brain-derived neurotrophic factor |
| RTM | Regression to the mean |
| AD | Alzheimer’s disease |
| LOAD | Late-onset Alzheimer’s disease |
| RCFT | Rey Complex Figure Test |
| MCI | Mild cognitive impairment |
| SPON1 | Sponding-1 |
| NPS | Neuropsychological |
| GDS-15 | Short Geriatric Depression Scale |
| CI | Confidence Interval |
| LDL-C | Low-density lipoprotein cholesterol |
| TC | Total cholesterol |
| VLDL | Very low-density lipoprotein |
| HDL-C | High-density lipoprotein cholesterol |
| CBF | Cerebral blood flow |
| CRF | Cardiorespiratory fitness |
| SNPs | Single-nucleotide polymorphisms |
References
- Burton, R.L.; O’Connell, M.E.; Morgan, D.G. Cognitive and Neuropsychiatric Correlates of Functional Impairment Across the Continuum of No Cognitive Impairment to Dementia. Arch. Clin. Neuropsychol. 2018, 33, 795–807. Available online: https://academic.oup.com/acn/article/33/7/795/4668722 (accessed on 3 April 2025). [CrossRef]
- Dexter, M.; Ossmy, O. The effects of typical ageing on cognitive control: Recent advances and future directions. Front. Aging Neurosci. 2023, 15, 1231410. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1231410/full (accessed on 3 April 2025). [CrossRef]
- Suri, S.; Heise, V.; Trachtenberg, A.J.; Mackay, C.E. The forgotten APOE allele: A review of the evidence and suggested mechanisms for the protective effect of APOE ε2. Neurosci. Biobehav. Rev. 2013, 37, 2878–2886. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0149763413002340 (accessed on 3 April 2025). [CrossRef]
- Fan, J.; Tao, W.; Li, X.; Li, H.; Zhang, J.; Wei, D.; Chen, Y.; Zhang, Z.L. The Contribution of Genetic Factors to Cognitive Impairment and Dementia: Apolipoprotein E Gene, Gene Interactions, and Polygenic Risk. Int. J. Mol. Sci. 2019, 20, 1177. Available online: https://www.mdpi.com/1422-0067/20/5/1177 (accessed on 4 April 2025). [CrossRef] [PubMed]
- Belloy, M.E.; Andrews, S.J.; Le Guen, Y.; Cuccaro, M.; Farrer, L.A.; Napolioni, V.; Greicius, M.D. APOE Genotype and Alzheimer Disease Risk Across Age, Sex, and Population Ancestry. JAMA Neurol. 2023, 80, 1284. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2811630 (accessed on 4 April 2025). [CrossRef] [PubMed]
- de Rojas, I.; Moreno-Grau, S.; Tesi, N.; Grenier-Boley, B.; Andrade, V.; Jansen, I.E.; Pedersen, N.L.; Stringa, N.; Zettergren, A.; Hernández, I.; et al. Common variants in Alzheimer’s disease and risk stratification by polygenic risk scores. Nat. Commun. 2021, 12, 3417. Available online: https://www.nature.com/articles/s41467-021-22491-8 (accessed on 4 April 2025). [CrossRef] [PubMed]
- Tuminello, E.R.; Han, S. The apolipoprotein e antagonistic pleiotropy hypothesis: Review and recommendations. Int. J. Alzheimer’s Dis. 2011, 2011, 726197. [Google Scholar] [CrossRef]
- Fernández-Calle, R.; Konings, S.C.; Frontiñán-Rubio, J.; García-Revilla, J.; Camprubí-Ferrer, L.; Svensson, M.; Martinson, I.; Boza-Serrano, A.; Venero, J.L.; Nielsen, H.M.; et al. APOE in the bullseye of neurodegenerative diseases: Impact of the APOE genotype in Alzheimer’s disease pathology and brain diseases. Mol. Neurodegener. 2022, 17, 62. Available online: https://molecularneurodegeneration.biomedcentral.com/articles/10.1186/s13024-022-00566-4 (accessed on 4 April 2025). [CrossRef]
- O’Donoghue, M.C.; Murphy, S.E.; Zamboni, G.; Nobre, A.C.; Mackay, C.E. APOE genotype and cognition in healthy individuals at risk of Alzheimer’s disease: A review. Cortex 2018, 104, 103–123. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0010945218301060 (accessed on 4 April 2025). [CrossRef]
- Morrison, C.; Oliver, M.D.; Berry, V.; Kamal, F.; Dadar, M. The influence of APOE status on rate of cognitive decline. GeroScience 2024, 46, 3263–3274. Available online: https://link.springer.com/10.1007/s11357-024-01069-4 (accessed on 4 April 2025). [CrossRef]
- Chen, J.; Shu, H.; Wang, Z.; Liu, D.; Shi, Y.; Xu, L.; Zhang, Z. Protective effect of APOE epsilon 2 on intrinsic functional connectivity of the entorhinal cortex is associated with better episodic memory in elderly individuals with risk factors for Alzheimer’s disease. Oncotarget 2016, 7, 58789. Available online: http://www.oncotarget.com/fulltext/11289 (accessed on 4 April 2025). [CrossRef][Green Version]
- Kim, Y.J.; Seo, S.W.; Park, S.B.; Yang, J.J.; Lee, J.S.; Lee, J.; Jang, Y.K.; Kim, S.T.; Lee, K.H.; Lee, J.M.; et al. Protective effects of APOE e2 against disease progression in subcortical vascular mild cognitive impairment patients: A three-year longitudinal study. Sci. Rep. 2017, 7, 1910. Available online: http://www.nature.com/articles/s41598-017-02046-y (accessed on 4 April 2025). [CrossRef]
- Sinclair, L.I.; Pleydell-Pearce, C.W.; Day, I.N.M. Possible positive effect of the APOE ε2 allele on cognition in early to mid-adult life. Neurobiol. Learn. Mem. 2017, 146, 37–46. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1074742717301582 (accessed on 5 April 2025). [CrossRef]
- Wang, S.-M.; Kang, D.W.; Um, Y.H.; Kim, S.; Kim, R.E.Y.; Kim, D.; Lee, C.U.; Lim, H.K. Cognitive Normal Older Adults with APOE-2 Allele Show a Distinctive Functional Connectivity Pattern in Response to Cerebral Aβ Deposition. Int. J. Mol. Sci. 2023, 24, 11250. Available online: https://www.mdpi.com/1422-0067/24/14/11250 (accessed on 5 April 2025). [CrossRef]
- Kim, H.; Devanand, D.P.; Carlson, S.; Goldberg, T.E. Apolipoprotein E Genotype e2: Neuroprotection and Its Limits. Front. Aging Neurosci. 2022, 14, 919712. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2022.919712/full (accessed on 5 April 2025). [CrossRef]
- Wood, M.E.; Xiong, L.Y.; Wong, Y.Y.; Buckley, R.F.; Swardfager, W.; Masellis, M.; Lim, A.S.P.; Nichols, E.; Joie, R.; Casaletto, K.B.; et al. Sex differences in associations between APOE ε2 and longitudinal cognitive decline. Alzheimer’s Dement. 2023, 19, 4651–4661. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13036 (accessed on 6 April 2025). [CrossRef]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. Available online: https://www.nature.com/articles/ng1934 (accessed on 7 April 2025). [CrossRef] [PubMed]
- Hobel, Z.; Isenberg, A.L.; Raghupathy, D.; Mack, W.; Pa, J. APOE ε4 Gene Dose and Sex Effects on Alzheimer’s Disease MRI Biomarkers in Older Adults with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2019, 71, 647–658. Available online: https://journals.sagepub.com/doi/10.3233/JAD-180859?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 8 April 2025). [CrossRef] [PubMed]
- Walters, S.; Contreras, A.G.; Eissman, J.M.; Mukherjee, S.; Lee, M.L.; Choi, S.-E.; Scollard, P.; Trittschuh, E.H.; Mez, J.B.; Bush, W.S.; et al. Associations of Sex, Race, and Apolipoprotein E Alleles With Multiple Domains of Cognition Among Older Adults. JAMA Neurol. 2023, 80, 929. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2806771 (accessed on 8 April 2025). [CrossRef]
- Srisaikaew, P.; Chad, J.A.; Mahakkanukrauh, P.; Anderson, N.D.; Chen, J.J. Effect of sex on the APOE4-aging interaction in the white matter microstructure of cognitively normal older adults using diffusion-tensor MRI with orthogonal-tensor decomposition (DT-DOME). Front. Neurosci. 2023, 17, 1049609. Available online: https://www.frontiersin.org/articles/10.3389/fnins.2023.1049609/full (accessed on 8 April 2025).
- Gilsanz, P.; Lee, C.; Corrada, M.M.; Kawas, C.H.; Quesenberry, C.P.; Whitmer, R.A. Reproductive period and risk of dementia in a diverse cohort of health care members. Neurology 2019, 92, e2005–e2014. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000007326 (accessed on 8 April 2025). [CrossRef] [PubMed]
- Moutinho, S. Women twice as likely to develop Alzheimer’s disease as men—But scientists do not know why. Nat. Med. 2025, 31, 704–707. Available online: https://www.nature.com/articles/s41591-025-03564-3 (accessed on 8 April 2025). [CrossRef]
- Bove, R.; Secor, E.; Chibnik, L.B.; Barnes, L.L.; Schneider, J.A.; Bennett, D.A.; De Jager, P.L. Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014, 82, 222–229. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000000033 (accessed on 8 April 2025). [CrossRef] [PubMed]
- Mervosh, N.; Devi, G. Estrogen, menopause, and Alzheimer’s disease: Understanding the link to cognitive decline in women. Front. Mol. Biosci. 2025, 12, 1634302. Available online: https://www.frontiersin.org/articles/10.3389/fmolb.2025.1634302/full (accessed on 10 April 2025). [CrossRef]
- Mosconi, L.; Nerattini, M.; Matthews, D.C.; Jett, S.; Andy, C.; Williams, S.; Yepez, C.B.; Zarate, C.; Carlton, C.; Fauci, F.; et al. In vivo brain estrogen receptor density by neuroendocrine aging and relationships with cognition and symptomatology. Sci. Rep. 2024, 14, 12680. Available online: https://www.nature.com/articles/s41598-024-62820-7 (accessed on 10 April 2025). [CrossRef]
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Varsavsky, I.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Sex differences in Alzheimer risk. Neurology 2017, 89, 1382–1390. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000004425 (accessed on 10 April 2025). [CrossRef]
- Mosconi, L.; Berti, V.; Quinn, C.; McHugh, P.; Petrongolo, G.; Osorio, R.S.; Connaughty, C.; Pupi, A.; Vallabhajosula, S.; Isaacson, R.S.; et al. Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PLoS ONE 2017, 12, e0185926. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29016679 (accessed on 10 April 2025). [CrossRef]
- Mishra, A.; Wang, Y.; Yin, F.; Vitali, F.; Rodgers, K.E.; Soto, M.; Mosconi, L.; Wang, T.; Brinton, R.D. A tale of two systems: Lessons learned from female mid-life aging with implications for Alzheimer’s prevention & treatment. Ageing Res. Rev. 2022, 74, 101542. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1568163721002890 (accessed on 12 April 2025). [PubMed]
- Jett, S.; Dyke, J.P.; Andy, C.; Schelbaum, E.; Jang, G.; Boneu Yepez, C.; Pahlajani, S.; Diaz, I.; Brinton, R.D.; Mosconi, L. Sex and menopause impact 31P-Magnetic Resonance Spectroscopy brain mitochondrial function in association with 11C-PiB PET amyloid-beta load. Sci. Rep. 2022, 12, 22087. Available online: https://www.nature.com/articles/s41598-022-26573-5 (accessed on 12 April 2025). [CrossRef] [PubMed]
- Babapour Mofrad, R.; Tijms, B.M.; Scheltens, P.; Barkhof, F.; van der Flier, W.M.; Sikkes, S.A.M.; Teunissen, C.E. Sex differences in CSF biomarkers vary by Alzheimer disease stage and APOE ε4 genotype. Neurology 2020, 95, e2378–e2388. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000010629 (accessed on 14 April 2025). [CrossRef]
- Sundermann, E.E.; Tran, M.; Maki, P.M.; Bondi, M.W. Sex differences in the association between apolipoprotein E ε4 allele and Alzheimer’s disease markers. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 438–447. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1016/j.dadm.2018.06.004 (accessed on 14 April 2025). [CrossRef]
- Arnold, M.; Nho, K.; Kueider-Paisley, A.; Massaro, T.; Huynh, K.; Brauner, B.; MahmoudianDehkordi, S.; Louie, G.; Moseley, M.A.; Thompson, J.W.; et al. Sex and APOE ε4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 2020, 11, 1148. Available online: https://www.nature.com/articles/s41467-020-14959-w (accessed on 14 April 2025). [CrossRef]
- Xu, X.; Kwon, J.; Yan, R.; Apio, C.; Song, S.; Heo, G.; Yang, Q.; Timsina, J.; Liu, M.; Budde, J.; et al. Sex Differences in Apolipoprotein E and Alzheimer Disease Pathology Across Ancestries. JAMA Netw. Open 2025, 8, e250562. Available online: http://www.ncbi.nlm.nih.gov/pubmed/40067298 (accessed on 14 April 2025). [CrossRef]
- Boccalini, C.; Peretti, D.E.; Scheffler, M.; Mu, L.; Griffa, A.; Testart, N.; Allali, G.; Prior, J.O.; Ashton, N.J.; Zetterberg, H.; et al. Sex differences in the association of Alzheimer’s disease biomarkers and cognition in a multicenter memory clinic study. Alzheimer’s Res. Ther. 2025, 17, 46. Available online: http://www.ncbi.nlm.nih.gov/pubmed/39966925 (accessed on 15 April 2025). [CrossRef]
- Nemes, S.; Logan, P.E.; Manchella, M.K.; Mundada, N.S.; La Joie, R.; Polsinelli, A.J.; Hammers, D.B.; Koeppe, R.A.; Foroud, T.M.; Nudelman, K.N.; et al. Sex and APOE ε4 carrier effects on atrophy, amyloid PET, and tau PET burden in early-onset Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, S49–S63. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/alz.13403 (accessed on 15 April 2025). [CrossRef] [PubMed]
- Hyman, B.T.; Gomez-Isla, T.; Briggs, M.; Chung, H.; Nichols, S.; Kohout, F.; Wallace, R. Apolipoprotein E and cognitive change in an elderly population. Ann. Neurol. 1996, 40, 55–66. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ana.410400111 (accessed on 15 April 2025). [CrossRef] [PubMed]
- Davies, G.; Armstrong, N.; Bis, J.C.; Bressler, J.; Chouraki, V.; Giddaluru, S.; Hofer, E.; Ibrahim-Verbaas, C.A.; Kirin, M.; Lahti, J.; et al. Genetic contributions to variation in general cognitive function: A meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53,949). Mol. Psychiatry 2015, 20, 183–192. Available online: https://www.nature.com/articles/mp2014188 (accessed on 15 April 2025). [CrossRef]
- Lamonja-Vicente, N.; Dacosta-Aguayo, R.; López-Olóriz, J.; Prades-Senovilla, L.; Roig-Coll, F.; Castells-Sánchez, A.; Soriano-Raya, J.J.; Clemente, I.; Miralbell, J.; Barrios, M.; et al. Sex-Specific Protective Effects of APOE ε2 on Cognitive Performance. J. Gerontol. Ser. A 2021, 76, 41–49. Available online: https://academic.oup.com/biomedgerontology/article/76/1/41/5913014 (accessed on 17 April 2025). [CrossRef] [PubMed]
- Reas, E.T.; Laughlin, G.A.; Bergstrom, J.; Kritz-Silverstein, D.; Barrett-Connor, E.; McEvoy, L.K. Effects of APOE on cognitive aging in community-dwelling older adults. Neuropsychology 2019, 33, 406–416. Available online: http://doi.apa.org/getdoi.cfm?doi=10.1037/neu0000501 (accessed on 17 April 2025). [CrossRef]
- Dessy, T.; Barhdadi, A.; Cyr, M.-C.; Sandoval, J.; Bherer, L.; Rouleau, J.; Provost, S.; Lemieux Perreault, L.P.; Sylvestre, M.P.; Gagliano Taliun, S.A.; et al. Disentangling the effects of sex and gender on APOE ε4-related neurocognitive impairment. Alzheimer’s Dement. 2025, 17, e70111. Available online: http://www.ncbi.nlm.nih.gov/pubmed/40352685 (accessed on 17 April 2025). [CrossRef]
- Burns, A.P.; Fortel, I.; Zhan, L.; Lazarov, O.; Mackin, R.S.; Demos, A.P.; Bendlin, B.; Leow, A. Longitudinal excitation-inhibition balance altered by sex and APOE-ε4. Commun. Biol. 2025, 8, 488. Available online: https://www.nature.com/articles/s42003-025-07876-5 (accessed on 17 April 2025). [CrossRef]
- Barabash, A.; Marcos, A.; Ancín, I.; Vázquez-Alvarez, B.; de Ugarte, C.; Gil, P.; Fernández, C.; Encinas, M.; López-Ibor, J.J.; Cabranes, J.A. APOE, ACT and CHRNA7 genes in the conversion from amnestic mild cognitive impairment to Alzheimer’s disease. Neurobiol. Aging 2009, 30, 1254–1264. Available online: https://linkinghub.elsevier.com/retrieve/pii/S019745800700423X (accessed on 18 April 2025). [CrossRef]
- Bretsky, P.; Guralnik, J.M.; Launer, L.; Albert, M.; Seeman, T.E. The role of APOE-ε4 in longitudinal cognitive decline. Neurology 2003, 60, 1077–1081. Available online: https://www.neurology.org/doi/10.1212/01.WNL.0000055875.26908.24 (accessed on 18 April 2025). [CrossRef]
- Chuang, Y.-F.; Hayden, K.M.; Norton, M.C.; Tschanz, J.; Breitner, J.C.S.; Welsh-Bohmer, K.A.; Zandi, P.P. Association between APOE ε4 Allele and Vascular Dementia: The Cache County Study. Dement. Geriatr. Cogn. Disord. 2010, 29, 248–253. Available online: https://karger.com/article/doi/10.1159/000285166 (accessed on 20 April 2025). [CrossRef]
- Konishi, K.; Bhat, V.; Banner, H.; Poirier, J.; Joober, R.; Bohbot, V.D. APOE2 Is Associated with Spatial Navigational Strategies and Increased Gray Matter in the Hippocampus. Front. Hum. Neurosci. 2016, 10, 349. Available online: http://journal.frontiersin.org/Article/10.3389/fnhum.2016.00349/abstract (accessed on 20 April 2025). [CrossRef]
- Sweigart, B.; Andersen, S.L.; Gurinovich, A.; Cosentino, S.; Schupf, N.; Perls, T.T.; Sebastiani, P. APOE E2/E2 Is Associated with Slower Rate of Cognitive Decline with Age. J. Alzheimer’s Dis. 2021, 83, 853–860. Available online: https://journals.sagepub.com/doi/full/10.3233/JAD-201205 (accessed on 20 April 2025). [CrossRef] [PubMed]
- Tiraboschi, P.; Hansen, L.A.; Masliah, E.; Alford, M.; Thal, L.J.; Corey-Bloom, J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology 2004, 62, 1977–1983. Available online: https://www.neurology.org/doi/10.1212/01.WNL.0000128091.92139.0F (accessed on 21 April 2025). [CrossRef] [PubMed]
- Conejero-Goldberg, C.; Gomar, J.J.; Bobes-Bascaran, T.; Hyde, T.M.; Kleinman, J.E.; Herman, M.M.; Chen, S.; Davies, P.; Goldberg, T.E. APOE2 enhances neuroprotection against Alzheimer’s disease through multiple molecular mechanisms. Mol. Psychiatry 2014, 19, 1243–1250. Available online: https://www.nature.com/articles/mp2013194 (accessed on 22 April 2025). [CrossRef] [PubMed]
- Serrano-Pozo, A.; Qian, J.; Monsell, S.E.; Betensky, R.A.; Hyman, B.T. APOE ε2 is associated with milder clinical and pathological Alzheimer’s disease. Ann. Neurol. 2015, 77, 917–929. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ana.24369 (accessed on 22 April 2025). [CrossRef]
- Lancaster, C.; Tabet, N.; Rusted, J. The APOE paradox: Do attentional control differences in mid-adulthood reflect risk of late-life cognitive decline. Neurobiol. Aging 2016, 48, 114–121. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0197458016301932 (accessed on 22 April 2025). [CrossRef]
- Marioni, R.E.; Campbell, A.; Scotland, G.; Hayward, C.; Porteous, D.J.; Deary, I.J. Differential effects of the APOE e4 allele on different domains of cognitive ability across the life-course. Eur. J. Hum. Genet. 2016, 24, 919–923. [Google Scholar] [CrossRef]
- Alexander, D.M.; Williams, L.M.; Gatt, J.M.; Dobson-Stone, C.; Kuan, S.A.; Todd, E.G.; Schofield, P.R.; Cooper, N.J.; Gordon, E. The contribution of apolipoprotein E alleles on cognitive performance and dynamic neural activity over six decades. Biol. Psychol. 2007, 75, 229–238. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0301051107000476 (accessed on 22 April 2025). [CrossRef]
- Batterham, P.J.; Bunce, D.; Cherbuin, N.; Christensen, H. Apolipoprotein E ε4 and Later-Life Decline in Cognitive Function and Grip Strength. Am. J. Geriatr. Psychiatry 2013, 21, 1010–1019. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1064748113000407 (accessed on 24 April 2025). [CrossRef]
- Schiepers, O.J.G.; Harris, S.E.; Gow, A.J.; Pattie, A.; Brett, C.E.; Starr, J.M.; Deary, I.J. APOE E4 status predicts age-related cognitive decline in the ninth decade: Longitudinal follow-up of the Lothian Birth Cohort 1921. Mol. Psychiatry 2012, 17, 315–324. Available online: https://www.nature.com/articles/mp2010137 (accessed on 24 April 2025). [CrossRef]
- Fernandez, S.; Burnham, S.C.; Milicic, L.; Savage, G.; Maruff, P.; Peretti, M.; Sohrabi, H.R.; Lim, Y.Y.; Weinborn, M.; Ames, D.; et al. SPON1 Is Associated with Amyloid-β and APOE ε4-Related Cognitive Decline in Cognitively Normal Adults. J. Alzheimer’s Dis. Rep. 2021, 5, 111–120. Available online: https://journals.sagepub.com/doi/10.3233/ADR-200246 (accessed on 24 April 2025). [CrossRef]
- Lim, Y.Y.; Villemagne, V.L.; Laws, S.M.; Ames, D.; Pietrzak, R.H.; Ellis, K.A.; Harrington, K.D.; Bourgeat, P.; Salvado, O.; Darby, D.; et al. BDNF Val66Met, Aβ amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2457–2464. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0197458013002042 (accessed on 24 April 2025). [CrossRef]
- Lim, Y.Y.; Hassenstab, J.; Cruchaga, C.; Goate, A.; Fagan, A.M.; Benzinger, T.L.S.; Maruff, P.; Snyder, P.J.; Masters, C.L.; Allegri, R.; et al. BDNF Val66Met moderates memory impairment, hippocampal function and tau in preclinical autosomal dominant Alzheimer’s disease. Brain 2016, 139, 2766–2777. Available online: https://academic.oup.com/brain/article-lookup/doi/10.1093/brain/aww200 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Zhang, Y.; Jin, X.; Lutz, M.W.; Ju, S.-Y.; Liu, K.; Guo, G.; Zeng, Y.; Yao, Y. Interaction between APOE ε4 and dietary protein intake on cognitive decline: A longitudinal cohort study. Clin. Nutr. 2021, 40, 2716–2725. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0261561421001436 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Levy, B.R.; Slade, M.D.; Pietrzak, R.H.; Ferrucci, L. When Culture Influences Genes: Positive Age Beliefs Amplify the Cognitive-Aging Benefit of APOE ε2. J. Gerontol. Ser. B 2020, 75, e198–e203. Available online: https://academic.oup.com/psychsocgerontology/article/75/8/e198/5896592 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Stringa, N.; van Schoor, N.M.; Milaneschi, Y.; Ikram, M.A.; Del Panta, V.; Koolhaas, C.M.; Voortman, T.; Bandinelli, S.; Wolters, F.J.; Huisman, M. Physical Activity as Moderator of the Association Between APOE and Cognitive Decline in Older Adults: Results from Three Longitudinal Cohort Studies. J. Gerontol. Ser. A 2020, 75, 1880–1886. Available online: https://academic.oup.com/biomedgerontology/article/75/10/1880/5763509 (accessed on 26 April 2025). [CrossRef]
- Kim, R.; Park, S.; Yoo, D.; Jun, J.-S.; Jeon, B. Association of Physical Activity and APOE Genotype with Longitudinal Cognitive Change in Early Parkinson Disease. Neurology 2021, 96, e2429–e2437. Available online: https://www.neurology.org/doi/10.1212/WNL.0000000000011852 (accessed on 26 April 2025). [CrossRef] [PubMed]
- Xiang, Q.; Andersen, S.L.; Perls, T.T.; Sebastiani, P. Studying the Interplay Between Apolipoprotein E and Education on Cognitive Decline in Centenarians Using Bayesian Beta Regression. Front. Genet. 2021, 11, 606831. Available online: https://www.frontiersin.org/articles/10.3389/fgene.2020.606831/full (accessed on 27 April 2025). [CrossRef]
- Ji, S.; Kang, J.; Han, C.; Xu, X.; Chen, M.; Chen, J.; Chhetri, J.K.; Pan, J.; Chan, P. Potential role of APOE ε4 allele as a modifier for the association of BDNF Val66Met polymorphisms and cognitive impairment in community-dwelling older adults. Front. Aging Neurosci. 2024, 16, 1330193. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1330193/full (accessed on 27 April 2025). [CrossRef]
- Vilor-Tejedor, N.; Operto, G.; Evans, T.E.; Falcon, C.; Crous-Bou, M.; Minguillón, C.; Cacciaglia, R.; Milà-Alomà, M.; Grau-Rivera, O.; Suárez-Calvet, M.; et al. Effect of BDNF Val66Met on hippocampal subfields volumes and compensatory interaction with APOE-ε4 in middle-age cognitively unimpaired individuals from the ALFA study. Brain Struct. Funct. 2020, 225, 2331–2345. Available online: https://link.springer.com/10.1007/s00429-020-02125-3 (accessed on 1 May 2025). [CrossRef] [PubMed]
- Edmunds, K.J.; Pandos, A.A.; Hoang, I.; Mamlouk, G.M.; Motovylyak, A.; Lose, S.R.; Asthana, S.; Stremlau, M.; Johnson, S.C.; van Praag, H.; et al. BDNF expression mediates verbal learning and memory in women in a cohort enriched with risk for Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2025, 17, e70062. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1002/dad2.70062 (accessed on 1 May 2025). [CrossRef]
- Cechova, K.; Andel, R.; Angelucci, F.; Chmatalova, Z.; Markova, H.; Laczó, J.; Vyhnalek, M.; Matoska, V.; Kaplan, V.; Nedelska, Z.; et al. Impact of APOE and BDNF Val66Met Gene Polymorphisms on Cognitive Functions in Patients with Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2020, 73, 247–257. Available online: https://journals.sagepub.com/doi/10.3233/JAD-190464?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 1 May 2025). [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. Available online: https://translationalneurodegeneration.biomedcentral.com/articles/10.1186/s40035-022-00279-0 (accessed on 2 May 2025). [CrossRef]
- Riedel, B.C.; Thompson, P.M.; Brinton, R.D. Age, APOE and sex: Triad of risk of Alzheimer’s disease. J. Steroid Biochem. Mol. Biol. 2016, 160, 134–147. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0960076016300589 (accessed on 2 May 2025). [CrossRef] [PubMed]
- Gong, L.; Xu, R.; Liu, D.; Lan, L.; Zhang, B.; Zhang, C. The Specific Impact of Apolipoprotein E Epsilon 2 on Cognition and Brain Function in Cognitively Normal Elders and Mild Cognitive Impairment Patients. Front. Aging Neurosci. 2020, 11, 374. Available online: https://www.frontiersin.org/article/10.3389/fnagi.2019.00374/full (accessed on 2 May 2025). [CrossRef]
- Insel, P.S.; Hansson, O.; Mattsson-Carlgren, N. Association Between Apolipoprotein E ε2 vs ε4, Age, and β-Amyloid in Adults Without Cognitive Impairment. JAMA Neurol. 2021, 78, 229. Available online: https://jamanetwork.com/journals/jamaneurology/fullarticle/2771412 (accessed on 3 May 2025). [CrossRef] [PubMed]
- Wilson, R.S. The apolipoprotein E varepsilon2 allele and decline in episodic memory. J. Neurol. Neurosurg. Psychiatry 2002, 73, 672–677. Available online: https://jnnp.bmj.com/lookup/doi/10.1136/jnnp.73.6.672 (accessed on 3 May 2025). [CrossRef]
- Small, B.J.; Rosnick, C.B.; Fratiglioni, L.; Bäckman, L. Apolipoprotein E and Cognitive Performance: A Meta-Analysis. Psychol. Aging 2004, 19, 592–600. Available online: https://doi.apa.org/doi/10.1037/0882-7974.19.4.592 (accessed on 4 May 2025). [CrossRef] [PubMed]
- Wisdom, N.M.; Callahan, J.L.; Hawkins, K.A. The effects of apolipoprotein E on non-impaired cognitive functioning: A meta-analysis. Neurobiol. Aging 2011, 32, 63–74. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0197458009000384 (accessed on 4 May 2025). [CrossRef]
- Zokaei, N.; Board, A.G.; Slavkova, E.; EMackay, C.; Nobre, A.C.; Husain, M. Superior short-term memory in APOE ε2 carriers across the age range. Behav. Brain Res. 2021, 397, 112918. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0166432820306173 (accessed on 5 May 2025). [CrossRef]
- Meyer, M.R.; Tschanz, J.T.; Norton, M.C.; Welsh-Bohmer, K.A.; Steffens, D.C.; Wyse, B.W.; Breitner, J.C. APOE genotype predicts when—Not whether—One is predisposed to develop Alzheimer disease. Nat. Genet. 1998, 19, 321–322. Available online: https://www.nature.com/articles/ng0898_321 (accessed on 5 May 2025). [CrossRef]
- Shinohara, M.; Kanekiyo, T.; Tachibana, M.; Kurti, A.; Shinohara, M.; Fu, Y.; Zhao, J.; Han, X.; Sullivan, P.M.; Rebeck, G.W.; et al. APOE2 is associated with longevity independent of Alzheimer’s disease. eLife 2020, 9, e62199. Available online: https://elifesciences.org/articles/62199 (accessed on 6 May 2025). [CrossRef]
- Muros, M.; Rodríguez-Ferrer, C. Apolipoprotein E polymorphism influence on lipids, apolipoproteins and Lp(a) in a Spanish population underexpressing apo E4. Atherosclerosis 1996, 121, 13–21. Available online: https://linkinghub.elsevier.com/retrieve/pii/0021915095066438 (accessed on 6 May 2025). [CrossRef]
- Bennet, A.M.; Di Angelantonio, E.; Ye, Z.; Wensley, F.; Dahlin, A.; Ahlbom, A.; Keavney, B.; Collins, R.; Wiman, B.; de Faire, U.; et al. Association of Apolipoprotein E Genotypes With Lipid Levels and Coronary Risk. JAMA 2007, 298, 1300. Available online: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.298.11.1300 (accessed on 7 May 2025). [CrossRef]
- Reiman, E.M.; Arboleda-Velasquez, J.F.; Quiroz, Y.T.; Huentelman, M.J.; Beach, T.G.; Caselli, R.J.; Chen, Y.; Su, Y.; Myers, A.J.; Hardy, J.; et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5000-person neuropathological study. Nat. Commun. 2020, 11, 667. Available online: https://www.nature.com/articles/s41467-019-14279-8 (accessed on 8 May 2025). [CrossRef]
- Wang, X.; Magkos, F.; Mittendorfer, B. Sex Differences in Lipid and Lipoprotein Metabolism: It’s Not Just about Sex Hormones. J. Clin. Endocrinol. Metab. 2011, 96, 885–893. Available online: https://academic.oup.com/jcem/article/96/4/885/2720832 (accessed on 8 May 2025). [CrossRef] [PubMed]
- Tehrani, F.R.; Erfani, H.; Cheraghi, L.; Tohidi, M.; Azizi, F. Lipid profiles and ovarian reserve status: A longitudinal study. Hum. Reprod. 2014, 29, 2522–2529. Available online: https://academic.oup.com/humrep/article-lookup/doi/10.1093/humrep/deu249 (accessed on 8 May 2025). [CrossRef] [PubMed]
- Tontisirin, N.; Muangman, S.L.; Suz, P.; Pihoker, C.; Fisk, D.; Moore, A.; Lam, A.M.; Vavilala, M.S. Early Childhood Gender Differences in Anterior and Posterior Cerebral Blood Flow Velocity and Autoregulation. Pediatrics 2007, 119, e610–e615. Available online: https://publications.aap.org/pediatrics/article/119/3/e610/70449/Early-Childhood-Gender-Differences-in-Anterior-and (accessed on 8 May 2025). [CrossRef]
- Satterthwaite, T.D.; Shinohara, R.T.; Wolf, D.H.; Hopson, R.D.; Elliott, M.A.; Vandekar, S.N.; Ruparel, K.; Calkins, M.E.; Roalf, D.R.; Gennatas, E.D.; et al. Impact of puberty on the evolution of cerebral perfusion during adolescence. Proc. Natl. Acad. Sci. USA 2014, 111, 8643–8648. Available online: https://pnas.org/doi/full/10.1073/pnas.1400178111 (accessed on 10 May 2025). [CrossRef] [PubMed]
- Rodriguez, G.; Warkentin, S.; Risberg, J.; Rosadini, G. Sex Differences in Regional Cerebral Blood Flow. J. Cereb. Blood Flow Metab. 1988, 8, 783–789. Available online: https://journals.sagepub.com/doi/10.1038/jcbfm.1988.133?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 10 May 2025). [CrossRef]
- Sunday, L.; Osuna, C.; Krause, D.N.; Duckles, S.P. Age alters cerebrovascular inflammation and effects of estrogen. Am. J. Physiol. Circ. Physiol. 2007, 292, H2333–H2340. Available online: https://www.physiology.org/doi/10.1152/ajpheart.01057.2006 (accessed on 10 May 2025). [CrossRef]
- Abi-Ghanem, C.; Robison, L.S.; Zuloaga, K.L. Androgens’ effects on cerebrovascular function in health and disease. Biol. Sex Differ. 2020, 11, 35. Available online: https://bsd.biomedcentral.com/articles/10.1186/s13293-020-00309-4 (accessed on 11 May 2025). [CrossRef]
- Banica, T.; Verroken, C.; Reyns, T.; Mahmoud, A.; T’Sjoen, G.; Fiers, T.; Kaufman, J.M.; Lapauw, B. Early Decline of Androgen Levels in Healthy Adult Men: An Effect of Aging Per Se? A Prospective Cohort Study. J. Clin. Endocrinol. Metab. 2021, 106, e1074–e1083. Available online: https://academic.oup.com/jcem/article/106/4/e1074/6056486 (accessed on 11 May 2025). [CrossRef]
- Cai, Z.; Li, H. An Updated Review: Androgens and Cognitive Impairment in Older Men. Front. Endocrinol. 2020, 11, 586909. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33281745 (accessed on 12 May 2025). [CrossRef]
- Lim, Y.Y.; Villemagne, V.L.; Laws, S.M.; Pietrzak, R.H.; Snyder, P.J.; Ames, D.; Ellis, K.A.; Harrington, K.; Rembach, A.; Martins, R.N.; et al. APOE and BDNF polymorphisms moderate amyloid β-related cognitive decline in preclinical Alzheimer’s disease. Mol. Psychiatry 2015, 20, 1322–1328. Available online: http://www.nature.com/articles/mp2014123 (accessed on 12 May 2025). [CrossRef]
- Cativiela-Campos, B.; Ruiz-Sobremazas, D.; Rodulfo-Cárdenas, R.; Barrasa, A.; Sánchez-Santed, F.; Colomina, M.T.; Aschner, M.; López-Granero, C. What are the consequences of PM air pollution exposure on elderly behavior? A systematic review. Environ. Pollut. 2025, 375, 126279. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0269749125006529 (accessed on 12 May 2025). [CrossRef] [PubMed]
- Roig-Coll, F.; Castells-Sánchez, A.; Lamonja-Vicente, N.; Torán-Monserrat, P.; Pera, G.; García-Molina, A.; Tormos, J.M.; Montero-Alía, P.; Alzamora, M.T.; Dacosta-Aguayo, R.; et al. Effects of Aerobic Exercise, Cognitive and Combined Training on Cognition in Physically Inactive Healthy Late-Middle-Aged Adults: The Projecte Moviment Randomized Controlled Trial. Front. Aging Neurosci. 2020, 12, 590168. Available online: https://www.frontiersin.org/articles/10.3389/fnagi.2020.590168/full (accessed on 12 May 2025). [CrossRef] [PubMed]
- Marrugat, J.; Subirana, I.; Comin, E.; Cabezas, C.; Vila, J.; Elosua, R.; Nam, B.H.; Ramos, R.; Sala, J.; Solanas, P.; et al. Validity of an adaptation of the Framingham cardiovascular risk function: The VERIFICA study. J. Epidemiol. Community Health 2007, 61, 40–47. Available online: http://jech.bmj.com/cgi/doi/10.1136/jech.2005.038505 (accessed on 12 May 2025). [CrossRef]
- López-Cancio, E.; Dorado, L.; Millán, M.; Reverté, S.; Suñol, A.; Massuet, A.; Mataró, M.; Galán, A.; Alzamora, M.; Pera, G.; et al. The population-based Barcelona-Asymptomatic Intracranial Atherosclerosis Study (ASIA): Rationale and design. BMC Neurol. 2011, 11, 22. Available online: http://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-11-22 (accessed on 12 May 2025). [CrossRef] [PubMed]
- Crespo-Cuevas, A.M.; Canento, T.; Hernández-Perez, M.; Cáceres, C.; González, A.; Ispierto, L.; Mataró, M.; Vilas, D.; Planas-Ballvé, A.; Martin, L.; et al. The Barcelona-Asymptomatic Intracranial Atherosclerosis (AsIA) study: Subclinical cervico-cerebral stenosis and middle cerebral artery pulsatility index as predictors of long-term incident cognitive impairment. Atherosclerosis 2020, 312, 104–109. Available online: https://linkinghub.elsevier.com/retrieve/pii/S002191502030469X (accessed on 13 May 2025). [CrossRef]
- Miralbell, J.; López-Cancio, E.; López-Oloriz, J.; Arenillas, J.F.; Barrios, M.; Soriano-Raya, J.J.; Galán, A.; Cáceres, C.; Alzamora, M.; Pera, G.; et al. Cognitive Patterns in Relation to Biomarkers of Cerebrovascular Disease and Vascular Risk Factors. Cerebrovasc. Dis. 2013, 36, 98–105. Available online: https://karger.com/article/doi/10.1159/000352059 (accessed on 14 May 2025). [CrossRef]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. Available online: https://www.tandfonline.com/doi/full/10.1300/J018v05n01_09 (accessed on 14 May 2025). [CrossRef]
- Barnett, A.G. Regression to the mean: What it is and how to deal with it. Int. J. Epidemiol. 2004, 34, 215–220. Available online: https://academic.oup.com/ije/article-lookup/doi/10.1093/ije/dyh299 (accessed on 14 May 2025). [CrossRef] [PubMed]
- Chiolero, A.; Paradis, G.; Rich, B.; Hanley, J.A. Assessing the Relationship between the Baseline Value of a Continuous Variable and Subsequent Change Over Time. Front. Public Health 2013, 1, 29. Available online: http://journal.frontiersin.org/article/10.3389/fpubh.2013.00029/abstract (accessed on 15 May 2025). [CrossRef]
- Oldham, P.D. A note on the analysis of repeated measurements of the same subjects. J. Chronic. Dis. 1962, 15, 969–977. Available online: https://linkinghub.elsevier.com/retrieve/pii/0021968162901169 (accessed on 15 May 2025). [CrossRef]
- Corrada, M.M.; Paganini-Hill, A.; Berlau, D.J.; Kawas, C.H. Apolipoprotein E genotype, dementia, and mortality in the oldest old: The 90+ Study. Alzheimer’s Dement. 2013, 9, 12–18. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/10.1016/j.jalz.2011.12.004 (accessed on 15 May 2025). [CrossRef]

| Baseline | Follow-Up | |||||
|---|---|---|---|---|---|---|
| Total (n = 386) | Men (n = 244) | Women (n = 142) | Total (n = 386) | Men (n = 244) | Women (n = 142) | |
| Sociodemographic and clinical data | ||||||
| Age (years) | 65.04 (6.83) | 66.14 (6.95) | 63.15 (6.20) | 71.82 (6.58) | 72.80 (6.82) | 70.13 (5.79) |
| Education (years) | 6.76 (4.22) | 6.83 (4.61) | 6.63 (3.46) | 6.76 (4.22) | 6.83 (4.61) | 6.63 (3.46) |
| GDS-15 | 2.34 (2.56) | 1.89 (1.99) | 3.10 (3.18) | 1.41 (2.26) | 1.01 (1.71) | 2.08 (2.83) |
| REGICOR | 7.76 (3.58) | 8.64 (3.83) | 6.25 (2.46) | No data | No data | No data |
| ε2 vs. non-ε2 (% ε2 carriers (n)) | 9.8 (38) | 7.8 (19) | 13.4 (19) | 9.8 (38) | 7.8 (19) | 13.4 (19) |
| ε4 vs. non-ε4 (% ε4 carriers (n)) | 17.9 (69) | 20.1 (49) | 14.1 (20) | 17.9 (69) | 20.1 (49) | 14.1 (20) |
| Neuropsychological domains (Z scores) | ||||||
| Visuospatial skills/speed | 0.06 (0.42) | 0.08 (0.42) | 0.01 (0.41) | 0.16 (1.35) | 0.19 (1.54) | −0.02 (0.90) |
| Verbal memory | 0.09 (0.92) | 0.02 (0.94) | 0.21 (0.87) | −0.01 (0.92) | −0.11 (0.89) | 0.16 (0.95) |
| Verbal fluency | 0.04 (0.84) | 0.12 (0.89) | −0.11 (0.71) | 0.10 (0.95) | 0.17 (0.97) | 0.00 (0.93) |
| Visuospatial Skills/Speed | Verbal Memory | Verbal Fluency | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOTAL | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm |
| APOE ε4 | 358 | 0.235 | −0.099/0.569 | 0.169 | 0.339 | 374 | −0.147 | −0.320/0.027 | 0.098 | 0.320 | 372 | −0.168 | −0.343/0.007 | 0.060 | 0.222 |
| APOE ε2 | 358 | 0.026 | −0.436/0.487 | 0.914 | 1 | 374 | −0.238 | −0.474/−0.001 | 0.049 | 0.180 | 372 | 0.105 | −0.137/0.348 | 0.395 | 0.840 |
| MEN | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm |
| APOE ε4 | 225 | 0.306 | −0.176/0.778 | 0.214 | 0.377 | 233 | −0.163 | −0.352/0.026 | 0.093 | 0.270 | 233 | −0.192 | −0.392/0.007 | 0.060 | 0.217 |
| APOE ε2 | 225 | −0.007 | −0.768/0.753 | 0.984 | 1 | 233 | −0.382 | −0.670/−0.095 | 0.010 | 0.027 | 233 | 0.135 | −0.176/0.446 | 0.395 | 0.828 |
| WOMEN | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm |
| APOE ε4 | 133 | −0.011 | −0.185/0.163 | 0.902 | 1 | 141 | −0.116 | −0.497/0.266 | 0.552 | 0.961 | 139 | −0.075 | −0.440/0.290 | 0.686 | 0.987 |
| APOE ε2 | 133 | 0.100 | −0.086/0.287 | 0.293 | 0.714 | 141 | −0.017 | −0.448/0.414 | 0.939 | 1 | 139 | 0.041 | −0.362/0.445 | 0.841 | 1 |
| Visuospatial Skills/Speed | Verbal Memory | Verbal Fluency | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TOTAL | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm |
| APOE ε4 | 358 | 0.267 | −0.068/0.602 | 0.119 | 0.273 | 374 | −0.180 | −0.391/0.030 | 0.094 | 0.323 | 372 | −0.178 | −0.396/0.040 | 0.111 | 0.340 |
| APOE ε2 | 358 | −0.020 | −0.483/0.443 | 0.933 | 1 | 374 | −0.028 | −0.313/0.258 | 0.850 | 0.999 | 372 | 0.283 | −0.016/0.582 | 0.064 | 0.212 |
| MEN | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm |
| APOE ε4 | 225 | 0.333 | −0.149/0.816 | 0.177 | 0.331 | 233 | −0.188 | −0.424/0.047 | 0.118 | 0.367 | 233 | −0.224 | −0.483/0.035 | 0.092 | 0.318 |
| APOE ε2 | 225 | −0.112 | −0.861/0.642 | 0.772 | 0.996 | 233 | −0.354 | −0.714/0.007 | 0.056 | 0.199 | 233 | 0.285 | −0.116/0.685 | 0.165 | 0.488 |
| WOMEN | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm | n | β | 95% CI | p | p-perm |
| APOE ε4 | 133 | 0.031 | −0.155/0.218 | 0.741 | 0.999 | 141 | −0.168 | −0.614/0.278 | 0.462 | 0.889 | 139 | −0.034 | −0.459/0.392 | 0.876 | 1 |
| APOE ε2 | 133 | 0.128 | −0.072/0.329 | 0.213 | 0.624 | 141 | 0.437 | −0.037/0.911 | 0.073 | 0.247 | 139 | 0.287 | −0.174/0.748 | 0.224 | 0.590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamonja-Vicente, N.; Dacosta-Aguayo, R.; López-Olóriz, J.; Prades-Senovilla, L.; Soriano-Raya, J.J.; Clemente, I.C.; Miralbell, J.; Barrios, M.; López-Cancio, E.; Cáceres, C.; et al. Sex and APOE ε2 Interactive Effects on the Longitudinal Change in Cognition in a Population-Based Cohort of Older Adults with Vascular Risk Factors. Int. J. Mol. Sci. 2025, 26, 10591. https://doi.org/10.3390/ijms262110591
Lamonja-Vicente N, Dacosta-Aguayo R, López-Olóriz J, Prades-Senovilla L, Soriano-Raya JJ, Clemente IC, Miralbell J, Barrios M, López-Cancio E, Cáceres C, et al. Sex and APOE ε2 Interactive Effects on the Longitudinal Change in Cognition in a Population-Based Cohort of Older Adults with Vascular Risk Factors. International Journal of Molecular Sciences. 2025; 26(21):10591. https://doi.org/10.3390/ijms262110591
Chicago/Turabian StyleLamonja-Vicente, Noemí, Rosalía Dacosta-Aguayo, Jorge López-Olóriz, Laia Prades-Senovilla, Juan José Soriano-Raya, Inmaculada C. Clemente, Júlia Miralbell, Maite Barrios, Elena López-Cancio, Cynthia Cáceres, and et al. 2025. "Sex and APOE ε2 Interactive Effects on the Longitudinal Change in Cognition in a Population-Based Cohort of Older Adults with Vascular Risk Factors" International Journal of Molecular Sciences 26, no. 21: 10591. https://doi.org/10.3390/ijms262110591
APA StyleLamonja-Vicente, N., Dacosta-Aguayo, R., López-Olóriz, J., Prades-Senovilla, L., Soriano-Raya, J. J., Clemente, I. C., Miralbell, J., Barrios, M., López-Cancio, E., Cáceres, C., Millán, M., Torán, P., Pera, G., Carmona-Cervelló, M., Herrero, C., Montero-Alia, P., Palau-Antoja, M., Hernández-Pérez, M., Canento, T., ... Via, M. (2025). Sex and APOE ε2 Interactive Effects on the Longitudinal Change in Cognition in a Population-Based Cohort of Older Adults with Vascular Risk Factors. International Journal of Molecular Sciences, 26(21), 10591. https://doi.org/10.3390/ijms262110591







