Targeting the BCL2 Family: Advances and Challenges in BH3 Mimetic-Based Therapies
Abstract
1. Introduction
2. Targeting the BCL2 Protein Family with BH3 Mimetics in Cancer Therapy
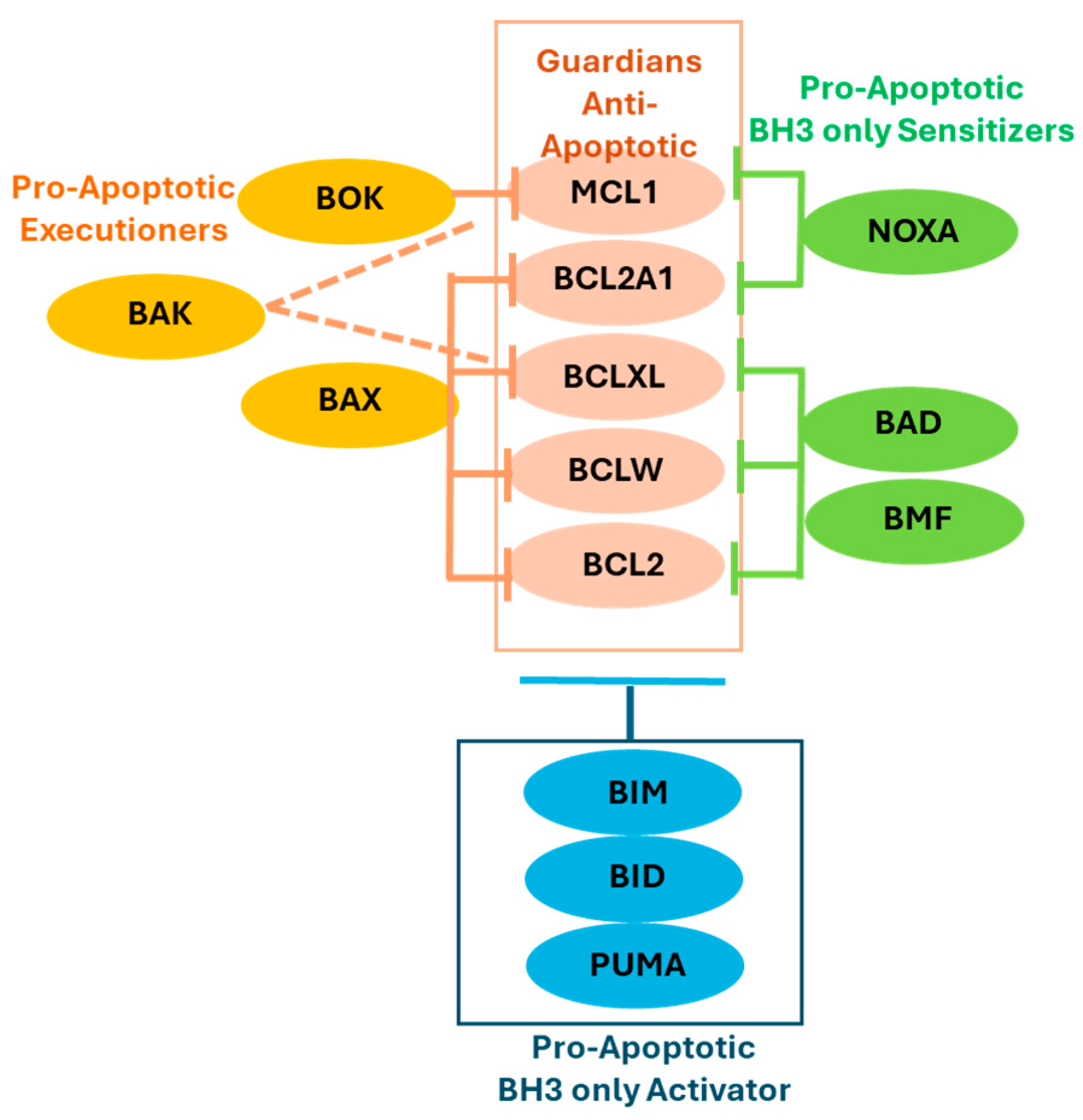
2.1. The BCL2 Protein Family and Its Role in Apoptosis
2.1.1. Anti-Apoptotic Guardians
2.1.2. Pro-Apoptotic Members
2.2. Roles of BCL2 Family Proteins in Cancer
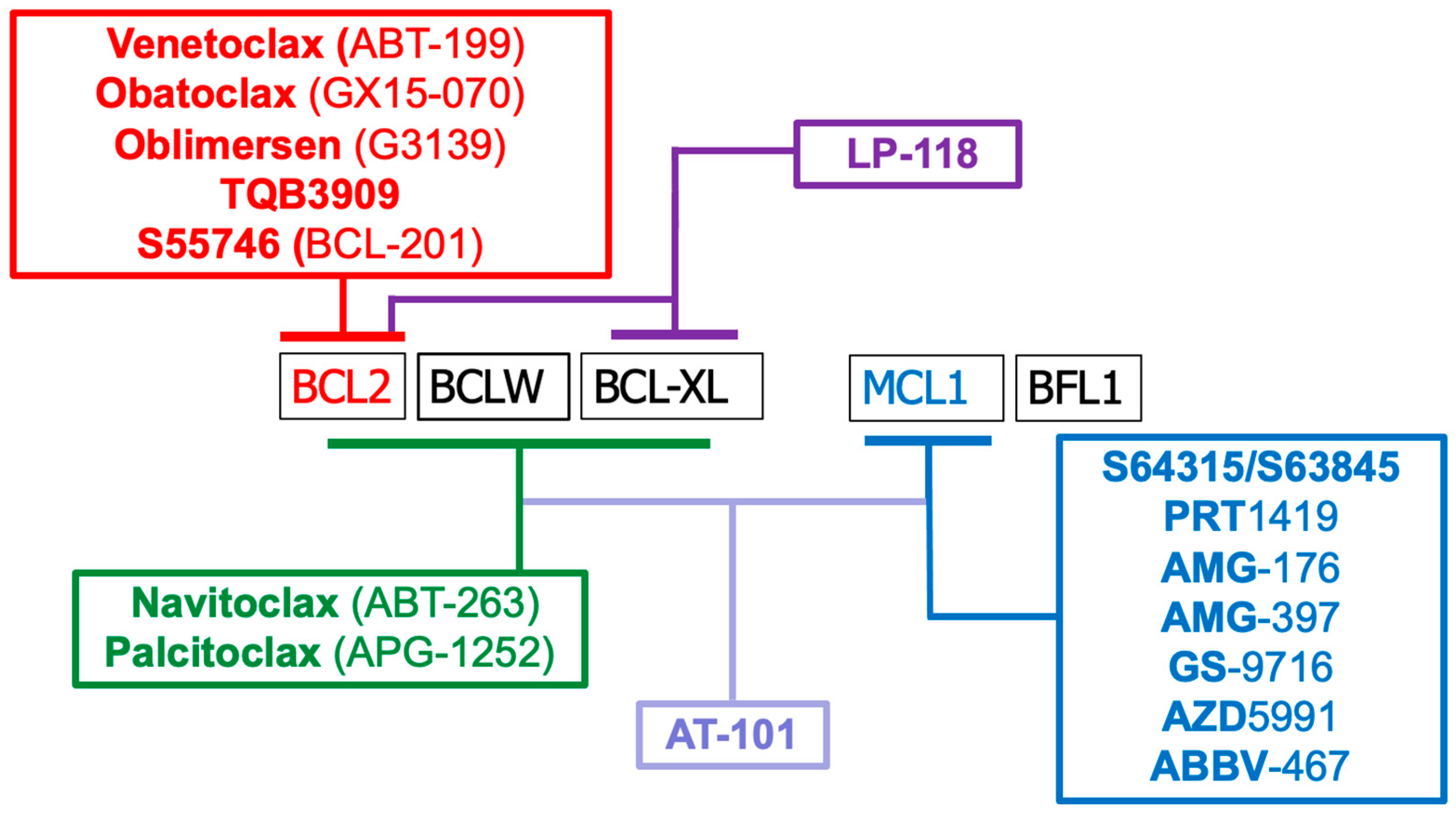
2.3. Modulating BCL2 Family of Proteins with BH3 Mimetics for Cancer Treatments
2.4. Clinical Outcomes of BH3 Mimetics, Adverse Effects, and Future Directions
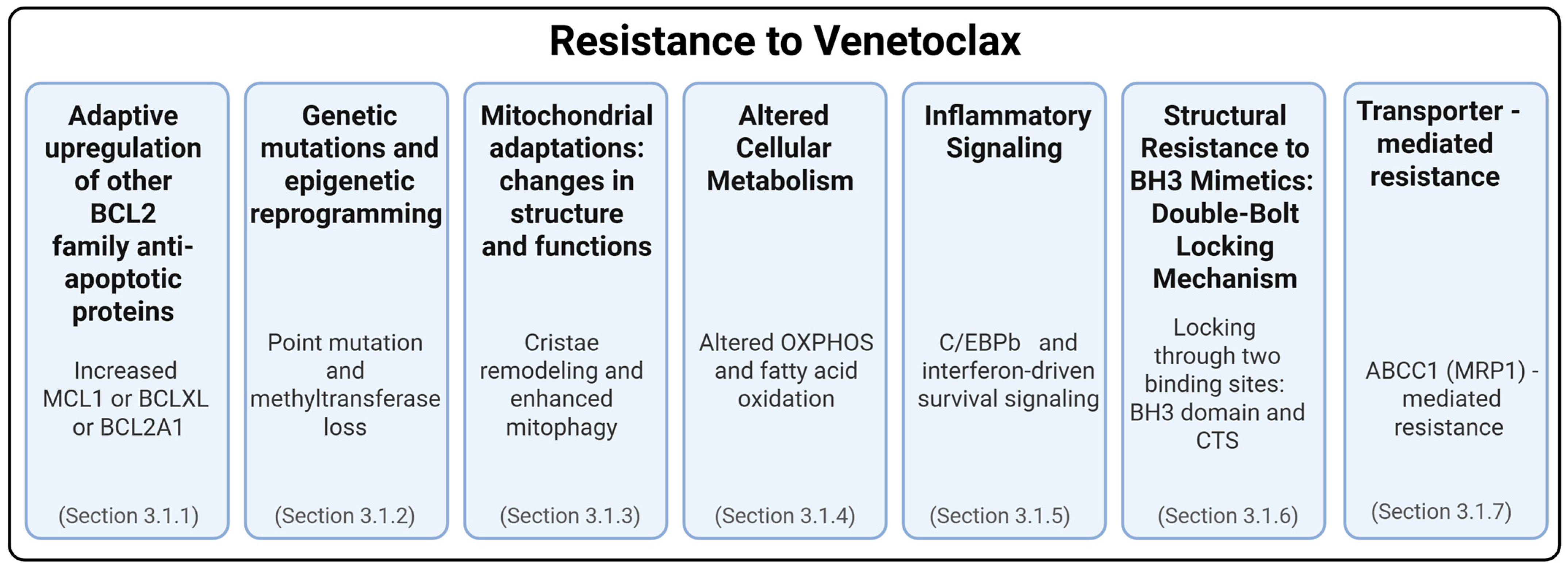
3. Resistance Mechanisms of BH3 Mimetics
3.1. Resistance to Venetoclax
3.1.1. Adaptive Upregulation of Other BCL2 Family Anti-Apoptotic Proteins
3.1.2. Genetic Mutations and Epigenetic Reprogramming in Venetoclax Resistance
3.1.3. Mitochondrial Adaptations: Changes in Structure and Functions
3.1.4. Altered Cellular Metabolism
3.1.5. Inflammatory Signaling
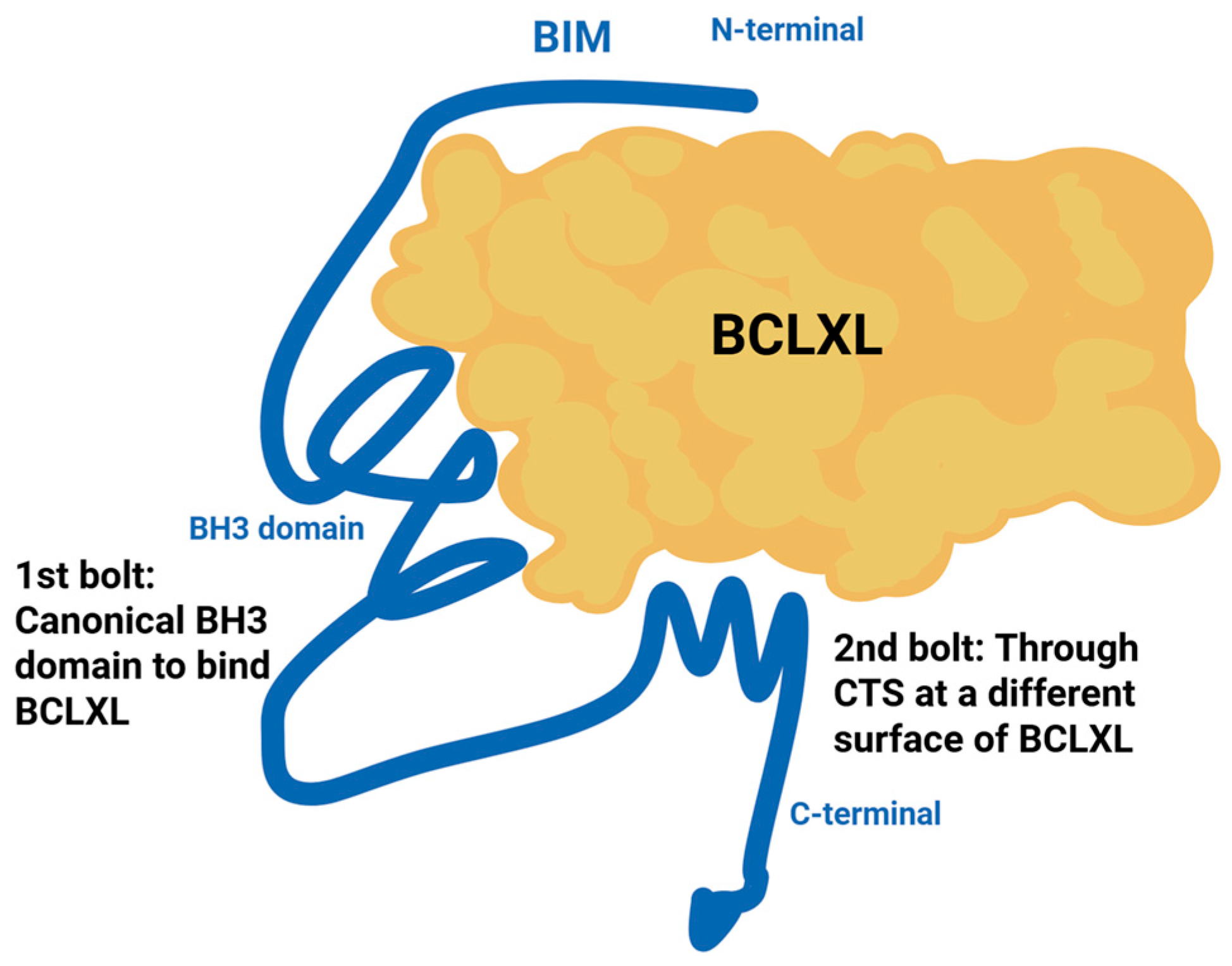
3.1.6. Structural Resistance to BH3 Mimetics: The Double-Bolt Locking Mechanism
3.1.7. Transporter-Mediated Resistance to BH3 Mimetics
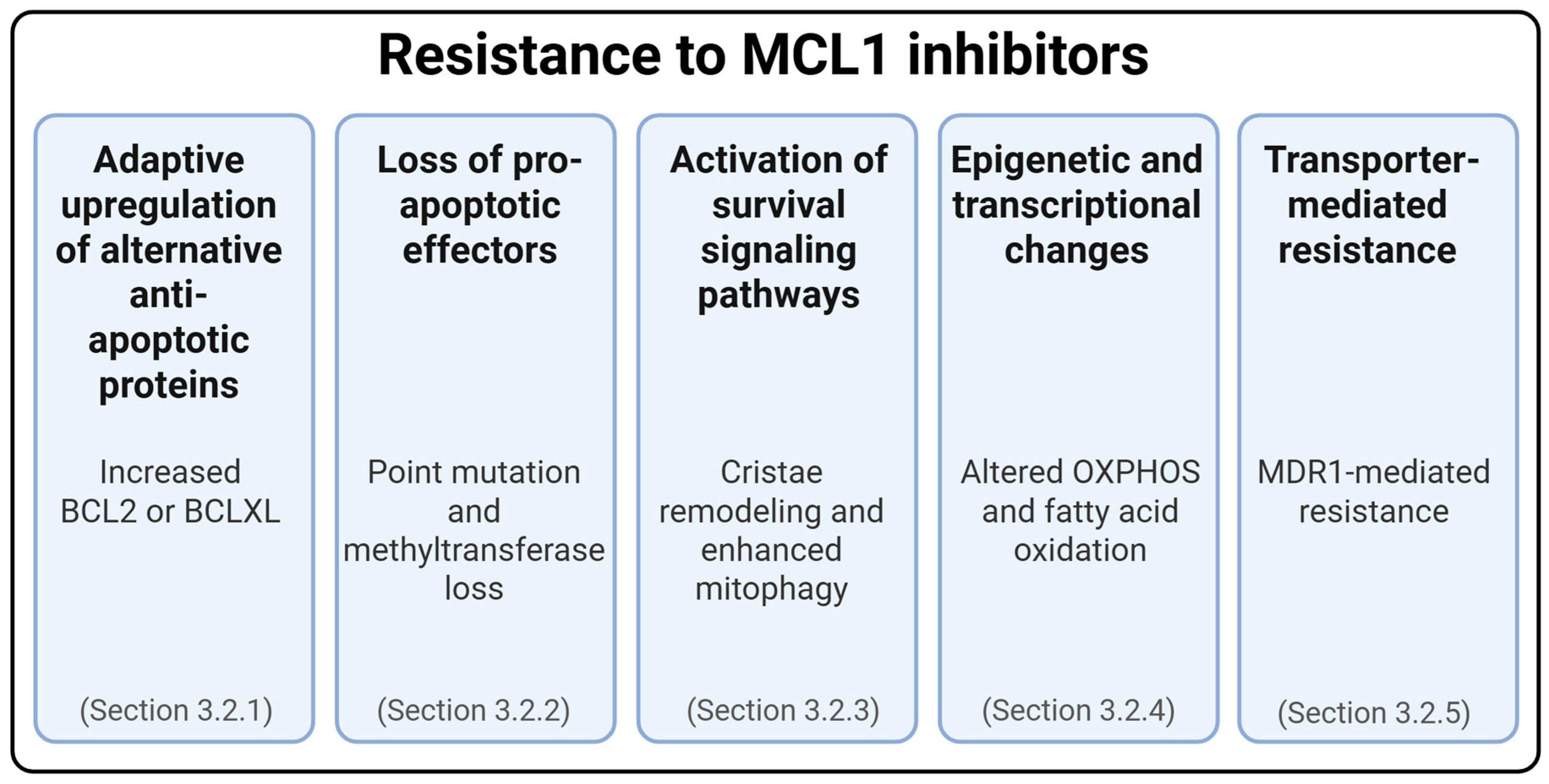
3.2. Resistance to MCL1 Inhibitors
3.2.1. Adaptive Upregulation of Alternative Anti-Apoptotic Proteins
3.2.2. Loss of Pro-Apoptotic Effectors
3.2.3. Activation of Survival Signaling Pathways
3.2.4. Epigenetic and Transcriptional Changes
3.2.5. Transporter-Mediated Cross-Resistance to MCL1 Inhibitors
3.3. Shared and Distinct Mechanisms of Resistance to Venetoclax and MCL1 Inhibitors
3.4. Resistance Mechanisms to BH3 Mimetics in Solid Tumors
4. Combination Approaches to Improve Efficacy of Treating Cancers
4.1. BH3 Mimetics in Combination with Other BH3 Mimetics
4.2. BH3 Mimetics with Targeted Therapy
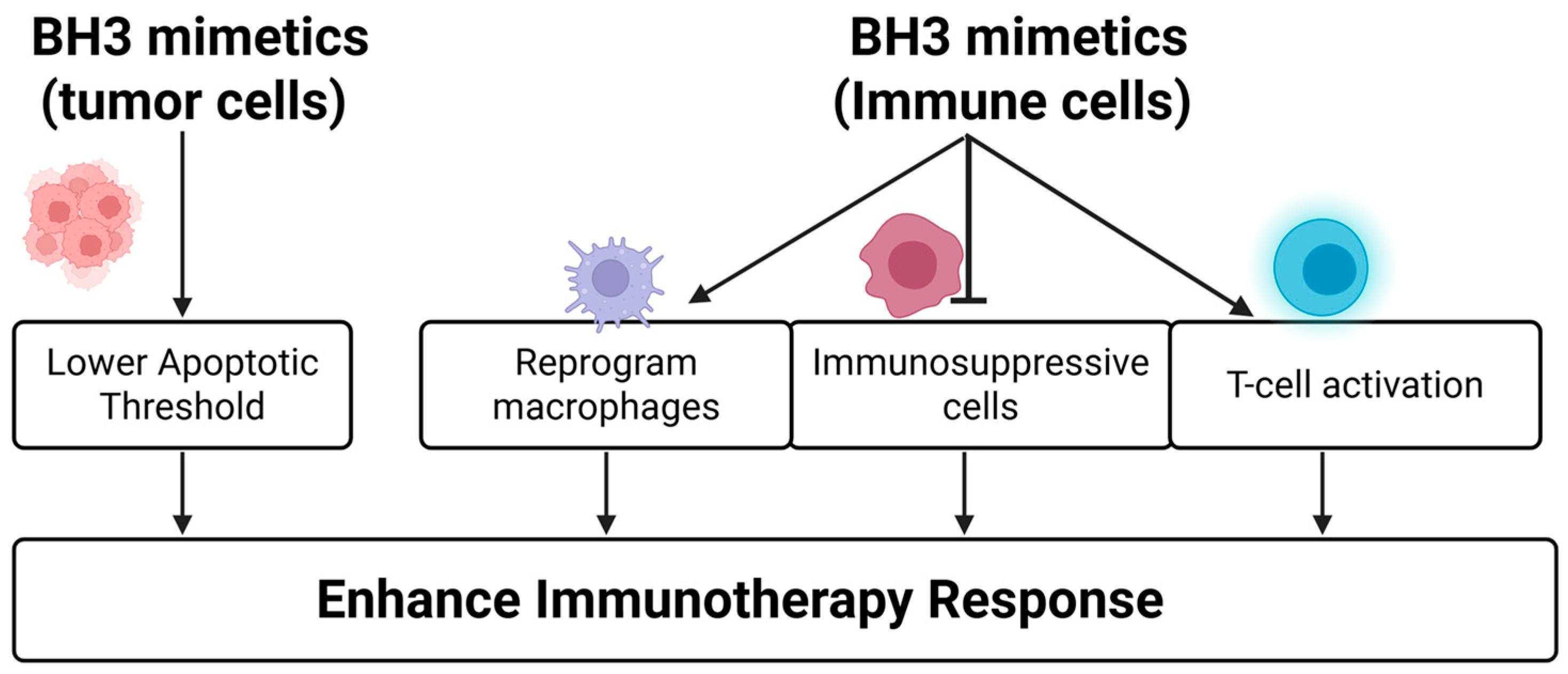
4.3. BH3 Mimetics Enhance Immunotherapies
- Venetoclax increased CD8+ T cell infiltration and effector function without affecting T cell viability, leading to improved responses to anti-PD-1 in preclinical models [143].
- The BCL2 inhibitor APG-2575 reprogrammed macrophages from an M2-like (immunosuppressive) to M1-like (pro-inflammatory) phenotype via NLRP3 activation, significantly enhancing CD8+ T cell infiltration and the anti-tumor activity of PD-1 blockade in solid tumor models [144].
- In lymphoma, venetoclax combined with anti-CD47 antibodies (e.g., TJC4) enhanced macrophage-mediated phagocytosis and demonstrated synergistic immune activation [146].
- In AML, venetoclax plus hypomethylating agents (HMAs) increases PD-1 expression on T cells—a reversible effect with nivolumab co-treatment—resulting in restored T cell function and reduced disease burden [147].
- MCL1 inhibitors such as S64315 have also shown immune-modulating effects by depleting myeloid-derived suppressor cells, thereby alleviating immunosuppression and enhancing ICI efficacy in melanoma models [145].
- BH3 mimetics with STING agonists: Particularly effective in TP53-mutant tumors, this combination restores apoptotic signaling via innate immune pathways, even in the absence of functional p53, and enhances tumor killing with minimal toxicity [149].
- BH3 mimetics with antiretrovirals: In HTLV-1–driven leukemia, MCL1 inhibition selectively eliminated infected T cells and slowed disease progression, suggesting a potential curative approach [150].
- Adoptive cell therapies: In CAR T and NK cell therapies, venetoclax sensitizes tumor cells to killing while sparing immune cells [151,152,153]. Engineering CAR T cells with BCL2 mutations protects them from venetoclax-induced apoptosis, enabling safe combination strategies even in resistant settings.
4.4. BH3 Mimetics with Chemotherapy
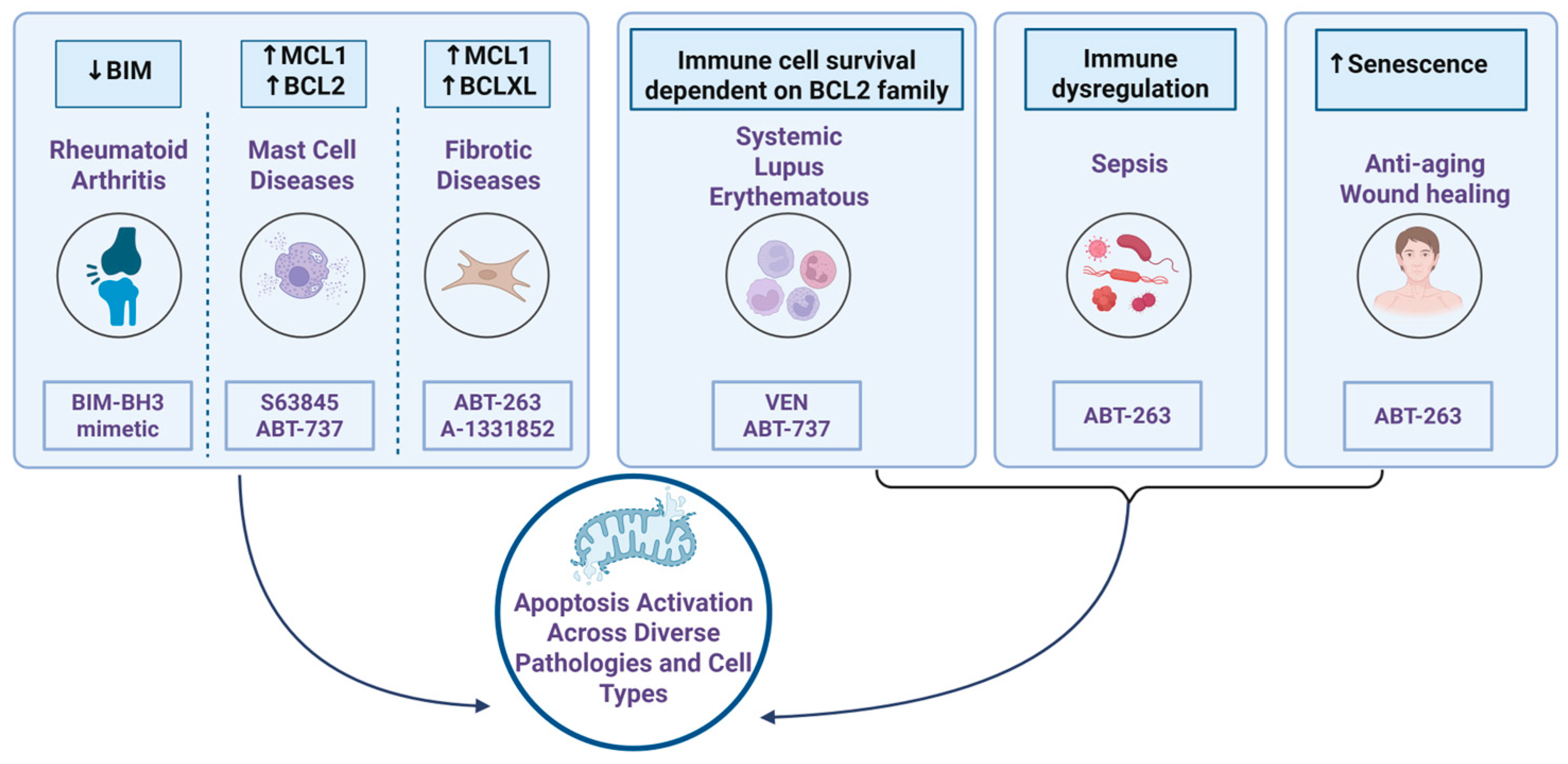
5. Role of BH3 Mimetics Beyond Cancer
5.1. Autoimmune Diseases
5.2. Fibrotic Disease
5.3. Mast Cell Associated Diseases
5.4. Targeting Senescent Cells: Regenerative and Anti-Aging Applications of BH3 Mimetics
5.5. Sepsis
6. Non-Apoptotic Roles of BCL2 Family Proteins
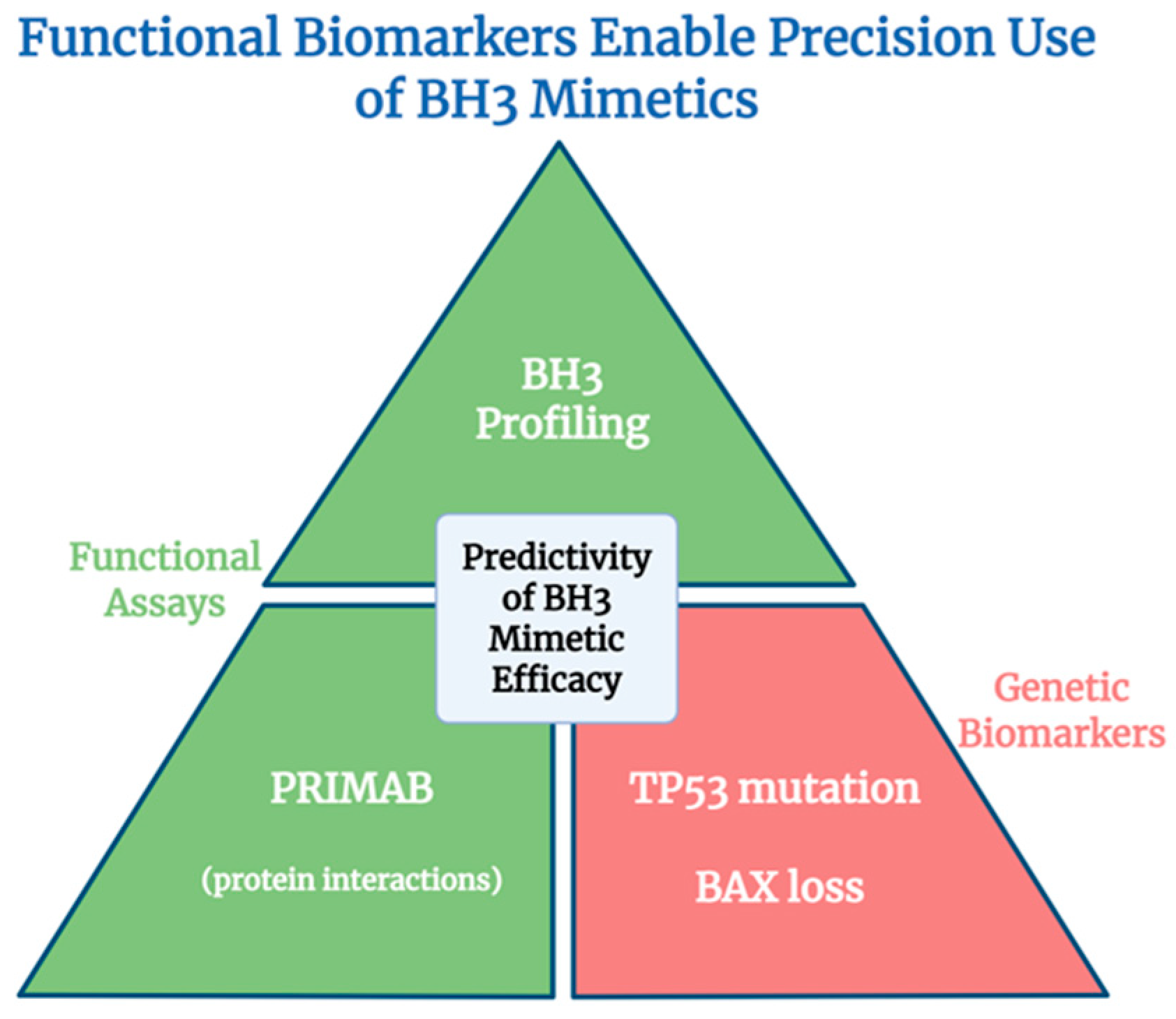
7. Target Selectivity and Biomarker-Driven Precision in BH3 Mimetic Therapy
- (1)
- Target selectivity—which anti-apoptotic BCL2 family protein the drug inhibits.
- (2)
- Apoptotic priming—whether the target cells are already poised to undergo apoptosis.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| BCL2 | B-cell lymphoma 2 |
| BCLXL | B-cell lymphoma 2 (BCL2) like-1 long |
| BCLW | Bcl-2-like protein 2 |
| BCL2A1 | Bcl-2-related protein A1 |
| BH | Bcl-2 homology |
| BID | BH3-interacting domain death agonist |
| BIM | Bcl-2-interacting mediator of cell death |
| CLL | Chronic lymphocytic leukemia |
| DLBCL | Diffuse large B cell lymphoma |
| ER | Endoplasmic reticulum |
| FAO | Fatty acid oxidation |
| ICI | Immune checkpoint inhibitors |
| MCL1 | Myeloid cell leukemia 1 |
| MOMP | Outer mitochondrial membrane permeabilization |
| NOXA | Phorbol-12-myristate-13-acetate-induced protein 1 |
| NSCLC | Non-small cell lung cancer |
| OXPHOS | Oxidative phosphorylation |
| PUMA | Bcl-2-binding component 3 |
| TNBC | Triple-negative breast cancer |
References
- Popgeorgiev, N.; Sa, J.D.; Jabbour, L.; Banjara, S.; Nguyen, T.T.M.; Akhavan, E.S.A.; Gadet, R.; Ralchev, N.; Manon, S.; Hinds, M.G.; et al. Ancient and conserved functional interplay between Bcl-2 family proteins in the mitochondrial pathway of apoptosis. Sci. Adv. 2020, 6, eabc4149. [Google Scholar] [CrossRef]
- Banjara, S.; Suraweera, C.D.; Hinds, M.G.; Kvansakul, M. The Bcl-2 Family: Ancient Origins, Conserved Structures, and Divergent Mechanisms. Biomolecules 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petros, A.M.; Olejniczak, E.T.; Fesik, S.W. Structural biology of the Bcl-2 family of proteins. Biochim. Biophys. Acta 2004, 1644, 83–94. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Miyashita, T.; Krajewski, S.; Takayama, S.; Aime-Sempe, C.; Kitada, S.; Sato, T.; Wang, H.G.; Harigai, M.; Hanada, M.; et al. Bcl-2 family proteins and the regulation of programmed cell death in leukemia and lymphoma. Cancer Treat. Res. 1996, 84, 31–72. [Google Scholar] [CrossRef]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 family: From apoptosis mechanisms to new advances in targeted therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef]
- Croce, C.M.; Vaux, D.; Strasser, A.; Opferman, J.T.; Czabotar, P.E.; Fesik, S.W. The BCL-2 protein family: From discovery to drug development. Cell Death Differ. 2025, 32, 1369–1381. [Google Scholar] [CrossRef] [PubMed]
- Gabellini, C.; Trisciuoglio, D.; Del Bufalo, D. Non-canonical roles of Bcl-2 and Bcl-xL proteins: Relevance of BH4 domain. Carcinogenesis 2017, 38, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rafiuddin-Shah, M.; Tu, H.C.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.; Cheng, E.H. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat. Cell Biol. 2006, 8, 1348–1358. [Google Scholar] [CrossRef]
- Hartman, M.L.; Czyz, M. BCL-w: Apoptotic and non-apoptotic role in health and disease. Cell Death Dis. 2020, 11, 260. [Google Scholar] [CrossRef] [PubMed]
- Holmgreen, S.P.; Huang, D.C.; Adams, J.M.; Cory, S. Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members. Cell Death Differ. 1999, 6, 525–532. [Google Scholar] [CrossRef]
- Lee, E.F.; Dewson, G.; Evangelista, M.; Pettikiriarachchi, A.; Gold, G.J.; Zhu, H.; Colman, P.M.; Fairlie, W.D. The functional differences between pro-survival and pro-apoptotic B cell lymphoma 2 (Bcl-2) proteins depend on structural differences in their Bcl-2 homology 3 (BH3) domains. J. Biol. Chem. 2014, 289, 36001–36017. [Google Scholar] [CrossRef]
- Rooswinkel, R.W.; van de Kooij, B.; de Vries, E.; Paauwe, M.; Braster, R.; Verheij, M.; Borst, J. Antiapoptotic potency of Bcl-2 proteins primarily relies on their stability, not binding selectivity. Blood 2014, 123, 2806–2815. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Youle, R.J. SnapShot: BCL-2 proteins. Cell 2009, 138, 404.e1. [Google Scholar] [CrossRef] [PubMed]
- Pervushin, N.V.; Kopeina, G.S.; Zhivotovsky, B. Bcl-B: An “unknown” protein of the Bcl-2 family. Biol. Direct 2023, 18, 69. [Google Scholar] [CrossRef]
- Zhang, H.; Holzgreve, W.; De Geyter, C. Bcl2-L-10, a novel anti-apoptotic member of the Bcl-2 family, blocks apoptosis in the mitochondria death pathway but not in the death receptor pathway. Hum. Mol. Genet. 2001, 10, 2329–2339. [Google Scholar] [CrossRef]
- Saddam, M.; Paul, S.K.; Habib, M.A.; Fahim, M.A.; Mimi, A.; Islam, S.; Paul, B.; Helal, M.M.U. Emerging biomarkers and potential therapeutics of the BCL-2 protein family: The apoptotic and anti-apoptotic context. Egypt. J. Med. Human Genet. 2024, 25, 12. [Google Scholar] [CrossRef]
- Dakkak, B.E.; Taneera, J.; El-Huneidi, W.; Abu-Gharbieh, E.; Hamoudi, R.; Semreen, M.H.; Soares, N.C.; Abu-Rish, E.Y.; Alkawareek, M.Y.; Alkilany, A.M.; et al. Unlocking the Therapeutic Potential of BCL-2 Associated Protein Family: Exploring BCL-2 Inhibitors in Cancer Therapy. Biomol. Ther. 2024, 32, 267–280. [Google Scholar] [CrossRef]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Vela, L.; Gonzalo, O.; Naval, J.; Marzo, I. Direct Interaction of Bax and Bak Proteins with Bcl-2 Homology Domain 3 (BH3)-only Proteins in Living Cells Revealed by Fluorescence Complementation*. J. Biol. Chem. 2013, 288, 4935–4946. [Google Scholar] [CrossRef] [PubMed]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- King, L.E.; Hohorst, L.; García-Sáez, A.J. Expanding roles of BCL-2 proteins in apoptosis execution and beyond. J. Cell Sci. 2023, 136, jcs260790. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Croce, C.M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc. Natl. Acad. Sci. USA 1986, 83, 5214–5218. [Google Scholar] [CrossRef]
- Del Gaizo Moore, V.; Letai, A. BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013, 332, 202–205. [Google Scholar] [CrossRef]
- Perciavalle, R.M.; Opferman, J.T. Delving deeper: MCL-1’s contributions to normal and cancer biology. Trends Cell Biol. 2013, 23, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.A.; Dash, R.; Azab, B.; Sarkar, S.; Das, S.K.; Kumar, S.; Oyesanya, R.A.; Dasgupta, S.; Dent, P.; Grant, S.; et al. Targeting Mcl-1 for the therapy of cancer. Expert. Opin. Investig. Drugs 2011, 20, 1397–1411. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, S.I.; Timofeeva, N.; Sarkar, A.; Gandhi, V. Targeting MCL-1 protein to treat cancer: Opportunities and challenges. Front. Oncol. 2023, 13, 1226289. [Google Scholar] [CrossRef]
- Kotschy, A.; Szlavik, Z.; Murray, J.; Davidson, J.; Maragno, A.L.; Le Toumelin-Braizat, G.; Chanrion, M.; Kelly, G.L.; Gong, J.N.; Moujalled, D.M.; et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature 2016, 538, 477–482. [Google Scholar] [CrossRef]
- Merino, D.; Kelly, G.L.; Lessene, G.; Wei, A.H.; Roberts, A.W.; Strasser, A. BH3-Mimetic Drugs: Blazing the Trail for New Cancer Medicines. Cancer Cell 2018, 34, 879–891. [Google Scholar] [CrossRef]
- Sale, M.J.; Minihane, E.; Monks, N.R.; Gilley, R.; Richards, F.M.; Schifferli, K.P.; Andersen, C.L.; Davies, E.J.; Vicente, M.A.; Ozono, E.; et al. Targeting melanoma’s MCL1 bias unleashes the apoptotic potential of BRAF and ERK1/2 pathway inhibitors. Nat. Commun. 2019, 10, 5167. [Google Scholar] [CrossRef]
- Alharbi, F.; Almanifi, E.; Ashrafuzzaman, M. Targeting BCL-2 family proteins using BH3 mimetic drugs for cancer therapy: A systematic review of randomized clinical trials. Med. Drug Discov. 2024, 24, 100199. [Google Scholar] [CrossRef]
- Chonghaile, T.N.; Roderick, J.E.; Glenfield, C.; Ryan, J.; Sallan, S.E.; Silverman, L.B.; Loh, M.L.; Hunger, S.P.; Wood, B.; DeAngelo, D.J.; et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014, 4, 1074–1087. [Google Scholar] [CrossRef] [PubMed]
- Delbridge, A.R.D.; Strasser, A. The BCL-2 protein family, BH3-mimetics and cancer therapy. Cell Death Differ. 2015, 22, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Kump, K.J.; Ahmad, E.; Foucar, C.; Avelar, R.A.; Murga-Zamalloa, C.; Lieberman, M.; Kandarpa, M.; Mady, A.S.A.; DiFeo, A.; Zhang, L.; et al. Understanding the Functional Dependence and Inhibition of the Bcl-2 Pro-Survival Proteins in a Wide Spectrum of Cancers toward Precision Medicine. ACS Pharmacol. Transl. Sci. 2025, 8, 2922–2935. [Google Scholar] [CrossRef]
- Guerra, V.A.; DiNardo, C.; Konopleva, M. Venetoclax-based therapies for acute myeloid leukemia. Best. Pract. Res. Clin. Haematol. 2019, 32, 145–153. [Google Scholar] [CrossRef]
- Lasica, M.; Anderson, M.A. Review of Venetoclax in CLL, AML and Multiple Myeloma. J. Pers. Med. 2021, 11, 463. [Google Scholar] [CrossRef]
- Pan, R.; Hogdal, L.J.; Benito, J.M.; Bucci, D.; Han, L.; Borthakur, G.; Cortes, J.; DeAngelo, D.J.; Debose, L.; Mu, H.; et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014, 4, 362–375. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Waggoner, M.; Katsetos, J.; Thomas, E.; Galinsky, I.; Fox, H. Practical Management of the Venetoclax-Treated Patient in Chronic Lymphocytic Leukemia and Acute Myeloid Leukemia. J. Adv. Pract. Oncol. 2022, 13, 400–415. [Google Scholar] [CrossRef]
- Glaviano, A.; Weisberg, E.; Lam, H.Y.; Tan, D.J.J.; Innes, A.J.; Ge, Y.; Lai, C.E.; Stock, W.; Glytsou, C.; Smit, L.; et al. Apoptosis-targeting BH3 mimetics: Transforming treatment for patients with acute myeloid leukaemia. Nat. Rev. Clin. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Parry, N.; Wheadon, H.; Copland, M. The application of BH3 mimetics in myeloid leukemias. Cell Death Dis. 2021, 12, 222. [Google Scholar] [CrossRef]
- Lin, V.S.; Xu, Z.F.; Huang, D.C.S.; Thijssen, R. BH3 Mimetics for the Treatment of B-Cell Malignancies-Insights and Lessons from the Clinic. Cancers 2020, 12, 3353. [Google Scholar] [CrossRef] [PubMed]
- Varkaris, A.; Wang, K.; Nouri, M.; Kozlova, N.; Schmidt, D.R.; Stavridi, A.; Arai, S.; Ambrosio, N.; Poluben, L.; Jimenez-Vacas, J.M.; et al. BH3 mimetics targeting BCL-XL have efficacy in solid tumors with RB1 loss and replication stress. Nat. Commun. 2025, 16, 4931. [Google Scholar] [CrossRef]
- Firestein, G.S.; Yeo, M.; Zvaifler, N.J. Apoptosis in rheumatoid arthritis synovium. J. Clin. Investig. 1995, 96, 1631–1638. [Google Scholar] [CrossRef]
- Gandhi, L.; Camidge, D.R.; Ribeiro de Oliveira, M.; Bonomi, P.; Gandara, D.; Khaira, D.; Hann, C.L.; McKeegan, E.M.; Litvinovich, E.; Hemken, P.M.; et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J. Clin. Oncol. 2011, 29, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Ploumaki, I.; Triantafyllou, E.; Koumprentziotis, I.A.; Karampinos, K.; Drougkas, K.; Karavolias, I.; Trontzas, I.; Kotteas, E.A. Bcl-2 pathway inhibition in solid tumors: A review of clinical trials. Clin. Transl. Oncol. 2023, 25, 1554–1578. [Google Scholar] [CrossRef] [PubMed]
- Fairlie, W.D.; Lee, E.F. Targeting the BCL-2-regulated apoptotic pathway for the treatment of solid cancers. Biochem. Soc. Trans. 2021, 49, 2397–2410. [Google Scholar] [CrossRef]
- López, J.; Llop-Hernández, À.; Verdura, S.; Serrano-Hervás, E.; Martinez-Balibrea, E.; Bosch-Barrera, J.; Teixidor, E.; López-Bonet, E.; Martin-Castillo, B.; Sardanyés, J.; et al. Mitochondrial priming and response to BH3 mimetics in “one-two punch” senogenic-senolytic strategies. Cell Death Discov. 2025, 11, 91. [Google Scholar] [CrossRef]
- El Abbassi, A.; Redouane, S.; Azoubi, Z.; Zougagh, N.; Mouslim, A.; Menggad, M. Screening of the Prodiginine Molecules as BH3-Mimetics against the Developed Bcl-2 Antiapoptotic Chemotherapeutic Resistance: A Molecular Docking and ADMET Study Supported by Molecular Dynamics Simulations. Curr. Comput. Aided Drug Des. 2025. [Google Scholar] [CrossRef]
- Huhn, A.J.; Guerra, R.M.; Harvey, E.P.; Bird, G.H.; Walensky, L.D. Selective Covalent Targeting of Anti-Apoptotic BFL-1 by Cysteine-Reactive Stapled Peptide Inhibitors. Cell Chem. Biol. 2016, 23, 1123–1134. [Google Scholar] [CrossRef]
- Wang, G.; Diepstraten, S.T.; Herold, M.J. Last but not least: BFL-1 as an emerging target for anti-cancer therapies. Biochem. Soc. Trans. 2022, 50, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: A phase 3 randomized placebo-controlled trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Guo, Y.; Wang, Z.; He, W.; Zhu, Y.; Zhao, X.; Sun, L.; Wang, Y. Clinical Pharmacology and Side Effects of Venetoclax in Hematologic Malignancies. Curr. Drug Metab. 2025, 25, 564–575. [Google Scholar] [CrossRef]
- Prosty, C.; Katergi, K.; Nguyen, A.; Luo, O.D.; Sorin, M.; Cherniak, V.; Sebag, M.; Demir, K.; McDonald, E.G.; Lee, T.C.; et al. Risk of infectious adverse events of venetoclax therapy for hematologic malignancies: A systematic review and meta-analysis of RCTs. Blood Adv. 2024, 8, 857–866. [Google Scholar] [CrossRef]
- Lee, E.F.; Harris, T.J.; Tran, S.; Evangelista, M.; Arulananda, S.; John, T.; Ramnac, C.; Hobbs, C.; Zhu, H.; Gunasingh, G.; et al. BCL-XL and MCL-1 are the key BCL-2 family proteins in melanoma cell survival. Cell Death Dis. 2019, 10, 342. [Google Scholar] [CrossRef]
- Mukherjee, N.; Amato, C.M.; Skees, J.; Todd, K.J.; Lambert, K.A.; Robinson, W.A.; Van Gulick, R.; Weight, R.M.; Dart, C.R.; Tobin, R.P.; et al. Simultaneously Inhibiting BCL2 and MCL1 Is a Therapeutic Option for Patients with Advanced Melanoma. Cancers 2020, 12, 2182. [Google Scholar] [CrossRef]
- Mukherjee, N.; Skees, J.; Todd, K.J.; West, D.A.; Lambert, K.A.; Robinson, W.A.; Amato, C.M.; Couts, K.L.; Van Gulick, R.; MacBeth, M.; et al. MCL1 inhibitors S63845/MIK665 plus Navitoclax synergistically kill difficult-to-treat melanoma cells. Cell Death Dis. 2020, 11, 443. [Google Scholar] [CrossRef]
- Wilson, W.H.; O’Connor, O.A.; Czuczman, M.S.; LaCasce, A.S.; Gerecitano, J.F.; Leonard, J.P.; Tulpule, A.; Dunleavy, K.; Xiong, H.; Chiu, Y.L.; et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: A phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010, 11, 1149–1159. [Google Scholar] [CrossRef]
- de Vos, S.; Leonard, J.P.; Friedberg, J.W.; Zain, J.; Dunleavy, K.; Humerickhouse, R.; Hayslip, J.; Pesko, J.; Wilson, W.H. Safety and efficacy of navitoclax, a BCL-2 and BCL-X(L) inhibitor, in patients with relapsed or refractory lymphoid malignancies: Results from a phase 2a study. Leuk. Lymphoma 2021, 62, 810–818. [Google Scholar] [CrossRef]
- Corcoran, R.B.; Do, K.T.; Kim, J.E.; Cleary, J.M.; Parikh, A.R.; Yeku, O.O.; Xiong, N.; Weekes, C.D.; Veneris, J.; Ahronian, L.G.; et al. Phase I/II Study of Combined BCL-xL and MEK Inhibition with Navitoclax and Trametinib in KRAS or NRAS Mutant Advanced Solid Tumors. Clin. Cancer Res. 2024, 30, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.L.; Taneja, N.; Neininger, A.C.; Wang, L.; Robertson, G.L.; Riffle, S.N.; Shi, L.; Knollmann, B.C.; Burnette, D.T.; Gama, V. MCL-1 Inhibition by Selective BH3 Mimetics Disrupts Mitochondrial Dynamics Causing Loss of Viability and Functionality of Human Cardiomyocytes. iScience 2020, 23, 101015. [Google Scholar] [CrossRef]
- Falchook, G.; Patel, M.; McKean, M.; Philipovskiy, A.; Chiaverelli, R.; Sun, W.; Fossler, M.; Novotny, W.; Kurman, M.; Rowe, S.; et al. Abstract CT172: A phase 1, open-label, dose-escalation study of PRT1419, a selective induced myeloid leukemia cell differentiation protein (MCL-1) inhibitor, in patients (pts) with advanced/metastatic solid tumors. Cancer Res. 2023, 83, CT172. [Google Scholar] [CrossRef]
- Chaulin, A.M. The Essential Strategies to Mitigate Cardiotoxicity Caused by Doxorubicin. Life 2023, 13, 2148. [Google Scholar] [CrossRef] [PubMed]
- Rauh, U.; Wei, G.; Serrano-Wu, M.; Kosmidis, G.; Kaulfuss, S.; Siegel, F.; Thede, K.; McFarland, J.; Lemke, C.T.; Werbeck, N.; et al. BRD-810 is a highly selective MCL1 inhibitor with optimized in vivo clearance and robust efficacy in solid and hematological tumor models. Nat. Cancer 2024, 5, 1479–1493. [Google Scholar] [CrossRef]
- Cornu, M.; Lemaitre, T.; Kieffer, C.; Voisin-Chiret, A.S. PROTAC 2.0: Expanding the frontiers of targeted protein degradation. Drug Discov. Today 2025, 30, 104376. [Google Scholar] [CrossRef]
- Mehrotra, N.; Kharbanda, S.; Singh, H. BH3 mimetics in cancer therapy and their future perspectives with nanodelivery. Nanomedicine 2021, 16, 1067–1070. [Google Scholar] [CrossRef]
- Shahar, N.; Larisch, S. Inhibiting the inhibitors: Targeting anti-apoptotic proteins in cancer and therapy resistance. Drug Resist. Updat. 2020, 52, 100712. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Yu, L.; Yang, L. Mechanisms of venetoclax resistance and solutions. Front. Oncol. 2022, 12, 1005659. [Google Scholar] [CrossRef]
- Allen, B.; Bottomly, D.; Kohnke, T.; Wang, A.; Lin, H.Y.; Johnson, K.; Kenna, I.; Streltsova, A.; Martin, E.; Chen, R.; et al. A CEBPB/IL-1beta/TNF-alpha feedback loop drives drug resistance to venetoclax and MDM2 inhibitors in monocytic leukemia. Blood 2025, 145, 2488–2506. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, S.; Qiao, X.; Knight, T.; Edwards, H.; Polin, L.; Kushner, J.; Dzinic, S.H.; White, K.; Wang, G.; et al. Inhibition of Bcl-2 Synergistically Enhances the Antileukemic Activity of Midostaurin and Gilteritinib in Preclinical Models of FLT3-Mutated Acute Myeloid Leukemia. Clin. Cancer Res. 2019, 25, 6815–6826. [Google Scholar] [CrossRef]
- Zhang, H.; Nakauchi, Y.; Kohnke, T.; Stafford, M.; Bottomly, D.; Thomas, R.; Wilmot, B.; McWeeney, S.K.; Majeti, R.; Tyner, J.W. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat. Cancer 2020, 1, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Li, L.; Nguyen, B.; Seo, J.; Wu, M.; Seale, T.; Levis, M.; Duffield, A.; Hu, Y.; Small, D. FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal Transduct. Target. Ther. 2021, 6, 186. [Google Scholar] [CrossRef] [PubMed]
- Ong, F.; Kim, K.; Konopleva, M.Y. Venetoclax resistance: Mechanistic insights and future strategies. Cancer Drug Resist. 2022, 5, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Ishizawa, J.; Ayoub, E.; Montoya, R.H.; Ostermann, L.B.; Muftuoglu, M.; Ruvolo, V.R.; Patsilevas, T.; Scruggs, D.A.; Khazaei, S.; et al. Enhanced TP53 reactivation disrupts MYC transcriptional program and overcomes venetoclax resistance in acute myeloid leukemias. Sci. Adv. 2023, 9, eadh1436. [Google Scholar] [CrossRef]
- Brown, F.C.; Wang, X.; Birkinshaw, R.; Chua, C.C.; Morley, T.; Kasapgil, S.; Pomilio, G.; Blombery, P.; Huang, D.C.S.; Czabotar, P.; et al. Acquired BCL2 variants associated with venetoclax resistance in acute myeloid leukemia. Blood Adv. 2025, 9, 127–131. [Google Scholar] [CrossRef]
- Chatzilygeroudi, T.; Karantanos, T.; Pappa, V. Unraveling Venetoclax Resistance: Navigating the Future of HMA/Venetoclax-Refractory AML in the Molecular Era. Cancers 2025, 17, 1586. [Google Scholar] [CrossRef]
- Nwosu, G.O.; Ross, D.M.; Powell, J.A.; Pitson, S.M. Venetoclax therapy and emerging resistance mechanisms in acute myeloid leukaemia. Cell Death Dis. 2024, 15, 413. [Google Scholar] [CrossRef]
- Hafezi, S.; Rahmani, M. Targeting BCL-2 in Cancer: Advances, Challenges, and Perspectives. Cancers 2021, 13, 1292. [Google Scholar] [CrossRef]
- Portelinha, A.; Wang, S.; Parsa, S.; Jiang, M.; Gorelick, A.N.; Mohanty, S.; Sharma, S.; de Stanchina, E.; Berishaj, M.; Zhao, C.; et al. SETD1B mutations confer apoptosis resistance and BCL2 independence in B cell lymphoma. J. Exp. Med. 2024, 221, e20231143. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, X.; Aryal, S.; Veasey, V.; Zhang, P.; Li, F.J.; Luan, Y.; Bhatia, R.; Zhou, Y.; Lu, R. CRISPR screen of venetoclax response-associated genes identifies transcription factor ZNF740 as a key functional regulator. Cell Death Dis. 2024, 15, 627. [Google Scholar] [CrossRef]
- Wood, S.; Willbanks, A.; Cheng, J.X. RNA Cytosine Methyltransferases NSUN1 and NSUN2 Mediate the Lineage-Associated Resistance to Venetoclax in Leukemia. Blood 2020, 136, 13–14. [Google Scholar] [CrossRef]
- Mroz, D.; Jaglowska, J.; Wevers, R.A.; Zietkiewicz, S. CLPB Deficiency, a Mitochondrial Chaperonopathy With Neutropenia and Neurological Presentation. J. Inherit. Metab. Dis. 2025, 48, e70025. [Google Scholar] [CrossRef]
- Chen, X.; Glytsou, C.; Zhou, H.; Narang, S.; Reyna, D.E.; Lopez, A.; Sakellaropoulos, T.; Gong, Y.; Kloetgen, A.; Yap, Y.S.; et al. Targeting Mitochondrial Structure Sensitizes Acute Myeloid Leukemia to Venetoclax Treatment. Cancer Discov. 2019, 9, 890–909. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Smith, S.B.; Yoon, Y. The short variant of the mitochondrial dynamin OPA1 maintains mitochondrial energetics and cristae structure. J. Biol. Chem. 2017, 292, 7115–7130. [Google Scholar] [CrossRef]
- Glytsou, C.; Chen, X.; Zacharioudakis, E.; Al-Santli, W.; Zhou, H.; Nadorp, B.; Lee, S.; Lasry, A.; Sun, Z.; Papaioannou, D.; et al. Mitophagy Promotes Resistance to BH3 Mimetics in Acute Myeloid Leukemia. Cancer Discov. 2023, 13, 1656–1677. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dorn, G.W., 2nd. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.S.; Wilkinson, S.; James, J.; Ryan, K.M.; Vousden, K.H. PUMA- and Bax-induced autophagy contributes to apoptosis. Cell Death Differ. 2009, 16, 1135–1145. [Google Scholar] [CrossRef]
- Lin, S.; Larrue, C.; Scheidegger, N.K.; Seong, B.K.A.; Dharia, N.V.; Kuljanin, M.; Wechsler, C.S.; Kugener, G.; Robichaud, A.L.; Conway, A.S.; et al. An In Vivo CRISPR Screening Platform for Prioritizing Therapeutic Targets in AML. Cancer Discov. 2022, 12, 432–449. [Google Scholar] [CrossRef]
- Nakao, F.; Setoguchi, K.; Semba, Y.; Yamauchi, T.; Nogami, J.; Sasaki, K.; Imanaga, H.; Terasaki, T.; Miyazaki, M.; Hirabayashi, S.; et al. Targeting a mitochondrial E3 ubiquitin ligase complex to overcome AML cell-intrinsic Venetoclax resistance. Leukemia 2023, 37, 1028–1038. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell 2018, 34, 724–740.e4. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.M.; Jones, C.L.; Pollyea, D.A.; Culp-Hill, R.; D’Alessandro, A.; Winters, A.; Krug, A.; Abbott, D.; Goosman, M.; Pei, S.; et al. Fatty acid metabolism underlies venetoclax resistance in acute myeloid leukemia stem cells. Nat. Cancer 2020, 1, 1176–1187. [Google Scholar] [CrossRef]
- Wang, B.; Reville, P.K.; Yassouf, M.Y.; Jelloul, F.Z.; Ly, C.; Desai, P.N.; Wang, Z.; Borges, P.; Veletic, I.; Dasdemir, E.; et al. Comprehensive characterization of IFNgamma signaling in acute myeloid leukemia reveals prognostic and therapeutic strategies. Nat. Commun. 2024, 15, 1821. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Osterlund, E.J.; Chi, X.; Pogmore, J.; Leber, B.; Andrews, D.W. Bim escapes displacement by BH3-mimetic anti-cancer drugs by double-bolt locking both Bcl-XL and Bcl-2. Elife 2019, 8, e37689. [Google Scholar] [CrossRef]
- Ebner, J.; Schmoellerl, J.; Piontek, M.; Manhart, G.; Troester, S.; Carter, B.Z.; Neubauer, H.; Moriggl, R.; Szakács, G.; Zuber, J.; et al. ABCC1 and glutathione metabolism limit the efficacy of BCL-2 inhibitors in acute myeloid leukemia. Nat. Commun. 2023, 14, 5709. [Google Scholar] [CrossRef] [PubMed]
- Willis, S.N.; Chen, L.; Dewson, G.; Wei, A.; Naik, E.; Fletcher, J.I.; Adams, J.M.; Huang, D.C. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes. Dev. 2005, 19, 1294–1305. [Google Scholar] [CrossRef]
- Tantawy, S.I.; Timofeeva, N.; Hernandez, A.; Sarkar, A.; Gandhi, V. Decoding the mechanism behind MCL-1 inhibitors: A pathway to understanding MCL-1 protein stability. Oncotarget 2023, 14, 653–655. [Google Scholar] [CrossRef] [PubMed]
- Diepstraten, S.T.; Young, S.; La Marca, J.E.; Wang, Z.; Kluck, R.M.; Strasser, A.; Kelly, G.L. Lymphoma cells lacking pro-apoptotic BAX are highly resistant to BH3-mimetics targeting pro-survival MCL-1 but retain sensitivity to conventional DNA-damaging drugs. Cell Death Differ. 2023, 30, 1005–1017. [Google Scholar] [CrossRef]
- Caenepeel, S.; Brown, S.P.; Belmontes, B.; Moody, G.; Keegan, K.S.; Chui, D.; Whittington, D.A.; Huang, X.; Poppe, L.; Cheng, A.C.; et al. AMG 176, a Selective MCL1 Inhibitor, Is Effective in Hematologic Cancer Models Alone and in Combination with Established Therapies. Cancer Discov. 2018, 8, 1582–1597, Correction in Cancer Discov. 2019, 9, 980. [Google Scholar] [CrossRef]
- Wei, G.; Margolin, A.A.; Haery, L.; Brown, E.; Cucolo, L.; Julian, B.; Shehata, S.; Kung, A.L.; Beroukhim, R.; Golub, T.R. Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell 2012, 21, 547–562. [Google Scholar] [CrossRef]
- Müller, D.; Mazzeo, P.; Koch, R.; Bösherz, M.-S.; Welter, S.; von Hammerstein-Equord, A.; Hinterthaner, M.; Cordes, L.; Belharazem, D.; Marx, A.; et al. Functional apoptosis profiling identifies MCL-1 and BCL-xL as prognostic markers and therapeutic targets in advanced thymomas and thymic carcinomas. BMC Med. 2021, 19, 300. [Google Scholar] [CrossRef]
- Fitzgerald, M.-C.; O’Halloran, P.J.; Kerrane, S.A.; Ní Chonghaile, T.; Connolly, N.M.C.; Murphy, B.M. The identification of BCL-XL and MCL-1 as key anti-apoptotic proteins in medulloblastoma that mediate distinct roles in chemotherapy resistance. Cell Death Dis. 2023, 14, 705. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Kellish, P.; Connis, N.; Thummuri, D.; Wiegand, J.; Zhang, P.; Zhang, X.; Budamagunta, V.; Hua, N.; Yang, Y.; et al. Co-targeting BCL-XL and MCL-1 with DT2216 and AZD8055 synergistically inhibit small-cell lung cancer growth without causing on-target toxicities in mice. Cell Death Discov. 2023, 9, 1. [Google Scholar] [CrossRef]
- Mukherjee, N.; Dart, C.R.; Amato, C.M.; Honig-Frand, A.; Lambert, J.R.; Lambert, K.A.; Robinson, W.A.; Tobin, R.P.; McCarter, M.D.; Couts, K.L.; et al. Expression Differences in BCL2 Family Members between Uveal and Cutaneous Melanomas Account for Varying Sensitivity to BH3 Mimetics. J. Investig. Dermatol. 2022, 142, 1912–1922.e7. [Google Scholar] [CrossRef]
- Opydo, M.; Mlyczyńska, A.; Mlyczyńska, E.; Rak, A.; Kolaczkowska, E. Synergistic Action of MCL-1 Inhibitor with BCL-2/BCL-XL or MAPK Pathway Inhibitors Enhances Acute Myeloid Leukemia Cell Apoptosis and Differentiation. Int. J. Mol. Sci. 2023, 24, 7180. [Google Scholar] [CrossRef]
- Ramsey, H.E.; Fischer, M.A.; Lee, T.; Gorska, A.E.; Arrate, M.P.; Fuller, L.; Boyd, K.L.; Strickland, S.A.; Sensintaffar, J.; Hogdal, L.J.; et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018, 8, 1566–1581. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, L.; Nkwocha, J.; Kmieciak, M.; Shang, S.; Cowart, L.A.; Yue, Y.; Horimoto, K.; Hawkridge, A.; Rijal, A.; et al. Src inhibition potentiates MCL-1 antagonist activity in acute myeloid leukemia. Signal Transduct. Target. Ther. 2025, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Certo, M.; Del Gaizo Moore, V.; Nishino, M.; Wei, G.; Korsmeyer, S.; Armstrong, S.A.; Letai, A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006, 9, 351–365. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Xiang, S.; Wang, J.; Qu, L.; Chen, X.; Guo, M.; Lu, X.; Chen, Y. Deciphering molecular specificity in MCL-1/BAK interaction and its implications for designing potent MCL-1 inhibitors. Cell Death Differ. 2025, 32, 991–999. [Google Scholar] [CrossRef]
- Lwin, T.; Lin, J.; Choi, Y.S.; Zhang, X.; Moscinski, L.C.; Wright, K.L.; Sotomayor, E.M.; Dalton, W.S.; Tao, J. Follicular dendritic cell-dependent drug resistance of non-Hodgkin lymphoma involves cell adhesion-mediated Bim down-regulation through induction of microRNA-181a. Blood 2010, 116, 5228–5236. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.A.; Kurtoglu, M.; Matulis, S.M.; Liu, J.; Siefker, D.; Gutman, D.M.; Kaufman, J.L.; Lee, K.P.; Lonial, S.; Boise, L.H. Distribution of Bim determines Mcl-1 dependence or codependence with Bcl-xL/Bcl-2 in Mcl-1-expressing myeloma cells. Blood 2011, 118, 1329–1339. [Google Scholar] [CrossRef]
- Arai, S.; Varkaris, A.; Nouri, M.; Chen, S.; Xie, L.; Balk, S.P. MARCH5 mediates NOXA-dependent MCL1 degradation driven by kinase inhibitors and integrated stress response activation. eLife 2020, 9, e54954. [Google Scholar] [CrossRef]
- Montero, J.; Gstalder, C.; Kim, D.J.; Sadowicz, D.; Miles, W.; Manos, M.; Cidado, J.R.; Paul Secrist, J.; Tron, A.E.; Flaherty, K.; et al. Destabilization of NOXA mRNA as a common resistance mechanism to targeted therapies. Nat. Commun. 2019, 10, 5157. [Google Scholar] [CrossRef]
- Wood, K.C. Overcoming MCL-1-driven adaptive resistance to targeted therapies. Nat. Commun. 2020, 11, 531. [Google Scholar] [CrossRef] [PubMed]
- Widden, H.; Placzek, W.J. The multiple mechanisms of MCL1 in the regulation of cell fate. Commun. Biol. 2021, 4, 1029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Riley-Gillis, B.; Han, L.; Jia, Y.; Lodi, A.; Zhang, H.; Ganesan, S.; Pan, R.; Konoplev, S.N.; Sweeney, S.R.; et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal Transduct. Target. Ther. 2022, 7, 51, Correction in Signal Transduct. Target. Ther. 2022, 7, 110. [Google Scholar] [CrossRef]
- Liu, S.; Qiao, X.; Wu, S.; Gai, Y.; Su, Y.; Edwards, H.; Wang, Y.; Lin, H.; Taub, J.W.; Wang, G.; et al. c-Myc plays a critical role in the antileukemic activity of the Mcl-1-selective inhibitor AZD5991 in acute myeloid leukemia. Apoptosis 2022, 27, 913–928. [Google Scholar] [CrossRef]
- Carter, B.Z.; Mak, P.Y.; Tao, W.; Warmoes, M.; Lorenzi, P.L.; Mak, D.; Ruvolo, V.; Tan, L.; Cidado, J.; Drew, L.; et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica 2022, 107, 58–76. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Mysliwski, M.; Serio, J.; Ropa, J.; Abulwerdi, F.A.; Chan, R.J.; Patel, J.P.; Tallman, M.S.; Paietta, E.; et al. Mutated Ptpn11 alters leukemic stem cell frequency and reduces the sensitivity of acute myeloid leukemia cells to Mcl1 inhibition. Leukemia 2015, 29, 1290–1300. [Google Scholar] [CrossRef]
- Bolomsky, A.; Miettinen, J.J.; Malyutina, A.; Besse, A.; Huber, J.; Fellinger, S.; Breid, H.; Parsons, A.; Klavins, K.; Hannich, J.T.; et al. Heterogeneous modulation of Bcl-2 family members and drug efflux mediate MCL-1 inhibitor resistance in multiple myeloma. Blood Adv. 2021, 5, 4125–4139. [Google Scholar] [CrossRef] [PubMed]
- Fire, E.; Gullá, S.V.; Grant, R.A.; Keating, A.E. Mcl-1-Bim complexes accommodate surprising point mutations via minor structural changes. Protein Sci. 2010, 19, 507–519. [Google Scholar] [CrossRef]
- McGill, G.G.; Horstmann, M.; Widlund, H.R.; Du, J.; Motyckova, G.; Nishimura, E.K.; Lin, Y.L.; Ramaswamy, S.; Avery, W.; Ding, H.F.; et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 2002, 109, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Haq, R.; Yokoyama, S.; Hawryluk, E.B.; Jönsson, G.B.; Frederick, D.T.; McHenry, K.; Porter, D.; Tran, T.N.; Love, K.T.; Langer, R.; et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc. Natl. Acad. Sci. USA 2013, 110, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.W.; Wei, A.H.; Huang, D.C.S. BCL2 and MCL1 inhibitors for hematologic malignancies. Blood 2021, 138, 1120–1136. [Google Scholar] [CrossRef]
- Desai, P.; Lonial, S.; Cashen, A.; Kamdar, M.; Flinn, I.; O’Brien, S.; Garcia, J.S.; Korde, N.; Moslehi, J.; Wey, M.; et al. A Phase 1 First-in-Human Study of the MCL-1 Inhibitor AZD5991 in Patients with Relapsed/Refractory Hematologic Malignancies. Clin. Cancer Res. 2024, 30, 4844–4855. [Google Scholar] [CrossRef]
- Wang, L.; Xi, C.; Liu, R.; Ye, T.; Xiang, N.; Deng, J.; Li, H. Dual targeting of Mcl-1 and Bcl-2 to overcome chemoresistance in cervical and colon cancer. Anticancer. Drugs 2024, 35, 219–226. [Google Scholar] [CrossRef]
- Lou, J.; Zhou, Q.; Lyu, X.; Cen, X.; Liu, C.; Yan, Z.; Li, Y.; Tang, H.; Liu, Q.; Ding, J.; et al. Discovery of a Covalent Inhibitor That Overcame Resistance to Venetoclax in AML Cells Overexpressing BFL-1. J. Med. Chem. 2024, 67, 10795–10830. [Google Scholar] [CrossRef]
- Gelmi, M.C.; Houtzagers, L.E.; Strub, T.; Krossa, I.; Jager, M.J. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int. J. Mol. Sci. 2022, 23, 6001. [Google Scholar] [CrossRef]
- Hartman, M.L.; Sztiller-Sikorska, M.; Czyz, M. Synergistic activity of S63845 and parthenolide to overcome acquired resistance to MEK1/2 inhibitor in melanoma cells: Mechanisms and therapeutic potential. Biomed. Pharmacother. 2025, 188, 118183. [Google Scholar] [CrossRef]
- Wu, S.; Liu, F.; Gai, Y.; Carter, J.; Edwards, H.; Huttemann, M.; Wang, G.; Li, C.; Taub, J.W.; Wang, Y.; et al. Combining the novel FLT3 and MERTK dual inhibitor MRX-2843 with venetoclax results in promising antileukemic activity against FLT3-ITD AML. Leuk. Res. 2024, 144, 107547. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Li, Q.; Wang, P. Sitravatinib combined with venetoclax exerts effective synergy to eliminate acute myeloid leukemia cells with FLT3-ITD mutations. Transl. Oncol. 2025, 59, 102467. [Google Scholar] [CrossRef]
- Cui, Y.; Shao, X.; Yang, H.; Xin, J.; Liu, Y.; Zhang, M.; Sun, C.; Chen, G.; Shen, G.; Meng, X.; et al. MDM2 inhibitor APG-115 synergizes with ABT-199 to induce cell apoptosis in chronic lymphocytic leukemia. Front. Pharmacol. 2024, 15, 1441383. [Google Scholar] [CrossRef]
- Tagoug, A.; Safra, I. The Impact of Panobinostat on Cell Death in Combination with S63845 in Multiple Myeloma Cells. Indian. J. Hematol. Blood Transfus. 2023, 39, 245–257. [Google Scholar] [CrossRef]
- Erdogdu, U.; Dolgikh, N.; Laszig, S.; Sarchen, V.; Meister, M.T.; Wanior, M.; Knapp, S.; Boedicker, C. Selective BH3 mimetics synergize with BET inhibition to induce mitochondrial apoptosis in rhabdomyosarcoma cells. Neoplasia 2022, 24, 109–119. [Google Scholar] [CrossRef]
- Sasi, B.K.; Tarantelli, C.; Martindale, S.; Civanelli, E.; Cannas, E.; Sartori, G.; Arribas, A.J.; Fernandes, S.M.; Shupe, S.J.; Machado, J.H.; et al. Novel PI3kdelta inhibitor roginolisib synergizes with venetoclax in hematologic malignancies. Haematologica 2025. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, C.; Zoppoli, P.; Cappelli, L.V.; Yoffe, L.; Moretti, M.; Izzo, M.; Mallia, S.; Kayembe, C.; Taylor, A.; Petrillo, G.; et al. BH3 mimetic drugs overcome the microenvironment-induced resistance to crizotinib in ALK+ anaplastic large cell lymphoma. Blood Adv. 2025, 9, 4757–4775. [Google Scholar] [CrossRef] [PubMed]
- Tarantelli, C.; Kayali, O.; Civanelli, E.; Cascione, L.; Mensah, A.A.; Folloni, C.; Arribas, A.J.; Rinaldi, A.; Cmiljanovic, V.; Mondello, P.; et al. Targeting of PIM Kinases Shows Single Agent Efficacy and Synergizes With BCL2 Inhibitors in Diffuse Large B Cell Lymphoma of the ABC Subtype. Hematol. Oncol. 2025, 43, e70055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Atencia Taboada, L.; Baglamis, S.; de Kroon, M.; Elshout, C.; Ramesh, P.; Helderman, R.; Torang, A.; Cameron, K.; van Driel, M.S.; et al. GSK-3 and BCL-XL inhibition mitigates the competitive advantage of APC-mutant colorectal cancer cells. Oncogenesis 2025, 14, 25. [Google Scholar] [CrossRef]
- O’Brien, C.; Ling, T.; Berman, J.M.; Culp-Hill, R.; Reisz, J.A.; Rondeau, V.; Jahangiri, S.; St-Germain, J.; Macwan, V.; Astori, A.; et al. Simultaneous inhibition of Sirtuin 3 and cholesterol homeostasis targets acute myeloid leukemia stem cells by perturbing fatty acid beta-oxidation and inducing lipotoxicity. Haematologica 2023, 108, 2343–2357. [Google Scholar] [CrossRef] [PubMed]
- Hurrish, K.H.; Su, Y.; Patel, S.; Ramage, C.L.; Zhao, J.; Temby, B.R.; Carter, J.L.; Edwards, H.; Buck, S.A.; Wiley, S.E.; et al. Enhancing anti-AML activity of venetoclax by isoflavone ME-344 through suppression of OXPHOS and/or purine biosynthesis in vitro. Biochem. Pharmacol. 2024, 220, 115981. [Google Scholar] [CrossRef]
- Gomez Solsona, B.; Horn, H.; Schmitt, A.; Xu, W.; Bucher, P.; Heinrich, A.; Kalmbach, S.; Kreienkamp, N.; Franke, M.; Wimmers, F.; et al. Inhibition of glutaminase-1 in DLBCL potentiates venetoclax-induced antitumor activity by promoting oxidative stress. Blood Adv. 2023, 7, 7433–7444. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Haribhai, D.; Mathew, R.; Duggan, R.; Ellis, P.A.; Wang, R.; Lasater, E.A.; Shi, Y.; Dave, N.; Riehm, J.J.; et al. Venetoclax Increases Intratumoral Effector T Cells and Antitumor Efficacy in Combination with Immune Checkpoint Blockade. Cancer Discov. 2021, 11, 68–79. [Google Scholar] [CrossRef]
- Luo, F.; Li, H.; Ma, W.; Cao, J.; Chen, Q.; Lu, F.; Qiu, M.; Zhou, P.; Xia, Z.; Zeng, K.; et al. The BCL-2 inhibitor APG-2575 resets tumor-associated macrophages toward the M1 phenotype, promoting a favorable response to anti-PD-1 therapy via NLRP3 activation. Cell Mol. Immunol. 2024, 21, 60–79. [Google Scholar] [CrossRef]
- Mukherjee, N.; Katsnelson, E.; Brunetti, T.M.; Michel, K.; Couts, K.L.; Lambert, K.A.; Robinson, W.A.; McCarter, M.D.; Norris, D.A.; Tobin, R.P.; et al. MCL1 inhibition targets Myeloid Derived Suppressors Cells, promotes antitumor immunity and enhances the efficacy of immune checkpoint blockade. Cell Death Dis. 2024, 15, 198. [Google Scholar] [CrossRef]
- Li, M.; Yu, H.; Qi, F.; Ye, Y.; Hu, D.; Cao, J.; Wang, D.; Mi, L.; Wang, Z.; Ding, N.; et al. Anti-CD47 immunotherapy in combination with BCL-2 inhibitor to enhance anti-tumor activity in B-cell lymphoma. Hematol. Oncol. 2022, 40, 596–608. [Google Scholar] [CrossRef]
- Zeng, Z.; Maiti, A.; Herbrich, S.; Cai, T.; Cavazos, A.; Manzella, T.; Ma, H.; Hayes, K.; Matthews, J.; DiNardo, C.D.; et al. Triple combination targeting methyltransferase, BCL-2, and PD-1 facilitates antileukemia responses in acute myeloid leukemia. Cancer 2023, 129, 531–540. [Google Scholar] [CrossRef]
- Ming, Z.; Lim, S.Y.; Stewart, A.; Pedersen, B.; Shklovskaya, E.; Menzies, A.M.; Carlino, M.S.; Kefford, R.F.; Lee, J.H.; Scolyer, R.A.; et al. IFN-gamma Signaling Sensitizes Melanoma Cells to BH3 Mimetics. J. Investig. Dermatol. 2023, 143, 1246–1256.e8. [Google Scholar] [CrossRef] [PubMed]
- Diepstraten, S.T.; Yuan, Y.; La Marca, J.E.; Young, S.; Chang, C.; Whelan, L.; Ross, A.M.; Fischer, K.C.; Pomilio, G.; Morris, R.; et al. Putting the STING back into BH3-mimetic drugs for TP53-mutant blood cancers. Cancer Cell 2024, 42, 850–868.e9. [Google Scholar] [CrossRef]
- Cooney, J.P.; Hirons, A.; Jansz, N.; Allison, C.C.; Hickey, P.; Teh, C.E.; Tan, T.; Dagley, L.F.; Yousef, J.; Yurick, D.; et al. Combination antiretroviral therapy and MCL-1 inhibition mitigate HTLV-1 infection in vivo. Cell 2025, 188, 4896–4912. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Guruprasad, P.; Ghilardi, G.; Pajarillo, R.; Sauter, C.T.; Patel, R.; Ballard, H.J.; Hong, S.J.; Chun, I.; Yang, N.; et al. Modulation of BCL-2 in Both T Cells and Tumor Cells to Enhance Chimeric Antigen Receptor T-cell Immunotherapy against Cancer. Cancer Discov. 2022, 12, 2372–2391. [Google Scholar] [CrossRef]
- Pan, R.; Ryan, J.; Pan, D.; Wucherpfennig, K.W.; Letai, A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell 2022, 185, 1521–1538.e18. [Google Scholar] [CrossRef]
- Saxena, K.; Hung, S.H.; Ryu, E.; Singh, S.; Zhang Tatarata, Q.; Zeng, Z.; Wang, Z.; Konopleva, M.Y.; Yee, C. BH3 mimetics augment cytotoxic T cell killing of acute myeloid leukemia via mitochondrial apoptotic mechanism. Cell Death Discov. 2025, 11, 120. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Zhang, J.; Yan, L.; Zhao, H.; Ding, L.; Bhatara, S.; Yang, X.; Yoshimura, S.; Yang, W.; et al. Single-cell systems pharmacology identifies development-driven drug response and combination therapy in B cell acute lymphoblastic leukemia. Cancer Cell 2024, 42, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Place, A.E.; Karol, S.E.; Forlenza, C.J.; Cooper, T.M.; Fraser, C.; Cario, G.; O’Brien, M.M.; Gerber, N.U.; Bourquin, J.P.; Reinhardt, D.; et al. Venetoclax Combined With Chemotherapy in Pediatric and Adolescent/Young Adult Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2025, 72, e31630. [Google Scholar] [CrossRef]
- Mantzaris, I.; Goldfinger, M.; Uriel, M.; Shastri, A.; Shah, N.; Gritsman, K.; Kornblum, N.S.; Shapiro, L.; Sica, R.A.; Munoz, A.; et al. Venetoclax plus daunorubicin and cytarabine for newly diagnosed acute myeloid leukemia: Results of a phase 1b study. Blood 2025, 145, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Arulananda, S.; O’Brien, M.; Evangelista, M.; Jenkins, L.J.; Poh, A.R.; Walkiewicz, M.; Leong, T.; Mariadason, J.M.; Cebon, J.; Balachander, S.B.; et al. A novel BH3-mimetic, AZD0466, targeting BCL-XL and BCL-2 is effective in pre-clinical models of malignant pleural mesothelioma. Cell Death Discov. 2021, 7, 122. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, J.; Liu, W.; Yang, Z.; Yu, F. Dual Targeting of Aurora-A and Bcl-xL Synergistically Reshapes the Immune Microenvironment and Induces Apoptosis in Breast Cancer. Cancer Sci. 2025, 116, 1823–1835. [Google Scholar] [CrossRef] [PubMed]
- Vaux, D.L.; Flavell, R.A. Apoptosis genes and autoimmunity. Curr. Opin. Immunol. 2000, 12, 719–724. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kwok, S.K.; Son, H.J.; Ryu, J.G.; Kim, E.K.; Oh, H.J.; Cho, M.L.; Ju, J.H.; Park, S.H.; Kim, H.Y. IL-17-mediated Bcl-2 expression regulates survival of fibroblast-like synoviocytes in rheumatoid arthritis through STAT3 activation. Arthritis Res. Ther. 2013, 15, R31. [Google Scholar] [CrossRef]
- Isomäki, P.; Söderström, K.O.; Punnonen, J.; Roivainen, A.; Luukkainen, R.; Merilahti-Palo, R.; Nikkari, S.; Lassila, O.; Toivanen, P. Expression of bcl-2 in rheumatoid arthritis. Br. J. Rheumatol. 1996, 35, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Scatizzi, J.C.; Hutcheson, J.; Pope, R.M.; Firestein, G.S.; Koch, A.E.; Mavers, M.; Smason, A.; Agrawal, H.; Haines, G.K., 3rd; Chandel, N.S.; et al. Bim-Bcl-2 homology 3 mimetic therapy is effective at suppressing inflammatory arthritis through the activation of myeloid cell apoptosis. Arthritis Rheum. 2010, 62, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Hutcheson, J.; Perlman, H. BH3-only proteins in rheumatoid arthritis: Potential targets for therapeutic intervention. Oncogene 2008, 27 (Suppl. S1), S168–S175. [Google Scholar] [CrossRef]
- Kielbassa, K.; Van der Weele, L.; Voskuyl, A.E.; de Vries, N.; Eldering, E.; Kuijpers, T.W. Differential expression pattern of Bcl-2 family members in B and T cells in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2023, 25, 225. [Google Scholar] [CrossRef]
- Khalil, N.M.; Yousef, R.N.; AlDeen, H.G.; Behiry, M.E.; Ashmawy, I.; Ramadan, A.; Awadallah, E.; Ali, A.; Alnaggar, A.R. Evaluation of the interaction between anti-apoptotic Bcl-2 protein family mRNA expression and autophagy gene Bcl-1 expression in Egyptian SLE patients. Lupus 2022, 31, 1186–1190. [Google Scholar] [CrossRef]
- Gordon, M.J.; Maldonado, E.; Danilov, A.V. Refractory Autoimmune Cytopenias Treated With Venetoclax. Hemasphere 2019, 3, e202. [Google Scholar] [CrossRef]
- Lu, P.; Fleischmann, R.; Curtis, C.; Ignatenko, S.; Clarke, S.H.; Desai, M.; Wong, S.L.; Grebe, K.M.; Black, K.; Zeng, J.; et al. Safety and pharmacodynamics of venetoclax (ABT-199) in a randomized single and multiple ascending dose study in women with systemic lupus erythematosus. Lupus 2018, 27, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Kuehl, T.; Lagares, D. BH3 mimetics as anti-fibrotic therapy: Unleashing the mitochondrial pathway of apoptosis in myofibroblasts. Matrix Biol. 2018, 68–69, 94–105. [Google Scholar] [CrossRef]
- Lagares, D.; Santos, A.; Grasberger, P.E.; Liu, F.; Probst, C.K.; Rahimi, R.A.; Sakai, N.; Kuehl, T.; Ryan, J.; Bhola, P.; et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med. 2017, 9, eaal3765. [Google Scholar] [CrossRef]
- Moncsek, A.; Al-Suraih, M.S.; Trussoni, C.E.; O’Hara, S.P.; Splinter, P.L.; Zuber, C.; Patsenker, E.; Valli, P.V.; Fingas, C.D.; Weber, A.; et al. Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(-/-)) mice. Hepatology 2018, 67, 247–259. [Google Scholar] [CrossRef]
- Kaufmann, T.; Simon, H.U. Pharmacological Induction of Granulocyte Cell Death as Therapeutic Strategy. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 231–247. [Google Scholar] [CrossRef]
- Karlberg, M.; Ekoff, M.; Huang, D.C.; Mustonen, P.; Harvima, I.T.; Nilsson, G. The BH3-mimetic ABT-737 induces mast cell apoptosis in vitro and in vivo: Potential for therapeutics. J. Immunol. 2010, 185, 2555–2562. [Google Scholar] [CrossRef]
- Reinhart, R.; Rohner, L.; Wicki, S.; Fux, M.; Kaufmann, T. BH3 mimetics efficiently induce apoptosis in mouse basophils and mast cells. Cell Death Differ. 2018, 25, 204–216. [Google Scholar] [CrossRef]
- Odinius, T.O.; Buschhorn, L.; Wagner, C.; Hauch, R.T.; Dill, V.; Dechant, M.; Buck, M.C.; Shoumariyeh, K.; Moog, P.; Schwaab, J.; et al. Comprehensive characterization of central BCL-2 family members in aberrant eosinophils and their impact on therapeutic strategies. J. Cancer Res. Clin. Oncol. 2022, 148, 331–340. [Google Scholar] [CrossRef]
- Shvedova, M.; Thanapaul, R.; Ha, J.; Dhillon, J.; Shin, G.H.; Crouch, J.; Gower, A.C.; Gritli, S.; Roh, D.S. Topical ABT-263 treatment reduces aged skin senescence and improves subsequent wound healing. Aging 2024, 17, 16–32. [Google Scholar] [CrossRef]
- Miura, Y.; Endo, K.; Komori, K.; Sekiya, I. Clearance of senescent cells with ABT-263 improves biological functions of synovial mesenchymal stem cells from osteoarthritis patients. Stem Cell Res. Ther. 2022, 13, 222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L.H.; Lu, J.; Xu, L.M.; Cheng, B.L.; Xiong, J.Y. ABT-263 enhanced bacterial phagocytosis of macrophages in aged mouse through Beclin-1-dependent autophagy. BMC Geriatr. 2021, 21, 225. [Google Scholar] [CrossRef]
- Gross, A.; Katz, S.G. Non-apoptotic functions of BCL-2 family proteins. Cell Death Differ. 2017, 24, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Wright, T.; Turnis, M.E.; Grace, C.R.; Li, X.; Brakefield, L.A.; Wang, Y.D.; Xu, H.; Kaminska, E.; Climer, L.K.; Mukiza, T.O.; et al. Anti-apoptotic MCL-1 promotes long-chain fatty acid oxidation through interaction with ACSL1. Mol. Cell 2024, 84, 1338–1353.e8. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, K.; McArthur, K.; Malelang, S.; Gibson, L.; Tee, A.; Elahee Doomun, S.N.; Rowe, C.L.; Arandjelovic, P.; Marchingo, J.M.; D’Silva, D.; et al. Relative importance of the anti-apoptotic versus apoptosis-unrelated functions of MCL-1 in vivo. Science 2025, 389, 1003–1011. [Google Scholar] [CrossRef]
- Adhikary, U.; Paulo, J.A.; Godes, M.; Roychoudhury, S.; Prew, M.S.; Ben-Nun, Y.; Yu, E.W.; Budhraja, A.; Opferman, J.T.; Chowdhury, D.; et al. Targeting MCL-1 triggers DNA damage and an anti-proliferative response independent from apoptosis induction. Cell Rep. 2023, 42, 113176. [Google Scholar] [CrossRef]
- Moyzis, A.G.; Lally, N.S.; Liang, W.; Najor, R.H.; Gustafsson, A.B. Mcl-1 Differentially Regulates Autophagy in Response to Changes in Energy Status and Mitochondrial Damage. Cells 2022, 11, 1469. [Google Scholar] [CrossRef]
- Gadet, R.; Jabbour, L.; Nguyen, T.T.M.; Lohez, O.; Mikaelian, I.; Gonzalo, P.; Luyten, T.; Chalabi-Dchar, M.; Wierinckx, A.; Marcillat, O.; et al. The endoplasmic reticulum pool of Bcl-xL prevents cell death through IP3R-dependent calcium release. Cell Death Discov. 2024, 10, 346. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Roman-Dominguez, A.; Sanz-Ros, J.; Romero-Garcia, N.; Huete-Acevedo, J.; Dromant, M.; Cuervo, A.M.; Borras, C.; Vina, J. Bcl-xL overexpression in T cells preserves muscle mitochondrial structure and function and prevents frailty in old mice. Sci. Adv. 2025, 11, eadr1378. [Google Scholar] [CrossRef] [PubMed]
- Kehr, S.; Vogler, M. It’s time to die: BH3 mimetics in solid tumors. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118987. [Google Scholar] [CrossRef] [PubMed]
- Bierbrauer, A.; Jacob, M.; Vogler, M.; Fulda, S. A direct comparison of selective BH3-mimetics reveals BCL-X(L), BCL-2 and MCL-1 as promising therapeutic targets in neuroblastoma. Br. J. Cancer 2020, 122, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Pioso, M.S.; Olesinski, E.A.; Yilma, B.; Ryan, J.A.; Mashaka, T.; Leutz, B.; Adamia, S.; Zhu, H.; Kuang, Y.; et al. Reduced Mitochondrial Apoptotic Priming Drives Resistance to BH3 Mimetics in Acute Myeloid Leukemia. Cancer Cell 2020, 38, 872–890.e6. [Google Scholar] [CrossRef]
- Potter, D.S.; Du, R.; Bhola, P.; Bueno, R.; Letai, A. Dynamic BH3 profiling identifies active BH3 mimetic combinations in non-small cell lung cancer. Cell Death Dis. 2021, 12, 741. [Google Scholar] [CrossRef]
- Mukherjee, N.; Strosnider, A.; Vagher, B.; Lambert, K.A.; Slaven, S.; Robinson, W.A.; Amato, C.M.; Couts, K.L.; Bemis, J.G.T.; Turner, J.A.; et al. BH3 mimetics induce apoptosis independent of DRP-1 in melanoma. Cell Death Dis. 2018, 9, 907. [Google Scholar] [CrossRef]
- Vera, M.B.; Morris-Hanon, O.; Nogueiras, G.I.; Ripari, L.B.; Esquivel, M.I.; Perez-Castro, C.; Romorini, L.; Sevlever, G.E.; Scassa, M.E.; Videla-Richardson, G.A. Noxa and Mcl-1 expression influence the sensitivity to BH3-mimetics that target Bcl-xL in patient-derived glioma stem cells. Sci. Rep. 2022, 12, 17729. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.J.; Rahman, F.; Karakas, B.; Shahid, M.; Lim, B.; Bouley, S.J.; Walker, J.A.; Lee, E.F.; Fairlie, W.D.; Kelly, K.R.; et al. Development of a Novel Biomarker Platform for Profiling Key Protein-Protein Interactions to Predict the Efficacy of BH3-Mimetic Drugs. Cancers 2025, 17, 1852. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.M.; Brown, F.C.; Chua, C.C.; Dengler, M.A.; Pomilio, G.; Anstee, N.S.; Litalien, V.; Thompson, E.; Morley, T.; MacRaild, S.; et al. Acquired mutations in BAX confer resistance to BH3-mimetic therapy in acute myeloid leukemia. Blood 2023, 141, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.J.; Spiliopoulou, P.; Siu, L.L. Circulating Biomarkers for Therapeutic Monitoring of Anti-cancer Agents. Oncologist 2022, 27, 352–362. [Google Scholar] [CrossRef]

| Name | Apoptotic Function | Non-Apoptotic Function |
|---|---|---|
| BCL2 | Anti-apoptotic | Calcium homeostasis at the ER, metabolism, autophagy |
| MCL1 | Anti-apoptotic | Calcium homeostasis at the ER, mitochondria shape, metabolism, and autophagy |
| BCLXL | Anti-apoptotic | Mitochondria shape, membrane permeability, calcium homeostasis at the ER, metabolism, autophagy, and DNA damage response |
| BCLW | Anti-apoptotic | Mitochondria shape |
| BCL2A1(BFL1) | Anti-apoptotic | Autophagy |
| BCLB | Primarily anti-apoptotic; may have cell-specific pro-apoptotic roles | Autophagy |
| BAD | Pro-apoptotic Initiator: Sensor | Autophagy, glucose, and lipid metabolism |
| BMF | Pro-apoptotic Initiator: Sensor | Autophagy |
| NOXA | Pro-apoptotic Initiator: Sensor | Glucose and lipid metabolism, autophagy |
| BID | Pro-apoptotic Initiator: Activator | Mitochondria shape, calcium homeostasis at the ER, unfolded protein response, DNA damage response, and glucose and lipid metabolism |
| BIM | Pro-apoptotic Initiator: Activator | Calcium homeostasis at the ER, unfolded protein response, and autophagy |
| PUMA | Pro-apoptotic Initiators: Activator | Calcium homeostasis at the ER, unfolded protein response, autophagy, and DNA damage response |
| BAX | Pro-apoptotic Executioner | Mitochondria shape, calcium homeostasis at the ER, metabolism, and unfolded protein response |
| BAK | Pro-apoptotic Executioner | Mitochondria shape, calcium homeostasis at the ER, metabolism, unfolded protein response, and autophagy |
| BOK | Pro-apoptotic Executioner | Calcium Homeostasis at the ER, unfolded protein response |
| BIK/BLK | Pro-apoptotic Executioner | Mitochondria shape, Calcium Homeostasis at the ER, Autophagy |
| HRK | Pro-apoptotic Initiators | Autophagy |
| BECLIN | Pro-autophagy | Autophagy |
| BNIP3 | Pro-apoptotic Initiator: Sensor | Mitochondrial membrane permeability, calcium homeostasis, and autophagy |
| Compound Name | Description | Clinical Trials | Status as of 2025 |
|---|---|---|---|
| AT-101 | (-)-Gossypol; pan-BCL2 inhibitor derived from a natural product. | Phase I/II trials in prostate cancer, CLL, and others. | No recent trials: limited efficacy observed in past trials. |
| Obatoclax (GX15-070) | Pan-BCL2 family inhibitor; targets BCL2, BCXL, and MCL1; induces apoptosis. | Phase I/II trials in hematologic and solid tumors. | Development discontinued due to toxicity. |
| Oblimersen sodium/G3139 | Antisense oligonucleotide targeting BCL2 mRNA to reduce BCL2 expression. | Multiple Phase III trials in CLL, melanoma, and others. | Clinical trials unsuccessful; development halted. |
| ABT-263 (Navitoclax) | Inhibits BCL2, BCLXL, and BCLW; induces apoptosis in cancer cells. | Phase I/II trials; major dose-limiting toxicity is thrombocytopenia. | Clinical trials ongoing. |
| ABT199 (Venetoclax) | Selective BCL2 inhibitor; FDA-approved for CLL, AML, and other cancers. | Multiple ongoing/completed Phase III trials. | FDA-approved for CLL, AML; Clinical trials ongoing for others. |
| Pelcitoclax (APG1252) | BCLXL/BCL2 dual inhibitor; aims to retain potency while reducing platelet toxicity. | Phase I/II trials in solid tumors and lymphomas in China and globally. | Clinical trial terminated. |
| LP-118 | Potent BCL2-selective inhibitor designed to overcome venetoclax resistance. | Phase I trials in hematologic malignancies. | Clinical trials active and ongoing. |
| TQB3909 | BCL2 inhibitor developed in China. | Phase I trials for hematologic cancers initiated in China. | Clinical trials ongoing but status unknown. |
| S55746 (BCL201) | Selective BCL2 inhibitor (developed by Servier/Novartis); orally bioavailable. | Phase I studies in hematologic malignancies; development status unclear. | Clinical trials completed. |
| MIK665 (S64315) | Selective MCL1 inhibitor (Servier/Novartis). | Phase I trials in AML, multiple myeloma, and solid tumors. | Majority of clinical trials completed. |
| PRT1419 | Potent MCL1 inhibitor (Prelude Therapeutics). | Phase I clinical trials in hematologic malignancies. | Majority of clinical trials completed. |
| Tapotoclax (AMG-176) | Selective MCL1 inhibitor developed by Amgen. | Phase I trials in relapsed/refractory hematologic malignancies. | Clinical trial terminated. |
| AMG-397 | Oral MCL1 inhibitor (Amgen); designed for convenience and improved delivery. | Phase I trials; | Clinical trial terminated. |
| GS-9716 | MCL1 inhibitor developed by Gilead Sciences. | Phase I trials in hematologic malignancies; | Limited information: one listed trial but not recruiting. |
| AZD5991 | Selective MCL1 inhibitor (AstraZeneca). | Phase I trials in hematologic cancers; | Clinical trial terminated due to cardiac toxicity concerns. |
| ABBV-467 | MCL1 inhibitor developed by AbbVie. | Phase I trials | Clinical trial terminated. |
| Compound Name | Description | Preclinical Studies |
|---|---|---|
| HA141 | Early synthetic small molecule BCL2 inhibitor; binds the BH3-binding groove. | Demonstrated apoptosis induction in leukemia and glioma cell lines; limited by poor solubility and toxicity. |
| ABT-737 | Potent inhibitor of BCL2, BCLXL, and BCLW; precursor to navitoclax. | Validated in multiple hematologic and solid tumor models; key tool in apoptosis research. |
| WEHI-539 | Selective BCLXL inhibitor. | Used to dissect BCLXL-specific functions; effective in synergy with other agents in lymphoma models. |
| A1155463/A1331852 | Selective, potent BCLXL inhibitors developed by AbbVie. | Induce apoptosis in BCLXL-dependent tumor cells; show synergy with chemotherapeutics. |
| PROTAC BCLXL degrader (XZ424) | PROTAC molecule designed to degrade BCLXL while sparing platelets by exploiting differential E3 ligase expression. | Effective in killing BCLXL-dependent cancer cells while minimizing thrombocytopenia in preclinical models. |
| UMI-77 | MCL1-selective BH3 mimetic. | Shown to induce apoptosis in pancreatic and lung cancer models. |
| S63845 | Selective and potent MCL1 inhibitor developed by Servier/Novartis. | Induces apoptosis in MCL1-dependent AML, multiple myeloma, and lymphoma models; widely studied preclinically. |
| ANJ810 | MCL1 inhibitor developed by Anji Pharmaceuticals. | Preclinical data show antitumor activity in hematologic cancers; limited publicly available data. |
| APG-3526/AS00491 | Selective BCL2 inhibitors developed by Ascentage Pharma. | Preclinical evidence suggests improved potency and resistance profile vs. venetoclax. |
| STP-369 | MCL1 inhibitor. | Demonstrated tumor regression in preclinical lymphoma and AML models. |
| Compound 56 | Covalent BCLXL inhibitor (structure-based design). | Reported to have improved selectivity and reduced platelet toxicity; still in the early preclinical phase. |
| A-97 derivatives | Series of small-molecule BCL2 inhibitors; A-97 is a potent BCL2 inhibitor. | Demonstrated pro-apoptotic activity in leukemia cells; derivatives under optimization. |
| Silibinin | Natural flavonoid with weak BH3-mimetic and anti-BCL2 activity. | Shown to induce apoptosis in various cancer cell lines; low potency; considered adjunctive or supportive. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, N.; Sheetz, J.; Shellman, Y.G. Targeting the BCL2 Family: Advances and Challenges in BH3 Mimetic-Based Therapies. Int. J. Mol. Sci. 2025, 26, 9859. https://doi.org/10.3390/ijms26209859
Mukherjee N, Sheetz J, Shellman YG. Targeting the BCL2 Family: Advances and Challenges in BH3 Mimetic-Based Therapies. International Journal of Molecular Sciences. 2025; 26(20):9859. https://doi.org/10.3390/ijms26209859
Chicago/Turabian StyleMukherjee, Nabanita, James Sheetz, and Yiqun G. Shellman. 2025. "Targeting the BCL2 Family: Advances and Challenges in BH3 Mimetic-Based Therapies" International Journal of Molecular Sciences 26, no. 20: 9859. https://doi.org/10.3390/ijms26209859
APA StyleMukherjee, N., Sheetz, J., & Shellman, Y. G. (2025). Targeting the BCL2 Family: Advances and Challenges in BH3 Mimetic-Based Therapies. International Journal of Molecular Sciences, 26(20), 9859. https://doi.org/10.3390/ijms26209859






