Toxicological Responses of Photosynthetic Genes in Chlorella vulgaris Exposed to Environmentally Relevant Concentrations of TiO2 Nanoparticles
Abstract
1. Introduction
2. Results
2.1. Standard Analysis of TiO2 Nanoparticles
2.2. Cell Inhibition Assay
2.3. Standardization of Primers
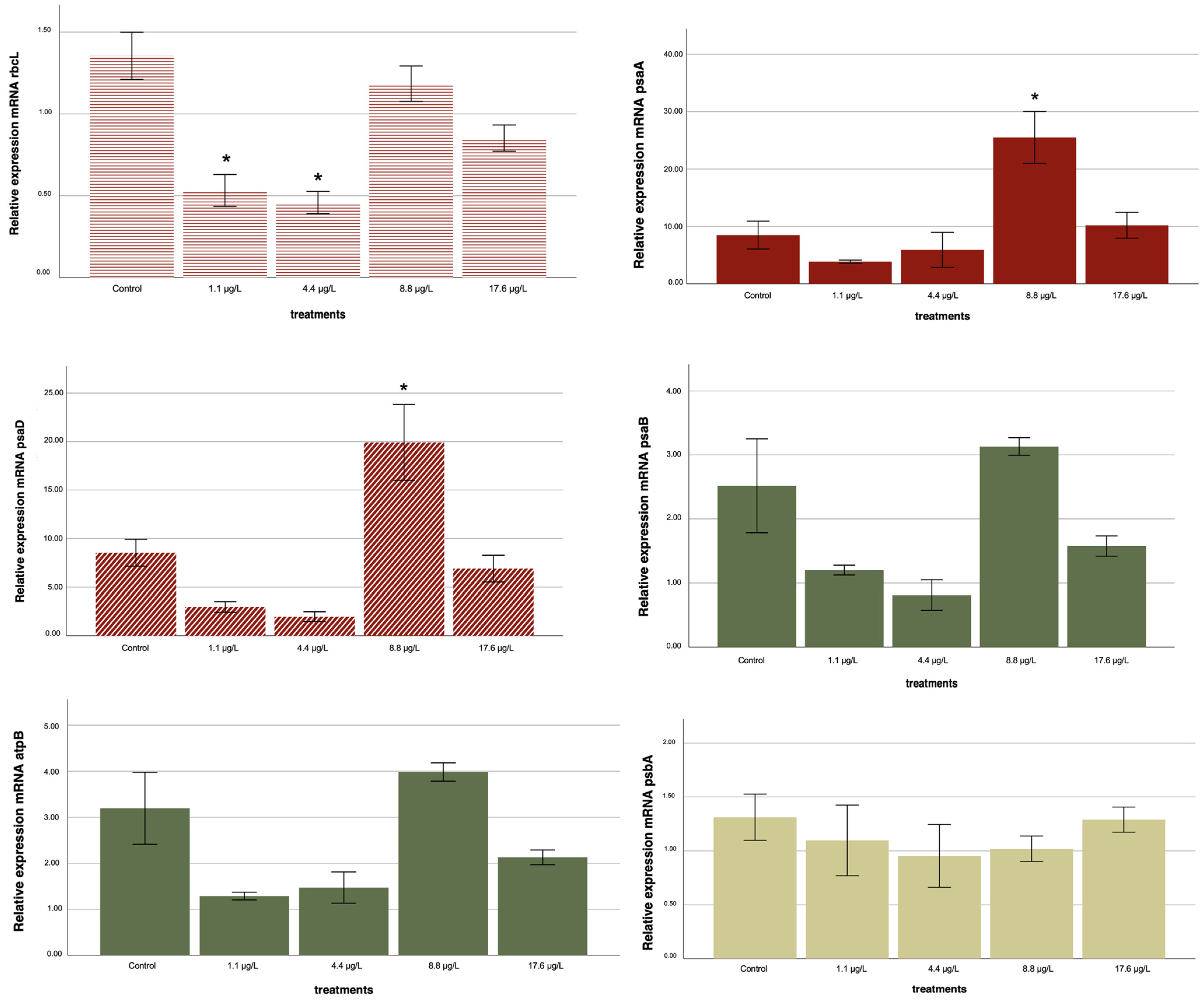
2.4. Gene Expression
3. Discussion
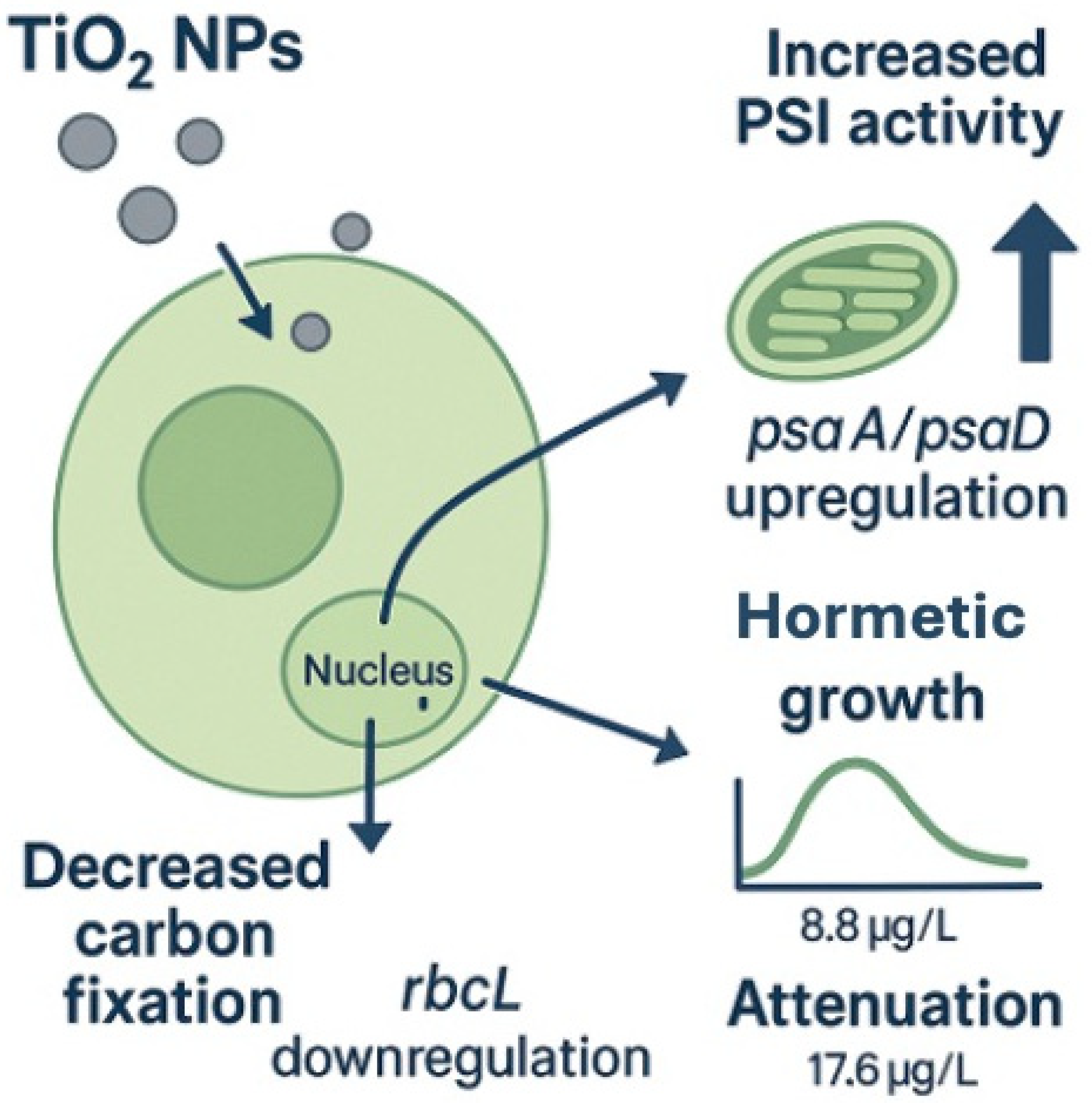
3.1. Adaptive Response: Positive Regulation
3.2. Toxic Effect: Negative Regulation
3.3. Hormetic Observations: Cell Density and Gene Regulation
3.4. Ecological Implications: A Balance Between Adaptation and Risk
3.5. Study Limitations
3.6. Perspectives on Metabolomics and Integrated Omics Approaches
4. Materials and Methods
4.1. Analysis of TiO2 Nanoparticle Standard
4.2. Biological Analysis
4.3. Obtaining RNA from Chlorella vulgaris
4.4. Primer Design
4.5. RT-qPCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Post Hoc Tests—Bonferroni | |||||||
|---|---|---|---|---|---|---|---|
| Dependent Variable | (I) Treatments | (J) Treatments | Mean Difference (I–J) | Std. Error | Sig | Lower Bound | Upper Bound |
| atpB | Control | 1.1 µg/L | 1.906 | 0.144 | 0.071 | −0.118 | 3.9308 |
| 4.4 µg/L | 1.722 | 0.589 | 0.123 | −0.302 | 3.7464 | ||
| 8.8 µg/L | −0.788 | 0.344 | 1.000 | −2.812 | 1.2364 | ||
| 17.6 µg/L | 1.065 | 0.276 | 0.889 | −0.959 | 3.0894 | ||
| rbcL | Control | 1.1 µg/L | 0.823 * | 0.169 | 0.002 | 0.301 | 1.3441 |
| 4.4 µg/L | 0.897 * | 0.118 | 0.001 | 0.375 | 1.4181 | ||
| 8.8 µg/L | 0.170 | 0.188 | 1.000 | −0.351 | 0.6918 | ||
| 17.6 µg/L | 0.503 | 0.139 | 0.062 | −0.019 | 10.244 | ||
| psaA | Control | 1.1 µg/L | 4.627 | 0.479 | 1.000 | −9.854 | 19.1084 |
| 4.4 µg/L | 2.575 | 5.293 | 1.000 | −11.907 | 17.0561 | ||
| 8.8 µg/L | −17.020 * | 7.830 | 0.018 | −31.501 | −2.5383 | ||
| 17.6 µg/L | 1.718 | 3.920 | 1.000 | −16.199 | 12.7634 | ||
| psaB | Control | 1.1 µg/L | 1.317 | 0.133 | 0.268 | −0.502 | 3.1367 |
| 4.4 µg/L | 1.705 | 0.411 | 0.073 | −0.115 | 3.5240 | ||
| 8.8 µg/L | −0.614 | 0.238 | 1.000 | −2.433 | 1.2057 | ||
| 17.6 µg/L | 0.942 | 0.273 | 0.934 | −0.877 | 2.7613 | ||
| psaD | Control | 1.1 µg/L | 5.570 | 0.959 | 0.752 | −4.476 | 15.6157 |
| 4.4 µg/L | 6.567 | 0.872 | 0.413 | −3.479 | 16.6127 | ||
| 8.8 µg/L | −11.375 * | 6.772 | 0.023 | −21.421 | −1.3293 | ||
| 17.6 µg/L | 1.638 | 2.399 | 1.000 | −8.408 | 11.6837 | ||
| psbA | Control | 1.1 µg/L | 0.215 | 0.567 | 1.000 | −0.952 | 1.3817 |
| 4.4 µg/L | 0.358 | 0.505 | 1.000 | −0.809 | 1.5247 | ||
| 8.8 µg/L | 0.292 | 0.204 | 1.000 | −0.875 | 1.4594 | ||
| 17.6 µg/L | 0.022 | 0.202 | 1.000 | −1.145 | 1.1890 | ||
References
- Yadav, G.; Ahmaruzzaman, M. Consumer Nanoproducts: A Brief Introduction. In Handbook of Consumer Nanoproducts; Mallakpour, S., Hussain, C.M., Eds.; Springer Nature: Singapore, 2022; pp. 3–16. ISBN 978-981-16-8698-6. [Google Scholar]
- Alizadeh, H.; Mostaan, M.A.; Malih, N.; Davoodi, J. Size and Shape Dependent Thermal Properties of Rutile TiO2 Nanoparticles: A Molecular Dynamics Simulation Study. Mol. Simul. 2020, 46, 341–349. [Google Scholar] [CrossRef]
- Westerhoff, P.; Song, G.; Hristovski, K.; Kiser, M.A. Occurrence and Removal of Titanium at Full Scale Wastewater Treatment Plants: Implications for TiO2 Nanomaterials. J. Environ. Monit. 2011, 13, 1195–1203. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Emke, E.; Tromp, P.; Hofman, J.A.; Carboni, A.; Schooneman, F.; de Voogt, P.; van Wezel, A.P. Is There Evidence for Man-Made Nanoparticles in the Dutch Environment? Sci. Total Environ. 2017, 576, 273–283. [Google Scholar] [CrossRef]
- Jin, A.; Wang, Y.; Lin, K.; Jiang, L. Nanoparticles Modified by Polydopamine: Working as “Drug” Carriers. Bioact. Mater. 2020, 5, 522–541. [Google Scholar] [CrossRef]
- Chomanee, J.; Thongboon, K.; Tekasakul, S.; Furuuchi, M.; Dejchanchaiwong, R.; Tekasakul, P. Physicochemical and Toxicological Characteristics of Nanoparticles in Aerosols in Southern Thailand during Recent Haze Episodes in Lower Southeast Asia. J. Environ. Sci. 2020, 94, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sun, T.Y.; Nowack, B. Environmental Concentrations of Engineered Nanomaterials: Review of Modeling and Analytical Studies. Environ. Pollut. 2013, 181, 287. [Google Scholar] [CrossRef] [PubMed]
- Cao, G. Nanostructures & Nanomaterials: Synthesis, Properties & Applications; Imperial College Press: London, UK, 2004; ISBN 1-86094-480-9. [Google Scholar]
- Sengupta, A.; Sarkar, C.K. Introduction to Nano: Basics to Nanoscience and Nanotechnology; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 3-662-47314-3. [Google Scholar]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- ISO/TS 80004-1; Nanotechnologies—Vocabulary—Part 1: Core Terms. ISO (the International Organization for Standardization): Geneva, Switzerland, 2015.
- Casarett, L.J. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 2008; Volume 71470514, ISBN 0-07-147051-4. [Google Scholar]
- Mirzaei, J.; Reznikov, M.; Hegmann, T. Quantum Dots as Liquid Crystal Dopants. J. Mater. Chem. 2012, 22, 22350–22365. [Google Scholar] [CrossRef]
- Baalousha, M.; Wang, J.; Nabi, M.M.; Loosli, F.; Valenca, R.; Mohanty, S.K.; Afrooz, N.; Cantando, E.; Aich, N. Stormwater Green Infrastructures Retain High Concentrations of TiO2 Engineered (Nano)-Particles. J. Hazard. Mater. 2020, 392, 122335. [Google Scholar] [CrossRef]
- Philippe, A.; Bazoobandi, A.; Goeppert, N. Quantification of Anthropogenic TiO2 Nanoparticles in Soils and Sediments Combining Size Fractionation and Trace Element Ratio. J. Anal. At. Spectrom. 2022, 37, 338–350. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Nanoparticle-Induced Ecotoxicological Risks in Aquatic Environments: Concepts and Controversies. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: Cambridge, MA, USA, 2019; pp. 129–141. [Google Scholar]
- Deng, X.-Y.; Cheng, J.; Hu, X.-L.; Wang, L.; Li, D.; Gao, K. Biological Effects of TiO2 and CeO2 Nanoparticles on the Growth, Photosynthetic Activity, and Cellular Components of a Marine Diatom Phaeodactylum tricornutum. Sci. Total Environ. 2017, 575, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; You, Y.; Wang, B.; Wu, S.; Zhang, Z.; Zheng, X.; Bao, M.; Zhan, J. Inactivation of Harmful Cyanobacteria by Ag/AgCl@ ZIF-8 Coating under Visible Light: Efficiency and Its Mechanisms. Appl. Catal. B Environ. 2019, 256, 117866. [Google Scholar] [CrossRef]
- Kim, H.J.; Phenrat, T.; Tilton, R.D.; Lowry, G.V. Effect of Kaolinite, Silica Fines and pH on Transport of Polymer-Modified Zero Valent Iron Nano-Particles in Heterogeneous Porous Media. J. Colloid Interface Sci. 2012, 370, 1. [Google Scholar] [CrossRef]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver Nanoparticle Toxicity Effect on Growth and Cellular Viability of the Aquatic Plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Stegemeier, J.P.; Colman, B.P.; Schwab, F.; Wiesner, M.R.; Lowry, G.V. Uptake and Distribution of Silver in the Aquatic Plant Landoltia punctata (Duckweed) Exposed to Silver and Silver Sulfide Nanoparticles. Environ. Sci. Technol. 2017, 51, 4936–4943. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Q.; Yousaf, B.; Ullah, H.; Ali, M.U.; Ok, Y.S.; Rinklebe, J. Environmental Transformation and Nano-Toxicity of Engineered Nano-Particles (ENPs) in Aquatic and Terrestrial Organisms. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2523–2581. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, X.; Li, Y.; Pichan, C.; Chen, Z. Molecular Interactions Between Silver Nanoparticles and Model Cell Membranes. Top. Catal. 2018, 61, 1148–1162. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Pérez, S.; Blasco, J. Toxicity of Silver and Gold Nanoparticles on Marine Microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef]
- Xiang, L.; Fang, J.; Cheng, H. Toxicity of Silver Nanoparticles to Green Algae M. aeruginosa and Alleviation by Organic Matter. Environ. Monit. Assess. 2018, 190, 667. [Google Scholar] [CrossRef]
- Ivask, A.; Kurvet, I.; Kasemets, K.; Blinova, I.; Aruoja, V.; Suppi, S.; Vija, H.; Käkinen, A.; Titma, T.; Heinlaan, M.; et al. Size-Dependent Toxicity of Silver Nanoparticles to Bacteria, Yeast, Algae, Crustaceans and Mammalian Cells In Vitro. PLoS ONE 2014, 9, e102108. [Google Scholar] [CrossRef]
- Weber, S.; Grande, P.M.; Blank, L.M.; Klose, H. Insights into Cell Wall Disintegration of Chlorella vulgaris. PLoS ONE 2022, 17, e0262500. [Google Scholar] [CrossRef]
- Takeda, H. Taxonomical Assignment of Chlorococal Algae from Their Cell Wall Composition. Phytochemistry 1993, 34, 1053–1055. [Google Scholar] [CrossRef]
- Middepogu, A.; Hou, J.; Gao, X.; Lin, D. Effect and Mechanism of TiO2 Nanoparticles on the Photosynthesis of Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2018, 161, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Ghouri, F.; Shahid, M.J.; Zhong, M.; Zia, M.A.; Alomrani, S.O.; Liu, J.; Sun, L.; Ali, S.; Liu, X.; Shahid, M.Q. Alleviated Lead Toxicity in Rice Plant by Co-Augmented Action of Genome Doubling and TiO2 Nanoparticles on Gene Expression, Cytological and Physiological Changes. Sci. Total Environ. 2024, 911, 168709. [Google Scholar] [CrossRef] [PubMed]
- Bagheenayat, N.; Barzin, G.; Jafarinia, M.; Pishkar, L.; Entezari, M. The Use of the Jip-Test to Investigate the Role of Nitric Oxide in Alleviation Drought Damage to Photosystem II in Salvia officinalis L. Biol. Bull. 2022, 49, S83–S91. [Google Scholar] [CrossRef]
- Rochaix, J.-D. Redox Regulation of Thylakoid Protein Kinases and Photosynthetic Gene Expression. Antioxid. Redox Signal. 2013, 18, 2184–2201. [Google Scholar] [CrossRef]
- Qian, H.; Chen, W.; Sheng, G.D.; Xu, X.; Liu, W.; Fu, Z. Effects of Glufosinate on Antioxidant Enzymes, Subcellular Structure, and Gene Expression in the Unicellular Green Alga Chlorella vulgaris. Aquat. Toxicol. 2008, 88, 301–307. [Google Scholar] [CrossRef]
- Cheloni, G.; Marti, E.; Slaveykova, V.I. Interactive Effects of Copper Oxide Nanoparticles and Light to Green Alga Chlamydomonas Reinhardtii. Aquat. Toxicol. 2016, 170, 120–128. [Google Scholar] [CrossRef]
- von Moos, N.; Maillard, L.; Slaveykova, V.I. Dynamics of Sub-Lethal Effects of Nano-CuO on the Microalga Chlamydomonas Reinhardtii during Short-Term Exposure. Aquat. Toxicol. 2015, 161, 267–275. [Google Scholar] [CrossRef]
- Saison, C.; Perreault, F.; Daigle, J.-C.; Fortin, C.; Claverie, J.; Morin, M.; Popovic, R. Effect of Core–Shell Copper Oxide Nanoparticles on Cell Culture Morphology and Photosynthesis (Photosystem II Energy Distribution) in the Green Alga, Chlamydomonas Reinhardtii. Aquat. Toxicol. 2010, 96, 109–114. [Google Scholar] [CrossRef]
- da Costa, C.H.; Perreault, F.; Oukarroum, A.; Melegari, S.P.; Popovic, R.; Matias, W.G. Effect of Chromium Oxide (III) Nanoparticles on the Production of Reactive Oxygen Species and Photosystem II Activity in the Green Alga Chlamydomonas Reinhardtii. Sci. Total Environ. 2016, 565, 951–960. [Google Scholar] [CrossRef]
- Simon, D.F.; Domingos, R.F.; Hauser, C.; Hutchins, C.M.; Zerges, W.; Wilkinson, K.J. Transcriptome Sequencing (RNA-Seq) Analysis of the Effects of Metal Nanoparticle Exposure on the Transcriptome of Chlamydomonas Reinhardtii. Appl. Environ. Microbiol. 2013, 79, 4774–4785. [Google Scholar] [CrossRef]
- Perreault, F.; Oukarroum, A.; Melegari, S.P.; Matias, W.G.; Popovic, R. Polymer Coating of Copper Oxide Nanoparticles Increases Nanoparticles Uptake and Toxicity in the Green Alga Chlamydomonas Reinhardtii. Chemosphere 2012, 87, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ren, W.; Ciric, L.; Bhatti, M. Phytotoxicological Effects of Phytosynthesized Nanoparticles: A Systematic Review and Meta-Analysis. Crit. Rev. Environ. Sci. Technol. 2024, 54, 1237–1257. [Google Scholar] [CrossRef]
- Komazec, B.; Vitko, S.; Balen, B.; Cindrić, M.; Biba, R.; Peharec Štefanić, P. Multi-Parameter Analysis of Photosynthetic and Molecular Responses in Chlorella vulgaris Exposed to Silver Nanoparticles and Ions. Toxics 2025, 13, 627. [Google Scholar] [CrossRef] [PubMed]
- Damyanov, M.; Kolackova, M.; Tarbajova, V.; Bytesnikova, Z.; Vintrlikova, N.; Svec, P.; Pekarkova, J.; Urbis, P.; Havrankova, J.; Zachej, S.; et al. Distinct mRNA and miRNA Responses in Chlamydomonas Reinhardtii Reveal Particle-Specific Adaptive Mechanisms to ZnO Nanoparticles and ZnO Bulk. Algal Res. 2025, 91, 104266. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The Two Faces of Nanomaterials: A Quantification of Hormesis in Algae and Plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef]
- Iadonisi, G.; Cantele, G.; Chiofalo, M.L. Electronic Structure of Nanosystems and Crystals. In Introduction to Solid State Physics and Crystalline Nanostructures; Iadonisi, G., Cantele, G., Chiofalo, M.L., Eds.; Springer: Milano, Italy, 2014; pp. 97–264. ISBN 978-88-470-2805-0. [Google Scholar]
- OECD TG 201; Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. OECD Publishing: Paris, France, 2011.
- Sendra, M.; Yeste, M.P.; Gatica, J.M.; Moreno-Garrido, I.; Blasco, J. Homoagglomeration and Heteroagglomeration of TiO2, in Nanoparticle and Bulk Form, onto Freshwater and Marine Microalgae. Sci. Total Environ. 2017, 592, 403–411. [Google Scholar] [CrossRef]
- Saxena, P.; Saharan, V.; Baroliya, P.K.; Gour, V.S.; Rai, M.K. Harish Mechanism of Nanotoxicity in Chlorella vulgaris Exposed to Zinc and Iron Oxide. Toxicol. Rep. 2021, 8, 724–731. [Google Scholar] [CrossRef]
- Chang, W.-C.; Zheng, H.-Q.; Chen, C.-N.N. Comparative Transcriptome Analysis Reveals a Potential Photosynthate Partitioning Mechanism between Lipid and Starch Biosynthetic Pathways in Green Microalgae. Algal Res. 2016, 16, 54–62. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Calabrese, E.J. Biphasic Dose Responses in Biology, Toxicology and Medicine: Accounting for Their Generalizability and Quantitative Features. Environ. Pollut. 2013, 182, 452–460. [Google Scholar] [CrossRef]
- Erofeeva, E.A. Hormesis and Nanomaterials. From Biostimulation to Toxicity. In Plant Biostimulation with Nanomaterials; Juárez-Maldonado, A., Benavides-Mendoza, A., Ojeda-Barrios, D.L., Tortella Fuentes, G., Seabra, A.B., Eds.; Springer Nature: Singapore, 2025; pp. 1–19. ISBN 978-981-96-4648-7. [Google Scholar]
- Qian, W.; Chen, C.C.; Huang, Y.; Zhu, X. Exposure Concentration Ratios and Biological Responses Play a Critical Role in Determining the Joint Toxicity of TiO2 Nanoparticles and As(V) to the Organism: The Case Study in Marine Algae Phaeodactylum Tricornutum. Sci. Total Environ. 2024, 909, 168508. [Google Scholar] [CrossRef]
- Solomonova, E.; Shoman, N.; Akimov, A.; Rylkova, O. Viability of the Microalgae Thalassiosira Weissflogii and Prorocentrum Cordatum to Titanium Nanoparticles. Water Air Soil Pollut. 2024, 235, 743. [Google Scholar] [CrossRef]
- Shaw, R.; Kumar, H.; Kapoor, M. Recent Findings in Adverse Effects of TiO2 NPs in Marine Algae and Zooplanktons: A Threat to Marine Ecosystems. Int. J. Marit. Eng. 2024, 1, 237–244. [Google Scholar] [CrossRef]
- Nava, V.; Leoni, B. A Critical Review of Interactions between Microplastics, Microalgae and Aquatic Ecosystem Function. Water Res. 2021, 188, 116476. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Tarre, V.; Kiparissides, A. The Effects of Illumination and Trophic Strategy on Gene Expression in Chlamydomonas Reinhardtii. Algal Res. 2021, 54, 102186. [Google Scholar] [CrossRef]
- Sivakumar, M.; Dhinakarasamy, I.; Chakraborty, S.; Clements, C.; Thirumurugan, N.K.; Chandrasekar, A.; Vinayagam, J.; Kumar, C.; Thirugnanasambandam, R.; Kumar, V.R.; et al. Effects of Titanium Oxide Nanoparticles on Growth, Biochemical Composition, and Photosystem Mechanism of Marine Microalgae Isochrysis Galbana COR-A3. Nanotoxicology 2025, 19, 156–179. [Google Scholar] [CrossRef]
- Romero, N.; Kergaravat, S.V.; Regaldo, L.; Hernández, S.R.; Seabra, A.B.; Ferreira, F.F.; Lourenço, I.M.; Castro, G.R.; Gagneten, A.M. Multiple Physiological Response Analyses of Chlorella vulgaris Exposed to Silver Nanoparticles, Ciprofloxacin, and Their Combination. Environ. Toxicol. Chem. 2025, 44, 1051–1065. [Google Scholar] [CrossRef]
- Khosrovyan, A.; Vodovnik, M.; Mortimer, M. Omics Approaches in Environmental Effect Assessment of Engineered Nanomaterials and Nanoplastics. Environ. Sci. Nano 2025, 12, 2551–2579. [Google Scholar] [CrossRef]
- OECD. Titanium Dioxide: Summary of the Dossier; OECD Publishing: Paris, France, 2016. [Google Scholar]
- Zheng, Z.-Y.; Yang, Y.-T.; Zhou, J.-X.; Peng, Z.-X.; Ni, H.-G. Possible Causes of Extreme Variation of Benzo[a]Pyrene Acute Toxicity Test on Daphnia magna. Toxics 2024, 12, 714. [Google Scholar] [CrossRef]
- Aigner, S.; Glaser, K.; Arc, E.; Holzinger, A.; Schletter, M.; Karsten, U.; Kranner, I. Adaptation to Aquatic and Terrestrial Environments in Chlorella vulgaris (Chlorophyta). Front. Microbiol. 2020, 11, 585836. [Google Scholar] [CrossRef]
- Almutairi, A.W.; El-Sayed, A.E.-K.B.; Reda, M.M. Evaluation of High Salinity Adaptation for Lipid Bio-Accumulation in the Green Microalga Chlorella vulgaris. Saudi J. Biol. Sci. 2021, 28, 3981–3988. [Google Scholar] [CrossRef]
- Wen, Y.; He, Y.; Ji, X.; Li, S.; Chen, L.; Zhou, Y.; Wang, M.; Chen, B. Isolation of an Indigenous Chlorella vulgaris from Swine Wastewater and Characterization of Its Nutrient Removal Ability in Undiluted Sewage. Bioresour. Technol. 2017, 243, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, G.G.; Perfetti-Bolaño, A.; Meléndrez, M.; Pozo, K.; Corsi, I.; Barra, R.O.; Urrutia, R. First Evidence of Anthropogenic TiO2 Nanoparticles Occurrence in Chilean Rivers. Environ. Adv. 2024, 16, 100536. [Google Scholar] [CrossRef]
- Steinberg, X.P.; Hepp, M.I.; Fernández García, Y.; Suganuma, T.; Swanson, S.K.; Washburn, M.; Workman, J.L.; Gutiérrez, J.L. Human CCAAT/Enhancer-Binding Protein β Interacts with Chromatin Remodeling Complexes of the Imitation Switch Subfamily. Biochemistry 2012, 51, 952–962. [Google Scholar] [CrossRef] [PubMed]




| (I) Group | (J) Treatment | Mean Difference (I–J) | Standard Error | Sig. | 95% Confidence Interval | |
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| Control | 1.1 µg/L | 5.6667 | 2.9059 | 0.633 | −15.3685 | 26.7018 |
| 4.4 µg/L | −24.6667 | 8.2731 | 0.372 | −98.8309 | 49.4976 | |
| 8.8 µg/L | −28.3333 | 4.5216 | 0.09 | −65.8700 | 9.2033 | |
| 17.6 µg/L | −61.000 * | 2.0000 | <0.001 | −72.9276 | −49.0724 | |
| Gen | Slope | R2 | Y-Inter | Eff% | Error |
|---|---|---|---|---|---|
| 18S | −3.448 | 0.997 | 18.29 | 95.004 | 0.051 |
| atpB | −3.2 | 0.981 | 23.149 | 105.341 | 0.113 |
| rbcL | −3.294 | 0.983 | 20.448 | 101.194 | 0.107 |
| psaA | −2.951 | 0.944 | 25.448 | 118.227 | 0.179 |
| psaB | −3.375 | 0.985 | 26.552 | 97.832 | 0.106 |
| psaD | −2.912 | 0.758 | 29.714 | 120.531 | 0.412 |
| psbA | −3.458 | 0.983 | 20.29 | 94.637 | 0.114 |
| Test of Homogeneity of Variances | |||||
|---|---|---|---|---|---|
| Dependent Variable | Levene Statistic | df1 | df2 | Sig. | |
| atpB | 2.433 | 4 | 10 | 0.116 | |
| rbcL | 0.912 | 4 | 10 | 0.493 | |
| psaA | 3.625 * | 4 | 10 | 0.045 | |
| psaB | 7.192 * | 4 | 10 | 0.005 | |
| psaD | 6.332 * | 4 | 10 | 0.008 | |
| psbA | 2.238 | 4 | 10 | 0.138 | |
| ANOVA | |||||
| Dependent Variable | Sum of Squares | df1 | Mean Square | F | Sig. |
| atpB | 15.913 | 4 | 3.978 | 8.300 | 0.003 * |
| rbcL | 1.853 | 4 | 0.463 | 14.568 | 0.000 * |
| psaA | 882.128 | 4 | 220.532 | 8.992 | 0.002 * |
| psaB | 10.981 | 4 | 2.745 | 7.092 | 0.006 * |
| psaD | 615.215 | 4 | 153.804 | 13.031 | 0.001 * |
| psbA | 1.901 | 4 | 0.077 | 0.484 | 0.748 |
| Gene Name | GenBank Accession or Source | Forward Prime (5′3′) | Reverse Prime (5′3′) | Tm | %GC | PCR Product |
|---|---|---|---|---|---|---|
| 18S | ♦ Designed in this study | AACGGCTACCACATCCAAGG | GTCCCACCCGAAATCCAACT | 55 | 55 | 250 pb |
| atpB | EF113499.1 | CCAATTCACCGTTCAGCACC | TTTCCCTACACCTGCACCAC | 55 | 55 | 144 pb |
| psaA | ♦ Designed in this study | AAATGCAGACGTTGGTGGTG | CGCTAAACTGCCGAGACCTA | 55 | 50 | 256 pb |
| psaB | GQ423926.1 | GCCCAATGGATTCAAGCAGC | AGAACCACGAGCGTCAAGAG | 55 | 55 | 255 pb |
| psaD | MG596028.1 | AGCGTGTATAACCCAGCTCG | AGTGTCCTCCTCATACGCCT | 55 | 55 | 267 pb |
| psbA | KX066373.1 | CGTTGCCGGTGTATTTGGTG | ACAACTGGCCAAGCAGCTAA | 55 | 55 | 240 pb |
| rbcL | MK295221.1 | GCACGCTGTAATTGACCGTC | TCAACAAGAGCTGGCATGTG | 55 | 55 | 285 pb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, G.G.; Rivas-Valdés, F.; Benavente, B.P.; Olivares, R.; Hepp, M.I.; Barra, R.O.; Urrutia, R. Toxicological Responses of Photosynthetic Genes in Chlorella vulgaris Exposed to Environmentally Relevant Concentrations of TiO2 Nanoparticles. Int. J. Mol. Sci. 2025, 26, 10271. https://doi.org/10.3390/ijms262110271
Gutiérrez GG, Rivas-Valdés F, Benavente BP, Olivares R, Hepp MI, Barra RO, Urrutia R. Toxicological Responses of Photosynthetic Genes in Chlorella vulgaris Exposed to Environmentally Relevant Concentrations of TiO2 Nanoparticles. International Journal of Molecular Sciences. 2025; 26(21):10271. https://doi.org/10.3390/ijms262110271
Chicago/Turabian StyleGutiérrez, Gester G., Fernando Rivas-Valdés, Bárbara P. Benavente, René Olivares, Matías I. Hepp, Ricardo O. Barra, and Roberto Urrutia. 2025. "Toxicological Responses of Photosynthetic Genes in Chlorella vulgaris Exposed to Environmentally Relevant Concentrations of TiO2 Nanoparticles" International Journal of Molecular Sciences 26, no. 21: 10271. https://doi.org/10.3390/ijms262110271
APA StyleGutiérrez, G. G., Rivas-Valdés, F., Benavente, B. P., Olivares, R., Hepp, M. I., Barra, R. O., & Urrutia, R. (2025). Toxicological Responses of Photosynthetic Genes in Chlorella vulgaris Exposed to Environmentally Relevant Concentrations of TiO2 Nanoparticles. International Journal of Molecular Sciences, 26(21), 10271. https://doi.org/10.3390/ijms262110271







