Protein Design Meets Single-Molecule Detection: Towards Programmable Nanopore Sensors
Abstract
1. Introduction
2. Native Nanopores
2.1. Multimeric β-Barrel Pores for High-Resolution Sequencing
2.2. Monomeric β-Barrel Pores for Versatile Protein Detection
2.3. α-Helical Pores for Large Protein Trapping
2.4. Metagenomic Mining
3. Engineered Nanopores
4. De Novo Design of Nanopores
4.1. De Novo Design of Transmembrane β-Barrel Nanopores
4.2. De Novo Design of α-Helical Nanopores-from Ion Channels to Tunable Pores
4.3. De Novo Designed Target Binding Proteins for Indirect Nanopore Sensing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ying, Y.-L.; Hu, Z.-L.; Zhang, S.; Qing, Y.; Fragasso, A.; Maglia, G.; Meller, A.; Bayley, H.; Dekker, C.; Long, Y.-T. Nanopore-based technologies beyond DNA sequencing. Nat. Nanotechnol. 2022, 17, 1136–1146. [Google Scholar] [CrossRef]
- Mayer, S.F.; Cao, C.; Dal Peraro, M. Biological nanopores for single-molecule sensing. iScience 2022, 25, 104145. [Google Scholar] [CrossRef]
- Liu, J.; Aksimentiev, A. Molecular Determinants of Current Blockade Produced by Peptide Transport Through a Nanopore. ACS Nanosci. Au 2024, 4, 21–29. [Google Scholar] [CrossRef]
- Deamer, D.; Akeson, M.; Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 2016, 34, 518–524. [Google Scholar] [CrossRef]
- Jain, M.; Olsen, H.E.; Paten, B.; Akeson, M. The Oxford Nanopore MinION: Delivery of nanopore sequencing to the genomics community. Genome Biol. 2016, 17, 239. [Google Scholar] [CrossRef]
- Dorey, A.; Howorka, S. Nanopore DNA sequencing technologies and their applications towards single-molecule proteomics. Nat. Chem. 2024, 16, 314–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Bollas, A.; Wang, Y.; Au, K.F. Nanopore sequencing technology, bioinformatics and applications. Nat. Biotechnol. 2021, 39, 1348–1365. [Google Scholar] [CrossRef]
- Xue, L.; Yamazaki, H.; Ren, R.; Wanunu, M.; Ivanov, A.P.; Edel, J.B. Solid-state nanopore sensors. Nat. Rev. Mater. 2020, 5, 931–951. [Google Scholar] [CrossRef]
- Fragasso, A.; Schmid, S.; Dekker, C. Comparing Current Noise in Biological and Solid-State Nanopores. ACS Nano 2020, 14, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.I.; Sandler, S.E.; Vendruscolo, M.; Keyser, U.F. Detection of protein oligomers with nanopores. Nat. Rev. Chem. 2025, 9, 224–240. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, C.; Wang, Z.; Chen, S.; Zhang, D.; Li, K.; Sun, K.; Zhao, C.; Wang, Y.; Xu, M.; et al. Real-time detection of 20 amino acids and discrimination of pathologically relevant peptides with functionalized nanopore. Nat. Methods 2024, 21, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Ritmejeris, J.; Chen, X.; Dekker, C. Single-molecule protein sequencing with nanopores. Nat. Rev. Bioeng. 2025, 3, 303–316. [Google Scholar] [CrossRef]
- Lu, C.; Bonini, A.; Viel, J.H.; Maglia, G. Toward single-molecule protein sequencing using nanopores. Nat. Biotechnol. 2025, 43, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Martin-Baniandres, P.; Lan, W.-H.; Board, S.; Romero-Ruiz, M.; Garcia-Manyes, S.; Qing, Y.; Bayley, H. Enzyme-less nanopore detection of post-translational modifications within long polypeptides. Nat. Nanotechnol. 2023, 18, 1335–1340. [Google Scholar] [CrossRef]
- Lucas, F.L.R.; Versloot, R.C.A.; Yakovlieva, L.; Walvoort, M.T.C.; Maglia, G. Protein identification by nanopore peptide profiling. Nat. Commun. 2021, 12, 5795. [Google Scholar] [CrossRef]
- Wei, X.; Penkauskas, T.; Reiner, J.E.; Kennard, C.; Uline, M.J.; Wang, Q.; Li, S.; Aksimentiev, A.; Robertson, J.W.F.; Liu, C. Engineering Biological Nanopore Approaches toward Protein Sequencing. ACS Nano 2023, 17, 16369–16395. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, S.; Zhou, X.; Yang, X.; Li, X.; Wang, Y.; Fan, P.; Xiao, Y.; Sun, W.; Zhang, P.; et al. Unambiguous discrimination of all 20 proteinogenic amino acids and their modifications by nanopore. Nat. Methods 2024, 21, 92–101. [Google Scholar] [CrossRef]
- Lucas, F.L.R.; Sarthak, K.; Lenting, E.M.; Coltan, D.; van der Heide, N.J.; Versloot, R.C.A.; Aksimentiev, A.; Maglia, G. The Manipulation of the Internal Hydrophobicity of FraC Nanopores Augments Peptide Capture and Recognition. ACS Nano 2021, 15, 9600–9613. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, Y.; Li, Z.; Zhou, K.; Liu, L.; Wu, H.-C. Peptide sequencing based on host–guest interaction-assisted nanopore sensing. Nat. Methods 2024, 21, 102–109. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.-C.; Hu, Z.-L.; Ying, Y.-L.; Long, Y.-T. Dynamic Features Driven by Stochastic Collisions in a Nanopore for Precise Single-Molecule Identification. J. Am. Chem. Soc. 2025, 147, 1781–1791. [Google Scholar] [CrossRef]
- Yao, G.; Tian, Y.; Ke, W.; Fang, J.; Ma, S.; Li, T.; Cheng, X.; Xia, B.; Wen, L.; Gao, Z. Direct Identification of Complex Glycans via a Highly Sensitive Engineered Nanopore. J. Am. Chem. Soc. 2024, 146, 13356–13366. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xiong, Y.; Cao, Y.; Zhang, C.; Li, Y.; Ning, H.; Liu, F.; Zhou, H.; Li, X.; Ye, X.; et al. Identification of tagged glycans with a protein nanopore. Nat. Commun. 2023, 14, 1737. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.-Z.; Li, M.-Y.; Ying, Y.-L.; Long, Y.-T. Is the Volume Exclusion Model Practicable for Nanopore Protein Sequencing? Anal. Chem. 2021, 93, 11364–11369. [Google Scholar] [CrossRef]
- Nivala, J.; Marks, D.B.; Akeson, M. Unfoldase-mediated protein translocation through an α-hemolysin nanopore. Nat. Biotechnol. 2013, 31, 247–250. [Google Scholar] [CrossRef]
- Motone, K.; Kontogiorgos-Heintz, D.; Wee, J.; Kurihara, K.; Yang, S.; Roote, G.; Fox, O.E.; Fang, Y.; Queen, M.; Tolhurst, M.; et al. Multi-pass, single-molecule nanopore reading of long protein strands. Nature 2024, 633, 662–669. [Google Scholar] [CrossRef]
- Silva, D.A.; Yu, S.; Ulge, U.Y.; Spangler, J.B.; Jude, K.M.; Labão-Almeida, C.; Ali, L.R.; Quijano-Rubio, A.; Ruterbusch, M.; Leung, I.; et al. De novo design of potent and selective mimics of IL-2 and IL-15. Nature 2019, 565, 186–191. [Google Scholar] [CrossRef]
- Huang, P.-S.; Boyken, S.E.; Baker, D. The coming of age of de novo protein design. Nature 2016, 537, 320–327. [Google Scholar] [CrossRef]
- Kortemme, T. De novo protein design—From new structures to programmable functions. Cell 2024, 187, 526–544. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, J.B.; Baranov, M.; Costello, Z.; Barber, K.W.; Wang, W.; Ismail, A.; Frappier, V.; Lord, D.M.; Ng-Thow-Hing, C.; Van Vlack, E.R.; et al. Illuminating protein space with a programmable generative model. Nature 2023, 623, 1070–1078. [Google Scholar] [CrossRef]
- Anishchenko, I.; Pellock, S.J.; Chidyausiku, T.M.; Ramelot, T.A.; Ovchinnikov, S.; Hao, J.; Bafna, K.; Norn, C.; Kang, A.; Bera, A.K.; et al. De novo protein design by deep network hallucination. Nature 2021, 600, 547–552. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Dauparas, J.; Anishchenko, I.; Bennett, N.; Bai, H.; Ragotte, R.J.; Milles, L.F.; Wicky, B.I.M.; Courbet, A.; de Haas, R.J.; Bethel, N.; et al. Robust deep learning–based protein sequence design using ProteinMPNN. Science 2022, 378, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.L.; Juergens, D.; Bennett, N.R.; Trippe, B.L.; Yim, J.; Eisenach, H.E.; Ahern, W.; Borst, A.J.; Ragotte, R.J.; Milles, L.F.; et al. De novo design of protein structure and function with RFdiffusion. Nature 2023, 620, 1089–1100. [Google Scholar] [CrossRef]
- Wicky, B.I.M.; Milles, L.F.; Courbet, A.; Ragotte, R.J.; Dauparas, J.; Kinfu, E.; Tipps, S.; Kibler, R.D.; Baek, M.; DiMaio, F.; et al. Hallucinating symmetric protein assemblies. Science 2022, 378, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Rubio, A.; Yeh, H.-W.; Park, J.; Lee, H.; Langan, R.A.; Boyken, S.E.; Lajoie, M.J.; Cao, L.; Chow, C.M.; Miranda, M.C.; et al. De novo design of modular and tunable protein biosensors. Nature 2021, 591, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Ferruz, N.; Schmidt, S.; Höcker, B. ProtGPT2 is a deep unsupervised language model for protein design. Nat. Commun. 2022, 13, 4348. [Google Scholar] [CrossRef]
- Chu, A.E.; Lu, T.; Huang, P.S. Sparks of function by de novo protein design. Nat. Biotechnol. 2024, 42, 203–215. [Google Scholar] [CrossRef]
- Cao, L.; Goreshnik, I.; Coventry, B.; Case, J.B.; Miller, L.; Kozodoy, L.; Chen, R.E.; Carter, L.; Walls, A.C.; Park, Y.-J.; et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 2020, 370, 426–431. [Google Scholar] [CrossRef]
- Peraro, M.D.; van der Goot, F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016, 14, 77–92. [Google Scholar] [CrossRef]
- Cosentino, K.; Ros, U.; García-Sáez, A.J. Assembling the puzzle: Oligomerization of α-pore forming proteins in membranes. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.W.F.; Ghimire, M.L.; Reiner, J.E. Nanopore sensing: A physical-chemical approach. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183644. [Google Scholar] [CrossRef]
- Song, L.; Hobaugh, M.R.; Shustak, C.; Cheley, S.; Bayley, H.; Gouaux, J.E. Structure of staphylococcal alpha-hemolysin, a heptameric transmembrane pore. Science 1996, 274, 1859–1866. [Google Scholar] [CrossRef]
- Meller, A.; Nivon, L.; Brandin, E.; Golovchenko, J.; Branton, D. Rapid nanopore discrimination between single polynucleotide molecules. Proc. Natl. Acad. Sci. USA 2000, 97, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, D.; Heron, A.J.; Klingelhoefer, J.; Mikhailova, E.; Maglia, G.; Bayley, H. Nucleobase recognition in ssDNA at the central constriction of the alpha-hemolysin pore. Nano Lett. 2010, 10, 3633–3637. [Google Scholar] [CrossRef]
- Kasianowicz, J.; Walker, B.; Krishnasastry, M.; Bayley, H. Genetically Engineered Pores as Metal ION Biosensors. MRS Online Proc. Libr. 1993, 330, 217–223. [Google Scholar] [CrossRef]
- Braha, O.; Gu, L.Q.; Zhou, L.; Lu, X.; Cheley, S.; Bayley, H. Simultaneous stochastic sensing of divalent metal ions. Nat. Biotechnol. 2000, 18, 1005–1007. [Google Scholar] [CrossRef]
- Gu, L.-Q.; Braha, O.; Conlan, S.; Cheley, S.; Bayley, H. Stochastic sensing of organic analytes by a pore-forming protein containing a molecular adapter. Nature 1999, 398, 686–690. [Google Scholar] [CrossRef]
- Versloot, R.C.A.; Straathof, S.A.P.; Stouwie, G.; Tadema, M.J.; Maglia, G. β-Barrel Nanopores with an Acidic-Aromatic Sensing Region Identify Proteinogenic Peptides at Low pH. ACS Nano 2022, 16, 7258–7268. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Cirauqui, N.; Marcaida, M.J.; Buglakova, E.; Duperrex, A.; Radenovic, A.; Dal Peraro, M. Single-molecule sensing of peptides and nucleic acids by engineered aerolysin nanopores. Nat. Commun. 2019, 10, 4918. [Google Scholar] [CrossRef] [PubMed]
- Sauciuc, A.; Morozzo della Rocca, B.; Tadema, M.J.; Chinappi, M.; Maglia, G. Translocation of linearized full-length proteins through an engineered nanopore under opposing electrophoretic force. Nat. Biotechnol. 2024, 42, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Anton, J.S.; Iacovache, I.; Bada Juarez, J.F.; Abriata, L.A.; Perrin, L.W.; Cao, C.; Marcaida, M.J.; Zuber, B.; Dal Peraro, M. Aerolysin Nanopore Structures Revealed at High Resolution in a Lipid Environment. J. Am. Chem. Soc. 2025, 147, 4984–4992. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, H.; Sarthak, K.; Ensslen, T.; Piguet, F.; Manivet, P.; Pelta, J.; Behrends, J.C.; Aksimentiev, A.; Oukhaled, A. Electrical recognition of the twenty proteinogenic amino acids using an aerolysin nanopore. Nat. Biotechnol. 2020, 38, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Derrington, I.M.; Butler, T.Z.; Collins, M.D.; Manrao, E.; Pavlenok, M.; Niederweis, M.; Gundlach, J.H. Nanopore DNA sequencing with MspA. Proc. Natl. Acad. Sci. USA 2010, 107, 16060–16065. [Google Scholar] [CrossRef]
- Butler, T.Z.; Pavlenok, M.; Derrington, I.M.; Niederweis, M.; Gundlach, J.H. Single-molecule DNA detection with an engineered MspA protein nanopore. Proc. Natl. Acad. Sci. USA 2008, 105, 20647–20652. [Google Scholar] [CrossRef]
- Jain, M.; Tyson, J.R.; Loose, M.; Ip, C.L.C.; Eccles, D.A.; O’Grady, J.; Malla, S.; Leggett, R.M.; Wallerman, O.; Jansen, H.J.; et al. MinION Analysis and Reference Consortium: Phase 2 data release and analysis of R9.0 chemistry. F1000Research 2017, 6, 760. [Google Scholar] [CrossRef]
- Goyal, P.; Krasteva, P.V.; Van Gerven, N.; Gubellini, F.; Van den Broeck, I.; Troupiotis-Tsailaki, A.; Jonckheere, W.; Pehau-Arnaudet, G.; Pinkner, J.S.; Chapman, M.R.; et al. Structural and mechanistic insights into the bacterial amyloid secretion channel CsgG. Nature 2014, 516, 250–253. [Google Scholar] [CrossRef]
- Fahie, M.A.; Yang, B.; Mullis, M.; Holden, M.A.; Chen, M. Selective Detection of Protein Homologues in Serum Using an OmpG Nanopore. Anal. Chem. 2015, 87, 11143–11149. [Google Scholar] [CrossRef]
- Mohammad, M.M.; Iyer, R.; Howard, K.R.; McPike, M.P.; Borer, P.N.; Movileanu, L. Engineering a Rigid Protein Tunnel for Biomolecular Detection. J. Am. Chem. Soc. 2012, 134, 9521–9531. [Google Scholar] [CrossRef]
- Fahie, M.A.; Candido, J.; Andree, G.; Chen, M. Tuning Protein Discrimination Through Altering the Sampling Interface Formed between the Analyte and the OmpG Nanopore. ACS Sens. 2021, 6, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Ha, J.-H.; Mayse, L.A.; Presti, M.F.; Wolfe, A.J.; Moody, K.J.; Loh, S.N.; Movileanu, L. A generalizable nanopore sensor for highly specific protein detection at single-molecule precision. Nat. Commun. 2023, 14, 1374. [Google Scholar] [CrossRef] [PubMed]
- Soskine, M.; Biesemans, A.; Maglia, G. Single-Molecule Analyte Recognition with ClyA Nanopores Equipped with Internal Protein Adaptors. J. Am. Chem. Soc. 2015, 137, 5793–5797. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.-K.; Kim, J.-S.; Lee, M.-K.; Ryu, K.-S.; Chi, S.-W. Probing the Neuraminidase Activity of Influenza Virus Using a Cytolysin A Protein Nanopore. Anal. Chem. 2020, 92, 14303–14308. [Google Scholar] [CrossRef]
- Tanaka, K.; Caaveiro, J.M.; Morante, K.; González-Mañas, J.M.; Tsumoto, K. Structural basis for self-assembly of a cytolytic pore lined by protein and lipid. Nat. Commun. 2015, 6, 6337. [Google Scholar] [CrossRef]
- Huang, G.; Voet, A.; Maglia, G. FraC nanopores with adjustable diameter identify the mass of opposite-charge peptides with 44 dalton resolution. Nat. Commun. 2019, 10, 835. [Google Scholar] [CrossRef]
- Straathof, S.; Di Muccio, G.; Yelleswarapu, M.; Alzate Banguero, M.; Wloka, C.; van der Heide, N.J.; Chinappi, M.; Maglia, G. Protein Sizing with 15 nm Conical Biological Nanopore YaxAB. ACS Nano 2023, 17, 13685–13699. [Google Scholar] [CrossRef]
- Jeong, K.-B.; Ryu, M.; Kim, J.-S.; Kim, M.; Yoo, J.; Chung, M.; Oh, S.; Jo, G.; Lee, S.-G.; Kim, H.M.; et al. Single-molecule fingerprinting of protein-drug interaction using a funneled biological nanopore. Nat. Commun. 2023, 14, 1461. [Google Scholar] [CrossRef]
- Zhang, C.; He, Y.; Wang, J.; Chen, T.; Baltar, F.; Hu, M.; Liao, J.; Xiao, X.; Li, Z.-R.; Dong, X. LucaPCycle: Illuminating microbial phosphorus cycling in deep-sea cold seep sediments using protein language models. Nat. Commun. 2025, 16, 4862. [Google Scholar] [CrossRef]
- Szalkai, B.; Grolmusz, V. MetaHMM: A webserver for identifying novel genes with specified functions in metagenomic samples. Genomics 2019, 111, 883–885. [Google Scholar] [CrossRef]
- Barrio-Hernandez, I.; Yeo, J.; Jänes, J.; Mirdita, M.; Gilchrist, C.L.M.; Wein, T.; Varadi, M.; Velankar, S.; Beltrao, P.; Steinegger, M. Clustering predicted structures at the scale of the known protein universe. Nature 2023, 622, 637–645. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhang, Y.; Wang, L.; Guo, F.; Yun, Q.; Zeng, T.; Yan, X.; Yu, L.; Cheng, L.; Wu, W.; et al. A single-molecule nanopore sequencing platform. bioRxiv 2024. [Google Scholar] [CrossRef]
- Stoddart, D.; Maglia, G.; Mikhailova, E.; Heron, A.J.; Bayley, H. Multiple base-recognition sites in a biological nanopore: Two heads are better than one. Angew. Chem. Int. Ed. Engl. 2010, 49, 556–559. [Google Scholar] [CrossRef]
- Schubeis, T.; Spehr, J.; Viereck, J.; Köpping, L.; Nagaraj, M.; Ahmed, M.; Ritter, C. Structural and functional characterization of the Curli adaptor protein CsgF. FEBS Lett. 2018, 592, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Van der Verren, S.E.; Van Gerven, N.; Jonckheere, W.; Hambley, R.; Singh, P.; Kilgour, J.; Jordan, M.; Wallace, E.J.; Jayasinghe, L.; Remaut, H. A dual-constriction biological nanopore resolves homonucleotide sequences with high fidelity. Nat. Biotechnol. 2020, 38, 1415–1420. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, H.; Zhang, X.; Zhang, X.; Huang, Y. Cryo-EM structure of the nonameric CsgG-CsgF complex and its implications for controlling curli biogenesis in Enterobacteriaceae. PLoS Biol. 2020, 18, e3000748. [Google Scholar] [CrossRef] [PubMed]
- Howorka, S.; Siwy, Z.S. Reading amino acids in a nanopore. Nat. Biotechnol. 2020, 38, 159–160. [Google Scholar] [CrossRef]
- Brinkerhoff, H.; Kang, A.S.W.; Liu, J.; Aksimentiev, A.; Dekker, C. Multiple rereads of single proteins at single–amino acid resolution using nanopores. Science 2021, 374, 1509–1513. [Google Scholar] [CrossRef]
- Yu, L.; Kang, X.; Li, F.; Mehrafrooz, B.; Makhamreh, A.; Fallahi, A.; Foster, J.C.; Aksimentiev, A.; Chen, M.; Wanunu, M. Unidirectional single-file transport of full-length proteins through a nanopore. Nat. Biotechnol. 2023, 41, 1130–1139. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, G.; Versloot, R.C.A.; Bruininks, B.M.H.; de Souza, P.C.T.; Marrink, S.J.; Maglia, G. Bottom-up fabrication of a proteasome-nanopore that unravels and processes single proteins. Nat. Chem. 2021, 13, 1192–1199. [Google Scholar] [CrossRef]
- Schweke, H.; Pacesa, M.; Levin, T.; Goverde, C.A.; Kumar, P.; Duhoo, Y.; Dornfeld, L.J.; Dubreuil, B.; Georgeon, S.; Ovchinnikov, S.; et al. An atlas of protein homo-oligomerization across domains of life. Cell 2024, 187, 999–1010.e1015. [Google Scholar] [CrossRef] [PubMed]
- Crnković, A.; Srnko, M.; Anderluh, G. Biological Nanopores: Engineering on Demand. Life 2021, 11, 27. [Google Scholar] [CrossRef]
- Bayley, H.; Cremer, P.S. Stochastic sensors inspired by biology. Nature 2001, 413, 226–230. [Google Scholar] [CrossRef]
- Ratinho, L.; Meyer, N.; Greive, S.; Cressiot, B.; Pelta, J. Nanopore sensing of protein and peptide conformation for point-of-care applications. Nat. Commun. 2025, 16, 3211. [Google Scholar] [CrossRef]
- Zambaldi, V.; La, D.; Chu, A.E.; Patani, H.; Danson, A.E.; Kwan, T.O.C.; Frerix, T.; Schneider, R.G.; Saxton, D.; Thillaisundaram, A.; et al. De novo design of high-affinity protein binders with AlphaProteo. arXiv 2024, arXiv:2409.08022. [Google Scholar] [CrossRef]
- Vorobieva, A.A. Principles and Methods in Computational Membrane Protein Design. J. Mol. Biol. 2021, 433, 167154. [Google Scholar] [CrossRef] [PubMed]
- Wimley, W.C. The versatile β-barrel membrane protein. Curr. Opin. Struct. Biol. 2003, 13, 404–411. [Google Scholar] [CrossRef]
- Shimizu, K.; Mijiddorj, B.; Usami, M.; Mizoguchi, I.; Yoshida, S.; Akayama, S.; Hamada, Y.; Ohyama, A.; Usui, K.; Kawamura, I.; et al. De novo design of a nanopore for single-molecule detection that incorporates a β-hairpin peptide. Nat. Nanotechnol. 2022, 17, 67–75. [Google Scholar] [CrossRef]
- Vorobieva, A.A.; White, P.; Liang, B.; Horne, J.E.; Bera, A.K.; Chow, C.M.; Gerben, S.; Marx, S.; Kang, A.; Stiving, A.Q.; et al. De novo design of transmembrane β barrels. Science 2021, 371, eabc8182. [Google Scholar] [CrossRef]
- Berhanu, S.; Majumder, S.; Müntener, T.; Whitehouse, J.; Berner, C.; Bera, A.K.; Kang, A.; Liang, B.; Khan, N.; Sankaran, B.; et al. Sculpting conducting nanopore size and shape through de novo protein design. Science 2024, 385, 282–288. [Google Scholar] [CrossRef]
- Kim, D.E.; Watson, J.L.; Juergens, D.; Majumder, S.; Sonigra, R.; Gerben, S.R.; Kang, A.; Bera, A.K.; Li, X.; Baker, D. Parametrically guided design of beta barrels and transmembrane nanopores using deep learning. bioRxiv 2025. [Google Scholar] [CrossRef]
- Lu, P.; Min, D.; DiMaio, F.; Wei, K.Y.; Vahey, M.D.; Boyken, S.E.; Chen, Z.; Fallas, J.A.; Ueda, G.; Sheffler, W.; et al. Accurate computational design of multipass transmembrane proteins. Science 2018, 359, 1042–1046. [Google Scholar] [CrossRef]
- Xu, C.; Lu, P.; Gamal El-Din, T.M.; Pei, X.Y.; Johnson, M.C.; Uyeda, A.; Bick, M.J.; Xu, Q.; Jiang, D.; Bai, H.; et al. Computational design of transmembrane pores. Nature 2020, 585, 129–134. [Google Scholar] [CrossRef]
- Niitsu, A.; Thomson, A.R.; Scott, A.J.; Sengel, J.T.; Jung, J.; Mahendran, K.R.; Sodeoka, M.; Bayley, H.; Sugita, Y.; Woolfson, D.N.; et al. Rational Design Principles for De Novo α-Helical Peptide Barrels with Dynamic Conductive Channels. J. Am. Chem. Soc. 2025, 147, 11741–11753. [Google Scholar] [CrossRef]
- Fujita, S.; Kawamura, I.; Kawano, R. Cell-Free Expression of De Novo Designed Peptides That Form β-Barrel Nanopores. ACS Nano 2023, 17, 3358–3367. [Google Scholar] [CrossRef]
- Leman, J.K.; Weitzner, B.D.; Lewis, S.M.; Adolf-Bryfogle, J.; Alam, N.; Alford, R.F.; Aprahamian, M.; Baker, D.; Barlow, K.A.; Barth, P.; et al. Macromolecular modeling and design in Rosetta: Recent methods and frameworks. Nat. Methods 2020, 17, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.E.; Jensen, D.R.; Feldman, D.; Tischer, D.; Saleem, A.; Chow, C.M.; Li, X.; Carter, L.; Milles, L.; Nguyen, H.; et al. De novo design of small beta barrel proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2207974120. [Google Scholar] [CrossRef]
- Dolorfino, M.; Samanta, R.; Vorobieva, A. ProteinMPNN Recovers Complex Sequence Properties of Transmembrane β-barrels. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Joh, N.H.; Wang, T.; Bhate, M.P.; Acharya, R.; Wu, Y.; Grabe, M.; Hong, M.; Grigoryan, G.; DeGrado, W.F. De novo design of a transmembrane Zn2+-transporting four-helix bundle. Science 2014, 346, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Duran, A.M.; Meiler, J. Computational design of membrane proteins using RosettaMembrane. Protein Sci. 2018, 27, 341–355. [Google Scholar] [CrossRef]
- Boyken, S.E.; Chen, Z.; Groves, B.; Langan, R.A.; Oberdorfer, G.; Ford, A.; Gilmore, J.M.; Xu, C.; DiMaio, F.; Pereira, J.H.; et al. De novo design of protein homo-oligomers with modular hydrogen-bond network–mediated specificity. Science 2016, 352, 680–687. [Google Scholar] [CrossRef] [PubMed]
- Crick, F.H. The Fourier transform of a coiled-coil. Acta Crystallogr. 1953, 6, 685–689. [Google Scholar] [CrossRef]
- Thomson, A.R.; Wood, C.W.; Burton, A.J.; Bartlett, G.J.; Sessions, R.B.; Brady, R.L.; Woolfson, D.N. Computational design of water-soluble α-helical barrels. Science 2014, 346, 485–488. [Google Scholar] [CrossRef]
- Albanese, K.I.; Petrenas, R.; Pirro, F.; Naudin, E.A.; Borucu, U.; Dawson, W.M.; Scott, D.A.; Leggett, G.J.; Weiner, O.D.; Oliver, T.A.A.; et al. Rationally seeded computational protein design of ɑ-helical barrels. Nat. Chem. Biol. 2024, 20, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Dawson, W.M.; Martin, F.J.O.; Rhys, G.G.; Shelley, K.L.; Brady, R.L.; Woolfson, D.N. Coiled coils 9-to-5: Rational de novo design of α-helical barrels with tunable oligomeric states. Chem. Sci. 2021, 12, 6923–6928. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Niitsu, A.; Kratochvil, H.T.; Lang, E.J.M.; Sengel, J.T.; Dawson, W.M.; Mahendran, K.R.; Mravic, M.; Thomson, A.R.; Brady, R.L.; et al. Constructing ion channels from water-soluble α-helical barrels. Nat. Chem. 2021, 13, 643–650. [Google Scholar] [CrossRef]
- Bennett, N.R.; Coventry, B.; Goreshnik, I.; Huang, B.; Allen, A.; Vafeados, D.; Peng, Y.P.; Dauparas, J.; Baek, M.; Stewart, L.; et al. Improving de novo protein binder design with deep learning. Nat. Commun. 2023, 14, 2625. [Google Scholar] [CrossRef]
- Vázquez Torres, S.; Leung, P.J.Y.; Venkatesh, P.; Lutz, I.D.; Hink, F.; Huynh, H.-H.; Becker, J.; Yeh, A.H.-W.; Juergens, D.; Bennett, N.R.; et al. De novo design of high-affinity binders of bioactive helical peptides. Nature 2024, 626, 435–442. [Google Scholar] [CrossRef]
- Pacesa, M.; Nickel, L.; Schellhaas, C.; Schmidt, J.; Pyatova, E.; Kissling, L.; Barendse, P.; Choudhury, J.; Kapoor, S.; Alcaraz-Serna, A.; et al. One-shot design of functional protein binders with BindCraft. Nature 2025, 646, 483–492. [Google Scholar] [CrossRef]
- Tinberg, C.E.; Khare, S.D.; Dou, J.; Doyle, L.; Nelson, J.W.; Schena, A.; Jankowski, W.; Kalodimos, C.G.; Johnsson, K.; Stoddard, B.L.; et al. Computational design of ligand-binding proteins with high affinity and selectivity. Nature 2013, 501, 212–216. [Google Scholar] [CrossRef]
- Dou, J.; Vorobieva, A.A.; Sheffler, W.; Doyle, L.A.; Park, H.; Bick, M.J.; Mao, B.; Foight, G.W.; Lee, M.Y.; Gagnon, L.A.; et al. De novo design of a fluorescence-activating β-barrel. Nature 2018, 561, 485–491. [Google Scholar] [CrossRef]
- Rotem, D.; Jayasinghe, L.; Salichou, M.; Bayley, H. Protein Detection by Nanopores Equipped with Aptamers. J. Am. Chem. Soc. 2012, 134, 2781–2787. [Google Scholar] [CrossRef]
- Thakur, A.K.; Movileanu, L. Real-time measurement of protein–protein interactions at single-molecule resolution using a biological nanopore. Nat. Biotechnol. 2019, 37, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A.; Silva, D.-A.; Rocklin, G.J.; Hicks, D.R.; Vergara, R.; Murapa, P.; Bernard, S.M.; Zhang, L.; Lam, K.-H.; Yao, G.; et al. Massively parallel de novo protein design for targeted therapeutics. Nature 2017, 550, 74–79. [Google Scholar] [CrossRef]
- Linsky, T.W.; Vergara, R.; Codina, N.; Nelson, J.W.; Walker, M.J.; Su, W.; Barnes, C.O.; Hsiang, T.-Y.; Esser-Nobis, K.; Yu, K.; et al. De novo design of potent and resilient hACE2 decoys to neutralize SARS-CoV-2. Science 2020, 370, 1208–1214. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Feng, L.; Zhang, C.; Lai, L. Target-Specific De Novo Peptide Binder Design with DiffPepBuilder. J. Chem. Inf. Model. 2024, 64, 9135–9149. [Google Scholar] [CrossRef]
- Polizzi, N.F.; DeGrado, W.F. A defined structural unit enables de novo design of small-molecule–binding proteins. Science 2020, 369, 1227–1233. [Google Scholar] [CrossRef]
- Koehl, A.; Jagota, M.; Erdmann-Pham, D.D.; Fung, A.; Song, Y.S. Transferability of Geometric Patterns from Protein Self-Interactions to Protein-Ligand Interactions. Pac. Symp. Biocomput. 2022, 27, 22–33. [Google Scholar]
- Lu, L.; Gou, X.; Tan, S.K.; Mann, S.I.; Yang, H.; Zhong, X.; Gazgalis, D.; Valdiviezo, J.; Jo, H.; Wu, Y.; et al. De novo design of drug-binding proteins with predictable binding energy and specificity. Science 2024, 384, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Wang, J.; Ahern, W.; Sturmfels, P.; Venkatesh, P.; Kalvet, I.; Lee, G.R.; Morey-Burrows, F.S.; Anishchenko, I.; Humphreys, I.R.; et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 2024, 384, eadl2528. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.R.; Pellock, S.J.; Norn, C.; Tischer, D.; Dauparas, J.; Anischenko, I.; Mercer, J.A.M.; Kang, A.; Bera, A.; Nguyen, H.; et al. Small-molecule binding and sensing with a designed protein family. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Dauparas, J.; Lee, G.R.; Pecoraro, R.; An, L.; Anishchenko, I.; Glasscock, C.; Baker, D. Atomic context-conditioned protein sequence design using LigandMPNN. Nat. Methods 2025, 22, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Leaver-Fay, A.; Tyka, M.; Lewis, S.M.; Lange, O.F.; Thompson, J.; Jacak, R.; Kaufman, K.W.; Renfrew, P.D.; Smith, C.A.; Sheffler, W.; et al. Methods in Enzymology; Johnson, M.L., Brand, L., Eds.; Academic Press: Cambridge, MA, USA, 2011; Volume 487, pp. 545–574. [Google Scholar]
- An, L.; Said, M.; Tran, L.; Majumder, S.; Goreshnik, I.; Lee, G.R.; Juergens, D.; Dauparas, J.; Anishchenko, I.; Coventry, B.; et al. Binding and sensing diverse small molecules using shape-complementary pseudocycles. Science 2024, 385, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
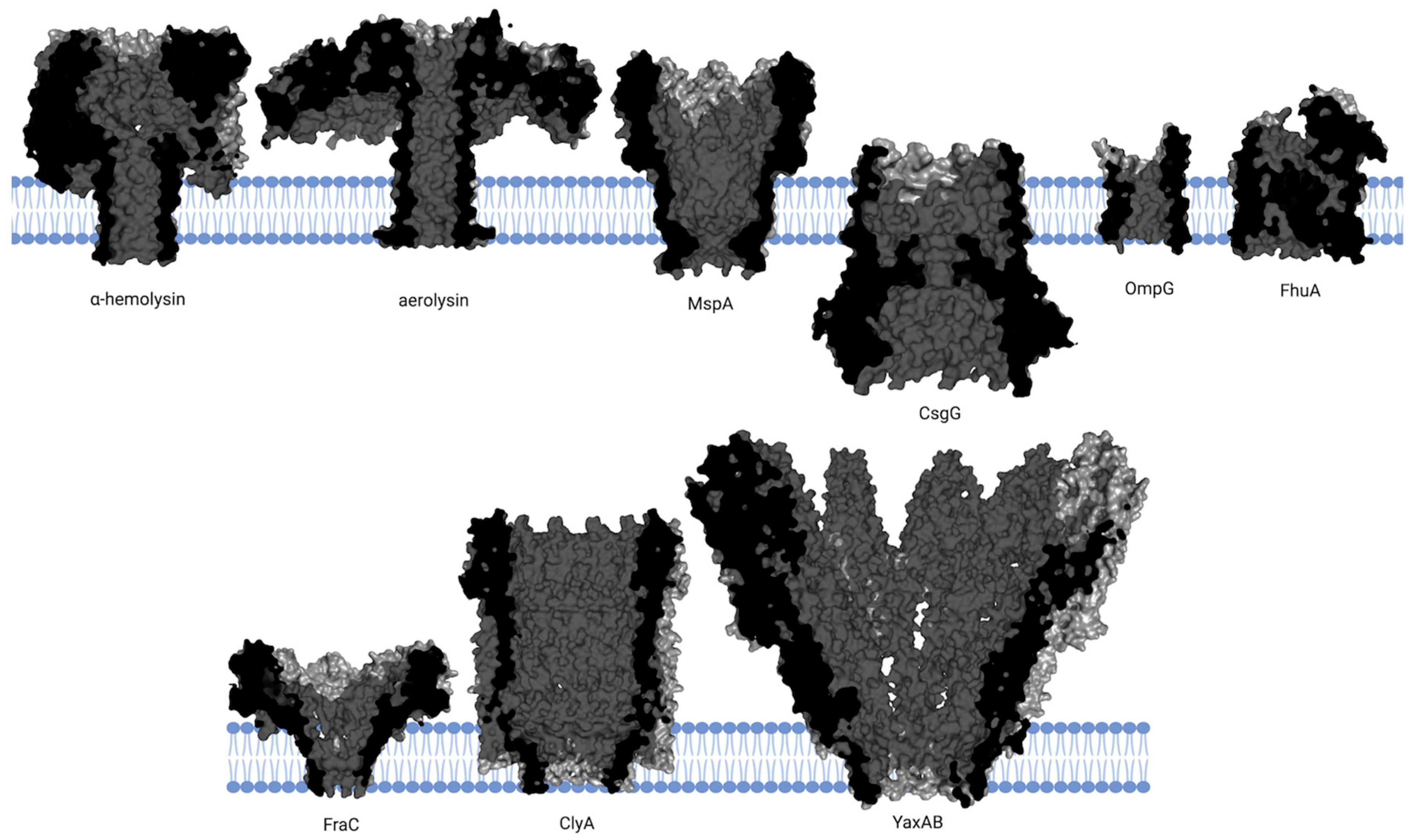
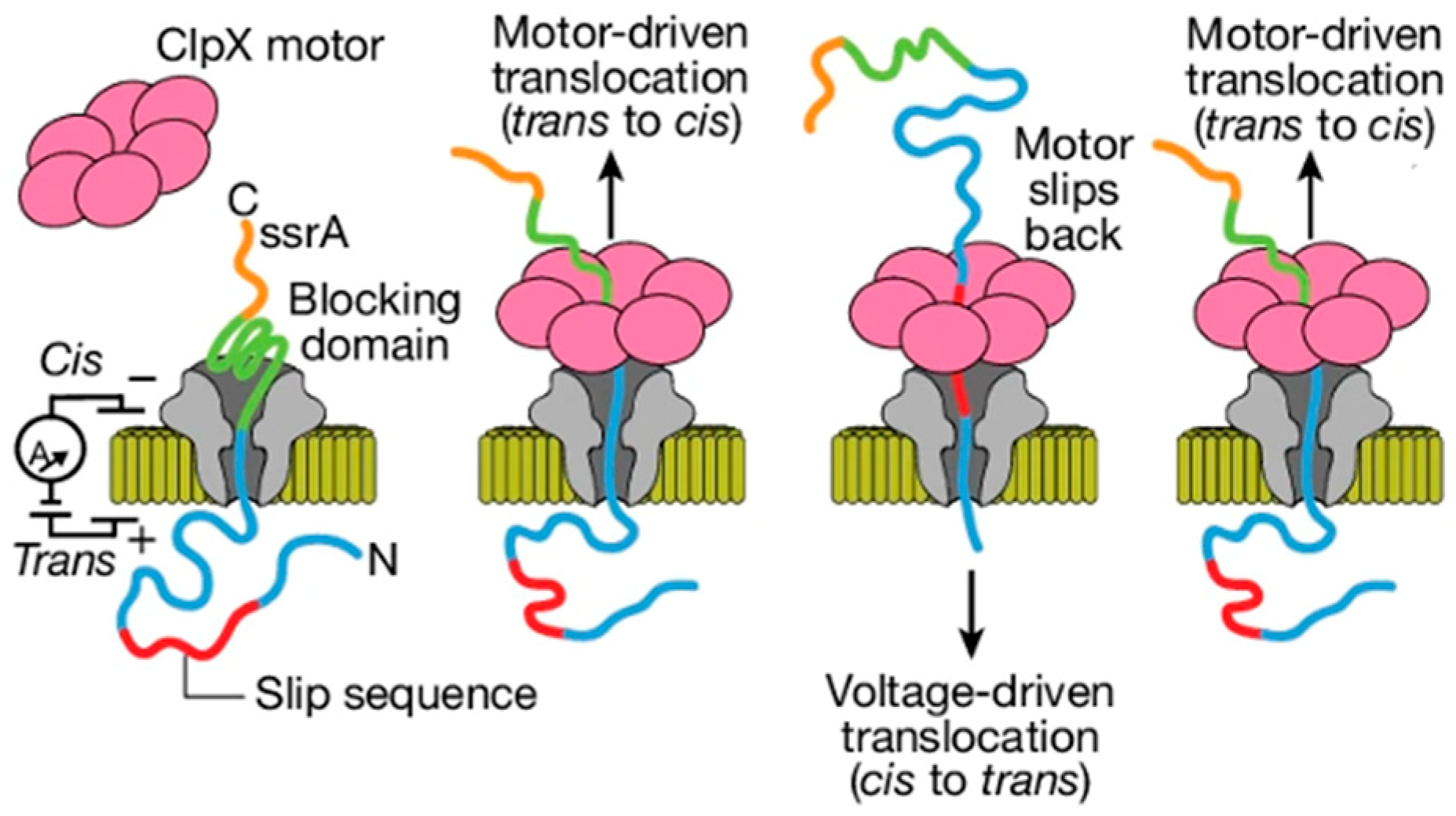

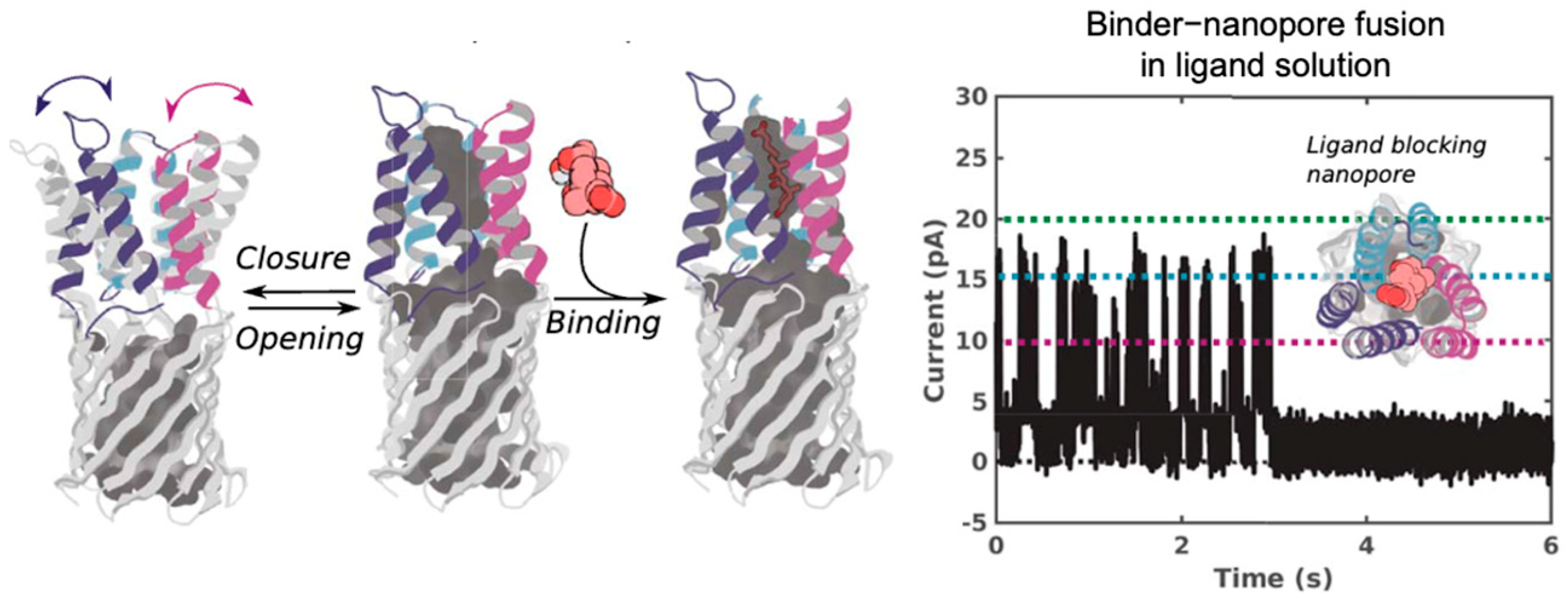
| Category | Structure | Performance | Application Scope | Key Advantages | Limitations |
|---|---|---|---|---|---|
| Native Nanopores | Mushroom- or funnel-shaped pores with varying diameters | Stable and reproducible single-molecule signals | DNA/RNA sequencing; protein detection | High signal stability; biologically robust; wide range of sizes | Limited natural pool; restricted tunability |
| Engineered Nanopores | Multi-domain fusion based on native scaffolds | Enhanced resolution and gating dynamics; superior single-molecule performance | Advanced DNA and protein analysis and sequencing | Achieves customized functional enhancement while maintaining native pore stability | Constrained by natural backbone; limited modification space |
| De novo designed Nanopores | Cylindrical pores with tunable geometry and pore diameter; incorporation of novel sensing components | Full control over architecture, stability, and binding properties | Specific molecular recognition and custom biosensing | Breaks limitations of native and engineered pores; enables novel functionalities | Difficult to achieve robust, stable, and reproducible pore function; design and validation remain complex |
| Design Method | Key Parameters/Strategy | Outcomes | References | |
|---|---|---|---|---|
| β-barrel nanopores | Rational design | β-strands; β-turn; stabilizing elements; hydrogen-bond network. | SV28 nanopore; SVG28 nanopore. | [88] |
| Rosetta design | Parameters: number of strands (n); shear number (s); barrel length (I) 2D blueprint → 3D backbone → sequence design. | 8–14-stranded barrels; triangular, oval, and rectangular cross-sections, electrical conductance comparable to natural pores. | [89,90] | |
| Parametric design & Deep learning | Parameters: strand number, shear number; strand length refined with RFjoint2 and RFdiffusion | Higher success rate; 16-stranded pore. | [91] | |
| α-helical nanopores | Parametric and Rosetta | 3 regions: aqueous, hydrophobic lipid core, internal core. | Atomic-level control over protein topology and transmembrane orientation. | [92] |
| Parametric and Rosetta | Tuning Crick equation parameters for pore size and shape. | TMHC6 (ion selective), TMH4C4 (small molecule permeable). | [93] | |
| Rational Design | Heptad repeat (abcdefg) | Stable self-assembling barrels | [94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, C. Protein Design Meets Single-Molecule Detection: Towards Programmable Nanopore Sensors. Int. J. Mol. Sci. 2025, 26, 10561. https://doi.org/10.3390/ijms262110561
Liu X, Xu C. Protein Design Meets Single-Molecule Detection: Towards Programmable Nanopore Sensors. International Journal of Molecular Sciences. 2025; 26(21):10561. https://doi.org/10.3390/ijms262110561
Chicago/Turabian StyleLiu, Xintong, and Chunfu Xu. 2025. "Protein Design Meets Single-Molecule Detection: Towards Programmable Nanopore Sensors" International Journal of Molecular Sciences 26, no. 21: 10561. https://doi.org/10.3390/ijms262110561
APA StyleLiu, X., & Xu, C. (2025). Protein Design Meets Single-Molecule Detection: Towards Programmable Nanopore Sensors. International Journal of Molecular Sciences, 26(21), 10561. https://doi.org/10.3390/ijms262110561






