IRES-Mediated Translation: Expanding the Toolkits of RNA Therapy
Abstract
1. Introduction
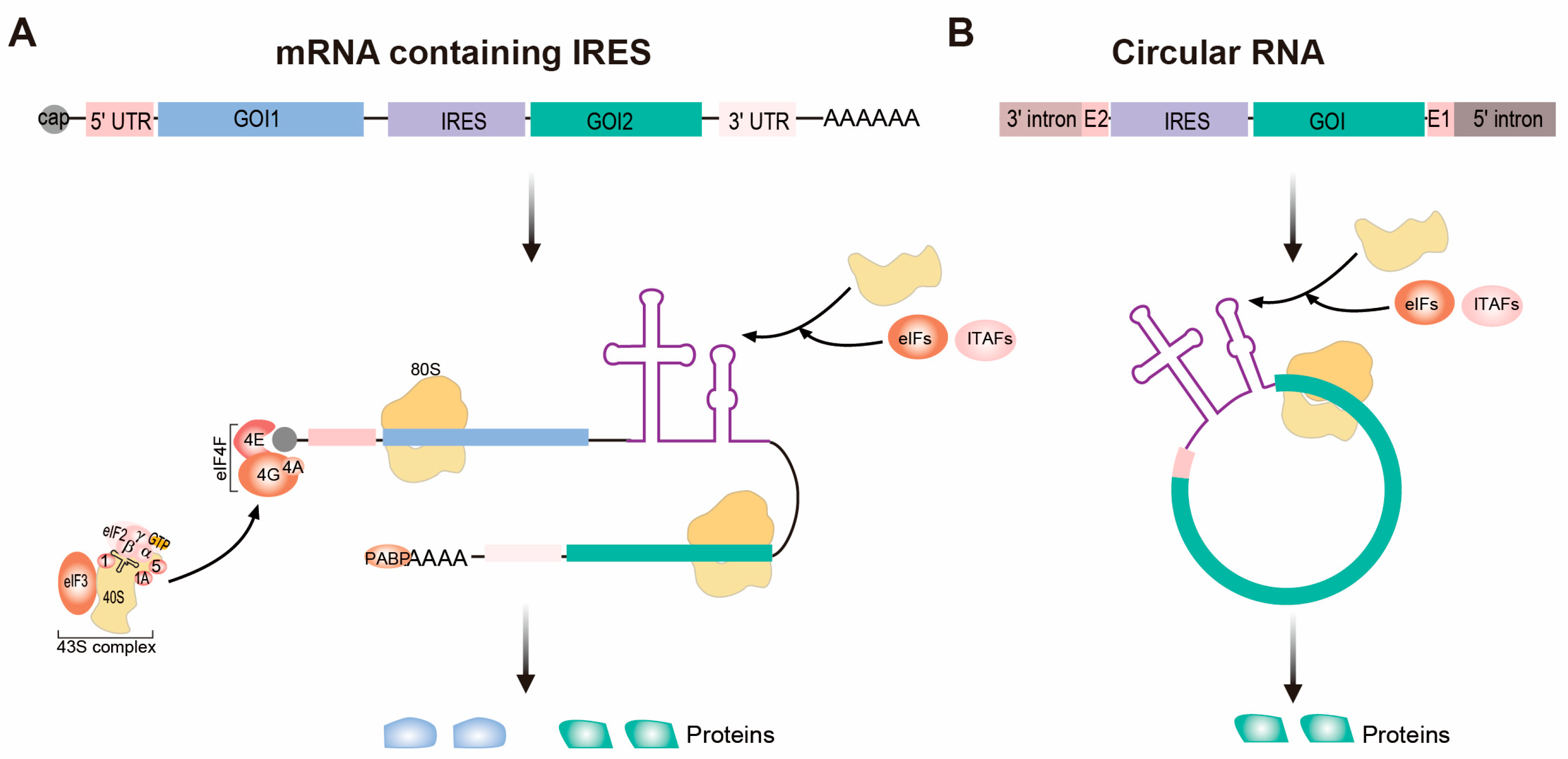
2. IRES-Based Platforms for Therapeutic Protein Expression
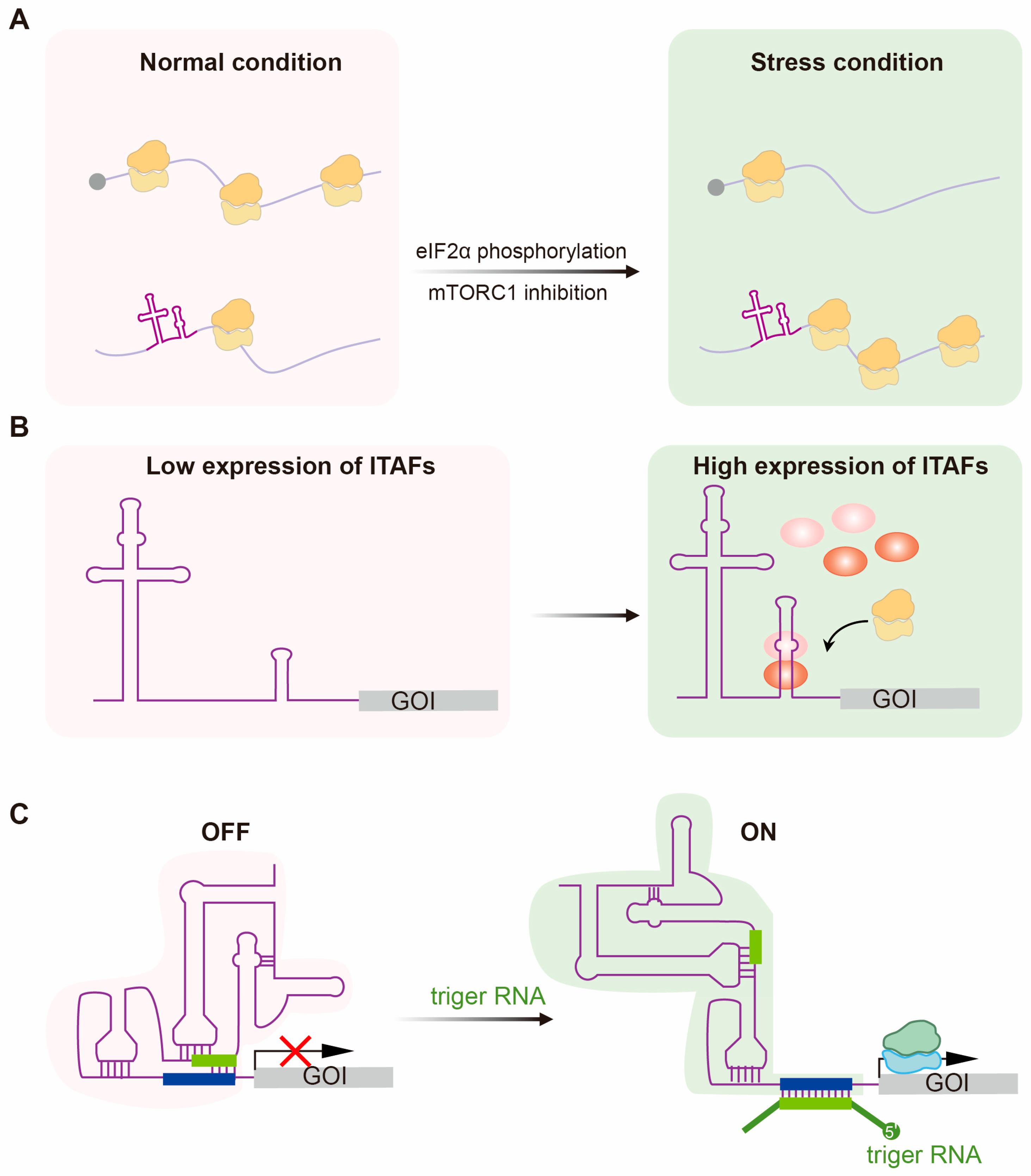
3. Modulation of IRES-Mediated Translation for Conditional Expression
3.1. Predominant Translation of IRES Under Stress Conditions
3.2. Cell-Specific Translation of IRESs
3.3. Responsive IRES Switches
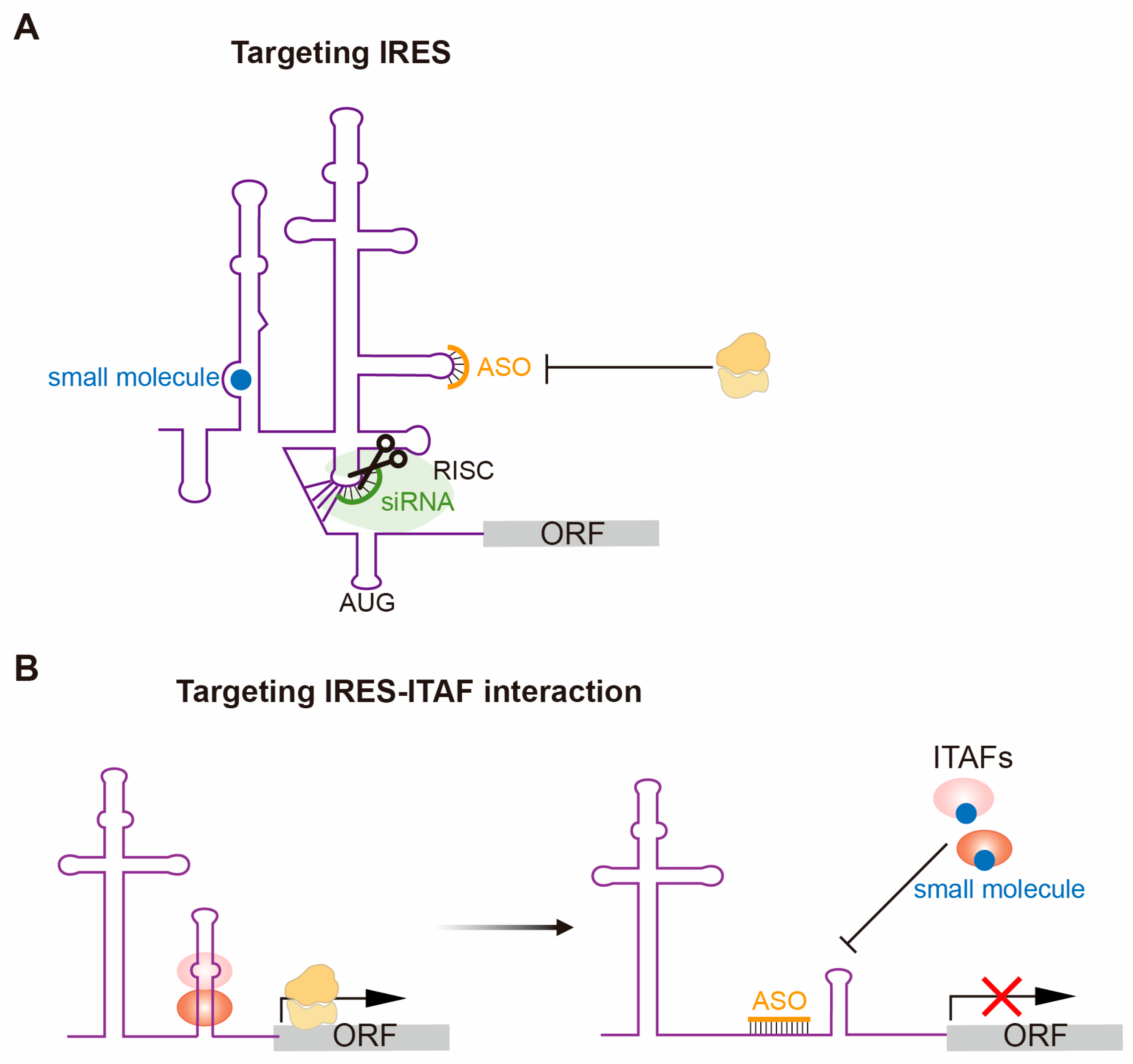
4. Therapeutically Targeting IRES-Dependent Translation
5. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for non-viral intracellular delivery of messenger RNA therapeutics. J. Control Release 2016, 240, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Kis, Z.; Kontoravdi, C.; Shah, N. Quality by Design for enabling RNA platform production processes. Trends Biotechnol. 2022, 40, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Vergnes, J.N. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2021, 384, 1577. [Google Scholar] [CrossRef]
- Zhang, N.N.; Li, X.F.; Deng, Y.Q.; Zhao, H.; Huang, Y.J.; Yang, G.; Huang, W.J.; Gao, P.; Zhou, C.; Zhang, R.R.; et al. A Thermostable mRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e1216. [Google Scholar] [CrossRef]
- Tseng, H.F.; Ackerson, B.K.; Sy, L.S.; Tubert, J.E.; Luo, Y.; Qiu, S.; Lee, G.S.; Bruxvoort, K.J.; Ku, J.H.; Florea, A.; et al. mRNA-1273 bivalent (original and Omicron) COVID-19 vaccine effectiveness against COVID-19 outcomes in the United States. Nat. Commun. 2023, 14, 5851. [Google Scholar] [CrossRef]
- Qin, S.; Tang, X.; Chen, Y.; Chen, K.; Fan, N.; Xiao, W.; Zheng, Q.; Li, G.; Teng, Y.; Wu, M.; et al. mRNA-based therapeutics: Powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 2022, 7, 166. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D.; Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, M.; Wang, Y.; You, J. Progress and prospects of mRNA-based drugs in pre-clinical and clinical applications. Signal Transduct. Target. Ther. 2024, 9, 322. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Brito Querido, J.; Díaz-López, I.; Ramakrishnan, V.; Brito Querido, J.; Díaz-López, I.; Ramakrishnan, V. The molecular basis of translation initiation and its regulation in eukaryotes. Nat. Rev. Mol. Cell Biol. 2023, 25, 168–186. [Google Scholar] [CrossRef]
- Sonenberg, N.; Hinnebusch, A.G. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell 2009, 136, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M.; Sonenberg, N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005, 6, 318–327. [Google Scholar] [CrossRef]
- Stern-Ginossar, N.; Thompson, S.R.; Mathews, M.B.; Mohr, I. Translational Control in Virus-Infected Cells. Cold Spring Harb. Perspect. Biol. 2019, 11, a033001. [Google Scholar] [CrossRef]
- Kwan, T.; Thompson, S.R. Noncanonical Translation Initiation in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2019, 11, a032672. [Google Scholar] [CrossRef]
- Meyer, K.D.; Patil, D.P.; Zhou, J.; Zinoviev, A.; Skabkin, M.A.; Elemento, O.; Pestova, T.V.; Qian, S.B.; Jaffrey, S.R. 5′ UTR m(6)A Promotes Cap-Independent Translation. Cell 2015, 163, 999–1010. [Google Scholar] [CrossRef]
- Zhou, J.; Wan, J.; Gao, X.; Zhang, X.; Jaffrey, S.R.; Qian, S.B. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature 2015, 526, 591–594. [Google Scholar] [CrossRef]
- Simon, A.E.; Miller, W.A. 3′ cap-independent translation enhancers of plant viruses. Annu. Rev. Microbiol. 2013, 67, 21–42. [Google Scholar] [CrossRef]
- Pelletier, J.; Sonenberg, N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 1988, 334, 320–325. [Google Scholar] [CrossRef]
- Jang, S.K.; Kräusslich, H.G.; Nicklin, M.J.; Duke, G.M.; Palmenberg, A.C.; Wimmer, E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J. Virol. 1988, 62, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.M.; Le Mercier, P.; Girard, M.; Kean, K.M. Comparison of picornaviral IRES-driven internal initiation of translation in cultured cells of different origins. Nucleic Acids Res. 1997, 25, 925–932. [Google Scholar] [CrossRef]
- Coldwell, M.J.; Mitchell, S.A.; Stoneley, M.; MacFarlane, M.; Willis, A.E. Initiation of Apaf-1 translation by internal ribosome entry. Oncogene 2000, 19, 899–905. [Google Scholar] [CrossRef]
- Stoneley, M.; Paulin, F.E.; Le Quesne, J.P.; Chappell, S.A.; Willis, A.E. C-Myc 5′ untranslated region contains an internal ribosome entry segment. Oncogene 1998, 16, 423–428. [Google Scholar] [CrossRef]
- Yamamoto, H.; Unbehaun, A.; Spahn, C.M.T. Ribosomal Chamber Music: Toward an Understanding of IRES Mechanisms. Trends Biochem. Sci. 2017, 42, 655–668. [Google Scholar] [CrossRef]
- Lozano, G.; Martinez-Salas, E. Structural insights into viral IRES-dependent translation mechanisms. Curr. Opin. Virol. 2015, 12, 113–120. [Google Scholar] [CrossRef]
- Jackson, R.J. The current status of vertebrate cellular mRNA IRESs. Cold Spring Harb. Perspect. Biol. 2013, 5, a011569. [Google Scholar] [CrossRef]
- Mailliot, J.; Martin, F. Viral internal ribosomal entry sites: Four classes for one goal. Wiley Interdiscip. Rev. RNA 2018, 9, e1458. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, T.R.; Abaeva, I.S.; Pestova, T.V.; Hellen, C.U. The mechanism of translation initiation on Type 1 picornavirus IRESs. EMBO J. 2014, 33, 76–92. [Google Scholar] [CrossRef] [PubMed]
- de Breyne, S.; Yu, Y.; Unbehaun, A.; Pestova, T.V.; Hellen, C.U. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc. Natl. Acad. Sci. USA 2009, 106, 9197–9202. [Google Scholar] [CrossRef] [PubMed]
- Lomakin, I.B.; Hellen, C.U.; Pestova, T.V. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol. Cell Biol. 2000, 20, 6019–6029. [Google Scholar] [CrossRef] [PubMed]
- Pestova, T.V.; Shatsky, I.N.; Hellen, C.U. Functional dissection of eukaryotic initiation factor 4F: The 4A subunit and the central domain of the 4G subunit are sufficient to mediate internal entry of 43S preinitiation complexes. Mol. Cell Biol. 1996, 16, 6870–6878. [Google Scholar] [CrossRef]
- Pestova, T.V.; Hellen, C.U.; Shatsky, I.N. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell Biol. 1996, 16, 6859–6869. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Abaeva, I.S.; Marintchev, A.; Pestova, T.V.; Hellen, C.U. Common conformational changes induced in type 2 picornavirus IRESs by cognate trans-acting factors. Nucleic Acids Res. 2011, 39, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Kafasla, P.; Morgner, N.; Poyry, T.A.; Curry, S.; Robinson, C.V.; Jackson, R.J. Polypyrimidine tract binding protein stabilizes the encephalomyocarditis virus IRES structure via binding multiple sites in a unique orientation. Mol. Cell 2009, 34, 556–568. [Google Scholar] [CrossRef]
- Otto, G.A.; Puglisi, J.D. The pathway of HCV IRES-mediated translation initiation. Cell 2004, 119, 369–380. [Google Scholar] [CrossRef]
- Quade, N.; Boehringer, D.; Leibundgut, M.; van den Heuvel, J.; Ban, N. Cryo-EM structure of Hepatitis C virus IRES bound to the human ribosome at 3.9-A resolution. Nat. Commun. 2015, 6, 7646. [Google Scholar] [CrossRef]
- Fernandez, I.S.; Bai, X.-C.; Murshudov, G.; Scheres, S.H.W.; Ramakrishnan, V. Initiation of Translation by Cricket Paralysis Virus IRES Requires Its Translocation in the Ribosome. Cell 2014, 157, 823–831. [Google Scholar] [CrossRef]
- Jan, E.; Sarnow, P. Factorless ribosome assembly on the internal ribosome entry site of cricket paralysis virus. J. Mol. Biol. 2002, 324, 889–902. [Google Scholar] [CrossRef]
- Wilson, J.E.; Pestova, T.V.; Hellen, C.U.T.; Sarnow, P. Initiation of protein synthesis from the A site of the ribosome. Cell 2000, 102, 511–520. [Google Scholar] [CrossRef]
- MacCallum, P.R.; Jack, S.C.; Egan, P.A.; McDermott, B.T.; Elliott, R.M.; Chan, S.W. Cap-dependent and hepatitis C virus internal ribosome entry site-mediated translation are modulated by phosphorylation of eIF2alpha under oxidative stress. J. Gen. Virol. 2006, 87, 3251–3262. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.M.; Park, J.H.; Keum, S.J.; Jang, S.K. eIF2A mediates translation of hepatitis C viral mRNA under stress conditions. EMBO J. 2011, 30, 2454–2464. [Google Scholar] [CrossRef]
- Welnowska, E.; Sanz, M.A.; Redondo, N.; Carrasco, L. Translation of viral mRNA without active eIF2: The case of picornaviruses. PLoS ONE 2011, 6, e22230. [Google Scholar] [CrossRef]
- Martinez-Salas, E.; Pineiro, D.; Fernandez, N. Alternative Mechanisms to Initiate Translation in Eukaryotic mRNAs. Comp. Funct. Genom. 2012, 2012, 391546. [Google Scholar] [CrossRef]
- Weingarten-Gabbay, S.; Elias-Kirma, S.; Nir, R.; Gritsenko, A.A.; Stern-Ginossar, N.; Yakhini, Z.; Weinberger, A.; Segal, E. Comparative genetics. Systematic discovery of cap-independent translation sequences in human and viral genomes. Science 2016, 351, aad4939. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.; Jordan, L.E.; Holcik, M. Distinct 5′ UTRs regulate XIAP expression under normal growth conditions and during cellular stress. Nucleic Acids Res. 2010, 38, 4665–4674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marash, L.; Liberman, N.; Henis-Korenblit, S.; Sivan, G.; Reem, E.; Elroy-Stein, O.; Kimchi, A. DAP5 promotes cap-independent translation of Bcl-2 and CDK1 to facilitate cell survival during mitosis. Mol. Cell 2008, 30, 447–459. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Miskimins, W.K. Far upstream element binding protein 1 activates translation of p27Kip1 mRNA through its internal ribosomal entry site. Int. J. Biochem. Cell Biol. 2011, 43, 1641–1648. [Google Scholar] [CrossRef]
- Yang, D.Q.; Halaby, M.J.; Zhang, Y. The identification of an internal ribosomal entry site in the 5′-untranslated region of p53 mRNA provides a novel mechanism for the regulation of its translation following DNA damage. Oncogene 2006, 25, 4613–4619. [Google Scholar] [CrossRef]
- Creancier, L.; Mercier, P.; Prats, A.C.; Morello, D. c-myc Internal ribosome entry site activity is developmentally controlled and subjected to a strong translational repression in adult transgenic mice. Mol. Cell. Biol. 2001, 21, 1833–1840. [Google Scholar] [CrossRef]
- Blau, L.; Knirsh, R.; Ben-Dror, I.; Oren, S.; Kuphal, S.; Hau, P.; Proescholdt, M.; Bosserhoff, A.-K.; Vardimon, L. Aberrant expression of c-Jun in glioblastoma by internal ribosome entry site (IRES)-mediated translational activation. Proc. Natl. Acad. Sci. USA 2012, 109, E2875–E2884. [Google Scholar] [CrossRef]
- Schepens, B.; Tinton, S.A.; Bruynooghe, Y.; Beyaert, R.; Cornelis, S. The polypyrimidine tract-binding protein stimulates HIF-1α IRES-mediated translation during hypoxia. Nucleic Acids Res. 2005, 33, 6884–6894. [Google Scholar] [CrossRef] [PubMed]
- Bornes, S.; Prado-Lourenco, L.; Bastide, A.; Zanibellato, C.; Iacovoni, J.S.; Lacazette, E.; Prats, A.C.; Touriol, C.; Prats, H. Translational induction of VEGF internal ribosome entry site elements during the early response to ischemic stress. Circ. Res. 2007, 100, 305–308. [Google Scholar] [CrossRef]
- Bonnal, S.; Schaeffer, C.; Créancier, L.; Clamens, S.; Moine, H.; Prats, A.C.; Vagner, S. A single internal ribosome entry site containing a G quartet RNA structure drives fibroblast growth factor 2 gene expression at four alternative translation initiation codons. J. Biol. Chem. 2003, 278, 39330–39336. [Google Scholar] [CrossRef]
- Komar, A.A.; Hatzoglou, M. Cellular IRES-mediated translation: The war of ITAFs in pathophysiological states. Cell Cycle 2011, 10, 229–240. [Google Scholar] [CrossRef]
- Leppek, K.; Das, R.; Barna, M. Functional 5′ UTR mRNA structures in eukaryotic translation regulation and how to find them. Nat. Rev. Mol. Cell Biol. 2018, 19, 158–174, Correction in Nat. Rev. Mol. Cell Biol. 2018, 19, 673. [Google Scholar] [CrossRef]
- O’Leary, E.; Jiang, Y.Y.; Kristensen, L.S.; Hansen, T.B.; Kjems, J. The therapeutic potential of circular RNAs. Nat. Rev. Genet. 2025, 26, 230–244. [Google Scholar] [CrossRef]
- Dolgin, E. Why rings of RNA could be the next blockbuster drug. Nature 2023, 622, 22–24. [Google Scholar] [CrossRef]
- Zhang, X.O.; Wang, H.B.; Zhang, Y.; Lu, X.; Chen, L.L.; Yang, L. Complementary sequence-mediated exon circularization. Cell 2014, 159, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Chen, L.L. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef]
- Wesselhoeft, R.A.; Kowalski, P.S.; Parker-Hale, F.C.; Huang, Y.; Bisaria, N.; Anderson, D.G. RNA Circularization Diminishes Immunogenicity and Can Extend Translation Duration In Vivo. Mol. Cell 2019, 74, 508–520.e4. [Google Scholar] [CrossRef] [PubMed]
- Wesselhoeft, R.A.; Kowalski, P.S.; Anderson, D.G. Engineering circular RNA for potent and stable translation in eukaryotic cells. Nat. Commun. 2018, 9, 2629. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, L.; Wang, X.; Li, J.; Pan, Q.; Kou, H.; Yin, J.; Gao, F.; Liao, X.; Zhang, C.; et al. Synergically enhanced anti-tumor immunity of in vivo panCAR by circRNA vaccine boosting. Cell Rep. Med. 2025, 6, 102250. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Li, X.; Wu, W.; Jiang, H.; Zheng, Y.; Zhou, J.; Ye, X.; Lu, J.; Wang, W.; et al. A single-dose circular RNA vaccine prevents Zika virus infection without enhancing dengue severity in mice. Nat. Commun. 2024, 15, 8932. [Google Scholar] [CrossRef]
- Qu, L.; Yi, Z.; Shen, Y.; Lin, L.; Chen, F.; Xu, Y.; Wu, Z.; Tang, H.; Zhang, X.; Tian, F.; et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 2022, 185, 1728–1744.e16. [Google Scholar] [CrossRef]
- Li, H.; Peng, K.; Yang, K.; Ma, W.; Qi, S.; Yu, X.; He, J.; Lin, X.; Yu, G. Circular RNA cancer vaccines drive immunity in hard-to-treat malignancies. Theranostics 2022, 12, 6422–6436. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, Z.A.; Kieft, J.S. Viral RNA structure-based strategies to manipulate translation. Nat. Rev. Microbiol. 2019, 17, 110–123. [Google Scholar] [CrossRef]
- Komar, A.A.; Hatzoglou, M. Exploring Internal Ribosome Entry Sites as Therapeutic Targets. Front. Oncol. 2015, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, S.M.; Chien, A.C.; Lee, C.G. Exploiting internal ribosome entry sites in gene therapy vector design. Curr. Gene Ther. 2004, 4, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Shaimardanova, A.A.; Kitaeva, K.V.; Abdrakhmanova, I.I.; Chernov, V.M.; Rutland, C.S.; Rizvanov, A.A.; Chulpanova, D.S.; Solovyeva, V.V. Production and Application of Multicistronic Constructs for Various Human Disease Therapies. Pharmaceutics 2019, 11, 580. [Google Scholar] [CrossRef]
- Renaud-Gabardos, E.; Hantelys, F.; Morfoisse, F.; Chaufour, X.; Garmy-Susini, B.; Prats, A.C. Internal ribosome entry site-based vectors for combined gene therapy. World J. Exp. Med. 2015, 5, 11–20. [Google Scholar] [CrossRef]
- Chan, H.Y.; V., S.; Xing, X.; Kraus, P.; Yap, S.P.; Ng, P.; Lim, S.L.; Lufkin, T. Comparison of IRES and F2A-based locus-specific multicistronic expression in stable mouse lines. PLoS ONE 2011, 6, e28885. [Google Scholar] [CrossRef]
- Lee, S.E.; Hyun, H.; Park, R.; Choi, Y.; Son, Y.J.; Park, Y.G.; Jeong, S.G.; Shin, M.Y.; Ha, H.J.; Hong, H.S.; et al. Production of transgenic pig as an Alzheimer’s disease model using a multi-cistronic vector system. PLoS ONE 2017, 12, e0177933. [Google Scholar] [CrossRef]
- Zitvogel, L.; Tahara, H.; Cai, Q.; Storkus, W.J.; Muller, G.; Wolf, S.F.; Gately, M.; Robbins, P.D.; Lotze, M.T. Construction and characterization of retroviral vectors expressing biologically active human interleukin-12. Hum. Gene Ther. 1994, 5, 1493–1506. [Google Scholar] [CrossRef]
- Couderc, B.; Zitvogel, L.; Douin-Echinard, V.; Djennane, L.; Tahara, H.; Favre, G.; Lotze, M.T.; Robbins, P.D. Enhancement of antitumor immunity by expression of CD70 (CD27 ligand) or CD154 (CD40 ligand) costimulatory molecules in tumor cells. Cancer Gene Ther. 1998, 5, 163–175. [Google Scholar] [PubMed]
- Isayeva, T.; Ren, C.; Ponnazhagan, S. Recombinant adeno-associated virus 2-mediated antiangiogenic prevention in a mouse model of intraperitoneal ovarian cancer. Clin. Cancer Res. 2005, 11, 1342–1347. [Google Scholar] [CrossRef]
- Prats, A.C.; Van den Berghe, L.; Rayssac, A.; Ainaoui, N.; Morfoisse, F.; Pujol, F.; Legonidec, S.; Bikfalvi, A.; Prats, H.; Pyronnet, S.; et al. CXCL4L1-fibstatin cooperation inhibits tumor angiogenesis, lymphangiogenesis and metastasis. Microvasc. Res. 2013, 89, 25–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rayssac, A.; Neveu, C.; Pucelle, M.; Van den Berghe, L.; Prado-Lourenco, L.; Arnal, J.F.; Chaufour, X.; Prats, A.C. IRES-based Vector Coexpressing FGF2 and Cyr61 Provides Synergistic and Safe Therapeutics of Lower Limb Ischemia. Mol. Ther. 2009, 17, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, K.Z.; Qiang, H.; Tang, Y.L.; Li, Q.A.; Li, M.A.; Dang, X.Q. Angiopoiesis and bone regeneration via co-expression of the hVEGF and hBMP genes from an adeno-associated viral vector in vitro and in vivo. Acta Pharmacol. Sin. 2010, 31, 821–830. [Google Scholar] [CrossRef][Green Version]
- Azzouz, M.; Martin-Rendon, E.; Barber, R.D.; Mitrophanous, K.A.; Carter, E.E.; Rohll, J.B.; Kingsman, S.M.; Kingsman, A.J.; Mazarakis, N.D. Multicistronic lentiviral vector-mediated striatal gene transfer of aromatic L-amino acid decarboxylase, tyrosine hydroxylase, and GTP cyclohydrolase I induces sustained transgene expression, dopamine production, and functional improvement in a rat model of Parkinson’s disease. J. Neurosci. 2002, 22, 10302–10312. [Google Scholar][Green Version]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef]
- Lee, R.J.; Springer, M.L.; Blanco-Bose, W.E.; Shaw, R.; Ursell, P.C.; Blau, H.M. VEGF gene delivery to myocardium: Deleterious effects of unregulated expression. Circulation 2000, 102, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.R.; Speakman, M.T.; Patterson, M.; Hale, S.S.; Isner, J.M.; Kedes, L.H.; Kloner, R.A. Evaluation of the effects of intramyocardial injection of DNA expressing vascular endothelial growth factor (VEGF) in a myocardial infarction model in the rat--angiogenesis and angioma formation. J. Am. Coll. Cardiol. 2000, 35, 1323–1330. [Google Scholar] [CrossRef]
- Masaki, I.; Yonemitsu, Y.; Yamashita, A.; Sata, S.; Tanii, M.; Komori, K.; Nakagawa, K.; Hou, X.; Nagai, Y.; Hasegawa, M.; et al. Angiogenic gene therapy for experimental critical limb ischemia: Acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circ. Res. 2002, 90, 966–973. [Google Scholar] [CrossRef]
- Jazwa, A.; Tomczyk, M.; Taha, H.M.; Hytonen, E.; Stoszko, M.; Zentilin, L.; Giacca, M.; Yla-Herttuala, S.; Emanueli, C.; Jozkowicz, A.; et al. Arteriogenic therapy based on simultaneous delivery of VEGF-A and FGF4 genes improves the recovery from acute limb ischemia. Vasc. Cell 2013, 5, 13. [Google Scholar] [CrossRef]
- Koch, A.; Aguilera, L.; Morisaki, T.; Munsky, B.; Stasevich, T.J. Quantifying the dynamics of IRES and cap translation with single-molecule resolution in live cells. Nat. Struct. Mol. Biol. 2020, 27, 1095–1104, Correction in Nat. Struct. Mol. Biol. 2020, 27, 1209–1210. [Google Scholar] [CrossRef]
- Douin, V.; Bornes, S.; Creancier, L.; Rochaix, P.; Favre, G.; Prats, A.C.; Couderc, B. Use and comparison of different internal ribosomal entry sites (IRES) in tricistronic retroviral vectors. BMC Biotechnol. 2004, 4, 16. [Google Scholar] [CrossRef]
- Koh, E.Y.; Ho, S.C.; Mariati; Song, Z.; Bi, X.; Bardor, M.; Yang, Y. An internal ribosome entry site (IRES) mutant library for tuning expression level of multiple genes in mammalian cells. PLoS ONE 2013, 8, e82100. [Google Scholar] [CrossRef][Green Version]
- Attal, J.; Theron, M.C.; Puissant, C.; Houdebine, L.M. Effect of intercistronic length on internal ribosome entry site (IRES) efficiency in bicistronic mRNA. Gene Expr. 1999, 8, 299–309. [Google Scholar] [PubMed][Green Version]
- Harries, M.; Phillipps, N.; Anderson, R.; Prentice, G.; Collins, M. Comparison of bicistronic retroviral vectors containing internal ribosome entry sites (IRES) using expression of human interleukin-12 (IL-12) as a readout. J. Gene Med. 2000, 2, 243–249. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, W.; Li, X.; Zhang, J.; Chen, S.; Zhang, J.L.; Yang, L.; Chen, L.L. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016, 15, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.K.; Cheng, R.; Demeter, J.; Chen, J.; Weingarten-Gabbay, S.; Jiang, L.; Snyder, M.P.; Weissman, J.S.; Segal, E.; Jackson, P.K.; et al. Structured elements drive extensive circular RNA translation. Mol. Cell 2021, 81, 4300–4318.e13. [Google Scholar] [CrossRef]
- Weigelt, C.M.; Sehgal, R.; Tain, L.S.; Cheng, J.; Esser, J.; Pahl, A.; Dieterich, C.; Gronke, S.; Partridge, L. An Insulin-Sensitive Circular RNA that Regulates Lifespan in Drosophila. Mol. Cell 2020, 79, 268–279.e5. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, X.; Mao, M.; Song, X.; Wu, P.; Zhang, Y.; Jin, Y.; Yang, Y.; Chen, L.L.; Wang, Y.; et al. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e9. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Sarnow, P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular RNAs. Science 1995, 268, 415–417. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.X.; Xue, W.; Zhang, Y.; Jiang, S.; Yin, Q.F.; Wei, J.; Yao, R.W.; Yang, L.; Chen, L.L. Coordinated circRNA Biogenesis and Function with NF90/NF110 in Viral Infection. Mol. Cell 2017, 67, 214–227.e7. [Google Scholar] [CrossRef]
- Wang, F.; Cai, G.; Wang, Y.; Zhuang, Q.; Cai, Z.; Li, Y.; Gao, S.; Li, F.; Zhang, C.; Zhao, B.; et al. Circular RNA-based neoantigen vaccine for hepatocellular carcinoma immunotherapy. MedComm 2024, 5, e667. [Google Scholar] [CrossRef]
- Yu, H.; Wen, Y.; Yu, W.; Lu, L.; Yang, Y.; Liu, C.; Hu, Z.; Fang, Z.; Huang, S. Optimized circular RNA vaccines for superior cancer immunotherapy. Theranostics 2025, 15, 1420–1438. [Google Scholar] [CrossRef]
- Kudla, G.; Murray, A.W.; Tollervey, D.; Plotkin, J.B. Coding-sequence determinants of gene expression in Escherichia coli. Science 2009, 324, 255–258. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Lin, A.; Xu, C.; Li, Z.; Liu, K.; Liu, B.; Ma, X.; Zhao, F.; Jiang, H.; et al. Algorithm for optimized mRNA design improves stability and immunogenicity. Nature 2023, 621, 396–403. [Google Scholar] [CrossRef]
- Leppek, K.; Byeon, G.W.; Kladwang, W.; Wayment-Steele, H.K.; Kerr, C.H.; Xu, A.F.; Kim, D.S.; Topkar, V.V.; Choe, C.; Rothschild, D.; et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 2022, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef]
- Niu, D.; Wu, Y.; Lian, J. Circular RNA vaccine in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 341. [Google Scholar] [CrossRef]
- Cao, X.F.; Cai, Z.Y.; Zhang, J.Y.; Zhao, F.Q. Engineering circular RNA medicines. Nat. Rev. Bioeng. 2025, 3, 270–287. [Google Scholar] [CrossRef]
- Chen, R.; Wang, S.K.; Belk, J.A.; Amaya, L.; Li, Z.; Cardenas, A.; Abe, B.T.; Chen, C.K.; Wender, P.A.; Chang, H.Y. Engineering circular RNA for enhanced protein production. Nat. Biotechnol. 2023, 41, 262–272. [Google Scholar] [CrossRef]
- Liu, C.X.; Yang, L.; Chen, L.L. Dynamic conformation: Marching toward circular RNA function and application. Mol. Cell 2024, 84, 3596–3609. [Google Scholar] [CrossRef]
- Choi, S.W.; Nam, J.W. Optimal design of synthetic circular RNAs. Exp. Mol. Med. 2024, 56, 1281–1292. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Sun, Y.; Liao, Y.; Jiang, G.; Sun, B.Y.; Yu, J.; Zhao, D. Unlocking the potential of circular RNA vaccines: A bioinformatics and computational biology perspective. EBioMedicine 2025, 114, 105638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, H. Artificial intelligence-driven circRNA vaccine development: Multimodal collaborative optimization and a new paradigm for biomedical applications. Brief. Bioinform. 2025, 26, bbaf263. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, S.; Khoutorsky, A.; Mathews, M.B.; Sonenberg, N. Translation deregulation in human disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.W.; Yi, Z.; Weissman, J.S.; Chen, J. The dark proteome: Translation from noncanonical open reading frames. Trends Cell Biol. 2022, 32, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Godet, A.C.; David, F.; Hantelys, F.; Tatin, F.; Lacazette, E.; Garmy-Susini, B.; Prats, A.C. IRES Trans-Acting Factors, Key Actors of the Stress Response. Int. J. Mol. Sci. 2019, 20, 924. [Google Scholar] [CrossRef]
- Wek, R.C. Role of eIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef]
- Ma, X.J.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Lang, K.J.; Kappel, A.; Goodall, G.J. Hypoxia-inducible factor-1alpha mRNA contains an internal ribosome entry site that allows efficient translation during normoxia and hypoxia. Mol. Biol. Cell 2002, 13, 1792–1801. [Google Scholar] [CrossRef]
- Bushell, M.; Stoneley, M.; Kong, Y.W.; Hamilton, T.L.; Spriggs, K.A.; Dobbyn, H.C.; Qin, X.; Sarnow, P.; Willis, A.E. Polypyrimidine tract binding protein regulates IRES-mediated gene expression during apoptosis. Mol. Cell 2006, 23, 401–412. [Google Scholar] [CrossRef]
- Ungureanu, N.H.; Cloutier, M.; Lewis, S.M.; de Silva, N.; Blais, J.D.; Bell, J.C.; Holcik, M. Internal ribosome entry site-mediated translation of Apaf-1, but not XIAP, is regulated during UV-induced cell death. J. Biol. Chem. 2006, 281, 15155–15163. [Google Scholar] [CrossRef]
- Hanson, P.J.; Zhang, H.M.; Hemida, M.G.; Ye, X.; Qiu, Y.; Yang, D. IRES-Dependent Translational Control during Virus-Induced Endoplasmic Reticulum Stress and Apoptosis. Front. Microbiol. 2012, 3, 92. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011, 9, 860–875. [Google Scholar] [CrossRef]
- Lee, K.M.; Chen, C.J.; Shih, S.R. Regulation Mechanisms of Viral IRES-Driven Translation. Trends Microbiol. 2017, 25, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, R.E. Translational control by viral proteinases. Virus Res. 2006, 119, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.; des Georges, A.; Dhote, V.; Langlois, R.; Liao, H.Y.; Grassucci, R.A.; Pestova, T.V.; Hellen, C.U.; Frank, J. Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature 2013, 503, 539–543. [Google Scholar] [CrossRef]
- Gonzalez-Almela, E.; Williams, H.; Sanz, M.A.; Carrasco, L. The Initiation Factors eIF2, eIF2A, eIF2D, eIF4A, and eIF4G Are Not Involved in Translation Driven by Hepatitis C Virus IRES in Human Cells. Front. Microbiol. 2018, 9, 207. [Google Scholar] [CrossRef]
- Fabbri, L.; Chakraborty, A.; Robert, C.; Vagner, S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat. Rev. Cancer 2021, 21, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Zhang, X.; Zhou, J.; Li, Q.; Chu, L.; Di, G.; Xu, Z.; Chen, Q.; Wang, M.; Jiang, X.; et al. An in vitro-transcribed circular RNA targets the mitochondrial inner membrane cardiolipin to ablate EIF4G2(+)/PTBP1(+) pan-adenocarcinoma. Nat. Cancer 2024, 5, 30–46. [Google Scholar] [CrossRef]
- Creancier, L.; Morello, D.; Mercier, P.; Prats, A.C. Fibroblast growth factor 2 internal ribosome entry site (IRES) activity ex vivo and in transgenic mice reveals a stringent tissue-specific regulation. J. Cell Biol. 2000, 150, 275–281. [Google Scholar] [CrossRef]
- Martineau, Y.; Le Bec, C.; Monbrun, L.; Allo, V.; Chiu, I.M.; Danos, O.; Moine, H.; Prats, H.; Prats, A.C. Internal ribosome entry site structural motifs conserved among mammalian fibroblast growth factor 1 alternatively spliced mRNAs. Mol. Cell. Biol. 2004, 24, 7622–7635. [Google Scholar] [CrossRef]
- Delluc-Clavieres, A.; Le Bec, C.; Van den Berghe, L.; Conte, C.; Allo, V.; Danos, O.; Prats, A.C. Efficient gene transfer in skeletal muscle with AAV-derived bicistronic vector using the FGF-1 IRES. Gene Ther. 2008, 15, 1090–1098. [Google Scholar] [CrossRef]
- Pilipenko, E.V.; Viktorova, E.G.; Guest, S.T.; Agol, V.I.; Roos, R.P. Cell-specific proteins regulate viral RNA translation and virus-induced disease. EMBO J. 2001, 20, 6899–6908. [Google Scholar] [CrossRef]
- Castello, A.; Fischer, B.; Eichelbaum, K.; Horos, R.; Beckmann, B.M.; Strein, C.; Davey, N.E.; Humphreys, D.T.; Preiss, T.; Steinmetz, L.M.; et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell 2012, 149, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, E.V.; Pestova, T.V.; Kolupaeva, V.G.; Khitrina, E.V.; Poperechnaya, A.N.; Agol, V.I.; Hellen, C.U. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 2000, 14, 2028–2045. [Google Scholar] [CrossRef]
- Plank, T.D.; Whitehurst, J.T.; Kieft, J.S. Cell type specificity and structural determinants of IRES activity from the 5′ leaders of different HIV-1 transcripts. Nucleic Acids Res. 2013, 41, 6698–6714. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, A.; Fukao, A.; Kosaka, M.; Funakami, Y.; Takizawa, N.; Takeuchi, O.; Duncan, K.E.; Fujiwara, T. Translation of Hepatitis A Virus IRES Is Upregulated by a Hepatic Cell-Specific Factor. Front. Genet. 2018, 9, 307. [Google Scholar] [CrossRef] [PubMed]
- Yanagiya, A.; Ohka, S.; Hashida, N.; Okamura, M.; Taya, C.; Kamoshita, N.; Iwasaki, K.; Sasaki, Y.; Yonekawa, H.; Nomoto, A. Tissue-specific replicating capacity of a chimeric poliovirus that carries the internal ribosome entry site of hepatitis C virus in a new mouse model transgenic for the human poliovirus receptor. J. Virol. 2003, 77, 10479–10487. [Google Scholar] [CrossRef][Green Version]
- Merrill, M.K.; Dobrikova, E.Y.; Gromeier, M. Cell-type-specific repression of internal ribosome entry site activity by double-stranded RNA-binding protein 76. J. Virol. 2006, 80, 3147–3156. [Google Scholar] [CrossRef]
- Zong, Y.; Lin, Y.; Wei, T.; Cheng, Q. Lipid Nanoparticle (LNP) Enables mRNA Delivery for Cancer Therapy. Adv. Mater. 2023, 35, e2303261. [Google Scholar] [CrossRef]
- Parhiz, H.; Shuvaev, V.V.; Pardi, N.; Khoshnejad, M.; Kiseleva, R.Y.; Brenner, J.S.; Uhler, T.; Tuyishime, S.; Mui, B.L.; Tam, Y.K.; et al. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control Release 2018, 291, 106–115. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Kare, A.J.; Ingham, E.S.; Paulmurugan, R.; Robinson, E.R.; Baikoghli, M.; Inayathullah, M.; Seo, J.W.; Wang, J.; Fite, B.Z.; et al. In situ T-cell transfection by anti-CD3-conjugated lipid nanoparticles leads to T-cell activation, migration, and phenotypic shift. Biomaterials 2022, 281, 121339. [Google Scholar] [CrossRef]
- Su, F.Y.; Zhao, Q.H.; Dahotre, S.N.; Gamboa, L.; Bawage, S.S.; Silva Trenkle, A.D.; Zamat, A.; Phuengkham, H.; Ahmed, R.; Santangelo, P.J.; et al. In vivo mRNA delivery to virus-specific T cells by light-induced ligand exchange of MHC class I antigen-presenting nanoparticles. Sci. Adv. 2022, 8, eabm7950. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Sun, Y.; Yu, X.; Lee, S.M.; Cheng, Q.; Wei, T.; Gong, J.; Robinson, J.; Zhang, D.; et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 2023, 18, 265–291. [Google Scholar] [CrossRef]
- Springer, A.D.; Dowdy, S.F. GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid. Ther. 2018, 28, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Si, K.; Dai, Z.; Li, Z.; Ye, Z.; Ding, B.; Feng, S.; Sun, B.; Shen, Y.; Xiao, Z. Engineered exosome-mediated messenger RNA and single-chain variable fragment delivery for human chimeric antigen receptor T-cell engineering. Cytotherapy 2023, 25, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Yu, X.; Liu, P.; Ren, H.; Wei, T.; Cheng, Q. Simplified Lipid Nanoparticles for Tissue- And Cell-Targeted mRNA Delivery Facilitate Precision Tumor Therapy in a Lung Metastasis Mouse Model. Adv. Mater. 2024, 36, e2409812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Salaita, K. Smart Nucleic Acids as Future Therapeutics. Trends Biotechnol. 2021, 39, 1289–1307. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, H.; Bailey, C.C.; Gao, G.; Farzan, M. Rational design of aptazyme riboswitches for efficient control of gene expression in mammalian cells. Elife 2016, 5, e18858. [Google Scholar] [CrossRef]
- Mustafina, K.; Fukunaga, K.; Yokobayashi, Y. Design of Mammalian ON-Riboswitches Based on Tandemly Fused Aptamer and Ribozyme. ACS Synth. Biol. 2020, 9, 19–25. [Google Scholar] [CrossRef]
- Ono, H.; Kawasaki, S.; Saito, H. Orthogonal Protein-Responsive mRNA Switches for Mammalian Synthetic Biology. ACS Synth. Biol. 2020, 9, 169–174. [Google Scholar] [CrossRef]
- Fujita, Y.; Hirosawa, M.; Hayashi, K.; Hatani, T.; Yoshida, Y.; Yamamoto, T.; Saito, H. A versatile and robust cell purification system with an RNA-only circuit composed of microRNA-responsive ON and OFF switches. Sci. Adv. 2022, 8, eabj1793. [Google Scholar] [CrossRef]
- DiAndreth, B.; Wauford, N.; Hu, E.; Palacios, S.; Weiss, R. PERSIST platform provides programmable RNA regulation using CRISPR endoRNases. Nat. Commun. 2022, 13, 2582. [Google Scholar] [CrossRef]
- Wang, S.; Emery, N.J.; Liu, A.P. A Novel Synthetic Toehold Switch for MicroRNA Detection in Mammalian Cells. ACS Synth. Biol. 2019, 8, 1079–1088. [Google Scholar] [CrossRef]
- Zhao, E.M.; Mao, A.S.; de Puig, H.; Zhang, K.; Tippens, N.D.; Tan, X.; Ran, F.A.; Han, I.; Nguyen, P.Q.; Chory, E.J.; et al. RNA-responsive elements for eukaryotic translational control. Nat. Biotechnol. 2022, 40, 539–545. [Google Scholar] [CrossRef]
- Ning, H.; Liu, G.; Li, L.; Liu, Q.; Huang, H.; Xie, Z. Rational design of microRNA-responsive switch for programmable translational control in mammalian cells. Nat. Commun. 2023, 14, 7193. [Google Scholar] [CrossRef]
- Kameda, S.; Ohno, H.; Saito, H. Synthetic circular RNA switches and circuits that control protein expression in mammalian cells. Nucleic Acids Res. 2023, 51, e24. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, A.; Vopalensky, V.; Pospisek, M. Understanding the potential of hepatitis C virus internal ribosome entry site domains to modulate translation initiation via their structure and function. Wiley Interdiscip. Rev. RNA 2015, 6, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Hanecak, R.; BrownDriver, V.; Fox, M.C.; Azad, R.F.; Furusako, S.; Nozaki, C.; Ford, C.; Sasmor, H.; Anderson, K.P. Antisense oligonucleotide inhibition of hepatitis C virus gene expression in transformed hepatocytes. J. Virol. 1996, 70, 5203–5212. [Google Scholar] [CrossRef]
- Wakita, T.; Wands, J.R. Specific-Inhibition of Hepatitis-C Virus Expression by Antisense Oligodeoxynucleotides-in-Vitro Model for Selection of Target Sequence. J. Biol. Chem. 1994, 269, 14205–14210. [Google Scholar] [CrossRef] [PubMed]
- Martinand-Mari, C.; Lebleu, B.; Robbins, I. Oligonucleotide-based strategies to inhibit human hepatitis C virus. Oligonucleotides 2003, 13, 539–548. [Google Scholar] [CrossRef]
- Nulf, C.J.; Corey, D. Intracellular inhibition of hepatitis C virus (HCV) internal ribosomal entry site (IRES)-dependent translation by peptide nucleic acids (PNAs) and locked nucleic acids (LNAs). Nucleic Acids Res. 2004, 32, 3792–3798. [Google Scholar] [CrossRef]
- McCaffrey, A.P.; Meuse, L.; Karimi, M.; Contag, C.H.; Kay, M.A. A potent and specific morpholino antisense inhibitor of hepatitis C translation in mice. Hepatology 2003, 38, 503–508. [Google Scholar] [CrossRef]
- Subramanian, N.; Mani, P.; Roy, S.; Gnanasundram, S.V.; Sarkar, D.P.; Das, S. Targeted delivery of hepatitis C virus-specific short hairpin RNA in mouse liver using Sendai virosomes. J. Gen. Virol. 2009, 90, 1812–1819. [Google Scholar] [CrossRef]
- Kanda, T.; Steele, R.; Ray, R.; Ray, R.B. Small interfering RNA targeted to hepatitis C virus 5′ nontranslated region exerts potent antiviral effect. J. Virol. 2007, 81, 669–676. [Google Scholar] [CrossRef]
- Dibrov, S.M.; Parsons, J.; Carnevali, M.; Zhou, S.; Rynearson, K.D.; Ding, K.J.; Sega, E.G.; Brunn, N.D.; Boerneke, M.A.; Castaldi, M.P.; et al. Hepatitis C Virus Translation Inhibitors Targeting the Internal Ribosomal Entry Site. J. Med. Chem. 2014, 57, 1694–1707. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Dallas, A.; Ilves, H.; Shorenstein, J.; MacLachlan, I.; Klumpp, K.; Johnston, B.H. Formulated Minimal-Length Synthetic Small Hairpin RNAs Are Potent Inhibitors of Hepatitis C Virus in Mice With Humanized Livers. Gastroenterology 2014, 146, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Lee, S.H.; Kim, E.J.; Cho, H.; Lee, W.; Kim, G.W.; Park, H.J.; Cho, S.W.; Lee, C.; Oh, J.W. Inhibition of Hepatitis C Virus in Mice by a Small Interfering RNA Targeting a Highly Conserved Sequence in Viral IRES Pseudoknot. PLoS ONE 2016, 11, e0146710. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parsons, J.; Castaldi, M.P.; Dutta, S.; Dibrov, S.M.; Wyles, D.L.; Hermann, T. Conformational inhibition of the hepatitis C virus internal ribosome entry site RNA. Nat. Chem. Biol. 2009, 5, 823–825. [Google Scholar] [CrossRef]
- Davila-Calderon, J.; Patwardhan, N.N.; Chiu, L.Y.; Sugarman, A.; Cai, Z.; Penutmutchu, S.R.; Li, M.L.; Brewer, G.; Hargrove, A.E.; Tolbert, B.S. IRES-targeting small molecule inhibits enterovirus 71 replication via allosteric stabilization of a ternary complex. Nat. Commun. 2020, 11, 4775. [Google Scholar] [CrossRef]
- Gao, M.; Duan, H.; Liu, J.; Zhang, H.; Wang, X.; Zhu, M.; Guo, J.; Zhao, Z.; Meng, L.; Peng, Y. The multi-targeted kinase inhibitor sorafenib inhibits enterovirus 71 replication by regulating IRES-dependent translation of viral proteins. Antivir. Res. 2014, 106, 80–85. [Google Scholar] [CrossRef]
- Tsai, F.J.; Lin, C.W.; Lai, C.C.; Lan, Y.C.; Lai, C.H.; Hung, C.H.; Hsueh, K.C.; Lin, T.H.; Chang, H.C.; Wan, L.; et al. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011, 128, 312–322. [Google Scholar] [CrossRef]
- Stone, J.K.; Rijnbrand, R.; Stein, D.A.; Ma, Y.; Yang, Y.; Iversen, P.L.; Andino, R. A morpholino oligomer targeting highly conserved internal ribosome entry site sequence is able to inhibit multiple species of picornavirus. Antimicrob. Agents Chemother. 2008, 52, 1970–1981. [Google Scholar] [CrossRef]
- Childs-Disney, J.L.; Yang, X.; Gibaut, Q.M.R.; Tong, Y.; Batey, R.T.; Disney, M.D. Targeting RNA structures with small molecules. Nat. Rev. Drug Discov. 2022, 21, 736–762. [Google Scholar] [CrossRef]
- Marques, R.; Lacerda, R.; Romao, L. Internal Ribosome Entry Site (IRES)-Mediated Translation and Its Potential for Novel mRNA-Based Therapy Development. Biomedicines 2022, 10, 1865. [Google Scholar] [CrossRef] [PubMed]
- Dorn, G.; Gmeiner, C.; de Vries, T.; Dedic, E.; Novakovic, M.; Damberger, F.F.; Maris, C.; Finol, E.; Sarnowski, C.P.; Kohlbrecher, J.; et al. Integrative solution structure of PTBP1-IRES complex reveals strong compaction and ordering with residual conformational flexibility. Nat. Commun. 2023, 14, 6429. [Google Scholar] [CrossRef] [PubMed]
- Kafasla, P.; Morgner, N.; Robinson, C.V.; Jackson, R.J. Polypyrimidine tract-binding protein stimulates the poliovirus IRES by modulating eIF4G binding. EMBO J. 2010, 29, 3710–3722. [Google Scholar] [CrossRef]
- Grover, R.; Ray, P.S.; Das, S. Polypyrimidine tract binding protein regulates IRES-mediated translation of p53 isoforms. Cell Cycle 2008, 7, 2189–2198. [Google Scholar] [CrossRef]
- Fan, X.; Zhao, Z.; Ma, L.; Huang, X.; Zhan, Q.; Song, Y. PTBP1 promotes IRES-mediated translation of cyclin B1 in cancer. Acta Biochim. Biophys. Sin. 2022, 54, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Le Quesne, J.P.; Stoneley, M.; Fraser, G.A.; Willis, A.E. Derivation of a structural model for the c-myc IRES. J. Mol. Biol. 2001, 310, 111–126. [Google Scholar] [CrossRef]
- Damiano, F.; Rochira, A.; Tocci, R.; Alemanno, S.; Gnoni, A.; Siculella, L. hnRNP A1 mediates the activation of the IRES-dependent SREBP-1a mRNA translation in response to endoplasmic reticulum stress. Biochem. J. 2013, 449, 543–553. [Google Scholar] [CrossRef]
- Lewis, S.M.; Veyrier, A.; Hosszu Ungureanu, N.; Bonnal, S.; Vagner, S.; Holcik, M. Subcellular relocalization of a trans-acting factor regulates XIAP IRES-dependent translation. Mol. Biol. Cell 2007, 18, 1302–1311. [Google Scholar] [CrossRef]
- Cammas, A.; Pileur, F.; Bonnal, S.; Lewis, S.M.; Lévêque, N.; Holcik, M.; Vagner, S. Cytoplasmic relocalization of heterogeneous nuclear ribonucleoprotein A1 controls translation initiation of specific mRNAs. Mol. Biol. Cell 2007, 18, 5048–5059. [Google Scholar] [CrossRef]
- Bonnal, S.; Pileur, F.; Orsini, C.; Parker, F.; Pujol, F.; Prats, A.C.; Vagner, S. Heterogeneous nuclear ribonucleoprotein A1 is a novel internal ribosome entry site trans-acting factor that modulates alternative initiation of translation of the fibroblast growth factor 2 mRNA. J. Biol. Chem. 2005, 280, 4144–4153. [Google Scholar] [CrossRef]
- Jo, O.D.; Martin, J.; Bernath, A.; Masri, J.; Lichtenstein, A.; Gera, J. Heterogeneous nuclear ribonucleoprotein A1 regulates cyclin D1 and c-myc internal ribosome entry site function through Akt signaling. J. Biol. Chem. 2008, 283, 23274–23287. [Google Scholar] [CrossRef] [PubMed]
- Benavides-Serrato, A.; Saunders, J.T.; Holmes, B.; Nishimura, R.N.; Lichtenstein, A.; Gera, J. Repurposing Potential of Riluzole as an ITAF Inhibitor in mTOR Therapy Resistant Glioblastoma. Int. J. Mol. Sci. 2020, 21, 344. [Google Scholar] [CrossRef] [PubMed]
- Holcik, M. Targeting translation for treatment of cancer—A novel role for IRES? Curr. Cancer Drug Targets 2004, 4, 299–311. [Google Scholar] [CrossRef]
- Vaklavas, C.; Meng, Z.; Choi, H.; Grizzle, W.E.; Zinn, K.R.; Blume, S.W. Small molecule inhibitors of IRES-mediated translation. Cancer Biol. Ther. 2015, 16, 1471–1485. [Google Scholar] [CrossRef]
- Peladeau, C.; Jasmin, B.J. Targeting IRES-dependent translation as a novel approach for treating Duchenne muscular dystrophy. RNA Biol. 2021, 18, 1238–1251. [Google Scholar] [CrossRef] [PubMed]
- Amaya, L.; Grigoryan, L.; Li, Z.; Lee, A.; Wender, P.A.; Pulendran, B.; Chang, H.Y. Circular RNA vaccine induces potent T cell responses. Proc. Natl. Acad. Sci. USA 2023, 120, e2302191120. [Google Scholar] [CrossRef]
- Terenin, I.M.; Smirnova, V.V.; Andreev, D.E.; Dmitriev, S.E.; Shatsky, I.N. A researcher’s guide to the galaxy of IRESs. Cell. Mol. Life Sci. 2017, 74, 1431–1455. [Google Scholar] [CrossRef]
- Shatsky, I.N.; Terenin, I.M.; Smirnova, V.V.; Andreev, D.E. Cap-Independent Translation: What’s in a Name? Trends Biochem. Sci. 2018, 43, 882–895. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Wu, Z. IRES-Mediated Translation: Expanding the Toolkits of RNA Therapy. Int. J. Mol. Sci. 2025, 26, 10542. https://doi.org/10.3390/ijms262110542
Gao X, Wu Z. IRES-Mediated Translation: Expanding the Toolkits of RNA Therapy. International Journal of Molecular Sciences. 2025; 26(21):10542. https://doi.org/10.3390/ijms262110542
Chicago/Turabian StyleGao, Xiang, and Zhenfang Wu. 2025. "IRES-Mediated Translation: Expanding the Toolkits of RNA Therapy" International Journal of Molecular Sciences 26, no. 21: 10542. https://doi.org/10.3390/ijms262110542
APA StyleGao, X., & Wu, Z. (2025). IRES-Mediated Translation: Expanding the Toolkits of RNA Therapy. International Journal of Molecular Sciences, 26(21), 10542. https://doi.org/10.3390/ijms262110542





