Atypical Presentations and Molecular Diagnosis of Ocular Bartonellosis
Abstract
1. Introduction
2. Results (Table 1)
| Case | Age/Gender | Symptoms | Diagnostic Findings | Treatment | Outcome |
|---|---|---|---|---|---|
| 1 | 20 M | Fever, ↓ VA RE, bilateral optic disc swelling, scotomas | Bartonella IgG+, choroiditis, retinitis, macular star, CSF mild pleocytosis | Ciprofloxacin + Azithromycin (allergic to sulfa) | Full recovery (20/20 both eyes) |
| 2 | 17 F | Fever, ↓ VA RE, central scotoma | Bartonella IgG+, optic disc swelling, macular star, focal haemorrhage, focal choroiditis | Rifampicin + TMP-SMX | Full recovery (20/20) |
| 3 | 71 F | Bilateral ↓ VA, resistant posterior uveitis | Bartonella DNA (PCR+, vitreous), microaneurysms, placoid exudates, choroidal hypoperfusion | Intravitreal gentamicin + ciprofloxacin → Rifampicin + TMP-SMX + low-dose steroids | Partial recovery (20/63 RE, 20/200 LE, fibrosis) |
| 4 | 11 F | ↓ VA RE, bilateral sectorial optic nerve swelling | Bartonella IgG+/IgM+, bilateral macular edoema (OCT), normal MRI/LP | Azithromycin (age contraindicated quinolones) | Full recovery (20/20 both eyes) |
| 5 | 30 F | Painful ↓ VA LE, papillary edoema, serous RD | Bartonella IgG+, focal choroiditis, negative systemic workup | Rifampicin + TMP-SMX (10 days) | Full recovery (20/20 in 8 weeks) |
2.1. Case 1 (Figure 1)

2.2. Case 2 (Figure 2)
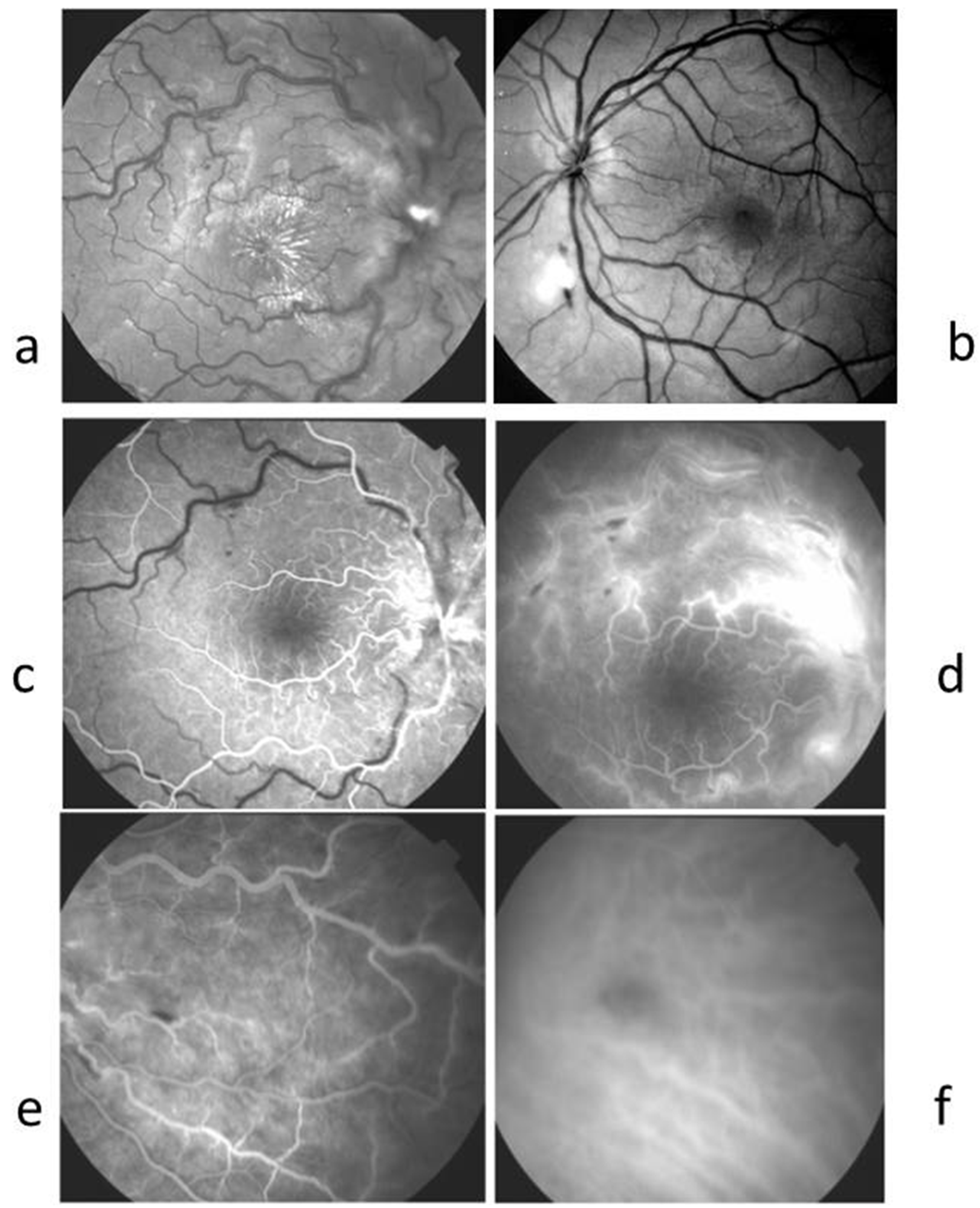
2.3. Case 3 (Figure 3)
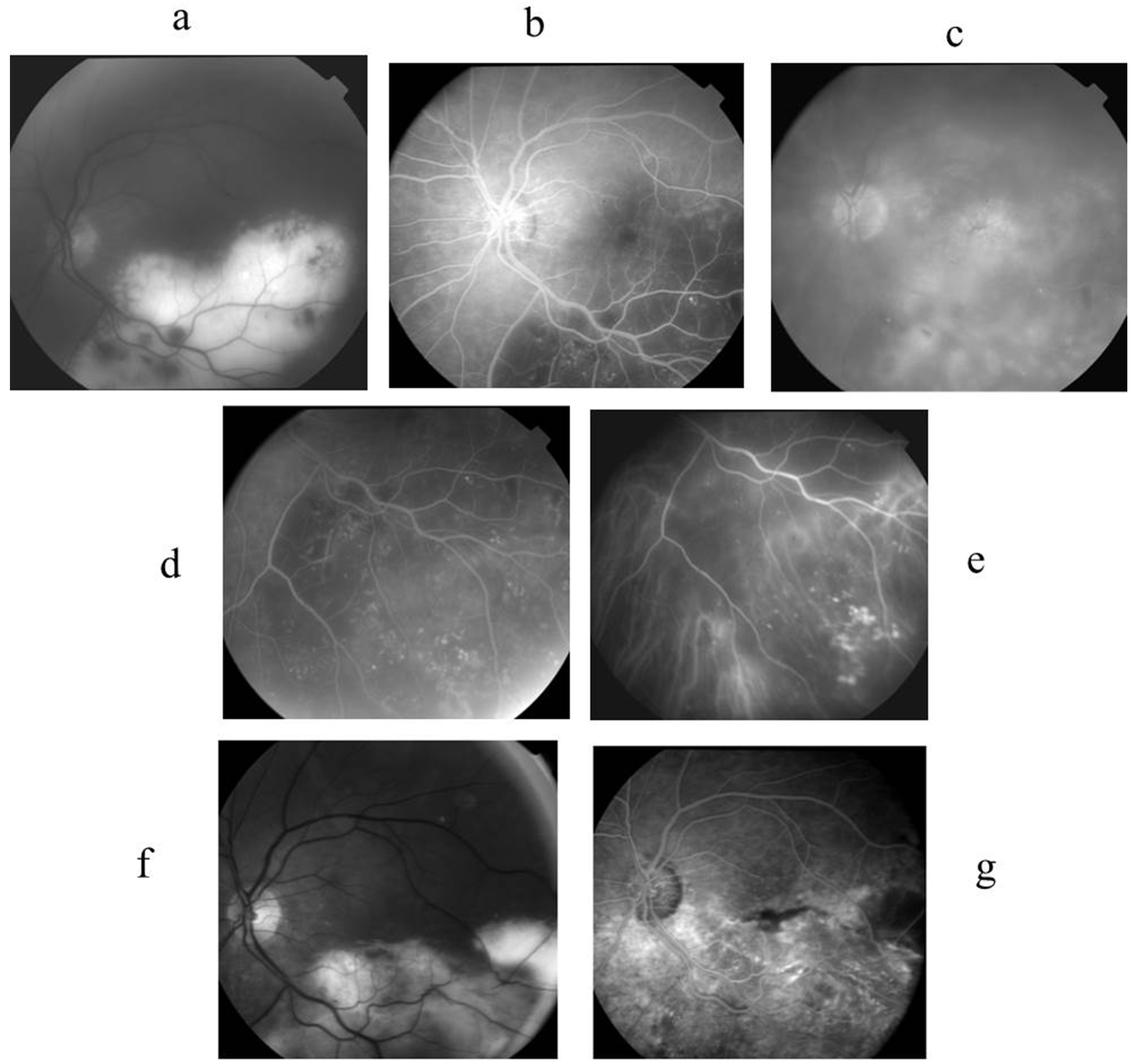
2.4. Case 4 (Figure 4)
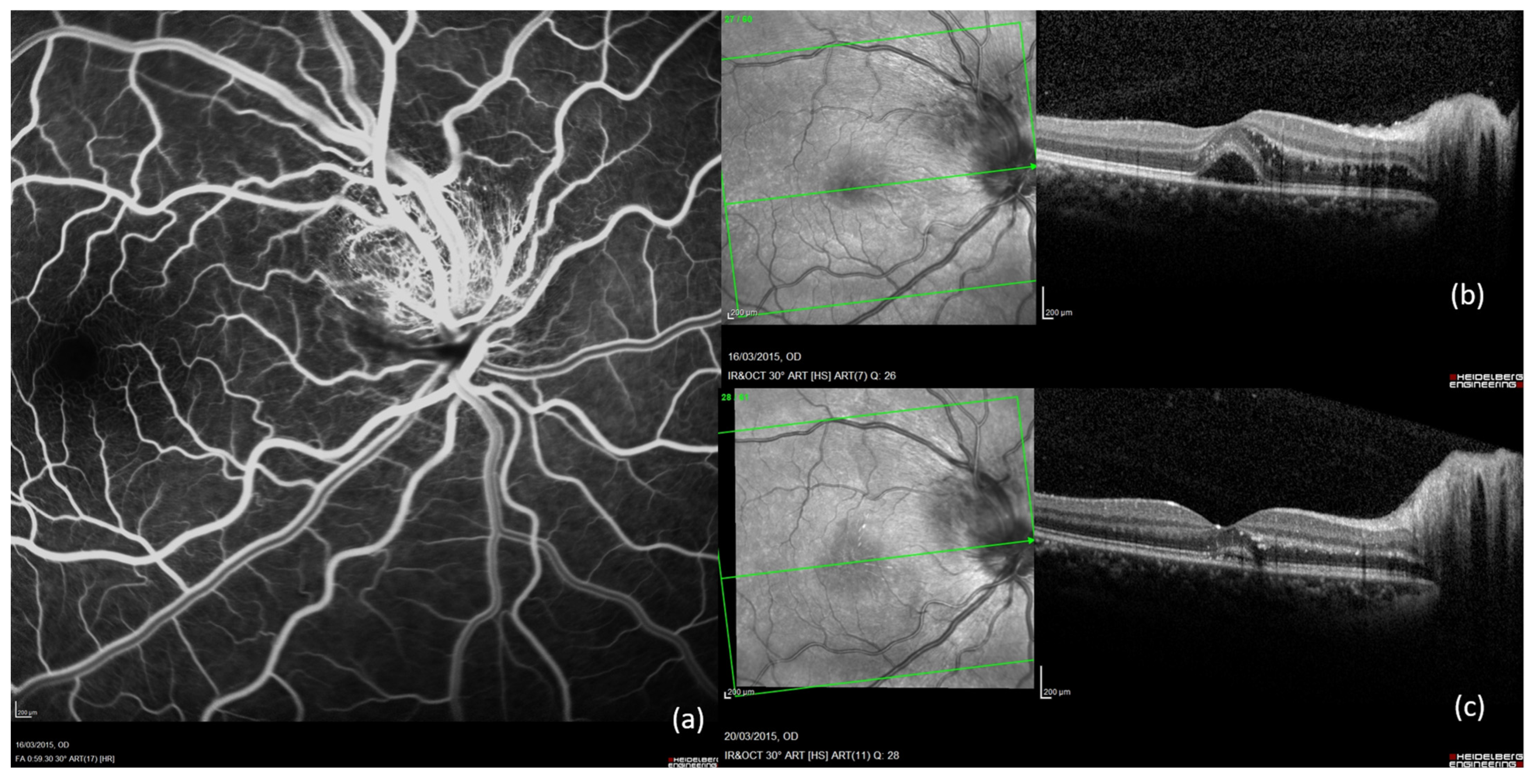
2.5. Case 5 (Figure 5)
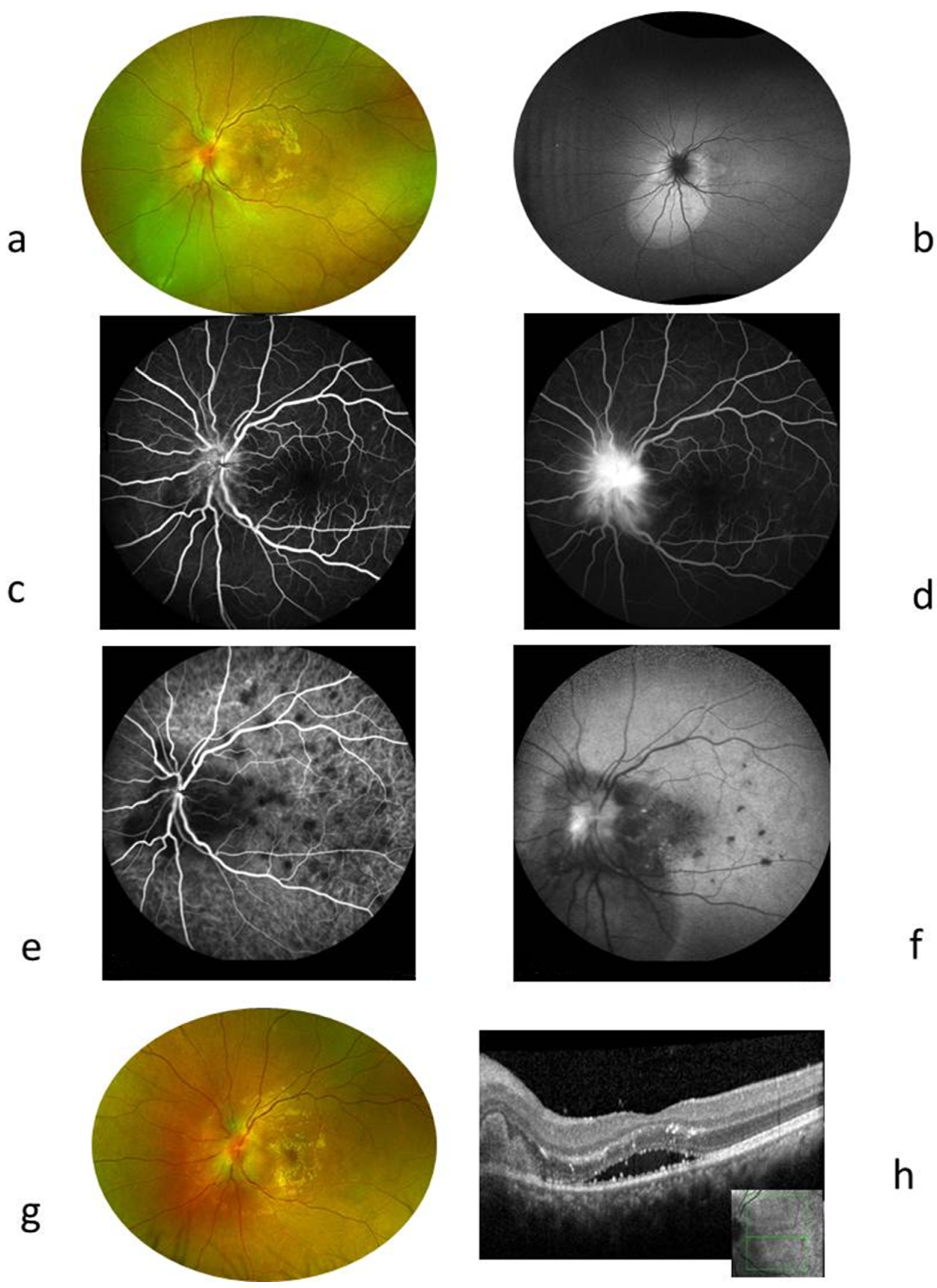
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Exclusion Criteria
4.3. Diagnostic Definitions
4.4. Ethics and Consent
4.5. Ophthalmic and Wide Workup Assessment
4.6. Molecular Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B. henselae | Bartonella henselae |
| SD-OCT | Spectral Domain Optical Coherence Tomography |
| FA | Fluorescein Angiography |
| ICGA | Indocyanine Green Angiography |
| CSD | Cat scratch disease |
| CRP | C-reactive protein |
| WBC | White blood cell |
| HIV | Human immunodeficiency virus |
| ACE | Angiotensin conversion enzyme |
| CT | Computed tomography scan |
| VDRL | Venereal Disease Research Laboratory |
References
- Debre, R.; Lamy, M.; Jammet, M.L.; Costil, L.; Mozziconacci, P. Cat scratch disease. Sem. Hop. 1950, 26, 1895–1904. [Google Scholar]
- Carithers, H.A. Cat-scratch disease. An overview based on a study of 1200 patients. Am. J. Dis. Child. 1985, 139, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Regnery, R.L.; Olson, J.G.; Perkins, B.A.; Bibb, W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 1992, 339, 1443–1445. [Google Scholar] [CrossRef]
- Casson, R.J.; O’Day, J.; Crompton, J.L. Leber’s idiopathic stellate neuroretinitis: Differential diagnosis and approach to management. Aust. N. Z. J. Ophthalmol. 1999, 27, 65–69. [Google Scholar] [CrossRef]
- Dutta Majumder, P.; Mochizuki, M.; González-López, J.J.; Gonzales, J.; Sharma, M.; Sharma, K.; Biswas, J. Laboratory Investigations in Infectious Uveitis. Ocul. Immunol. Inflamm. 2023, 31, 1405–1415. [Google Scholar] [CrossRef]
- Thomas, J.; Nguyen, N.V.; Fashina, T.; Huang, Y.; Yeh, S.; Conrady, C.D. An update on immunological and molecular tests and their impact in infectious uveitis. Front. Ophthalmol. 2023, 3, 1132131. [Google Scholar] [CrossRef] [PubMed]
- Adra, C.N.; Eggerding, F.A. Diagnostic molecular microbiology in posterior uveitis: Principles and applications. Int. Ophthalmol. Clin. 1995, 35, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Dutta Majumder, P.; Palkar, A.; Annamalai, R.; Biswas, J. Laboratory investigations in uveitis: Current practice and future directions. Can. J. Ophthalmol. J. Can. Ophtalmol. 2018, 53, 193–198. [Google Scholar] [CrossRef]
- Teoh, S.C.; Dick, A.D. Diagnostic techniques for inflammatory eye disease: Past, present and future: A review. BMC Ophthalmol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Celiker, H.; Kazokoglu, H.; Eraslan, M.; Cerman, E.; Karabas, L. Bartonella henselae Neuroretinitis in Patients Without Cat Scratch. Jpn. J. Infect. Dis. 2018, 71, 397–401. [Google Scholar] [CrossRef]
- Depeyre, C.; Mancel, E.; Besson-Leaud, L.; Goursaud, R. Abrupt visual loss in children. Three case studies of ocular bartonellosis. J. Fr. Ophtalmol. 2005, 28, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Alaan, K.; Fisher, M.; Ellis, B. Cat scratch disease of the eye: A case report and literature review. W. Va. Med. J. 2014, 110, 16–18. [Google Scholar]
- Nassif, D.S. Acute Unilateral Vision Loss in a Female Adolescent Due to Ocular Bartonellosis. Pediatr. Emerg. Care 2019, 35, e65–e66. [Google Scholar] [CrossRef]
- Täger, F.M.; Jahnsen, K.J.; Mediavilla, R.M.; Burgos, L.R. Ocular bartonellosis: Report of three clinical cases. Rev. Chil. Infectol. Organo Of. Soc. Chil. Infectol. 2008, 25, 58–63. [Google Scholar] [CrossRef]
- Díaz-Valle, D.; Toledano Fernández, N.; Arteaga Sánchez, A.; Miguélez Sánchez, R.; Pascual Allen, D. Severe retinal phlebitis in ocular bartonellosis. Arch. Soc. Esp. Oftalmol. 2003, 78, 223–226. [Google Scholar] [CrossRef]
- Valor, C.; Huber, K. Atypical presentation of cat scratch disease: Parinaud’s oculoglandular syndrome with facial nerve paresis. BMJ Case Rep. 2018, 2018, bcr2018224378. [Google Scholar] [CrossRef] [PubMed]
- Carithers, H.A.; Margileth, A.M. Cat-scratch disease. Acute encephalopathy and other neurologic manifestations. Am. J. Dis. Child. 1991, 145, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Kalogeropoulos, D.; Asproudis, I.; Stefaniotou, M.; Moschos, M.M.; Mentis, A.; Malamos, K.; Kalogeropoulos, C. Bartonella henselae- and quintana-associated uveitis: A case series and approach of a potentially severe disease with a broad spectrum of ocular manifestations. Int. Ophthalmol. 2019, 39, 2505–2515. [Google Scholar] [CrossRef]
- Habot-Wilner, Z.; Trivizki, O.; Goldstein, M.; Kesler, A.; Shulman, S.; Horowitz, J.; Amer, R.; David, R.; Ben-Arie-Weintrob, Y.; Bakshi, E.; et al. Cat-scratch disease: Ocular manifestations and treatment outcome. Acta Ophthalmol. 2018, 96, e524–e532. [Google Scholar] [CrossRef]
- Biancardi, A.L.; Curi, A.L.L. Cat-scratch disease. Ocul. Immunol. Inflamm. 2014, 22, 148–154. [Google Scholar] [CrossRef]
- Deschasse, C.; Bielefeld, P.; Muselier, A.; Bour, J.B.; Besancenot, J.F.; Garcher, C.C.; Bron, A.M. Eye and cat scratch disease: A case series. J. Fr. Ophtalmol. 2016, 39, 164–170. [Google Scholar] [CrossRef]
- Berguiga, M.; Abouzeid, H.; Bart, P.-A.; Guex-Crosier, Y. Severe occlusive vasculitis as a complication of cat scratch disease. Klin. Monatsbl. Augenheilkd. 2008, 225, 486–487. [Google Scholar] [CrossRef]
- Fenollar, F.; Sire, S.; Wilhelm, N.; Raoult, D. Bartonella vinsonii subsp. arupensis as an agent of blood culture-negative endocarditis in a human. J. Clin. Microbiol. 2005, 43, 945–947, Erratum in J. Clin. Microbiol. 2005, 43, 4923. [Google Scholar] [CrossRef]
- Maurin, M.; Rolain, J.M.; Raoult, D. Comparison of in-house and commercial slides for detection by immunofluorescence of immunoglobulins G and M against Bartonella henselae and Bartonella quintana. Clin. Diagn. Lab. Immunol. 2002, 9, 1004–1009. [Google Scholar] [CrossRef]
- Gass, J.D. Diseases of the optic nerve that may simulate macular disease. Trans. Sect. Ophthalmol. Am. Acad. Ophthalmol. Otolaryngol. 1977, 83, 763–770. [Google Scholar]
- Reed, J.B.; Scales, D.K.; Wong, M.T.; Lattuada, C.P.; Dolan, M.J.; Schwab, I.R. Bartonella henselae neuroretinitis in cat scratch disease. Diagnosis, management, and sequelae. Ophthalmology 1998, 105, 459–466. [Google Scholar] [CrossRef]
- Ormerod, L.D.; Skolnick, K.A.; Menosky, M.M.; Pavan, P.R.; Pon, D.M. Retinal and choroidal manifestations of cat-scratch disease. Ophthalmology 1998, 105, 1024–1031. [Google Scholar] [CrossRef]
- Eiger-Moscovich, M.; Amer, R.; Oray, M.; Tabbara, K.F.; Tugal-Tutkun, I.; Kramer, M. Retinal artery occlusion due to Bartonella henselae infection: A case series. Acta Ophthalmol. 2016, 94, e367–e370. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.M.; Davis, J.L.; Gass, D.M. Branch retinal arterial occlusions in multifocal retinitis with optic nerve edema. Arch. Ophthalmol. 1995, 113, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Rothova, A.; Kerkhoff, F.; Hooft, H.J.; Ossewaarde, J.M. Bartonella serology for patients with intraocular inflammatory disease. Retina 1998, 18, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Soheilian, M.; Markomichelakis, N.; Foster, C.S. Intermediate uveitis and retinal vasculitis as manifestations of cat scratch disease. Am. J. Ophthalmol. 1996, 122, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Golnik, K.C.; Marotto, M.E.; Fanous, M.M.; Heitter, D.; King, L.P.; Halpern, J.I.; Holley, P.H. Ophthalmic manifestations of Rochalimaea species. Am. J. Ophthalmol. 1994, 118, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.; Goldstein, E.; Hung, V.S.; Koehler, J.E.; Richardson, W. Use of retinal biopsy to diagnose Bartonella (formerly Rochalimaea) henselae retinitis in an HIV-infected patient. Arch. Ophthalmol. 1998, 116, 937–940. [Google Scholar] [CrossRef]
- Lohmann, C.P.; Gabler, B.; Kroher, G.; Spiegel, D.; Linde, H.J.; Reischl, U. Disciforme keratitis caused by Bartonella henselae: An unusual ocular complication in cat scratch disease. Eur. J. Ophthalmol. 2000, 10, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.V.; Michels, K.S.; Lauer, A.K.; Samples, J.R. Bartonella henselae infection associated with neuroretinitis, central retinal artery and vein occlusion, neovascular glaucoma, and severe vision loss. Am. J. Ophthalmol. 2004, 137, 187–189. [Google Scholar] [CrossRef]
- Mirakhur, B.; Shah, S.S.; Ratner, A.J.; Goldstein, S.M.; Bell, L.M.; Kim, J.O. Cat Scratch Disease Presenting as Orbital Abscess and Osteomyelitis. J. Clin. Microbiol. 2003, 41, 3991–3993. [Google Scholar] [CrossRef][Green Version]
- Khurana, R.N.; Albini, T.; Green, R.L.; Rao, N.A.; Lim, J.I. Bartonella henselae infection presenting as a unilateral panuveitis simulating Vogt-Koyanagi-Harada syndrome. Am. J. Ophthalmol. 2004, 138, 1063–1065. [Google Scholar] [CrossRef]
- Koehler, J.E. Bartonella-associated infections in HIV-infected patients. AIDS Clin. Care 1995, 7, 97–102. [Google Scholar]
- Fish, R.H.; Hogan, R.N.; Nightingale, S.D.; Anand, R. Peripapillary angiomatosis associated with cat-scratch neuroretinitis. Arch. Ophthalmol. 1992, 110, 323. [Google Scholar] [CrossRef]
- Cunningham, E.T.; McDonald, H.R.; Schatz, H.; Johnson, R.N.; Ai, E.; Grand, M.G. Inflammatory mass of the optic nerve head associated with systemic Bartonella henselae infection. Arch. Ophthalmol. 1997, 115, 1596–1597. [Google Scholar] [CrossRef]
- Drancourt, M.; Berger, P.; Terrada, C.; Bodaghi, B.; Conrath, J.; Raoult, D.; LeHoang, P. High prevalence of fastidious bacteria in 1520 cases of uveitis of unknown etiology. Medicine 2008, 87, 167–176. [Google Scholar] [CrossRef]
- Lyon, E.; Wittwer, C.T. LightCycler technology in molecular diagnostics. J. Mol. Diagn. JMD 2009, 11, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Foucault, C.; Raoult, D.; Brouqui, P. Randomized open trial of gentamicin and doxycycline for eradication of Bartonella quintana from blood in patients with chronic bacteremia. Antimicrob. Agents Chemother. 2003, 47, 2204–2207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Patel, S.J.; Petrarca, R.; Shah, S.M.; Zimmer-Galler, I.; Janjua, K.A.; Do, D.V.; Nguyen, Q.D. Atypical Bartonella hensalae chorioretinitis in an immunocompromised patient. Ocul. Immunol. Inflamm. 2008, 16, 45–49. [Google Scholar] [CrossRef]
- Barza, M. Factors affecting the intraocular penetration of antibiotics. The influence of route, inflammation, animal species and tissue pigmentation. Scand. J. Infect. Dis. Suppl. 1978, 14, 151–159. [Google Scholar]
- Tabbara, K.F.; al-Kharashi, S.A.; al-Mansouri, S.M.; al-Omar, O.M.; Cooper, H.; el-Asrar, A.M.; Foulds, G. Ocular levels of azithromycin. Arch. Ophthalmol. 1998, 116, 1625–1628. [Google Scholar] [CrossRef][Green Version]
- Hancock, H.A.; Guidry, C.; Read, R.W.; Ready, E.L.; Kraft, T.W. Acute Aminoglycoside Retinal Toxicity In Vivo and In Vitro. Investig. Opthalmol. Vis. Sci. 2005, 46, 4804. [Google Scholar] [CrossRef]
- Fardeau, C.; Lee, C.P.L.; Merle-Béral, H.; Cassoux, N.; Bodaghi, B.; Davi, F.; Lehoang, P. Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin primary intraocular lymphoma. Am. J. Ophthalmol. 2009, 147, 886–894.e1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alafaleq, M.; Fardeau, C. Atypical Presentations and Molecular Diagnosis of Ocular Bartonellosis. Int. J. Mol. Sci. 2025, 26, 10421. https://doi.org/10.3390/ijms262110421
Alafaleq M, Fardeau C. Atypical Presentations and Molecular Diagnosis of Ocular Bartonellosis. International Journal of Molecular Sciences. 2025; 26(21):10421. https://doi.org/10.3390/ijms262110421
Chicago/Turabian StyleAlafaleq, Munirah, and Christine Fardeau. 2025. "Atypical Presentations and Molecular Diagnosis of Ocular Bartonellosis" International Journal of Molecular Sciences 26, no. 21: 10421. https://doi.org/10.3390/ijms262110421
APA StyleAlafaleq, M., & Fardeau, C. (2025). Atypical Presentations and Molecular Diagnosis of Ocular Bartonellosis. International Journal of Molecular Sciences, 26(21), 10421. https://doi.org/10.3390/ijms262110421







