Abstract
We report a comprehensive investigation of the photophysical properties of Hoechst 33258 (HOE) embedded in polyvinyl alcohol (PVA) films. HOE displays a bright, highly polarized, blue fluorescence emission centered at 430 nm, indicating effective immobilization within the polymer matrix of PVA. Its fluorescence quantum yield is notably high (~0.74), as determined relative to a quinine sulfate standard. In addition, we observed that HOE-doped PVA films exhibit room temperature phosphorescence (RTP) that remains visible for several seconds after UV excitation ceases. The slightly negative phosphorescence anisotropy implies that the triplet–singlet radiative transition is orthogonal to the singlet–singlet transition governing fluorescence. Notably, we observed that direct triplet-state excitation at longer wavelengths (beyond the primary absorption band) produces highly polarized RTP. We believe this possibility of direct triplet-state excitation opens new avenues for studying RTP in polymer-immobilized molecules.
1. Introduction
Hoechst 33258 (HOE), a bisbenzimide derivative, was first synthesized by Hoechst AG in the early 1970s [1,2]. This fluorescent dye has since become a widely used tool for deoxyribonucleic acid (DNA) staining in various scientific applications [3]. It selectively binds to the minor groove of AT-rich double-stranded DNA, exhibiting a blue fluorescence emission upon ultraviolet (UV) excitation [4,5,6]. Notably, Hoechst dyes are commonly used as substitutes for DAPI [7]. While they share DAPI’s high specificity for DNA and preference for A-T base pairs, Hoechst dyes offer advantages such as lower cytotoxicity and greater binding flexibility [8]. Consequently, Hoechst dyes are often preferred for staining DNA in both living and fixed cells. The fluorescent properties of Hoechst dyes allow for their use in fluorescence microscopy, flow cytometry, and immunochemistry [9,10]. Recent studies have expanded their application to developing composite probes, intracellular targeting, and creating new molecular sensors [11,12,13,14,15]. Moreover, Hoechst dyes’ ability to visualize and quantify cellular DNA makes them useful in advanced imaging techniques, drug delivery, and live-cell imaging studies [16,17,18,19]. These attributes make Hoechst dyes a valuable tool in contemporary biomedical research.
Despite its wide range of applications, the room temperature phosphorescence (RTP) of Hoechst 33258 has not been explored. RTP offers distinct advantages compared to fluorescence, including significantly larger Stokes shifts, which allow for better spectral separation of excitation and emission wavelengths. Additionally, RTP features longer emission lifetimes, which facilitate time-resolved measurements and reduce background interference, ultimately leading to enhanced signal-to-noise ratios and improved detection sensitivity [20,21,22,23]. These properties have made RTP increasingly attractive for various applications such as encryption [24,25], decryption [26], anticounterfeiting [27], and bioimaging [28]. Most frequently, RTP materials are inorganic or organometallic [29]. However, these materials often face limitations due to their scarcity, high cost, and cytotoxicity [30,31]. Consequently, researchers have increasingly turned their attention to pure organic RTP materials, which offer flexible molecular designs and tunable properties [32].
Phosphorescence involves the transition from the lowest singlet state (S1) to the excited triplet state (Tn) through intersystem crossing (ISC), and then returning to the ground state (S0) [33]. For RTP to be effective, two conditions must be met: first, the triplet excited state must be efficiently populated from S1 to Tn through ISC. Strong spin–orbit coupling (SOC) is essential for facilitating this process. Second, it is crucial to suppress non-radiative deactivation pathways in the triplet excited state to prevent quenching by external factors such as oxygen and humidity. This can be achieved by reducing the thermal motion of molecules through methods such as crystallization, polymerization, and matrix rigidification [33,34,35,36].
Poly vinyl alcohol (PVA) films meet such criteria and have emerged as a prominent polymer matrix for RTP studies due to their excellent film-forming properties, biocompatibility, and ease of processing [37,38,39,40]. Our laboratory, along with the broader scientific literature, has reported RTP of small organic molecules embedded in PVA films [41,42,43,44,45,46]. Additionally, PVA’s compatibility with a wide range of phosphorescent materials underscores its versatility and effectiveness in RTP applications [47,48,49,50,51,52,53,54,55]. For instance, Chen et al. reported on aggregation-induced RTP from self-quenching-resistant nitrogen-doped carbon dot powder within PVA, taking advantage of its moisture-resistant properties [56]. Moreover, the RTP of metal–organic hybrids has been documented, with Yang et al. reporting RTP from the embedding of a Zn-IPA Metal–Organic Framework (MOF) into a PVA matrix [48]. Recently, Zeonex, an excellent polymer matrix and superior optical material, has been used in RTP [57,58,59,60].
Hence, in this study, we investigate the photophysical properties of Hoechst 33258 in PVA films. Building on our previous work with DAPI-doped PVA, which demonstrated the potential for RTP [61], we posit that the matrix rigidification afforded by embedding Hoechst or DAPI in polymeric films may extend to biological matrices [4,5,6]. Although RTP has been achieved in various aqueous solutions, the use of naturally occurring biological structures, such as DNA, as a matrix remains largely unexplored. Past efforts have instead relied on inorganic substances (e.g., quaternary ammonium) or techniques (e.g., silica encapsulation) to ensure robust matrix formation [62,63,64]. Notably, leveraging DNA as a matrix for RTP could open new avenues for applications beyond simple DNA detection—particularly in advanced biomedical imaging techniques—by offering highly stable, low-toxicity systems with potentially longer-lived phosphorescent signals.
2. Results
2.1. Absorption and Fluorescence Spectra
The absorption spectrum of HOE-doped PVA film is shown in Figure 1. It consists of a long-wavelength band with a peak absorption corresponding to the S0–S1 transition at ~350 nm and weaker peaks below 310 nm corresponding to higher excited states.
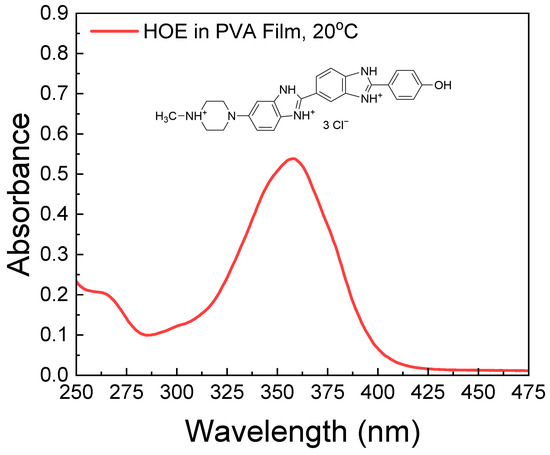
Figure 1.
Absorption spectrum of HOE-doped PVA film at 20 °C. The chemical structure of HOE is shown above the spectrum.
By taking into account the extinction coefficient of HOE at its maximum peak (47,000 M−1 cm−1) [65], and the thickness of the film (200 μm), the concentration of HOE used in the film is approximately 0.5 mM. This concentration is optimal for absorption measurements and provides an excellent signal-to-noise ratio for emission measurements. We prepared a series of films with absorbances from 0.1 to 1.0 (corresponding to concentrations from 0.1 mM to 1 mM) and observed no differences in the shapes of the spectra. This indicated the absence of HOE aggregates in the films.
The fluorescence excitation and emission spectra of HOE-doped PVA film are shown in Figure 2, where the excitation spectrum matches the absorption spectrum. The bright blue fluorescence emission spans from 375 nm to 575 nm with a peak at 430 nm. The small spectral overlap (Stoke’s shift) of HOE-doped PVA prevents the excitation energy migration process which may lower fluorescence anisotropy.
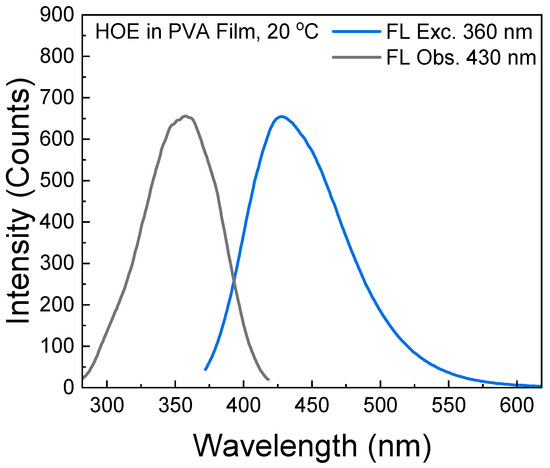
Figure 2.
Excitation and emission fluorescence spectra of HOE-doped PVA film.
2.2. Fluorescence Anisotropy
The polarized components from the fluorescence excitation spectra of HOE-doped PVA film, along with the anisotropy values calculated using Equation (1), are shown in Figure 3. At longer excitation wavelengths, the anisotropy reaches 0.3, indicating that the HOE molecules are immobilized. The high value of the fluorescence anisotropy spectrum is also shown in the fluorescence emission; see Figure 4.
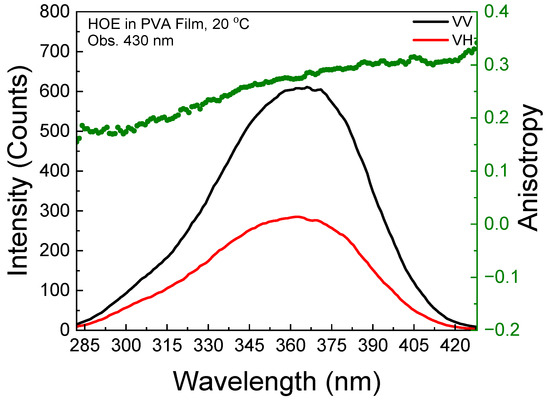
Figure 3.
Fluorescence excitation anisotropy spectrum of HOE-doped PVA film (green dots correspond to anisotropy values).
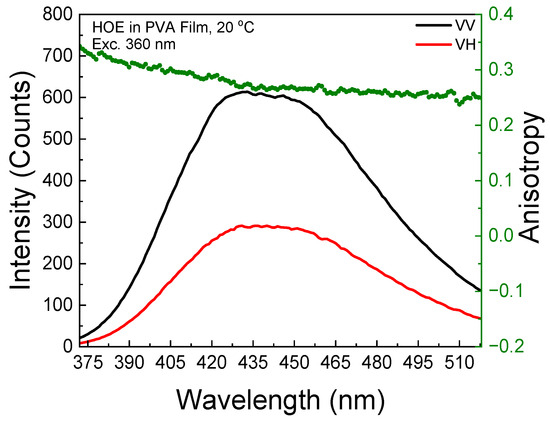
Figure 4.
Fluorescence emission anisotropy spectrum of HOE-doped PVA film (green dots correspond to anisotropy values).
2.3. Fluorescence Quantum Yield
The fluorescence efficiency of HOE-doped PVA film was estimated using quinine sulfate (QS), a commonly used quantum yield (QY) standard. QS was dissolved in 1N H2SO4 and the solution was placed in a 1 mm thick microcuvette. A strip of HOE-doped PVA film was placed into the microcuvette and filled with benzene to match the refractive index of PVA. Both absorption and fluorescence spectra of QS matched those of HOE. With the excitation set at 351 nm, where absorbances of QS and HOE are identical (see Figure 5, top), the QY of HOE-doped PVA film was calculated as a product of integrated intensities ratio and squares of the ratio of refractive indexes (HOE/QS); see details in the Section 3. The QY of HOE-doped PVA was estimated to be 0.74.
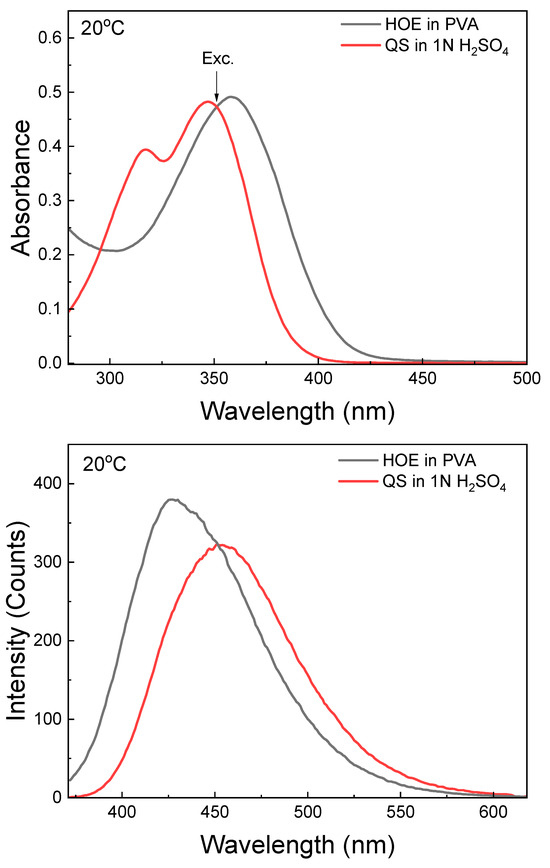
Figure 5.
Comparison of fluorescence emissions of HOE in PVA and quinine sulfate in 1N H2SO4. Top: absorbances; bottom: fluorescence intensities. The excitation was at equal absorbances at 351 nm.
2.4. Fluorescence Lifetime
The fluorescence intensity decay of HOE-doped PVA was measured in time-domain mode with an excitation from a pulsed 370 nm laser diode and observation set at 430 nm. The decay was approximated with two exponents yielding an average lifetime of 2 ns; see Figure 6.
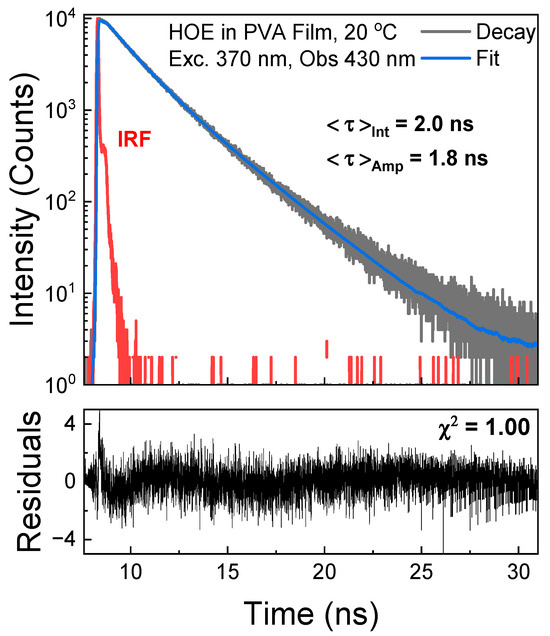
Figure 6.
Fluorescence intensity decay of HOE-doped PVA film. The decay was fitted to a double exponential model with recovered parameters and , and amplitudes and . Instrument response function (IRF) is in red.
2.5. Phosphorescence Spectra
The RTP of HOE-doped PVA was measured with the gating parameters described in the Section 3. The RTP of the HOE-doped PVA film was green and persisted for a couple of seconds after being exposed on the UV illuminator; see Figure 7, top. The RTP spectrum spans from 400 nm to about 650 nm; see Figure 7. The RTP excitation spectrum differs from the fluorescence excitation spectrum; compare Figure 7 and Figure 2. First, its maximum is shifted towards shorter wavelengths. Second, unlike the fluorescence, the phosphorescence can be excited at longer wavelengths up to 480 nm, suggesting the possibility of direct triplet-state excitation. The illumination at wavelengths longer than 420 nm primarily excites the triplet state.
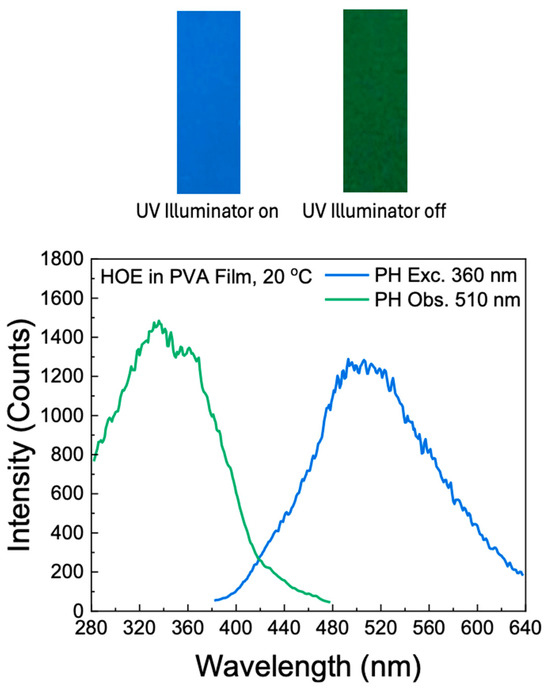
Figure 7.
Top: a strip of HOE-doped PVA film on the UV illuminator; bottom: phosphorescence excitation and emission spectra of HOE-doped PVA film.
2.6. Phosphorescence Lifetime
The phosphorescence lifetime of the HOE-doped PVA film was measured with the Varian Eclipse spectrofluorometer in phosphorescence lifetime mode with gating parameters detailed in the Section 3. The phosphorescence intensity decay was fitted with two exponents; see Figure 8.
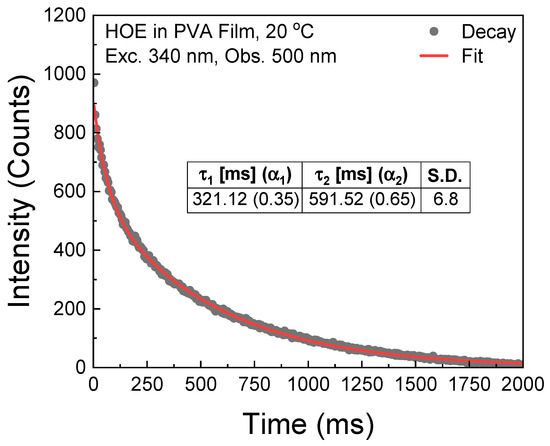
Figure 8.
Phosphorescence intensity decay of HOE-doped PVA film.
2.7. RTP Anisotropy
The phosphorescence excitation anisotropy is slightly negative within the S0–S1 absorption range as expected since the phosphorescence transition is orthogonal to the absorption/fluorescence transition. However, above 400 nm, there is a sharp increase, indicating the direct excitation to the triplet state; see Figure 9.
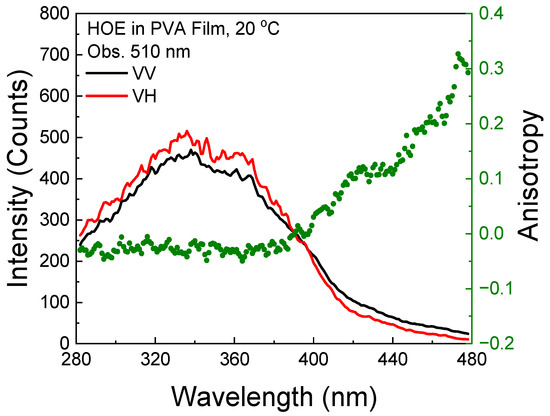
Figure 9.
Phosphorescence excitation anisotropy spectrum of HOE-doped PVA film (green dots correspond to anisotropy values).
The phosphorescence emission anisotropy exhibited slightly higher values at shorter observation wavelengths, indicating the presence of residual delayed fluorescence; see Figure 10. In the phosphorescence wavelength region, the anisotropy is slightly negative.
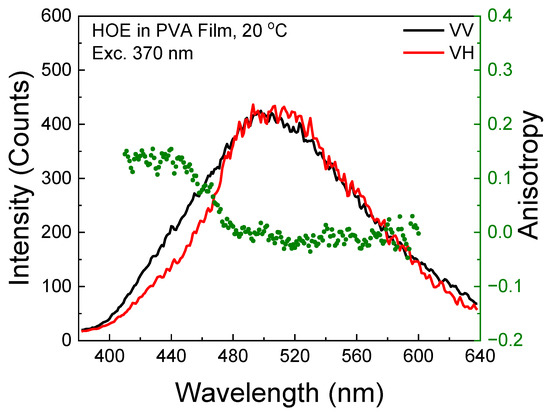
Figure 10.
Phosphorescence emission spectrum of HOE-doped PVA film (green dots correspond to anisotropy values).
2.8. RTP with Direct Triplet-State Excitation
The RTP spectrum with an excitation of 460 nm (well outside of HOE-doped PVA film absorption band; see Figure 1) is shown in Figure 11. Although the spectrum is noisy as it is measured at the limit of the instrument sensitivity, it does not involve the intersystem crossing process. With a stronger excitation from a 480 nm laser diode, the RTP becomes bright and easily visible to the naked eye; see Figure 12.
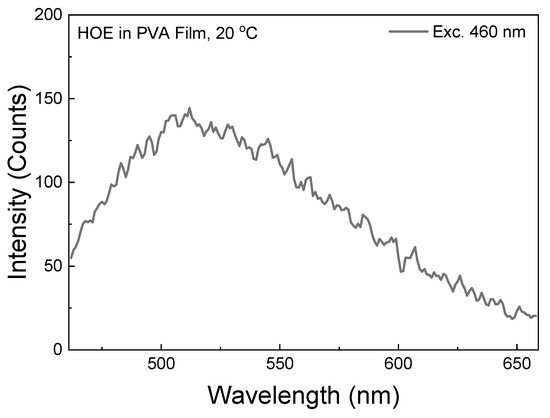
Figure 11.
RTP emission spectrum of HOE-doped PVA measured with 460 nm excitation. The gating parameters were the same as for other RTP measurements.

Figure 12.
HOE-doped PVA film phosphorescence upon 480 nm excitation from a laser diode.
3. Materials and Methods
The bisbenzimide (Hoechst 33258) was from Molecular Probes (Eugene, OR, USA). Polyvinyl alcohol (PVA) [MW 130,000, 98% hydrolyzed] was obtained from Sigma-Aldrich (St. Louis, MO, USA).
3.1. Film Preparation
A bulk solution was prepared in a 1 L Erlenmeyer flask using a 10% (w/w) PVA solution in deionized water. The solution was heated and stirred at 95 °C until the PVA completely dissolved and reached a honey-like consistency. Then, it was allowed to cool. A small volume of Hoechst 33258 stock solution was added to 20 mL of PVA solution, throroughly mixed, and then poured into an 85 mm Petri dish. Blank PVA films were made using a similar method to serve as control for background signal correction. The PVA films were set aside to dry for approximately one week. Once the films were dried, a razor blade was used to peel the PVA films from the Petri dishes. Using calipers, the thicknesses of the PVA films were measured to be ~200 microns.
3.2. Absorption Spectra
The Varian Cary 60 UV-Vis Spectrophotometer (Agilent Technologies, Inc., Santa Clara, CA, USA) was used for the room temperature absorption measurements. Unless otherwise specified, the absorbance spectra were corrected using the blank PVA film as a baseline.
3.3. Fluorescence Spectra
The Varian Cary Eclipse Spectrofluorometer (Agilent Technologies, Inc., Santa Clara, CA, USA) was used to conduct steady-state fluorescence measurements. A front-face configuration was achieved using a custom attachment on the Varian Cary Eclipse spectrofluorometer [66]. Anisotropy measurements were performed using a UV grid polarizer and a plastic sheet polarizer added to the excitation and emission sides, respectively.
3.4. Fluorescence Anisotropy
The fluorescence excitation and emission anisotropies were calculated from the measured polarized intensity components IVH and IVV as
where the IVV and IVH components are the fluorescence intensities when excited with vertical polarization (V) and observed with horizontal (H) or vertical polarization, respectively. G (G-Factor) was used to account for the uneven transmissions of IVH and IVV components through the detection path. Equation (1) was utilized for both phosphorescent and fluorescent emission measurements.
3.5. Fluorescence Quantum Yield
To estimate the fluorescence quantum yield (QY), the HOE sample was compared to a solution of quinine sulfate (QS) in 1N H2SO4—a well-established QY standard (QY = 0.54 [67]). The measurements were conducted using a front-face configuration with 1 mm path-length microcuvettes. Benzene was added to the cuvette containing the HOE-doped PVA film as it has the same refractive index as the PVA film. The different refractive indices for QS in 1N H2SO4 (n = 1.35) and for HOE-doped PVA film (n = 1.48) were accounted for in the QY calculation.
3.6. Fluorescence Lifetime
Fluorescence lifetime measurements were conducted using an FT200 fluorometer (PicoQuant GmBH, Berlin, Germany), which is equipped with a time-correlated single photon counting module (PicoHarp 300, PicoQuant GmBH, Berlin, Germany) and with an ability to resolve 4 ps. A 370 nm pulsed laser diode (LDH-P-C-375B, PicoQuant GmBH, Berlin, Germany) was used with a repetition rate of 4 MHz (PDL 800-B, PicoQuant GmBH, Berlin, Germany). The time-dependent data were analyzed using FluoFit4 (PicoQuant GmBH, Berlin, Germany).
3.7. Phosphorescence Spectra Measurements
Phosphorescence emission and excitation spectra were measured with the Varian Cary Eclipse using its time-gated phosphorescence detection mode. This setting eliminates short-lived emission components, including but not limited to Raman scattering, Rayleigh scattering, and fluorescence background signals. Unless otherwise specified, the parameters used in this setting were as follows: Total Decay Time—1.0 s, Number of flashes—10, Delay Time—0.5 ms, and Gate Time—5 ms. The phosphorescence intensity decay was measured up to 2 s. The total phosphorescence excitation and emission anisotropies were calculated from the measured polarized phosphorescence intensity components IVH and IVV, as described by Equation (1).
4. Conclusions
In this study, we comprehensively characterized the fluorescence and phosphorescence properties of HOE-doped PVA films. The bright, highly polarized, blue fluorescence (peaking at 430 nm) is associated with a notably high quantum yield (~0.74), indicating the effective immobilization of HOE within the polymer matrix. Time-resolved measurements revealed a relatively short fluorescence lifetime of ~2 ns, whereas RTP displayed a longer lifetime of ~0.5 s. When excited within HOE’s primary absorption band, the phosphorescence showed slightly negative anisotropy, suggesting that the triplet–singlet radiative transition is orthogonal to the singlet–singlet transition. Notably, we observed that long-wavelength excitation beyond the main absorption band can directly populate the triplet state, producing a highly polarized phosphorescence similar to that of fluorescence. The RTP brightness of HOE-doped PVA film samples is relatively high, comparable to DAPI and other reported organic compounds. With a long-wavelength excitation outside the main absorption band, stronger excitations from lasers can be used to obtain a very high RTP emission, as shown in Figure 12. We believe that this possibility of a direct triplet-state excitation route broadens the potential for novel spectroscopic and imaging applications of HOE.
Author Contributions
Conceptualization, B.L., D.P., Z.G. and I.G.; methodology, B.L., Z.G. and I.G.; software, B.L. and A.J.; validation, B.L., A.J. and I.G.; formal analysis, B.L., A.J. and I.G.; investigation, B.L., A.J., D.P., T.T.P. and I.G.; resources, Z.G.; data curation, B.L. and A.J.; writing—original draft preparation, B.L., D.P., R.S. and I.G.; writing—review and editing, B.L., D.P., R.S., Z.G. and I.G.; visualization, B.L., A.J., D.P. and T.T.P.; supervision, B.L., Z.G. and I.G.; project administration, B.L., Z.G. and I.G.; funding acquisition, Z.G., and I.G. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by [I.G.].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors. The data are not publicly available due to privacy.
Acknowledgments
Z.G. acknowledges the support from “Tex” Moncrief Jr.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bontemps, J.; Houssier, C.; Fredericq, E. Physico-chemical study of the complexes of “33258 Hoechst” with DNA and nucleohistone. Nucleic Acids Res. 1975, 2, 971–984. [Google Scholar] [CrossRef] [PubMed]
- Weisblum, B.; Haenssler, E. Fluorometric properties of the bibenzimidazole derivative Hoechst 33258, a fluorescent probe specific for AT concentration in chromosomal DNA. Chromosoma 1974, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.K.; Usman, N.; Frederick, C.A.; Wang, A.H. The molecular structure of the complex of Hoechst 33258 and the DNA dodecamer d(CGCGAATTCGCG). Nucleic Acids Res. 1988, 16, 2671–2690. [Google Scholar] [CrossRef] [PubMed]
- Latt, S.A.; Stetten, G.; Juergens, L.A.; Willard, H.F.; Scher, C.D. Recent developments in the detection of deoxyribonucleic acid synthesis by 33258 Hoechst fluorescence. J. Histochem. Cytochem. 1975, 23, 493–505. [Google Scholar] [CrossRef]
- Latt, S.A.; Stetten, G. Spectral studies on 33258 Hoechst and related bisbenzimidazole dyes useful for fluorescent detection of deoxyribonucleic acid synthesis. J. Histochem. Cytochem. 1976, 24, 24–33. [Google Scholar] [CrossRef]
- Han, F.; Taulier, N.; Chalikian, T.V. Association of the minor groove binding drug Hoechst 33258 with d(CGCGAATTCGCG)2: Volumetric, calorimetric, and spectroscopic characterizations. Biochemistry 2005, 44, 9785–9794. [Google Scholar] [CrossRef]
- Karg, T.J.; Golic, K.G. Photoconversion of DAPI and Hoechst dyes to green and red-emitting forms after exposure to UV excitation. Chromosoma 2018, 127, 235–245. [Google Scholar] [CrossRef]
- Portugal, J.; Waring, M.J. Assignment of DNA binding sites for 4′,6-diamidine-2-phenylindole and bisbenzimide (Hoechst 33258). A comparative footprinting study. Biochim. Biophys. Acta Gene Struct. Expr. 1988, 949, 158–168. [Google Scholar] [CrossRef]
- Wiederschain, G. The Handbook–A Guide to Fluorescent Probes and Labeling Techniques. Biochemistry 2011, 76, 1235–1237. [Google Scholar] [CrossRef]
- Kubbies, M. Flow cytometric recognition of clastogen induced chromatin damage in G0/G1 lymphocytes by non-stoichiometric Hoechst fluorochrome binding. Cytometry 1990, 11, 386–394. [Google Scholar] [CrossRef]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The Use of Hoechst Dyes for DNA Staining and Beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, C.; Zhou, X.; Qin, J. Label-free aptamer-based sensors for L-argininamide by using nucleic acid minor groove binding dyes. Chem. Commun. 2011, 47, 3192–3194. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Nishihira, A.; Wakabayashi, M.; Kuwahara, M.; Sawai, H. Biomolecular sensor based on fluorescence-labeled aptamer. Bioorg. Med. Chem. Lett. 2006, 16, 4381–4384. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, N.; Kellish, P.; King, A.; Arya, D.P. Impact of Linker Length and Composition on Fragment Binding and Cell Permeation: Story of a Bisbenzimidazole Dye Fragment. Biochemistry 2017, 56, 6434–6447. [Google Scholar] [CrossRef]
- Hara, D.; Umehara, Y.; Son, A.; Asahi, W.; Misu, S.; Kurihara, R.; Kondo, T.; Tanabe, K. Tracking the Oxygen Status in the Cell Nucleus with a Hoechst-Tagged Phosphorescent Ruthenium Complex. ChemBioChem 2018, 19, 956–962. [Google Scholar] [CrossRef]
- Dasari, M.; Acharya, A.P.; Kim, D.; Lee, S.; Lee, S.; Rhea, J.; Molinaro, R.; Murthy, N. H-gemcitabine: A new gemcitabine prodrug for treating cancer. Bioconjug. Chem. 2013, 24, 4–8. [Google Scholar] [CrossRef]
- Dasari, M.; Lee, S.; Sy, J.; Kim, D.; Lee, S.; Brown, M.; Michael Davis, M.; Murthy, N. Hoechst-IR: An imaging agent that detects necrotic tissue in vivo by binding extracellular DNA. Org. Lett. 2010, 12, 3300–3303. [Google Scholar] [CrossRef]
- Nakamura, A.; Takigawa, K.; Kurishita, Y.; Kuwata, K.; Ishida, M.; Shimoda, Y.; Hamachi, I.; Tsukiji, S. Hoechst tagging: A modular strategy to design synthetic fluorescent probes for live-cell nucleus imaging. Chem. Commun. 2014, 50, 6149–6152. [Google Scholar] [CrossRef]
- Szczurek, A.T.; Prakash, K.; Lee, H.K.; Żurek-Biesiada, D.J.; Best, G.; Hagmann, M.; Dobrucki, J.W.; Cremer, C.; Birk, U. Single molecule localization microscopy of the distribution of chromatin using Hoechst and DAPI fluorescent probes. Nucleus 2014, 5, 331–340. [Google Scholar] [CrossRef]
- Ji, M.; Ma, X. Recent progress with the application of organic room-temperature phosphorescent materials. Ind. Chem. Mater. 2023, 1, 582–594. [Google Scholar] [CrossRef]
- Mu, Y.; Yang, Z.; Chen, J.; Yang, Z.; Li, W.; Tan, X.; Mao, Z.; Yu, T.; Zhao, J.; Zheng, S.; et al. Mechano-induced persistent room-temperature phosphorescence from purely organic molecules. Chem. Sci. 2018, 9, 3782–3787. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Ma, X.; Tian, H. Recent Advances of Pure Organic Room Temperature Phosphorescence Based on Functional Polymers. Acc. Mater. Res. 2023, 4, 827–838. [Google Scholar] [CrossRef]
- Gu, L.; Shi, H.; Bian, L.; Gu, M.; Ling, K.; Wang, X.; Ma, H.; Cai, S.; Ning, W.; Fu, L.; et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics 2019, 13, 406–411. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, H.; Fu, L.; Jian, K.; Zhao, X. Carbon Dot-Based Room-Temperature Phosphorescent Materials for Information Encryption. ACS Appl. Nano Mater. 2023, 6, 15597–15605. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, J.; Sun, J.; Xu, B.; Gao, Z.; Wang, X.; Yan, L.; Zhu, C.; Meng, X. Ultralong-lived room temperature phosphorescence from N and P codoped self-protective carbonized polymer dots for confidential information encryption and decryption. J. Mater. Chem. C. 2021, 9, 4847–4853. [Google Scholar] [CrossRef]
- Su, Y.; Phua, S.Z.F.; Li, Y.; Zhou, X.; Jana, D.; Liu, G.; Lim, W.Q.; Ong, W.K.; Yang, C.; Zhao, Y. Ultralong room temperature phosphorescence from amorphous organic materials toward confidential information encryption and decryption. Sci. Adv. 2018, 4, eaas9732. [Google Scholar] [CrossRef]
- Guo, J.; Wu, M.; Zhang, L.; Peng, J.; Li, X.; Chen, C.; Ma, H. Multicolor room temperature phosphorescence cellulose with source-boosting effect for information encryption. Mater. Today Chem. 2024, 40, 102211. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Yang, J.; Fang, M.; Ding, D.; Tang, B.Z.; Li, Z. High Performance of Simple Organic Phosphorescence Host–Guest Materials and their Application in Time-Resolved Bioimaging. Adv. Mater. 2021, 33, 2007811. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, H.; Yang, J.; Fang, M.; Ding, D.; Tang, B.Z.; Li, Z. Construction of room temperature phosphorescent materials with ultralong lifetime by in-situ derivation strategy. Nat. Commun. 2023, 14, 4164. [Google Scholar] [CrossRef]
- Abdukayum, A.; Chen, J.-T.; Zhao, Q.; Yan, X.-P. Functional near infrared-emitting Cr3+/Pr3+ co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 2013, 135, 14125–14133. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, K.Y.; Tong, X.; Liu, Y.; Hu, C.; Liu, S.; Yu, Q.; Zhao, Q.; Huang, W. Phosphorescent polymeric thermometers for in vitro and in vivo temperature sensing with minimized background interference. Adv. Funct. Mater. 2016, 26, 4386–4396. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z. Molecular packing: Another key point for the performance of organic and polymeric optoelectronic materials. Acc. Chem. Res. 2020, 53, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; He, Z.; Tang, B.Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 2020, 5, 869–885. [Google Scholar] [CrossRef]
- Ma, X.-K.; Liu, Y. Supramolecular purely organic room-temperature phosphorescence. Acc. Chem. Res. 2021, 54, 3403–3414. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, K.Y.; Tong, X.; Liu, Y.; Hu, C.; Liu, S.; Yu, Q.; Zhao, Q.; Huang, W. Ultralong purely organic aqueous phosphorescence supramolecular polymer for targeted tumor cell imaging. Nat. Commun. 2020, 11, 4655. [Google Scholar]
- Zhang, Z.Y.; Xu, W.W.; Xu, W.S.; Niu, J.; Sun, X.H.; Liu, Y. A synergistic enhancement strategy for realizing ultralong and efficient room-temperature phosphorescence. Angew. Chem. Int. Ed. 2020, 59, 18748–18754. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yang, C.; Zhao, Y. Long-lived organic room-temperature phosphorescence from amorphous polymer systems. Acc. Chem. Res. 2022, 55, 1160–1170. [Google Scholar] [CrossRef]
- Fang, M.-M.; Yang, J.; Li, Z. Recent advances in purely organic room temperature phosphorescence polymer. Chin. J. Polym. Sci. 2019, 37, 383–393. [Google Scholar] [CrossRef]
- Xiong, S.; Xiong, Y.; Wang, D.; Pan, Y.; Chen, K.; Zhao, Z.; Wang, D.; Tang, B.Z. Achieving Tunable Organic Afterglow and UV-Irradiation-Responsive Ultralong Room-Temperature Phosphorescence from Pyridine-Substituted Triphenylamine Derivatives. Adv. Mater. 2023, 35, 2301874. [Google Scholar] [CrossRef]
- Gao, H.; Ma, X. Recent progress on pure organic room temperature phosphorescent polymers. Aggregate 2021, 2, e38. [Google Scholar] [CrossRef]
- Alexander, E.; Chavez, J.; Ceresa, L.; Seung, M.; Pham, D.; Gryczynski, Z.; Gryczynski, I. Room temperature phosphorescence of coumarin 106 with direct triplet state excitation. Dye. Pigment. 2023, 217, 111389. [Google Scholar] [CrossRef]
- Kenry; Chen, C.; Liu, B. Enhancing the performance of pure organic room-temperature phosphorescent luminophores. Nat. Commun. 2019, 10, 2111. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, D.; Seo, S.; Jung, J.; Kim, J. Tailoring intermolecular interactions for efficient room-temperature phosphorescence from purely organic materials in amorphous polymer matrices. Angew. Chem. Int. Ed. Engl. 2014, 53, 11177–11181. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Nitsch, J.; Marder, T.B. Persistent Room-Temperature Phosphorescence from Purely Organic Molecules and Multi-Component Systems. Adv. Opt. Mater. 2021, 9, 2100411. [Google Scholar] [CrossRef]
- Nidhankar, A.D.; Goudappagouda Wakchaure, V.C.; Babu, S.S. Efficient metal-free organic room temperature phosphors. Chem. Sci. 2021, 12, 4216–4236. [Google Scholar] [CrossRef] [PubMed]
- Gryczynski, Z.; Kimball, J.; Fudala, R.; Chavez, J.; Ceresa, L.; Szabelski, M.; Borejdo, J.; Gryczynski, I. Photophysical properties of 2-Phenylindole in poly (vinyl alcohol) film at room temperature. Enhanced phosphorescence anisotropy with direct triplet state excitation. Methods Appl. Fluoresc. 2019, 8, 014008. [Google Scholar] [CrossRef]
- Zhou, B.; Qi, Z.; Yan, D. Highly Efficient and Direct Ultralong All-Phosphorescence from Metal–Organic Framework Photonic Glasses. Angew. Chem. Int. Ed. 2022, 61, e202208735. [Google Scholar] [CrossRef]
- Yang, X.; Yan, D. Strongly enhanced long-lived persistent room temperature phosphorescence based on the formation of metal–organic hybrids. Adv. Opt. Mater. 2016, 4, 897–905. [Google Scholar] [CrossRef]
- Gao, R.; Kodaimati, M.S.; Yan, D. Recent advances in persistent luminescence based on molecular hybrid materials. Chem. Soc. Rev. 2021, 50, 5564–5589. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Su, Y.; Zheng, Y.; Tang, W.; Yang, C.; Tang, H.; Qu, L.; Li, Y.; Zhao, Y. Simple vanilla derivatives for long-lived room-temperature polymer phosphorescence as invisible security inks. Research 2021, 2021, 9837412. [Google Scholar] [CrossRef]
- Al-Attar, H.A.; Monkman, A.P. Room-temperature phosphorescence from films of isolated water-soluble conjugated polymers in hydrogen-bonded matrices. Adv. Funct. Mater. 2012, 22, 3824–3832. [Google Scholar] [CrossRef]
- Tian, Z.; Li, D.; Ushakova, E.V.; Maslov, V.G.; Zhou, D.; Jing, P.; Shen, D.; Qu, S.; Rogach, A.L. Multilevel data encryption using thermal-treatment controlled room temperature phosphorescence of carbon dot/polyvinylalcohol composites. Adv. Sci. 2018, 5, 1800795. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, W.; Wu, P.; Ma, C.; Liu, Y.; Xu, M.; Liu, S. Long-lived room-temperature phosphorescent nitrogen doped CQDs/PVA composites: Fabrication, characterization and application. Eng. Sci. 2018, 4, 111–118. [Google Scholar] [CrossRef]
- Xu, M.; Wu, X.; Yang, Y.; Ma, C.; Li, W.; Yu, H.; Chen, Z.; Li, J.; Zhang, K.; Liu, S. Designing hybrid chiral photonic films with circularly polarized room-temperature phosphorescence. Acs Nano 2020, 14, 11130–11139. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Ma, X.; Tian, H. Multicolor photoluminescence of a hybrid film via the dual-emitting strategy of an inorganic fluorescent Au nanocluster and an organic room-temperature phosphorescent copolymer. Ind. Eng. Chem. Res. 2018, 57, 2866–2872. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Hu, C.; Zhang, H.; Lei, B.; Liu, Y. Room temperature phosphorescence from moisture-resistant and oxygen-barred carbon dot aggregates. 10.1039/C7TC01615H. J. Mater. Chem. C 2017, 5, 6243–6250. [Google Scholar] [CrossRef]
- Skhirtladze, L.; Leitonas, K.; Bucinskas, A.; Woon, K.L.; Volyniuk, D.; Keruckienė, R.; Mahmoudi, M.; Lapkowski, M.; Ariffin, A.; Grazulevicius, J.V. Turn on of room temperature phosphorescence of donor-acceptor-donor type compounds via transformation of excited states by rigid hosts for oxygen sensing. Sens. Actuators B Chem. 2023, 380, 133295. [Google Scholar] [CrossRef]
- Skuodis, E.; Leitonas, K.; Panchenko, A.; Volyniuk, L.; Simokaitienė, J.; Keruckienė, R.; Volyniuk, D.; Minaev, B.F.; Gražulevičius, J.V. Very sensitive probes for quantitative and organoleptic detection of oxygen based on conformer-induced room-temperature phosphorescence enhancement of the derivative of triazatruxene and phenothiazine. Sens. Actuators B Chem. 2022, 373, 132727. [Google Scholar] [CrossRef]
- Keruckiene, R.; Volyniuk, D.; Leitonas, K.; Grazulevicius, J.V. Dual emission fluorescence/room-temperature phosphorescence of phenothiazine and benzotrifluoride derivatives and its application for optical sensing of oxygen. Sens. Actuators B Chem. 2020, 321, 128533. [Google Scholar] [CrossRef]
- Keruckiene, R.; Lekavicius, D.; Guzauskas, M.; Simokaitiene, J.; Grazulevicius, J.V. Effects of the introduction of tertiary amino linkages on the properties of phenothiazine derivatives as delayed emission fluorophores. ChemPhotoChem 2024, 8, e202400002. [Google Scholar] [CrossRef]
- Alexander, E.; Lee, B.; Pham, D.; Garcia-Rodriguez, S.; Gryczynski, Z.; Gryczynski, I. Photophysical properties of DAPI in PVA films. Possibility of room temperature phosphorescence. Anal Biochem. 2024, 689, 115498. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Xu, X.; Zhu, X.; Shen, L.; Wan, W.; Hu, M. Based on FRET to construct color-tunable ultralong lifetime room temperature phosphorescent carbon dots in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 304, 123404. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Jia, L. A simple method for the determination of benzoic acid based on room temperature phosphorescence of 1-bromopyrene/γ-cyclodextrin complex in water. Talanta 2017, 162, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Zou, W.S. 3-Aminopropyltriethoxysilane-functionalized manganese doped ZnS quantum dots for room-temperature phosphorescence sensing ultratrace 2,4,6-trinitrotoluene in aqueous solution. Talanta 2011, 85, 469–475. [Google Scholar] [CrossRef]
- Haugland, R.P. Molecular Probes. Handbook of Fluorescent Probes and Research Chemicals; Molecular Probes: Eugene, OR, USA, 1992–1994. [Google Scholar]
- Gryczynski, Z.; Gryczynski, I. Practical Fluorescence Spectroscopy; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxford, UK, 2019. [Google Scholar]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).