Abstract
In this study, Enterococcus faecalis strains isolated from raw cow’s milk were examined for genetic diversity, ability to produce biogenic amines (including histamine, tyramine, putrescine, cadaverine, 2-phenylethylamine) and the presence of corresponding amino acid decarboxylase genes. Identification of 29 strains obtained from Polish farms was carried out by polymerase chain reaction (PCR) and matrix-assisted laser desorption and ionization time of flight (MALDI-TOF MS) methods, and their genetic relationships were assessed by the Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR) technique. Amine production capacity was assessed in vitro on synthetic medium, while the presence of decarboxylase genes (hdcA, tyrS, tyrDC, Odc, ldc) was detected by molecular assays, with the use of optimized primers enabling the detection of tyrDC in strains previously considered negative. The results showed high variability between strains and the ability of some isolates to produce high concentrations of tyrDC (max. > 1000 mg/kg); the presence of the tyrDC gene was strongly correlated with high production, although tyrDC-positive strains with low production were also reported, suggesting the influence of regulatory or environmental factors. The study underscores the need for precise molecular tools and systematic monitoring of biogenic amines to ensure the safety and quality of dairy products.
1. Introduction
Ensuring the product safety at a microbiological level is a priority challenge for the food industry, to avoid consumer risks [1]. Food-safety management systems therefore rely on preventive practices—strict sanitation, control of cross-contamination and of processing parameters (temperature, pH, water activity), and careful selection of starter/adjunct cultures—to minimize contamination and the accumulation of harmful compounds. Among these, proper sanitization with strict standards of cleanliness, attention to cross-contamination, and the control of process parameters (e.g., temperature, pH) are needed [2]. These measures are intended to prevent the spread of foodborne pathogens and the accumulation of harmful microbial metabolites [3].
One group of these undesirable compounds, as an indicator of food spoilage [4], is represented by biogenic amines (BAs), which are responsible for adverse effects and are involved in several pathogenic syndromes in consumer health, depending on their concentration [5]. They are derived from bacterial decarboxylation of the corresponding amino acids through decarboxylase enzymes [6,7]. BAs are low-molecular-weight nitrogen compounds formed mainly as a result of bacterial decarboxylation of amino acids using specific decarboxylases. Typical BAs found in food are histamine and tyramine (heterocyclic and aromatic, respectively) and aliphatic amines such as putrescine and cadaverine. Polyamines (spermidine, spermine) have distinct biosynthesis pathways and functions and are often discussed separately, although putrescine is sometimes classified in both groups [8,9,10]. The most dangerous BAs found in foods are represented by histamine and tyramine due to the symptoms that they may cause (“fish poisoning” and “cheese reaction” syndrome). Their toxicity also depends on individual sensitivity or allergy, and the simultaneous monoamine oxidase inhibitory drugs or ethanol intake [11]. High levels of BAs in foods can lead to symptoms like hypertension, headaches, and allergic reactions. Recent studies have highlighted the toxicological effects of BAs and their synergistic potential, which could intensify adverse health outcomes [12].
Tyramine is the most common BA associated with enterococci, particularly in dairy and meat products, where it accumulates due to tyrosine decarboxylase activity [13]. Putrescine is another significant BA found in fermented foods, such as cheeses and soybean-based products, primarily synthesized via the ornithine decarboxylase or agmatine deiminase pathways [14]. Histamine and cadaverine, though less frequently linked to enterococci than other lactic acid bacteria, have been detected in specific fermentation processes [15]. 2-phenylethylamine has also been identified in several strains of enterococci used in artisanal cheese production [16].
The formation and level of BA in food depend on the development of microflora and factors such as storage time and temperature, food properties (water activity, pH), availability of free amino acids, and the presence of microorganisms with decarboxylase genes [4,17]. The decarboxylase enzyme activity is a characteristic of many groups of bacteria, including Enterobacteriaceae, Lactobacillaceae, Clostridium sp., Streptococcus sp., Pseudomonas sp. and Enterococcus sp. [7,18], leading to the formation of BAs in several food products.
Due to their ability to survive in harsh conditions, enterococci are a widespread microbiota in many products. Their presence in foods is controversial: on one hand, some enterococci are considered probiotics, with health benefits, while on the other hand, they are characterized by a high frequency of antibiotic resistance, the presence of many virulence factors, or the production of BAs [19,20,21,22]. Several species of Enterococcus (especially Enterococcus faecalis, Enterococcus faecium, Enterococcus durans, and Enterococcus mundtii) are well-documented producers of tyramine, accumulating it during fermentation through tyrosine decarboxylase activity. They may also contribute to putrescine formation through ornithine decarboxylase (ODC) and agmatine deiminase (ADI, aguBDAC) pathways. Ladero et al. [18] suggested that the capability to produce tyramine can be considered a species-level trait in E. faecalis, but is also widespread in E. faecium, E. durans and E. mundtii. Moreover, it is reported that they can produce other biogenic amines, including putrescine [5,22]. The biosynthesis of putrescine in E. faecalis occurs mainly through two distinct metabolic pathways: the ornithine decarboxylase (ODC) pathway and the agmatine deiminase (ADI) pathway. While the ODC pathway converts ornithine into putrescine via ornithine decarboxylase (encoded by the odc gene), the ADI pathway transforms agmatine into putrescine through a set of enzymes encoded by the aguBDAC operon [23,24]. Regulation involves response regulators such as aguR, as well as co-regulation between agmatine/tyramine clusters [25]. Although enterococci contribute to BA formation in dried fermented meat products and some cheeses, their frequent occurrence of antibiotic resistance determinants and virulence factors makes them problematic as starter or probiotic strains [26]. Therefore, modern strain selection criteria explicitly include the inability to produce BAs (absence of decarboxylase genes and no measurable formation of amines under appropriate conditions) in addition to the absence of transferable resistance and virulence traits.
Therefore, this study aimed to investigate the ability of Enterococcus faecalis strains isolated from cow raw milk to produce selected biogenic amines (histamine, tyramine, putrescine, cadaverine, and 2-phenylethylamine), and to determine the presence of genetic determinants encoding amino acid decarboxylase enzymes responsible for BA production. To improve the accuracy of gene detection, different sets of primers targeting the same decarboxylase genes were used, particularly for tyrDC, to assess their effectiveness and highlight the relevance of proper primer selection in molecular screening.
2. Results and Discussion
Enterococci are recognized as an important component of the natural microbiota of milk and dairy products [27,28]. They may include strains capable of producing biogenic amines. In many traditional fermented products such strains occur naturally and contribute to the fermentation process rather than representing classical spoilage microorganisms. Most enterococcal strains reported in the literature are tyramine producers [29]. While several authors have reported correlation between the number of enterococci and the concentration of tyramine [5,25] and putrescine [5,18,25] in dairy products, the presence of biogenic-amine-producing strains does not by itself imply a health hazard—the potential risk depends on the amount of biogenic amines produced and whether their concentration exceeds thresholds that are significant from a toxicological point of view. For this reason, accurate molecular characterization of strains and systematic monitoring of biogenic amine levels are necessary to assess both quality and safety.
Although biogenic amines can cause negative effects in sensitive individuals, raw milk that undergoes routine handling and transportation does not usually promote bacterial growth to a degree sufficient to produce high concentrations of amines. The main risk is associated with further processing (e.g., traditional fermentation) or prolonged improper storage, which may allow the proliferation of bacteria that produce amines. Therefore, risk assessments should be based on quantitative data on amines and contextual information on the use and storage of milk. Mainly responsible for it are 3 species of enterococci: E. faecium, Enterococcus durans and E. faecalis [18]. Studies on the ability of E. faecalis isolated from raw milk to produce biogenic amines are an important step in assessing the potential health risk posed by the presence of this microorganism in dairy products.
2.1. Genotypic Similarity of E. faecalis Strains Based on ERIC-PCR Analysis
The dendrogram obtained from ERIC-PCR analysis, constructed using the UPGMA (unweighted pair group method with arithmetic mean) algorithm, shows the hierarchical structure of genetic similarity between the E. faecalis strains studied (Figure 1). Similarity levels ranged from 18.7% to 100%, indicating the presence of both closely related and genetically distinct strains. The highest similarity (100%) was observed between strain pairs 12EN and 15EN, 61EN and 62EN, 64EN and 69EN, and 79EN, 80EN and 81EN, suggesting potential clonal origin or very recent common ancestry. At a similarity threshold of approximately 60%, the dendrogram reveals the presence of distinct genetic clusters, highlighting potential differences in evolutionary divergence among the strains. Notably, no clear relationship was observed between clustering patterns and the geographic origin of the isolates (Warmian–Masurian vs. Kuyavian–Pomeranian Voivodeships), suggesting that genetic variability may be influenced by other factors such as environmental conditions, farm-level practices, or selective pressure.
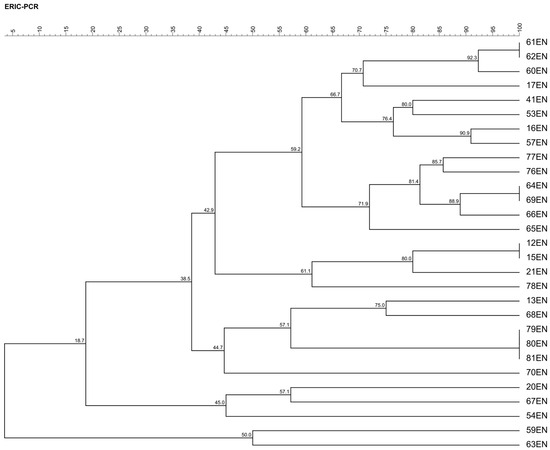
Figure 1.
The UPGMA dendrogram obtained from ERIC-PCR profiles shows similarity in the restriction pattern.
This observed genetic diversity prompts further investigation into the functional implications of these variations. Genetic analysis of E. faecalis isolated from raw milk can provide valuable insights into potential virulence determinants and the presence of genes responsible for biogenic amine synthesis [30].
2.2. Production of Biogenic Amines Among Enterococcus faecalis Strains
The potential production of BAs was tested for each selected E. faecalis strain in a synthetic microbial medium, added with specific amino acid precursors. Histidine, tyrosine, 2-phenylalanine, ornithine, and lysine were added to observe the accumulation of histamine, tyramine, 2-phenylethylamine, putrescine, and cadaverine, respectively. The HPLC results for the 29 E. faecalis strains (Table 1) showed a widespread ability to produce tyramine and 2-phenylethylamine among the samples, while histamine, putrescine, and cadaverine were under the detection limit (3 mg/L) in all of them. Significant differences were observed in the levels of biogenic amines between different bacterial strains of the same species. Tyramine production characterized most of the tested strains (82.8%) with levels ranging from 791.96 ± 94.81 mg/L to 1081.27 ± 84.97 mg/L. The highest concentrations of tyramine are found in strains 76EN (1081.27 ± 84.97 mg/L), 60EN (1080.08 ± 103.60 mg/L) and 20EN (1057.76 ± 112.50 mg/L), indicating a strong presence of tyrosine decarboxylase activity within these strains. On the other hand, the 17.2% tested strains (54EN, 63EN, 68EN, 70EN and 81EN) show minimal tyramine levels (below 15 mg/L), suggesting variability in the enzyme activity or gene expression among these samples. E. faecalis, able to produce high concentrations of tyramine, can pose a health risk to consumers. Consumption of products containing high levels of tyramine can lead to hypertensive reactions in susceptible individuals, which is especially true for dairy products. The literature emphasizes that the toxic effects of individual biogenic amines are evident even at relatively low concentrations [31]. According to Bover-Cid et al. [32], histamine can cause adverse effects at different doses, depending on individual sensitivity. The toxic threshold levels for histamine range from 8–40 mg (mild poisoning) to 1500–4000 mg (severe poisoning), with histamine-containing fish poisoning (HFP) often associated with levels between 600 and 3000 mg/kg in fish products [32]. According to Regulation (EC) 2073/2005 [33], the maximum permissible levels of histamine in fresh fish are 100–200 mg/kg and in salted fish products up to 400 mg/kg [33]. In the case of tyramine, hypertensive reactions have been reported after consumption of 200–2000 mg in healthy individuals, while migraine sufferers may react to doses as low as 100 mg [32]. In addition, according to recent studies, newer generation monoamine oxidase inhibitors (MAOIs) (RIMAs) tolerate up to 50–100 mg, while in the average population, a dose equivalent to 200–800 mg of tyramine causes hypertensive crises [34]. The proposed upper limit for the sum of biogenic amines (histamine, tyramine, putrescine, cadaverine, etc.) in food is 750–900 mg/kg [35]. However, even in strains carrying this gene, the level of tyramine production can vary due to specific regulatory factors and the availability of precursors in the culture medium. The literature repeatedly describes the need to monitor levels of biogenic amines in food products, especially meat and dairy, to reduce the health risks associated with their consumption [18]. Research has shown that enterococci strains exhibit varying tyramine production capacities based on growth conditions [36]. For example, pre-culture in tyrosine-enriched environments enhances decarboxylase activity. Factors such as pH between 5 and 6.5 and high moisture content in the medium are optimal for tyramine production. Conversely, lack of precursors or unfavorable environmental conditions can lead to diminished or no tyramine production despite the presence of the gene [36]. Further studies, including analysis of regulatory sequences and gene expression, could help explain the mechanisms responsible for such a large variation in tyramine production in strains with apparently similar genetic profiles.

Table 1.
Detection of tyramine and 2-phenylethylamine accumulation in E. faecalis strains. Data are reported in mg/L as a mean of three repetitions.
2-phenylethylamine is another BA that the analyzed enterococci produced at concentrations ranging from 104.94 ± 11.55 mg/L to 186.58 ± 26.40 mg/L, though its levels are much lower compared to tyramine. The highest concentrations are found in strains 76EN (186.58 ± 26.40 mg/L) and 77EN (185.44 ± 26.02 mg/L), followed by 60EN (177.06 ± 32.90 mg/L) and 20EN (178.54 ± 37.56 mg/L). It is important to note that the same strains that were not characterized by a tyramine production were also unable to accumulate a huge amount of 2-phenylethylamine. This observation is consistent with recent findings indicating that both tyramine and 2-phenylethylamine can be synthesized by the same enzyme, tyrosine decarboxylase (TyrDC). A knockout study conducted by Pérez et al. [37] demonstrated that deletion of the tdcA gene in E. durans abolished the production of both tyramine and 2-phenylethylamine, providing direct evidence that TyrDC catalyzes the decarboxylation of phenylalanine as well. Although the presence of the tyrDC gene is a prerequisite for this biosynthetic capability, its expression and enzymatic activity are known to be modulated by strain-specific regulatory mechanisms and environmental conditions [38,39]. Consequently, even tyrDC-positive strains may vary in their actual production levels. The literature suggests that 2-phenylethylamine production is less common among lactic acid bacterial strains and usually occurs at lower levels than tyramine [5]. Moreover, genotypic and phenotypic characteristics of these bacteria may vary depending on environmental conditions, which can significantly influence their biochemical activity and potential toxicity [38,40]. For instance, the microbial composition and their activity in raw milk or artisanal cheeses can be influenced by environmental conditions, such as temperature, humidity, and seasonal changes in animal feed [41]. These factors can modulate the ability of Enterococcus species to produce biogenic amines, as well as their antimicrobial resistance profiles [42].
Although no accumulation of putrescine was detected in any of the analyzed E. faecalis strains under the tested conditions, this absence must be interpreted with caution. The biosynthesis of putrescine in E. faecalis primarily occurs via the agmatine deiminase pathway, which requires the presence of agmatine in the environment as a substrate [24]. In our study, the medium was supplemented with ornithine, thus favoring the detection of the ornithine decarboxylase pathway. Consequently, the lack of putrescine detection may reflect pathway-specific activation rather than a true inability of these strains to produce this compound. Previous research has identified the aguBDAC operon responsible for agmatine conversion to putrescine and confirmed its activity in various dairy and fermented food isolates [23,25]. Therefore, the true putrescine-producing potential of these strains remains to be determined.
The complete absence of histamine, cadaverine, and ornithine-derived putrescine in all strains indicates focus to tyramine and 2-phenylethylamine as the main biogenic amines, with potential implications for food safety given the high levels of tyramine observed in many strains. The data indicate that E. faecalis strains are potential sources of tyramine and 2-phenylethylamine, with the variability in production possibly influenced by genetic factors, environmental conditions, or strain-specific metabolic regulation. Future research on BAs in dairy products should focus on developing innovative control strategies and understanding the underlying mechanisms of BA production and degradation. One promising area involves the use of enzymatic reduction by lactic acid bacteria, such as Lacticaseibacillus casei, which has demonstrated the ability to degrade BAs like histamine and putrescine in dairy environments [43].
2.3. Presence of Genes Associated with the Accumulation of Biogenic Amines Among Selected Strains
In our study, none of the selected enterococcal strains tested positive for the presence of histidine decarboxylase (hdcA), lysine decarboxylase (ldc), or ornithine decarboxylase (Odc) genes using primers HdC1/HdC2, Cad2F/Cad2R, and ODF/ODR, respectively. Only genes involved in tyramine synthesis (tyrDC and tyrS) were detected in the tested strains, as shown in Table 2. According to the literature, the ability to produce tyramine and 2-phenylethylamine accumulation is observed among enterococci; however, these strains are not able to produce cadaverine and putrescine [44]. The results of our study are consistent with those of Inoğlu and Tuncer [45], in which none of the analyzed enterococci strains possessed the hdc, ldc, and Odc genes. It is important to note, however, that the main genetic cluster responsible for putrescine biosynthesis in E. faecalis is the agmatine deiminase pathway, which includes the aguA, aguB, aguC, aguD, and aguR genes. These genes encode enzymes and regulatory elements necessary for converting agmatine to putrescine and were not targeted in our current PCR screening [24].

Table 2.
Presence of tyramine-associated genes (tyrS, tyrDC) among Enterococcus faecalis strains.
In total, twenty-one strains (72.41%) carried both tyrS and tyrDC. The remaining eight strains (27.59%)—i.e., 41EN, 57EN, 59EN, 60EN, 66EN, 68EN, 78EN and 79EN—possessed only the tyrDC gene, while lacking tyrS. Among these eight tyrS-deficient strains, as many as seven had high tyramine production (>900 mg/L). The only exception was strain 68EN, whose tyramine production capacity was much lower than <20 mg/L. Additionally, five strains (54EN, 63EN, 68EN, 70EN, and 81EN) produced only trace amounts of tyramine (<10 mg/L) despite carrying the tyrDC gene, which indicates that the mere presence of this gene does not guarantee its expression or functional activity. Similarly, a study by Bargossi et al. [46] showed that although tyrDC gene was common in enterococci, strains differed significantly in decarboxylase activity. In other bacteria, the gene may be silent. Eom et al. [47] observed isolates with intact histidine and tyrosine decarboxylase genes, but no expression of these enzymes and no histamine or tyramine production, respectively. These findings underscore the complexity of the genetic and environmental regulation of biogenic amine biosynthesis. The observed variability in tyramine production among genetically similar strains suggests that additional regulatory mechanisms, including alternative transcription factors or strain-specific expression controls, may influence tyrDC activation [5,37,48]. Environmental factors are known to play a key role in regulating tyrDC gene expression and tyramine biosynthesis. Environmental and nutritional factors further modulate tyrDC expression and activity. For example, acidic conditions combined with abundant tyrosine strongly induce the pathway. Perez et al. [49] showed that low pH, together with ample substrate, triggers tyrDC transcription and boosts tyramine output. Strains incubated at elevated temperatures (e.g., 45 °C) produced substantially higher amounts of tyramine compared to those grown at lower temperatures, such as 25 °C [50]. Osmotic stress is another important modulatory factor. Liu et al. [51] demonstrated that NaCl stress led to upregulation of both tyrDC and the downstream tyrP gene encoding the tyrosine/tyramine antiporter in E. faecalis, resulting in increased tyramine accumulation under specific stress conditions. These findings indicate that parameters such as pH, temperature, salt concentration, and precursor (tyrosine) availability can dramatically alter the transcriptional activity of the tyrDC gene and the enzymatic function of tyrosine decarboxylase. As a result, these factors introduce additional layers of physiological control over tyramine formation in enterococci [47,49].
The locus influencing the ability to produce tyramine usually includes genes encoding tyrosine decarboxylase (tyrDC), tyrosyl tRNA synthetase (tyrS, located upstream of the tyrDC gene), putative tyrosine/tyramine permease (tyrP, located downstream of the tyrDC gene) and Na+/H+ antiporter (nhaC) [52]. The selection of this specific gene locus for analysis was based on its well-documented and central role in tyramine biosynthesis in enterococci. The tyrDC gene encodes the key enzyme responsible for tyramine production, while its adjacent genes (including the tyrS gene) form a conserved tdc cluster frequently co-transcribed and functionally associated with regulation, transport, and adaptation to environmental stress. Investigating this region allowed not only for confirmation of tyramine-producing potential but also for a deeper understanding of genetic determinants potentially affecting strain-specific variation in amine biosynthesis. The researchers indicate the organization of different tdc clusters, their distribution, and high sequence similarity, indicating a horizontal transfer of this cluster from a common source [53]. Moreover, horizontal gene transfer (HGT) has been shown to play a key role in the spread of genes responsible to produce biogenic amines—increasing the metabolic flexibility and adaptability of bacterial populations in cheese microbiomes—and this spread is often mediated by mobile genetic elements such as plasmids and integrons [54,55].
The results of this study indicate that the key element in tyramine biosynthesis in the enterococci strains studied is the tyrDC gene, regardless of the primer set used (DEC5/DEC3 or Tdc-F2/Tdc-R2; Table 3). Detection of the tyrDC gene using two different primer pairs (DEC5/DEC3 and Tdc-F2/Tdc-R2) indicates a high probability of the actual presence of this gene in the analyzed material. The use of two independent primer systems, each targeting distinct regions of the tyrDC gene, increases the overall reliability of detection and minimizes the risk of false-positive or false-negative results due to non-specific binding, sequence variability, or template degradation. Using both sets in parallel helps compensate for possible mismatches or partial DNA degradation that might prevent one primer pair from binding effectively—under such conditions, the alternate pair may still succeed. Concordant amplification by both primer sets, therefore, serves as confirmatory evidence for tyrDC presence, reducing the likelihood of spurious results or amplification dropouts. This approach allows for a more precise identification of microorganisms capable of tyramine production, a trait of toxicological relevance in the context of food safety. The presence of the tyrDC gene in all 29 strains analyzed strongly suggests that the enzyme tyrosine decarboxylase, encoded by this gene, plays a key role in the biosynthesis of this biogenic amine, which is consistent with previous studies [45,46,56,57,58]. Further studies, including analysis of regulatory sequences and gene expression, could help explain the mechanisms responsible for such a large variation in tyramine production in strains with apparently similar genetic profiles.

Table 3.
The primer sequences used to identify genes encoding decarboxylase enzyme to produce biogenic amines.
2.4. Limitations and Future Work
A key limitation of the present study is the focus on the ornithine decarboxylase (ODC) pathway to assess putrescine biosynthesis, without consideration of the agmatine deiminase pathway. As demonstrated by Landete et al. [24] the ADI pathway constitutes the primary route for putrescine formation in E. faecalis, driven by the aguBDAC gene cluster. Moreover, putrescine production via this route is inducible by the presence of agmatine in the growth medium. Future studies should therefore incorporate agmatine supplementation and PCR amplification of the agu operon to fully evaluate the putrescine-producing capacity of E. faecalis strains. This will provide a more complete understanding of their potential impact on biogenic amine accumulation in dairy products.
3. Materials and Methods
3.1. Selected Strains Used in This Study
The 29 Enterococcus faecalis strains used in this study belonged to the collection of the Department of Food Microbiology, Meat Technology and Chemistry at the University of Warmia and Mazury in Olsztyn (Poland). These strains were previously isolated from raw milk samples collected from various farms located in the Warmian–Masurian and Kuyavian–Pomeranian Voivodeships in Poland. These strains were previously isolated from raw bulk milk samples (100 mL each), collected aseptically during routine milking by instructed personnel into sterile containers. All samples were obtained in 2022 from clinically healthy cows. According to farmers’ declarations, no antibiotic treatments had been applied within 30 days prior to sampling. The cows were kept under pasture housing conditions. Table S1 (Supplementary Material) lists the farm identifiers, geographical areas, sampling dates, host species, health status, recent antibiotic use, housing, and sampling method. Different presumptive Enterococcus isolates were initially obtained using Slanetz–Bartley agar (Merck, Darmstadt, Germany), and species identification confirmed them as E. faecalis using two methods—PCR and MALDI-TOF MS (bioMérieux, Marcy-l’Étoile, Frnace). The isolates were stored at −80 °C in microbanks (Biomaxima, Lublin, Poland) and cultured on Tryptic Soy Agar (TSA; Merck, Darmstadt, Germany) at 37 °C in 24 h before further analyses.
3.1.1. PCR Identification
All presumptive enterococci isolates were genotypically identified by PCR. Genomic DNA was purified using the Genomic Mini kit (A&A Biotechnology, Gdańsk, Poland), according to the manufacturer instructions. The obtained genomic DNA was stored at −20 °C for further analysis. Then, the identification at the species level was performed by PCR, using specific primers described by Zarzecka et al. [65] (Table 4). PCR reaction products were separated by electrophoresis in a 1.5% agarose gel (m/v), stained with 0.5 μg/mL ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA), and visualized under UV light using the G-BOX gel analysis and documentation system (Syngene, Cambridge, UK).

Table 4.
The primer sequences used to identify the presumed enterococci [65].
3.1.2. MALDI-TOF MS Identification
All isolates were also identified using MALDI-TOF MS (matrix-assisted laser desorption and ionization time of flight). Measurements were performed using the VITEK® MS system (Applied Maths/bioMérieux, Sint-Martens-Latem, Belgium) according to the methodology described by Wiśniewski et al. [66] and Zakrzewski et al. [67] with an acceleration voltage of 200 kV, a mass range of 2–20 kDa, a laser frequency of 50 Hz, and an extraction delay time of 200 ns. Mass fingerprints were analyzed via the VITEK® MS v2.0 MALDI-TOF system (RUO; SARAMIS v4.13).
Isolates were tested in duplicate using the direct transfer protocol. After 48 h of incubation at 30 °C in TSA (Merck, Darmstadt, Germany), they were transferred to the target plate, treated with 1 µL of MALDI matrix VitekMS-CHCA, and analyzed. Confidence levels were expressed as percentages.
3.2. Genotyping of Strains Using ERIC-PCR
The strains were genotyped using ERIC-PCR (Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction) following the protocol outlined by Zarzecka et al. [68] and Wiśniewski et al. [69]. The reaction was conducted using a Mastercycler Nexus GX2 Thermocycler (Eppendorf, Hamburg, Germany) in a 25 μL reaction volume, including 12.5 μL of DreamTaq Green PCR Master Mix (2×) (ThermoFisher Scientific, Waltham, MA, USA), 1.25 μL of both 10 pmol primers ERIC1 (5′-ATG TAA GCT CCT GGG GAT TCA-3′) and ERIC2 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′) (Genomed, Gdańsk, Poland), 1.5 μL of DNA template (50 ng/μL), and nuclease-free water to reach the final volume. The PCR reaction conditions included an initial denaturation at 95 °C for 7 min to effectively separate DNA strands, followed by 30 cycles consisting of denaturation at 94 °C for 1 min, primer attachment at 52 °C for 1 min, and elongation at 65 °C for 8 min. Finally, a final elongation at 65 °C for 15 min was performed to ensure that all PCR products were fully synthesized.
The PCR products were separated using electrophoresis on a 1.5% agarose gel prepared with TBE buffer (1% Tris-borate-EDTA) and stained with 0.5 μg/mL ethidium bromide (Sigma-Aldrich, St. Louis, MO, USA). The bands were visualized using the G-BOX F3 system (Syngene International Limited, Bengaluru, KA, India). Cluster analysis was conducted using BioNumerics version 7.6.3 (Applied Maths/bioMérieux), employing the Dice coefficient for similarity calculations and generating dendrograms with the unweighted pair group method with arithmetic mean (UPGMA).
3.3. Screening of Biogenic Amine Production
Determination of the potential of enterococci strains to produce biogenic amines (histamine, tyramine, putrescine, cadaverine, and 2-phenylethylamine) was carried out by a screening method using the medium proposed by Bover-Cid and Holzapfel [70]. Overnight cultures incubated at 30 °C in Brain Hearth Infusion (BHI) broth (Merck, Darmstadt, Germany) were used at a concentration of 6 log CFU/mL in Bover-Cid and Holzapfel broth, added with the specific BA precursors (Table 5) and incubated at 37 °C, under aerobic conditions. This step was performed three times. Biogenic amine production was confirmed by high-performance liquid chromatography (HPLC) analysis, according to the method reported by Barbieri et al. [71]. Briefly, the obtained samples were centrifuged at 6000 rpm for 10 min, and the resulting cell-free supernatants were analyzed using an Agilent Instrument 1260 Infinity HPLC instrument (Agilent technologies, Inc., Santa Clara, CA, USA) with an automatic injector (G1329B ALS 1260, 20 μL loop) equipped with a UV detector (G1314F VWD 1260) set at 254 nm. Before injection, samples were derivatized with dansyl chloride (Sigma Aldrich, St. Louis, MO, USA) according to the method of Martuscelli et al. [72]. A C18 Waters Spherisorb ODS-2 column (150 × 4.6 mm, 3 μm) was used for chromatographic separation. The amounts of amines were reported in mg/L, based on a calibration curve obtained using aqueous dansyl-chloride-derivatized amine standards (Sigma-Aldrich, St. Luise, MO, USA). The detection limit of the analysis under the applied conditions was 3 mg/L. All samples were analyzed in triplicate.

Table 5.
Summary of tested amino acid precursors for the formation of specific biogenic amines.
3.4. Detection of the Genes Related to the Production of Biogenic Amines
The PCR method was used to determine the presence of genes related to the potential production of biogenic amines, such as hdcA, tyrS, and tyrDC, Odc, and ldc encoding histidine, tyrosine, ornithine, and lysine decarboxylase, respectively. The primers used in this study are listed in Table 3. The amplification reaction was performed in a thermocycler (Mastercycler nexus GX2/GX2e; Eppendorf, Hamburg, Germany). A 25 µL reaction mixture was used, which contained 2 µL of template DNA (50 ng/µL), 1X Easy-Taq buffer, 10 mM of dNTPs (deoxyribonucleotides), 0.4 µM of each primer, and 1.5 U Easy-Taq enzyme. The rest of the volume was made up of nuclease-free water. Amplification reactions were performed according to the parameters shown in Table 6. Each PCR cycle included positive and negative controls. Genomic DNA from E. faecalis ATCC 29212 was used as a positive control for the tyrDC gene, while genomic DNA from bacterial strains belonging to the strain collection in which the other genes analyzed in our study were identified was used as positive controls for the respective targets. Negative controls (nuclease-free water) were included in each run to monitor for contamination. PCR products were subjected to an electrophoresis analysis on a 1.5% agarose gel. Then the image of the resulting bands was visualized under UV light after ethidium bromide staining using the G-BOX F3 fluorescent stained gel documentation and analysis system (Syngene International Limited, Bengaluru, KA, India).

Table 6.
Specific amplification reaction parameters for each selected gene.
4. Conclusions
Enterococcus faecalis strains exhibit strain-specific biogenic amine production, with tyramine synthesis strongly associated with the presence of the tyrDC gene. However, because biogenic amine production depends on multiple factors, including regulatory mechanisms and environmental conditions, culture-based detection methods may yield false-negative results. Therefore, the presence of biosynthetic genes such as tyrDC is considered the most reliable indicator of a strain’s potential to accumulate biogenic amines in food products. In vitro assays revealed significantly higher tyramine levels compared to 2-phenylethylamine, reflecting the enzyme’s greater affinity for tyrosine. The application of two complementary primer sets (DEC5/DEC3 and Tdc-F2/Tdc-R2) enhanced detection reliability across genetically diverse strains, minimizing false negatives. While our controlled conditions clarify the strains’ intrinsic amine-producing potential, actual accumulation in dairy matrices may vary with pH, temperature, microbial interactions, and substrate availability, underscoring the need for in-product validation. Although putrescine was not detected in this study, the possibility of its biosynthesis via the agmatine deiminase pathway remains open. Further investigations involving agmatine supplementation and detection of agu genes are needed to fully characterize putrescine production potential. These findings support the implementation of routine molecular screening and targeted process controls to mitigate biogenic amine risks in fermented foods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262110480/s1.
Author Contributions
Conceptualization, P.W.; methodology, P.W. and F.B.; validation, P.W.; formal analysis, P.W. and F.B.; investigation, P.W. and F.B.; resources, P.W.; data curation, P.W. and F.B.; writing—original draft preparation, P.W.; writing—review and editing, F.B.; visualization, P.W.; funding acquisition, P.W. All authors have read and agreed to the published version of the manuscript.
Funding
The results were obtained through the PROM Program for International Exchange of Doctoral Students and Academic Staff in the Food Microbiology Laboratory at the Faculty of Agricultural and Food Sciences (Alma Mater Studiorum, Bologna University), which the Polish National Agency for Academic Exchange. The APC was funded by the Minister of Science under “the Regional Initiative of Excellence Program”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and Supplementary Materials.
Acknowledgments
We are grateful to Anna Zadernowska, Chiara Montanari, and Giulia Tabanelli, for their invaluable support in conducting the research and for their expert supervision. The authors have not stated any conflicts of interest.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bohrer, B.M. Review: Nutrient Density and Nutritional Value of Meat Products and Non-Meat Foods High in Protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jacxsens, L.; Membré, J.-M.; Nauta, M.; Peterz, M. Relevance of Microbial Finished Product Testing in Food Safety Management. Food Control 2016, 60, 31–43. [Google Scholar] [CrossRef]
- Barlow, S.M.; Boobis, A.R.; Bridges, J.; Cockburn, A.; Dekant, W.; Hepburn, P.; Houben, G.F.; König, J.; Nauta, M.J.; Schuermans, J. The Role of Hazard- and Risk-Based Approaches in Ensuring Food Safety. Trends Food Sci. Technol. 2015, 46, 176–188. [Google Scholar] [CrossRef]
- Ruiz-Capillas, C.; Herrero, A. Impact of Biogenic Amines on Food Quality and Safety. Foods 2019, 8, 62. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic Amine Production by Lactic Acid Bacteria: A Review. Foods 2019, 8, 17. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, Y.; Peng, Q.; Han, Y. Biogenic Amines in Wine: A Review. Int. J. Food Sci. Technol. 2015, 50, 1523–1532. [Google Scholar] [CrossRef]
- Özogul, F.; Kacar, Ç.; Hamed, I. Inhibition Effects of Carvacrol on Biogenic Amines Formation by Common Food-Borne Pathogens in Histidine Decarboxylase Broth. LWT 2015, 64, 50–55. [Google Scholar] [CrossRef]
- Cunha, S.C.; Lopes, R.; Fernandes, J.O. Biogenic Amines in Liqueurs: Influence of Processing and Composition. J. Food Compos. Anal. 2017, 56, 147–155. [Google Scholar] [CrossRef]
- Kalač, P. Health Effects and Occurrence of Dietary Polyamines: A Review for the Period 2005–Mid 2013. Food Chem. 2014, 161, 27–39. [Google Scholar] [CrossRef]
- Akyol, O.; Tessier, K.; Akyol, S. Accuracy and Uniformity of the Nomenclature of Biogenic Amines and Polyamines in Metabolomics Studies: A Preliminary Study. BMB 2021, 49, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Dala-Paula, B.M.; Custódio, F.B.; Gloria, M.B. Health Concerns Associated with Biogenic Amines in Food and Interaction with Amine Oxidase Drugs. Curr. Opin. Food Sci. 2023, 54, 101090. [Google Scholar] [CrossRef]
- Del Rio, B.; Fernandez, M.; Redruello, B.; Ladero, V.; Alvarez, M.A. New Insights into the Toxicological Effects of Dietary Biogenic Amines. Food Chem. 2024, 435, 137558. [Google Scholar] [CrossRef]
- Jeon, A.R.; Lee, J.H.; Mah, J.-H. Biogenic Amine Formation and Bacterial Contribution in Cheonggukjang, a Korean Traditional Fermented Soybean Food. LWT 2018, 92, 282–289. [Google Scholar] [CrossRef]
- Hu, M.; Dong, J.; Tan, G.; Li, X.; Zheng, Z.; Li, M. Metagenomic Insights into the Bacteria Responsible for Producing Biogenic Amines in Sufu. Food Microbiol. 2021, 98, 103762. [Google Scholar] [CrossRef]
- Kim, K.H.; Chun, B.H.; Kim, J.; Jeon, C.O. Identification of Biogenic Amine-Producing Microbes during Fermentation of Ganjang, a Korean Traditional Soy Sauce, through Metagenomic and Metatranscriptomic Analyses. Food Control 2021, 121, 107681. [Google Scholar] [CrossRef]
- Ladero, V.; Martín, M.C.; Redruello, B.; Mayo, B.; Flórez, A.B.; Fernández, M.; Alvarez, M.A. Genetic and Functional Analysis of Biogenic Amine Production Capacity among Starter and Non-Starter Lactic Acid Bacteria Isolated from Artisanal Cheeses. Eur. Food Res. Technol. 2015, 241, 377–383. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Domínguez, R. Role of Autochthonous Starter Cultures in the Reduction of Biogenic Amines in Traditional Meat Products. Curr. Opin. Food Sci. 2017, 14, 61–65. [Google Scholar] [CrossRef]
- Ladero, V.; Fernández, M.; Calles-Enríquez, M.; Sánchez-Llana, E.; Cañedo, E.; Martín, M.C.; Alvarez, M.A. Is the Production of the Biogenic Amines Tyramine and Putrescine a Species-Level Trait in Enterococci? Food Microbiol. 2012, 30, 132–138. [Google Scholar] [CrossRef]
- Graham, K.; Stack, H.; Rea, R. Safety, Beneficial and Technological Properties of Enterococci for Use in Functional Food Applications—A Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3836–3861. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.M.; Manso, J.A.; Figueiredo, A.C.; Antunes, L.; Cruz, R.; Manadas, B.; Bur, D.; Pereira, P.J.B.; Faro, C.; Simões, I. Functional and Structural Characterization of Synthetic Cardosin B-Derived Rennet. Appl. Microbiol. Biotechnol. 2017, 101, 6951–6968. [Google Scholar] [CrossRef]
- Nami, Y.; Vaseghi Bakhshayesh, R.; Mohammadzadeh Jalaly, H.; Lotfi, H.; Eslami, S.; Hejazi, M.A. Probiotic Properties of Enterococcus Isolated From Artisanal Dairy Products. Front. Microbiol. 2019, 10, 300. [Google Scholar] [CrossRef]
- Montanari, C.; Barbieri, F.; Lorenzini, S.; Gottardi, D.; Šimat, V.; Özogul, F.; Gardini, F.; Tabanelli, G. Survival, Growth, and Biogenic Amine Production of Enterococcus faecium FC12 in Response to Extracts and Essential Oils of Rubus Fruticosus and Juniperus Oxycedrus. Front. Nutr. 2023, 9, 1092172. [Google Scholar] [CrossRef]
- Suárez, C.; Espariz, M.; Blancato, V.S.; Magni, C. Expression of the Agmatine Deiminase Pathway in Enterococcus faecalis Is Activated by the AguR Regulator and Repressed by CcpA and PTSMan Systems. PLoS ONE 2013, 8, e76170. [Google Scholar] [CrossRef]
- Landete, J.M.; Arena, M.E.; Pardo, I.; Manca de Nadara, M.C.; Ferrer, S. The Role of Two Families of Bacterial Enzymes in Putrescine Synthesis from Agmatine via Agmatine Deiminase. Int. Microbiol. 2010, 13, 169–177. [Google Scholar] [CrossRef]
- Perez, M.; Ladero, V.; Del Rio, B.; Redruello, B.; De Jong, A.; Kuipers, O.; Kok, J.; Martin, M.C.; Fernandez, M.; Alvarez, M.A. The Relationship among Tyrosine Decarboxylase and Agmatine Deiminase Pathways in Enterococcus faecalis. Front. Microbiol. 2017, 8, 2107. [Google Scholar] [CrossRef]
- Nalazek-Rudnicka, K.; Wasik, A. Development and Validation of an LC–MS/MS Method for the Determination of Biogenic Amines in Wines and Beers. Monatsh. Chem. 2017, 148, 1685–1696. [Google Scholar] [CrossRef]
- Dapkevicius, M.D.L.E.; Sgardioli, B.; Câmara, S.P.A.; Poeta, P.; Malcata, F.X. Current Trends of Enterococci in Dairy Products: A Comprehensive Review of Their Multiple Roles. Foods 2021, 10, 821. [Google Scholar] [CrossRef]
- Hanzelová, Z.; Dudriková, E.; Lovayová, V.; Výrostková, J.; Regecová, I.; Zigo, F.; Bartáková, K. Occurrence of Enterococci in the Process of Artisanal Cheesemaking and Their Antimicrobial Resistance. Life 2024, 14, 890. [Google Scholar] [CrossRef]
- Sarantinopoulos, P.; Andrighetto, C.; Georgalaki, M.D.; Rea, M.C.; Lombardi, A.; Cogan, T.M.; Kalantzopoulos, G.; Tsakalidou, E. Biochemical Properties of Enterococci Relevant to Their Technological Performance. Int. Dairy J. 2001, 11, 621–647. [Google Scholar] [CrossRef]
- Nasiri, M.; Hanifian, S. Enterococcus faecalis and Enterococcus faecium in Pasteurized Milk: Prevalence, Genotyping, and Characterization of Virulence Traits. LWT 2022, 153, 112452. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Processing Contaminants: Biogenic Amines. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 381–391. ISBN 978-0-12-378613-5. [Google Scholar]
- Commission Regulation (EC) Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs 2005. Available online: https://eur-lex.europa.eu/legal-content/PL/TXT/PDF/?uri=CELEX:32005R2073 (accessed on 16 March 2025).
- Van Hoogdalem, E.; Smith, K.L.; Hartstra, J.; Constant, J. Rethinking, Reducing, and Refining the Classical Oral Tyramine Challenge Test of Monoamine Oxidase (MAO) Inhibitors. Clin. Transl. Sci. 2023, 16, 2058–2069. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F. Chapter 1. Biogenic Amines Formation, Toxicity, Regulations in Food. In Food Chemistry, Function and Analysis; Saad, B., Tofalo, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–17. ISBN 978-1-78801-436-6. [Google Scholar]
- Sadighara, P.; Aghebat-Bekheir, S.; Shafaroodi, H.; Basaran, B.; Sadighara, M. Tyramine, a Biogenic Agent in Cheese: Amount and Factors Affecting Its Formation, a Systematic Review. Food Prod. Process. Nutr. 2024, 6, 30. [Google Scholar] [CrossRef]
- Perez, M.; Calles-Enríquez, M.; Del Rio, B.; Redruello, B.; De Jong, A.; Kuipers, O.P.; Kok, J.; Martin, M.C.; Ladero, V.; Fernandez, M.; et al. Construction and Characterization of a Double Mutant of Enterococcus faecalis That Does Not Produce Biogenic Amines. Sci. Rep. 2019, 9, 16881. [Google Scholar] [CrossRef]
- Jiménez, E.; Ladero, V.; Chico, I.; Maldonado-Barragán, A.; López, M.; Martín, V.; Fernández, L.; Fernández, M.; Álvarez, M.A.; Torres, C.; et al. Antibiotic Resistance, Virulence Determinants and Production of Biogenic Amines among Enterococci from Ovine, Feline, Canine, Porcine and Human Milk. BMC Microbiol. 2013, 13, 288. [Google Scholar] [CrossRef]
- Bargossi, E.; Tabanelli, G.; Montanari, C.; Gatto, V.; Chinnici, F.; Gardini, F.; Torriani, S. Growth, Biogenic Amine Production and tyrDC Transcription of Enterococcus faecalis in Synthetic Medium Containing Defined Amino Acid Concentrations. J. Appl. Microbiol. 2017, 122, 1078–1091. [Google Scholar] [CrossRef]
- Amuasi, G.R.; Dsani, E.; Owusu-Nyantakyi, C.; Owusu, F.A.; Mohktar, Q.; Nilsson, P.; Adu, B.; Hendriksen, R.S.; Egyir, B. Enterococcus Species: Insights into Antimicrobial Resistance and Whole-Genome Features of Isolates Recovered from Livestock and Raw Meat in Ghana. Front. Microbiol. 2023, 14, 1254896. [Google Scholar] [CrossRef]
- Yerlikaya, O.; Akbulut, N. Potential Use of Probiotic Enterococcus faecium and Enterococcus durans Strains in Izmir Tulum Cheese as Adjunct Culture. J. Food Sci. Technol. 2019, 56, 2175–2185. [Google Scholar] [CrossRef]
- Silvetti, T.; Morandi, S.; Brasca, M. Does Enterococcus faecalis from Traditional Raw Milk Cheeses Serve as a Reservoir of Antibiotic Resistance and Pathogenic Traits? FPD 2019, 16, fpd.2018.2542. [Google Scholar] [CrossRef]
- Klementová, L.; Purevdorj, K.; Butor, I.; Jančová, P.; Bábková, D.; Buňka, F.; Buňková, L. Reduction of Histamine, Putrescine and Cadaverine by the Bacteria Lacticaseibacillus casei Depending on Selected Factors in the Real Condition of the Dairy Product. Food Microbiol. 2024, 117, 104391. [Google Scholar] [CrossRef]
- Suzzi, G. Biogenic Amines in Dry Fermented Sausages: A Review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Inoğlu, Z.N.; Tuncer, Y. Safety Assessment of Enterococcus faecium and Enterococcus faecalis Strains Isolated from Turkish Tulum Cheese. J. Food Saf. 2013, 33, 369–377. [Google Scholar] [CrossRef]
- Bargossi, E.; Tabanelli, G.; Montanari, C.; Lanciotti, R.; Gatto, V.; Gardini, F.; Torriani, S. Tyrosine Decarboxylase Activity of Enterococci Grown in Media with Different Nutritional Potential: Tyramine and 2-Phenylethylamine Accumulation and tyrDC Gene Expression. Front. Microbiol. 2015, 6, 138455. [Google Scholar] [CrossRef]
- Eom, J.S.; Seo, B.Y.; Choi, H.S. Biogenic Amine Degradation by Bacillus Species Isolated from Traditional Fermented Soybean Food and Detection of Decarboxylase-Related Genes. J. Microbiol. Biotechnol. 2015, 25, 1519–1527. [Google Scholar] [CrossRef]
- Lucas, P.; Landete, J.; Coton, M.; Coton, E.; Lonvaud-Funel, A. The Tyrosine Decarboxylase Operon of Lactobacillus brevis IOEB 9809: Characterization and Conservation in Tyramine-Producing Bacteria. FEMS Microbiol. Lett. 2003, 229, 65–71. [Google Scholar] [CrossRef]
- Perez, M.; Calles-Enríquez, M.; Nes, I.; Martin, M.C.; Fernandez, M.; Ladero, V.; Alvarez, M.A. Tyramine Biosynthesis Is Transcriptionally Induced at Low pH and Improves the Fitness of Enterococcus faecalis in Acidic Environments. Appl. Microbiol. Biotechnol. 2015, 99, 3547–3558. [Google Scholar] [CrossRef]
- Park, Y.K.; Jin, Y.H.; Lee, J.-H.; Byun, B.Y.; Lee, J.; Jeong, K.C.; Mah, J.-H. The Role of Enterococcus faecium as a Key Producer and Fermentation Condition as an Influencing Factor in Tyramine Accumulation in Cheonggukjang. Foods 2020, 9, 915. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Du, L.; Wang, D.; Zhu, Y.; Geng, Z.; Xu, X.; Xu, W. Effect of NaCI Treatments on Tyramine Biosynthesis of Enterococcus faecalis. J. Food Prot. 2015, 78, 940–945. [Google Scholar] [CrossRef]
- Marcobal, A.; De Las Rivas, B.; Landete, J.M.; Tabera, L.; Muñoz, R. Tyramine and Phenylethylamine Biosynthesis by Food Bacteria. Crit. Rev. Food Sci. Nutr. 2012, 52, 448–467. [Google Scholar] [CrossRef]
- Fernández, M.; Linares, D.M.; Alvarez, M.A. Sequencing of the Tyrosine Decarboxylase Cluster of Lactococcus lactis IPLA 655 and the Development of a PCR Method for Detecting Tyrosine Decarboxylating Lactic Acid Bacteria. J. Food Prot. 2004, 67, 2521–2529. [Google Scholar] [CrossRef]
- Bonham, K.S.; Wolfe, B.E.; Dutton, R.J. Extensive Horizontal Gene Transfer in Cheese-Associated Bacteria. eLife 2017, 6, e22144. [Google Scholar] [CrossRef]
- Vinayamohan, P.G.; Pellissery, A.J.; Venkitanarayanan, K. Role of Horizontal Gene Transfer in the Dissemination of Antimicrobial Resistance in Food Animal Production. Curr. Opin. Food Sci. 2022, 47, 100882. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Gupta, H.; Iyer, R.; Kumar, N.; Malik, R.K. Tyramine-Producing Enterococci Are Equally Detected on Tyramine Production Medium, by Quantification of Tyramine by HPLC, or by Tdc Gene-Targeted PCR. Dairy Sci. Technol. 2009, 89, 601–611. [Google Scholar] [CrossRef]
- Kučerová, K.; Svobodová, H.; Tůma, Š.; Ondráčková, I.; Plocková, M. Production of Biogenic Amines by Enterococci. Czech J. Food Sci. 2009, 27, SII50–SII55. [Google Scholar] [CrossRef]
- Kalhotka, L.; Manga, I.; Přichystalová, J.; Hůlová, M.; Vyletělová, M.; Šustová, K. Decarboxylase Activity Test of the Genus Enterococcus Isolated from Goat Milk and Cheese. Acta Vet. Brno 2012, 81, 145–151. [Google Scholar] [CrossRef]
- Fernández, M.; Del Río, B.; Linares, D.M.; Martín, M.C.; Alvarez, M.A. Real-Time Polymerase Chain Reaction for Quantitative Detection of Histamine-Producing Bacteria: Use in Cheese Production. J. Dairy Sci. 2006, 89, 3763–3769. [Google Scholar] [CrossRef]
- Coton, M.; Coton, E.; Lucas, P.; Lonvaud, A. Identification of the Gene Encoding a Putative Tyrosine Decarboxylase of Carnobacterium Divergens 508. Development of Molecular Tools for the Detection of Tyramine-Producing Bacteria. Food Microbiol. 2004, 21, 125–130. [Google Scholar] [CrossRef]
- Torriani, S.; Gatto, V.; Sembeni, S.; Tofalo, R.; Suzzi, G.; Belletti, N.; Gardini, F.; Bover-Cid, S. Rapid Detection and Quantification of Tyrosine Decarboxylase Gene (Tdc) and Its Expression in Gram-Positive Bacteria Associated with Fermented Foods Using PCR-Based Methods. J. Food Prot. 2008, 71, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bargossi, E.; Gardini, F.; Gatto, V.; Montanari, C.; Torriani, S.; Tabanelli, G. The Capability of Tyramine Production and Correlation between Phenotypic and Genetic Characteristics of Enterococcus faecium and Enterococcus faecalis Strains. Front. Microbiol. 2015, 6, 1371. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Ramadan, M.F. Genetic Screening of Biogenic Amines Production Capacity from Some Lactic Acid Bacteria Strains. Food Control 2016, 68, 220–228. [Google Scholar] [CrossRef]
- Landete, J.M.; De Las Rivas, B.; Marcobal, A.; Muñoz, R. Molecular Methods for the Detection of Biogenic Amine-Producing Bacteria on Foods. Int. J. Food Microbiol. 2007, 117, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, U.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Effects of Osmotic and High Pressure Stress on Expression of Virulence Factors among Enterococcus spp. Isolated from Food of Animal Origin. Food Microbiol. 2022, 102, 103900. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.J.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Antimicrobial Resistance and Virulence Characterization of Listeria monocytogenes Strains Isolated from Food and Food Processing Environments. Pathogens 2022, 11, 1099. [Google Scholar] [CrossRef]
- Zakrzewski, A.J.; Zarzecka, U.; Chajęcka-Wierzchowska, W.; Zadernowska, A. A Comparison of Methods for Identifying Enterobacterales Isolates from Fish and Prawns. Pathogens 2022, 11, 410. [Google Scholar] [CrossRef]
- Zarzecka, U.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Microorganisms from Starter and Protective Cultures—Occurrence of Antibiotic Resistance and Conjugal Transfer of Tet Genes in Vitro and during Food Fermentation. LWT 2022, 153, 112490. [Google Scholar] [CrossRef]
- Wiśniewski, P.; Zakrzewski, A.; Chajęcka-Wierzchowska, W.; Zadernowska, A. Possibility of Transfer and Activation of “silent” Tetracycline Resistance Genes among Enterococcus faecalis under High-Pressure Processing. Food Microbiol. 2024, 120, 104481. [Google Scholar] [CrossRef]
- Bover-Cid, S.; Holzapfel, W.H. Improved Screening Procedure for Biogenic Amine Production by Lactic Acid Bacteria. Int. J. Food Microbiol. 1999, 53, 33–41. [Google Scholar] [CrossRef]
- Barbieri, F.; Tabanelli, G.; Montanari, C.; Dall’Osso, N.; Šimat, V.; Smole Možina, S.; Baños, A.; Özogul, F.; Bassi, D.; Fontana, C.; et al. Mediterranean Spontaneously Fermented Sausages: Spotlight on Microbiological and Quality Features to Exploit Their Bacterial Biodiversity. Foods 2021, 10, 2691. [Google Scholar] [CrossRef]
- Martuscelli, M.; Crudele, M.A.; Gardini, F.; Suzzi, G. Biogenic Amine Formation and Oxidation by Staphylococcus xylosus Strains from Artisanal Fermented Sausages. Lett. Appl. Microbiol. 2000, 31, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wen, X.; Wen, Z.; Chen, S.; Wang, L.; Wei, X. Evaluation of the Biogenic Amines Formation and Degradation Abilities of Lactobacillus curvatus From Chinese Bacon. Front. Microbiol. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).