Liquid Biopsy Biomarkers for Cervical Cancer: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Protocol and Register
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
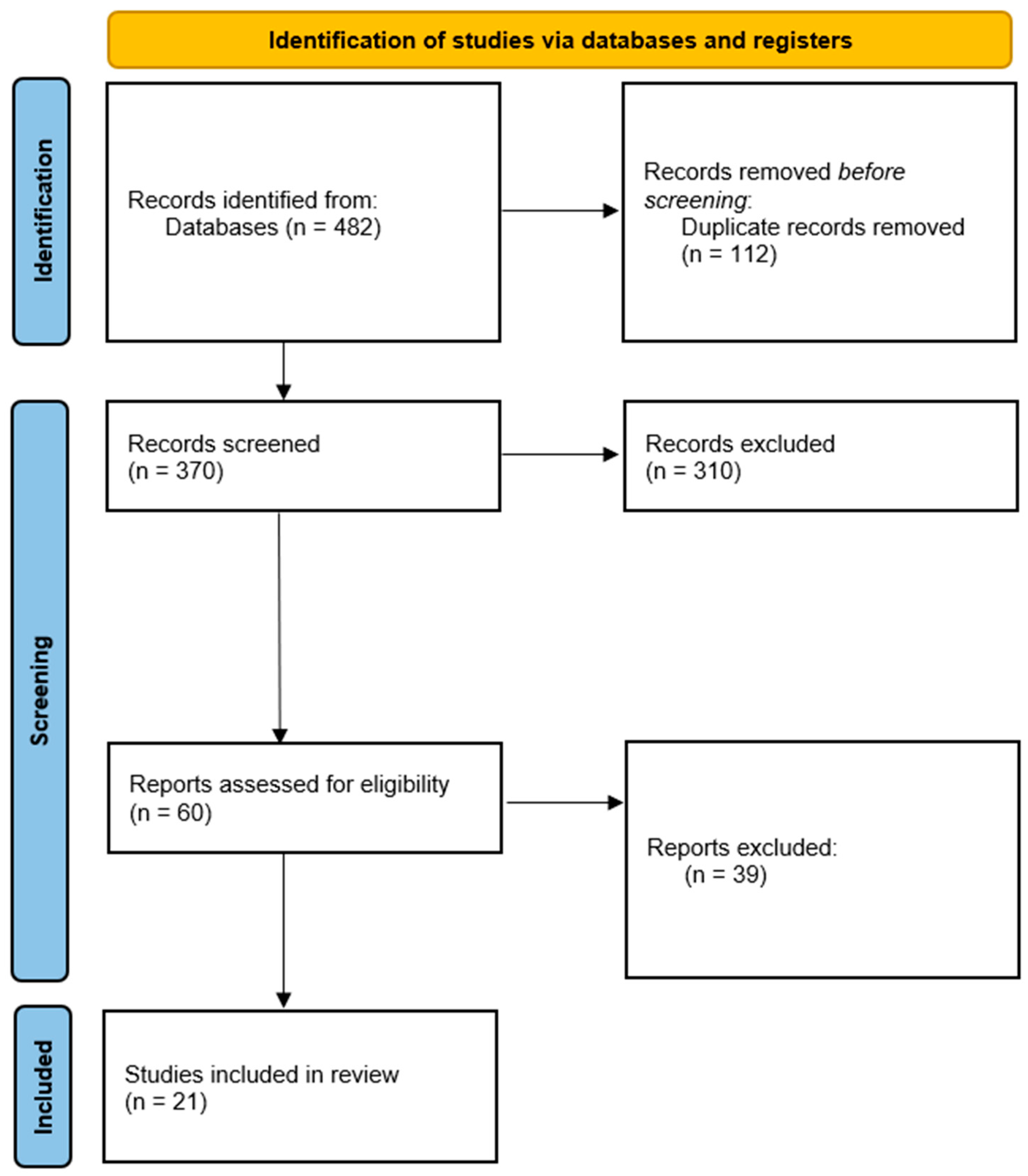
2.4. Study Selection
2.5. Data Extraction Process
2.6. Risk of Bias Assessment
2.7. Synthesis Methods
3. Results and Discussion
3.1. General Characteristics of the Studies
3.2. Main Findings by Biomarker
3.3. Narrative and Quantitative Synthesis
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| cfHPV-DNA | Cell-free HPV DNA |
| miRNAs | microRNAs |
| ddPCR | droplet digital PCR |
| RT-qPCR | Reverse Transcription quantitative PCR |
| NGS | Next-Generation Sequencing |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Wang, H.; Qin, R.; Han, Z.; Li, R. Global cervical cancer elimination: Quantifying the status, progress, and gaps. BMC Med. 2025, 23, 67. [Google Scholar] [CrossRef]
- Cruz-Valdez, A.; Palacio-Mejia, L.S.; Quezada-Sanchez, A.D.; Hernandez-Avila, J.E.; Galicia-Carmona, T.; Cetina-Perez, L.D.C.; Arango-Bravo, E.A.; Isla-Ortiz, D.; Aranda-Flores, C.E.; Uscanga-Sanchez, S.R.; et al. Cervical cancer prevention program in Mexico disrupted due to COVID-19 pandemic: Challenges and opportunities. Front. Oncol. 2023, 13, 1008560. [Google Scholar] [CrossRef]
- Garrido, F.; Wild, C.M.; Mittelberger, J.; Dobler, F.; Schneider, M.; Ansorge, N.; Kopke, M.; Strieder, A.; Ditsch, N.; Jeschke, U.; et al. The Role of Chemokines in Cervical Cancers. Medicina 2021, 57, 1141. [Google Scholar] [CrossRef]
- Millan-Catalan, O.; Perez-Yepez, E.A.; Martinez-Gutierrez, A.D.; Rodriguez-Morales, M.; Lopez-Urrutia, E.; Coronel-Martinez, J.; Cantu de Leon, D.; Jacobo-Herrera, N.; Peralta-Zaragoza, O.; Lopez-Camarillo, C.; et al. A microRNA Profile Regulates Inflammation-Related Signaling Pathways in Young Women with Locally Advanced Cervical Cancer. Cells 2024, 13, 896. [Google Scholar] [CrossRef]
- Jimenez-Wences, H.; Martinez-Carrillo, D.N.; Peralta-Zaragoza, O.; Campos-Viguri, G.E.; Hernandez-Sotelo, D.; Jimenez-Lopez, M.A.; Munoz-Camacho, J.G.; Garzon-Barrientos, V.H.; Illades-Aguiar, B.; Fernandez-Tilapa, G. Methylation and expression of miRNAs in precancerous lesions and cervical cancer with HPV16 infection. Oncol. Rep. 2016, 35, 2297–2305. [Google Scholar] [CrossRef]
- Agorastos, T.; Chatzistamatiou, K.; Katsamagkas, T.; Koliopoulos, G.; Daponte, A.; Constantinidis, T.; Constantinidis, T.C.; HERMES Study Group. Primary screening for cervical cancer based on high-risk human papillomavirus (HPV) detection and HPV 16 and HPV 18 genotyping, in comparison to cytology. PLoS ONE 2015, 10, e0119755. [Google Scholar] [CrossRef]
- Okunade, K.S.; Adejimi, A.A.; John-Olabode, S.O.; Oshodi, Y.A.; Oluwole, A.A. An Overview of HPV Screening Tests to Improve Access to Cervical Cancer Screening Among Underserved Populations: From Development to Implementation. Risk Manag. Healthc. Policy 2022, 15, 1823–1830. [Google Scholar] [CrossRef] [PubMed]
- Zarankiewicz, N.; Zielińska, M.; Kosz, K.; Kuchnicka, A.; Ciseł, B. High-risk HPV test in cervical cancer prevention—Present and future. J. Pre-Clin. Clin. Res. 2020, 14, 80–84. [Google Scholar] [CrossRef]
- Mittelstadt, S.; Kelemen, O.; Admard, J.; Gschwind, A.; Koch, A.; Worz, S.; Oberlechner, E.; Engler, T.; Bonzheim, I.; Staebler, A.; et al. Detection of circulating cell-free HPV DNA of 13 HPV types for patients with cervical cancer as potential biomarker to monitor therapy response and to detect relapse. Br. J. Cancer 2023, 128, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Poinho, M.; Dias, L.; Pinheiro, L.S.; Gomes, F.N.O.; Rondon, H.; de Oliveira, M.P.; Souza, J.S.; Figueiredo, H.F.; Lira, D.L.; Levi, J.E.; et al. The Role of cf-HPV DNA as an Innovative Biomarker for Predicting the Recurrence or Persistence of Cervical Cancer. Viruses 2025, 17, 409. [Google Scholar] [CrossRef]
- Vitkauskaite, A.; Urboniene, D.; Celiesiute, J.; Jariene, K.; Skrodeniene, E.; Nadisauskiene, R.J.; Vaitkiene, D. Circulating inflammatory markers in cervical cancer patients and healthy controls. J. Immunotoxicol. 2020, 17, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Kepsha, M.A.; Timofeeva, A.V.; Chernyshev, V.S.; Silachev, D.N.; Mezhevitinova, E.A.; Sukhikh, G.T. MicroRNA-Based Liquid Biopsy for Cervical Cancer Diagnostics and Treatment Monitoring. Int. J. Mol. Sci. 2024, 25, 13271. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Nishio, E.; Tsukamoto, T.; Kukimoto, I.; Iwata, A. Performance of an ancillary test for cervical cancer that measures miRNAs and cytokines in serum and cervical mucus. Cancer Sci. 2024, 115, 2795–2807. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, M.I.; Cardona, Y.T.; Agudelo, M.C.; Lopez, C.; Florez-Acosta, J.J.; Agudelo-Gamboa, S.; Garai, J.; Li, L.; Orozco-Castano, C.A.; Zabaleta, J.; et al. miRNAs signature as potential biomarkers for cervical precancerous lesions in human papillomavirus positive women. Sci. Rep. 2023, 13, 9822. [Google Scholar] [CrossRef] [PubMed]
- Aranda Flores, C.E.; Gomez Gutierrez, G.; Ortiz Leon, J.M.; Cruz Rodriguez, D.; Sorbye, S.W. Self-collected versus clinician-collected cervical samples for the detection of HPV infections by 14-type DNA and 7-type mRNA tests. BMC Infect. Dis. 2021, 21, 504. [Google Scholar] [CrossRef]
- Ramirez, A.T.; Valls, J.; Baena, A.; Rojas, F.D.; Ramirez, K.; Alvarez, R.; Cristaldo, C.; Henriquez, O.; Moreno, A.; Reynaga, D.C.; et al. Performance of cervical cytology and HPV testing for primary cervical cancer screening in Latin America: An analysis within the ESTAMPA study. Lancet Reg. Health Am. 2023, 26, 100593. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, version 6.4 (Updated August 2023); Cochrane: London, UK, 2023.
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in nonrandomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomized trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomized or nonrandomized studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; the PRISMA-DTA Group. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef]
- Ruiz Esparza Garrido, R.; Gutierrez, M.; Angel Velazquez Flores, M. Circulating cervical cancer biomarkers potentially useful in medical attention (Review). Mol. Clin. Oncol. 2023, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Zubillaga-Guerrero, M.I.; Illades-Aguiar, B.; Flores-Alfaro, E.; Castro-Coronel, Y.; Jimenez-Wences, H.; Patino, E.I.L.; Perez, K.I.G.; Del Carmen Alarcon-Romero, L. An increase of microRNA-16-1 is associated with the high proliferation of squamous intraepithelial lesions in the presence of the integrated state of HR-HPV in liquid cytology samples. Oncol. Lett. 2020, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Bonin-Jacob, C.M.; Almeida-Lugo, L.Z.; Puga, M.A.M.; Machado, A.P.; Padovani, C.T.J.; Noceti, M.C.; Ferreira, A.M.T.; Fernandes, C.; Resende, J.C.P.; Bovo, A.C.; et al. IL-6 and IL-10 in the serum and exfoliated cervical cells of patients infected with high-risk human papillomavirus. PLoS ONE 2021, 16, e0248639. [Google Scholar] [CrossRef]
- Ma, G.; Song, G.; Zou, X.; Shan, X.; Liu, Q.; Xia, T.; Zhou, X.; Zhu, W. Circulating plasma microRNA signature for the diagnosis of cervical cancer. Cancer Biomark. 2019, 26, 491–500. [Google Scholar] [CrossRef]
- Du, S.; Zhao, Y.; Lv, C.; Wei, M.; Gao, Z.; Meng, X. Applying Serum Proteins and MicroRNA as Novel Biomarkers for Early-Stage Cervical Cancer Detection. Sci. Rep. 2020, 10, 9033. [Google Scholar] [CrossRef]
- You, W.; Wang, Y.; Zheng, J. Plasma miR-127 and miR-218 Might Serve as Potential Biomarkers for Cervical Cancer. Reprod. Sci. 2015, 22, 1037–1041. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Wang, F.; Xu, D.; Guo, Y.; Cui, W. Serum miRNAs panel (miR-16-2*, miR-195, miR-2861, miR-497) as novel noninvasive biomarkers for detection of cervical cancer. Sci. Rep. 2015, 5, 17942. [Google Scholar] [CrossRef]
- Aftab, M.; Poojary, S.S.; Seshan, V.; Kumar, S.; Agarwal, P.; Tandon, S.; Zutshi, V.; Das, B.C. Urine miRNA signature as a potential noninvasive diagnostic and prognostic biomarker in cervical cancer. Sci. Rep. 2021, 11, 10323. [Google Scholar] [CrossRef]
- Ning, R.; Meng, S.; Wang, L.; Jia, Y.; Tang, F.; Sun, H.; Zhang, Z.; Zhang, C.; Fan, X.; Xiao, B.; et al. 6 Circulating miRNAs can be used as Noninvasive Biomarkers for the Detection of Cervical Lesions. J. Cancer 2021, 12, 5106–5113. [Google Scholar] [CrossRef]
- Jeannot, E.; Becette, V.; Campitelli, M.; Calmejane, M.A.; Lappartient, E.; Ruff, E.; Saada, S.; Holmes, A.; Bellet, D.; Sastre-Garau, X. Circulating human papillomavirus DNA detected using droplet digital PCR in the serum of patients diagnosed with early stage human papillomavirus-associated invasive carcinoma. J. Pathol. Clin. Res. 2016, 2, 201–209. [Google Scholar] [CrossRef]
- Jeannot, E.; Latouche, A.; Bonneau, C.; Calmejane, M.A.; Beaufort, C.; Ruigrok-Ritstier, K.; Bataillon, G.; Larbi Cherif, L.; Dupain, C.; Lecerf, C.; et al. Circulating HPV DNA as a Marker for Early Detection of Relapse in Patients with Cervical Cancer. Clin. Cancer Res. 2021, 27, 5869–5877. [Google Scholar] [CrossRef]
- Sivars, L.; Hellman, K.; Crona Guterstam, Y.; Holzhauser, S.; Nordenskjold, M.; Falconer, H.; Palsdottir, K.; Tham, E. Circulating cell-free tumor human papillomavirus DNA is a promising biomarker in cervical cancer. Gynecol. Oncol. 2022, 167, 107–114. [Google Scholar] [CrossRef]
- Bonlokke, S.; Stougaard, M.; Sorensen, B.S.; Booth, B.B.; Hogdall, E.; Nyvang, G.B.; Lindegaard, J.C.; Blaakaer, J.; Bertelsen, J.; Fuglsang, K.; et al. The Diagnostic Value of Circulating Cell-Free HPV DNA in Plasma from Cervical Cancer Patients. Cells 2022, 11, 2170. [Google Scholar] [CrossRef]
- Bonlokke, S.; Steiniche, T.; Sorensen, B.S.; Nyvang, G.B.; Lindegaard, J.C.; Blaakaer, J.; Bertelsen, J.; Fuglsang, K.; Strube, M.L.; Lenz, S.; et al. Circulating cell-free HPV DNA is a strong marker for disease severity in cervical cancer. Mol. Oncol. 2024, 18, 1231–1244. [Google Scholar] [CrossRef] [PubMed]
- Lalondrelle, S.; Lee, J.; Cutts, R.J.; Garcia Murillas, I.; Matthews, N.; Turner, N.; Harrington, K.; Vroobel, K.; Moretti, E.; Bhide, S.A. Predicting Response to Radical Chemoradiotherapy with Circulating HPV DNA (cHPV-DNA) in Locally Advanced Uterine Cervix Cancer. Cancers 2023, 15, 1387. [Google Scholar] [CrossRef] [PubMed]
- Thangarajah, F.; Busshoff, J.; Salamon, J.; Pruss, M.S.; Lenz, C.; Morgenstern, B.; Hellmich, M.; Schlosser, H.A.; Lenz, M.; Domrose, C.; et al. Digital droplet PCR-based quantification of ccfHPV-DNA as liquid biopsy in HPV-driven cervical and vulvar cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 12597–12604. [Google Scholar] [CrossRef] [PubMed]
- Beaussire-Trouvay, L.; Duhamel, O.; Perdrix, A.; Leveque, E.; Vion, R.; Rovelet-Lecrux, A.; Sarafan-Vasseur, N.; Di Fiore, F.; Crouzet, A.; Leheurteur, M.; et al. Prognostic value of HPV circulating tumor DNA detection and quantification in locally advanced cervical cancer. Front. Oncol. 2024, 14, 1382008. [Google Scholar] [CrossRef]
- Cai, C.; Peng, X.; Zhang, Y. Serum IL-6 Level Predicts the Prognosis and Diagnosis in Cervical Cancer Patients. Int. J. Womens Health 2022, 14, 655–663. [Google Scholar] [CrossRef]
- Vitkauskaite, A.; Urboniene, D.; Celiesiute, J.; Jariene, K.; Paskauskas, S.; Vaitkiene, D.; Vitkauskiene, A. Expression of Inflammation Depending on the Stage of Cervical Cancer. Medicina 2024, 60, 349. [Google Scholar] [CrossRef] [PubMed]
- Domenici, L.; Tonacci, A.; Aretini, P.; Garibaldi, S.; Perutelli, A.; Bottone, P.; Muzii, L.; Benedetti Panici, P. Inflammatory Biomarkers as Promising Predictors of Prognosis in Cervical Cancer Patients. Oncology 2021, 99, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, N.P.G.; Gally, T.B.; Borges, G.F.; Campos, L.C.G.; Kaneto, C.M. Systematic review of circulating MICRORNAS as biomarkers of cervical carcinogenesis. BMC Cancer 2022, 22, 862. [Google Scholar] [CrossRef] [PubMed]
| # | Author (Year) | Country | Design | n | Sample | Biomarker | Platform | Main Findings | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ma et al. (2019) | China | Prospective | 184 | Plasma | miRNAs | RT-qPCR | A 5-miRNA signature discriminates against CC vs. controls | [28] |
| 2 | Du et al. (2020) | China | Prospective | 260 | Serum | Proteins + miRNAs | RT-qPCR/ELISA | Combined panel detects early CC | [29] |
| 3 | Zubillaga-Guerrero et al. (2020) | Mexico | Prospective | 80 | Liquid cytology | miR-16-1 | RT-qPCR | Overexpression of miR-16-1 is associated with HR-HPV integration. | [26] |
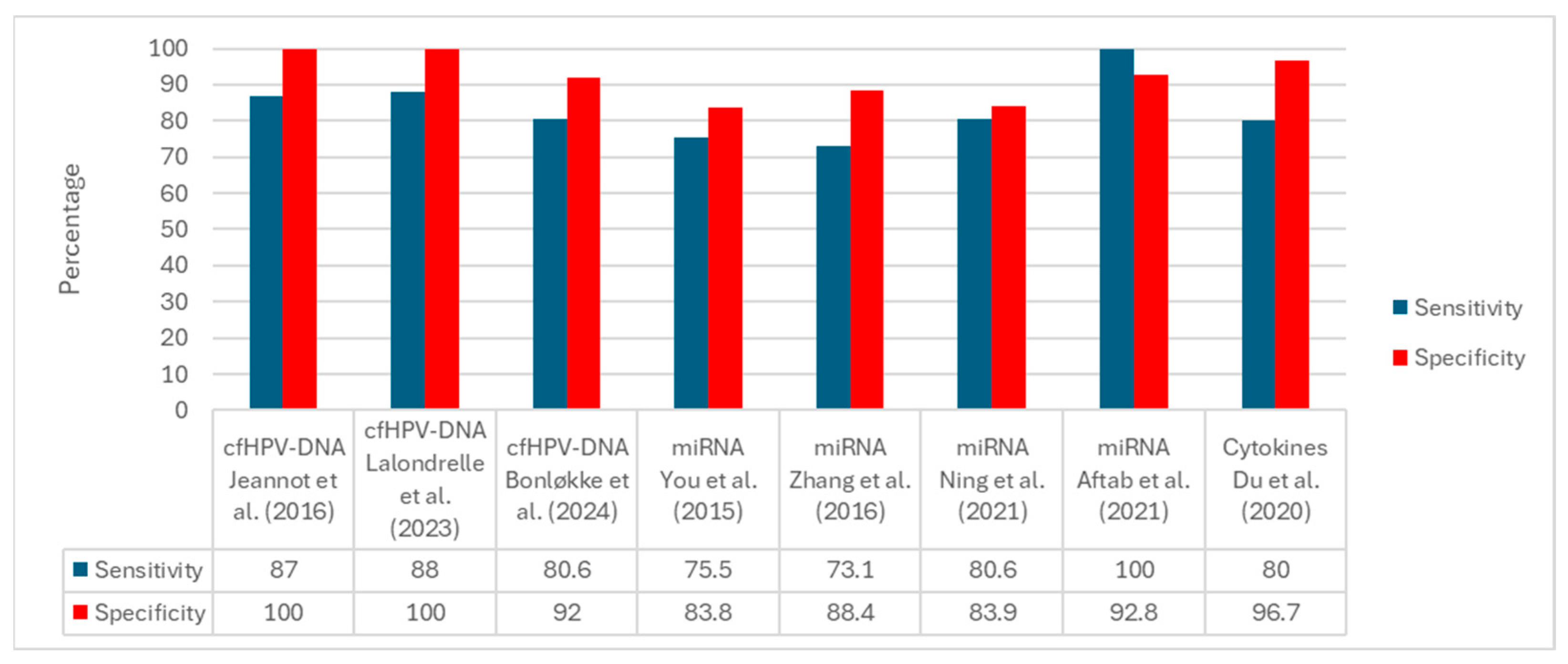
| 4 | You et al. (2015) | China | Prospective | 68 | Plasma | miRNAs | RT-qPCR | miR-127 and miR-205 show diagnostic utility | [30] |
| 5 | Zhang et al. (2016) | China | Prospective | 563 | Serum | miRNAs | RT-qPCR | Noninvasive serum panel for diagnosis | [31] |
| 6 | Aftab et al. (2021) | Pakistan | Prospective | 100 | Urine | miRNAs | RT-qPCR | The urinary miRNA profile is a valuable noninvasive test. | [32] |
| 7 | Ning et al. (2021) | China | Prospective | 380 | Plasma | miRNAs | RT-qPCR | Six-miRNA panel identifies high-grade lesions. | [33] |
| 8 | Poinho et al. (2025) | Brazil | Prospective | 39 | Plasma | cfHPV-DNA | qPCR | Circulating cfHPV-DNA is associated with diagnosis and recurrence in CC. | [11] |
| 9 | Jeannot et al. (2016) | France | Retrospective | 70 | Serum | cfHPV-DNA | ddPCR | Circulating HPV DNA was detected in early-stage CC. | [34] |
| 10 | Jeannot et al. (2021) | France | Prospective cohort | 94 | Plasma | cfHPV-DNA | ddPCR | Post-treatment persistence predicts recurrence. | [35] |
| 11 | Sivars et al. (2022) | Sweden | Prospective cohort | 54 | Plasma | cfHPV-DNA | ddPCR | High levels correlate with worse survival. | [36] |
| 12 | Bonløkke et al. (2022) | Denmark | Retrospective | 60 | Plasma | cfHPV-DNA | ddPCR | cfHPV-DNA discriminates patients with advanced CC. | [37] |
| 13 | Bonløkke et al. (2024) | Denmark | Prospective cohort | 179 | Plasma | cfHPV-DNA | ddPCR + NGS | cfHPV-DNA predicts which patients require primary oncologic therapy. | [38] |
| 14 | Mittelstadt et al. (2023) | Germany | Prospective cohort | 69 | Plasma | cfHPV-DNA | NGS | cfHPV-DNA correlates with stage and recurrence. | [10] |
| 15 | Lalondrelle et al. (2023) | UK | Prospective | 22 | Plasma | cfHPV-DNA | NGS | cfHPV-DNA as an early predictor of CRT response. | [39] |
| 16 | Thangarajah et al. (2023) | Germany | Prospective | 19 | Plasma | cfHPV-DNA | ddPCR | Quantification of cfHPV-DNA is useful for monitoring. | [40] |
| 17 | Beaussire-Trouvay et al. (2024) | France | Prospective | 97 | Plasma | cfHPV-DNA | ddPCR | Prognostic value in locally advanced CC. | [41] |
| 18 | Bonin-Jacob et al. (2021) | Brazil | Prospective | 410 | Serum | Cytokines | ELISA | IL-6 and IL-10 are elevated in advanced stages. | [27] |
| 19 | Cai et al. (2022) | China | Prospective cohort | 682 | Serum | Cytokines | ELISA | Serum IL-6 predicts poor prognosis. | [42] |
| 20 | Vitkauskaite et al. (2024) | Lithuania | Cross-sectional | 182 | Serum | Cytokines | Multiplex | Cytokines are associated with reduced survival. | [43] |
| 21 | Domenici et al. (2021) | Italy | Prospective cohort | 159 | Whole blood-Tissue | Cytokines | IHC | Overexpression of IL-6 is associated with a poor prognosis. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pineda-Migranas, J.A.; Bravata-Alcántara, J.C.; Cortés-Ortíz, I.A.; Cortés-Malagón, E.M.; de los Ángeles Romero-Tlalolini, M.; Sierra-Martínez, M.; Acosta-Altamirano, G. Liquid Biopsy Biomarkers for Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 10503. https://doi.org/10.3390/ijms262110503
Pineda-Migranas JA, Bravata-Alcántara JC, Cortés-Ortíz IA, Cortés-Malagón EM, de los Ángeles Romero-Tlalolini M, Sierra-Martínez M, Acosta-Altamirano G. Liquid Biopsy Biomarkers for Cervical Cancer: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(21):10503. https://doi.org/10.3390/ijms262110503
Chicago/Turabian StylePineda-Migranas, Jesús Alejandro, Juan Carlos Bravata-Alcántara, Iliana Alejandra Cortés-Ortíz, Enoc Mariano Cortés-Malagón, María de los Ángeles Romero-Tlalolini, Mónica Sierra-Martínez, and Gustavo Acosta-Altamirano. 2025. "Liquid Biopsy Biomarkers for Cervical Cancer: A Systematic Review" International Journal of Molecular Sciences 26, no. 21: 10503. https://doi.org/10.3390/ijms262110503
APA StylePineda-Migranas, J. A., Bravata-Alcántara, J. C., Cortés-Ortíz, I. A., Cortés-Malagón, E. M., de los Ángeles Romero-Tlalolini, M., Sierra-Martínez, M., & Acosta-Altamirano, G. (2025). Liquid Biopsy Biomarkers for Cervical Cancer: A Systematic Review. International Journal of Molecular Sciences, 26(21), 10503. https://doi.org/10.3390/ijms262110503






