Prokaryotic Expression and Binding Characteristics of Odor-Binding Protein GqinOBP10 in Gynaephora qinghaiensis
Abstract
1. Introduction
2. Results
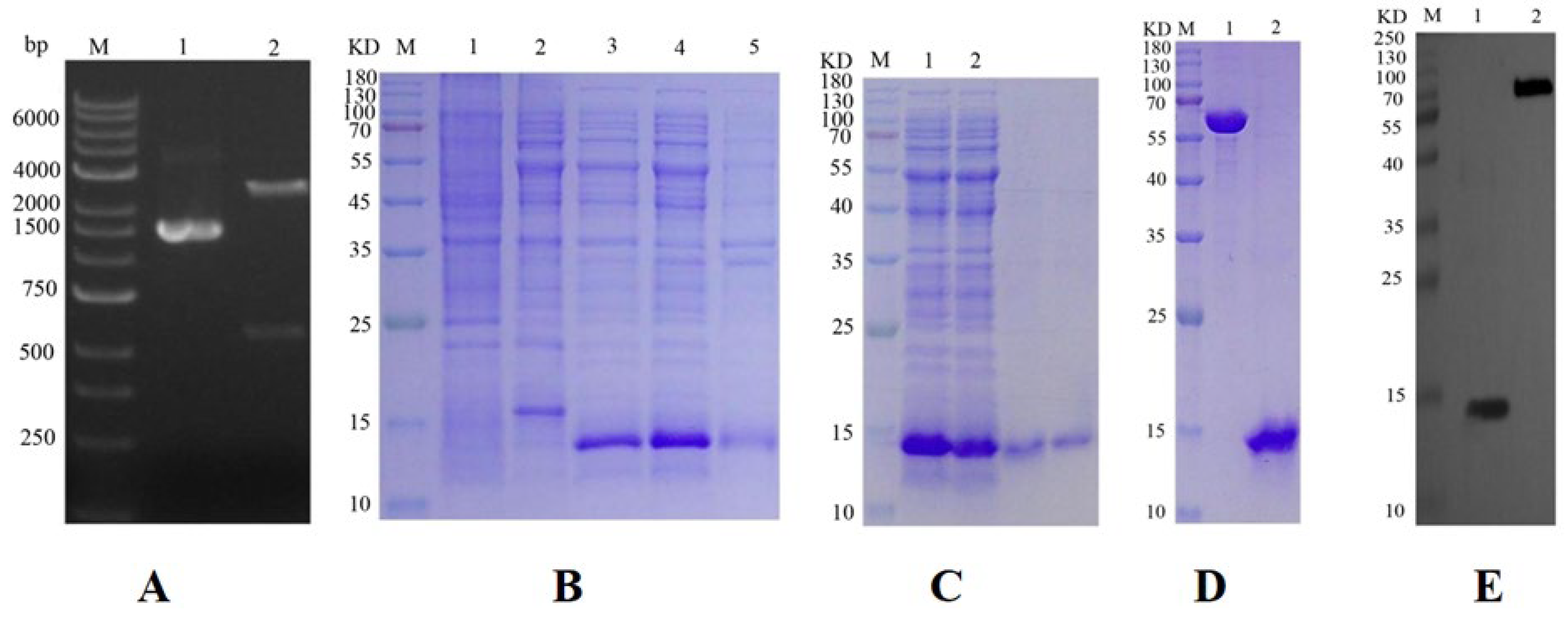
2.1. Construction of Prokaryotic Expression Vector
2.2. Expression and Purification of Target Protein
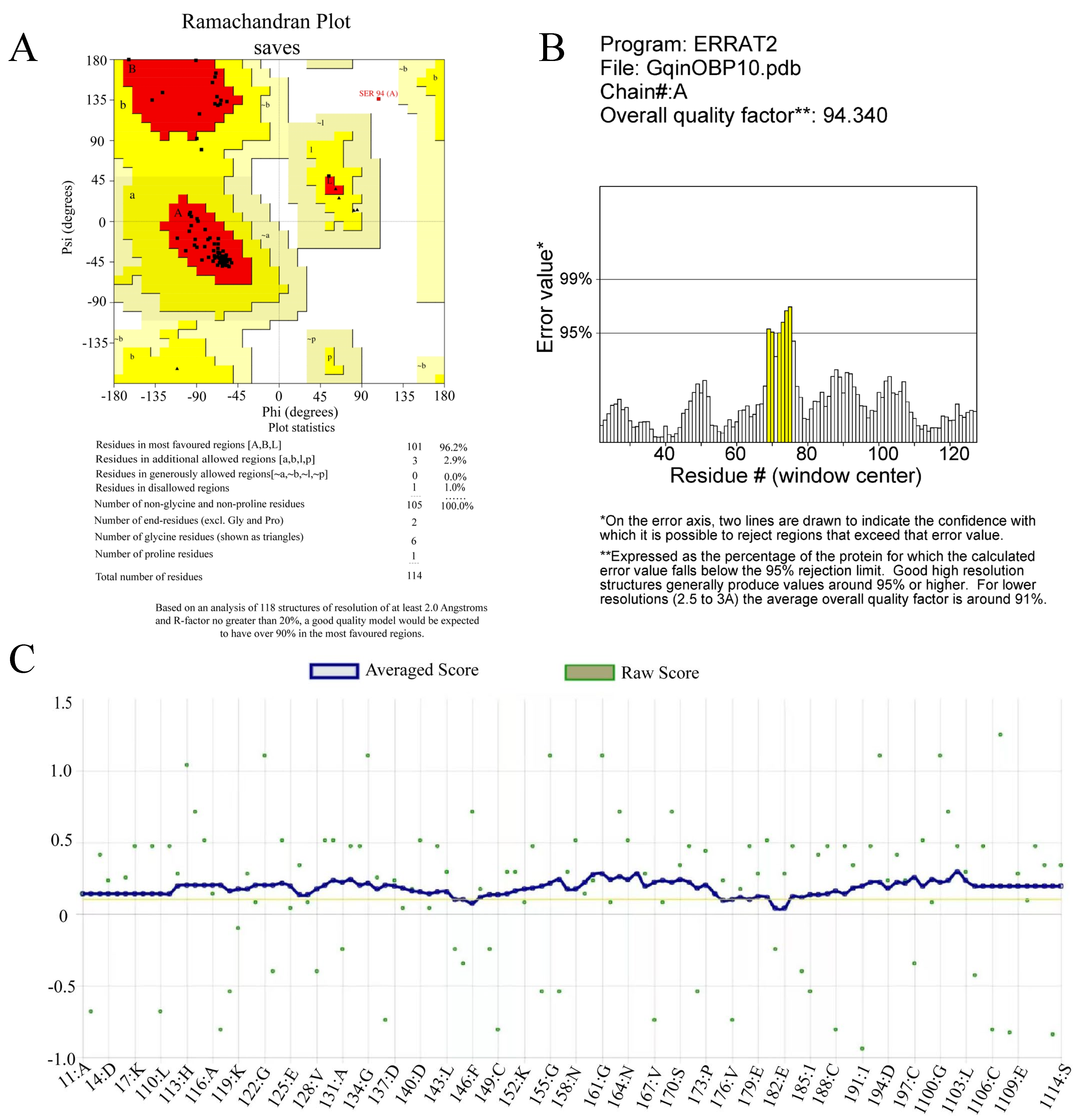
2.3. Target Protein Quality Analysis
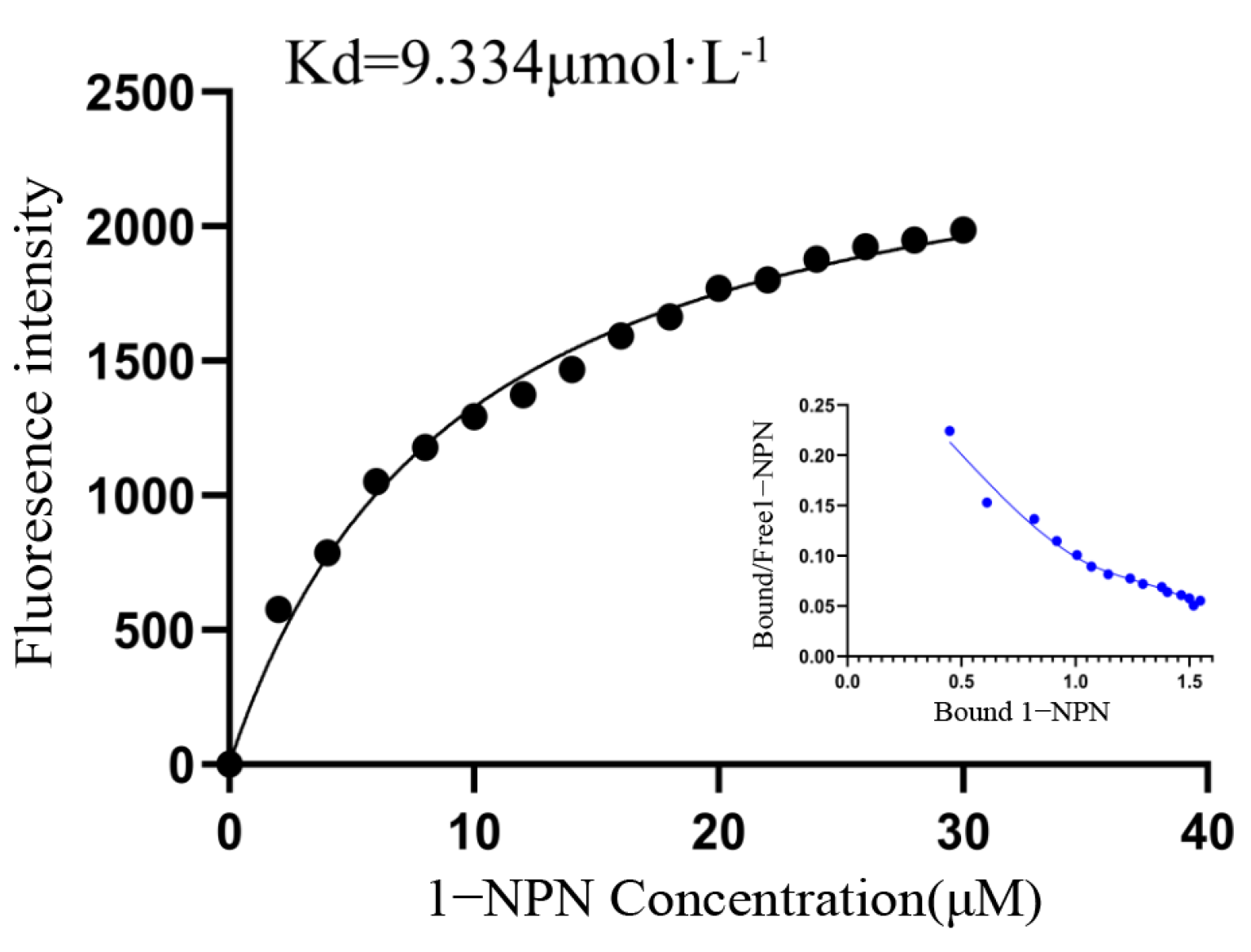
2.4. Analysis of Fluorescence Competitive Binding Characteristics of GqinOBP10 and Odor Ligands
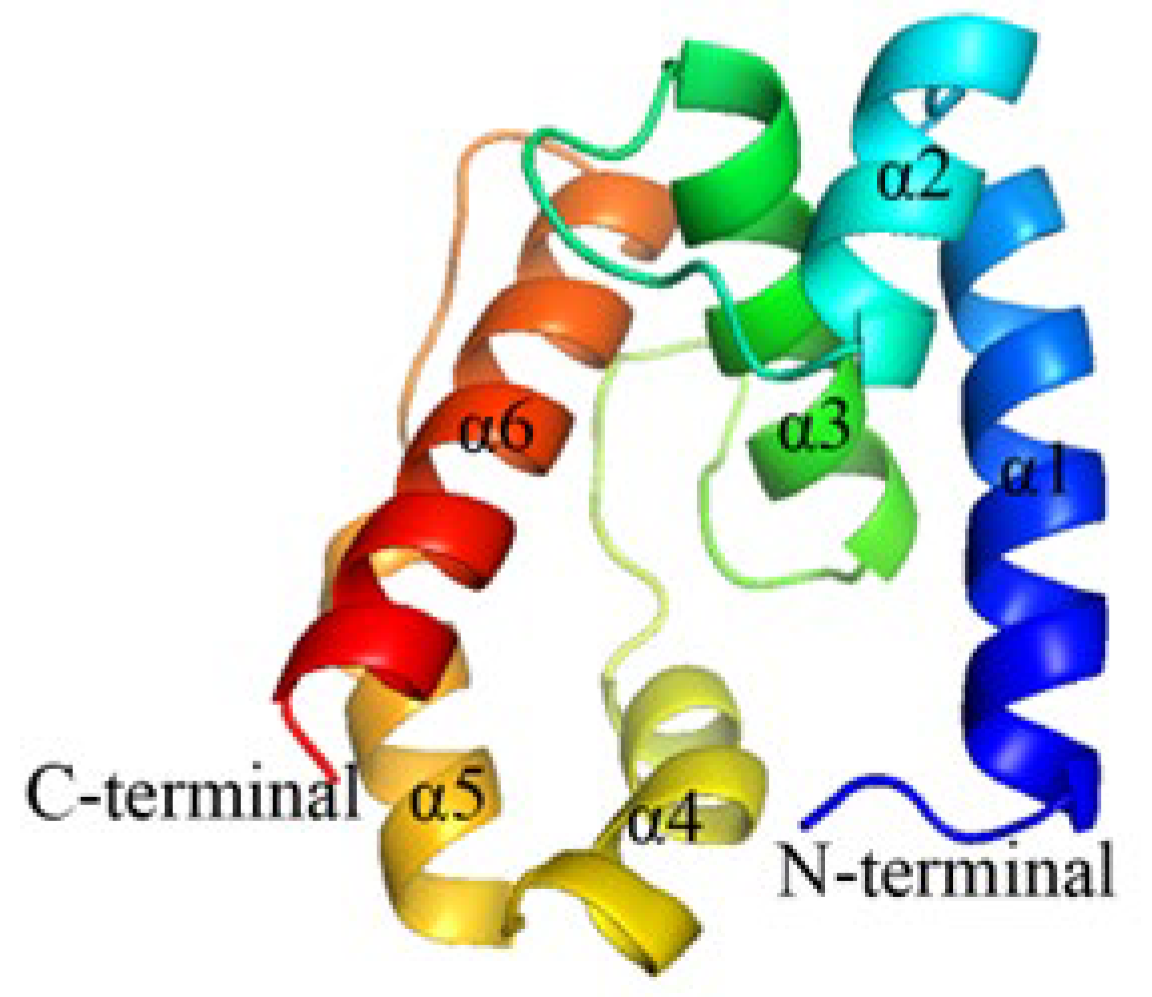
2.5. Construction and Evaluation of OBP10 Protein 3D Model
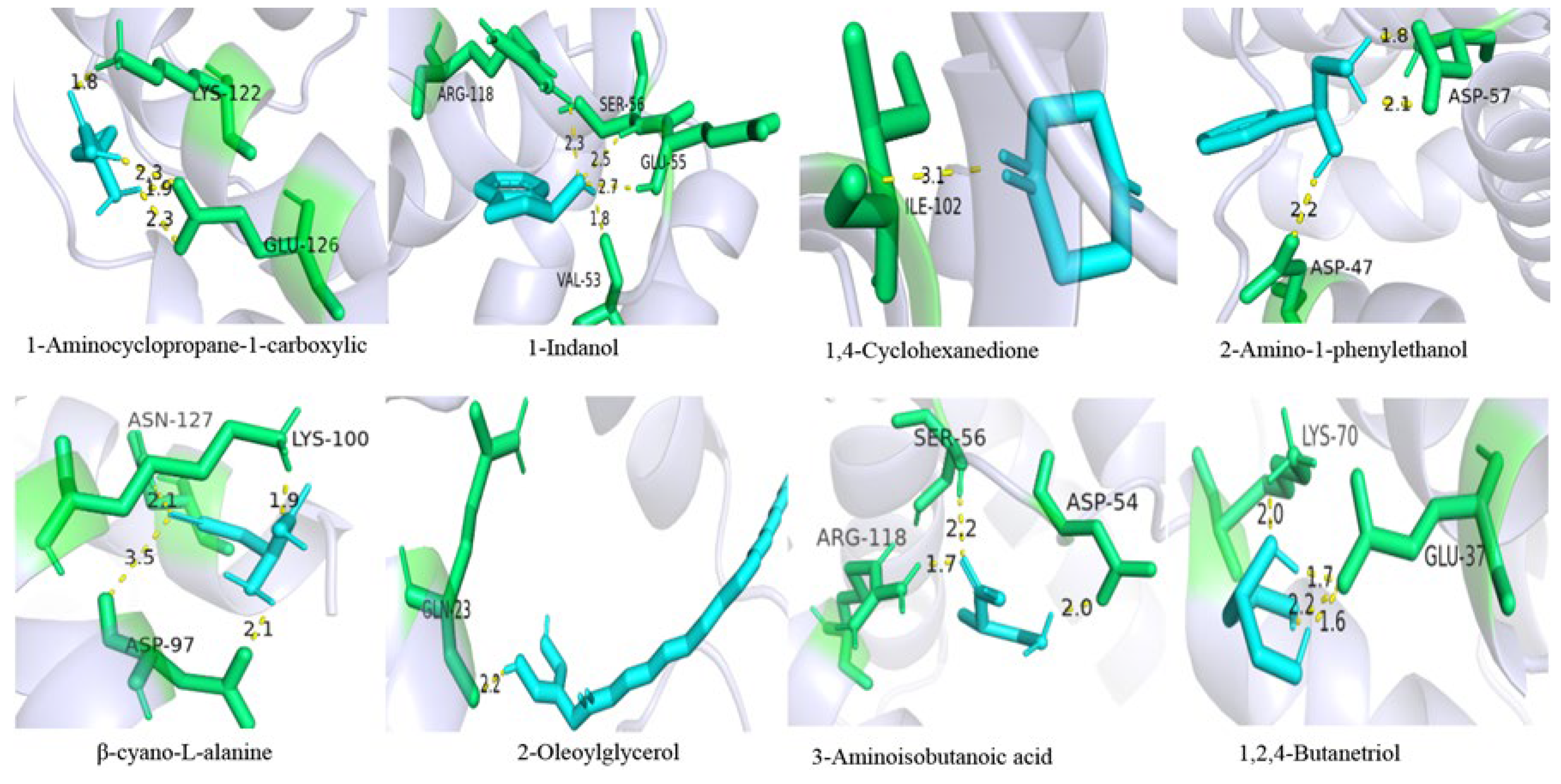
2.6. Molecular Docking of GqinOBP10 with Ligands
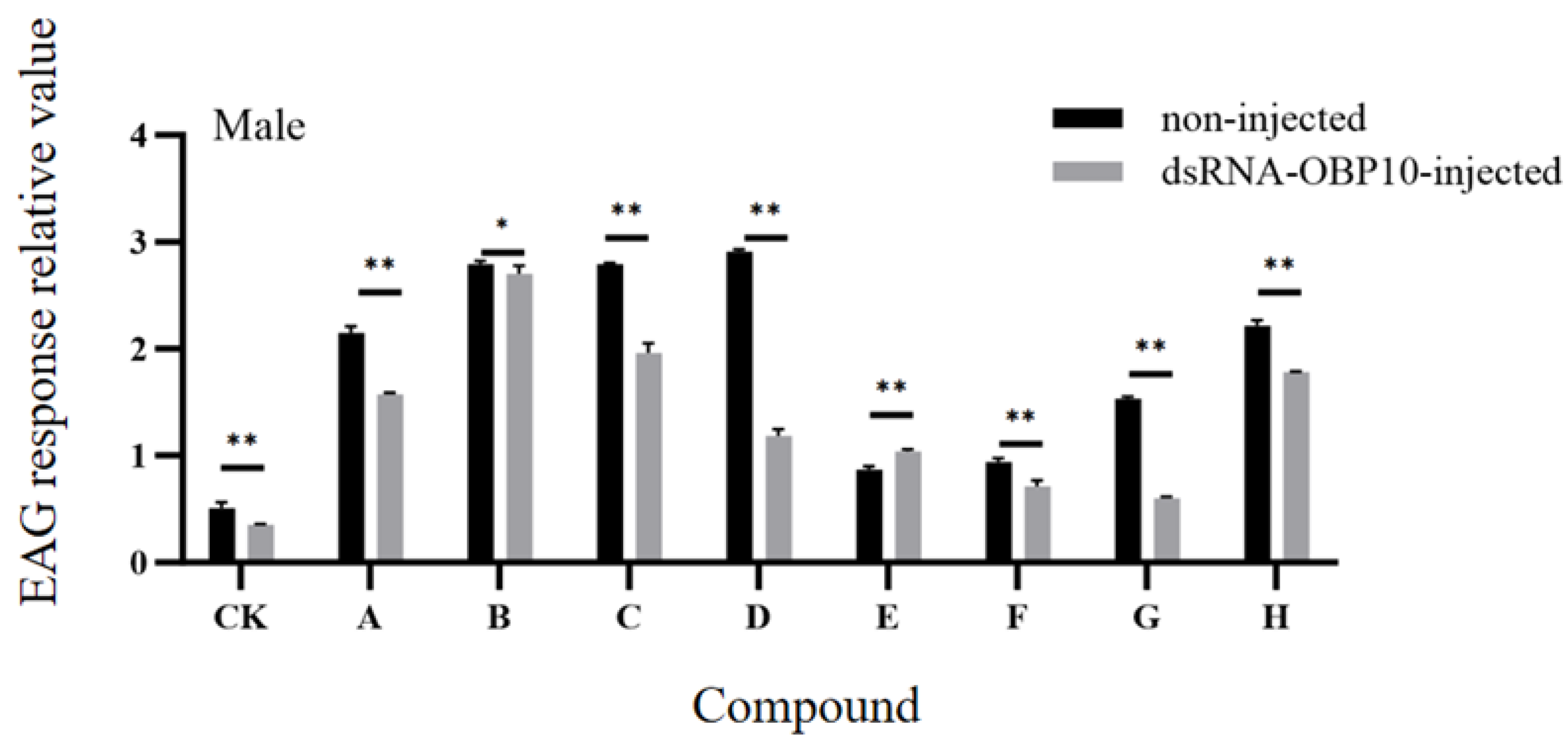
2.7. Effect of RNA Interference on the Expression Level of GqinOBP10
2.8. EAG Response After RNA Interference
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Cloning, Prokaryotic Expression, and Purification of OBP10 Gene
4.3. Western Blot Analysis of Purified GqinOBP10
4.4. Determination of Fluorescence Competitive Binding Characteristics of GqinOBP10
4.5. Determination of Binding Constant of GqinOBP10 and 1-NPN
4.6. Determination of Binding Ability of GqinOBP10 to Ligand Substances
4.7. Homology Modeling and Molecular Docking
4.8. RNA Interference
4.9. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.Z.; Li, Y.Y.; Zhang, R.Y.; Zhang, J.S. Investigation of parasitism rate of two parasitic natural enemies of Gynaephora qinghaiensis in pupal stage and analysis of their interaction with hosts. Chin. J. Appl. Entomol. 2024, 61, 206–216. [Google Scholar]
- Yan, L.; Wang, G.; Liu, Z.C. Number of Instars and Stadium Duration of Gynaephora menyuanensis (Lepidoptera: Lymantriidae) from Qinghai-Tibetan Plateau in China. Ann. Entomol. Soc. Am. 2006, 99, 1012–1018. [Google Scholar] [CrossRef]
- Wang, H.Z.; Zhong, X.; Zhang, G.R.; Liu, X.; Gu, L. Transcriptome characterization and gene expression analysis related to immune response in Gynaephora qinghaiensis pupae. J. Asia-Pac. Entomol. 2020, 23, 458–469. [Google Scholar] [CrossRef]
- Nan, Y.B.; Tang, D.J.; Yang, Y.C.; Kong, Y.S.; Zhou, Y.T. Identification and Expression Profiling of Chemosensory Protein Genes in Gynaephora qinghaiensis (Lepidoptera, Lymantriidae). Acta Agrestia Sin. 2023, 31, 3299–3309. [Google Scholar]
- Yuan, M.L.; Zhang, Q.L.; Wang, Z.F.; Guo, Z.L.; Bao, G.S. Molecular phylogeny of grassland caterpillars (Lepidoptera: Lymantriinae: Gynaephora) endemic to the Qinghai-Tibetan plateau. PLoS ONE 2015, 10, e0127257. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, J.; Chen, W.; Sun, Y.; Li, M.; Yu, H.; Yi, S.; Meng, B. Mapping of Gynaephora alpherakii inhabitability area in the National Park of Qilian Mountain, China. Agronomy 2023, 13, 594. [Google Scholar] [CrossRef]
- Yang, B.; Liu, Y.; Wang, B.; Wang, G. Olfaction-based Behaviorally Manipulated Technology of Pest Insects Research Progress, Opportunities and Challenges. Bull. Nat. Nat. Sci. Found. Chin. 2020, 34, 441–446. [Google Scholar]
- Zang, J.H.; Li, L.; Li, N.; Li, Y.Y.; Pang, B.P. Expression Profiling and Functional Analysis of Candidate Odorant Receptors in Galeruca daurica. Insects 2022, 13, 563, Erratum in Insects 2025, 16, 672. [Google Scholar] [CrossRef]
- Ghabbari, M.; Guarino, S.; Caleca, V.; Saiano, F.; Sinacori, M.; Baser, N.; Mediouni-Ben Jemâa, J.; Lo Verde, G. Behavior-modifying and insecticidal effects of plant extracts on adults of Ceratitis capitata (Wiedemann) (Diptera Tephritidae). J. Pest. Sci. 2018, 91, 907–917. [Google Scholar] [CrossRef]
- Leal, W. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Pelosi, P.; Calvello, M.; Ban, L.P. Diversity of odorant-binding proteins and chemosensory proteins in insects. Chem. Senses 2005, 30, 291–292. [Google Scholar] [CrossRef]
- Zhang, S.X.; Zhang, Y.; Xu, S.Q.; Wang, G.X.; Hu, Z.J.; Zhao, C.X.; Cui, W.Z. Mapping and expression analysis of GOBP/PBP subfamily gene cluster during pupal and adult stages of the silkworm, Bombyx mori. Acta Entomol. Sin. 2010, 53, 1069–1076. [Google Scholar]
- Ren, Z.Z.; Liu, X.L.; Hu, M.Y. Structure and Function of Insect Olfactory Proteins. Chin. J. Biochem. Mol. Biol. 2010, 26, 531–537. [Google Scholar]
- Leal, W.S.; Nikonova, L.; Peng, G.H. Disulfide structure of the pheromone binding protein from the silkworm moth, Bombyx mori. FEBS Lett. 1999, 464, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Scaloni, A.; Monti, M.; Angeli, S.; Pelosi, P. Structural analysis and disulfide-bridge pairing of two odorant-binding proteins from Bombyx mori. Biochem. Biophys. Res. Commun. 1999, 266, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, J.D.; Ha, T.S.; Jones, D.N.M.; Smith, D.P. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell 2008, 133, 1255–1265. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Liu, Y.; Fan, J.Y.; Ma, Y.J.; Yuan, C.Y.; Lou, B.H.; Wang, J.J. Odorant binding protein 2 reduces imidacloprid susceptibility of Diaphorina citri. Pestic Biochem. Physiol. 2020, 168, 104642. [Google Scholar] [CrossRef]
- Conchou, L.; Lucas, P.; Meslin, C.; Proffft, M.; Staudt, M.; Ronou, M. Insect odorscapes: From plant volatiles to natural olfactory scenes. Front. Physiol. 2019, 10, 972. [Google Scholar] [CrossRef]
- Hansson, B.S.; Stensmyr, M.C. Evolution of insect olfaction. Neuron 2011, 72, 698–711. [Google Scholar] [CrossRef]
- Rihani, K.; Ferveur, J.F.; Briand, L. The 40-Year Mystery of Insect Odorant-Binding Proteins. Biomolecules 2021, 11, 509. [Google Scholar] [CrossRef]
- Taszakowski, A.; Maslowski, A.; Daane, K.M.; Brozek, J. Closer view of antennal sensory organs of two Leptoglossus species (Insecta, Hemiptera, Coreidae). Sci. Rep. 2023, 13, 617. [Google Scholar] [CrossRef]
- Tao, Y.Y.; Zhu, X.Q.; Yang, W.; Yang, H.; Yang, C.P.; Guan, F.R.; Han, Q.L. Molecular Characterization, Expression and Binding Specificity Analysis of the Odorant-Binding Proteins of Scleroderma sichuanensis Xiao (Hymenoptera: Bethylidae). J. Kans. Entomol. Soc. 2020, 92, 459–479. [Google Scholar] [CrossRef]
- Shen, R.X.; Wang, Y.T.; Wu, J.H.; Zhang, N.; Zhang, H.D.; Xing, D.; Chen, Y.; Li, C.-X.; Zhao, T.-Y. Deltamethrin interacts with Culex quinquefasciatus odorant-binding protein: A novel potential resistance mechanism. Parasites Vectors 2022, 15, 2. [Google Scholar] [CrossRef]
- Zhang, R.X.; Wang, B.; Grossi, G.; Falabella, P.; Liu, Y.; Yan, S.C.; Lu, J.; Xi, J.H.; Wang, G.R. Molecular basis of alarm pheromone detection in aphids. Curr. Biol. 2017, 27, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.; Guidolin, A.; Sed, Z.; Cornel, A.J.; Leal, W.S. Knockdown of a mosquito odorant-binding protein involved in the sensitive detection of oviposition attractants. J. Chem. Ecol. 2010, 36, 245–248. [Google Scholar] [CrossRef]
- Swarup, S.; Williams, T.I.; Anholt, R.R. Functional dissection of odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav. 2011, 10, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yin, X.H.; Hu, S.Y.; Miao, H.N.; Wang, Z.; Li, H.; Zhang, Y.G.; Liang, P.; Gu, S.H. Overexpression of Two Odorant Binding Proteins Confers Chlorpyrifos Resistance in the Green Peach Aphid Myzus persicae. J. Agric. Food Chem. 2024, 72, 20101–20113. [Google Scholar] [CrossRef]
- Zhang, J.J.; Mao, K.K.; Ren, Z.J.; Jin, R.H.; Zhng, Y.H.; Cai, T.W.; He, S.; Li, J.; Wan, H. Odorant binding protein 3 is associated with nitenpyram and sulfoxaflor resistance in Nilaparvata lugens. Int. J. Biol. Macromol. 2022, 209, 1352–1358. [Google Scholar] [CrossRef]
- Chen, X.F.; Lei, Y.B.; Liang, C.H.; Lei, Q.; Wang, J.J.; Jiang, H.B. Odorant Binding Protein Expressed in Legs Enhances Malathion Tolerance in Bactrocera dorsalis (Hendel). J. Agric. Food Chem. 2024, 72, 4376–4383. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.T.; Zhang, Y.J.; Chen, J.L.; Li, X.C.; Liang, P.; Gao, X.W.; Zhou, J.J.; Gu, S.H. Coordinative mediation of the response to alarm pheromones by three odorant binding proteins in the green peach aphid Myzus persicae. Insect Biochem Mol Biol. 2021, 130, 103528. [Google Scholar] [CrossRef]
- Deng, S.; Yin, J.; Zhong, T.; Cao, Y.Z.; Li, K.B. Function and immunocytochemical localization of two novel odorant-binding proteins in olfactory sensilla of the scarab beetle Holotrichia oblita Faldermann (Coleoptera: Scarabaeidae). Chem. Senses 2012, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Nan, Y.B.; Zhang, Y.; He, Q.Y.; Chen, S.; Zhou, Y.T. Bioinformatics analysis on the Odorant Binding Protein Genes in Gynaephora qinghaiensis. Acta Agrestia Sin. 2023, 31, 688–698. [Google Scholar]
- Liu, Z.L.; Gao, S.J.; Han, H.B.; Tang, D.J.; Kong, Y.S.; Pan, X.N.; Zhou, Y.T. Identification and tissue expression profiling of odorant receptor genes in sex pheromone glands of female Gynaephora qinghaiensis (Lepidoptera: Lymantriidae). Plant Prot. 2025, 51, 69–79. [Google Scholar]
- Nan, Y.B.; Liu, Z.L.; Kou, G.X.; Wang, K.X.; Li, R.R.; Zhou, Y.T. Observations on the ultrastructure of adult Gynaephora qinghaiensis antennal sensilla and tissue expression analysis of GqinOBPs. Plant Prot. 2024, 50, 15–25+71. [Google Scholar]
- Nan, Y.B.; Wang, K.X.; Pan, X.N.; He, Q.Y.; Liu, Z.L.; Zhou, Y.T. Analysis of Larval Sensor Structure and GqinOBPs Expression of Gynaephora qinghaiensis. Acta Agrestia Sin. 2023, 31, 3626–3635. [Google Scholar]
- Liu, Z.L.; Kou, G.X.; Nan, Y.B.; Wang, K.X.; Zhou, Y.T. Identification and Expression Profiling of Ionotropic receptors Gene in Gynaephora qinghaiensis (Lepidoptera: Lymantriidae). Acta Agrestia Sin. 2024, 32, 1392–1400. [Google Scholar]
- Zhou, J.J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 83, 241–272. [Google Scholar] [PubMed]
- Zhou, J.J.; He, X.L.; Pickett, J.A.; Field, L.M. Identification of odorant-binding proteins of the yellow fever mosquito Aedes aegypti: Genome annotation and comparative analyses. Insect Mol Biol. 2008, 17, 147–163, Erratum in Insect Mol. Biol. 2008, 17, 445. [Google Scholar] [CrossRef]
- Xu, H.; Turlings, T.C. Plant volatiles as mate-finding cues for insects. Trends Plant Sci. 2018, 23, 100–111. [Google Scholar] [CrossRef]
- Duan, S.G.; Li, D.Z.; Wang, M.Q. Chemosensory proteins used as target for screening behaviourally active compounds in the rice pest Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Insect Mol. Biol. 2019, 28, 123–135. [Google Scholar] [CrossRef]
- Zhong, T.; Yin, J.; Deng, S.; Li, K.; Cao, Y. Fluorescence competition assay for the assessment of green leaf volatiles and trans-β-farnesene bound to three odorant-binding proteins in the wheat aphid Sitobion avenae (Fabricius). J. Insect Physiol. 2012, 58, 771–781. [Google Scholar] [CrossRef]
- Qin, J.M.; Cai, L.J.; Zheng, L.S.; Chen, X.J.; You, M.S. Identification and ligand binding characteristics of antennal binding protein Pxyl OBP31 in the diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae). Acta Ent. Sin. 2016, 59, 812–822. [Google Scholar]
- Zhou, S.S. Molecular Docking of Two Chemoreceptor Proteins to Host Volatiles. Mod. Agric. Res. 2023, 29, 45–48. [Google Scholar]
- Jiang, Q.Y.; Wang, W.X.; Zhang, Z.; Zhang, L. Binding specificity of locust odorant binding protein and its key binding site for initial recognition of alcohols. Insect Biochem Mol. 2009, 39, 440–447. [Google Scholar] [CrossRef]
- Li, G.W.; Zhang, Y.; Li, Y.P.; Wu, J.X.; Xu, X.L. Cloning, expression, and functional analysis of three odorant-binding proteins of the oriental fruit moth, Grapholita molesta (Busck) (Lepidoptera: Tortricidae). Arch. Insect Biochem. Physiol. 2016, 91, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.H.; Zhang, H.; Zhao, W.Q.; Liao, Z.G. Molecular docking of Plutella xylostella odorant binding protein PxylOBP33 with its related semiochemicals. Environ. Entomol. 2023, 45, 1291–1305. [Google Scholar]
- Guo, L.; Xie, S.A.; Yang, P.; Gong, X.F.; Chen, D.; Lv, S.J. Molecular docking of AzanOBP3 in jewel beetle Agrilus zanthoxylumi with its host volatiles. J. Plant Prot. 2021, 48, 376–387. [Google Scholar]
- Li, D.Z.; Yu, G.Q.; Yi, S.C.; Zhang, Y.; Kong, D.X.; Wang, M.Q. Structure-based analysis of the ligand-binding mechanism for DhelOBP21, a C-minus odorant binding protein, from Dastarcus helophoroides (Fairmaire; Coleoptera: Bothrideridae). Int. J. Biol. Sci. 2015, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Jiang, Z.M. Computer Simulation of Molecular Docking of α-Lactalbumin with Saponins. Dairy Sci. Technol. 2022, 45, 1–6. [Google Scholar]
- Bart, B.; Julie, T.; Arnold, D.L.; Liliane, S.; Roger, H. Fruitless RNAi knockdown in males interferes with copulation success in Schistocerca gregaria. Insect Biochem. Mol. Biol. 2011, 41, 340–347. [Google Scholar] [CrossRef]
- Dekebo, A.; Jung, C. Olfactory responses of Aethina tumida Murray (Coleoptera: Nitidulidae) to some major volatile compounds from hive materials and workers of Apis mellifera. J. Asia Pac. Entomol. 2020, 23, 504–508. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, X.F.; Xu, L.; Keesey, I.W.; Lei, Z.R.; Smagghe, G.; Wang, J.J. An antennae-Specific Odorant-Binding Protein is involved in Bactrocera dorsalis Olfaction. Front. Ecol. Evol. 2020, 8, 63. [Google Scholar] [CrossRef]
- Zhao, X.C.; Yan, Y.H.; Wang, R.; Wang, C.Z. Techniques used in insect neurobiology research: Electroantennogram recording. J. Appl. Entomol. 2004, 41, 270–274. [Google Scholar]
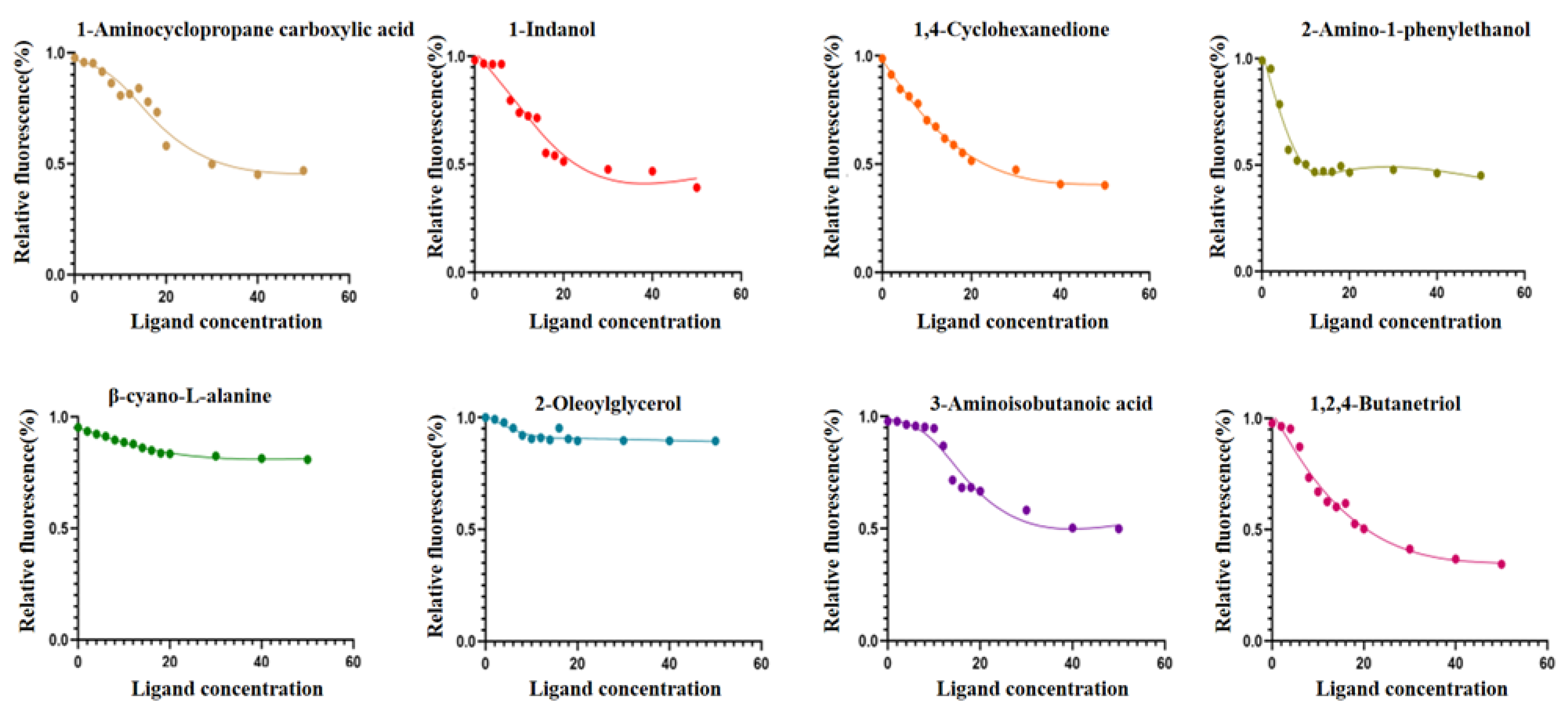

| Ligand | CAS NO. | Purity | Chemical Construction | Molecular Formula | IC50 (μmol·L−1) | Ki (μmol·L−1) |
|---|---|---|---|---|---|---|
| 1-Aminocyclopropane-1-carboxylic acid | 22059-21-8 | ≥98% | 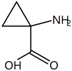 | C4H7NO2 | 18.7 | 18.59 |
| 1-Indanol | 6351-10-6 | ≥98% |  | C9H10O | 12.01 | 11.94 |
| 1,4-Cyclohexanedione | 637-88-7 | ≥98% | 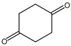 | C6H8O2 | 13.12 | 13.04 |
| 2-Amino-1-phenylethanol | 7568-93-6 | ≥98% |  | C6H8O2 | 4.506 | 4.48 |
| β-cyano-L-alanine | 6232-19-5 | ≥98% | 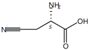 | C4H6N2O2 | 12.29 | 12.21 |
| 2-Oleoylglycerol | 3443-84-3 | ≥98% | 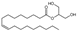 | C21H40O4 | 5.997 | 5.96 |
| 3-Aminoisobutanoic acid | 144-90-1 | ≥98% | 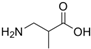 | C4H9NO2 | 14.97 | 14.88 |
| 1,2,4-butanetriol | 3068-00-6 | ≥98% |  | C4H9NO2 | 11.65 | 11.59 |
| Classification | Ligand | Binding Energy | Hydrophobic Interaction | Van der Waals Force | Hydrogen Bond |
|---|---|---|---|---|---|
| Carboxylic acid | 1-Aminocyclopropane-1-carboxylic acid | −3.48 | Lys122 | −2.11 | Lys122; Glu126 |
| Alcohol | 1-Indanol | −4.79 | Arg118; Tyr121; Lys122 | −4.92 | Val53; Glu55; Ser56; Arg118 |
| Ketone | 1,4-Cyclohexanedione | −4.04 | Phe80; Thr85; Val101; Ile102; Tyr124 | −3.86 | Ile102 |
| Alcohol | 2-Amino-1-phenylethanol | −4.36 | Gln52 | −3.37 | Asp47; Asp57; Asp57 |
| Carboxylic acid | β-cyano-L-alanine | −3.73 | Asp97; Asn127 | −2.73 | Asp97; Asp97; Lys100; Asn127 |
| Ester | 2-Oleoylglycerol | −6.92 | Leu27; Leu68; Ile71; Phe80; Thr85; Tyr121 | −10.16 | Gln23 |
| Carboxylic acid | 3-Aminoisobutanoic acid | −3.08 | Asp54; Glu55; Ser56 | −3.05 | Asp54; Ser56; Arg118 |
| Alcohol | 1,2,4-butanetriol | −1.34 | Lys69 | −2.42 | Glu37; Glu37; Lys70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Tang, D.; Lai, Y.; Gao, S.; Han, H.; Zhou, Y. Prokaryotic Expression and Binding Characteristics of Odor-Binding Protein GqinOBP10 in Gynaephora qinghaiensis. Int. J. Mol. Sci. 2025, 26, 10502. https://doi.org/10.3390/ijms262110502
Liu Z, Tang D, Lai Y, Gao S, Han H, Zhou Y. Prokaryotic Expression and Binding Characteristics of Odor-Binding Protein GqinOBP10 in Gynaephora qinghaiensis. International Journal of Molecular Sciences. 2025; 26(21):10502. https://doi.org/10.3390/ijms262110502
Chicago/Turabian StyleLiu, Zhanling, Dejing Tang, Youpeng Lai, Shujing Gao, Haibin Han, and Yuantao Zhou. 2025. "Prokaryotic Expression and Binding Characteristics of Odor-Binding Protein GqinOBP10 in Gynaephora qinghaiensis" International Journal of Molecular Sciences 26, no. 21: 10502. https://doi.org/10.3390/ijms262110502
APA StyleLiu, Z., Tang, D., Lai, Y., Gao, S., Han, H., & Zhou, Y. (2025). Prokaryotic Expression and Binding Characteristics of Odor-Binding Protein GqinOBP10 in Gynaephora qinghaiensis. International Journal of Molecular Sciences, 26(21), 10502. https://doi.org/10.3390/ijms262110502





