Synthesis and Properties of a Novel Four-Coordinate 8-Hdroxy-Quinolate-Based Complex
Abstract
1. Introduction
2. Results
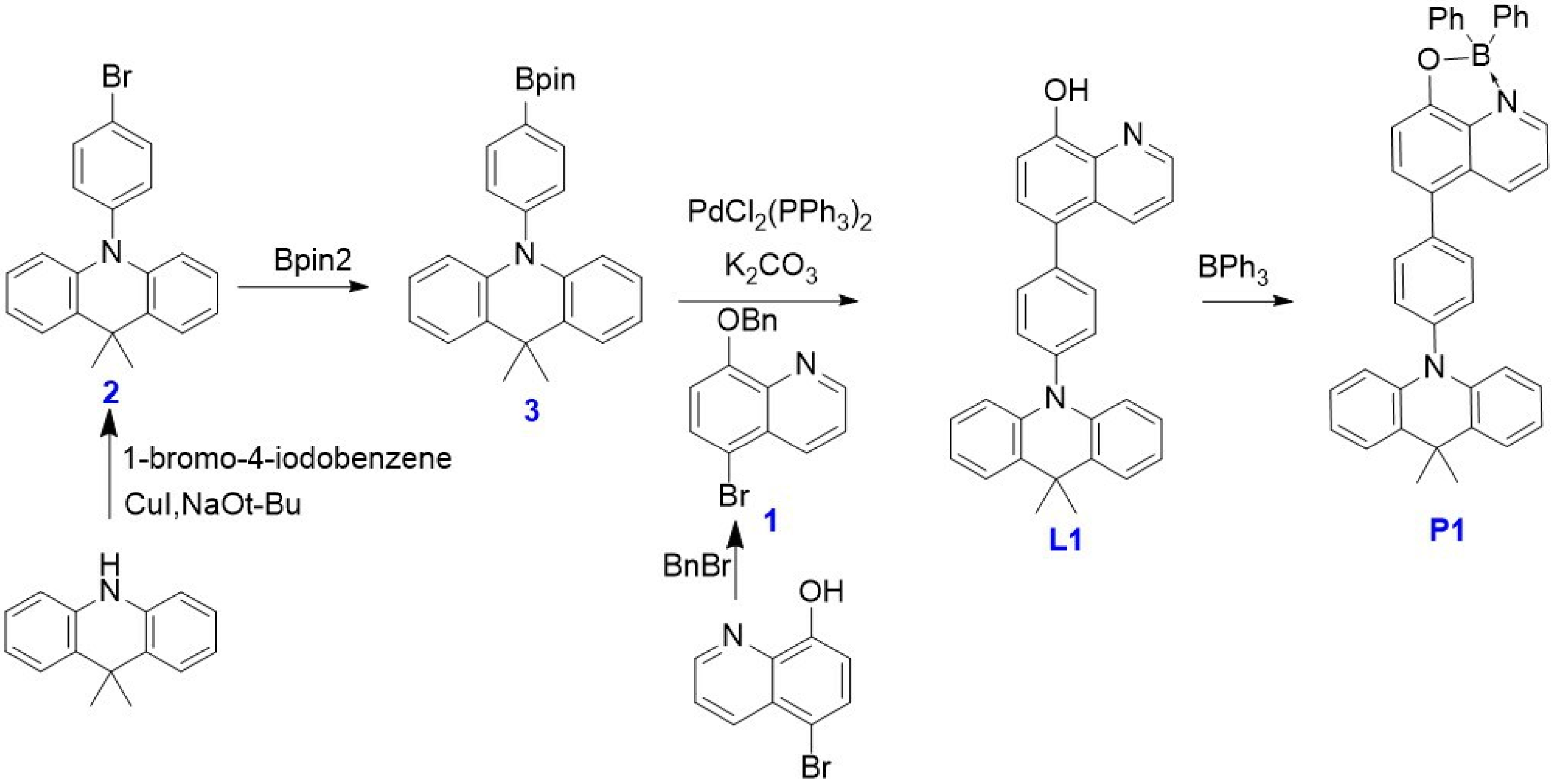
2.1. Design and Synthesis of the Four-Coordinate Emitter
2.2. Photophysical Properties
2.3. Theoretical Calculation
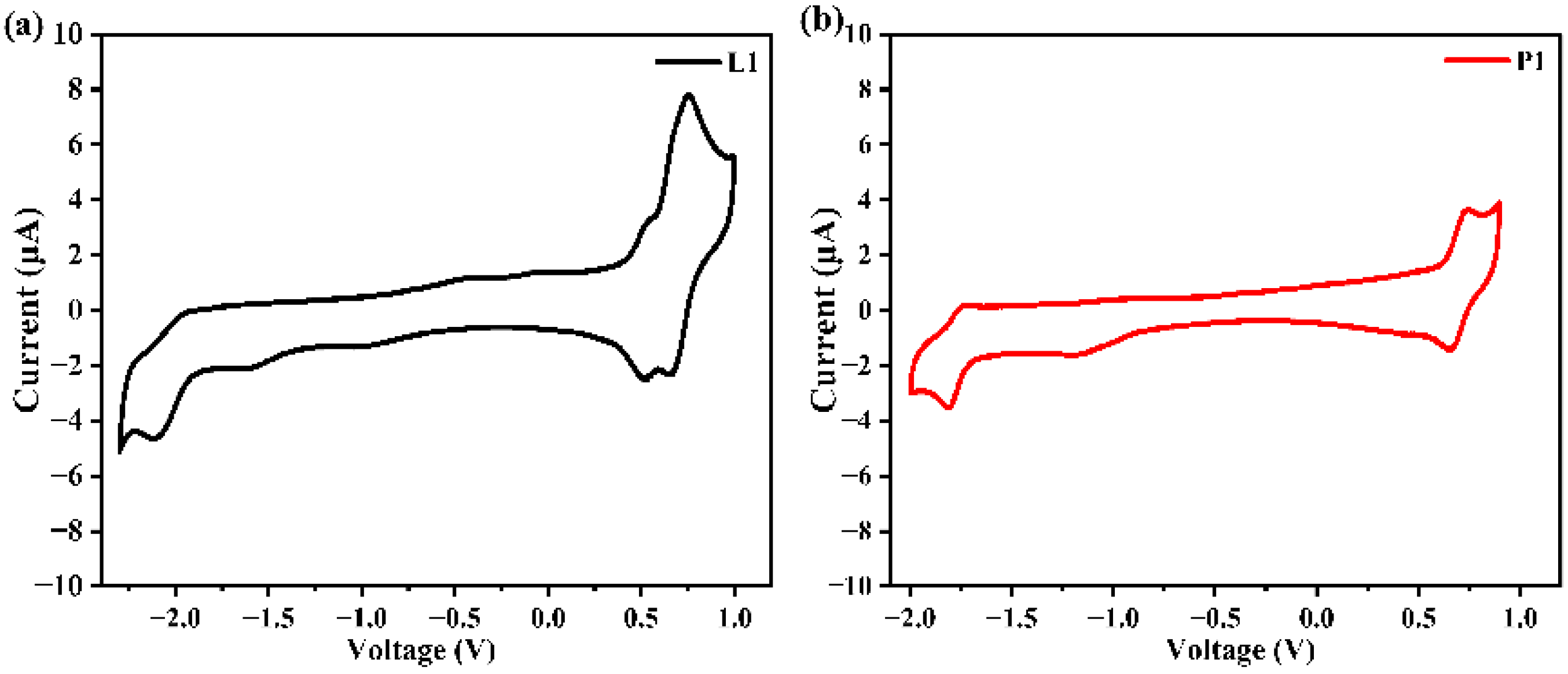
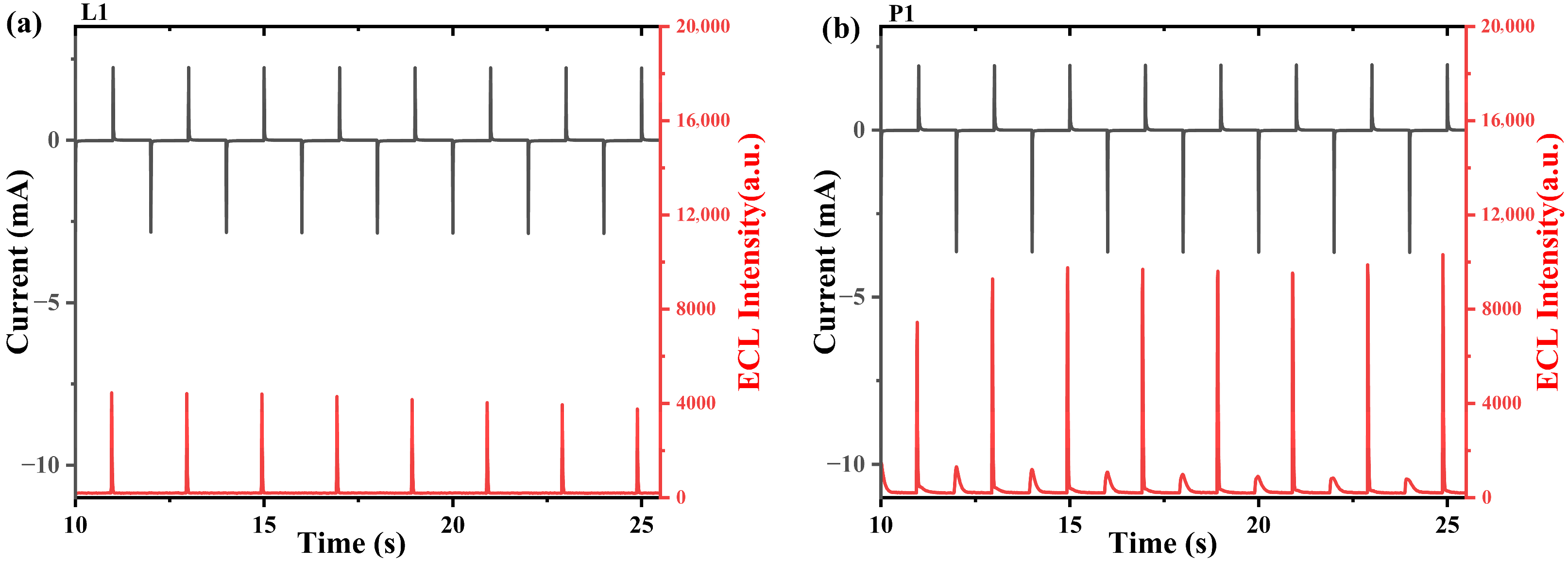
2.4. Electrochemistry and Annihilation ECL
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Synthesis Process
3.3. Calculations
3.4. Electrochemistry and ECL Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hesari, M.; Ding, Z. Review—Electrogenerated Chemiluminescence: Light Years Ahead. J. Electrochem. Soc. 2016, 163, H3116. [Google Scholar] [CrossRef]
- Dong, J.; Feng, J. Electrochemiluminescence from Single Molecule to Imaging. Anal. Chem. 2023, 95, 374–387. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Liu, X.; Wang, X. A review of innovative electrochemical strategies for bioactive molecule detection and cell imaging: Current advances and challenges. Anal. Chim. Acta 2024, 1285, 341920. [Google Scholar] [CrossRef]
- Shen, K.-Y.; Zhan, J.; Shen, L.; Xiong, Z.; Zhu, H.-T.; Wang, A.-J.; Yuan, P.-X.; Feng, J.-J. Hydrogen Bond Organic Frameworks as Radical Reactors for Enhancement in ECL Efficiency and Their Ultrasensitive Biosensing. Anal. Chem. 2023, 95, 4735–4743. [Google Scholar] [CrossRef]
- Bruno, J.G.; Kiel, J.L. Use of magnetic beads in selection and detection of biotoxin aptamers by electrochemiluminescence and enzymatic methods. Biotechniques 2002, 32, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Kijek, T.M.; Rossi, C.A.; Moss, D.; Parker, R.W.; Henchal, E.A. Rapid and sensitive immunomagnetic-electrochemiluminescent detection of staphyloccocal enterotoxin B. J. Immunol. Methods 2000, 236, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Shelton Daniel, R.; Karns Jeffrey, S. Quantitative Detection of Escherichia coli O157 in Surface Waters by Using Immunomagnetic Electrochemiluminescence. Appl. Environ. Microbiol. 2001, 67, 2908–2915. [Google Scholar] [CrossRef]
- Kerr, E.; Doeven, E.H.; Barbante, G.J.; Hogan, C.F.; Hayne, D.J.; Donnelly, P.S.; Francis, P.S. New perspectives on the annihilation electrogenerated chemiluminescence of mixed metal complexes in solution. Chem. Sci. 2016, 7, 5271–5279. [Google Scholar] [CrossRef]
- Fernandez-Hernandez, J.M.; Longhi, E.; Cysewski, R.; Polo, F.; Josel, H.-P.; De Cola, L. Photophysics and Electrochemiluminescence of Bright Cyclometalated Ir(III) Complexes in Aqueous Solutions. Anal. Chem. 2016, 88, 4174–4178. [Google Scholar] [CrossRef]
- Wong, J.M.; Zhang, R.; Xie, P.; Yang, L.; Zhang, M.; Zhou, R.; Wang, R.; Shen, Y.; Yang, B.; Wang, H.-B.; et al. Revealing Crystallization-Induced Blue-Shift Emission of a Di-Boron Complex by Enhanced Photoluminescence and Electrochemiluminescence. Angew. Chem. Int. Ed. 2020, 59, 17461–17466. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yang, L.; Wang, R.; Wisner, J.A.; Ding, Z.; Wang, H.-B. Intensified electrochemiluminescence and photoluminescence via supramolecular anion recognition interactions. Chem. Sci. 2024, 15, 12291–12300. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, J.; Wang, H. Synthesis and Electrochemiluminescence of a Di-Boron Thermally Activated Delayed Fluorescence Emitter. Molecules 2025, 30, 1718. [Google Scholar] [CrossRef]
- Hesari, M.; Ding, Z. A Grand Avenue to Au Nanocluster Electrochemiluminescence. Anal. Chim. Acta 2017, 50, 218–230. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, Z.; Xu, J.; Han, F.; Zhao, X.; Han, D.; Liu, Y.; Kang, Z.; Niu, L. Carbon Nitride Quantum Dots Enhancing the Anodic Electrochemiluminescence of Ruthenium(II) Tris(2,2′-bipyridyl) via Inhibiting the Oxygen Evolution Reaction. Anal. Chem. 2020, 92, 15352–15360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zeng, C.; Zhao, Y.; Ma, J.; Yao, X.; Huo, S.; Feng, Y.; Wang, M.; Lu, X. Precise Modulation of Intramolecular Aggregation-induced Electrochemiluminescence by Tetraphenylethylene-based Supramolecular Architectures. Angew. Chem. Int. Ed. 2023, 62, e202312692. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- Li, X.; Zhang, G.; Song, Q. Recent advances in the construction of tetracoordinate boron compounds. Chem. Commun. 2023, 59, 3812–3820. [Google Scholar] [CrossRef]
- Mellerup, S.K.; Wang, S. Boron-based stimuli responsive materials. Chem. Soc. Rev. 2019, 48, 3537–3549, Correction in Chem. Soc. Rev. 2019, 48, 3674. https://doi.org/10.1039/C9CS90043H. [Google Scholar] [CrossRef]
- Wakamiya, A.; Taniguchi, T.; Yamaguchi, S. Intramolecular B–N Coordination as a Scaffold for Electron-Transporting Materials: Synthesis and Properties of Boryl-Substituted Thienylthiazoles. Angew. Chem. Int. Ed. 2006, 45, 3170–3173. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.X.; Horioka, A.; Ishimatsu, R.; Mamada, M.; Adachi, C.; Tahara, K.; Hoshino, Y.; Ono, T. Advanced Molecular Design for Efficient Multicolor Electrochemiluminescence and Amplified Spontaneous Emission Based on Tetra-BF2 Complexes. Adv. Opt. Mater. 2024, 12, 2302803. [Google Scholar] [CrossRef]
- Hu, D.; Huang, R.; Fang, Y. Recent Advances in Tetra-Coordinate Boron-Based Photoactive Molecules for Luminescent Sensing, Imaging, and Anticounterfeiting. Precis. Chem. 2025, 3, 10–26. [Google Scholar] [CrossRef]
- Jin, C.; Yang, X.; Zhao, W.; Zhao, Y.; Wang, Z.; Tan, J. Synthesis, properties and emerging applications of multi-boron coordinated chromophores. Coord. Chem. Rev. 2024, 513, 215892. [Google Scholar] [CrossRef]
- Anthony, J.E. The Larger Acenes: Versatile Organic Semiconductors. Angew. Chem. Int. Ed. 2008, 47, 452–483. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, L.; Jiang, Y.; Li, C.; Gu, H.; Zhao, K.; Zhang, J.; Meng, H.; Ren, Y. Hyperconjugation Engineering of π-Extended Azaphosphinines for Designing Tunable Thermally Activated Delayed Fluorescence Emitters. J. Am. Chem. Soc. 2025, 147, 3650–3661. [Google Scholar] [CrossRef]
- Fukazawa, A.; Yamada, H.; Yamaguchi, S. Phosphonium- and borate-bridged zwitterionic ladder stilbene and its extended analogues. Angew. Chem. (Int. Ed. Engl.) 2008, 47, 5582–5585. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Wang, Y. Four-coordinate organoboron compounds for organic light-emitting diodes (OLEDs). Chem. Soc. Rev. 2013, 42, 8416–8433. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, S.L.; Ugolotti, J.; Britovsek, G.J.P.; Jones, T.S.; White, A.J.P. The effect of fluorination on the luminescent behaviour of 8-hydroxyquinoline boron compounds. New J. Chem. 2008, 32, 1379–1387. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Yuan, J.; Ye, Y.; He, Y.; Huang, B.; Gao, X. 8-Hydroxy Quinoline Derivatives as Auxiliary Ligands for Red-Emitting Cyclic-Platinum Phosphorescent Complexes: Synthesis and Properties. Helv. Chim. Acta 2017, 100, e1600308. [Google Scholar] [CrossRef]
- Rao, Y.L.; Wang, S. Four-coordinate organoboron compounds with a π-conjugated chelate ligand for optoelectronic applications. Inorg. Chem. 2011, 50, 12263–12274. [Google Scholar] [CrossRef] [PubMed]
- Pohl, R.; Montes, V.A.; Shinar, J.; Anzenbacher, P. Red−Green−Blue Emission from Tris(5-aryl-8-quinolinolate)Al(III) Complexes. J. Org. Chem. 2004, 69, 1723–1725. [Google Scholar] [CrossRef]
- Park, H.-J.; Han, S.H.; Lee, J.Y. Molecular Design of Thermally Activated Delayed-Fluorescent Emitters Using 2,2′-Bipyrimidine as the Acceptor in Donor–Acceptor Structures. Chem.—Asian J. 2017, 12, 2494–2500. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wu, L.-Y.; Cai, J.-H.; Li, G.-R.; Gan, N.-N.; Wang, Z.-H. Room-temperature borylation and one-pot two-step borylation/Suzuki–Miyaura cross-coupling reaction of aryl chlorides. RSC Adv. 2018, 8, 13643–13648. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhan, F.; Lou, W.; Wang, D.; Deng, C.; Cao, L.; Yang, Y.; Zhang, Q.; She, Y. Efficient deep-blue organic light-emitting diodes employing difluoroboron-enabled thermally activated delayed fluorescence emitters. J. Mater. Chem. C 2020, 8, 17464–17473. [Google Scholar] [CrossRef]
- Carrara, S.; Aliprandi, A.; Hogan, C.F.; De Cola, L. Aggregation-Induced Electrochemiluminescence of Platinum(II) Complexes. J. Am. Chem. Soc. 2017, 139, 14605–14610. [Google Scholar] [CrossRef] [PubMed]
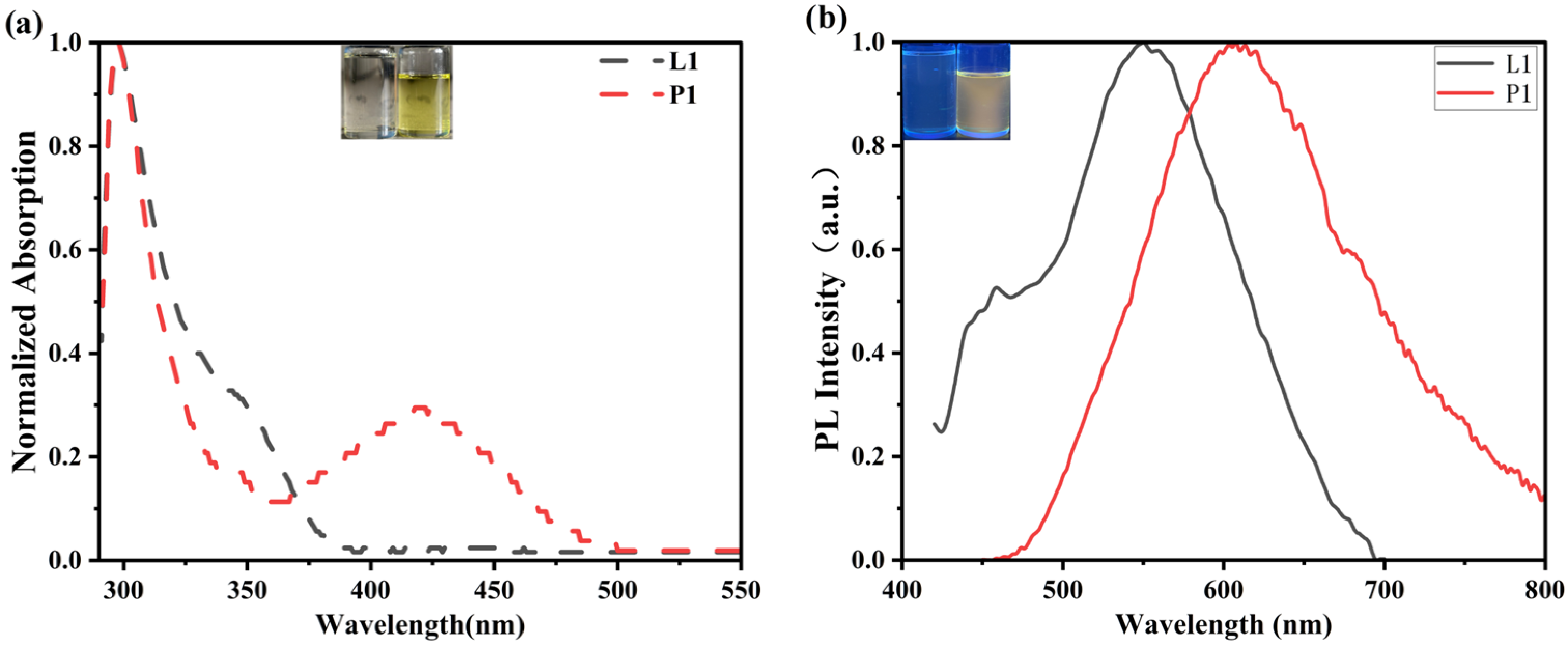

| Compounds | λabs [nm] | λFL [nm] | ΦPL 1 [%] | ΦECL 2 [%] | ΔEg 3 [eV] |
|---|---|---|---|---|---|
| L1 | 298 | 553 | 0.24% | / | 3.26 |
| P1 | 298, 420 | 608 | 3.4% | <0.2% | 2.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Zhang, Z.; Wang, R.; Wang, H. Synthesis and Properties of a Novel Four-Coordinate 8-Hdroxy-Quinolate-Based Complex. Int. J. Mol. Sci. 2025, 26, 10528. https://doi.org/10.3390/ijms262110528
Cheng J, Zhang Z, Wang R, Wang H. Synthesis and Properties of a Novel Four-Coordinate 8-Hdroxy-Quinolate-Based Complex. International Journal of Molecular Sciences. 2025; 26(21):10528. https://doi.org/10.3390/ijms262110528
Chicago/Turabian StyleCheng, Jun, Zilong Zhang, Ruiyao Wang, and Hongbo Wang. 2025. "Synthesis and Properties of a Novel Four-Coordinate 8-Hdroxy-Quinolate-Based Complex" International Journal of Molecular Sciences 26, no. 21: 10528. https://doi.org/10.3390/ijms262110528
APA StyleCheng, J., Zhang, Z., Wang, R., & Wang, H. (2025). Synthesis and Properties of a Novel Four-Coordinate 8-Hdroxy-Quinolate-Based Complex. International Journal of Molecular Sciences, 26(21), 10528. https://doi.org/10.3390/ijms262110528






