Overcoming Treatment Challenges in HIV-Associated Mycobacterial Diseases: New Therapeutic Frontiers
Abstract
1. Introduction
2. Epidemiology & Clinical Features of Major Mycobacterial Co-Infections in HIV
2.1. Tuberculosis (TB) in HIV
2.1.1. Epidemiology of TB in HIV
2.1.2. Clinical Features of TB in HIV
2.2. Mycobacterium avium Complex (MAC) in HIV
2.2.1. Epidemiology of MAC in HIV
2.2.2. Clinical Features of MAC in HIV
2.3. Leprosy (M. leprae) in HIV
2.3.1. Epidemiology of M. leprae in HIV
2.3.2. Clinical Features of M. leprae in HIV
2.4. Current Standard-of-Care Treatment
2.4.1. Tuberculosis in HIV Co-Infection
2.4.2. Mycobacterium avium Complex (MAC) Disease in HIV
2.4.3. Leprosy (Hansen’s Disease) in HIV
3. Drug–Drug Interactions Between Antiretroviral Therapy (ART) and Mycobacterial Medications
3.1. Rifamycins
3.2. Protease Inhibitors
3.3. NNRTIs
3.4. Integrase Strand Transfer Inhibitors (INSTIs)
3.5. Macrolides
3.6. Others
3.7. Newer and Second-Line Agents
4. Clinical Challenges and Complications
4.1. Drug Resistance in Mycobacterial Co-Infections
4.1.1. MDR-TB (Multidrug-Resistant Tuberculosis)
4.1.2. XDR-TB (Extensively Drug-Resistant Tuberculosis)
4.1.3. MAC Resistance
5. Immune Reconstitution Inflammatory Syndrome (IRIS)
6. Side Effects, Overlapping Toxicities, and Adherence Issues
6.1. Drug–Drug Interactions and Toxicities
6.1.1. Hepatotoxicity Associated with Rifampin
6.1.2. Nephrotoxicity and Ototoxicity of Second-Line Anti-TB Drugs
6.1.3. Impact of ART on Ototoxicity
6.1.4. Adherence Issues
7. Host-Directed and Adjunctive Therapeutics
7.1. Host-Directed Therapies (HDTs)
7.1.1. Vitamin D Supplementation
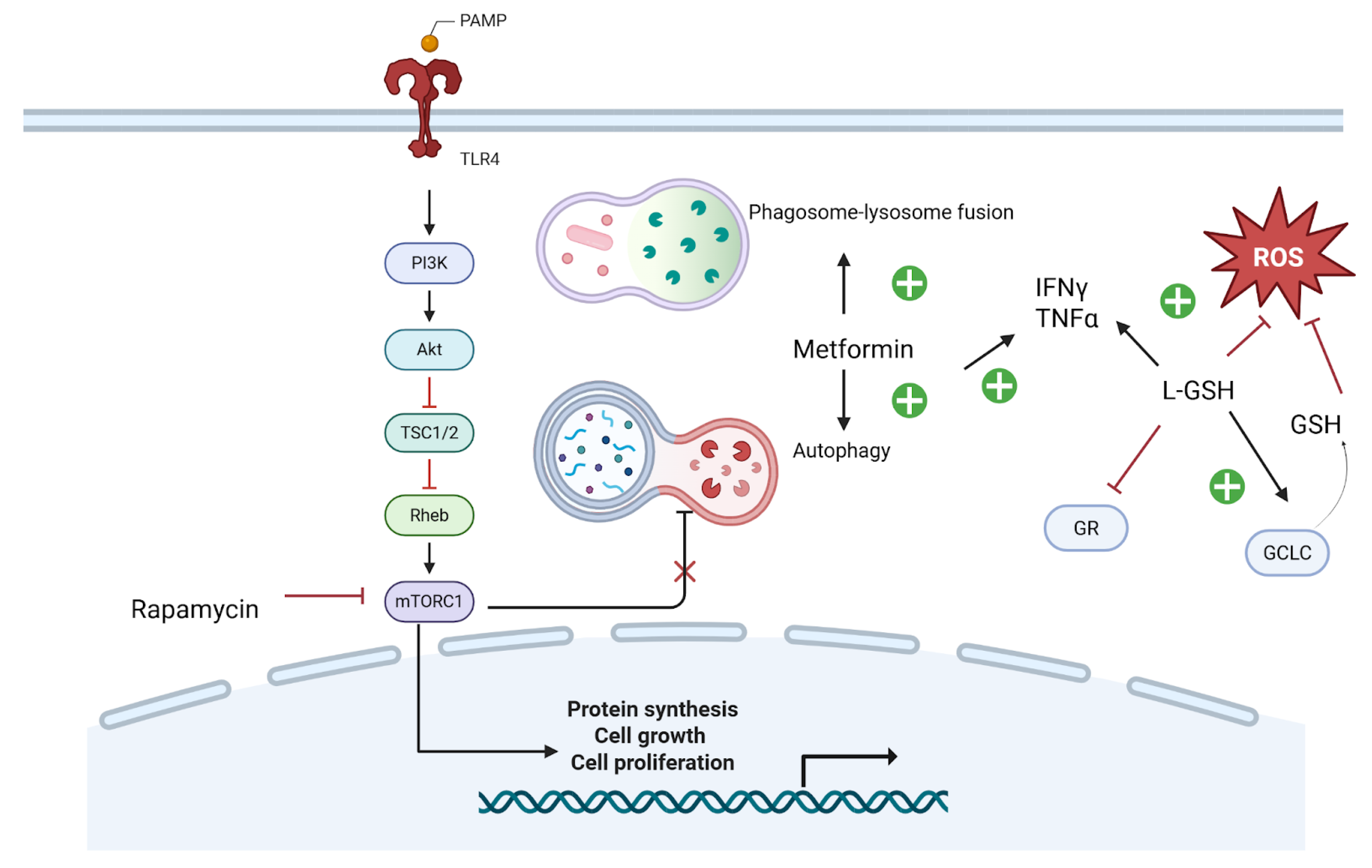
7.1.2. Metformin
7.1.3. Glutathione
7.1.4. mTOR Inhibitors
7.1.5. Anti-Inflammatory Agents
7.2. Immunotherapies and Cytokine Modulators
7.2.1. IFN-γ
7.2.2. Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF)
7.2.3. Interleukin-7
7.3. Novel Antimicrobials
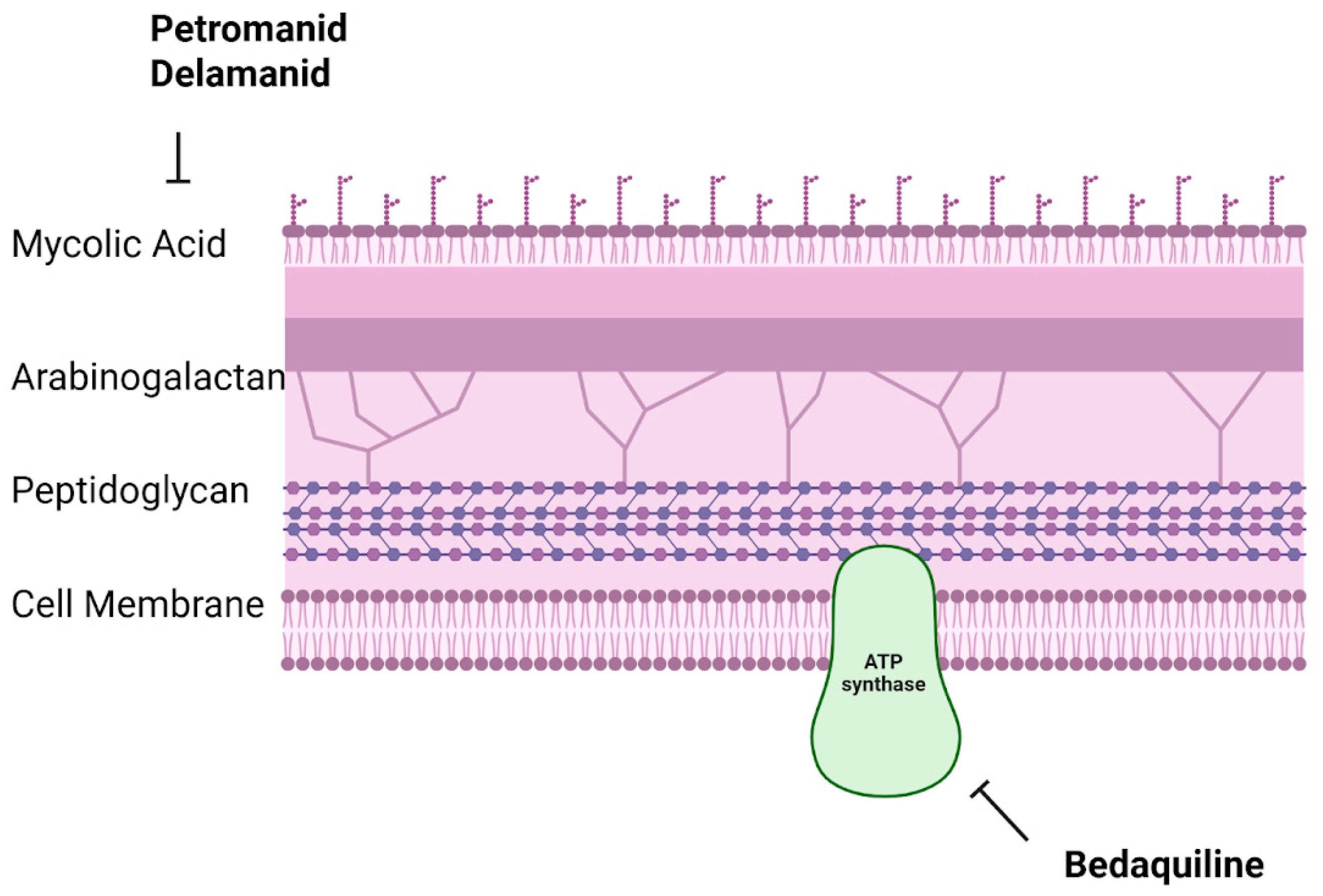
7.3.1. Bedaquiline
7.3.2. Pretomanid
7.3.3. Delamanid
7.4. Vaccines and Immunotherapy Developments
7.4.1. MTBVAC
7.4.2. VPM1002
7.4.3. Therapeutic Vaccines
7.4.4. Deregulated Host Transcription Factors as Therapeutic Targets in HIV-Mycobacterial Coinfection
7.5. Recent Clinical Trials and Future Directions
7.5.1. Shortening TB and MAC Therapy Regimens
7.5.2. Innovative Host-Directed Therapies and Immunotherapy Trials
7.5.3. Integrated Models of TB and HIV Care
7.5.4. Potential Role of Novel Vaccine Candidates (e.g., M72/AS01E)
- Vitamin D—ART compatibility: none expected; IRIS: neutral; Feasibility/monitoring: inexpensive, monitor calcium/renal function if high-dose.
- Metformin—ART compatibility: generally compatible; IRIS: potential anti-inflammatory benefit; Feasibility/monitoring: watch renal function and GI tolerance.
- mTOR modulation—ART compatibility: check DDIs (CYP3A, P-gp); IRIS: may blunt hyper-inflammation; Feasibility/monitoring: drug levels/toxicity if applicable.
- Glutathione augmentation—ART compatibility: none expected; IRIS: may reduce oxidative injury; Feasibility/monitoring: formulation access, hepatic/renal status.
- Cytokines (IFN-γ, GM-CSF)—ART compatibility: none direct; IRIS: use cautiously in high-inflammation states; Feasibility/monitoring: hematologic/immune monitoring.
- Therapeutic vaccines—ART compatibility: none direct; IRIS: consider timing around ART initiation; Feasibility/monitoring: trial availability, immunogenicity readouts.
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, K. Mycobacterial Disease Associated with HIV Infection. J. Gen. Intern. Med. 1991, 6, S19–S23. [Google Scholar] [CrossRef]
- Garcia, G.F.; Moura, A.S.; Ferreira, C.S.; Rocha, M.O.d.C. Clinical and Radiographic Features of HIV-Related Pulmonary Tuberculosis According to the Level of Immunosuppression. Rev. Soc. Bras. Med. Trop. 2007, 40, 622–626. [Google Scholar] [CrossRef][Green Version]
- Schutz, C.; Meintjes, G.; Almajid, F.; Wilkinson, R.J.; Pozniak, A. Clinical Management of Tuberculosis and HIV-1 Co-Infection. Eur. Respir. J. 2010, 36, 1460–1481. [Google Scholar] [CrossRef]
- Disseminated Mycobacterium avium Complex: Adult and Adolescent OIs | NIH. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/disseminated (accessed on 22 March 2025).
- Akram, S.M.; Attia, F.N. Mycobacterium avium Complex. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Buziashvili, M.; Djibuti, M.; Tukvadze, N.; DeHovitz, J.; Baliashvili, D. Incidence Rate and Risk Factors for Developing Active Tuberculosis Among People Living with HIV in Georgia 2019–2020 Cohort. Open Forum Infect. Dis. 2024, 11, ofae466. [Google Scholar] [CrossRef] [PubMed]
- Goletti, D.; Pisapia, R.; Fusco, F.M.; Aiello, A.; Van Crevel, R. Epidemiology, Pathogenesis, Clinical Presentation and Management of TB in Patients with HIV and Diabetes. Int. J. Tuberc. Lung Dis. 2023, 27, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Abaleka, F.I.; Nigussie, B.; Onal, O.; Al-Zakhari, R.; Yimer, E. A Case of Isolated Pulmonary Mycobacterium avium Complex Being the First Presentation of a Newly Diagnosed HIV/AIDS. Cureus 2020, 12, e9223. [Google Scholar] [CrossRef] [PubMed]
- Ustianowski, A.P.; Lawn, S.D.; Lockwood, D.N. Interactions between HIV Infection and Leprosy: A Paradox. Lancet Infect. Dis. 2006, 6, 350–360. [Google Scholar] [CrossRef]
- Sarno, E.N.; Illarramendi, X.; Nery, J.A.C.; Sales, A.M.; Gutierrez-Galhardo, M.C.; Penna, M.L.F.; Sampaio, E.P.; Kaplan, G. HIV-M. Leprae Interaction: Can HAART Modify the Course of Leprosy? Public Health Rep. 2008, 123, 206–212. [Google Scholar] [CrossRef]
- da Silva, T.P.; Bittencourt, T.L.; de Oliveira, A.L.; Prata, R.B.d.S.; Menezes, V.; Ferreira, H.; Nery, J.A.d.C.; de Oliveira, E.B.; Sperandio da Silva, G.M.; Sarno, E.N.; et al. Macrophage Polarization in Leprosy–HIV Co-Infected Patients. Front. Immunol. 2020, 11, 1493. [Google Scholar] [CrossRef]
- Pires, C.A.A.; Neto, F.O.M.J.; de Albuquerque, N.C.; Macedo, G.M.M.; Batista, K.d.N.M.; Xavier, M.B. Leprosy Reactions in Patients Coinfected with HIV: Clinical Aspects and Outcomes in Two Comparative Cohorts in the Amazon Region, Brazil. PLoS Neglected Trop. Dis. 2015, 9, e0003818. [Google Scholar] [CrossRef]
- Verma, N.; Arora, V.; Awasthi, R.; Chan, Y.; Jha, N.K.; Thapa, K.; Jawaid, T.; Kamal, M.; Gupta, G.; Liu, G.; et al. Recent Developments, Challenges and Future Prospects in Advanced Drug Delivery Systems in the Management of Tuberculosis. J. Drug Deliv. Sci. Technol. 2022, 75, 103690. [Google Scholar] [CrossRef]
- CDCTB. Tuberculosis (TB)—Treatment of LTBI and TB for Persons with HIV. Available online: https://www.cdc.gov/tb/topic/treatment/tbhiv.htm (accessed on 21 March 2025).
- Naidoo, A.; Naidoo, K.; Padayatchi, N.; Dooley, K.E. Use of Integrase Inhibitors in HIV-Associated Tuberculosis in High-Burden Settings: Implementation Challenges and Research Gaps. Lancet HIV 2022, 9, e130–e138. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.T.; Landovitz, R.J.; Sax, P.E.; Smith, D.M.; Springer, S.A.; Günthard, H.F.; Thompson, M.A.; Bedimo, R.J.; Benson, C.A.; Buchbinder, S.P.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV in Adults: 2024 Recommendations of the International Antiviral Society–USA Panel. JAMA 2025, 333, 609–628. [Google Scholar] [CrossRef] [PubMed]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef]
- Efsen, A.M.W.; Schultze, A.; Miller, R.F.; Panteleev, A.; Skrahin, A.; Podlekareva, D.N.; Miro, J.M.; Girardi, E.; Furrer, H.; Losso, M.H.; et al. Management of MDR-TB in HIV Co-Infected Patients in Eastern Europe: Results from the TB:HIV Study. J. Infect. 2018, 76, 44–54. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarkar, A. Extensively Drug-Resistant Tuberculosis (XDR-TB): A Daunting Challenge to the Current End TB Strategy and Policy Recommendations. Indian J. Tuberc. 2017, 64, 153–160. [Google Scholar] [CrossRef]
- World Health Organization. WHO Expert Committee on Leprosy: Eighth Report; WHO technical report series, no. 968; World Health Organization: Geneva, Switzerland, 2012; ISBN 978-92-4-120968-7. [Google Scholar]
- Wang, M.-G.; Wu, S.-Q.; He, J.-Q. Efficacy of Bedaquiline in the Treatment of Drug-Resistant Tuberculosis: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2021, 21, 970. [Google Scholar] [CrossRef]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef]
- Abay, S.M.; Deribe, K.; Reda, A.A.; Biadgilign, S.; Datiko, D.; Assefa, T.; Todd, M.; Deribew, A. The Effect of Early Initiation of Antiretroviral Therapy in TB/HIV-Coinfected Patients: A Systematic Review and Meta-Analysis. J. Int. Assoc. Provid. AIDS Care JIAPAC 2015, 14, 560–570. [Google Scholar] [CrossRef]
- Mfinanga, S.G.; Kirenga, B.J.; Chanda, D.M.; Mutayoba, B.; Mthiyane, T.; Yimer, G.; Ezechi, O.; Connolly, C.; Kapotwe, V.; Muwonge, C.; et al. Early versus Delayed Initiation of Highly Active Antiretroviral Therapy for HIV-Positive Adults with Newly Diagnosed Pulmonary Tuberculosis (TB-HAART): A Prospective, International, Randomised, Placebo-Controlled Trial. Lancet Infect. Dis. 2014, 14, 563–571. [Google Scholar] [CrossRef]
- CDC. CDC Releases New Provisional Guidance for the Use of Pretomanid as Part of a Regimen [Bedaquiline, Pretomanid, and Linezolid (BPaL)] to Treat Drug-Resistant Tuberculosis Disease. Available online: https://archive.cdc.gov/www_cdc_gov/tb/php/dear-colleague-letters/2022-bpal-guidance.html (accessed on 24 June 2025).
- Yangco, B.G.; Buchacz, K.; Baker, R.; Palella, F.J.; Armon, C.; Brooks, J.T. Is Primary Mycobacterium avium Complex Prophylaxis Necessary in Patients with CD4 < 50 Cells/μL Who Are Virologically Suppressed on cART? AIDS Patient Care STDs 2014, 28, 280–283. [Google Scholar] [CrossRef]
- Choi, S.W.; Shin, J.-S.; Park, S.-J.; Jung, E.; Park, Y.-G.; Jo, Y.H.; Kim, S.-J.; Yeo, S.-G.; Lee, J.; Kim, J.-H.; et al. Ambroxol Induces Autophagy and Potentiates Rifampin Activity Against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e01019-18. [Google Scholar] [CrossRef]
- Pooranagangadevi, N.; Padmapriyadarsini, C. Treatment of Tuberculosis and the Drug Interactions Associated With HIV-TB Co-Infection Treatment. Front. Trop. Dis. 2022, 3, 834013. [Google Scholar] [CrossRef]
- Sultana, Z.Z.; Hoque, F.U.; Beyene, J.; Akhlak-Ul-Islam, M.; Khan, M.H.R.; Ahmed, S.; Hawlader, D.H.; Hossain, A. HIV Infection and Multidrug Resistant Tuberculosis: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2021, 21, 51, Correction in BMC Infect. Dis. 2021, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.R.; Padayatchi, N.; Kvasnovsky, C.; Werner, L.; Master, I.; Horsburgh, C.R. Treatment Outcomes for Extensively Drug-Resistant Tuberculosis and HIV Co-Infection. Emerg. Infect. Dis. 2013, 19, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Honeyborne, I.; Lipman, M.; Zumla, A.; McHugh, T.D. The Changing Treatment Landscape for MDR/XDR-TB—Can Current Clinical Trials Revolutionise and Inform a Brave New World? Int. J. Infect. Dis. 2019, 80, S23–S28. [Google Scholar] [CrossRef]
- Gardner, E.M.; Burman, W.J.; DeGroote, M.A.; Hildred, G.; Pace, N.R. Conventional and Molecular Epidemiology of Macrolide Resistance among New Mycobacterium avium Complex Isolates Recovered from HIV-Infected Patients. Clin. Infect. Dis. 2005, 41, 1041–1044. [Google Scholar] [CrossRef]
- Currier, J.S.; McCutchan, A. Alternative Regimens for the Prophylaxis of Mycobacterium avium Complex in Acquired Immune Deficiency Syndrome (AIDS). Am. J. Med. 1997, 102, 28–31. [Google Scholar] [CrossRef]
- Griffith, D.E.; Brown-Elliott, B.A.; Langsjoen, B.; Zhang, Y.; Pan, X.; Girard, W.; Nelson, K.; Caccitolo, J.; Alvarez, J.; Shepherd, S.; et al. Clinical and Molecular Analysis of Macrolide Resistance in Mycobacterium avium Complex Lung Disease. Am. J. Respir. Crit. Care Med. 2006, 174, 928–934. [Google Scholar] [CrossRef]
- Kadota, T.; Matsui, H.; Hirose, T.; Suzuki, J.; Saito, M.; Akaba, T.; Kobayashi, K.; Akashi, S.; Kawashima, M.; Tamura, A.; et al. Analysis of Drug Treatment Outcome in Clarithromycin-Resistant Mycobacterium avium Complex Lung Disease. BMC Infect. Dis. 2016, 16, 31. [Google Scholar] [CrossRef]
- Field, S.K.; Cowie, R.L. Treatment of Mycobacterium avium-Intracellulare Complex Lung Disease with a Macrolide, Ethambutol, and Clofazimine. Chest 2003, 124, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.-D.; Duong, H.; Lee, M.-C.; Chen, J.-H.; Huang, W.-C.; Chen, H.-E.; Lin, J.-C.; Wang, J.-Y.; Lee, C.-H. Two-Drug versus Three-Drug Regimens for Treating Mycobacterium avium Complex Infection: A Systematic Review and Meta-Analysis. J. Infect. Public Health 2025, 18, 102711. [Google Scholar] [CrossRef] [PubMed]
- Church, L.W.P.; Chopra, A.; Judson, M.A. Paradoxical Reactions and the Immune Reconstitution Inflammatory Syndrome. Microbiol. Spectr. 2017, 5, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Burman, W.J.; Jones, B.E. Treatment of HIV-Related Tuberculosis in the Era of Effective Antiretroviral Therapy. Am. J. Respir. Crit. Care Med. 2001, 164, 7–12. [Google Scholar] [CrossRef]
- Hoffmann, C.J.; Churchyard, G.J. Chapter 29—Pulmonary Tuberculosis in Adults. In Tuberculosis; Schaaf, H.S., Zumla, A.I., Grange, J.M., Raviglione, M.C., Yew, W.W., Starke, J.R., Pai, M., Donald, P.R., Eds.; W.B. Saunders: Edinburgh, UK, 2009; pp. 332–341. ISBN 978-1-4160-3988-4. [Google Scholar]
- New York State Department of Health AIDS Institute. Management of Immune Reconstitution Inflammatory Syndrome (IRIS). 7 March 2024 (Updated 11 July 2025). Available online: https://www.hivguidelines.org/guideline/hiv-iris/ (accessed on 9 July 2025).
- Meintjes, G.; Lawn, S.D.; Scano, F.; Maartens, G.; French, M.A.; Worodria, W.; Elliott, J.H.; Murdoch, D.; Wilkinson, R.J.; Seyler, C.; et al. Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome: Case Definitions for Use in Resource-Limited Settings. Lancet Infect. Dis. 2008, 8, 516–523. [Google Scholar] [CrossRef]
- Pennington, N.; Quental, A.; Bernstein, E.L. Mycobacterium avium Complex (MAC) and Immune Reconstitution Inflammatory Syndrome (IRIS) in a Patient with Myelodysplastic Syndrome. Chest 2024, 166, A5595–A5596. [Google Scholar] [CrossRef]
- Kandikattu, D.P. What Is Rifampicin Toxicity? Available online: https://www.icliniq.com/articles/drug-and-supplements/rifampicin-toxicity (accessed on 24 June 2025).
- Ayelign, B.; Workneh, M.; Molla, M.D.; Dessie, G. Role of Vitamin-D Supplementation In TB/HIV Co-Infected Patients. Infect. Drug Resist. 2020, 13, 111–118. [Google Scholar] [CrossRef]
- Sazali, M.F.; Rahim, S.S.S.A.; Mohammad, A.H.; Kadir, F.; Payus, A.O.; Avoi, R.; Jeffree, M.S.; Omar, A.; Ibrahim, M.Y.; Atil, A.; et al. Improving Tuberculosis Medication Adherence: The Potential of Integrating Digital Technology and Health Belief Model. Tuberc. Respir. Dis. 2022, 86, 82–93. [Google Scholar] [CrossRef]
- Kibu, O.D.; Siysi, V.V.; Albert Legrand, S.E.; Asangbeng Tanue, E.; Nsagha, D.S. Treatment Adherence among HIV and TB Patients Using Single and Double Way Mobile Phone Text Messages: A Randomized Controlled Trial. J. Trop. Med. 2022, 2022, 2980141. [Google Scholar] [CrossRef]
- Shenoi, S.; Heysell, S.; Moll, A.; Friedland, G. Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis: Consequences for the Global HIV Community. Curr. Opin. Infect. Dis. 2009, 22, 11–17. [Google Scholar] [CrossRef]
- Arai, M.; Uchiba, M.; Komura, H.; Mizuochi, Y.; Harada, N.; Okajima, K. Metformin, an Antidiabetic Agent, Suppresses the Production of Tumor Necrosis Factor and Tissue Factor by Inhibiting Early Growth Response Factor-1 Expression in Human Monocytes in Vitro. J. Pharmacol. Exp. Ther. 2010, 334, 206–213. [Google Scholar] [CrossRef]
- Lachmandas, E.; Eckold, C.; Böhme, J.; Koeken, V.A.C.M.; Marzuki, M.B.; Blok, B.; Arts, R.J.W.; Chen, J.; Teng, K.W.W.; Ratter, J.; et al. Metformin Alters Human Host Responses to Mycobacterium tuberculosis in Healthy Subjects. J. Infect. Dis. 2019, 220, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.E.; Dorhoi, A.; Hotchkiss, R.S.; Bartenschlager, R. Host-Directed Therapies for Bacterial and Viral Infections. Nat. Rev. Drug Discov. 2018, 17, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, L.; Orr, P.; Turner-Brannen, E.; Slivinski, C.R.; Nickerson, P.W.; Mookherjee, N. Effect of Vitamin D Supplementation on Mycobacterium tuberculosis-Induced Innate Immune Responses in a Canadian Dené First Nations Cohort. PLoS ONE 2012, 7, e40692. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- Naicker, N.; Sigal, A.; Naidoo, K. Metformin as Host-Directed Therapy for TB Treatment: Scoping Review. Front. Microbiol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Nabipur, L.; Mouawad, M.; Venketaraman, V. Additive Effects of Glutathione in Improving Antibiotic Efficacy in HIV–M.Tb Co-Infection in the Central Nervous System: A Systematic Review. Viruses 2025, 17, 127. [Google Scholar] [CrossRef]
- Venketaraman, V.; Dayaram, Y.K.; Talaue, M.T.; Connell, N.D. Glutathione and Nitrosoglutathione in Macrophage Defense against Mycobacterium tuberculosis. Infect. Immun. 2005, 73, 1886–1889. [Google Scholar] [CrossRef]
- Morris, D.; Guerra, C.; Donohue, C.; Oh, H.; Khurasany, M.; Venketaraman, V. Unveiling the Mechanisms for Decreased Glutathione in Individuals with HIV Infection. J. Immunol. Res. 2012, 2012, 734125. [Google Scholar] [CrossRef]
- Bhatt, K.; Bhagavathula, M.; Verma, S.; Timmins, G.S.; Deretic, V.P.; Ellner, J.J.; Salgame, P. Rapamycin Modulates Pulmonary Pathology in a Murine Model of Mycobacterium tuberculosis Infection. Dis. Model. Mech. 2021, 14, dmm049018. [Google Scholar] [CrossRef]
- Patel, A.; Nguyen, L.; Shea, C.; Singh, S.; Venketaraman, V. The Role of mTOR in Mycobacterium tuberculosis Infection. Biomedicines 2024, 12, 2238. [Google Scholar] [CrossRef]
- Krencz, I.; Sebestyen, A.; Khoor, A. mTOR in Lung Neoplasms. Pathol. Oncol. Res. 2020, 26, 35–48. [Google Scholar] [CrossRef]
- Critchley, J.A.; Young, F.; Orton, L.; Garner, P. Corticosteroids for Prevention of Mortality in People with Tuberculosis: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2013, 13, 223–237. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yim, J.-J.; Yoon, B.-W. Adjuvant Interferon-γ Treatment in Two Cases of Refractory Tuberculosis of the Brain. Clin. Neurol. Neurosurg. 2012, 114, 732–734. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.M.; Jyonouchi, H.; Kotenko, S.V.; Smirnov, S.V.; Patel, R.; Aguila, H.; McSherry, G.; Dashefsky, B.; Holland, S.M. Adjunctive Treatment of Disseminated Mycobacterium avium Complex Infection with Interferon Alpha-2b in a Patient with Complete Interferon-Gamma Receptor R1 Deficiency. Eur. J. Pediatr. 2007, 166, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Nguyen, T.; Alsulami, S.; Platt, C. Interferon-Gamma Therapy Augments Antimicrobial Treatment of Mycobacterium avium Complex Infection in a Patient with MSMD. Ann. Allergy. Asthma. Immunol. 2024, 133, S154. [Google Scholar] [CrossRef]
- Frumkin, L.R. Role of Granulocyte Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor in the Treatment of Patients with HIV Infection. Curr. Opin. Hematol. 1997, 4, 200. [Google Scholar] [CrossRef]
- Hammer, S.; Gillis, J.; Pinkston, P.; Rose, R. Effect of Zidovudine and Granulocyte-Macrophage Colony-Stimulating Factor on Human Immunodeficiency Virus Replication in Alveolar Macrophages. Blood 1990, 75, 1215–1219. [Google Scholar] [CrossRef]
- Esser, R.; Glienke, W.; von Briesen, H.; Rubsamen-Waigmann, H.; Andreesen, R. Differential Regulation of Proinflammatory and Hematopoietic Cytokines in Human Macrophages after Infection with Human Immunodeficiency Virus. Blood 1996, 88, 3474–3481. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.A.; Angel, J.B. Granulocyte-Macrophage Colony-Stimulating Factor as an Immune-Based Therapy in HIV Infection. J. Immune Based Ther. Vaccines 2005, 3, 3. [Google Scholar] [CrossRef][Green Version]
- de Silva, T.I.; Cope, A.; Goepel, J.; Greig, J.M. The Use of Adjuvant Granulocyte-Macrophage Colony-Stimulating Factor in HIV-Related Disseminated Atypical Mycobacterial Infection. J. Infect. 2007, 54, e207–e210. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Singh, V.K.; Actor, J.K.; Hunter, R.L.; Jagannath, C.; Subbian, S.; Khan, A. GM-CSF Dependent Differential Control of Mycobacterium tuberculosis Infection in Human and Mouse Macrophages: Is Macrophage Source of GM-CSF Critical to Tuberculosis Immunity? Front. Immunol. 2020, 11, 1599. [Google Scholar] [CrossRef] [PubMed]
- Francois, B.; Jeannet, R.; Daix, T.; Walton, A.H.; Shotwell, M.S.; Unsinger, J.; Monneret, G.; Rimmelé, T.; Blood, T.; Morre, M.; et al. Interleukin-7 Restores Lymphocytes in Septic Shock: The IRIS-7 Randomized Clinical Trial. JCI Insight 2018, 3, e98960. [Google Scholar] [CrossRef]
- Sereti, I.; Dunham, R.M.; Spritzler, J.; Aga, E.; Proschan, M.A.; Medvik, K.; Battaglia, C.A.; Landay, A.L.; Pahwa, S.; Fischl, M.A.; et al. IL-7 Administration Drives T Cell–Cycle Entry and Expansion in HIV-1 Infection. Blood 2009, 113, 6304–6314. [Google Scholar] [CrossRef]
- French, M.A. The Immunopathogenesis of Immune Reconstitution Inflammatory Syndrome Has Become Clearer, but More Complex. J. Infect. Dis. 2023, 228, 106–110. [Google Scholar] [CrossRef]
- Khoshnood, S.; Goudarzi, M.; Taki, E.; Darbandi, A.; Kouhsari, E.; Heidary, M.; Motahar, M.; Moradi, M.; Bazyar, H. Bedaquiline: Current Status and Future Perspectives. J. Glob. Antimicrob. Resist. 2021, 25, 48–59. [Google Scholar] [CrossRef]
- Nguyen, T.V.A.; Anthony, R.M.; Bañuls, A.-L.; Nguyen, T.V.A.; Vu, D.H.; Alffenaar, J.-W.C. Bedaquiline Resistance: Its Emergence, Mechanism, and Prevention. Clin. Infect. Dis. 2018, 66, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Pretomanid in Drug-Resistant Tuberculosis: A Profile of Its Use. Drugs Ther. Perspect. 2020, 36, 273–279. [Google Scholar] [CrossRef]
- Shibeshi, W.; Sheth, A.N.; Admasu, A.; Berha, A.B.; Negash, Z.; Yimer, G. Nephrotoxicity and Ototoxic Symptoms of Injectable Second-Line Anti-Tubercular Drugs among Patients Treated for MDR-TB in Ethiopia: A Retrospective Cohort Study. BMC Pharmacol. Toxicol. 2019, 20, 31. [Google Scholar] [CrossRef]
- Nguyen, T.V.A.; Nguyen, Q.H.; Nguyen, T.N.T.; Anthony, R.M.; Vu, D.H.; Alffenaar, J.-W.C. Pretomanid Resistance: An Update on Emergence, Mechanisms and Relevance for Clinical Practice. Int. J. Antimicrob. Agents 2023, 62, 106953. [Google Scholar] [CrossRef]
- von Groote-Bidlingmaier, F.; Patientia, R.; Sanchez, E.; Balanag, V.; Ticona, E.; Segura, P.; Cadena, E.; Yu, C.; Cirule, A.; Lizarbe, V.; et al. Efficacy and Safety of Delamanid in Combination with an Optimised Background Regimen for Treatment of Multidrug-Resistant Tuberculosis: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Parallel Group Phase 3 Trial. Lancet Respir. Med. 2019, 7, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Matsumoto, M.; Ishida, H.; Ohguro, K.; Yoshitake, M.; Gupta, R.; Geiter, L.; Hafkin, J. Delamanid: From Discovery to Its Use for Pulmonary Multidrug-Resistant Tuberculosis (MDR-TB). Tuberculosis 2018, 111, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Broset, E.; Saubi, N.; Guitart, N.; Aguilo, N.; Uranga, S.; Kilpeläinen, A.; Eto, Y.; Hanke, T.; Gonzalo-Asensio, J.; Martín, C.; et al. MTBVAC-Based TB-HIV Vaccine Is Safe, Elicits HIV-T Cell Responses, and Protects against Mycobacterium tuberculosis in Mice. Mol. Ther. Methods Clin. Dev. 2019, 13, 253–264. [Google Scholar] [CrossRef]
- Lacámara, S.; Martin, C. MTBVAC: A Tuberculosis Vaccine Candidate Advancing Towards Clinical Efficacy Trials in TB Prevention. Arch. Bronconeumol. 2023, 59, 821–828. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E.; Kulkarni, P.S.; Shaligram, U.; Cotton, M.F.; Rentsch, C.A.; Eisele, B.; Grode, L.; Kaufmann, S.H.E. The Recombinant Bacille Calmette–Guérin Vaccine VPM1002: Ready for Clinical Efficacy Testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Loxton, A.G.; Knaul, J.K.; Grode, L.; Gutschmidt, A.; Meller, C.; Eisele, B.; Johnstone, H.; van der Spuy, G.; Maertzdorf, J.; Kaufmann, S.H.E.; et al. Safety and Immunogenicity of the Recombinant Mycobacterium Bovis BCG Vaccine VPM1002 in HIV-Unexposed Newborn Infants in South Africa. Clin. Vaccine Immunol. 2017, 24, e00439-16. [Google Scholar] [CrossRef]
- Chen, Z.; Julg, B. Therapeutic Vaccines for the Treatment of HIV. Transl. Res. 2020, 223, 61–75. [Google Scholar] [CrossRef]
- Bouzeyen, R.; Javid, B. Therapeutic Vaccines for Tuberculosis: An Overview. Front. Immunol. 2022, 13, 878471. [Google Scholar] [CrossRef]
- Rais, M.; Abdelaal, H.; Reese, V.A.; Ferede, D.; Larsen, S.E.; Pecor, T.; Erasmus, J.H.; Archer, J.; Khandhar, A.P.; Cooper, S.K.; et al. Immunogenicity and Protection against Mycobacterium avium with a Heterologous RNA Prime and Protein Boost Vaccine Regimen. Tuberculosis 2023, 138, 102302. [Google Scholar] [CrossRef]
- Jeong, E.-K.; Lee, H.-J.; Jung, Y.-J. Host-Directed Therapies for Tuberculosis. Pathogens 2022, 11, 1291. [Google Scholar] [CrossRef]
- Krug, S.; Parveen, S.; Bishai, W.R. Host-Directed Therapies: Modulating Inflammation to Treat Tuberculosis. Front. Immunol. 2021, 12, 660916. [Google Scholar] [CrossRef]
- Ranjbar, S.; Jasenosky, L.D.; Chow, N.; Goldfeld, A.E. Regulation of Mycobacterium tuberculosis-dependent HIV-1 transcription reveals a new role for NFAT5 in the toll-like receptor pathway. PLoS Pathog. 2012, 8, e1002620. [Google Scholar] [CrossRef]
- Bryk, R.; Mundhra, S.; Jiang, X.; Wood, M.; Pfau, D.; Weber, E.; Park, S.; Zhang, L.; Wilson, C.; Van der Westhuyzen, R.; et al. Potentiation of rifampin activity in a mouse model of tuberculosis by activation of host transcription factor EB. PLoS Pathog. 2020, 16, e1008567. [Google Scholar] [CrossRef] [PubMed]
- Yew, W.-W.; Chan, D.P.; Chang, K.-C.; Zhang, Y. How Does Metformin Act as a Host-Directed Agent in Tuberculosis Associated with Diabetes Mellitus? J. Thorac. Dis. 2020, 12, 1124–1126. [Google Scholar] [CrossRef] [PubMed]
- Mabhula, A.; Singh, V. Drug-Resistance in Mycobacterium tuberculosis: Where We Stand. MedChemComm 2019, 10, 1342–1360. [Google Scholar] [CrossRef]
- Singh, V. Tuberculosis Treatment-Shortening. Drug Discov. Today 2024, 29, 103955. [Google Scholar] [CrossRef]
- Jo, K.-W.; Kim, S.; Lee, J.Y.; Lee, S.-D.; Kim, W.S.; Kim, D.S.; Shim, T.S. Treatment Outcomes of Refractory MAC Pulmonary Disease Treated with Drugs with Unclear Efficacy. J. Infect. Chemother. 2014, 20, 602–606. [Google Scholar] [CrossRef]
- Zweijpfenning, S.M.H.; Aarnoutse, R.; Boeree, M.J.; Magis-Escurra, C.; Stemkens, R.; Geurts, B.; van Ingen, J.; Hoefsloot, W. Safety and Efficacy of Clofazimine as an Alternative for Rifampicin in Mycobacterium avium Complex Pulmonary Disease Treatment: Outcomes of a Randomized Trial. Chest 2024, 165, 1082–1092. [Google Scholar] [CrossRef]
- Saunders, M.J.; Evans, C.A. COVID-19, Tuberculosis and Poverty: Preventing a Perfect Storm. Eur. Respir. J. 2020, 56, 2001348. [Google Scholar] [CrossRef]
- Dlatu, N.; Longo-Mbenza, B.; Apalata, T. Models of Integration of TB and HIV Services and Factors Associated with Perceived Quality of TB-HIV Integrated Service Delivery in O. R Tambo District, South Africa. BMC Health Serv. Res. 2023, 23, 804. [Google Scholar] [CrossRef]
- Meeren, O.V.D.; Hatherill, M.; Nduba, V.; Wilkinson, R.J.; Muyoyeta, M.; Van Brakel, E.; Ayles, H.M.; Henostroza, G.; Thienemann, F.; Scriba, T.J.; et al. 120. A Randomized Double-Blind Trial Assessing the Efficacy of M72/AS01E Vaccine Against Pulmonary Tuberculosis Disease in Adults with Latent Mycobacterium tuberculosis Infection. Open Forum Infect. Dis. 2018, 5, S5–S6. [Google Scholar] [CrossRef]
- Bill & Melinda Gates Medical Research Institute. Bill & Melinda Gates Medical Research Institute Initiates Phase 3 Clinical Trial of Tuberculosis Vaccine Candidate. Press Release, 19 March 2024. Available online: https://www.gatesmri.org/mri-initiates-phase-3-clinical-trial-tuberculosis-vaccine-candidate/ (accessed on 16 June 2025).
- Ahmed, S.; Raqib, R.; Guðmundsson, G.H.; Bergman, P.; Agerberth, B.; Rekha, R.S. Host-Directed Therapy as a Novel Treatment Strategy to Overcome Tuberculosis: Targeting Immune Modulation. Antibiotics 2020, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.P.J.; Meintjes, G.; Wilkinson, R.J. HIV-1 Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome. Semin. Immunopathol. 2016, 38, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Pundkar, A.; Agarwal, A.; Gadkari, C.; Nagpal, A.K.; Kuttan, N. A Comprehensive Review of HIV-Associated Tuberculosis: Clinical Challenges and Advances in Management. Cureus 2024, 16, e68784. [Google Scholar] [CrossRef] [PubMed]
- Mhango, D.V.; Mzinza, D.T.; Jambo, K.C.; Mwandumba, H.C. HIV-Associated Tuberculosis: Clinical Challenges and Advances in Management. Curr. Opin. Infect. Dis. 2021, 34, 25–33. [Google Scholar] [CrossRef]

| HIV Disease Stage | Tuberculosis (TB) | Disseminated MAC | Leprosy (M. leprae) |
|---|---|---|---|
| Early HIV (CD4 > ~350/μL) | TB often presents with “classic” pulmonary disease (upper-lobe cavities, cough, fevers, night sweats), resembling HIV-negative TB [1,2]. Treatment success is high for drug-susceptible TB with standard first-line therapy (isoniazid, a rifamycin, pyrazinamide, ethambutol), with regimen choice/duration guided by susceptibility testing and major guidelines [3]. Drug-resistant TB requires second-line, all-oral regimens per WHO guidance (add WHO DR-TB guideline separately if you want WHO cited explicitly). Outcomes are good when immune function is largely intact [2,3]. | Rare at preserved CD4; pulmonary MAC typically occurs with underlying lung disease. Disseminated disease is uncommon when CD4 is relatively preserved [4,5]. | Leprosy risk is not increased by early HIV. Clinical presentation and response to standard multidrug therapy are unchanged. Outcomes mirror those in HIV-negative patients, assuming no severe immunosuppression. |
| Advanced HIV (CD4 < 200/μL) | TB risk and severity rise as CD4 falls. High rates of extrapulmonary and disseminated TB occur when CD4 < 50/μL. TB may present atypically (e.g., diffuse infiltrates, extrapulmonary sites). Mortality is higher (up to ~3-fold during TB therapy) without ART. Outcomes are poor if untreated, but can improve substantially with timely TB treatment and ART. | Incidence of disseminated MAC escalates at low CD4 counts (<50/μL). Presents with non-specific systemic symptoms (fever, weight loss, diarrhea, anemia) and multi-organ involvement. Without ART, MAC requires chronic suppressive therapy and carries high relapse risk. | Co-infection remains uncommon even in advanced HIV. Severe immunosuppression may blunt granulomatous inflammation, potentially leading to anergic (diffuse, lepromatous) leprosy forms—though studies have not found markedly altered leprosy spectra. Standard leprosy therapy still achieves high cure rates. However, advanced HIV patients are vulnerable to neuropathy and other infections that can complicate leprosy management. |
| After ART Initiation (IRIS phase) | Paradoxical TB-IRIS can occur within weeks of ART start in ~10–20% of co-treated patients, especially those with high TB antigen burden. Patients may acutely worsen (fever, enlarging lymph nodes or lesions) despite effective TB therapy. TB-IRIS is usually self-limited but can be severe (e.g., CNS TB-IRIS). Corticosteroids are used for moderate/severe cases to dampen inflammation. Most patients eventually improve with continued TB treatment and ART. | Unmasking MAC-IRIS is less common but can occur if subclinical MAC was present prior to ART. It typically manifests as fever, lymphadenitis, or focal disease flares shortly after ART commencement. Management includes NSAIDs for mild symptoms or a short prednisone course for severe inflammation. Importantly, ART is continued alongside ongoing MAC therapy. Prognosis: MAC-IRIS is generally manageable and rarely life-threatening; controlling HIV with ART ultimately improves MAC outcomes. | IRIS-leprosy: Immune reconstitution can trigger leprosy reversal reactions (Type 1 inflammation) or erythema nodosum leprosum (Type 2) in patients on or completing leprosy therapy. New leprosy diagnoses have occurred post-ART (unmasking of previously silent infections). These reactions cause acute nerve inflammation, requiring prompt anti-inflammatory treatment (corticosteroids) to prevent permanent nerve damage. Despite IRIS, leprosy treatment should be continued. With appropriate management of reactions, outcomes remain favorable (HIV does not significantly worsen long-term leprosy prognosis). |
| Infection | Without ART (Untreated or Late HIV) | With Effective ART (After Initiation) |
|---|---|---|
| Tuberculosis (TB) | High burden and high mortality: TB thrives as CD4 declines, often disseminating in late-stage HIV. Without ART, patients face a ~15–20× higher TB risk and up to three-fold higher mortality during TB treatment. Standard 6-month TB therapy may succeed initially, but without HIV control, recurrent TB or new infections are common. Drug interactions go unmanaged, as rifampicin lowers levels of protease inhibitors and some NNRTIs potentially compromising HIV therapy if continued. Adherence is challenging due to heavy pill burden and side effects on top of untreated HIV. | Improved outcomes, with IRIS risk: Initiating ART dramatically reduces TB mortality and future reactivation risk, and allows immune reconstitution to contain TB. However, early ART can precipitatea transient inflammatory worsening of TB symptoms (TB-IRS). Management of co-treatment is crucial: rifampicin-based TB therapy requires adjusting the ART regimen (e.g., using rifabutin or double-dose integrase inhibitors) to avoid drug–drug interactions. With ART, TB treatment success rates improve, relapse rates drop, and long-term survival is significantly higher than in patients who remain ART-naïve. |
| MAC | Disseminated disease almost inevitable in late AIDS: Without ART, patients with CD4 < 50/μL are highly susceptible to MAC; prophylactic azithromycin is indicated in this scenario. If MAC infection occurs, therapy (macrolide + ethambutol ± third agent) must be continued indefinitely or until immune recovery, because relapse is likely when immune defenses remain low. Prognosis is poor in the absence of ART asMAC was historically a major cause of wasting and death in advanced AIDS. Drug interactions are less problematic than TB (macrolides have moderate interactions), but untreated HIV typically precludes MAC cure, as the infection will recur once antibiotics stop. | Prevention and faster clearance: Effective ART raises CD4 counts, drastically cutting MAC incidence, such thatroutine primary prophylaxis is no longer needed if HIV is promptly controlled. In co-infections, starting ART enables eventual discontinuation of MAC therapy after ≥12 months, once CD4 > 100/μL is sustained. ART also improves weight gain and anemia associated with disseminated MAC. Immune reconstitution can cause mild MAC-IRIS (fever, lymph node inflammation), but these events are manageable and outweighed by the benefit of immune recovery. Overall, patients on ART have far better MAC outcomes: higher cure rates, lower risk of relapse, and improved survival, transforming MAC from a fatal illness into a treatable infection in the ART era. |
| Leprosy | Similar course, but diagnosis often missed: In ART-naïve HIV patients, leprosy follows its usual spectrumranging from paucibacillary to multibacillary disease with no clear increase in frequency. Advanced immunosuppression might allow higher bacillary loads (lepromatous leprosy), but paradoxically, anergic leprosy is not dramatically more common than in HIV-negative cases. The standard 6–12 month MDT (rifampicin, dapsone, clofazimine) is effective and well-tolerated even without ART, and HIV co-infection alone doesn’t justify extending therapy. However, clinicians may fail to diagnose leprosy in HIV patients, confusing neuropathic symptoms with HIV neuropathy or other dermatological conditions. Without ART, any concurrent infections or malnutrition can complicate leprosy management. | IRIS and enhanced inflammatory responses: ART does not impair leprosy drug efficacy coinfected patients respond as well as those without HIV. But immune reconstitution frequently triggers leprosy IRIS reactions. Patients on ART may develop sudden nerve pain, skin inflammation, or new lesions (often Type 1 reversal reactions) within months of starting therapy. Managing these episodes with corticosteroids is critical to prevent permanent nerve damage. The timing of leprosy diagnosis can also shift: previously unrecognized cases may “unmask” after ART initiates, due to the recovering immune system mounting a response. Despite these challenges, prognosis with ART remains positive leprosy treatment outcomes and relapse rates are comparable to HIV-negative cases. In fact, effective HIV control likely aids long-term leprosy immunity. The key is vigilant monitoring for IRIS and interdisciplinary care (dermatology/HIV) during the initial ART period. |
| Drug Class | Key Drugs | Interaction Mechanism/Effect | Clinical Management | Metabolic Effect |
|---|---|---|---|---|
| Rifamycins | Rifampin, Rifabutin, Rifapentine |
| Prefer rifabutin (150 mg 3×/week with boosted PIs; 300 mg daily otherwise). Avoid rifampin/rifapentine when compatible ART switch is not feasible; or switch ART to EFV or double-dose DTG/RAL. | Inducer (CYP3A4, UGT1A1, P-gp) |
| Protease Inhibitors (PIs) | Lopinavir, Atazanavir, Darunavir, Ritonavir, Cobicistat |
| Contraindicate rifampin with standard PI regimens. Substitute rifabutin and reduce rifabutin dose (150 mg 3×/week) when PIs must be retained. Consider switching to INSTI-based ART if rifampin cannot be avoided. | PIs: CYP3A4 inhibitors/substrates |
| NNRTIs | Efavirenz (EFV), Nevirapine (NVP), Rilpivirine (RPV), Etravirine (ETR), Doravirine (DOR), Delavirdine |
| Maintain EFV 600 mg with rifampin (guideline-preferred co-therapy). Switch NVP to EFV or use rifabutin. Avoid RPV, ETR, DOR with rifampin; DOR 100 mg BID permitted with rifabutin. Never combine delavirdine with any rifamycin. | EFV: CYP3A inducer; Others: CYP3A substrates; Delavirdine: CYP3A inhibitor |
| INSTIs | Dolutegravir (DTG), Raltegravir (RAL), Bictegravir (BIC), Elvitegravir/cobicistat (EVG/c), Cabotegravir (CAB) |
| Double DTG to 50 mg BID or RAL to 800 mg BID when co-administered with rifampin. Do NOT use BIC or EVG/c with rifampin; switch to DTG/RAL or replace rifampin with rifabutin. Avoid long-acting CAB/RPV with rifamycins; postpone injections until rifamycin therapy complete. | Rifampin: UGT1A1/CYP3A inducer; EVG/c: CYP3A inhibitor (via cobicistat) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikjeh, O.; Rejali, S.; Sasaninia, K.; Venketaraman, V. Overcoming Treatment Challenges in HIV-Associated Mycobacterial Diseases: New Therapeutic Frontiers. Int. J. Mol. Sci. 2025, 26, 10325. https://doi.org/10.3390/ijms262110325
Nikjeh O, Rejali S, Sasaninia K, Venketaraman V. Overcoming Treatment Challenges in HIV-Associated Mycobacterial Diseases: New Therapeutic Frontiers. International Journal of Molecular Sciences. 2025; 26(21):10325. https://doi.org/10.3390/ijms262110325
Chicago/Turabian StyleNikjeh, Omid, Seyedehparmis Rejali, Kayvan Sasaninia, and Vishwanath Venketaraman. 2025. "Overcoming Treatment Challenges in HIV-Associated Mycobacterial Diseases: New Therapeutic Frontiers" International Journal of Molecular Sciences 26, no. 21: 10325. https://doi.org/10.3390/ijms262110325
APA StyleNikjeh, O., Rejali, S., Sasaninia, K., & Venketaraman, V. (2025). Overcoming Treatment Challenges in HIV-Associated Mycobacterial Diseases: New Therapeutic Frontiers. International Journal of Molecular Sciences, 26(21), 10325. https://doi.org/10.3390/ijms262110325







