Characterization of Corneal Defects in ATG7-Deficient Mice
Abstract
1. Introduction
2. Results
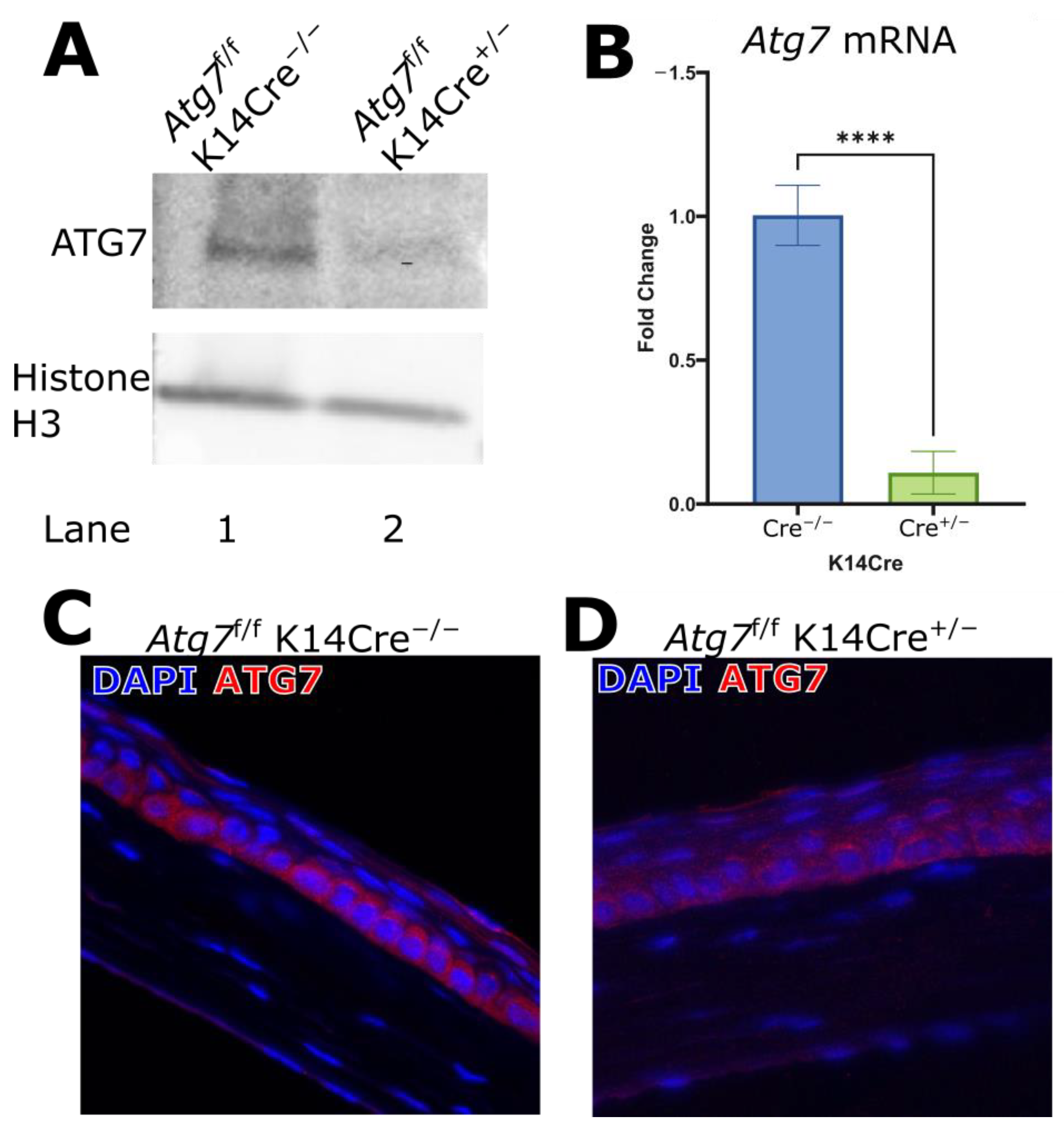
2.1. ATG7 Deficiency in the Corneal K14 Compartment
2.2. Autophagosome Formation in ATG7-Deficient Corneal Epithelium
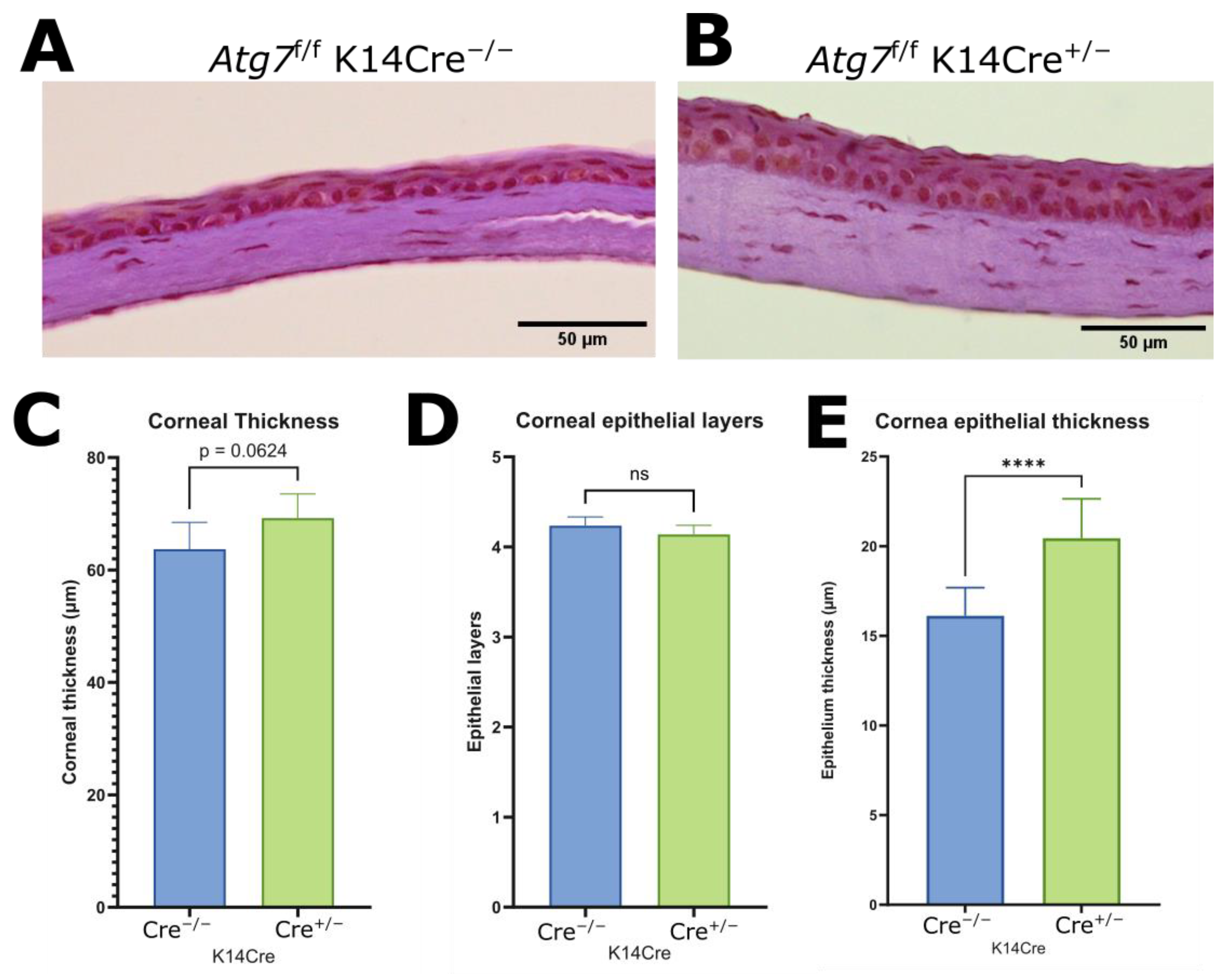
2.3. Increased Corneal Epithelial Thickness of ATG7-Deficient Mouse Cornea
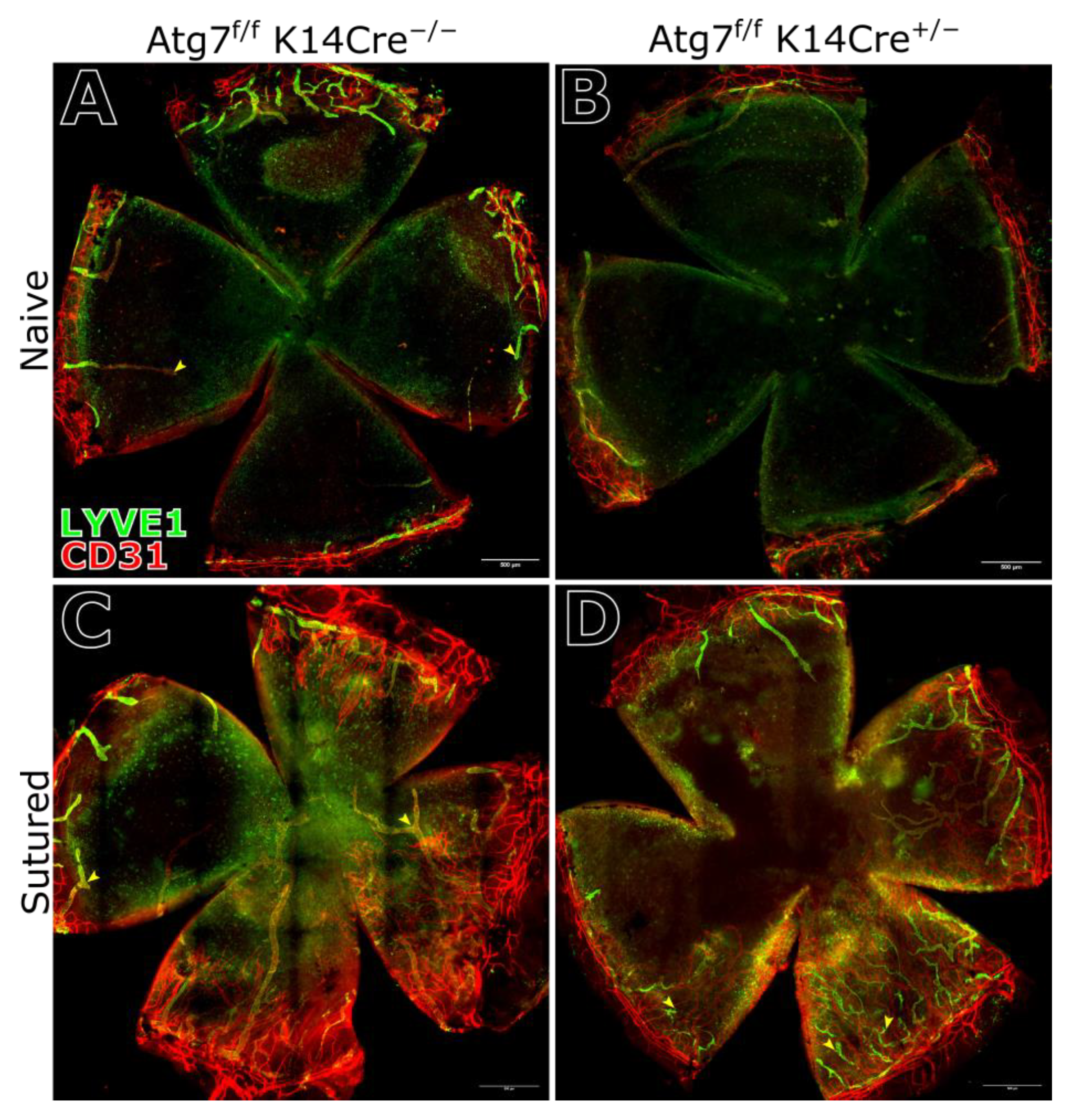
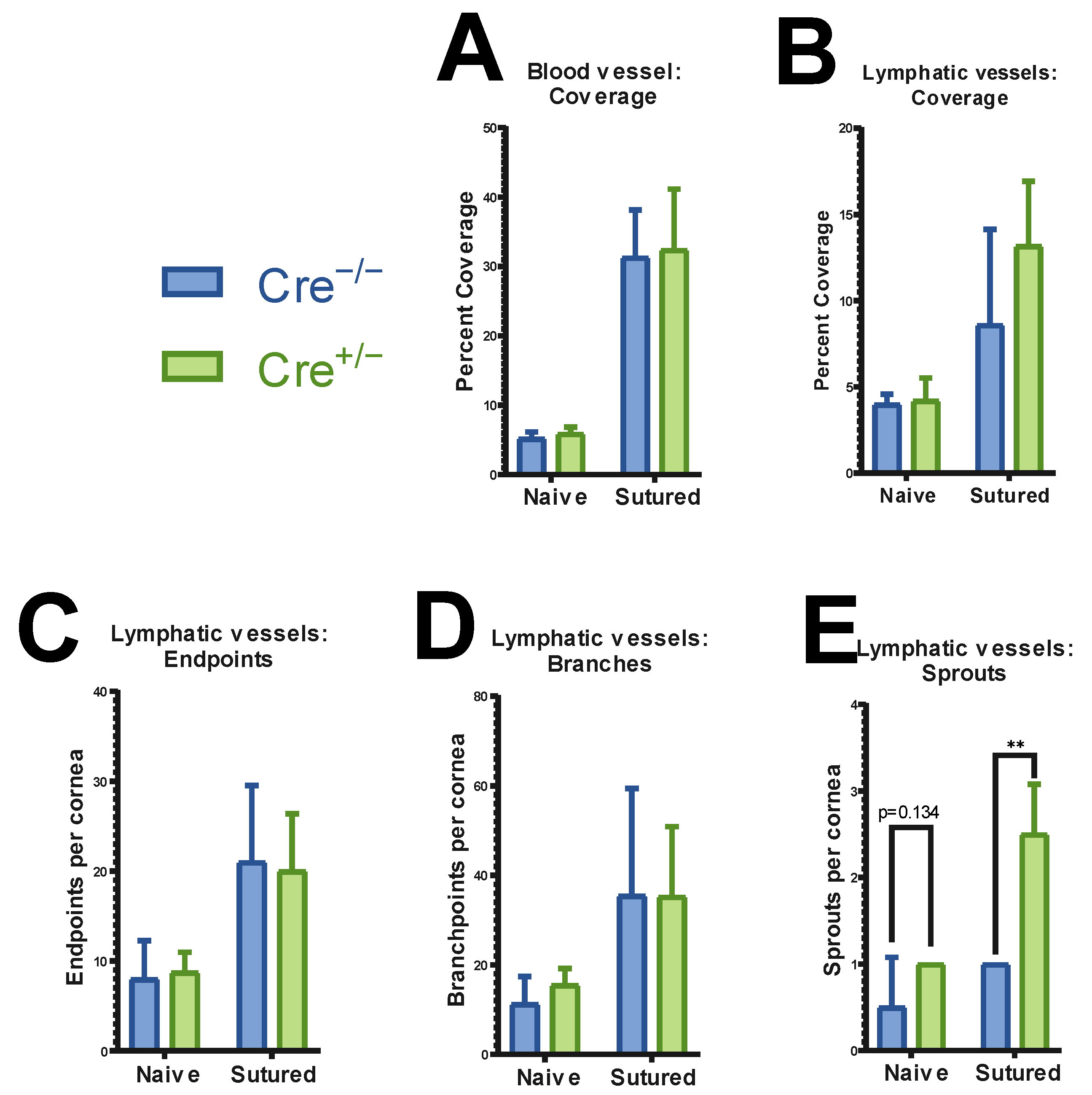
2.4. Corneal Vasculature Features of ATG7-Deficient Mouse Cornea
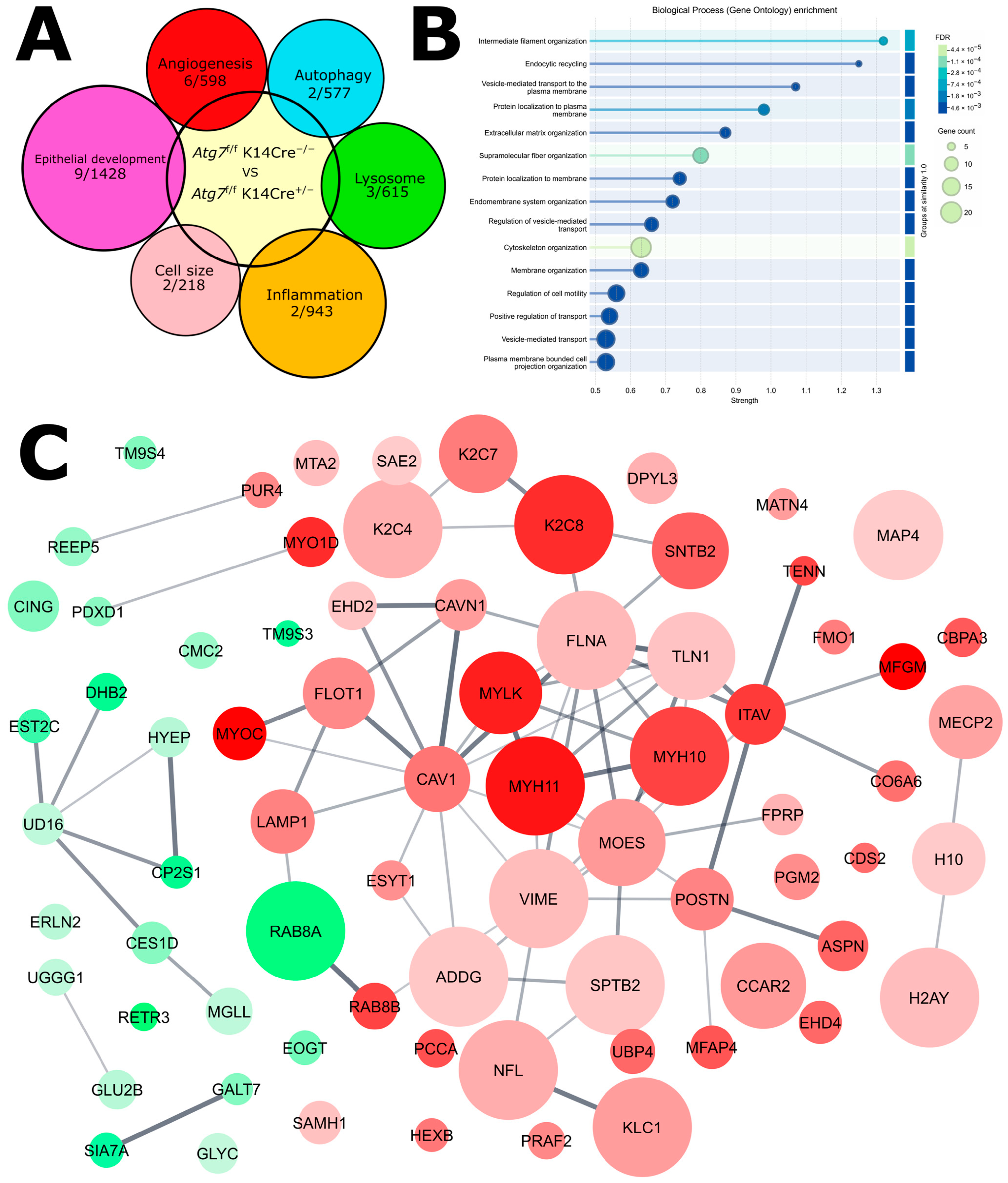
2.5. Proteomic Analysis Reveals Compensatory Mechanism for Autophagy-Deficiency
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. RNA Extraction and RT-PCR Analysis
4.3. Western Blotting
4.4. Transmission Electron Microscopy
4.5. Proteomics
4.5.1. Labeling for SP3
4.5.2. Quantitation
4.5.3. Data Processing
4.6. Immune Cell and Vessel Imaging
4.7. ATG7 Stain and LC3B Puncta Quantification
4.8. Corneal Suture Model
4.9. Statistical Analysis and Graphing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Hershko, A.; Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 1998, 67, 425–479. [Google Scholar] [CrossRef]
- Saftig, P.; Klumperman, J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009, 10, 623–635. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Ichimura, Y.; Kirisako, T.; Takao, T.; Satomi, Y.; Shimonishi, Y.; Ishihara, N.; Mizushima, N.; Tanida, I.; Kominami, E.; Ohsumi, M.; et al. A ubiquitin-like system mediates protein lipidation. Nature 2000, 408, 488–492. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef]
- Swamynathan, S.K.; Swamynathan, S. Corneal epithelial development and homeostasis. Differentiation 2023, 132, 4–14. [Google Scholar] [CrossRef]
- Kuma, A.; Hatano, M.; Matsui, M.; Yamamoto, A.; Nakaya, H.; Yoshimori, T.; Ohsumi, Y.; Tokuhisa, T.; Mizushima, N. The role of autophagy during the early neonatal starvation period. Nature 2004, 432, 1032–1036. [Google Scholar] [CrossRef]
- Rossiter, H.; Konig, U.; Barresi, C.; Buchberger, M.; Ghannadan, M.; Zhang, C.F.; Mlitz, V.; Gmeiner, R.; Sukseree, S.; Fodinger, D.; et al. Epidermal keratinocytes form a functional skin barrier in the absence of Atg7 dependent autophagy. J. Dermatol. Sci. 2013, 71, 67–75. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Jutten, B.; Keulers, T.G.; Savelkouls, K.G.; Peeters, H.J.; van den Beucken, T.; van Schooten, F.J.; Godschalk, R.W.; Vooijs, M.; Rouschop, K.M. Canonical autophagy does not contribute to cellular radioresistance. Radiother. Oncol. 2015, 114, 406–412. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Gipson, I.K. The epithelial basement membrane zone of the limbus. Eye 1989, 3 Pt 2, 132–140. [Google Scholar] [CrossRef]
- Lee, Y.J.; Hah, Y.J.; Kang, Y.N.; Kang, K.J.; Hwang, J.S.; Chung, W.J.; Cho, K.B.; Park, K.S.; Kim, E.S.; Seo, H.Y.; et al. The autophagy-related marker LC3 can predict prognosis in human hepatocellular carcinoma. PLoS ONE 2013, 8, e81540. [Google Scholar] [CrossRef]
- Kempuraj, D.; Mohan, R.R. Autophagy in Extracellular Matrix and Wound Healing Modulation in the Cornea. Biomedicines 2022, 10, 339. [Google Scholar] [CrossRef]
- Ayilam Ramachandran, R.; Sanches, J.M.; Robertson, D.M. The roles of autophagy and mitophagy in corneal pathology: Current knowledge and future perspectives. Front. Med. 2023, 10, 1064938. [Google Scholar] [CrossRef]
- Shetty, R.; Sharma, A.; Pahuja, N.; Chevour, P.; Padmajan, N.; Dhamodaran, K.; Jayadev, C.; Nuijts, R.M.M.A.; Ghosh, A.; Nallathambi, J. Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PLoS ONE 2017, 12, e0184628. [Google Scholar] [CrossRef]
- Jeyabalan, N.; Pillai, A.M.; Khamar, P.; Shetty, R.; Mohan, R.R.; Ghosh, A. Autophagy in dry eye disease: Therapeutic implications of autophagy modulators on the ocular surface. Indian. J. Ophthalmol. 2023, 71, 1285–1291. [Google Scholar] [CrossRef]
- Chen, X.; Qin, W.; Wang, L.; Jin, Y.; Tu, J.; Yuan, X. Autophagy gene Atg7 regulates the development of radiation-induced skin injury and fibrosis of skin. Skin. Res. Technol. 2023, 29, e13337. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.; Kim, S.K.; Park, H.; Kim, J.E.; Lee, S. Autophagy: Guardian of Skin Barrier. Biomedicines 2022, 10, 1817. [Google Scholar] [CrossRef]
- Yi, K.; Yang, Y.P.; Yuan, Y.; Xiang, Y.Q.; Zhou, S.B. Impaired Autophagy Causes Severe Corneal Neovascularization. Cells 2022, 11, 3895. [Google Scholar] [CrossRef]
- Yuan, X.Y.; Wilhelmus, K.R. Corneal neovascularization during experimental fungal keratitis. Mol. Vision. 2009, 15, 1988–1996. [Google Scholar]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef]
- Chen, K.; Soleimani, M.; Koganti, R.; Cheraqpour, K.; Habeel, S.; Djalilian, A.R. Cell-based therapies for limbal stem cell deficiency: A literature review. Ann. Eye Sci. 2023, 8, 1–12. [Google Scholar] [CrossRef]
- Ma, D.H.K.; Chen, J.K.; Zhang, F.; Lin, K.Y.; Yao, J.Y.; Yu, J.S. Regulation of corneal angiogenesis in limbal stem cell deficiency. Prog. Retin. Eye Res. 2006, 25, 563–590. [Google Scholar] [CrossRef]
- Qiang, L.; Sample, A.; Shea, C.R.; Soltani, K.; Macleod, K.F.; He, Y.Y. Autophagy gene regulates ultraviolet radiation-induced inflammation and skin tumorigenesis. Autophagy 2017, 13, 2086–2103. [Google Scholar] [CrossRef]
- Du, J.; Liu, W.; Song, Y.; Zhang, Y.; Dong, C.; Xiong, S.; Huang, Z.; Wang, T.; Ding, J.; He, Q.; et al. Activating autophagy promotes skin regeneration induced by mechanical stretch during tissue expansion. Burns Trauma. 2024, 12, tkad057. [Google Scholar] [CrossRef]
- Clahsen, T.; Hadrian, K.; Notara, M.; Schlereth, S.L.; Howaldt, A.; Prokosch, V.; Volatier, T.; Hos, D.; Schroedl, F.; Kaser-Eichberger, A.; et al. The novel role of lymphatic vessels in the pathogenesis of ocular diseases. Prog. Retin. Eye Res. 2023, 96, 101157. [Google Scholar] [CrossRef]
- Dias-Teixeira, K.L.; Sharifian Gh, M.; Romano, J.; Norouzi, F.; Laurie, G.W. Autophagy in the normal and diseased cornea. Exp. Eye Res. 2022, 225, 109274. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Saint-Geniez, M.; Hamrah, P.; Jin, Y.; Rashid, S.; Pytowski, B.; Persaud, K.; Wu, Y.; Streilein, J.W.; et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc. Natl. Acad. Sci. USA 2006, 103, 11405–11410. [Google Scholar] [CrossRef]
- Tanida, I.; Yamasaki, M.; Komatsu, M.; Ueno, T. The FAP motif within human ATG7, an autophagy-related E1-like enzyme, is essential for the E2-substrate reaction of LC3 lipidation. Autophagy 2012, 8, 88–97. [Google Scholar] [CrossRef]
- Lim, Y.M.; Lim, H.; Hur, K.Y.; Quan, W.; Lee, H.Y.; Cheon, H.; Ryu, D.; Koo, S.H.; Kim, H.L.; Kim, J.; et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat. Commun. 2014, 5, 4934. [Google Scholar] [CrossRef]
- Demir, K.; Kirsch, N.; Beretta, C.A.; Erdmann, G.; Ingelfinger, D.; Moro, E.; Argenton, F.; Carl, M.; Niehrs, C.; Boutros, M. RAB8B Is Required for Activity and Caveolar Endocytosis of LRP6. Cell Rep. 2013, 4, 1224–1234. [Google Scholar] [CrossRef]
- Sato, T.; Iwano, T.; Kunii, M.; Matsuda, S.; Mizuguchi, R.; Jung, Y.; Hagiwara, H.; Yoshihara, Y.; Yuzaki, M.; Harada, R.; et al. Rab8a and Rab8b are essential for several apical transport pathways but insufficient for ciliogenesis. J. Cell Sci. 2014, 127, 422–431. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Iavarone, F.; Maddaluno, M.; Grumati, P.; Settembre, C. RETREG3/FAM134C phosphorylation by CSNK2 regulates reticulophagy during starvation. Autophagy Rep. 2022, 1, 519–522. [Google Scholar] [CrossRef]
- Qian, X.; Sun, J.; Cui, Y. UVRAG: Orchestrating the initiation of reticulophagy. Autophagy 2024, 20, 712–713. [Google Scholar] [CrossRef]
- Trentesaux, C.; Fraudeau, M.; Pitasi, C.L.; Lemarchand, J.; Jacques, S.; Duche, A.; Letourneur, F.; Naser, E.; Bailly, K.; Schmitt, A.; et al. Essential role for autophagy protein ATG7 in the maintenance of intestinal stem cell integrity. Proc. Natl. Acad. Sci. USA 2020, 117, 11136–11146. [Google Scholar] [CrossRef]
- Amitai-Lange, A.; Altshuler, A.; Bubley, J.; Dbayat, N.; Tiosano, B.; Shalom-Feuerstein, R. Lineage tracing of stem and progenitor cells of the murine corneal epithelium. Stem Cells 2015, 33, 230–239. [Google Scholar] [CrossRef]
- Yang, J.J.; Cui, Y.X. TM9SF3 is a mammalian Golgiphagy receptor that safeguards Golgi integrity and glycosylation fidelity. Autophagy 2025. [Google Scholar] [CrossRef]
- Yang, J.; Dong, Y.; Xu, J.; Qian, X.; Cai, Y.; Chen, Y.; Zhang, P.; Gao, D.; Cui, Z.; Cui, Y. TM9SF3 is a Golgi-resident ATG8-binding protein essential for Golgi-selective autophagy. Dev. Cell 2025. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Tian, X.; Shao, G.; Lin, Q.; Sun, A. Golgiphagy: A novel selective autophagy to the fore. Cell Biosci. 2024, 14, 130. [Google Scholar] [CrossRef]
- Iwamoto, T.; Shimizu, S.; Tajima-Sakurai, H.; Yamaguchi, H.; Nishida, Y.; Arakawa, S.; Watada, H. Inhibition of Insulin Secretion Induces Golgi Morphological Changes. Juntendo Iji Zasshi 2023, 69, 42–49. [Google Scholar] [CrossRef]
- Song, W.; Postoak, J.L.; Yang, G.; Guo, X.; Pua, H.H.; Bader, J.; Rathmell, J.C.; Kobayashi, H.; Haase, V.H.; Leaptrot, K.L.; et al. Lipid kinase PIK3C3 maintains healthy brown and white adipose tissues to prevent metabolic diseases. Proc. Natl. Acad. Sci. USA 2023, 120, e2214874120. [Google Scholar] [CrossRef]
- Bragulla, H.H.; Homberger, D.G. Structure and functions of keratin proteins in simple, stratified, keratinized and cornified epithelia. J. Anat. 2009, 214, 516–559. [Google Scholar] [CrossRef]
- Lackner, C.; Gogg-Kamerer, M.; Zatloukal, K.; Stumptner, C.; Brunt, E.M.; Denk, H. Ballooned hepatocytes in steatohepatitis: The value of keratin immunohistochemistry for diagnosis. J. Hepatol. 2008, 48, 821–828. [Google Scholar] [CrossRef]
- Nah, J.; Yoo, S.M.; Jung, S.; Jeong, E.I.; Park, M.; Kaang, B.K.; Jung, Y.K. Phosphorylated CAV1 activates autophagy through an interaction with BECN1 under oxidative stress. Cell Death Dis. 2017, 8, e282210. [Google Scholar] [CrossRef]
- Codrici, E.; Albulescu, L.; Popescu, I.D.; Mihai, S.; Enciu, A.M.; Albulescu, R.; Tanase, C.; Hinescu, M.E. Caveolin-1-Knockout Mouse as a Model of Inflammatory Diseases. J. Immunol. Res. 2018, 2018, 2498576. [Google Scholar] [CrossRef]
- Yuan, K.F.; Huang, C.H.; Fox, J.; Gaid, M.; Weaver, A.; Li, G.P.; Singh, B.B.; Gao, H.W.; Wu, M. Elevated Inflammatory Response in Caveolin-1-deficient Mice with Infection Is Mediated by STAT3 Protein and Nuclear Factor κB (NF-κB). J. Biol. Chem. 2011, 286, 21814–21825. [Google Scholar] [CrossRef]
- Minakaki, G.; Menges, S.; Kittel, A.; Emmanouilidou, E.; Schaeffner, I.; Barkovits, K.; Bergmann, A.; Rockenstein, E.; Adame, A.; Marxreiter, F.; et al. Autophagy inhibition promotes SNCA/alpha-synuclein release and transfer via extracellular vesicles with a hybrid autophagosome-exosome-like phenotype. Autophagy 2018, 14, 98–119. [Google Scholar] [CrossRef]
- Kuo, H.J.; Tran, N.T.; Clary, S.A.; Morris, N.P.; Glanville, R.W. Characterization of EHD4, an EH domain-containing protein expressed in the extracellular matrix. J. Biol. Chem. 2001, 276, 43103–43110. [Google Scholar] [CrossRef]
- Torrino, S.; Shen, W.W.; Blouin, C.M.; Mani, S.K.; de Lesegno, C.V.; Bost, P.; Grassart, A.; Köster, D.; Valades-Cruz, C.A.; Chambon, V.; et al. EHD2 is a mechanotransducer connecting caveolae dynamics with gene transcription. J. Cell Biol. 2018, 217, 4092–4105. [Google Scholar] [CrossRef]
- Kajita, M.; Sugimura, K.; Ohoka, A.; Burden, J.; Suganuma, H.; Ikegawa, M.; Shimada, T.; Kitamura, T.; Shindoh, M.; Ishikawa, S.; et al. Filamin acts as a key regulator in epithelial defence against transformed cells. Nat. Commun. 2014, 5, 4428. [Google Scholar] [CrossRef]
- Baldassarre, M.; Razinia, Z.; Brahme, N.N.; Buccione, R.; Calderwood, D.A. Filamin A controls matrix metalloproteinase activity and regulates cell invasion in human fibrosarcoma cells. J. Cell Sci. 2012, 125, 3858–3869. [Google Scholar] [CrossRef]
- Kim, J.; Yang, C.; Kim, E.J.; Jang, J.; Kim, S.J.; Kang, S.M.; Kim, M.G.; Jung, H.; Park, D.; Kim, C. Vimentin filaments regulate integrin-ligand interactions by binding to the cytoplasmic tail of integrin β3. J. Cell Sci. 2016, 129, 2030–2042. [Google Scholar] [CrossRef]
- Feng, X.Z.; Du, W.Q.; Ding, M.R.; Zhao, W.K.; Xirefu, X.; Ma, M.S.; Zhuang, Y.H.; Fu, X.Y.; Shen, J.F.; Zhang, J.P.; et al. Myosin 1D and the branched actin network control the condensation of p62 bodies. Cell Res. 2022, 32, 659–669. [Google Scholar] [CrossRef]
- Noda, N.N. Cytoskeleton grows p62 condensates for autophagy. Cell Res. 2022, 32, 607–608. [Google Scholar] [CrossRef]
- Li, X.L.; Matsuoka, Y.; Bennett, V. Adducin preferentially recruits spectrin to the fast growing ends of actin filaments in a complex requiring the MARCKS-related domain and a newly defined oligomerization domain. J. Biol. Chem. 1998, 273, 19329–19338. [Google Scholar] [CrossRef]
- Xu, K.; Zhong, G.S.; Zhuang, X.W. Actin, Spectrin, and Associated Proteins Form a Periodic Cytoskeletal Structure in Axons. Science 2013, 339, 452–456. [Google Scholar] [CrossRef]
- Holzner, S.; Bromberger, S.; Wenzina, J.; Neumüller, K.; Holper, T.M.; Petzelbauer, P.; Bauer, W.; Weber, B.; Schossleitner, K. Phosphorylated cingulin localises GEF-H1 at tight junctions to protect vascular barriers in blood endothelial cells. J. Cell Sci. 2021, 134, jcs258557. [Google Scholar] [CrossRef]
- Aijaz, S.; D’Atri, F.; Citi, S.; Balda, M.S.; Matter, K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev. Cell 2005, 8, 777–786. [Google Scholar] [CrossRef]
- Kim, H.T.; Yin, W.G.; Jin, Y.J.; Panza, P.; Gunawan, F.; Grohmann, B.; Buettner, C.; Sokol, A.M.; Preussner, J.; Guenther, S.; et al. deficiency leads to defective extracellular matrix remodeling and pulmonary disease. Nat. Commun. 2018, 9, 4600. [Google Scholar] [CrossRef]
- Wang, R.P.; Arbel, E.; Tang, D.D. Smooth Muscle Myosin Localizes at the Leading Edge and Regulates the Redistribution of Actin-regulatory Proteins during Migration. Cells 2022, 11, 2334. [Google Scholar] [CrossRef]
- Motegi, S.; Leitner, W.W.; Lu, M.; Tada, Y.; Sárdy, M.; Wu, C.J.; Chavakis, T.; Udey, M.C. Pericyte-Derived MFG-E8 Regulates Pathologic Angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2024–2034. [Google Scholar] [CrossRef]
- Cui, Q.; Liu, H.C.; Liu, W.M.; Ma, F.; Lv, Y.; Ma, J.C.; Wu, R.Q.; Ren, Y.F. Milk fat globule epidermal growth factor 8 alleviates liver injury in severe acute pancreatitis by restoring autophagy flux and inhibiting ferroptosis in hepatocytes. World J. Gastroenterol. 2024, 30, 728. [Google Scholar] [CrossRef]
- Ren, Y.F.; Cui, Q.; Zhang, J.; Liu, W.M.; Xu, M.; Lv, Y.; Wu, Z.; Zhang, Y.Y.; Wu, R.Q. Milk Fat Globule-EGF Factor 8 Alleviates Pancreatic Fibrosis by Inhibiting ER Stress-Induced Chaperone-Mediated Autophagy in Mice. Front. Pharmacol. 2021, 12, 707259. [Google Scholar] [CrossRef]
- Hupfer, A.; Brichkina, A.; Koeniger, A.; Keber, C.; Denkert, C.; Pfefferle, P.; Helmprobst, F.; Pagenstecher, A.; Visekruna, A.; Lauth, M. Matrix stiffness drives stromal autophagy and promotes formation of a protumorigenic niche. Proc. Natl. Acad. Sci. USA 2021, 118, e2105367118. [Google Scholar] [CrossRef]
- Zha, S.Y.; Li, Z.; Chen, S.Y.; Liu, F.; Wang, F. MeCP2 inhibits cell functionality through FoxO3a and autophagy in endothelial progenitor cells. Aging 2019, 11, 6714–6733. [Google Scholar] [CrossRef]
- Vuu, Y.M.; Roberts, C.T.; Rastegar, M. MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism. Int. J. Mol. Sci. 2023, 24, 4218. [Google Scholar] [CrossRef]
- Esposito, A.; Seri, T.; Breccia, M.; Indrigo, M.; De Rocco, G.; Nuzzolillo, F.; Denti, V.; Pappacena, F.; Tartaglione, G.; Serrao, S.; et al. Unraveling autophagic imbalances and therapeutic insights in Mecp2-deficient models. Embo Mol. Med. 2024, 16, 2795–2826. [Google Scholar] [CrossRef]
- Hu, K.; Ma, R.T.; Huang, M.J.; Cao, X.Y.; Ding, Y.; Li, Y.X.; Chen, Y.F.; Xiao, L.J.; Ling, S.; Huang, Y.L.; et al. Mecp2 promotes the anti-inflammatory effect of alpinetin via epigenetic modification crosstalk. J. Cell. Mol. Med. 2024, 28, e18510. [Google Scholar] [CrossRef]
- Kaplan, N.; Wang, S.J.; Wang, J.Y.; Yang, W.D.; Ventrella, R.; Majekodunmi, A.; White, B.E.P.; Getsios, S.; Mitchell, B.J.; Peng, H.; et al. Ciliogenesis and autophagy are coordinately regulated by EphA2 in the cornea to maintain proper epithelial architecture. Ocul. Surf. 2021, 21, 193–205. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Song, Y.W.; Shi, Q.Q.; Fu, L. Research progress on FASN and MGLL in the regulation of abnormal lipid metabolism and the relationship between tumor invasion and metastasis. Front. Med. 2021, 15, 649–656. [Google Scholar] [CrossRef]
- Gruden, E.; Kienzl, M.; Danner, L.; Kaspret, D.M.; Pammer, A.; Ristic, D.; Kindler, O.; Doyle, A.D.; Wright, B.L.; Taschler, U.; et al. The Endocannabinoid System Drives Eosinophil Infiltration During Eosinophilic Esophagitis. Cell. Mol. Gastroenterol. Hepatol. 2025, 19, 101515. [Google Scholar] [CrossRef]
- Dietrich, T.; Bock, F.; Yuen, D.; Hos, D.; Bachmann, B.O.; Zahn, G.; Wiegand, S.; Chen, L.; Cursiefen, C. Cutting Edge: Lymphatic Vessels, Not Blood Vessels, Primarily Mediate Immune Rejections After Transplantation. J. Immunol. 2010, 184, 535–539. [Google Scholar] [CrossRef]
- Sukseree, S.; Mildner, M.; Rossiter, H.; Pammer, J.; Zhang, C.F.; Watanapokasin, R.; Tschachler, E.; Eckhart, L. Autophagy in the Thymic Epithelium Is Dispensable for the Development of Self-Tolerance in a Novel Mouse Model. PLoS ONE 2012, 7, e38933. [Google Scholar] [CrossRef]
- Searle, B.C.; Pino, L.K.; Egertson, J.D.; Ting, Y.S.; Lawrence, R.T.; MacLean, B.X.; Villén, J.; MacCoss, M.J. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat. Commun. 2018, 9, 5128. [Google Scholar] [CrossRef]
- Gessulat, S.; Schmidt, T.; Zolg, D.P.; Samaras, P.; Schnatbaum, K.; Zerweck, J.; Knaute, T.; Rechenberger, J.; Delanghe, B.; Huhmer, A.; et al. Prosit: Proteome-wide prediction of peptide tandem mass spectra by deep learning. Nat. Methods 2019, 16, 509–518. [Google Scholar] [CrossRef]
- Arganda-Carreras, I.; Kaynig, V.; Rueden, C.; Eliceiri, K.W.; Schindelin, J.; Cardona, A.; Sebastian Seung, H. Trainable Weka Segmentation: A machine learning tool for microscopy pixel classification. Bioinformatics 2017, 33, 2424–2426. [Google Scholar] [CrossRef]
- Arzt, M.; Deschamps, J.; Schmied, C.; Pietzsch, T.; Schmidt, D.; Tomancak, P.; Haase, R.; Jug, F. LABKIT: Labeling and Segmentation Toolkit for Big Image Data. Front. Comp. Sci 2022, 4, 777728. [Google Scholar] [CrossRef]
- Zhang, W.; Schönberg, A.; Bassett, F.; Hadrian, K.; Hos, D.; Becker, M.; Bock, F.; Cursiefen, C. Different Murine High-Risk Corneal Transplant Settings Vary Significantly in Their (Lymph)angiogenic and Inflammatory Cell Signatures. Investig. Ophthalmol. Vis. Sci. 2022, 63, 18. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Tang, D.D.; Chen, M.J.; Huang, X.H.; Zhang, G.C.; Zeng, L.; Zhang, G.S.; Wu, S.J.; Wang, Y.W. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bandla, C.; Kundu, D.J.; Kamatchinathan, S.; Bai, J.W.; Hewapathirana, S.; John, N.S.; Prakash, A.; Walzer, M.; Wang, S.B.; et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res. 2024, 53, D543–D553. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volatier, T.; Mourier, A.; Mann, J.; Meshko, B.; Hadrian, K.; Cursiefen, C.; Notara, M. Characterization of Corneal Defects in ATG7-Deficient Mice. Int. J. Mol. Sci. 2025, 26, 9989. https://doi.org/10.3390/ijms26209989
Volatier T, Mourier A, Mann J, Meshko B, Hadrian K, Cursiefen C, Notara M. Characterization of Corneal Defects in ATG7-Deficient Mice. International Journal of Molecular Sciences. 2025; 26(20):9989. https://doi.org/10.3390/ijms26209989
Chicago/Turabian StyleVolatier, Thomas, Andreas Mourier, Johanna Mann, Berbang Meshko, Karina Hadrian, Claus Cursiefen, and Maria Notara. 2025. "Characterization of Corneal Defects in ATG7-Deficient Mice" International Journal of Molecular Sciences 26, no. 20: 9989. https://doi.org/10.3390/ijms26209989
APA StyleVolatier, T., Mourier, A., Mann, J., Meshko, B., Hadrian, K., Cursiefen, C., & Notara, M. (2025). Characterization of Corneal Defects in ATG7-Deficient Mice. International Journal of Molecular Sciences, 26(20), 9989. https://doi.org/10.3390/ijms26209989






