Structure-Based Identification of Natural Inhibitors Targeting the Gc Glycoprotein of Oropouche Virus: An In Silico Approach
Abstract
1. Introduction
2. Results and Discussion
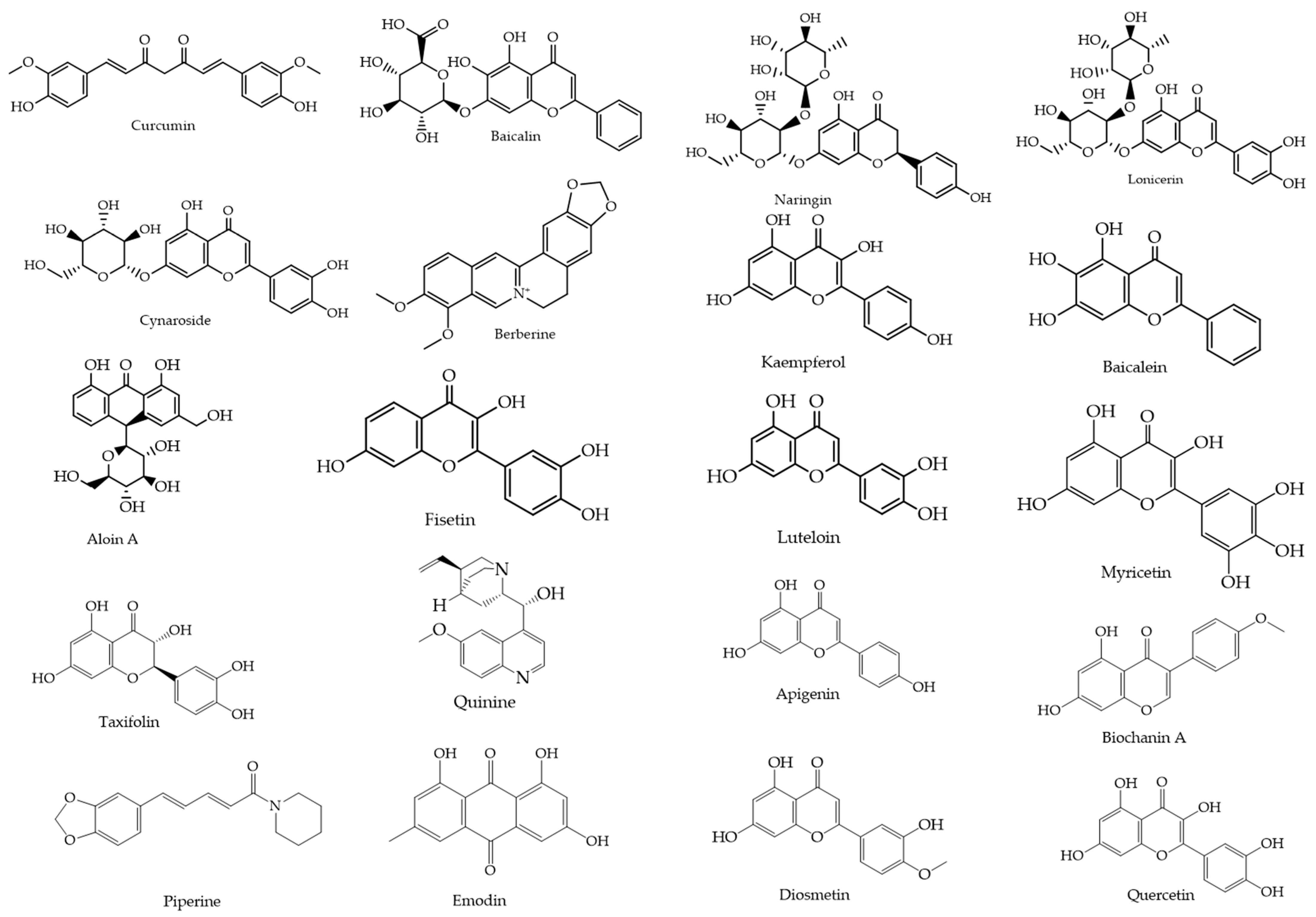
2.1. Data Collection
2.1.1. Selection of Luteolin as Baseline Scaffold
2.1.2. Similarity-Based Compound Retrieval Using PubChem: 2D and 3D Approaches
2.2. Molecular Docking
Target Selection
2.3. Docking Results
2.4. Pharmacokinetic and Toxicity Properties
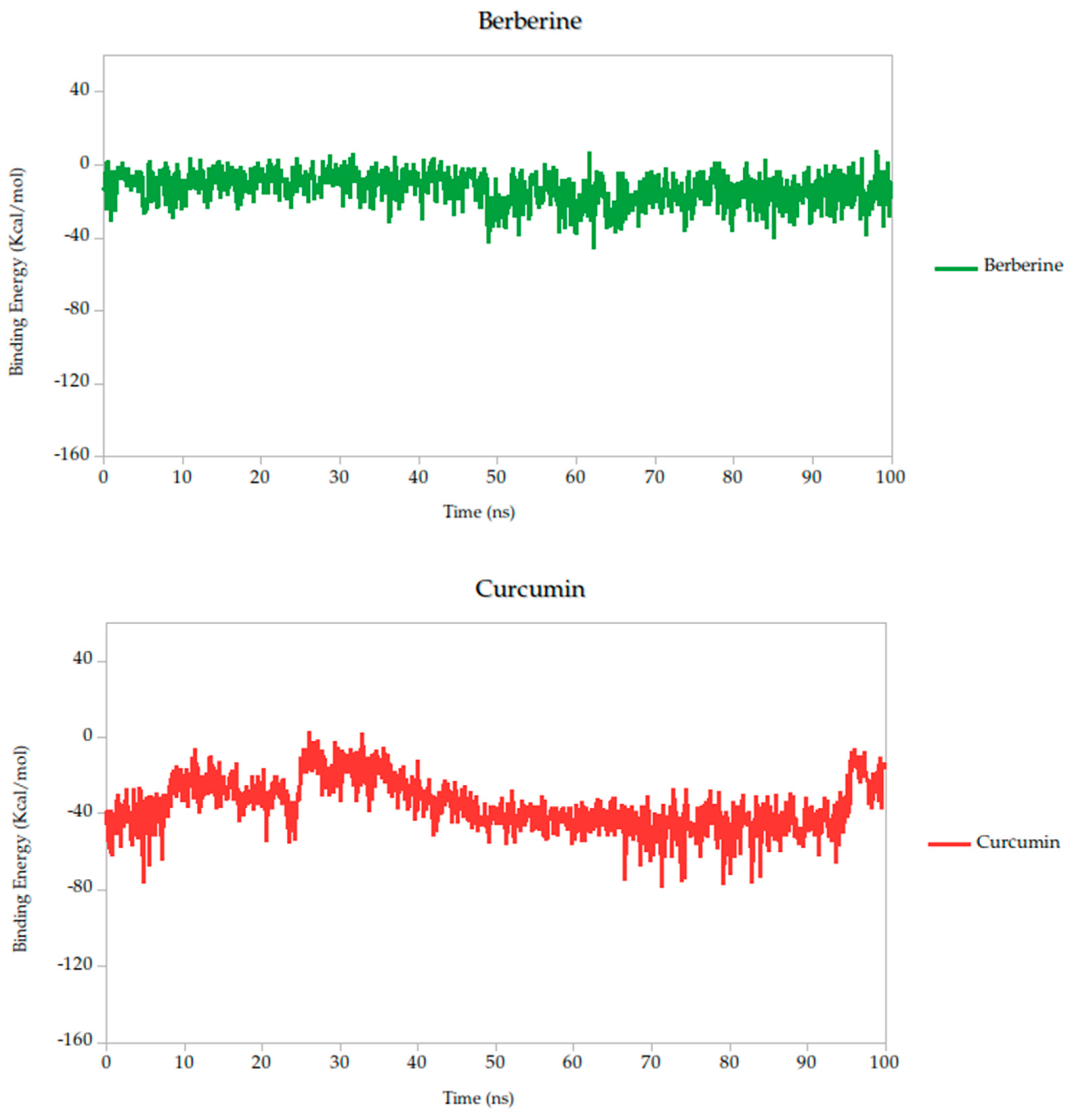
2.5. Molecular Dynamics Simulation
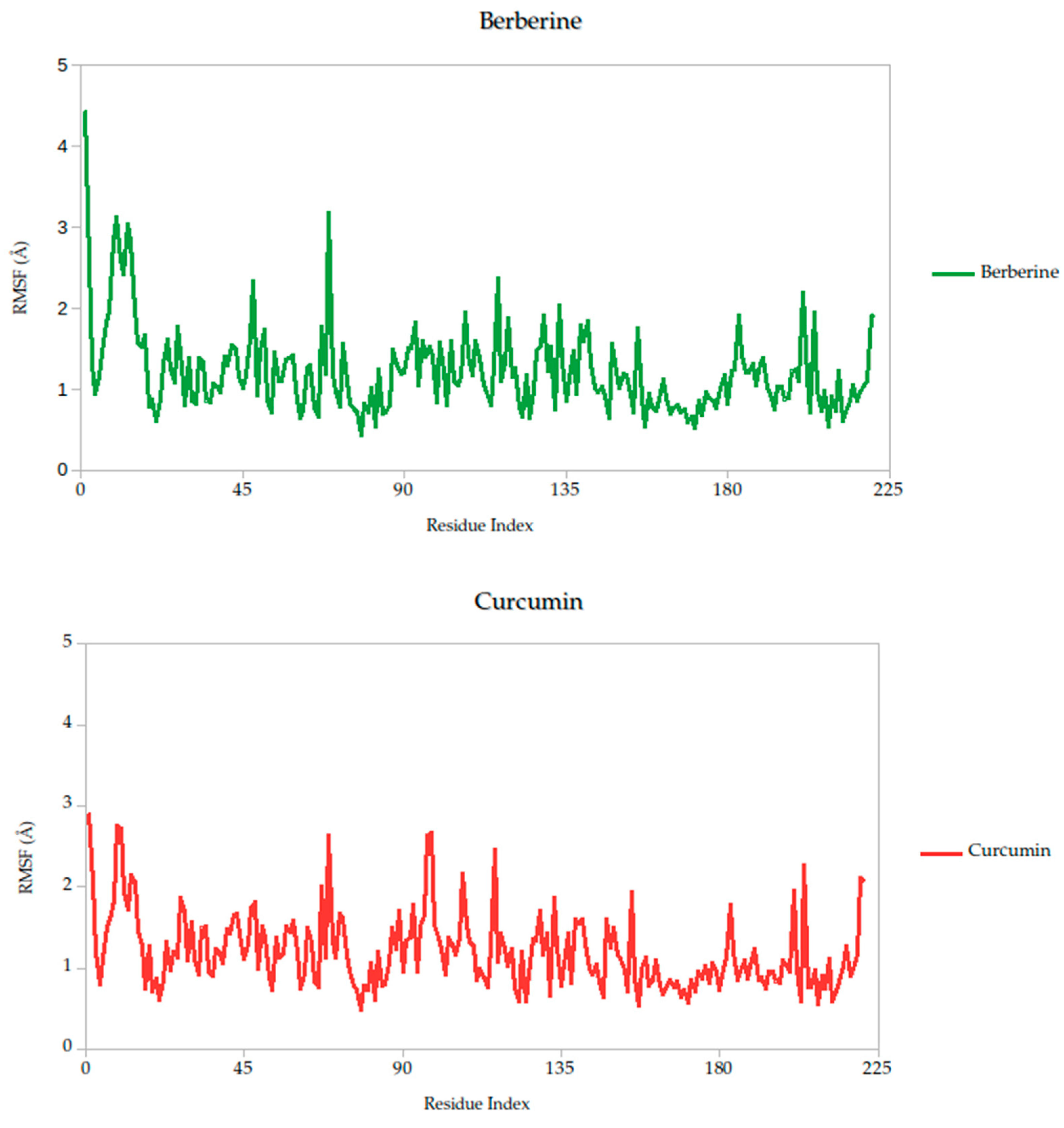
2.5.1. Residue-Level Flexibility (RMSF) and Mechanistic Implications
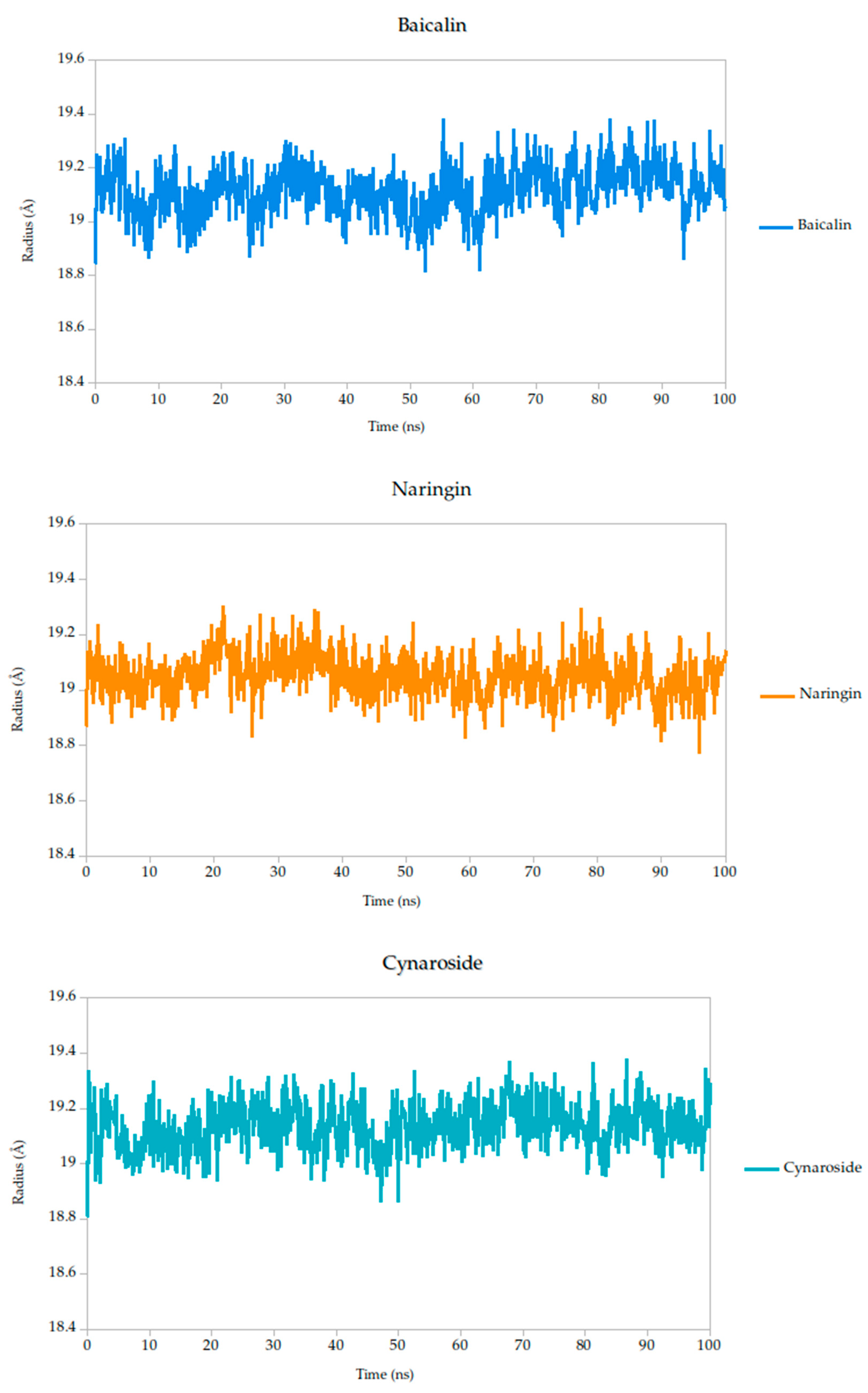
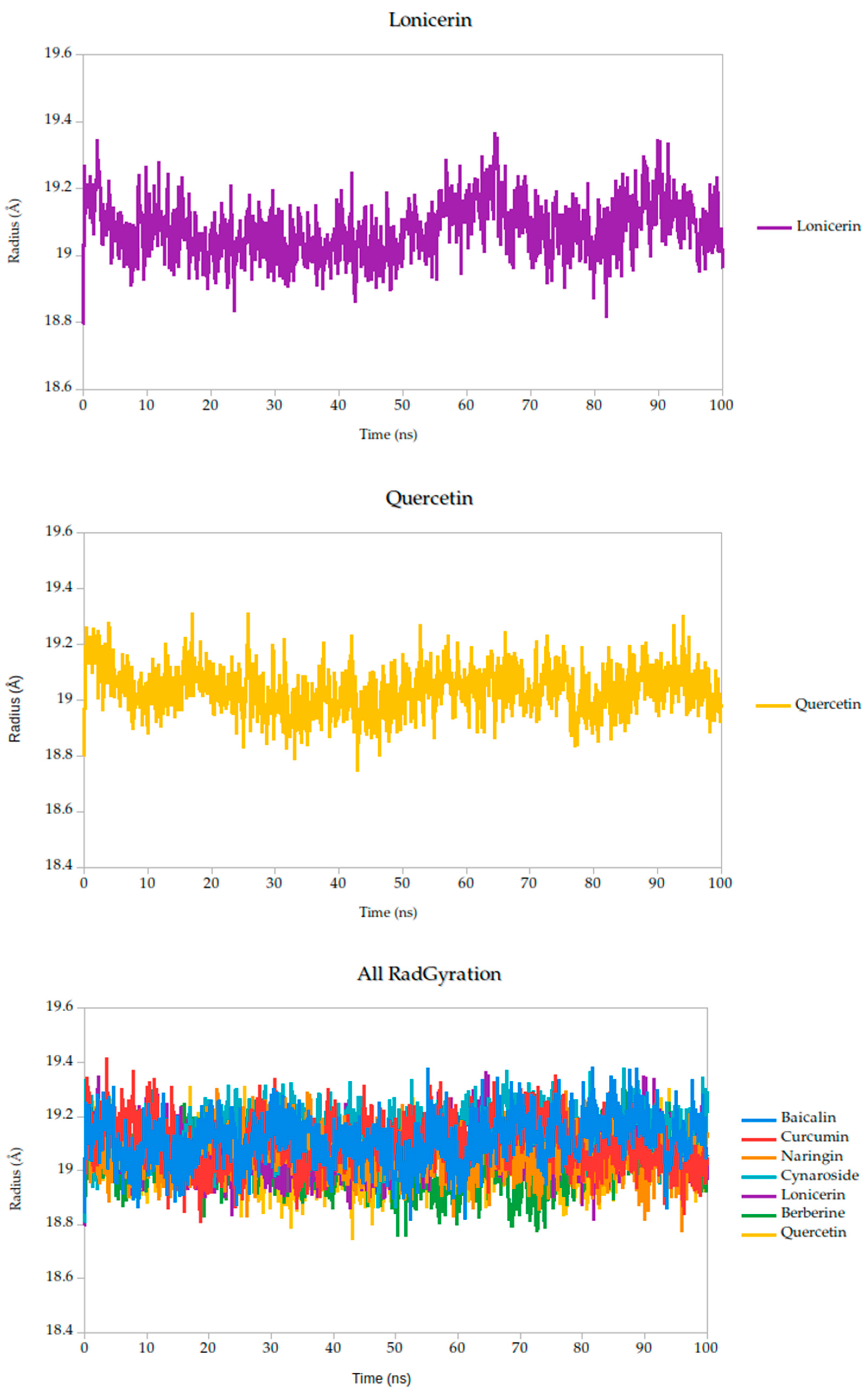
2.5.2. Radius of Gyration Analysis and Structural Implications
3. Materials and Methods
3.1. Compound Selection and Data Collection
Similarity-Based Compound Retrieval Using PubChem: 2D and 3D Approaches
3.2. Ligand Preparation and Optimization
3.3. ADMET Predictions
3.4. Molecular Docking Studies
3.5. Molecular Dynamics Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche Fever: A Review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Wu, Z.; Feng, S.; Lu, K.; Zhu, W.; Sun, H.; Niu, G. Oropouche virus: A neglected global arboviral threat. Virus Res. 2024, 341, 199318. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L. Oropouche Virus: An Emerging Orthobunyavirus. J. Gen. Virol. 2024, 105, 902027. [Google Scholar] [CrossRef]
- Romero-Alvarez, D.; Escobar, L.E.; Auguste, A.J.; Del Valle, S.Y.; Manore, C.A. Transmission risk of Oropouche fever across the Americas. Infect. Dis. Poverty. 2023, 12, 17. [Google Scholar] [CrossRef]
- Riccò, M.; Corrado, S.; Bottazzoli, M.; Marchesi, F.; Gili, R.; Bianchi, F.P.; Frisicale, E.M.; Guicciardi, S.; Fiacchini, D.; Tafuri, S.; et al. (Re-)Emergence of Oropouche Virus (OROV) Infections: Systematic Review and Meta-Analysis of Observational Studies. Viruses 2024, 16, 1498. [Google Scholar] [CrossRef] [PubMed]
- Scachetti, G.C.; Forato, J.; Claro, I.M.; Hua, X.; Salgado, B.B.; Vieira, A.; Simeoni, C.L.; Barbosa, A.R.C.; Rosa, I.L.; de Souza, G.F.; et al. Re-emergence of Oropouche virus between 2023 and 2024 in Brazil: An observational epidemiological study. Lancet Infect. Dis. 2025, 25, 266–275. [Google Scholar] [CrossRef]
- Bai, F.; Denyoh, P.M.D.; Urquhart, C.; Shrestha, S.; Yee, D.A. A Comprehensive Review of the Neglected and Emerging Oropouche Virus. Viruses 2025, 17, 439. [Google Scholar] [CrossRef]
- Bandeira, A.C.; Pereira, F.M.; Leal, A.; Santos, S.P.; Barbosa, A.C.; Souza, M.S.P.L.; de Souza, D.R.; Guimaraes, N.; Fonseca, V.; Giovanetti, M.; et al. Fatal Oropouche Virus Infections in Nonendemic Region, Brazil, 2024. Emerg. Infect Dis. 2024, 30, 11370–11374. [Google Scholar] [CrossRef]
- Da Rosa, J.F.T.; De Souza, W.M.; De Paula Pinheiro, F.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche virus: Clinical, epidemiological, and molecular aspects of a neglected orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Porwal, S.; Malviya, R.; Sridhar, S.B.; Shareef, J.; Wadhwa, T. Mysterious Oropouche virus: Transmission, symptoms, and control. Infect. Med. 2025, 4, 100177. [Google Scholar] [CrossRef]
- Guagliardo, S.A.J.; Connelly, C.R.; Lyons, S.; Martin, S.W.; Sutter, R.; Hughes, H.R.; Brault, A.C.; Lambert, A.J.; Gould, C.V.; Staples, J.E. Reemergence of Oropouche Virus in the Americas and Risk for Spread in the United States and Its Territories. Emerg. Infect. Dis. 2024, 30, 2241–2249. [Google Scholar] [CrossRef]
- Bernstein, A.S.; Ando, A.W.; Loch-Temzelides, T.; Vale, M.M.; Li, B.V.; Li, H.; Busch, J.; Chapman, C.A.; Kinnaird, M.; Nowak, K.; et al. The costs and benefits of primary prevention of zoonotic pandemics. Sci. Adv. 2022, 8, eabl4183. [Google Scholar] [CrossRef]
- Yoosuf, B.T.; Gaidhane, A.M.; Vadia, N.; Menon, S.V.; Chennakesavulu, K.; Panigrahi, R.; Bushi, G.; Rani, A.; Sah, S.; Shabil, M.; et al. Epidemiology, transmission dynamics, treatment strategies, and future perspectives on Oropouche virus. Diagn. Microbiol. Infect. Dis. 2025, 113, 116882. [Google Scholar] [CrossRef]
- Vijukumar, A.; Kumar, A.; Kumar, H. Potential Therapeutics and Vaccines: Current Progress and Challenges in Developing Antiviral Treatments or Vaccines for Oropouche Virus. Diagn. Microbiol. Infect. Dis. 2025, 111, 116699. [Google Scholar] [CrossRef]
- Gould, E.; Pettersson, J.; Higgs, S.; Charrel, R.; de Lamballerie, X. Emerging arboviruses: Why today? One Health 2017, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Srivastava, S.; Kumar, S.; Golmei, P.; Rahaman, S.A.; Mehta, R.; Ferraz, C.; Apostolopoulos, V.; Rodriguez-Morales, A.J. Oropouche fever outbreak in Brazil: An emerging concern in Latin America. Lancet Microbe 2024, 5, 100904. [Google Scholar] [CrossRef]
- Ortiz, D.I.; Piche-Ovares, M.; Romero-Vega, L.M.; Wagman, J.; Troyo, A. The Impact of Deforestation, Urbanization, and Changing Land Use Patterns on the Ecology of Mosquito and Tick-Borne Diseases in Central America. Insects 2022, 13, 20. [Google Scholar] [CrossRef]
- Castro, M.C.; Lima Neto, A.S. Unprecedented spread and genetic evolution of the Oropouche virus. Nat. Med. 2024, 28, 3420–3421. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.B.; Nunes, M.R.; Casseb, L.M.; Carvalho, V.L.; da Silva, E.V.P.; Silva, M.; Casseb, S.M.; Vasconcelos, P.F. Molecular epidemiology of oropouche virus, Brazil. Emerg. Infect. Dis. 2011, 17, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Moreira, H.M.; Sgorlon, G.; Queiroz, J.A.S.; Roca, T.P.; Ribeiro, J.; Teixeira, K.S.; Passos-Silva, A.M.; Araújo, A.; Gasparelo, N.W.F.; Santos, A.d.O.D.; et al. Outbreak of Oropouche virus in frontier regions in western Amazon. Microbiol. Spectr. 2024, 12, e01629-23. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; White, J.L.; Hughes, H.R.; Anne Guagliardo, S.J.; Velez, J.O.; Fitzpatrick, K.A.; Davis, E.H.; Stanek, D.; Kopp, E.; Dumoulin, P.; et al. Oropouche Virus Disease Among U.S. Travelers—United States, Centers for Disease Control and Prevention. MMWR 2024, 73, 769–773. [Google Scholar]
- de Armas Fernández, J.R.; Peña García, C.E.; Acosta Herrera, B.; Betancourt Plaza, I.; Gutiérrez de la Cruz, Y.; Resik Aguirre, S.; Kourí Cardellá, V.; Guzmán Tirado, M.G. Report of an unusual association of Oropouche Fever with Guillain-Barré syndrome in Cuba, 2024. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 11233–11237. [Google Scholar] [CrossRef]
- Martins, F.E.d.N.; Chiang, J.O.; Nunes, B.T.D.; Ribeiro, B.d.F.R.; Martins, L.C.; Casseb, L.M.N.; Henriques, D.F.; de Oliveira, C.S.; Maciel, E.L.N.; Cravo, L.d.C.C.; et al. Newborns with microcephaly in Brazil and potential vertical transmission of Oropouche virus: A case series. Lancet Infect Dis. 2024, 1, 155–165. [Google Scholar]
- Schwartz, D.A.; Dashraath, P.; Baud, D. Oropouche Virus (OROV) in Pregnancy: An Emerging Cause of Placental and Fetal Infection Associated with Stillbirth and Microcephaly following Vertical Transmission. Viruses 2024, 16, 9435. [Google Scholar] [CrossRef]
- Files, M.A.; Hansen, C.A.; Herrera, V.C.; Schindewolf, C.; Barrett, A.D.T.; Beasley, D.W.C.; Bourne, N.; Milligan, G.N. Baseline mapping of Oropouche virology, epidemiology, therapeutics, and vaccine research and development. npj Vaccines 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Benitez, A.J.; Alvarez, M.; Perez, L.; Gravier, R.; Serrano, S.; Hernandez, D.M.; Perez, M.M.; Gutierrez-Bugallo, G.; Martinez, Y.; Companioni, A.; et al. Oropouche Fever, Cuba, May 2024. Emerg. Infect. Dis. 2024, 30, 10155–10159. [Google Scholar] [CrossRef] [PubMed]
- Castilletti, C.; Mori, A.; Pomari, E.; Matucci, A.; Martelli, G.; Curiale, S.; Angheben, A.; Gobbi, F.G. First diagnoses of Oropouche virus in Europe: How can we strengthen communication and preparedness globally? Lancet Infect. Dis. 2024, 24, e602–e603. [Google Scholar] [CrossRef]
- da Silva-Júnior, E.F. Oropouche virus—The “Newest” invisible public enemy? Bioorganic Med. Chem. 2024, 109, 117797. [Google Scholar] [CrossRef]
- Ribas Freitas, A.R.; Schwartz, D.A.; Lima Neto, A.S.; Rodrigues, R.; Cavalcanti, L.P.G.; Alarcón-Elbal, P.M. Oropouche Virus (OROV): Expanding Threats, Shifting Patterns, and the Urgent Need for Collaborative Research in Latin America. Viruses 2025, 17, 353. [Google Scholar] [CrossRef]
- Poojari, C.S.; Bommer, T.; Hub, J.S. Viral fusion proteins of classes II and III recognize and reorganize complex biological membranes. Commun. Biol. 2025, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, D.; Li, C.; Deng, M.; Li, G.; Chen, S.; Zhao, M.; Qin, L.; Zhang, K. The Role of Orthobunyavirus Glycoprotein Gc in the Viral Life Cycle: From Viral Entry to Egress. Molecules 2025, 30, 503. [Google Scholar] [CrossRef]
- Hellert, J.; Aebischer, A.; Haouz, A.; Guardado-Calvo, P.; Reiche, S.; Beer, M.; Rey, F.A. Structure, function, and evolution of the Orthobunyavirus membrane fusion glycoprotein. Cell Rep. 2023, 42, 112142. [Google Scholar] [CrossRef]
- Wernike, K.; Brocchi, E.; Cordioli, P.; Sénéchal, Y.; Schelp, C.; Wegelt, A.; Aebischer, A.; Roman-Sosa, G.; Reimann, I.; Beer, M. A novel panel of monoclonal antibodies against Schmallenberg virus nucleoprotein and glycoprotein Gc allows specific orthobunyavirus detection and reveals antigenic differences. Vet. Res. 2015, 46, 27. [Google Scholar] [CrossRef]
- Roney, M.; Mohd Aluwi, M.F.F. The importance of in-silico studies in drug discovery. Intell. Pharm. 2024, 2, 578–579. [Google Scholar] [CrossRef]
- Murgueitio, M.S.; Bermudez, M.; Mortier, J.; Wolber, G. In silico virtual screening approaches for anti-viral drug discovery. Drug Discov. Today Technol. 2012, 9, e219–e225. [Google Scholar] [CrossRef]
- Cabrera, N.; Cuesta, S.A.; Mora, J.R.; Calle, L.; Márquez, E.A.; Kaunas, R.; Paz, J.L. In Silico Searching for Alternative Lead Compounds to Treat Type 2 Diabetes through a QSAR and Molecular Dynamics Study. Pharmaceutics 2022, 14, 232. [Google Scholar] [CrossRef] [PubMed]
- Valencia, J.; Rubio, V.; Puerto, G.; Vasquez, L.; Bernal, A.; Mora, J.R.; Cuesta, S.A.; Paz, J.L.; Insuasty, B.; Abonia, R.; et al. QSAR Studies, Molecular Docking, Molecular Dynamics, Synthesis, and Biological Evaluation of Novel Quinolinone-Based Thiosemicarbazones against Mycobacterium tuberculosis. Antibiotics 2023, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Bernal, A.; Márquez, E.A.; Flores-Sumoza, M.; Cuesta, S.A.; Mora, J.R.; Paz, J.L.; Mendoza-Mendoza, A.; Rodríguez-Macías, J.; Salazar, F.; Insuasty, D.; et al. Molecular Modeling of Vasodilatory Activity: Unveiling Novel Candidates Through Density Functional Theory, QSAR, and Molecular Dynamics. Int. J. Mol. Sci. 2024, 25, 12649. [Google Scholar] [CrossRef]
- Domingos, L.T.S.; de Moraes, D.C.; Santos, M.F.C.; Curvelo, J.A.R.; Bayona-Pacheco, B.; Marquez, E.A.; Martinez, A.W.B.; Berlinck, R.G.S.; Ferreira-Pereira, A. Batzelladine D, a Marine Natural Product, Reverses the Fluconazole Resistance Phenotype Mediated by Transmembrane Transporters in Candida albicans and Interferes with Its Biofilm: An In Vitro and In Silico Study. Mar. Drugs 2024, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, S.A.; Mora, J.R.; Márquez, E.A. In Silico Screening of the DrugBank Database to Search for Possible Drugs against SARS-CoV-2. Molecules 2021, 26, 1100. [Google Scholar] [CrossRef]
- Cortes, E.; Mora, J.; Márquez, E. Modelling the Anti-Methicillin-Resistant Staphylococcus Aureus (MRSA) Activity of Cannabinoids: A QSAR and Docking Study. Crystals 2020, 10, 692. [Google Scholar] [CrossRef]
- Elbouzidi, A.; Ouassou, H.; Aherkou, M.; Kharchoufa, L.; Meskali, N.; Baraich, A.; Mechchate, H.; Bouhrim, M.; Idir, A.; Hano, C.; et al. LC–MS/MS Phytochemical Profiling, Antioxidant Activity, and Cytotoxicity of the Ethanolic Extract of Atriplex halimus L. against Breast Cancer Cell Lines: Computational Studies and Experimental Validation. Pharmaceuticals 2022, 15, 1156. [Google Scholar] [CrossRef] [PubMed]
- Angourani, H.R.; Zarei, A.; Moghadam, M.M.; Ramazani, A.; Mastinu, A. Investigation on the Essential Oils of the Achillea Species: From Chemical Analysis to the In Silico Uptake against SARS-CoV-2 Main Protease. Life 2023, 13, 378. [Google Scholar] [CrossRef] [PubMed]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; Huang, C.; Li, Y.; Yu, H.; Wang, Y.; Duan, J.; Ling, Y. A Novel Chemometric Method for the Prediction of Human Oral Bioavailability. Int. J. Mol. Sci. 2012, 13, 6964–6982. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. Application of the SwissDrugDesign Online Resources in Virtual Screening. Int. J. Mol. Sci. 2019, 20, 4612. [Google Scholar] [CrossRef]
- Dev Sharma, P.; Alhudhaibi, A.M.; Al Noman, A.; Abdallah, E.M.; Taha, T.H.; Sharma, H. Systems Biology-Driven Discovery of Host-Targeted Therapeutics for Oropouche Virus: Integrating Network Pharmacology, Molecular Docking, and Drug Repurposing. Pharmaceuticals 2025, 18, 613. [Google Scholar] [CrossRef]
- Moradi, M.; Golmohammadi, R.; Najafi, A.; Moosazadeh Moghaddam, M.; Fasihi-Ramandi, M.; Mirnejad, R. A contemporary review on the important role of in silico approaches for managing different aspects of COVID-19 crisis. Inform. Med. Unlocked 2022, 28, 100862. [Google Scholar] [CrossRef]
- Chang, Y.; Hawkins, B.A.; Du, J.J.; Groundwater, P.W.; Hibbs, D.E.; Lai, F. A Guide to In Silico Drug Design. Pharmaceutics 2023, 15, 49. [Google Scholar] [CrossRef]
- dos Santos Peinado, R.; Saivish, M.V.; de Lima Menezes, G.; Fulco, U.L.; da Silva, R.A.; Korostov, K.; Eberle, R.J.; Melo, P.A.; Nogueira, M.L.; Pacca, C.C.; et al. The search for an antiviral lead molecule to combat the neglected emerging Oropouche virus. Curr. Res. Microb. Sci. 2024, 6, 100238. [Google Scholar] [CrossRef]
- Mandova, T.; Saivish, M.V.; Menezes, G.d.L.; Bezerra, K.S.; Fulco, U.L.; da Silva, R.A.; Da Costa, F.B.; Nogueira, M.L. Antiviral Activity and Molecular Dynamics Simulation of Hops Compounds against Oropouche Virus (Peribunyaviridae). Pharmaceutics 2023, 15, 2769. [Google Scholar] [CrossRef]
- Rosmalena, R.; Elya, B.; Dewi, B.E.; Fithriyah, F.; Desti, H.; Angelina, M.; Hanafi, M.; Lotulung, P.D.; Prasasty, V.D.; Seto, D. The Antiviral Effect of Indonesian Medicinal Plant Extracts Against Dengue Virus In Vitro and In Silico. Pathogens 2019, 8, 85. [Google Scholar] [CrossRef]
- Menezes, G.d.L.; Saivish, M.V.; Sacchetto, L.; da Silva, G.C.D.; Teixeira, I.d.S.; Mistrão, N.F.B.; Nogueira, M.L.; Oliveira, J.I.N.; Bezerra, K.S.; da Silva, R.A.; et al. Exploring Quercetin Hydrate’s Potential as an Antiviral Treatment for Oropouche Virus. Biophysica 2023, 3, 485–500. [Google Scholar] [CrossRef]
- Rehman, M.F.u.; Akhter, S.; Batool, A.I.; Selamoglu, Z.; Sevindik, M.; Eman, R.; Mustaqeem, M.; Akram, M.S.; Kanwal, F.; Lu, C.; et al. Effectiveness of Natural Antioxidants against SARS-CoV-2? Insights from the In-Silico World. Antibiotics 2021, 10, 1011. [Google Scholar] [CrossRef]
- Ali, M.A.; Sheikh, H.; Yaseen, M.; Faruqe, M.O.; Ullah, I.; Kumar, N.; Bhat, M.A.; Mollah, M.N.H. Exploring the Therapeutic Potential of Petiveria alliacea L. Phytochemicals: A Computational Study on Inhibiting SARS-CoV-2’s Main Protease (Mpro). Molecules 2024, 29, 2524. [Google Scholar] [CrossRef]
- Moballegh Nasery, M.; Abadi, B.; Poormoghadam, D.; Zarrabi, A.; Keyhanvar, P.; Khanbabaei, H.; Ashrafizadeh, M.; Mohammadinejad, R.; Tavakol, S.; Sethi, G. Curcumin Delivery Mediated by Bio-Based Nanoparticles: A Review. Molecules 2020, 25, 689. [Google Scholar] [CrossRef]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef]
- Rahban, M.; Habibi-Rezaei, M.; Mazaheri, M.; Saso, L.; Moosavi-Movahedi, A.A. Anti-Viral Potential and Modulation of Nrf2 by Curcumin: Pharmacological Implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Šudomová, M.; Berchová-Bímová, K.; Marzocco, S.; Liskova, A.; Kubatka, P.; Hassan, S.T.S. Berberine in Human Oncogenic Herpesvirus Infections and Their Linked Cancers. Viruses 2021, 13, 1014. [Google Scholar] [CrossRef]
- Jiménez Bolaño, D.C.; Insuasty, D.; Rodríguez Macías, J.D.; Grande-Tovar, C.D. Potential Use of Tomato Peel, a Rich Source of Lycopene, for Cancer Treatment. Molecules 2024, 29, 3079. [Google Scholar] [CrossRef]
- Lu, P.; Zhang, T.; Ren, Y.; Rao, H.; Lei, J.; Zhao, G.; Wang, M.; Gong, D.; Cao, Z. A Literature Review on the Antiviral Mechanism of Luteolin. Nat. Prod. Commun. 2023, 18, 1934578X231171521. [Google Scholar] [CrossRef]
- Yan, H.; Ma, L.; Wang, H.; Wu, S.; Huang, H.; Gu, Z.; Jiang, J.; Li, Y. Luteolin decreases the yield of influenza A virus in vitro by interfering with the coat protein I complex expression. J. Nat. Med. 2019, 73, 387–396. [Google Scholar] [CrossRef]
- Men, X.; Li, S.; Cai, X.; Fu, L.; Shao, Y.; Zhu, Y. Antiviral Activity of Luteolin against Pseudorabies Virus In Vitro and In Vivo. Animals 2023, 13, 761. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, X.; Gou, J.; Zhu, X.; Yin, D.; Yin, L.; Shen, X.; Dai, Y.; Pan, X. Antiviral activity of luteolin against porcine epidemic diarrhea virus in silico and in vitro. BMC Vet. Res. 2024, 20, 288. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Wang, Z.; Song, X.; Ren, Z.; Wang, X.; Wang, Y.; Zheng, K. Luteolin inhibits herpes simplex virus 1 infection by activating cyclic guanosine monophosphate-adenosine monophosphate synthase-mediated antiviral innate immunity. Phytomedicine 2023, 120, 155020. [Google Scholar] [CrossRef]
- Xu, K.; Liu, B.; Ma, Y.; Du, J.; Li, G.; Gao, H.; Zhang, Y.; Ning, Z. Physicochemical Properties and Antioxidant Activities of Luteolin-Phospholipid Complex. Molecules 2009, 14, 3486–3493. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef] [PubMed]
- Hellert, J.; Aebischer, A.; Wernike, K.; Haouz, A.; Brocchi, E.; Reiche, S.; Guardado-Calvo, P.; Beer, M.; Rey, F.A. Orthobunyavirus spike architecture and recognition by neutralizing antibodies. Nat. Commun. 2019, 10, 879. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, M.A.; Iqbal, M.N.; Saddick, S.; Ali, I.; Khan, F.S.; Kanwal, S.; Ahmed, D.; Ibrahim, M.; Afzal, U.; Awais, M. Identification of lead compounds against scm (Fms10) in enterococcus faecium using computer aided drug designing. Life 2021, 11, 77. [Google Scholar] [CrossRef]
- Bojarska, J.; Remko, M.; Breza, M.; Madura, I.D.; Kaczmarek, K.; Zabrocki, J.; Wolf, W.M. A supramolecular approach to structure-based design with a focus on synthons hierarchy in ornithine-derived ligands: Review, synthesis, experimental and in silico studies. Molecules 2020, 25, 1135. [Google Scholar] [CrossRef]
- Ivanova, L.; Karelson, M. The Impact of Software Used and the Type of Target Protein on Molecular Docking Accuracy. Molecules 2022, 27, 9041. [Google Scholar] [CrossRef]
- Windhaber, S.; Xin, Q.; Lozach, P.-Y. Orthobunyaviruses: From Virus Binding to Penetration into Mammalian Host Cells. Viruses 2021, 13, 872. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Hernandez, O.; Saurith-Coronell, O.; Rodríguez-Macías, J.; Márquez, E.; Mora, J.R.; Paz, J.L.; Flores-Sumoza, M.; Mendoza-Mendoza, A.; Flores-Morales, V.; Marrero-Ponce, Y.; et al. In Silico Identification of Potential Clovibactin-like Antibiotics Binding to Unique Cell Wall Precursors in Diverse Gram-Positive Bacterial Strains. Int. J. Mol. Sci. 2025, 26, 1724. [Google Scholar] [CrossRef]
- Parikesit, A.A.; Zahroh, H.; Nugroho, A.S.; Hapsari, A.; Sumo, U.; Tambunan, F. The Computation of Cyclic Peptide with Prolin-Prolin Bond as Fusion Inhibitor of DENV Envelope Protein through Molecular Docking and Molecular Dynamics Simulation. arXiv 2013, arXiv:1511.01388. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, D.; Giri, R. Targeting the nsp2 Cysteine Protease of Chikungunya Virus Using FDA Approved Library and Selected Cysteine Protease Inhibitors. Pathogens 2019, 8, 128. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 596–704. [Google Scholar] [CrossRef]
- Ratanakomol, T.; Roytrakul, S.; Wikan, N.; Smith, D.R. Berberine Inhibits Dengue Virus through Dual Mechanisms. Molecules 2021, 26, 18501. [Google Scholar] [CrossRef]
- Kumar, M.; Chung, S.-M.; Enkhtaivan, G.; Patel, R.V.; Shin, H.-S.; Mistry, B.M. Molecular Docking Studies and Biological Evaluation of Berberine–Benzothiazole Derivatives as an Anti-Influenza Agent via Blocking of Neuraminidase. Int. J. Mol. Sci. 2021, 22, 2368. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Mukherjee, S.; Manisegaran, M.; Campanile, M.; Del Vecchio, P.; Petraccone, L.; Winter, R. Binding Properties of RNA Quadruplex of SARS-CoV-2 to Berberine Compared to Telomeric DNA Quadruplex. Int. J. Mol. Sci. 2022, 23, 5690. [Google Scholar] [CrossRef]
- Varghese, F.S.; van Woudenbergh, E.; Overheul, G.J.; Eleveld, M.J.; Kurver, L.; van Heerbeek, N.; van Laarhoven, A.; Miesen, P.; den Hartog, G.; de Jonge, M.I.; et al. Berberine and Obatoclax Inhibit SARS-Cov-2 Replication in Primary Human Nasal Epithelial Cells In Vitro. Viruses 2021, 13, 282. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Yang, L.; Wang, H.-Q.; Zhao, X.-Q.; Liu, T.; Li, Y.-H.; Zeng, Q.-X.; Li, Y.-H.; Song, D.-Q. Synthesis and Evolution of Berberine Derivatives as a New Class of Antiviral Agents against Enterovirus 71 through the MEK/ERK Pathway and Autophagy. Molecules 2018, 23, 2084. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.A.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef]
- Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses 2019, 11, 1019. [Google Scholar] [CrossRef]
- Al-Karmalawy, A.A.; Dahab, M.A.; Metwaly, A.M.; Elhady, S.S.; Elkaeed, E.B.; Eissa, I.H.; Darwish, K.M. Molecular Docking and Dynamics Simulation Revealed the Potential Inhibitory Activity of ACEIs Against SARS-CoV-2 Targeting the hACE2 Receptor. Front. Chem. 2021, 9, 661230. [Google Scholar] [CrossRef]
- Roy, A.V.; Chan, M.; Banadyga, L.; He, S.; Zhu, W.; Chrétien, M.; Mbikay, M. Quercetin inhibits SARS-CoV-2 infection and prevents syncytium formation by cells co-expressing the viral spike protein and human ACE2. Virol. J. 2024, 21, 19. [Google Scholar] [CrossRef]
- Land, H.; Humble, M.S. YASARA: A Tool to Obtain Structural Guidance in Biocatalytic Investigations. Methods Mol. Biol. 2018, 1685, 43–67. [Google Scholar] [CrossRef] [PubMed]
- Ozvoldik, K.; Stockner, T.; Krieger, E. YASARA Model-Interactive Molecular Modeling from Two Dimensions to Virtual Realities. J. Chem. Inf. Model. 2023, 63, 20177–26182. [Google Scholar] [CrossRef]
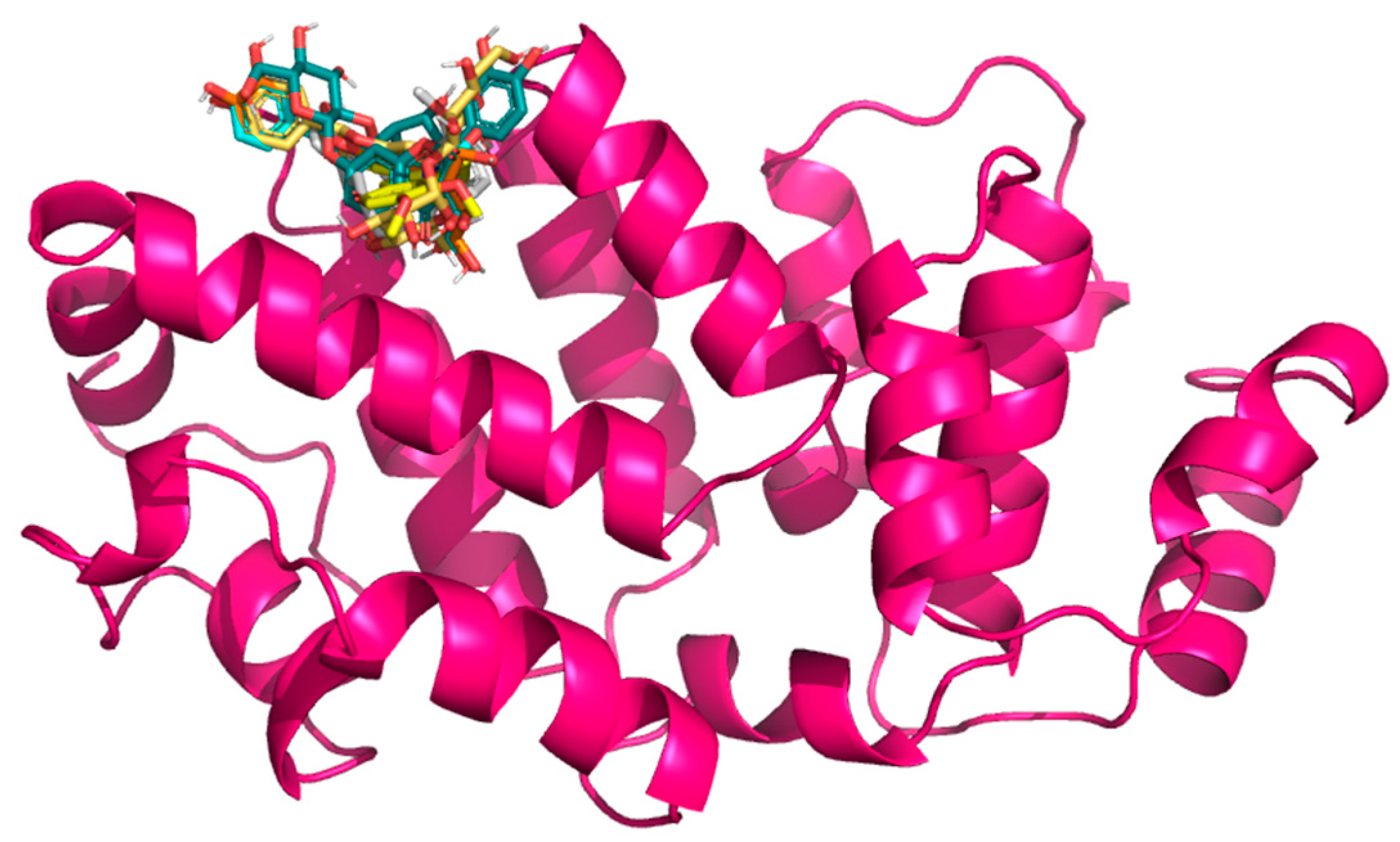
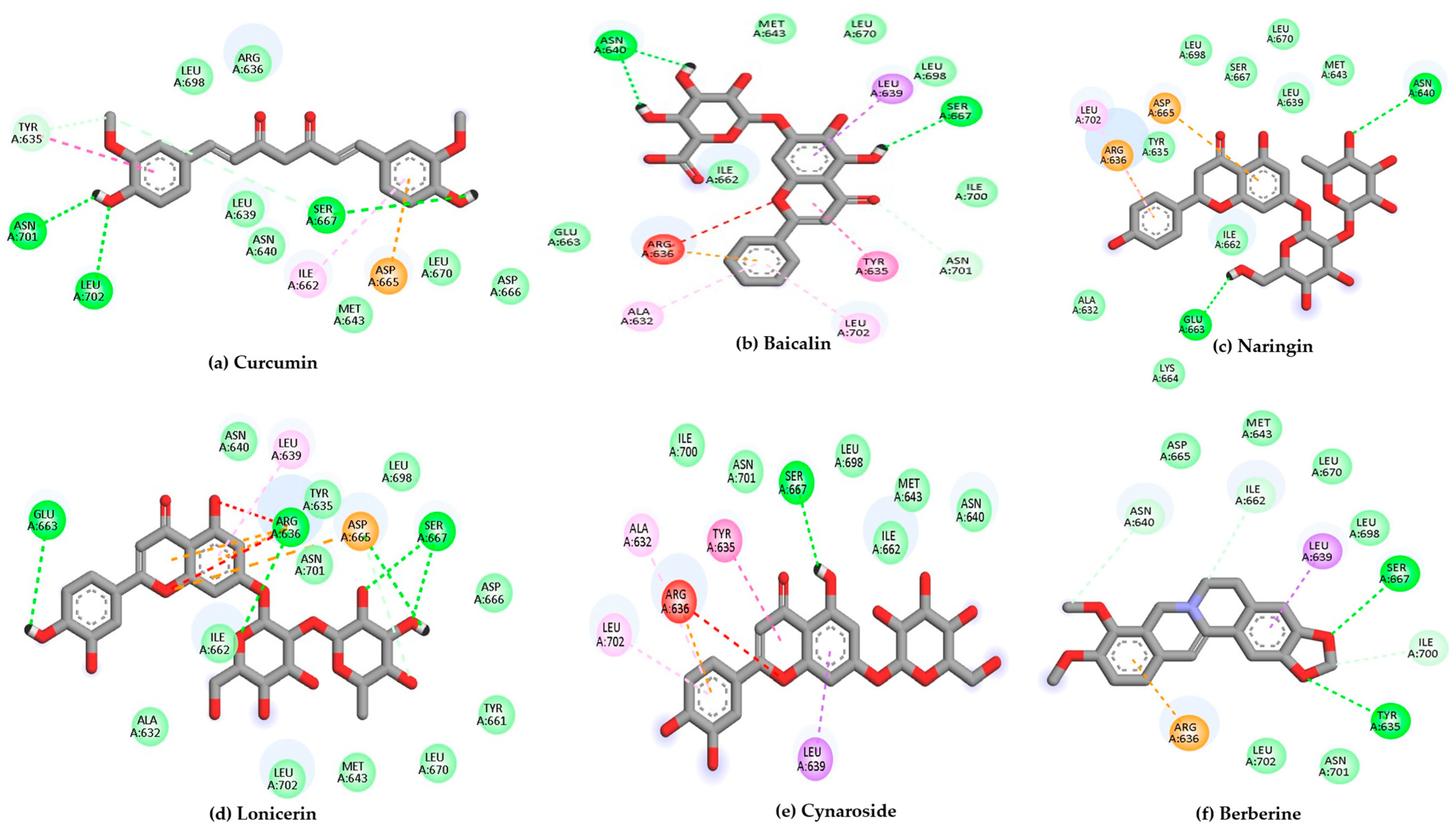

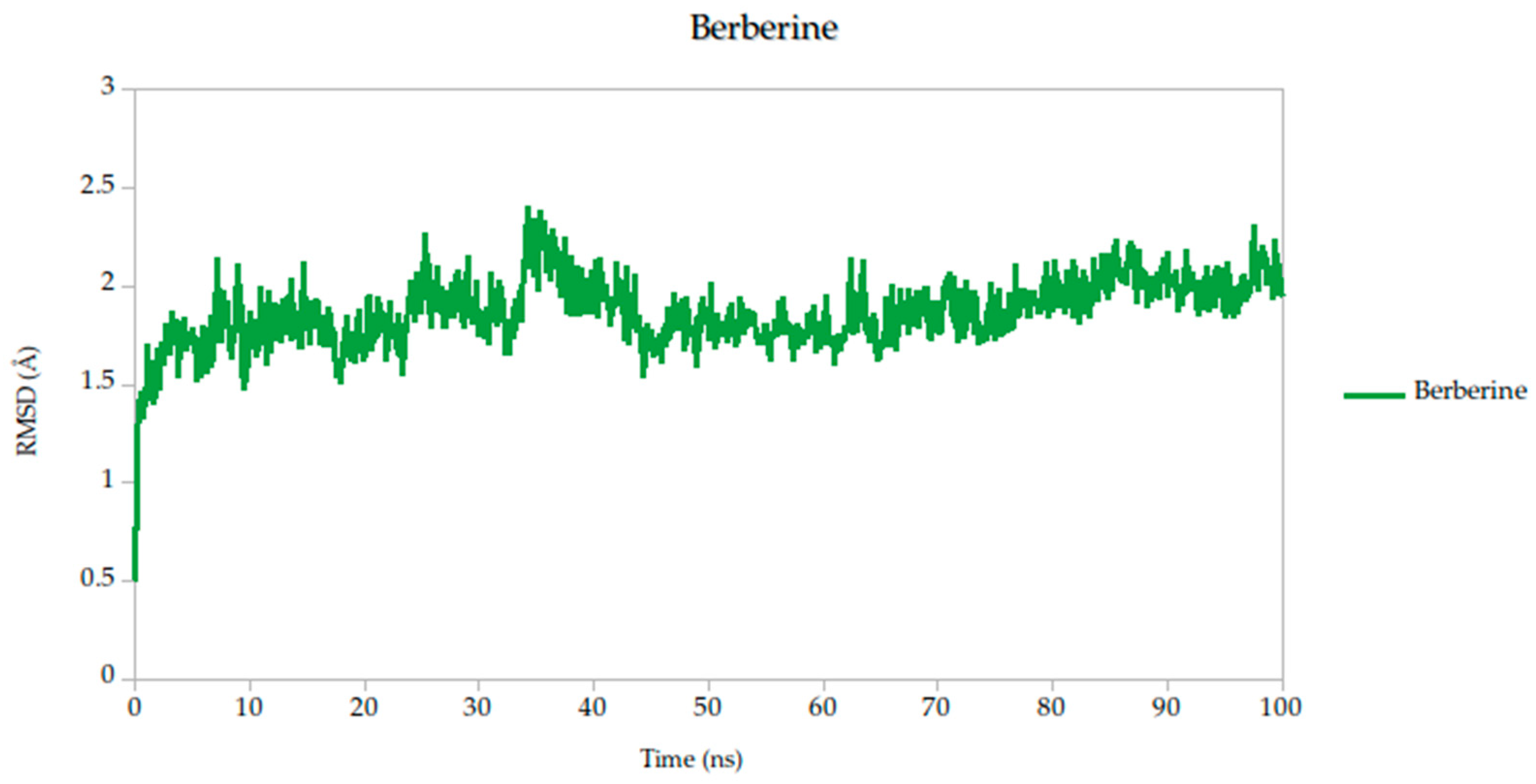
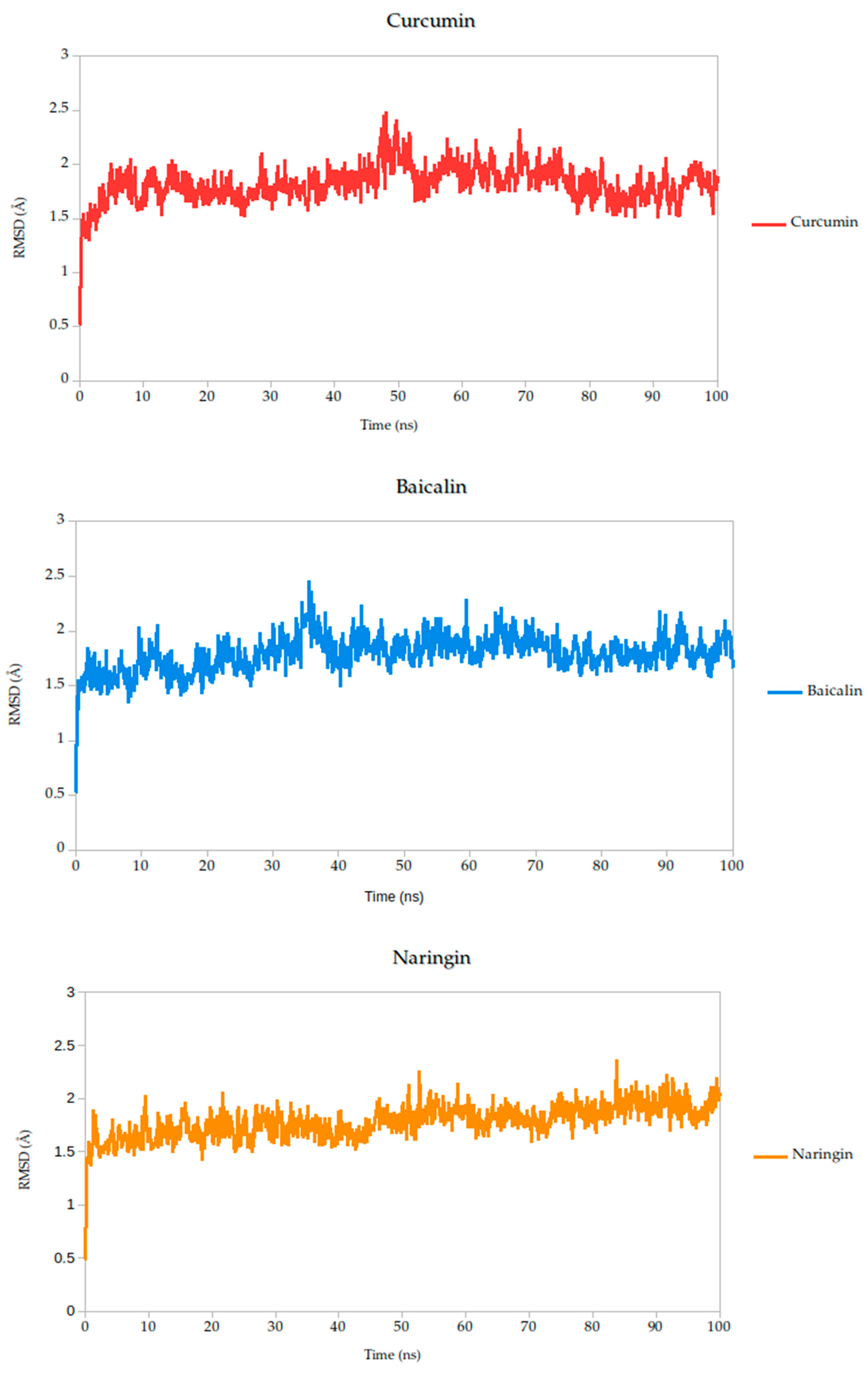
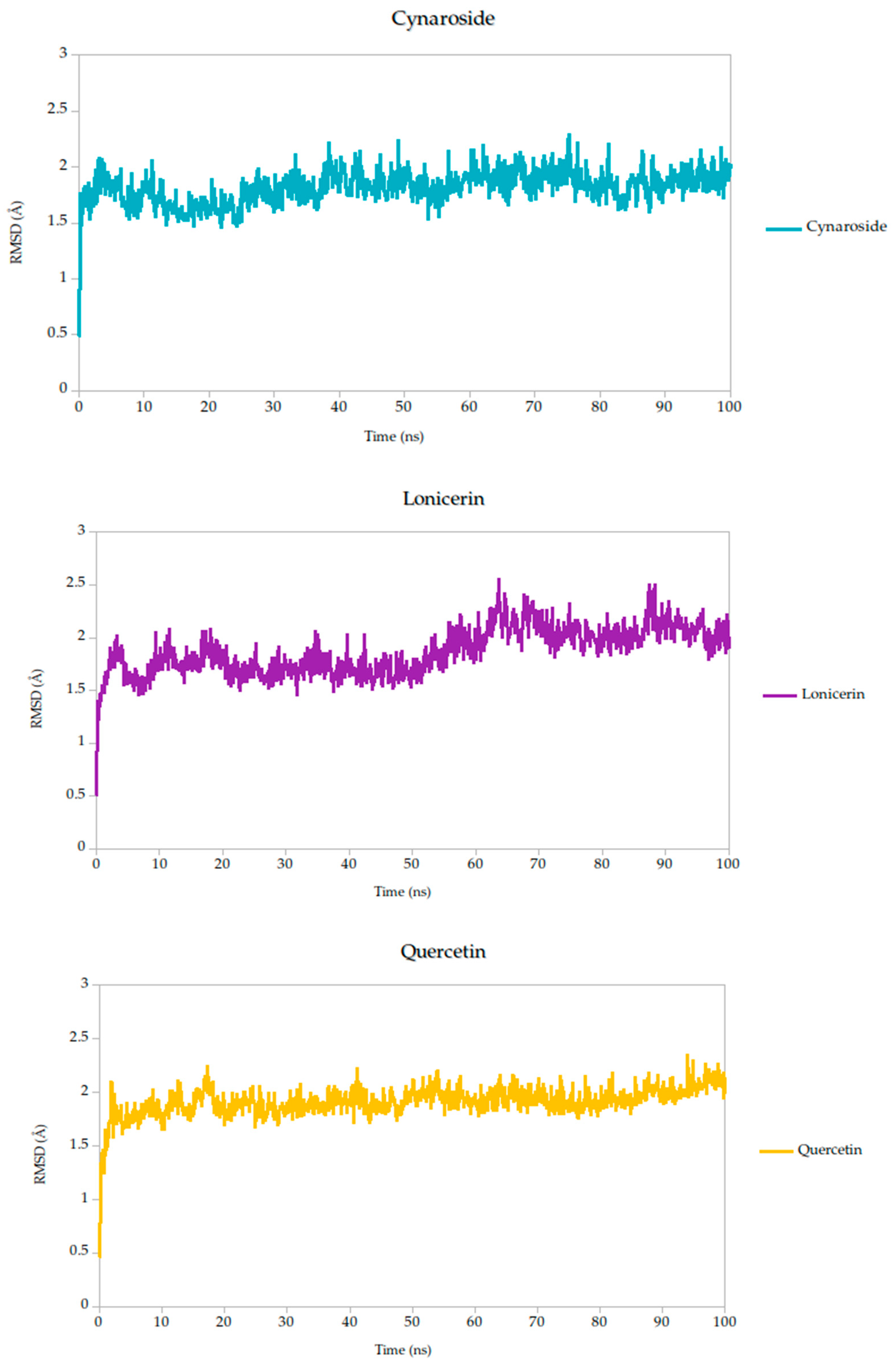
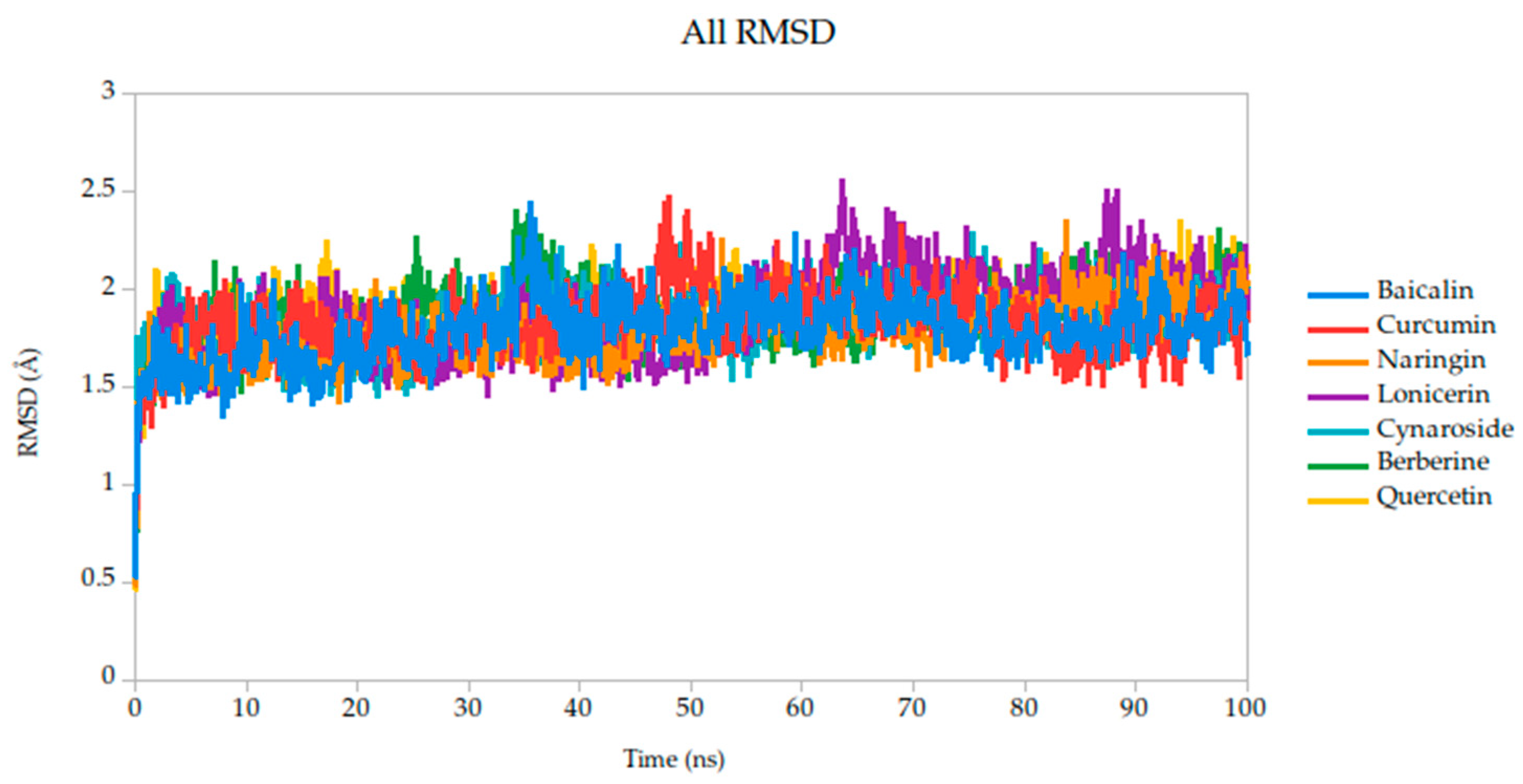






| Target | Ligands | Binding Energy (kcal/mol) | Hydrogen Bonds/Carbon Hydrogen Bonds | Other Interactions |
|---|---|---|---|---|
| (6H3X) | Curcumin | −7.9 | Tyr635 Arg636, Asn640, Met643, Asp666, Leu670, Leu698, Asn701, Leu702 | Ile662, Asp665 |
| Baicalin | −7.4 | Asn640, Met643, Ile662, Glu663, Ser667, Leu670, Leu698, Ile700, Asn701 | Ala632, Tyr635, Arg636, Leu639, Leu702 | |
| Naringin | −7.3 | Ala632, Tyr635, Leu639, Asn640, Met643, Ile662, Glu663, Lys664, Ser667, Leu670, Leu698 | Arg636, Asp665, Leu702 | |
| Berberine | −7.3 | Tyr635, Asn640, Met643, Ile662, Asp665, Ser667, Leu670, Leu698, Ile700, Asn701, Leu702 | Arg636, Leu639 | |
| Lonicerin | −7.1 | Arg636, Asn640, Ile662, Asp665, Leu639, Leu702 | Ala632, Tyr635, Met643, Glu663, Ser667, Leu698 | |
| Cynaroside | −7.1 | Asn640, Ile662, Ser667 | Ala632, Tyr635, Met643, Lys647, Glu663, Leu698, Ile700, Asn701 | |
| Kaempferol | −6.9 | Tyr635, Leu639, Asn640, Asp665, Ser667, Ile700 | Met643, Tyr661, Asn701 | |
| Baicalein | −6.9 | Tyr635, Asn640, Asp665, Asp666, Ser667, Leu670, Asn701 | Leu639, Met643, Ile662, Leu702 | |
| Aloin A | −6.9 | Tyr635, Asn640, Met643, Ile662, Glu663, Lys664, Asp665, Asp666, Ser667, Leu670, Leu698 | Arg636, Leu639 | |
| Fisetin | −6.8 | Tyr635, Asn640, Ser667 | Leu639, Met643, Ile662, Asp665, Leu670, Leu698, Asn701, Leu702 | |
| Luteolin | −6.8 | Tyr635, Asn640, Ser667 | Leu639, Met643, Ile662, Leu698, Asn701, Leu702 | |
| Myricetin | −6.8 | Tyr635, Arg636, Ile662, Asp665, Ser667 | Leu639, Asn640, Met643, Ile700, Asn701 | |
| Quercetin * | −6.8 | Tyr635, Ile662, Asp665, Ser667 | Leu639, Asn640, Met643, Tyr661, Ile700, Asn701, Leu702 | |
| Taxifolin | −6.8 | Asn640, Ile662, Ser667, Leu702 | Tyr635, Leu639, Met643, Asp665, Ile700, Asn701 | |
| Quinine | −6.7 | Tyr635, Asn640, Asp665, Ser667, Leu698, Asn701, Leu702 | Arg636, Leu639, Met643, Ile662 | |
| Apigenin | −6.7 | Tyr635, Ser667 | Leu639, Asn640, Met643, Leu670, Leu698, Asn701, Leu702 | |
| Biochanin A | −6.6 | Tyr635, Met643, Ile662, Ser667, Leu670, Leu698, Ile700, Asn701, Leu702 | Ala632, Arg636, Leu639 | |
| Emodin | −6.6 | Tyr635, Arg636, Asn640, Ile662, Ser667, Leu670, Leu702 | Leu639, Met643, Asp665 | |
| Diosmetin | −6.6 | Tyr635, Asn640, Ser667 | Leu639, Met643, Lys647, Ile662, Leu698, Asn701, Leu702 | |
| Piperine | −6.3 | Tyr635, Leu639, Asp665, Ser667, Leu670, Leu698 | Ala632, Arg636, Ile662, Leu702 |
| Properties | Curcumin | Baicalin | Naringin | Lonicerin | Cynaroside | Berberine | Quercetin | |
|---|---|---|---|---|---|---|---|---|
| Physico-chemical Properties | MW (g/mol) | 368.38 | 446.36 | 580.53 | 594.52 | 448.38 | 336.36 | 302.24 |
| Num. Heavy atoms | 27 | 32 | 41 | 42 | 32 | 25 | 22 | |
| Num. Arom. Heavy atoms | 12 | 16 | 12 | 16 | 16 | 16 | 16 | |
| Num. Rotatable atoms | 8 | 4 | 6 | 6 | 4 | 2 | 1 | |
| Num. H-bond acceptors | 6 | 11 | 14 | 15 | 11 | 4 | 7 | |
| Num. H-bond donnors | 2 | 6 | 8 | 9 | 7 | 0 | 5 | |
| Lipophilicity | Consensus Log P | 3.03 | 0.25 | −0.87 | −1.03 | 0.16 | 2.53 | 1.23 |
| Water solubility | Log S (ESOL) | −3.94 | −3.41 | −2.98 | −3.64 | −3.65 | −4.55 | −3.16 |
| Pharmacokinetics | GI absorption | High | Low | Low | Low | Low | High | High |
| BBB permeant | No | No | No | No | No | Yes | No | |
| Druglikeness | Lipinski. Violation | 0 | 2 | 3 | 3 | 2 | 0 | 0 |
| Medicinal Chemistry | Synth. accessibility | 2.97 | 5.09 | 6.16 | 6.37 | 5.17 | 3.14 | 3.23 |
| Toxicity | Hepatotoxicity (probability) | Inactive (0.61) | Inactive (0.75) | Inactive (0.81) | Inactive (0.81) | Inactive (0.82) | Inactive (0.82) | Inactive (0.69) |
| Neurotoxicity (probability) | Inactive (0.81) | Inactive (0.9) | Inactive (0.88) | Inactive (0.88) | Inactive (0.88) | Active (0.55) | Inactive (0.89) | |
| Nephrotoxicity (probability) | Active (0.59) | Active (0.68) | Active (0.77) | Active (0.77) | Active (0.76) | Inactive (0.5) | Active (0.62) | |
| Mutagenicity (probability) | Inactive (0.88) | Inactive (0.68) | Inactive (0.73) | Inactive (0.73) | Inactive (0.76) | Active (0.62) | Active (0.51) | |
| Cytotoxcity (probability) | Inactive (0.88) | Inactive (0.91) | Inactive (0.66) | Inactive (0.66) | Inactive (0.69) | Active (0.96) | Inactive (0.99) | |
| Predicted LD50 (mg/kg) | 2000 | 5000 | 5000 | 5000 | 5000 | 200 | 159 | |
| Tox-Level | 4 | 5 | 5 | 5 | 5 | 3 | 3 |
| Curcumin | Baicalin | Naringin | Lonicerin | Cynaroside | Berberine | Quercetin | |
|---|---|---|---|---|---|---|---|
| Binding Energy average (kcal/mol) | −38.14 | −62.84 | −58.01 | −92.91 | −50.81 | −13.10 | −39.79 |
| σ Binding Energy (kcal/mol) | 14.25 | 15.61 | 15.75 | 14.10 | 17.49 | 8.89 | 11.51 |
| RMSD min (Å) | 0.52 | 0.52 | 0.48 | 0.50 | 0.48 | 0.50 | 0.46 |
| RMSD max (Å) | 2.48 | 2.44 | 2.36 | 2.55 | 2.29 | 2.39 | 2.35 |
| RMSD mean (Å) | 1.82 | 1.79 | 1.78 | 1.87 | 1.82 | 1.86 | 1.91 |
| σ RMSD | 0.16 | 0.16 | 0.15 | 0.22 | 0.14 | 0.17 | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Echeverría, C.; Saurith-Coronell, O.; Rodriguez-Macías, J.; Márquez Brazón, E.A.; Mora, J.R.; Fuentes-Gandara, F.; Paz, J.L.; Salazar, F. Structure-Based Identification of Natural Inhibitors Targeting the Gc Glycoprotein of Oropouche Virus: An In Silico Approach. Int. J. Mol. Sci. 2025, 26, 10541. https://doi.org/10.3390/ijms262110541
Vargas-Echeverría C, Saurith-Coronell O, Rodriguez-Macías J, Márquez Brazón EA, Mora JR, Fuentes-Gandara F, Paz JL, Salazar F. Structure-Based Identification of Natural Inhibitors Targeting the Gc Glycoprotein of Oropouche Virus: An In Silico Approach. International Journal of Molecular Sciences. 2025; 26(21):10541. https://doi.org/10.3390/ijms262110541
Chicago/Turabian StyleVargas-Echeverría, Carlos, Oscar Saurith-Coronell, Juan Rodriguez-Macías, Edgar A. Márquez Brazón, José R. Mora, Fabio Fuentes-Gandara, José L. Paz, and Franklin Salazar. 2025. "Structure-Based Identification of Natural Inhibitors Targeting the Gc Glycoprotein of Oropouche Virus: An In Silico Approach" International Journal of Molecular Sciences 26, no. 21: 10541. https://doi.org/10.3390/ijms262110541
APA StyleVargas-Echeverría, C., Saurith-Coronell, O., Rodriguez-Macías, J., Márquez Brazón, E. A., Mora, J. R., Fuentes-Gandara, F., Paz, J. L., & Salazar, F. (2025). Structure-Based Identification of Natural Inhibitors Targeting the Gc Glycoprotein of Oropouche Virus: An In Silico Approach. International Journal of Molecular Sciences, 26(21), 10541. https://doi.org/10.3390/ijms262110541







