In Vivo Local Administration of Para-Amino-Bebblistatin to the Injured Spinal Cord Fails to Improve the NaChBac-Expressing DRGs Transplantation
Abstract
1. Introduction
2. Results
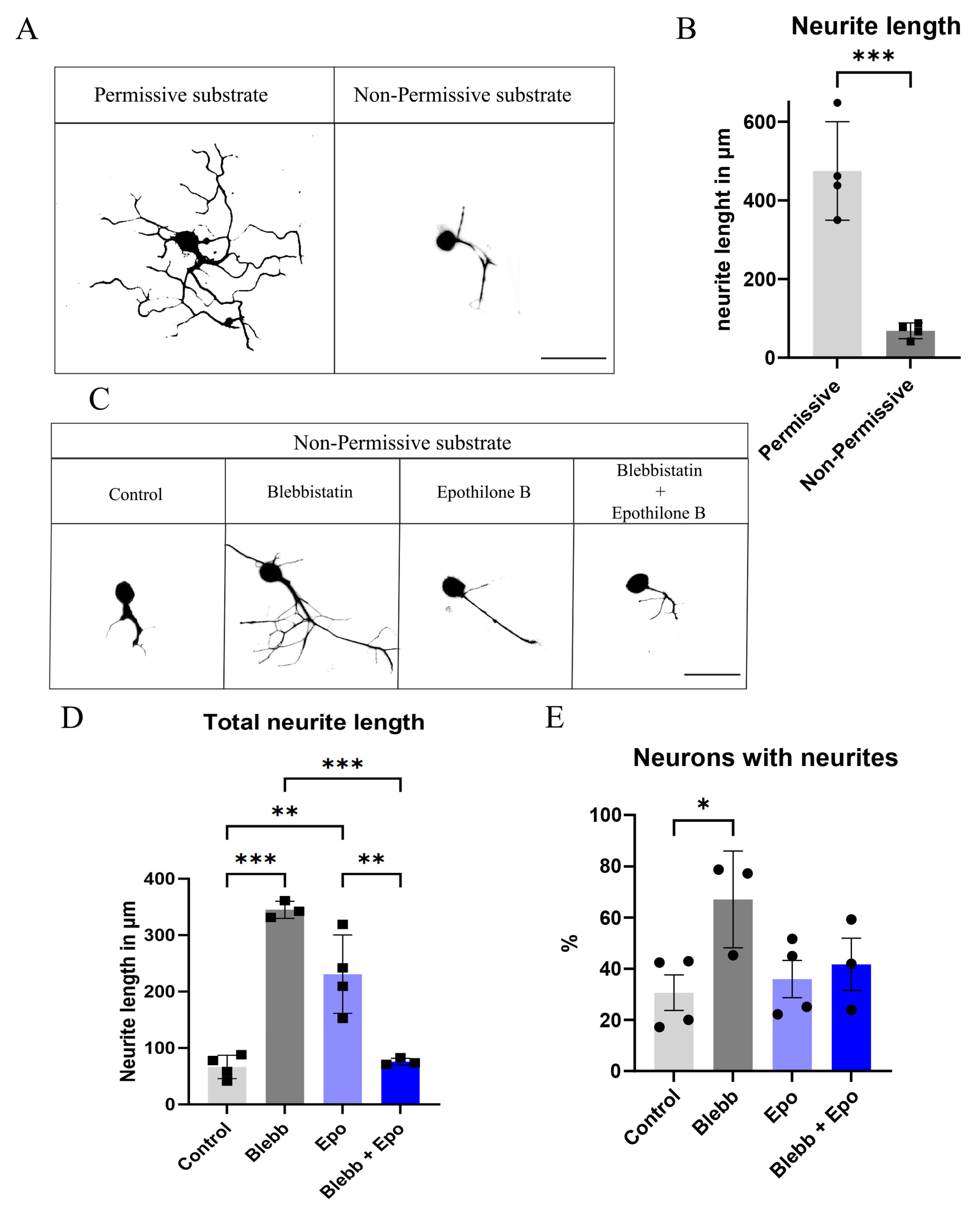
2.1. Blebbistatin or Epothilone B Alone Rescues the Neurite Length of Neurons Plated over CSPGs, Whereas a Combination Reverts This Effect
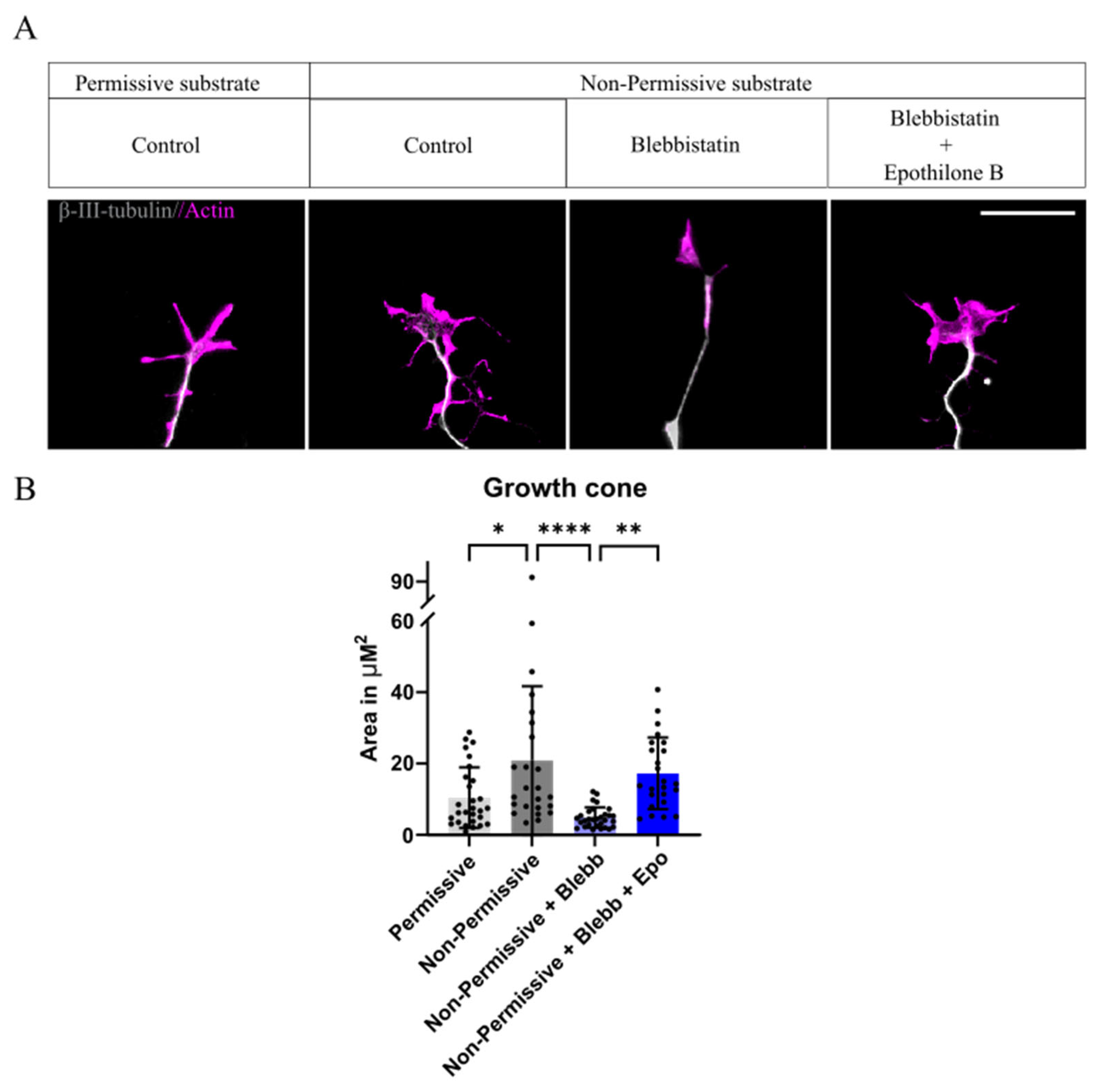
2.2. Growth Cone Area Increases with the Presence of CSPGs, Blebbistatin Reverts Back to Normal Conditions, Whereas the Combination with Epothilone B Fails to Do So
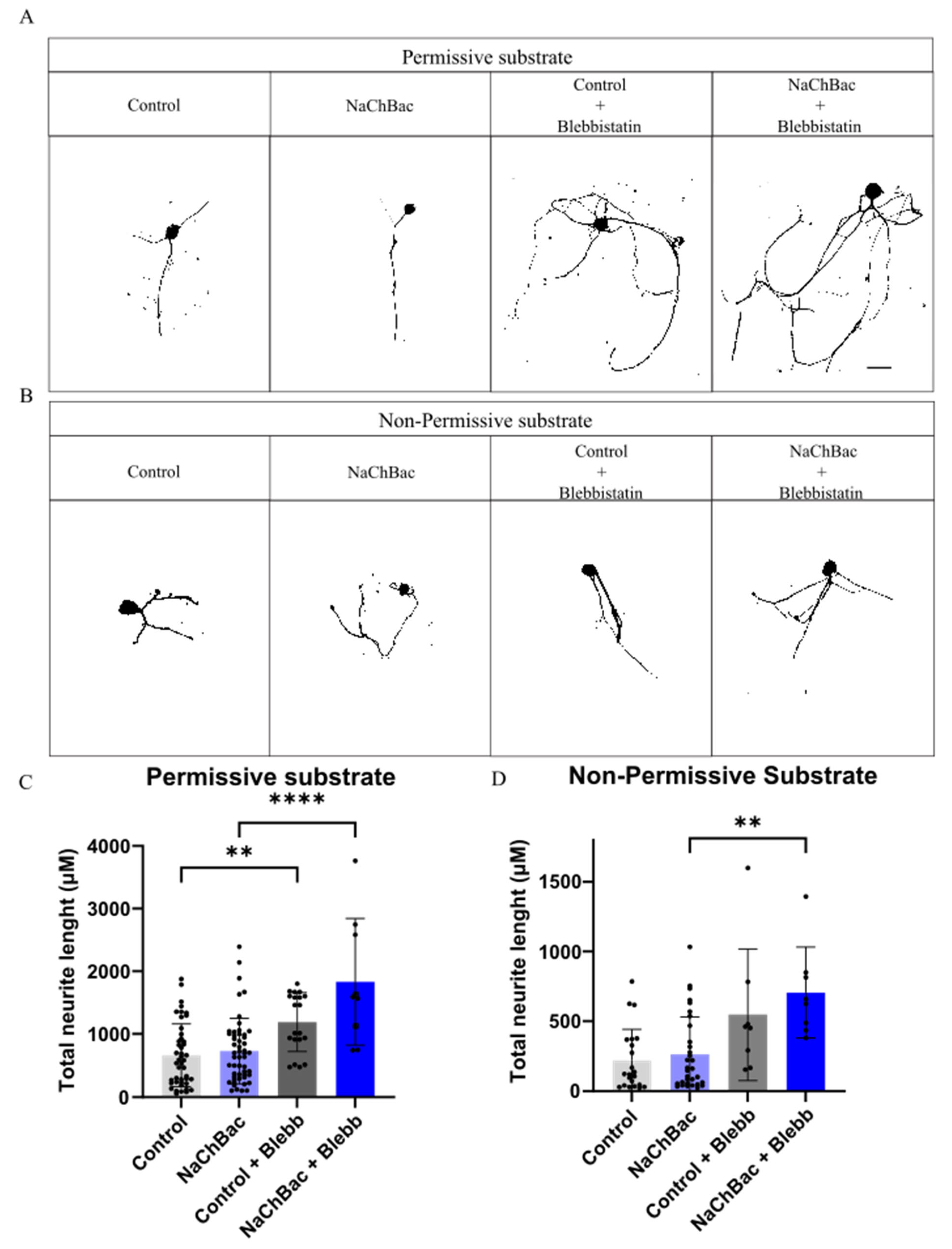
2.3. Blebbistatin Increases Neurite Length in Neurons Expressing NaChBac Under Non-Permissive Substrate
2.4. Administration of Para-Amino-Blebbistatin Does Not Alter Functional Locomotion After SCI
3. Discussion
4. Conclusions and Future Perspectives
5. Materials and Methods
5.1. Dorsal Root Ganglia Isolation and Culture
5.2. Coatings
5.3. Neurite Assay Experiments
5.4. Image Analysis
5.5. Spinal Cord Injury
5.6. Functional Evaluation
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hilton, B.J.; Bradke, F. Can injured adult CNS axons regenerate by recapitulating development? Development 2017, 144, 3417–3429. [Google Scholar] [CrossRef] [PubMed]
- Hoke, A.; Brushart, T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp. Neurol. 2010, 223, 1–4. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Aguayo, A.J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 1981, 214, 931–933. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.; Paniagua Soriano, G.; Sanchez Huertas, C.; Villalba Riquelme, E.M.; Lopez Mocholi, E.; Martinez Rojas, B.; Alastrue Agudo, A.; Dupraz, S.; Ferrer Montiel, A.V.; Moreno Manzano, V. Transplantation of dorsal root ganglia overexpressing the NaChBac sodium channel improves locomotion after complete SCI. Mol. Ther. 2024, 32, 1739–1759. [Google Scholar] [CrossRef]
- Neumann, S.; Bradke, F.; Tessier-Lavigne, M.; Basbaum, A.I. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 2002, 34, 885–893. [Google Scholar] [CrossRef]
- Davies, S.J.; Fitch, M.T.; Memberg, S.P.; Hall, A.K.; Raisman, G.; Silver, J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 1997, 390, 680–683. [Google Scholar] [CrossRef]
- Davies, S.J.; Goucher, D.R.; Doller, C.; Silver, J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 1999, 19, 5810–5822. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Xu, H.; Yue, L.; Shi, Q.; Clapham, D.E. A prokaryotic voltage-gated sodium channel. Science 2001, 294, 2372–2375. [Google Scholar] [CrossRef]
- Kelsch, W.; Lin, C.W.; Mosley, C.P.; Lois, C. A critical period for activity-dependent synaptic development during olfactory bulb adult neurogenesis. J. Neurosci. 2009, 29, 11852–11858. [Google Scholar] [CrossRef]
- Priya, R.; Paredes, M.F.; Karayannis, T.; Yusuf, N.; Liu, X.; Jaglin, X.; Graef, I.; Alvarez-Buylla, A.; Fishell, G. Activity Regulates Cell Death within Cortical Interneurons through a Calcineurin-Dependent Mechanism. Cell Rep. 2018, 22, 1695–1709. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Jiang, C.; Chen, Z.; Ni, S.; Fan, H.; Wang, Z.; Tian, F.; An, J.; Yang, H.; et al. Rho Kinase Inhibitor Y27632 Improves Recovery After Spinal Cord Injury by Shifting Astrocyte Phenotype and Morphology via the ROCK/NF-kappaB/C3 Pathway. Neurochem. Res. 2022, 47, 3733–3744. [Google Scholar] [CrossRef] [PubMed]
- Ruschel, J.; Hellal, F.; Flynn, K.C.; Dupraz, S.; Elliott, D.A.; Tedeschi, A.; Bates, M.; Sliwinski, C.; Brook, G.; Dobrindt, K.; et al. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science 2015, 348, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.M.; Hingorani Jai Prakash, S.; Bockemuhl, T.; Benner, J.M.; Schaffran, B.; Moreno-Manzano, V.; Buschges, A.; Bradke, F. Rehabilitation enhances epothilone-induced locomotor recovery after spinal cord injury. Brain Commun. 2023, 5, fcad005. [Google Scholar] [CrossRef]
- Courtine, G.; Sofroniew, M.V. Spinal cord repair: Advances in biology and technology. Nat. Med. 2019, 25, 898–908. [Google Scholar] [CrossRef]
- Hur, E.M.; Yang, I.H.; Kim, D.H.; Byun, J.; Saijilafu; Xu, W.L.; Nicovich, P.R.; Cheong, R.; Levchenko, A.; Thakor, N.; et al. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc. Natl. Acad. Sci. 2011, 108, 5057–5062. [Google Scholar] [CrossRef]
- Sandner, B.; Puttagunta, R.; Motsch, M.; Bradke, F.; Ruschel, J.; Blesch, A.; Weidner, N. Systemic epothilone D improves hindlimb function after spinal cord contusion injury in rats. Exp. Neurol. 2018, 306, 250–259. [Google Scholar] [CrossRef]
- Wang, X.W.; Yang, S.G.; Zhang, C.; Hu, M.W.; Qian, J.; Ma, J.J.; Zhang, Y.; Yang, B.B.; Weng, Y.L.; Ming, G.L.; et al. Knocking Out Non-muscle Myosin II in Retinal Ganglion Cells Promotes Long-Distance Optic Nerve Regeneration. Cell Rep. 2020, 31, 107537. [Google Scholar] [CrossRef]
- Heo, K.; Ho, T.S.; Zeng, X.; Turnes, B.L.; Arab, M.; Jayakar, S.; Chen, K.; Kimourtzis, G.; Condro, M.C.; Fazzari, E.; et al. Non-muscle myosin II inhibition at the site of axon injury increases axon regeneration. Nat. Commun. 2025, 16, 2975. [Google Scholar] [CrossRef]
- Franze, K.; Guck, J. The biophysics of neuronal growth. Rep. Prog. Phys. 2010, 73, 094601. [Google Scholar] [CrossRef]
- Leite, S.C.; Pinto-Costa, R.; Sousa, M.M. Actin dynamics in the growth cone: A key player in axon regeneration. Curr. Opin. Neurobiol. 2021, 69, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Dupraz, S.; Hilton, B.J.; Husch, A.; Santos, T.E.; Coles, C.H.; Stern, S.; Brakebusch, C.; Bradke, F. RhoA Controls Axon Extension Independent of Specification in the Developing Brain. Curr. Biol. 2019, 29, 3874–3886.e9. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, S.; Gordon-Weeks, P.R. Cytoskeletal dynamics in growth-cone steering. J. Cell Sci. 2009, 122, 3595–3604. [Google Scholar] [CrossRef] [PubMed]
- Situ, Z.; Lu, M.; Chen, W.; Xie, Z.; Chen, S.L.; Dang, L.; Li, M.D. Boosting the Release of Leaving Group from Blebbistatin Derivative Photocages via Enhancing Intramolecular Charge Transfer and Stabilizing Cationic Intermediate. J. Phys. Chem. Lett. 2023, 14, 11580–11586. [Google Scholar] [CrossRef]
- Stern, S.; Hilton, B.J.; Burnside, E.R.; Dupraz, S.; Handley, E.E.; Gonyer, J.M.; Brakebusch, C.; Bradke, F. RhoA drives actin compaction to restrict axon regeneration and astrocyte reactivity after CNS injury. Neuron 2021, 109, 3436–3455.e9. [Google Scholar] [CrossRef]
- Jang, E.H.; Sim, A.; Im, S.K.; Hur, E.M. Effects of Microtubule Stabilization by Epothilone B Depend on the Type and Age of Neurons. Neural. Plast. 2016, 2016, 5056418. [Google Scholar] [CrossRef]
- Chen, B.; Li, Y.; Yu, B.; Zhang, Z.; Brommer, B.; Williams, P.R.; Liu, Y.; Hegarty, S.V.; Zhou, S.; Zhu, J.; et al. Reactivation of Dormant Relay Pathways in Injured Spinal Cord by KCC2 Manipulations. Cell 2018, 174, 521–535.e13. [Google Scholar] [CrossRef]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hingorani, S.; Paniagua Soriano, G.; Huertas, C.S.; Manzano, V.M. In Vivo Local Administration of Para-Amino-Bebblistatin to the Injured Spinal Cord Fails to Improve the NaChBac-Expressing DRGs Transplantation. Int. J. Mol. Sci. 2025, 26, 10479. https://doi.org/10.3390/ijms262110479
Hingorani S, Paniagua Soriano G, Huertas CS, Manzano VM. In Vivo Local Administration of Para-Amino-Bebblistatin to the Injured Spinal Cord Fails to Improve the NaChBac-Expressing DRGs Transplantation. International Journal of Molecular Sciences. 2025; 26(21):10479. https://doi.org/10.3390/ijms262110479
Chicago/Turabian StyleHingorani, Sonia, Guillem Paniagua Soriano, Carlos Sánchez Huertas, and Victoria Moreno Manzano. 2025. "In Vivo Local Administration of Para-Amino-Bebblistatin to the Injured Spinal Cord Fails to Improve the NaChBac-Expressing DRGs Transplantation" International Journal of Molecular Sciences 26, no. 21: 10479. https://doi.org/10.3390/ijms262110479
APA StyleHingorani, S., Paniagua Soriano, G., Huertas, C. S., & Manzano, V. M. (2025). In Vivo Local Administration of Para-Amino-Bebblistatin to the Injured Spinal Cord Fails to Improve the NaChBac-Expressing DRGs Transplantation. International Journal of Molecular Sciences, 26(21), 10479. https://doi.org/10.3390/ijms262110479





