A Novel Surface Plasmon Resonance Imaging (SPRi) Biosensor for the Determination of Bovine Interleukin-10: Development, Validation, and Application in Biological Fluids
Abstract
1. Introduction
2. Results
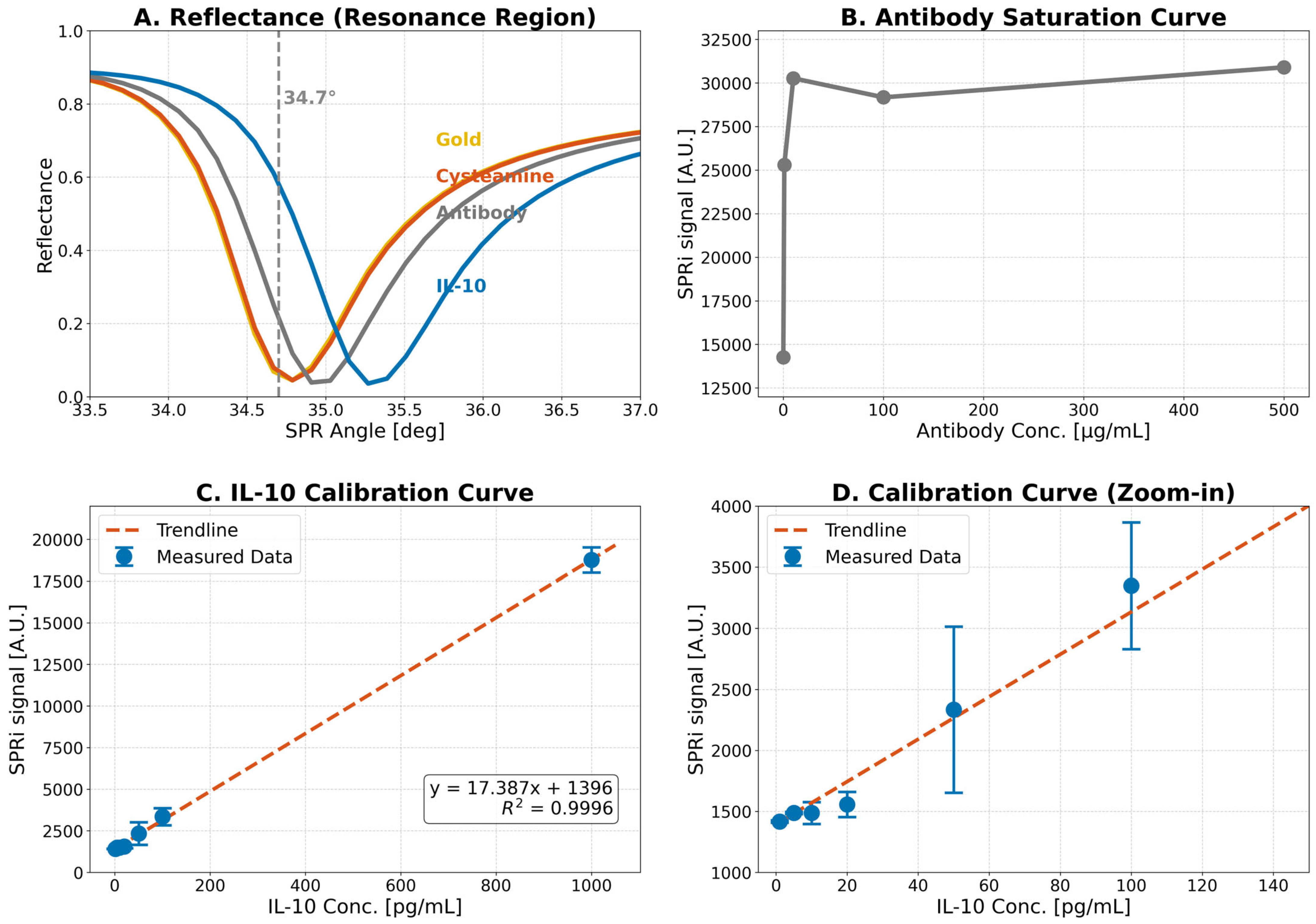
2.1. Optimization of Assay Conditions
2.2. Precision, Accuracy, and Repeatability
2.3. Repeatability
2.4. Selectivity
2.5. Application to Real Samples
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Biosensor Platform and Instrumentation
4.3. Biological Samples and Preparation
4.4. Biosensor Preparation Protocol
4.5. SPRi Measurement Procedure
4.6. Method Validation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- Bannerman, D.D. Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intramammary infection of dairy cows. J. Anim. Sci. 2009, 87, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bochniarz, M.; Zdzisinska, B.; Wawron, W.; Szczubial, M.; Dabrowski, R. Milk and serum IL-4, IL-6, IL-10, and amyloid A concentrations in cows with subclinical mastitis caused by coagulase-negative staphylococci. J. Dairy Sci. 2017, 100, 9674–9680. [Google Scholar] [CrossRef]
- Hussain, T.; Shah, S.Z.; Zhao, D.; Sreevatsan, S.; Zhou, X. The role of IL-10 in Mycobacterium avium subsp. paratuberculosis infection. Cell Commun. Signal. 2016, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Geva, E.; Lessing, J.B.; Lerner-Geva, L.; Azem, F.; Yovel, I.; Ben-Yosef, D.; Barkai, U.; Amit, A. Interleukin-10 in preovulatory follicular fluid of patients undergoing in-vitro fertilization and embryo transfer. Am. J. Reprod. Immunol. 1997, 37, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Hortal, M.; Fabregat, A.; Lledo, B.; Ortiz, J.A.; Moliner, B.; Bernabeu, A.; Bernabeu, R. IL-6/IL-10 and IL-1beta/IL-4 ratios associated with poor ovarian response in women undergoing in-vitro fertilization. Eur. J. Obstet. Gynecol. Reprod. Biol. 2023, 280, 68–72. [Google Scholar] [CrossRef]
- Pytel, A.T.; Zyzynska-Galenska, K.; Gajewski, Z.; Papis, K. Factors defining developmental competence of bovine oocytes collected for in vitro embryo productiondagger. Biol. Reprod. 2024, 111, 1–10. [Google Scholar] [CrossRef]
- Piccinni, M.P.; Vicenti, R.; Logiodice, F.; Fabbri, R.; Kullolli, O.; Pallecchi, M.; Paradisi, R.; Danza, G.; Macciocca, M.; Lombardelli, L.; et al. Description of the Follicular Fluid Cytokine and Hormone Profiles in Human Physiological Natural Cycles. J. Clin. Endocrinol. Metab. 2021, 106, e721–e738. [Google Scholar] [CrossRef]
- Hillyer, L.M.; Woodward, B. Interleukin-10 concentration determined by sandwich enzyme-linked immunosorbent assay is unrepresentative of bioactivity in murine blood. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R1514–R1519. [Google Scholar] [CrossRef]
- Alrabiah, N.A.; Evans, A.C.; Fahey, A.G.; Cantwell, N.; Lonergan, P.; McCormack, J.; Browne, J.A.; Fair, T. Immunological aspects of ovarian follicle ovulation and corpus luteum formation in cattle. Reproduction 2021, 162, 209–225. [Google Scholar] [CrossRef]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Zelazowska-Rutkowska, B.; Gorska, E.; Hermanowicz, A.; Gorodkiewicz, E. New Biosensor for Determination of Neuropilin-1 with Detection by Surface Plasmon Resonance Imaging. Sensors 2023, 23, 4118. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, P.; Chen, Y. Surface Plasmon Resonance Biosensors: A Review of Molecular Imaging with High Spatial Resolution. Biosensors 2024, 14, 84. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Tokarzewicz, A.; Gorodkiewicz, E. Regeneration of surface plasmone resonance chips for multiple use. Bulg. Chem. Commun. 2015, 47, 477–482. [Google Scholar]
- Baek, S.H.; Song, H.W.; Lee, S.; Kim, J.E.; Kim, Y.H.; Wi, J.S.; Ok, J.G.; Park, J.S.; Hong, S.; Kwak, M.K.; et al. Gold Nanoparticle-Enhanced and Roll-to-Roll Nanoimprinted LSPR Platform for Detecting Interleukin-10. Front. Chem. 2020, 8, 285. [Google Scholar] [CrossRef]
- Nanda, A.; Kalyani, T.; Kotal, H.; Jana, S.K. Highly Sensitive Electrochemical Immunosensor for Ultra-Low-Level Detection of Interleukin-10 Using A Cost-Effective Gold Nanoparticle-Modified Electrode. Anal. Bioanal. Electrochem. 2024, 16, 60–78. [Google Scholar] [CrossRef]
- Singampalli, K.L.; Neal-Harris, C.; Yee, C.; Lin, J.S.; Lillehoj, P.B. Highly Reusable Electrochemical Immunosensor for Ultrasensitive Protein Detection. Adv. Sens. Res. 2024, 3, 2400004. [Google Scholar] [CrossRef]
- Sipka, A.; Babasyan, S.; Asbie, S.; Freer, H.; Wagner, B. Optimization of a bovine cytokine multiplex assay using a new bovine and cross-reactive equine monoclonal antibodies. Vet. Immunol. Immunopathol. 2024, 273, 110789. [Google Scholar] [CrossRef]
- Bogdan, S.; Puscion-Jakubik, A.; Klimiuk, K.; Socha, K.; Kochanowicz, J.; Gorodkiewicz, E. The Levels of Leptin, Cystatin C, Neuropilin-1 and Tau Protein in Relation to Dietary Habits in Patients with Alzheimer’s Disease. J. Clin. Med. 2023, 12, 6855. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, B.; Lukaszewski, Z.; Hermanowicz-Szamatowicz, K.; Gorodkiewicz, E. A Multiple-Array SPRi Biosensor as a Tool for Detection of Gynecological-Oncological Diseases. Biosensors 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Lozano, O.; Sierra, M.; Soler, M.; Estevez, M.C.; Chiscano-Camon, L.; Ruiz-Sanmartin, A.; Ruiz-Rodriguez, J.C.; Ferrer, R.; Gonzalez-Lopez, J.J.; Esperalba, J.; et al. Label-Free Plasmonic Biosensor for Rapid, Quantitative, and Highly Sensitive COVID-19 Serology: Implementation and Clinical Validation. Anal. Chem. 2022, 94, 975–984. [Google Scholar] [CrossRef]
- Li, L.; Cheng, C.; Yang, H.; Ye, H.; Luo, X.; Xi, M. Label-Free Localized Surface Plasmon Resonance Biosensor Used to Detect Serum Interleukin-10 in Patients with Endometrial Cancer. Acta Phys. Pol. A 2020, 138, 338–344. [Google Scholar] [CrossRef]
- Hilali, N.; Ruankham, W.; Aarón Morales Frías, I.; Bellagambi, F.G.; Hangouët, M.; Martin, M.; Bausells, J.; Mohammadi, H.; Amine, A.; Zine, N.; et al. Copper-free click chemistry assisted antibodies for immunodetection of interleukin-10 in saliva. Microchem. J. 2023, 193, 108933. [Google Scholar] [CrossRef]
- Ben Halima, H.; Zine, N.; Nemeir, I.A.; Pfeiffer, N.; Heuberger, A.; Bausells, J.; Elaissari, A.; Jaffrezic-Renault, N.; Errachid, A. An ImmunoFET Coupled with an Immunomagnetic Preconcentration Technique for the Sensitive EIS Detection of HF Biomarkers. Micromachines 2024, 15, 296. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Lee, Z.J.; Naqvi, A.; Karnam, J.B.; Muthukumar, S.; Prasad, S. Multiplex sensing of IL-10 and CRP towards predicting critical illness in COVID-19 infections. Biosens. Bioelectron. X 2023, 13, 100307. [Google Scholar] [CrossRef]
- Bassel, L.L.; Co, C.; Macdonald, A.; Sly, L.; McCandless, E.E.; Hewson, J.; Tiwari, R.; Sharif, S.; Siracusa, L.; Clark, M.E.; et al. Pulmonary and systemic responses to aerosolized lysate of Staphylococcus aureus and Escherichia coli in calves. BMC Vet. Res. 2020, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Brownlee, B.J.; Parate, K.; Pola, C.C.; Chen, B.; Hostetter, J.M.; Jones, D.; Jackman, J.; Iverson, B.D.; Claussen, J.C. IFN-gamma and IL-10 Immunosensor with Vertically Aligned Carbon Nanotube Interdigitated Electrodes Toward Pen-Side Cattle Paratuberculosis Monitoring. Glob. Chall. 2024, 8, 2400021. [Google Scholar] [CrossRef] [PubMed]
- Sipka, A.; Mann, S.; Babasyan, S.; Freer, H.; Wagner, B. Development of a bead-based multiplex assay to quantify bovine interleukin-10, tumor necrosis factor-alpha, and interferon-gamma concentrations in plasma and cell culture supernatant. JDS Commun. 2022, 3, 207–211. [Google Scholar] [CrossRef]
- Chaisri, W.; Intanon, M.; Saipinta, D.; Srithanasuwan, A.; Pangprasit, N.; Jaraja, W.; Chuasakhonwilai, A.; Suriyasathaporn, W. Variation in Interleukin-4, -6, and -10 in Mastitis Milk: Associations with Infections, Pathogens, Somatic Cell Counts, and Oxidative Stress. Vet. Sci. 2024, 11, 350. [Google Scholar] [CrossRef]
- Peker, C.; Musal, B. Assessment of inflammatory cytokine concentrations during diagnosis and after treatment of postpartum dairy cows with clinical and subclinical endometritis. Large Anim. Rev. 2022, 28, 213–220. [Google Scholar]
- Wenz, J.R.; Fox, L.K.; Muller, F.J.; Rinaldi, M.; Zeng, R.; Bannerman, D.D. Factors associated with concentrations of select cytokine and acute phase proteins in dairy cows with naturally occurring clinical mastitis. J. Dairy Sci. 2010, 93, 2458–2470. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Brodzki, A.; Wawron, W.; Marczuk, J. Inflammatory cytokines and acute-phase proteins concentrations in the peripheral blood and uterus of cows that developed endometritis during early postpartum. Theriogenology 2015, 84, 11–18. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Krakowski, L.; Marczuk, J. Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet. Res. Commun. 2015, 39, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Islam, R.; Kumar, H.; Nandi, S.; Rai, R.B. Determination of anti-inflammatory cytokine in periparturient cows for prediction of postpartum reproductive diseases. Theriogenology 2013, 79, 974–979. [Google Scholar] [CrossRef]
- Dudemaine, P.L.; Fecteau, G.; Lessard, M.; Labrecque, O.; Roy, J.P.; Bissonnette, N. Increased blood-circulating interferon-gamma, interleukin-17, and osteopontin levels in bovine paratuberculosis. J. Dairy Sci. 2014, 97, 3382–3393. [Google Scholar] [CrossRef]
- Zhang, Z.; Doel, C.; Bashiruddin, J.B. Interleukin-10 production at the early stage of infection with foot-and-mouth disease virus related to the likelihood of persistent infection in cattle. Vet. Res. 2015, 46, 132. [Google Scholar] [CrossRef][Green Version]
- Rispoli, L.A.; Edwards, J.L.; Pohler, K.G.; Russell, S.; Somiari, R.I.; Payton, R.R.; Schrick, F.N. Heat-induced hyperthermia impacts the follicular fluid proteome of the periovulatory follicle in lactating dairy cows. PLoS ONE 2019, 14, e0227095. [Google Scholar] [CrossRef]
- Snider, A.P.; Gomes, R.S.; Summers, A.F.; Tenley, S.C.; Abedal-Majed, M.A.; McFee, R.M.; Wood, J.R.; Davis, J.S.; Cupp, A.S. Identification of Lipids and Cytokines in Plasma and Follicular Fluid Before and After Follicle-Stimulating Hormone Stimulation as Potential Markers for Follicular Maturation in Cattle. Animals 2023, 13, 3289. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman Alrabiah, N.; Simintiras, C.A.; Evans, A.C.O.; Lonergan, P.; Fair, T. Biochemical alterations in the follicular fluid of bovine peri-ovulatory follicles and association with final oocyte maturation. Reprod. Fertil. 2022, 4, e220090. [Google Scholar] [CrossRef] [PubMed]
- Gorodkiewicz, E.; Sankiewicz, A.; Laudański, P. Surface plasmon resonance imaging biosensors for aromatase based on a potent inhibitor and a specific antibody: Sensor development and application for biological material. Open Chem. 2014, 12, 557–567. [Google Scholar] [CrossRef]
- Mrozek, P.; Gorodkiewicz, E.; Falkowski, P.; Hoscilo, B. Sensitivity Analysis of Single- and Bimetallic Surface Plasmon Resonance Biosensors. Sensors 2021, 21, 4348. [Google Scholar] [CrossRef] [PubMed]


| Interferent Concentration 1 | SPRi Signal | CIL-10 [pg/mL] | Note |
|---|---|---|---|
| 500 pg/mL | 1418.541 | 0.756 | <LOQ |
| 1417.639 | 0.605 | <LOQ | |
| 1417.572 | 0.594 | <LOQ | |
| 2000 pg/mL | 1416.75 | 0.458 | <LOQ |
| 1418.798 | 0.798 | <LOQ | |
| 1417.866 | 0.643 | <LOQ |
| Sample 1 | SPRi Signal [AU] | Dilution | CIL-10 [pg/mL] | Body Fluid |
|---|---|---|---|---|
| 1 | 4252.8635 | 2 | 328.62 | follicular fluid |
| 2 | 7815.8005 | 2 | 738.46 | follicular fluid |
| 3 | 8443.117 | 2 | 810.62 | follicular fluid |
| 4 | 5348.612 | 2 | 454.66 | follicular fluid |
| 5 | 7521.1405 | 2 | 704.57 | follicular fluid |
| 6 | 7826.7345 | 2 | 739.72 | follicular fluid |
| 7 | 4185.5195 | 2 | 320.87 | follicular fluid |
| 8 | 5597.174 | 2 | 483.25 | follicular fluid |
| 9 | 7168.9685 | 2 | 664.06 | follicular fluid |
| A | 5018.086 | 1 | 208.32 | serum |
| B | 1816.113 | 1 | 24.16 | serum |
| C | 3811.059 | 1 | 138.90 | serum |
| D | 4448.985 | 1 | 175.59 | serum |
| E | 1519.105 | 1 | 7.08 | serum |
| F | 7277.147 | 1 | 338.25 | serum |
| G | 6977.786 | 1 | 321.03 | serum |
| H | 4709.417 | 1 | 190.57 | serum |
| I | 4199.57 | 1 | 161.25 | serum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytel, A.; Tobolski, D.; Skup, P.; Gargaś, J.; Flis, S.; Gajewski, Z.; Gorodkiewicz, E.; Papis, K. A Novel Surface Plasmon Resonance Imaging (SPRi) Biosensor for the Determination of Bovine Interleukin-10: Development, Validation, and Application in Biological Fluids. Int. J. Mol. Sci. 2025, 26, 10395. https://doi.org/10.3390/ijms262110395
Pytel A, Tobolski D, Skup P, Gargaś J, Flis S, Gajewski Z, Gorodkiewicz E, Papis K. A Novel Surface Plasmon Resonance Imaging (SPRi) Biosensor for the Determination of Bovine Interleukin-10: Development, Validation, and Application in Biological Fluids. International Journal of Molecular Sciences. 2025; 26(21):10395. https://doi.org/10.3390/ijms262110395
Chicago/Turabian StylePytel, Aleksandra, Dawid Tobolski, Piotr Skup, Justyna Gargaś, Sylwia Flis, Zdzisław Gajewski, Ewa Gorodkiewicz, and Krzysztof Papis. 2025. "A Novel Surface Plasmon Resonance Imaging (SPRi) Biosensor for the Determination of Bovine Interleukin-10: Development, Validation, and Application in Biological Fluids" International Journal of Molecular Sciences 26, no. 21: 10395. https://doi.org/10.3390/ijms262110395
APA StylePytel, A., Tobolski, D., Skup, P., Gargaś, J., Flis, S., Gajewski, Z., Gorodkiewicz, E., & Papis, K. (2025). A Novel Surface Plasmon Resonance Imaging (SPRi) Biosensor for the Determination of Bovine Interleukin-10: Development, Validation, and Application in Biological Fluids. International Journal of Molecular Sciences, 26(21), 10395. https://doi.org/10.3390/ijms262110395







