Application of a Non-Targeted Metabolomics Study in Plasmodium berghei-Infected Rats: Towards Unravelling Metabolic Alterations During Malaria Infection
Abstract
1. Introduction
2. Results
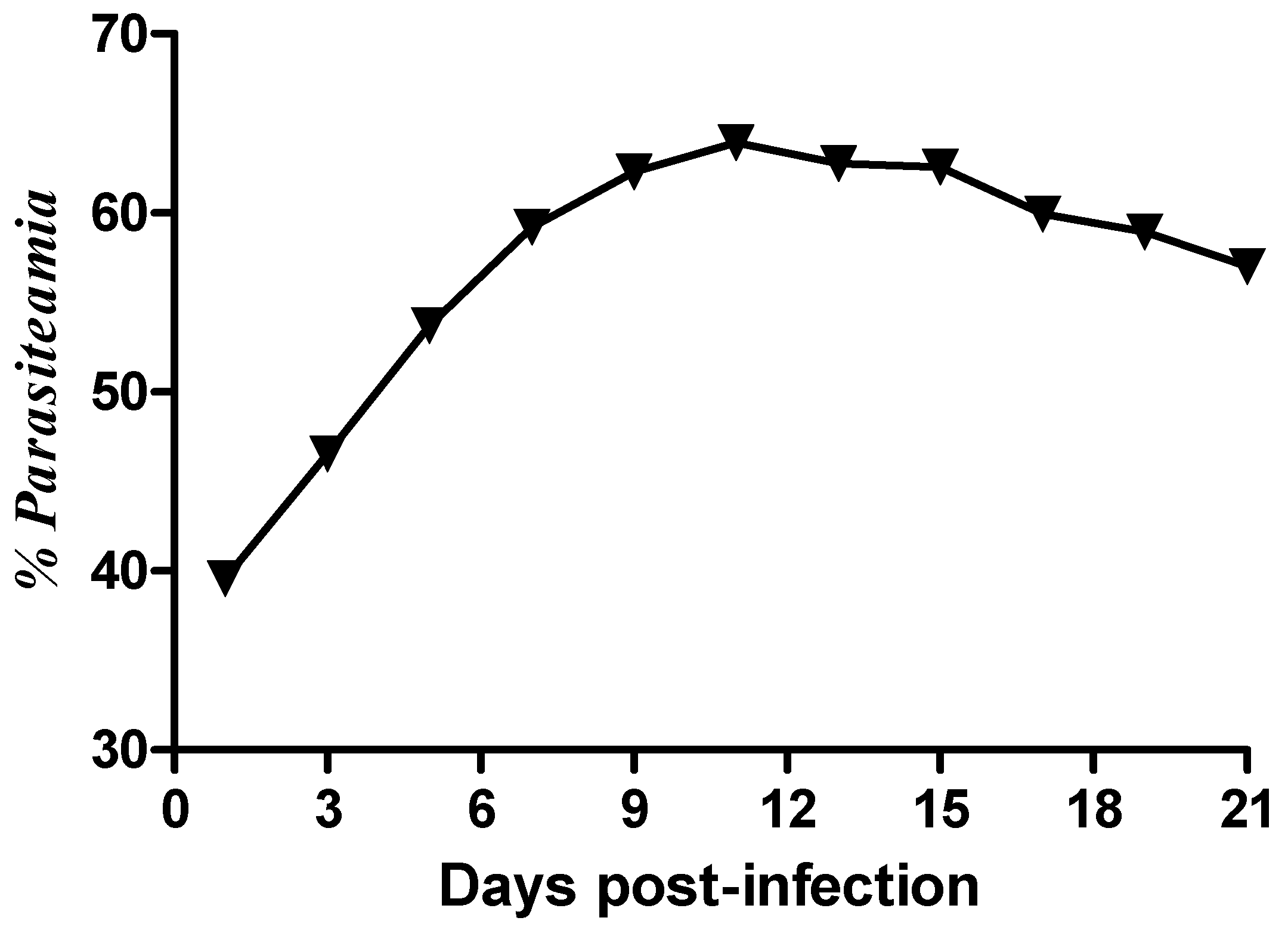
2.1. Parasitaemia
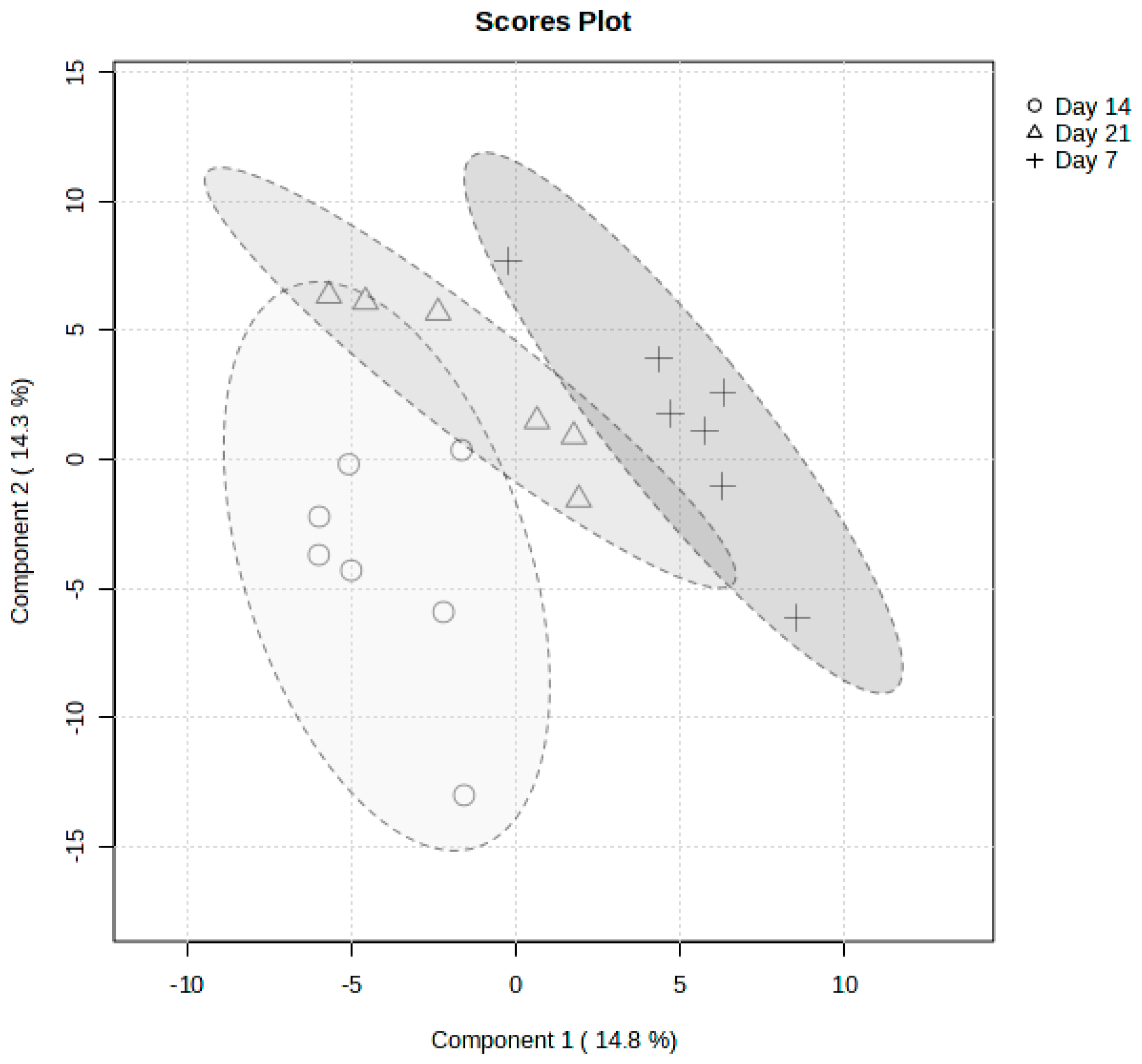
2.2. Metabolite Changes Between Days of Sacrifices Post Infection
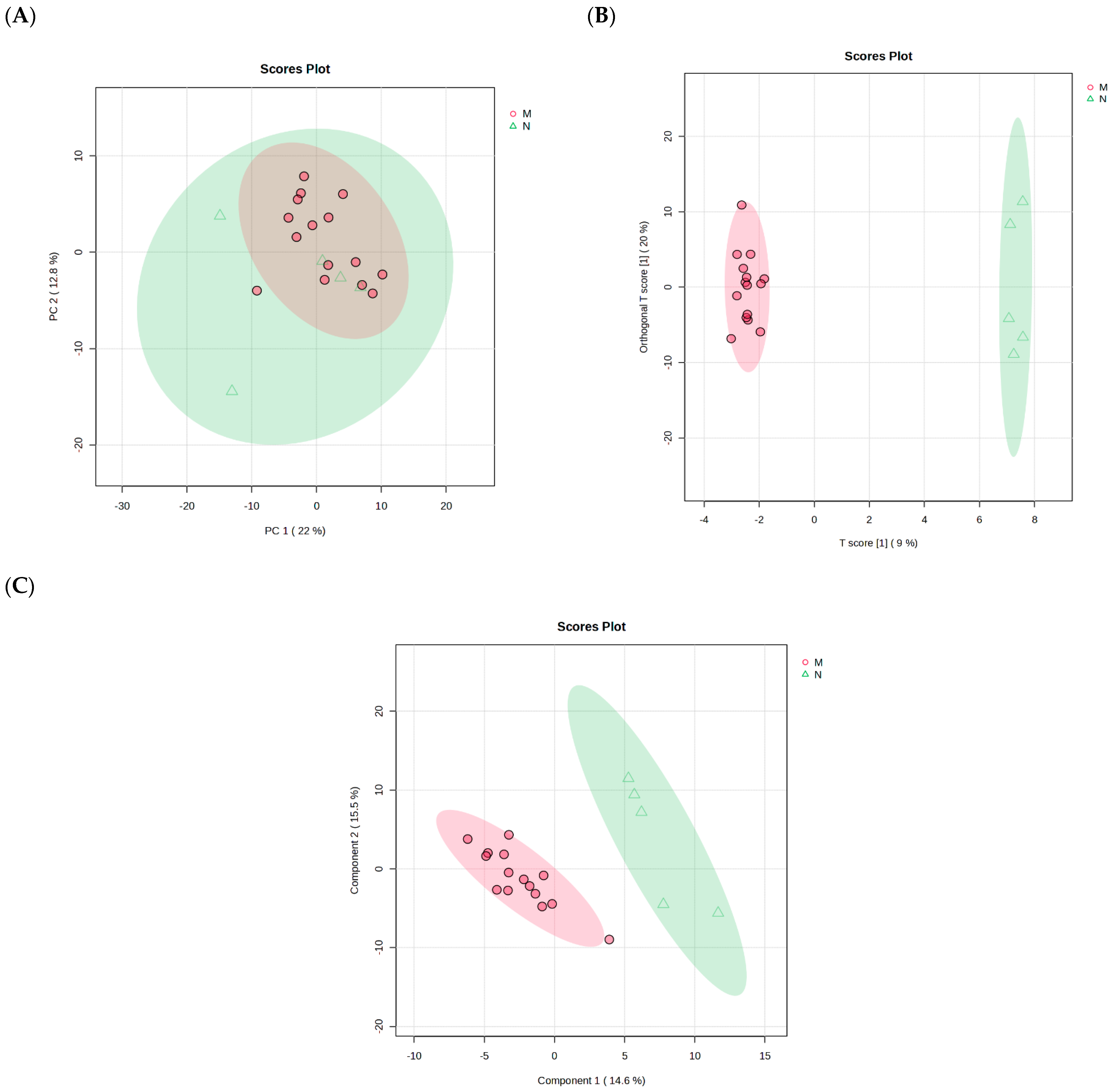
2.3. Pooled of Plasmodium berghei-Infected and Non-Infected Control Group
2.4. Identification of Differential Metabolites
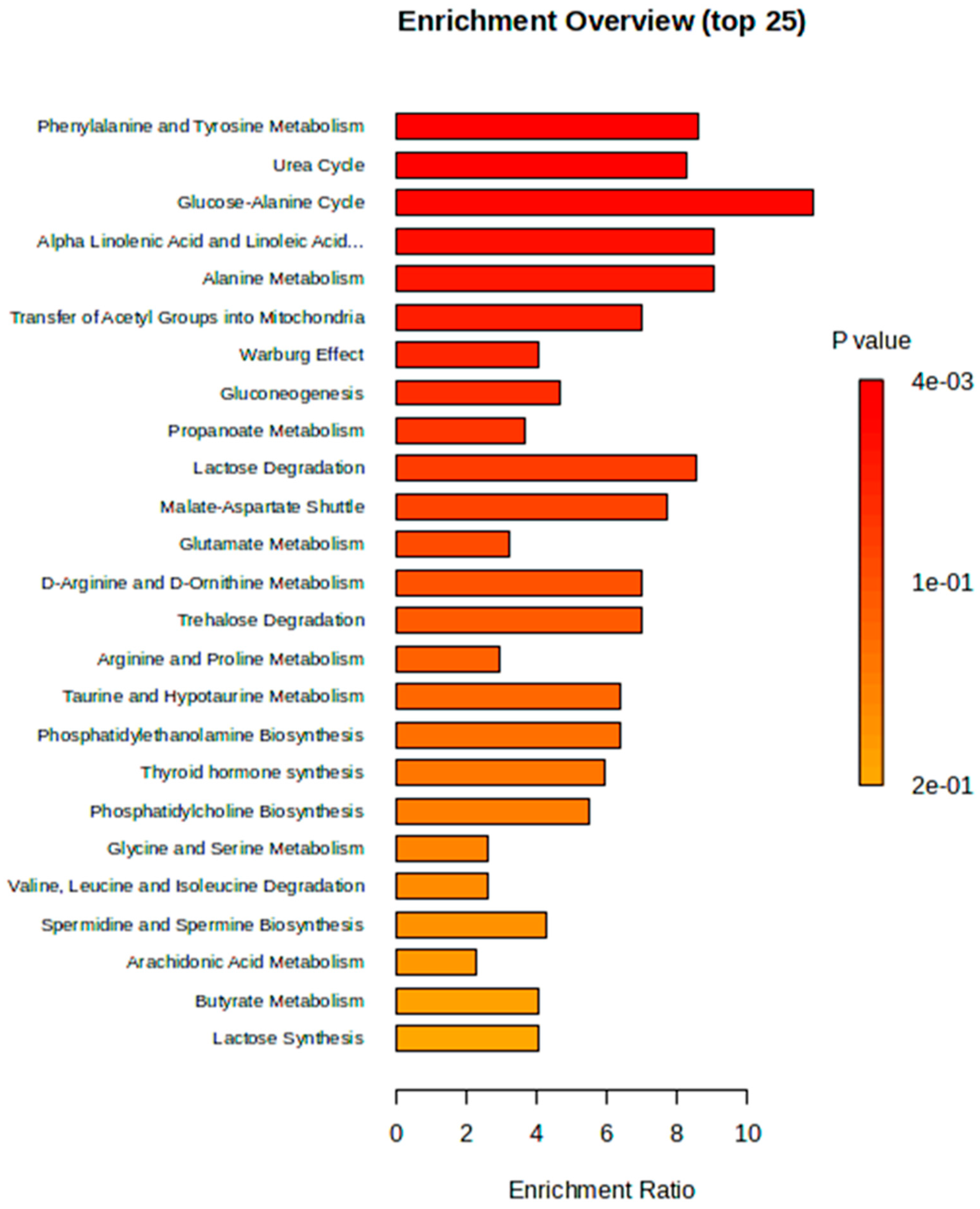
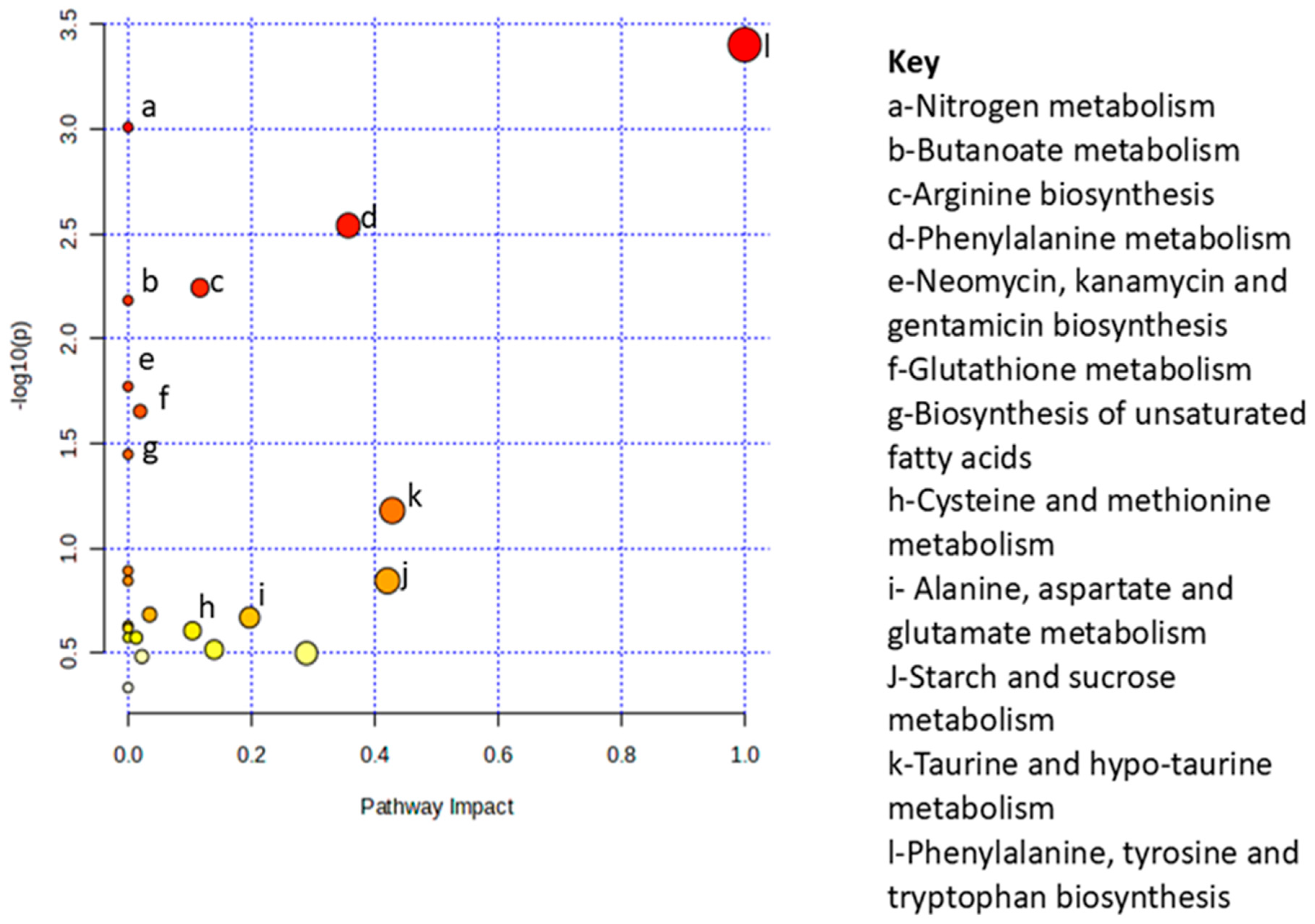
2.5. Metabolic Pathway and Enrichment Analysis
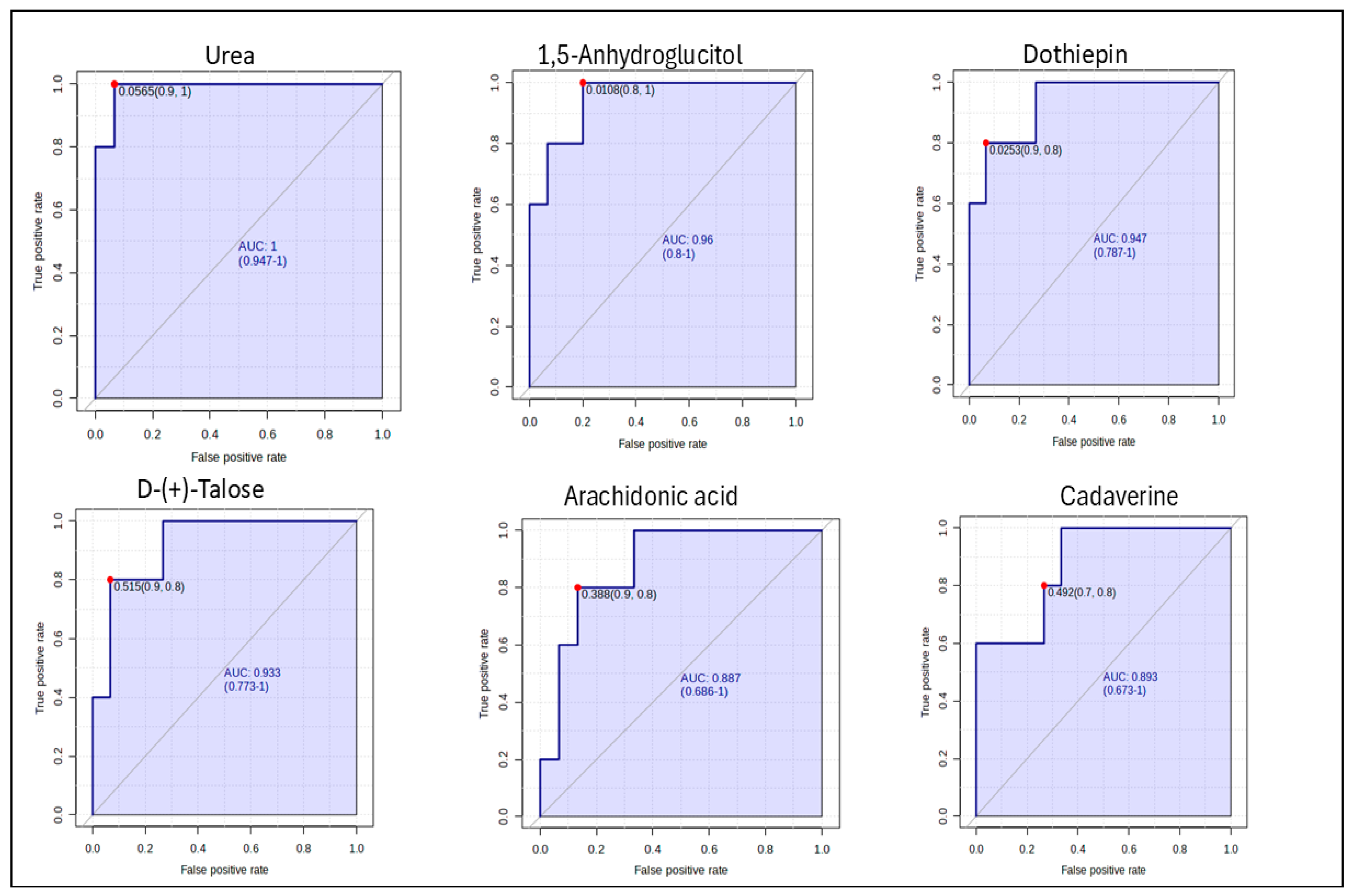
2.6. Determination of Potential Biomarkers
3. Discussion
4. Materials and Methods
4.1. Ethical Consideration
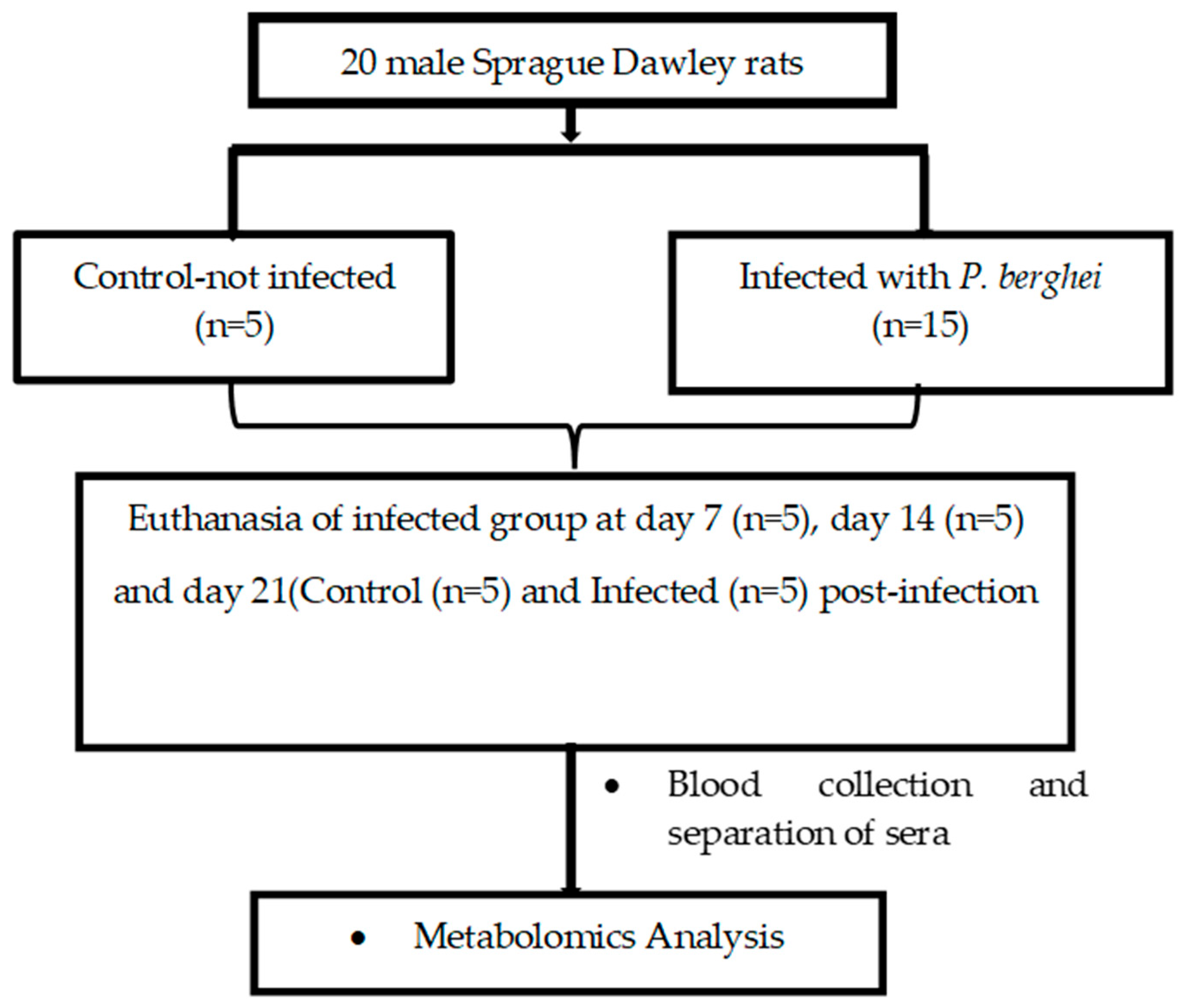
4.2. Experimental Design
4.3. Infection of SD-Rats with Plasmodium berghei Parasite
4.4. Assessment of Parasitemia
4.5. Terminal Studies
4.6. Serum Analysis
4.7. Bench Top Gas Chromatography Coupled to a Time-of-Flight Mass Spectrometer (BT GC-TOFMS) Untargeted-Approach
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| P | Plasmodium |
| SD | Sprague Dawley |
| p.i. | post-infection |
| RBCs | red blood cells |
| IDC | intraerythrocytic developmental cycle |
| IQR | Inter Quartile Range |
| BT GC-TOFMS | Bench Top Gas Chromatography coupled to a Time-Of-Flight Mass Spectrometer |
| PCA | Partial Component Analysis |
| PLS-DA | Partial Least Squares-Discriminant Analysis |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| AUC | Area under the curve |
| MSEA | Metabolite Set-Enrichment Analysis |
| VIP | Variable Importance in Projection |
| MetPA | Metabolic Pathway Analysis |
Appendix A
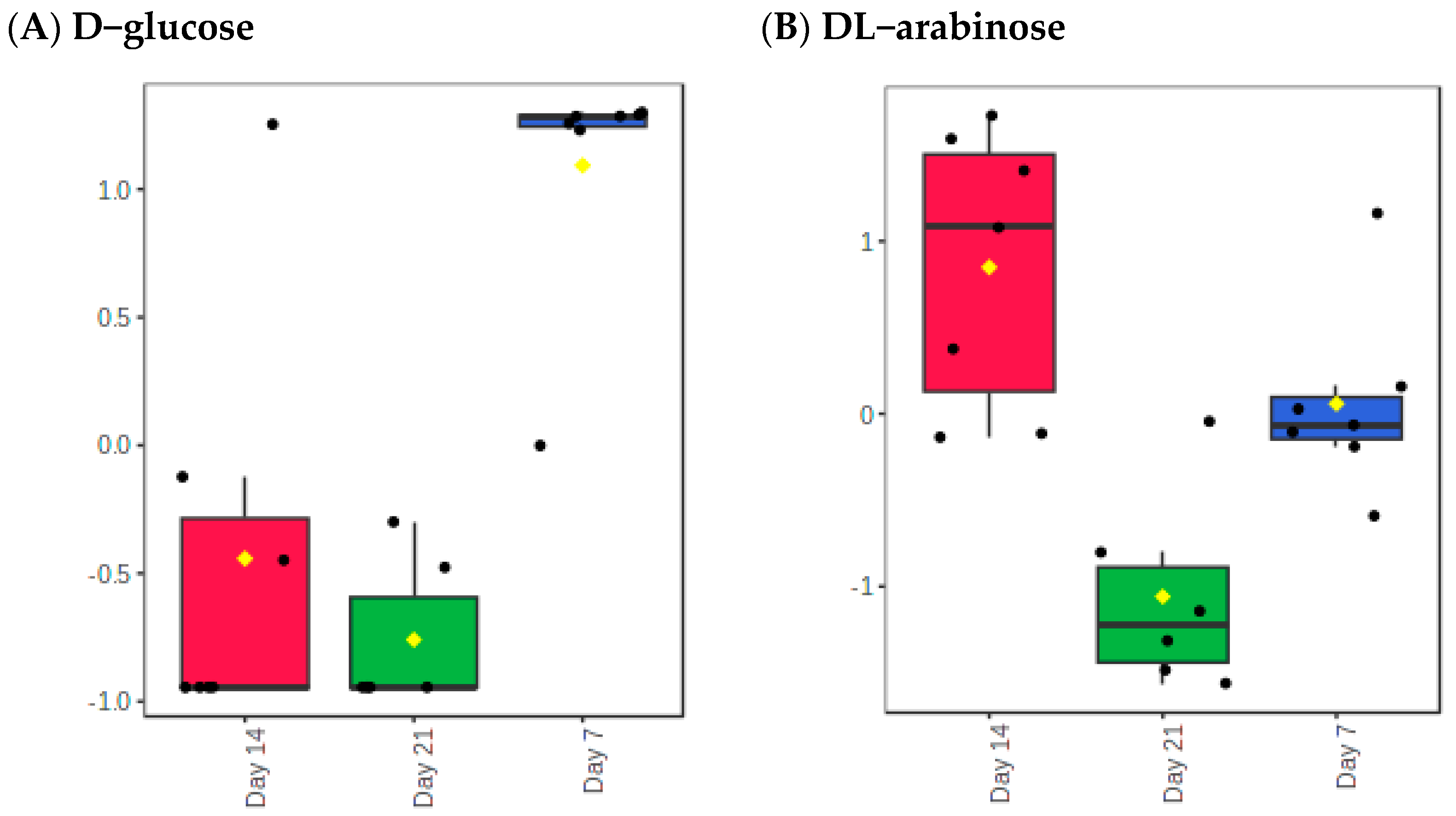
| Metabolite | f-Value | p-Value | FDR | Fisher’s LSD |
|---|---|---|---|---|
| D-glucose | 19.263 | 4 × 10−5 | 0.008927 | Day 7–Day 14; Day 7–Day 21 |
| DL-Arabinose | 13.941 | 0.000253 | 0.027114 | Day 14–Day 21; Day 14–Day 7; Day 7–Day 21 |
| Metabolic Pathways | Total | Expected | Hits | Raw P | Holm P | FDR |
|---|---|---|---|---|---|---|
| Glucose-Alanine Cycle | 13 | 0.156 | 2 | 0.00954 | 0.935 | 0.522 |
| Alpha Linolenic Acid and Linoleic Acid Metabolism | 17 | 0.204 | 2 | 0.0162 | 1 | 0.522 |
| Alanine Metabolism | 17 | 0.204 | 2 | 0.0162 | 1 | 0.522 |
| Transfer of Acetyl Groups into Mitochondria | 22 | 0.263 | 2 | 0.0266 | 1 | 0.522 |
| Warburg Effect | 57 | 0.683 | 3 | 0.0266 | 1 | 0.522 |
| Phenylalanine and Tyrosine Metabolism | 27 | 0.323 | 2 | 0.0391 | 1 | 0.585 |
| Urea Cycle | 28 | 0.335 | 2 | 0.0418 | 1 | 0.585 |
| Gluconeogenesis | 33 | 0.395 | 2 | 0.0565 | 1 | 0.692 |
| Propanoate Metabolism | 42 | 0.503 | 2 | 0.0868 | 1 | 0.85 |
| Lactose Degradation | 9 | 0.108 | 1 | 0.103 | 1 | 0.85 |
| Glutamate Metabolism | 48 | 0.575 | 2 | 0.109 | 1 | 0.85 |
| Malate-Aspartate Shuttle | 10 | 0.12 | 1 | 0.114 | 1 | 0.85 |
| Trehalose Degradation | 11 | 0.132 | 1 | 0.125 | 1 | 0.85 |
| Taurine and Hypotaurine Metabolism | 12 | 0.144 | 1 | 0.135 | 1 | 0.85 |
| Phosphatidylethanolamine Biosynthesis | 12 | 0.144 | 1 | 0.135 | 1 | 0.85 |
| Glycine and Serine Metabolism | 59 | 0.707 | 2 | 0.154 | 1 | 0.85 |
| Valine, Leucine and Isoleucine Degradation | 59 | 0.707 | 2 | 0.154 | 1 | 0.85 |
| Phosphatidylcholine Biosynthesis | 14 | 0.168 | 1 | 0.156 | 1 | 0.85 |
| Arachidonic Acid Metabolism | 67 | 0.802 | 2 | 0.189 | 1 | 0.855 |
| Spermidine and Spermine Biosynthesis | 18 | 0.216 | 1 | 0.196 | 1 | 0.855 |
| Butyrate Metabolism | 19 | 0.228 | 1 | 0.206 | 1 | 0.855 |
| Lactose Synthesis | 19 | 0.228 | 1 | 0.206 | 1 | 0.855 |
| Glutathione Metabolism | 20 | 0.24 | 1 | 0.216 | 1 | 0.855 |
| Threonine and 2-Oxobutanoate Degradation | 20 | 0.24 | 1 | 0.216 | 1 | 0.855 |
| Betaine Metabolism | 21 | 0.251 | 1 | 0.226 | 1 | 0.855 |
| Glycolysis | 23 | 0.275 | 1 | 0.244 | 1 | 0.855 |
| Glycerolipid Metabolism | 25 | 0.299 | 1 | 0.263 | 1 | 0.855 |
| Cysteine Metabolism | 26 | 0.311 | 1 | 0.272 | 1 | 0.855 |
| Plasmalogen Synthesis | 26 | 0.311 | 1 | 0.272 | 1 | 0.855 |
| Mitochondrial Beta-Oxidation of Long Chain Saturated Fatty Acids | 28 | 0.335 | 1 | 0.29 | 1 | 0.855 |
| Phospholipid Biosynthesis | 29 | 0.347 | 1 | 0.298 | 1 | 0.855 |
| Folate Metabolism | 29 | 0.347 | 1 | 0.298 | 1 | 0.855 |
| Lysine Degradation | 30 | 0.359 | 1 | 0.307 | 1 | 0.855 |
| Ammonia Recycling | 31 | 0.371 | 1 | 0.316 | 1 | 0.855 |
| Citric Acid Cycle | 32 | 0.383 | 1 | 0.324 | 1 | 0.855 |
| Amino Sugar Metabolism | 33 | 0.395 | 1 | 0.332 | 1 | 0.855 |
| Beta-Alanine Metabolism | 34 | 0.407 | 1 | 0.341 | 1 | 0.855 |
| Nicotinate and Nicotinamide Metabolism | 35 | 0.419 | 1 | 0.349 | 1 | 0.855 |
| Aspartate Metabolism | 35 | 0.419 | 1 | 0.349 | 1 | 0.855 |
| Fatty Acid Biosynthesis | 35 | 0.419 | 1 | 0.349 | 1 | 0.855 |
| Galactose Metabolism | 38 | 0.455 | 1 | 0.373 | 1 | 0.891 |
| Sphingolipid Metabolism | 40 | 0.479 | 1 | 0.388 | 1 | 0.899 |
| Methionine Metabolism | 42 | 0.503 | 1 | 0.404 | 1 | 0.899 |
| Histidine Metabolism | 42 | 0.503 | 1 | 0.404 | 1 | 0.899 |
| Pyruvate Metabolism | 47 | 0.563 | 1 | 0.44 | 1 | 0.958 |
| Arginine and Proline Metabolism | 52 | 0.623 | 1 | 0.474 | 1 | 1 |
| Pyrimidine Metabolism | 57 | 0.683 | 1 | 0.507 | 1 | 1 |
| Tryptophan Metabolism | 59 | 0.707 | 1 | 0.519 | 1 | 1 |
| Bile Acid Biosynthesis | 65 | 0.778 | 1 | 0.555 | 1 | 1 |
| Tyrosine Metabolism | 70 | 0.838 | 1 | 0.583 | 1 | 1 |
| Purine Metabolism | 73 | 0.874 | 1 | 0.599 | 1 | 1 |
References
- WHO. Community Deployment of Intermittent Preventive Treatment of Malaria in Pregnancy with Sulfadoxine-Pyrimethamine: A Field Guide; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Mohamed, S.A. Prevalence of Malaria and Associated Factors Among Febrile Children Aged 5years and Below at Banadir Hospital, Somalia. Master’s Thesis, Degree-Granting University, Nairobi, Kenya, 2023. [Google Scholar]
- Fernandes, D.M. The STUDY of Complications of Plasmodium Vivax Malaria Mono Infections; Rajiv Gandhi University of Health Sciences (India): Bengaluru, India, 2013. [Google Scholar]
- Srivastava, R. Study of Early Detection of Renal Impairment in Patients with Malaria; Rajiv Gandhi University of Health Sciences (India): Bengaluru, India, 2015. [Google Scholar]
- Soulard, V.; Bosson-Vanga, H.; Lorthiois, A.; Roucher, C.; Franetich, J.-F.; Zanghi, G.; Bordessoulles, M.; Tefit, M.; Thellier, M.; Morosan, S. Plasmodium falciparum full life cycle and Plasmodium ovale liver stages in humanized mice. Nat. Commun. 2015, 6, 7690. [Google Scholar] [CrossRef]
- Esho, R.V. Novel Phenotypic Features of the Malaria Parasite Through Time-Domain Nuclear Magnetic Resonance. Master’s Thesis, University of Minho Portugal, Braga, Portugal, 2023. [Google Scholar]
- Ashley, E.A.; Phyo, A.P. Drugs in development for malaria. Drugs 2018, 78, 861–879. [Google Scholar] [CrossRef]
- Herdman, M.T.; Sriboonvorakul, N.; Leopold, S.J.; Douthwaite, S.; Mohanty, S.; Hassan, M.M.U.; Maude, R.J.; Kingston, H.W.; Plewes, K.; Charunwatthana, P. The role of previously unmeasured organic acids in the pathogenesis of severe malaria. Crit. Care 2015, 19, 1–11, Erratum in Crit. Care 2015, 19, 382. [Google Scholar] [PubMed]
- Leopold, S.J.; Apinan, S.; Ghose, A.; Kingston, H.W.; Plewes, K.A.; Hossain, A.; Dutta, A.K.; Paul, S.; Barua, A.; Sattar, A. Amino acid derangements in adults with severe falciparum malaria. Sci. Rep. 2019, 9, 6602. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.L.; Tran, T.M.; Bond, C.; Opoka, R.O.; Datta, D.; Liechty, E.A.; Bangirana, P.; Namazzi, R.; Idro, R.; Cusick, S. Plasma amino acid concentrations in children with severe malaria are associated with mortality and worse long-term kidney and cognitive outcomes. J. Infect. Dis. 2022, 226, 2215–2225. [Google Scholar] [CrossRef]
- Leitner, W.W.; Bergmann-Leitner, E.S.; Angov, E. Comparison of Plasmodium berghei challenge models for the evaluation of pre-erythrocytic malaria vaccines and their effect on perceived vaccine efficacy. Malar. J. 2010, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Otun, S.O.; Graca, R.; Onisuru, O.; Achilonu, I. Evaluation of Plasmodium berghei models in malaria research. J. Cell. Signal. 2024, 5, 96–113. [Google Scholar] [CrossRef]
- Foote, S.J.; Iraqi, F.; Kemp, S.J. Controlling malaria and African trypanosomiasis: The role of the mouse. Brief. Funct. Genom. 2005, 4, 214–224. [Google Scholar] [CrossRef][Green Version]
- Li, J.V.; Wang, Y.; Saric, J.; Nicholson, J.K.; Dirnhofer, S.; Singer, B.H.; Tanner, M.; Wittlin, S.; Holmes, E.; Utzinger, J.R. Global metabolic responses of NMRI mice to an experimental Plasmodium berghei infection. J. Proteome Res. 2008, 7, 3948–3956. [Google Scholar] [CrossRef]
- Chahine, Z.M. Exploring Novel Drug Targets to Combat the Human Malaria Parasite, Plasmodium falciparum. Ph.D. Thesis, University of California, Riverside, Riverside, CA, USA, 2024. [Google Scholar]
- Olszewski, K.L.; Morrisey, J.M.; Wilinski, D.; Burns, J.M.; Vaidya, A.B.; Rabinowitz, J.D.; Llinás, M. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe 2009, 5, 191–199. [Google Scholar] [CrossRef]
- Colvin, H.N.; Cordy, R.J. Insights into malaria pathogenesis gained from host metabolomics. PLoS Pathog. 2020, 16, e1008930. [Google Scholar] [CrossRef]
- Yu, X.; Feng, G.; Zhang, Q.; Cao, J. From metabolite to metabolome: Metabolomics applications in plasmodium research. Front. Microbiol. 2021, 11, 626183. [Google Scholar] [CrossRef]
- Umo, E.S.; Mukaratirwa, S.; Murambiwa, P. Cytokine and Haemtological Profiles in Sprague-Dawley Rats Experimentally Infected with Trichnella zimbabwensis and Plasmodium berghei Anka. Master Thesis, University of KwaZulu-Natal, Durban, South Africa, 2017. [Google Scholar]
- Paul, A.S.; Egan, E.S.; Duraisingh, M.T. Host–parasite interactions that guide red blood cell invasion by malaria parasites. Curr. Opin. Hematol. 2015, 22, 220–226. [Google Scholar] [CrossRef]
- Sibiya, H.P.; Mabandla, M.V.; Musabayane, C.T. The Effects of Transdermally Delivered Oleanolic Acid on Malaria Parasites and Blood Glucose Homeostasis in P. berghei-Infected Male Sprague-Dawley Rats. PLoS ONE 2016, 11, e0167132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krugliak, M.; Zhang, J.; Ginsburg, H. Intraerythrocytic Plasmodium falciparum utilizes only a fraction of the amino acids derived from the digestion of host cell cytosol for the biosynthesis of its proteins. Mol. Biochem. Parasitol. 2002, 119, 249–256. [Google Scholar] [CrossRef]
- Henshall, I.G.; Spielmann, T. Critical interdependencies between Plasmodium nutrient flux and drugs. Trends Parasitol. 2023, 39, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How malaria parasites acquire nutrients from their host. Front. Cell Dev. Biol. 2021, 9, 649184. [Google Scholar] [CrossRef]
- De Niz, M.; Meibalan, E.; Mejia, P.; Ma, S.; Brancucci, N.M.; Agop-Nersesian, C.; Mandt, R.; Ngotho, P.; Hughes, K.R.; Waters, A.P. Plasmodium gametocytes display homing and vascular transmigration in the host bone marrow. Sci. Adv. 2018, 4, eaat3775. [Google Scholar] [CrossRef]
- MacRae, J.I.; Dixon, M.W.; Dearnley, M.K.; Chua, H.H.; Chambers, J.M.; Kenny, S.; Bottova, I.; Tilley, L.; McConville, M.J. Mitochondrial metabolism of sexual and asexual blood stages of the malaria parasite Plasmodium falciparum. BMC Biol. 2013, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.N.; Brown, S.H.; Rug, M.; Ridgway, M.C.; Mitchell, T.W.; Maier, A.G. Changes in lipid composition during sexual development of the malaria parasite Plasmodium falciparum. Malar. J. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Na, J.; Khan, A.; Kim, J.K.; Wadood, A.; Choe, Y.L.; Walker, D.I.; Jones, D.P.; Lim, C.S.; Park, Y.H. Discovery of metabolic alterations in the serum of patients infected with Plasmodium spp. by high-resolution metabolomics. Metabolomics 2020, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and ammonia metabolism and the control of renal nitrogen excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef]
- Mwaba, C.; Munsaka, S.; Bvulani, B.; Mwakazanga, D.; Chiluba, B.C.; Fitzwanga, K.; Mpabalwani, E. Malaria is the leading cause of acute kidney injury among a Zambian paediatric renal service cohort retrospectively evaluated for aetiologies, predictors of the need for dialysis, and outcomes. PLoS ONE 2023, 18, e0293037. [Google Scholar] [CrossRef]
- Akanbi, O.M. The influence of malaria infection on kidney and liver function in children in Akoko area of Ondo state, Nigeria. J. Parasitol. Vector Biol. 2015, 7, 163–168. [Google Scholar]
- Zaki, H.Y.; Abdalla, B.E.; Hayder, B. Biochemical Profiles of Children with Severe Plasmodium falciparum malaria in central Sudan: A case control study. Al Neelain Med. J. 2013, 3, 15–23. [Google Scholar]
- Ramos, S.; Ademolue, T.W.; Jentho, E.; Wu, Q.; Guerra, J.; Martins, R.; Pires, G.; Weis, S.; Carlos, A.R.; Mahú, I. A hypometabolic defense strategy against malaria. Cell Metab. 2022, 34, 1183–1200.e12. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, S.; Lee, J.; Park, J.-C.; Kim, K.H.; Ko, J.M.; Park, S.-H.; Kim, S.-K.; Mook-Jung, I.; Lee, J.Y. Neurotoxicity of phenylalanine on human iPSC-derived cerebral organoids. Mol. Genet. Metab. 2022, 136, 132–144. [Google Scholar] [CrossRef]
- Planche, T.; Dzeing, A.; Ngou-Milama, E.; Kombila, M.; Stacpoole, P. Metabolic complications of severe malaria. Malar. Drugs Dis. Post-Genom. Biol. 2005, 295, 105–136. [Google Scholar]
- Selvin, E.; Rawlings, A.; Lutsey, P.; Maruthur, N.; Pankow, J.S.; Steffes, M.; Coresh, J. Association of 1, 5-anhydroglucitol with cardiovascular disease and mortality. Diabetes 2016, 65, 201–208. [Google Scholar] [CrossRef]
- Migała, M.; Chałubińska-Fendler, J.; Zielińska, M. 1, 5-Anhydroglucitol as a marker of acute hyperglycemia in cardiovascular events. Rev. Diabet. Stud. RDS 2022, 18, 68. [Google Scholar] [CrossRef]
- Rubach, M.P.; Mukemba, J.; Florence, S.; Lopansri, B.K.; Hyland, K.; Volkheimer, A.D.; Yeo, T.W.; Anstey, N.M.; Weinberg, J.B.; Mwaikambo, E.D. Impaired systemic tetrahydrobiopterin bioavailability and increased oxidized biopterins in pediatric falciparum malaria: Association with disease severity. PLoS Pathog. 2015, 11, e1004655. [Google Scholar] [CrossRef]
- Day, N.P.; Phu, N.H.; Mai, N.T.H.; Chau, T.T.H.; Loc, P.P.; Van Chuong, L.; Sinh, D.X.; Holloway, P.; Hien, T.T.; White, N.J. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Crit. Care Med. 2000, 28, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Pukrittayakamee, S.; Krishna, S.; Kuile, F.T.; Wilaiwan, O.; Williamson, D.; White, N.J. Alanine metabolism in acute falciparum malaria. Trop. Med. Int. Health 2002, 7, 911–918. [Google Scholar] [CrossRef][Green Version]
- Lopansri, B.K.; Anstey, N.M.; Stoddard, G.J.; Mwaikambo, E.D.; Boutlis, C.S.; Tjitra, E.; Maniboey, H.; Hobbs, M.R.; Levesque, M.C.; Weinberg, J.B. Elevated plasma phenylalanine in severe malaria and implications for pathophysiology of neurological complications. Infect. Immun. 2006, 74, 3355–3359. [Google Scholar] [CrossRef]
- Medana, I.M.; Day, N.P.; Salahifar-Sabet, H.; Stocker, R.; Smythe, G.; Bwanaisa, L.; Njobvu, A.; Kayira, K.; Turner, G.D.; Taylor, T.E. Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J. Infect. Dis. 2003, 188, 844–849. [Google Scholar] [CrossRef]
- Leopold, S.J.; Watson, J.A.; Jeeyapant, A.; Simpson, J.A.; Phu, N.H.; Hien, T.T.; Day, N.P.; Dondorp, A.M.; White, N.J. Investigating causal pathways in severe falciparum malaria: A pooled retrospective analysis of clinical studies. PLoS Med. 2019, 16, e1002858. [Google Scholar] [CrossRef]
- Uttaro, A.D. Acquisition and biosynthesis of saturated and unsaturated fatty acids by trypanosomatids. Mol. Biochem. Parasitol. 2014, 196, 61–70. [Google Scholar] [CrossRef] [PubMed]
- de Aquino, G.P.; Gomes, M.A.M.; Salinas, R.K.; Laranjeira-Silva, M.F. Lipid and fatty acid metabolism in trypanosomatids. Microb. Cell 2021, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Skorokhod, O.; Barrera, V.; Mandili, G.; Costanza, F.; Valente, E.; Ulliers, D.; Schwarzer, E. Malaria Pigment Hemozoin Impairs GM-CSF Receptor Expression and Function by 4-Hydroxynonenal. Antioxidants 2021, 10, 1259. [Google Scholar] [CrossRef]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Diehl, K.H.; Hull, R.; Morton, D.; Pfister, R.; Rabemampianina, Y.; Smith, D.; Vidal, J.M.; van de Vorstenbosch, C. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001, 21, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin, B. Methodes D′analyse Sédimentaire et Reconstitution du Bassin: Le Jurassique Terminal-Berriasien des Chaines Subalpines Méridionales. Ph.D. Thesis, Université de Caen, Caen, France, 1977. [Google Scholar]
- Eberhard, M.L.; Lammie, P.J. Laboratory diagnosis of filariasis. Clin. Lab. Med. 1991, 11, 977–1010. [Google Scholar] [CrossRef] [PubMed]
- Swanepoel, A.C.; Bester, J.; Emmerson, O.; Soma, P.; Beukes, D.; van Reenen, M.; Loots, D.T.; Preez, I.D. Serum metabolome changes in relation to prothrombotic state induced by combined oral contraceptives with drospirenone and ethinylestradiol. OMICS A J. Integr. Biol. 2020, 24, 404–414. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Broadhurst, D.; Goodacre, R.; Reinke, S.N.; Kuligowski, J.; Wilson, I.D.; Lewis, M.R.; Dunn, W.B. Guidelines and considerations for the use of system suitability and quality control samples in mass spectrometry assays applied in untargeted clinical metabolomic studies. Metabolomics 2018, 14, 72. [Google Scholar] [CrossRef] [PubMed]
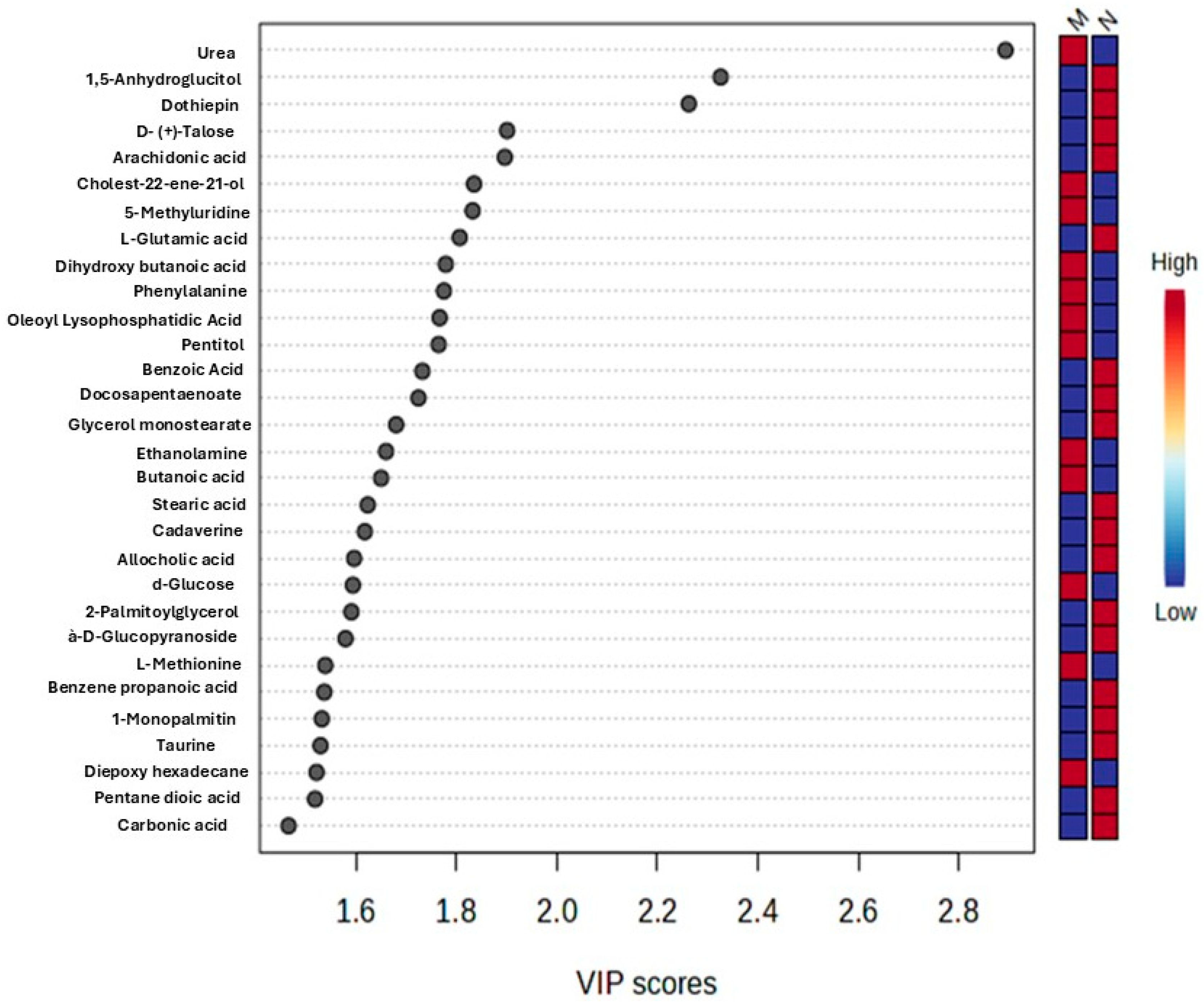

| Query | Class | VIP | p−Value | FDR | HMDB | PubChem | KEGG |
|---|---|---|---|---|---|---|---|
| Urea | Organic compound | 2.8929 | 7.52 × 10−7 | 0.000157 | HMDB0000294 | 1176 | C00086 |
| 1,5-Anhydroglucitol | Carbohydrate | 2.3261 | 0.000634 | 0.066291 | HMDB0002712 | 64960 | C07326 |
| Dothiepin | Xenobiotic | 2.2627 | 0.001014 | 0.070658 | None | None | None |
| D−(+)−Talose | Carbohydrate | 1.9011 | 0.008721 | 0.28696 | None | None | None |
| Arachidonic acid | Fatty acid | 1.8966 | 0.008919 | 0.28696 | HMDB0001043 | 444899 | C00219 |
| Cholest-22-ene-21-ol | Xenobiotic | 1.8353 | 0.011972 | 0.28696 | None | None | None |
| 5-Methyluridine | pyrimidine nucleoside | 1.8326 | 0.012128 | 0.28696 | HMDB0000884 | 445408 | None |
| L−Glutamic acid | Amino acid | 1.8068 | 0.013658 | 0.28696 | HMDB0000148 | 33032 | C00025 |
| Dihydroxy butanoic acid | Fatty acid | 1.7791 | 0.015475 | 0.28696 | None | None | None |
| Phenylalanine | Amino acid | 1.7754 | 0.015729 | 0.28696 | HMDB0000159 | 6140 | C00079 |
| Oleoyl Lysophosphatidic Acid | Fatty acid | 1.7668 | 0.016337 | 0.28696 | None | None | None |
| Pentitol | Carbohydrates | 1.7649 | 0.016476 | 0.28696 | HMDB0000508 | None | C00474 |
| Benzoic Acid | Organic compound | 1.7326 | 0.018952 | 0.29268 | HMDB0001870 | 243 | C00180 |
| Docosapentaenoate | Fatty acid | 1.7246 | 0.019605 | 0.29268 | HMDB0001976 | 6441454 | None |
| Glycerol monostearate | Fatty acid | 1.6804 | 0.023575 | 0.31479 | None | None | None |
| Ethanolamine | Organic compound | 1.6603 | 0.025571 | 0.31479 | HMDB0000149 | 700 | C00189 |
| Butanoic acid | Fatty acid | 1.6503 | 0.026609 | 0.31479 | HMDB0000039 | 264 | C00246 |
| Stearic acid | Fatty acids | 1.6238 | 0.029544 | 0.31479 | HMDB0000827 | 5281 | C01530 |
| Cadaverine | Organic compound | 1.6179 | 0.030226 | 0.31479 | HMDB0002322 | 273 | C01672 |
| Allocholic acid | Organic compound | 1.5967 | 0.032792 | 0.31479 | HMDB0000505 | 160636 | C17737 |
| d−Glucose | Carbohydrate | 1.5942 | 0.033103 | 0.31479 | HMDB0000122 | 5793 | C00031 |
| 2-Palmitoylglycerol | Fatty acid | 1.5913 | 0.033473 | 0.31479 | None | None | None |
| à−D−Glucopyranoside | Carbohydrate | 1.5795 | 0.034993 | 0.31479 | None | None | None |
| L-Methionine | Amino acid | 1.5393 | 0.040596 | 0.31479 | HMDB0000696 | 6137 | C00073 |
| Benzene propanoic acid | Carboxylic acid | 1.5373 | 0.040896 | 0.31479 | HMDB0000158 | 6057 | C00082 |
| 1−Monopalmitin | Fatty acid | 1.5324 | 0.041622 | 0.31479 | None | None | None |
| Taurine | Amino acid | 1.5298 | 0.042014 | 0.31479 | HMDB0000251 | 1123 | C00245 |
| Diepoxy hexadecane | Xenobiotic | 1.5219 | 0.043225 | 0.31479 | None | None | None |
| Pentane dioic acid | Organic compound | 1.519 | 0.04368 | 0.31479 | HMDB0000661 | 743 | C00489 |
| Carbonic acid | Xenobiotic | 1.4662 | 0.04578 | 0.31479 | HMDB0000596 | C00288 | None |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbuli, Z.N.; Ndlovu, I.S.; Masola, B.; Mukaratirwa, S. Application of a Non-Targeted Metabolomics Study in Plasmodium berghei-Infected Rats: Towards Unravelling Metabolic Alterations During Malaria Infection. Int. J. Mol. Sci. 2025, 26, 10324. https://doi.org/10.3390/ijms262110324
Mbuli ZN, Ndlovu IS, Masola B, Mukaratirwa S. Application of a Non-Targeted Metabolomics Study in Plasmodium berghei-Infected Rats: Towards Unravelling Metabolic Alterations During Malaria Infection. International Journal of Molecular Sciences. 2025; 26(21):10324. https://doi.org/10.3390/ijms262110324
Chicago/Turabian StyleMbuli, Zoxolo Nokulunga, Innocent Siyanda Ndlovu, Bubuya Masola, and Samson Mukaratirwa. 2025. "Application of a Non-Targeted Metabolomics Study in Plasmodium berghei-Infected Rats: Towards Unravelling Metabolic Alterations During Malaria Infection" International Journal of Molecular Sciences 26, no. 21: 10324. https://doi.org/10.3390/ijms262110324
APA StyleMbuli, Z. N., Ndlovu, I. S., Masola, B., & Mukaratirwa, S. (2025). Application of a Non-Targeted Metabolomics Study in Plasmodium berghei-Infected Rats: Towards Unravelling Metabolic Alterations During Malaria Infection. International Journal of Molecular Sciences, 26(21), 10324. https://doi.org/10.3390/ijms262110324







