Augmented Flow-Induced Outward Remodelling Occurs with Ageing in Mice
Abstract
1. Introduction
2. Results
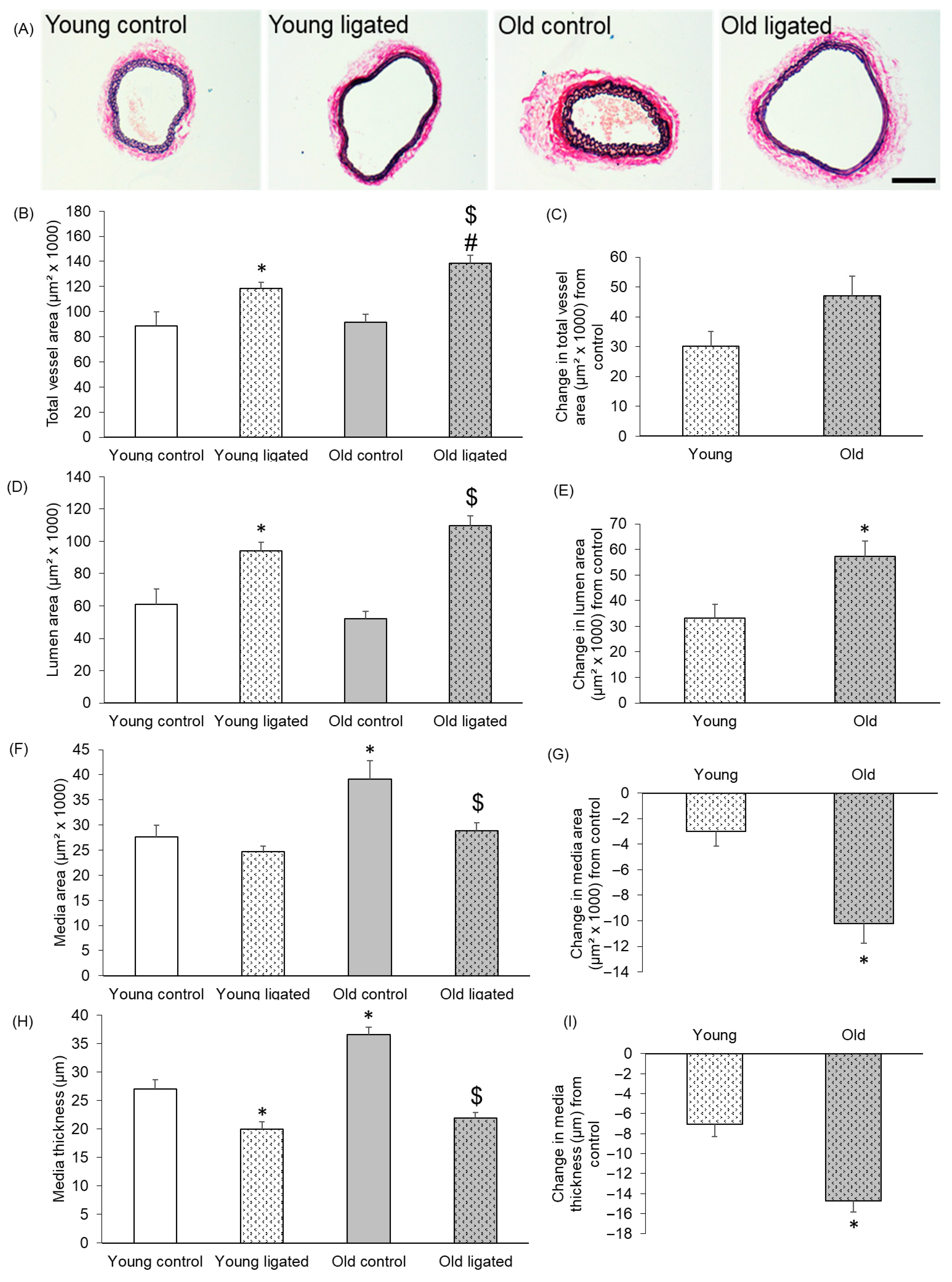
2.1. Remodelling of Right Carotid Artery Was Augmented in Old Mice Compare to Young
2.2. Medial Cell Number and Density, Proliferation and Apoptosis Did Not Differ in Old and Young Mice
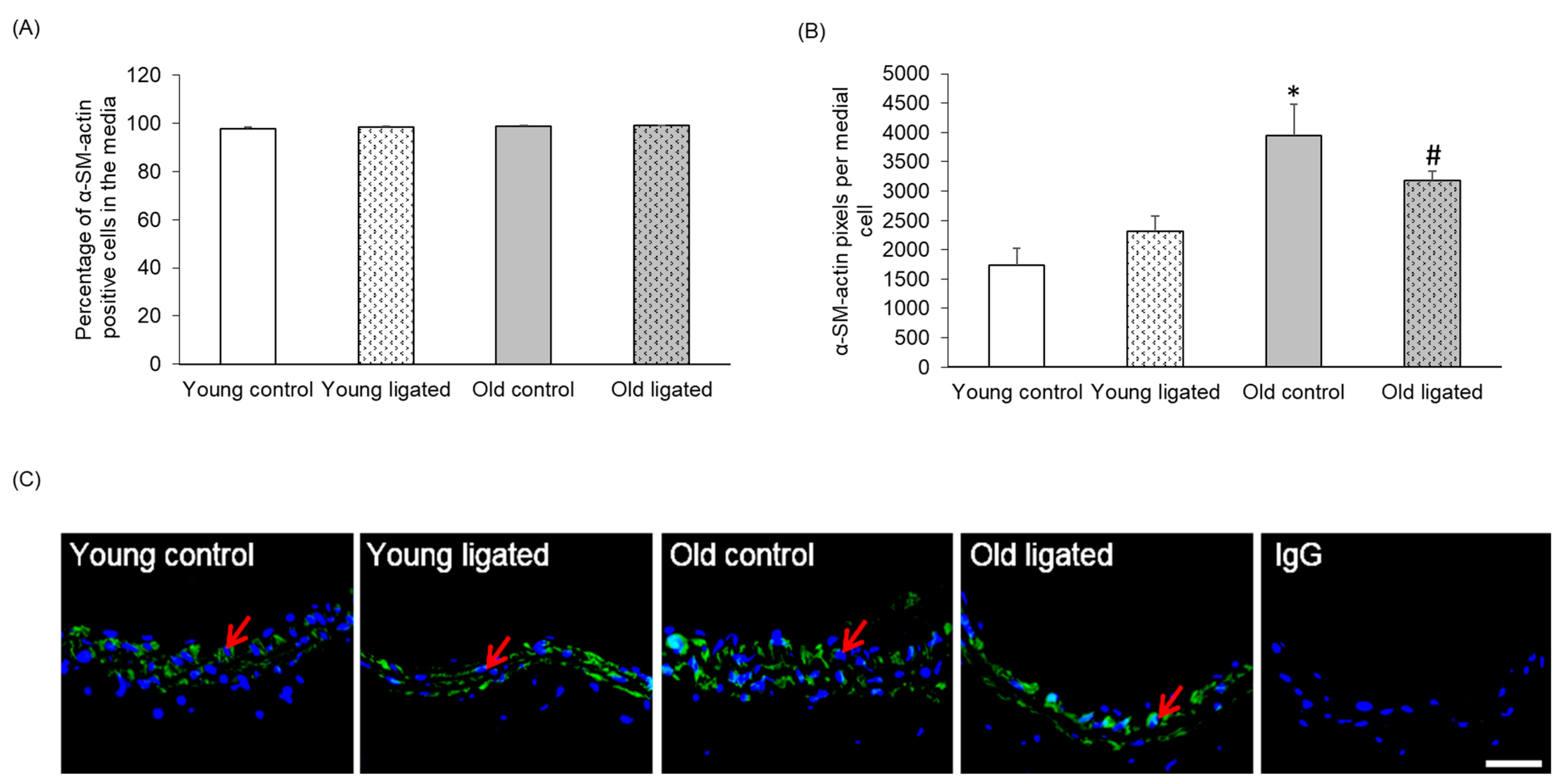
2.3. α-SM-Actin Content per Medial Cell Was Enhanced with Age but Unaffected by Elevated Blood Flow
2.4. Medial Elastin Content Was Lower in Old Mice with Elevated Blood Flow
2.5. Medial Collagen Content Was Increased with Age but Unaffected by Elevated Blood Flow
2.6. MMP-2 Protein Was Increased by Elevated Blood Flow in Young, but Not Old, Mice
2.7. AXIN-2-Positive Cells Increased with Elevated Blood Flow in Young, but Not Old, Mice
2.8. Medial AXIN-2 and MMP-2 Abundance Correlated in Young, but Not Old, Mice
2.9. Reduction in Adventitial Collagen Following Elevated Blood Flow Was Greater with Age
2.10. Macrophages Were Not Detected 21 Days After Ligation
3. Discussion
3.1. Comparison of Right Carotid Arteries from Young and Old Mice
3.2. Remodelling of the Right Carotid Artery in Response to Elevated Blood Flow in Young Mice
3.3. Remodelling of the Right Carotid Artery in Response to Elevated Blood Flow in Old Mice
3.4. Study Limitations
4. Methods
4.1. Murine Carotid Ligation Model
4.2. Histology
4.3. Immunohistochemistry
4.4. In Situ End Labelling—ISEL
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Glagov, S.; Weisenberg, E.; Zarins, C.K.; Stankunavicius, R.; Kolettis, G.J. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1987, 316, 1371–1375. [Google Scholar] [CrossRef]
- Pasterkamp, G.; Smits, P.C. Imaging of atherosclerosis. Remodelling of coronary arteries. J. Cardiovasc. Risk 2002, 9, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, G.; Schoneveld, A.H.; van der Wal, A.C.; Haudenschild, C.C.; Clarijs, R.J.; Becker, A.E.; Hillen, B.; Borst, C. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: The remodeling paradox. J. Am. Coll. Cardiol. 1998, 32, 655–662. [Google Scholar] [CrossRef]
- Smits, P.C.; Pasterkamp, G.; Quarles van Ufford, M.A.; Eefting, F.D.; Stella, P.R.; de Jaegere, P.P.; Borst, C. Coronary artery disease: Arterial remodelling and clinical presentation. Heart 1999, 82, 461–464. [Google Scholar] [CrossRef][Green Version]
- Pasterkamp, G.; Schoneveld, A.H.; Hijnen, D.J.; de Kleijn, D.P.; Teepen, H.; van der Wal, A.C.; Borst, C. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis 2000, 150, 245–253. [Google Scholar] [CrossRef]
- Katranas, S.A.; Kelekis, A.L.; Antoniadis, A.P.; Chatzizisis, Y.S.; Giannoglou, G.D. Association of remodeling with endothelial shear stress, plaque elasticity, and volume in coronary arteries: A pilot coronary computed tomography angiography study. Angiology 2014, 65, 413–419. [Google Scholar] [CrossRef]
- Wexberg, P.; Gyongyosi, M.; Sperker, W.; Kiss, K.; Yang, P.; Hassan, A.; Pasterkamp, G.; Glogar, D. Pre-existing arterial remodeling is associated with in-hospital and late adverse cardiac events after coronary interventions in patients with stable angina pectoris. J. Am. Coll. Cardiol. 2000, 36, 1860–1869. [Google Scholar] [CrossRef][Green Version]
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal aortic aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Mao, C.; Jia, Y.; Fu, Y.; Kong, W. Extracellular matrix dynamics in vascular remodeling. Am. J. Physiol. Cell Physiol. 2020, 319, C481–C499. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.C.; Papke, C.L.; He, R.; Milewicz, D.M. Pathogenesis of thoracic and abdominal aortic aneurysms. Ann. N. Y. Acad. Sci. 2006, 1085, 339–352. [Google Scholar] [CrossRef]
- Ailawadi, G.; Eliason, J.L.; Upchurch, G.R., Jr. Current concepts in the pathogenesis of abdominal aortic aneurysm. J. Vasc. Surg. 2003, 38, 584–588. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Moreno, P.; Nabel, E.G.; Hachinski, V.; Fuster, V. Cellular senescence, vascular disease, and aging: Part 2 of a 2-part review: Clinical vascular disease in the elderly. Circulation 2011, 123, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Bennett, M. Aging and atherosclerosis: Mechanisms, functional consequences, and potential therapeutics for cellular senescence. Circ. Res. 2012, 111, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Beeche, C.; Tavolinejad, H.; Zhao, B.; Segers, P.; Duda, J.; Gee, J.; Witschey, W.R.; Penn Medicine, B.; Chirinos, J.A. Geometric Aging of the Thoracic Aorta: Insights From 2 Large Cohorts. Hypertension 2025, 82, 1866–1877. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, A.; Marcellini, M.; Spagnoli, L.G. Aging influences development and progression of early aortic atherosclerotic lesions in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1123–1136. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Lyon, C.J.; Xia, X.; Liu, J.Z.; Tangirala, R.K.; Yin, F.; Boyadjian, R.; Bikineyeva, A.; Pratico, D.; Harrison, D.G.; et al. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ. Res. 2009, 104, e42–e54. [Google Scholar] [CrossRef]
- Yagi, K.; Komura, S.; Sasaguri, Y.; Yoshino, K.; Ohishi, N. Atherogenic change in the thoracic aorta of the senescence-accelerated mouse. Atherosclerosis 1995, 118, 233–236. [Google Scholar] [CrossRef]
- Fenton, M.; Huang, H.L.; Hong, Y.; Hawe, E.; Kurz, D.J.; Erusalimsky, J.D. Early atherogenesis in senescence-accelerated mice. Exp. Gerontol. 2004, 39, 115–122. [Google Scholar] [CrossRef]
- Olive, M.; Harten, I.; Mitchell, R.; Beers, J.K.; Djabali, K.; Cao, K.; Erdos, M.R.; Blair, C.; Funke, B.; Smoot, L.; et al. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2301–2309. [Google Scholar] [CrossRef]
- Stehbens, W.E.; Delahunt, B.; Shozawa, T.; Gilbert-Barness, E. Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc. Pathol. 2001, 10, 133–136. [Google Scholar] [CrossRef]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Hye, R.J.; Makaroun, M.S.; Barone, G.W.; Bandyk, D.; Moneta, G.L.; Makhoul, R.G. The aneurysm detection and management study screening program: Validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch. Intern. Med. 2000, 160, 1425–1430. [Google Scholar] [CrossRef]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Littooy, F.N.; Bandyk, D.; Krupski, W.C.; Barone, G.W.; Acher, C.W.; Ballard, D.J. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann. Intern. Med. 1997, 126, 441–449. [Google Scholar] [CrossRef]
- Brown, B.A.; Williams, H.; Bond, A.R.; Angelini, G.D.; Johnson, J.L.; George, S.J. Carotid artery ligation induced intimal thickening and proliferation is unaffected by ageing. J. Cell Commun. Signal 2018, 12, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Nuki, Y.; Matsumoto, M.M.; Tsang, E.; Young, W.L.; van Rooijen, N.; Kurihara, C.; Hashimoto, T. Roles of macrophages in flow-induced outward vascular remodeling. J. Cereb. Blood Flow. Metab. 2009, 29, 495–503. [Google Scholar] [CrossRef]
- Ota, R.; Kurihara, C.; Tsou, T.L.; Young, W.L.; Yeghiazarians, Y.; Chang, M.; Mobashery, S.; Sakamoto, A.; Hashimoto, T. Roles of matrix metalloproteinases in flow-induced outward vascular remodeling. J. Cereb. Blood Flow. Metab. 2009, 29, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Pohl, U.; Holtz, J.; Busse, R.; Bassenge, E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension 1986, 8, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bevan, J.A.; Joyce, E.H.; Wellman, G.C. Flow-dependent dilation in a resistance artery still occurs after endothelium removal. Circ. Res. 1988, 63, 980–985. [Google Scholar] [CrossRef]
- Kamiya, A.; Togawa, T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am. J. Physiol. 1980, 239, H14–H21. [Google Scholar] [CrossRef]
- Godin, D.; Ivan, E.; Johnson, C.; Magid, R.; Galis, Z.S. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation 2000, 102, 2861–2866. [Google Scholar] [CrossRef]
- Harmon, K.J.; Couper, L.L.; Lindner, V. Strain-dependent vascular remodeling phenotypes in inbred mice. Am. J. Pathol. 2000, 156, 1741–1748. [Google Scholar] [CrossRef][Green Version]
- Bonta, P.I.; Matlung, H.L.; Vos, M.; Peters, S.L.; Pannekoek, H.; Bakker, E.N.; de Vries, C.J. Nuclear receptor Nur77 inhibits vascular outward remodelling and reduces macrophage accumulation and matrix metalloproteinase levels. Cardiovasc. Res. 2010, 87, 561–568. [Google Scholar] [CrossRef]
- Tronc, F.; Mallat, Z.; Lehoux, S.; Wassef, M.; Esposito, B.; Tedgui, A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: Interaction with NO. Arterioscler. Thromb. Vasc. Biol. 2000, 20, E120–E126. [Google Scholar] [CrossRef] [PubMed]
- Monson, K.L.; Matsumoto, M.M.; Young, W.L.; Manley, G.T.; Hashimoto, T. Abrupt increase in rat carotid blood flow induces rapid alteration of artery mechanical properties. J. Mech. Behav. Biomed. Mater. 2011, 4, 9–15. [Google Scholar] [CrossRef][Green Version]
- Johnson, J.L.; Dwivedi, A.; Somerville, M.; George, S.J.; Newby, A.C. Matrix metalloproteinase (MMP)-3 activates MMP-9 mediated vascular smooth muscle cell migration and neointima formation in mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e35–e44. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Froehlich, J.; Galis, Z.S.; Lakatta, E.G. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension 1999, 33, 116–123. [Google Scholar] [CrossRef]
- Wang, M.; Takagi, G.; Asai, K.; Resuello, R.G.; Natividad, F.F.; Vatner, D.E.; Vatner, S.F.; Lakatta, E.G. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension 2003, 41, 1308–1316. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Jiang, L.Q.; Spinetti, G.; Pintus, G.; Monticone, R.; Kolodgie, F.D.; Virmani, R.; Lakatta, E.G. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension 2007, 50, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, J.; Telljohann, R.; Jiang, L.; Wu, J.; Monticone, R.E.; Kapoor, K.; Talan, M.; Lakatta, E.G. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension 2012, 60, 459–466. [Google Scholar] [CrossRef]
- Harada, K.; Chen, Z.; Ishibashi, S.; Osuga, J.; Yagyu, H.; Ohashi, K.; Yahagi, N.; Shionoiri, F.; Sun, L.; Yazaki, Y.; et al. Apoptotic cell death in atherosclerotic plaques of hyperlipidemic knockout mice. Atherosclerosis 1997, 135, 235–239. [Google Scholar] [CrossRef]
- Rich, L.; Whittaker, P. Collagen and picrosirius red staining: A polarized light assessment of fibrillar hue and spatial distribution. Braz. J. Morphol. Sci. 2005, 22, 97–104. [Google Scholar]
- Meissburger, B.; Stachorski, L.; Roder, E.; Rudofsky, G.; Wolfrum, C. Tissue inhibitor of matrix metalloproteinase 1 (TIMP1) controls adipogenesis in obesity in mice and in humans. Diabetologia 2011, 54, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wiesmann, M.; Rohan, M.; Chan, V.; Jefferson, A.B.; Guo, L.; Sakamoto, D.; Caothien, R.H.; Fuller, J.H.; Reinhard, C.; et al. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. USA 2001, 98, 14973–14978. [Google Scholar] [CrossRef] [PubMed]
- Jho, E.H.; Zhang, T.; Domon, C.; Joo, C.K.; Freund, J.N.; Costantini, F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002, 22, 1172–1183. [Google Scholar] [CrossRef]
- Lustig, B.; Jerchow, B.; Sachs, M.; Weiler, S.; Pietsch, T.; Karsten, U.; van de Wetering, M.; Clevers, H.; Schlag, P.M.; Birchmeier, W.; et al. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002, 22, 1184–1193. [Google Scholar] [CrossRef]
- Hemmeryckx, B.; Hoylaerts, M.F.; Deloose, E.; Van Hove, C.E.; Fransen, P.; Bult, H.; Lijnen, H.R. Age-associated pro-inflammatory adaptations of the mouse thoracic aorta. Thromb. Haemost. 2013, 110, 785–794. [Google Scholar] [CrossRef]
- Wheeler, J.B.; Mukherjee, R.; Stroud, R.E.; Jones, J.A.; Ikonomidis, J.S. Relation of murine thoracic aortic structural and cellular changes with aging to passive and active mechanical properties. J. Am. Heart Assoc. 2015, 4, e001744. [Google Scholar] [CrossRef]
- Wang, M.; Lakatta, E.G. Altered regulation of matrix metalloproteinase-2 in aortic remodeling during aging. Hypertension 2002, 39, 865–873. [Google Scholar] [CrossRef]
- Miller, S.J.; Watson, W.C.; Kerr, K.A.; Labarrere, C.A.; Chen, N.X.; Deeg, M.A.; Unthank, J.L. Development of progressive aortic vasculopathy in a rat model of aging. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2634–H2643. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Depre, C.; Ghosh, K.; Resuello, R.G.; Natividad, F.F.; Rossi, F.; Peppas, A.; Shen, Y.T.; Vatner, D.E.; Vatner, S.F. Mechanism of gender-specific differences in aortic stiffness with aging in nonhuman primates. Circulation 2007, 116, 669–676. [Google Scholar] [CrossRef]
- Qiu, H.; Zhu, Y.; Sun, Z.; Trzeciakowski, J.P.; Gansner, M.; Depre, C.; Resuello, R.R.; Natividad, F.F.; Hunter, W.C.; Genin, G.M.; et al. Short communication: Vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ. Res. 2010, 107, 615–619. [Google Scholar] [CrossRef]
- Johnson, J.L.; George, S.J.; Newby, A.C.; Jackson, C.L. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc. Natl. Acad. Sci. USA 2005, 102, 15575–15580. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Fritsche-Danielson, R.; Behrendt, M.; Westin-Eriksson, A.; Wennbo, H.; Herslof, M.; Elebring, M.; George, S.J.; McPheat, W.L.; Jackson, C.L. Effect of broad-spectrum matrix metalloproteinase inhibition on atherosclerotic plaque stability. Cardiovasc. Res. 2006, 71, 586–595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Wang, B.; Ren, C.; Hu, J.; Greenberg, D.A.; Chen, T.; Xie, L.; Jin, K. Age-related Impairment of Vascular Structure and Functions. Aging Dis. 2017, 8, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Moshkovsky, A.R.; Kirschner, M.W. The nonredundant nature of the Axin2 regulatory network in the canonical Wnt signaling pathway. Proc. Natl. Acad. Sci. USA 2022, 119, e2108408119. [Google Scholar] [CrossRef]
- Puspitasari, Y.M.; Diaz-Canestro, C.; Sudano, I.; Flammer, A.; Bonetti, N.R.; Wuest, P.; Liberale, L.; Constantino, S.; Paneni, F.; Ruschitzka, F.; et al. The role of matrix metalloproteinase-2 on age-dependent arterial stiffness. Eur. Heart J. 2020, 41, 37.78. [Google Scholar] [CrossRef]
- Diaz-Canestro, C.; Puspitasari, Y.M.; Liberale, L.; Guzik, T.J.; Flammer, A.J.; Bonetti, N.R.; Wust, P.; Costantino, S.; Paneni, F.; Akhmedov, A.; et al. MMP-2 knockdown blunts age-dependent carotid stiffness by decreasing elastin degradation and augmenting eNOS activation. Cardiovasc. Res. 2022, 118, 2385–2396. [Google Scholar] [CrossRef]
- Cancemi, P.; Aiello, A.; Accardi, G.; Caldarella, R.; Candore, G.; Caruso, C.; Ciaccio, M.; Cristaldi, L.; Di Gaudio, F.; Siino, V.; et al. The Role of Matrix Metalloproteinases (MMP-2 and MMP-9) in Ageing and Longevity: Focus on Sicilian Long-Living Individuals (LLIs). Mediat. Inflamm. 2020, 2020, 8635158. [Google Scholar] [CrossRef]
- Marchand, A.; Atassi, F.; Gaaya, A.; Leprince, P.; Le Feuvre, C.; Soubrier, F.; Lompre, A.M.; Nadaud, S. The Wnt/beta-catenin pathway is activated during advanced arterial aging in humans. Aging Cell 2011, 10, 220–232. [Google Scholar] [CrossRef]
- Korshunov, V.A.; Berk, B.C. Flow-induced vascular remodeling in the mouse: A model for carotid intima-media thickening. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2185–2191. [Google Scholar] [CrossRef]
- van Keulen, J.K.; Timmers, L.; van Kuijk, L.P.; Retnam, L.; Hoefer, I.E.; Pasterkamp, G.; Lim, S.K.; de Kleijn, D.P. The Nuclear Factor-kappa B p50 subunit is involved in flow-induced outward arterial remodeling. Atherosclerosis 2009, 202, 424–430. [Google Scholar] [CrossRef]
- Dumont, O.; Pinaud, F.; Guihot, A.L.; Baufreton, C.; Loufrani, L.; Henrion, D. Alteration in flow (shear stress)-induced remodelling in rat resistance arteries with aging: Improvement by a treatment with hydralazine. Cardiovasc. Res. 2008, 77, 600–608. [Google Scholar] [CrossRef]
- Monk, B.A.; George, S.J. The Effect of Ageing on Vascular Smooth Muscle Cell Behaviour--A Mini-Review. Gerontology 2015, 61, 416–426. [Google Scholar] [CrossRef]
- McEnaney, R.M.; McCreary, D.D.; Skirtich, N.O.; Andraska, E.A.; Sachdev, U.; Tzeng, E. Elastic Laminar Reorganization Occurs with Outward Diameter Expansion during Collateral Artery Growth and Requires Lysyl Oxidase for Stabilization. Cells 2021, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, H.; Byrne, M.; Krane, S.; Jaenisch, R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. USA 1997, 94, 1852–1856. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.N.; Matlung, H.L.; Bonta, P.; de Vries, C.J.; van Rooijen, N.; Vanbavel, E. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc. Res. 2008, 78, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.C.; Langille, B.L. Developmental remodeling of the internal elastic lamina of rabbit arteries: Effect of blood flow. Circ. Res. 1996, 78, 799–805. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, B.; Williams, H.; Egbulonu, S.; Bond, A.; Johnson, J.; George, S. Augmented Flow-Induced Outward Remodelling Occurs with Ageing in Mice. Int. J. Mol. Sci. 2025, 26, 10274. https://doi.org/10.3390/ijms262110274
Brown B, Williams H, Egbulonu S, Bond A, Johnson J, George S. Augmented Flow-Induced Outward Remodelling Occurs with Ageing in Mice. International Journal of Molecular Sciences. 2025; 26(21):10274. https://doi.org/10.3390/ijms262110274
Chicago/Turabian StyleBrown, Bethan, Helen Williams, Samson Egbulonu, Andrew Bond, Jason Johnson, and Sarah George. 2025. "Augmented Flow-Induced Outward Remodelling Occurs with Ageing in Mice" International Journal of Molecular Sciences 26, no. 21: 10274. https://doi.org/10.3390/ijms262110274
APA StyleBrown, B., Williams, H., Egbulonu, S., Bond, A., Johnson, J., & George, S. (2025). Augmented Flow-Induced Outward Remodelling Occurs with Ageing in Mice. International Journal of Molecular Sciences, 26(21), 10274. https://doi.org/10.3390/ijms262110274






