Understanding the Microenvironment of Intervertebral Disc Degeneration: A Comprehensive Review of Pathophysiological Insights and Therapeutic Implications
Abstract
1. Introduction
2. Methodological Approach of the Narrative Review
3. Microenvironment of a Healthy Intervertebral Disc
3.1. Nucleus Pulposus
3.2. Annulus Fibrosus
3.3. Cartilage Endplate
4. Cellular and Molecular Mechanisms Underlying Intervertebral Disc Degeneration
4.1. Phenotypic Shift in Nucleus Pulposus Cells
4.2. Phenotypic Alterations of Annulus Fibrosus Cells
4.3. Structural and Cellular Changes in the Cartilage Endplate
4.4. Biomechanical Causes of Intervertebral Disc Degeneration
4.5. Cellular Senescence
4.6. Autophagy Impairment
4.7. Programmed Cell Death Pathways in IVDD
4.7.1. Apoptosis
4.7.2. Necroptosis
4.7.3. Pyroptosis
4.7.4. Ferroptosis
4.8. Hypoxia, Nutrient Deprivation, and Aberrant Angiogenesis
4.9. Nerve Ingrowth and Pain Sensitization
4.10. Oxidative Stress and Organelle Dysfunction
4.11. Inflammatory Signaling
4.12. Immune Cell Infiltration
5. Extracellular Matrix Remodeling
5.1. Alterations of ECM Components During Disc Degeneration
5.2. Matrix-Degrading Enzymes
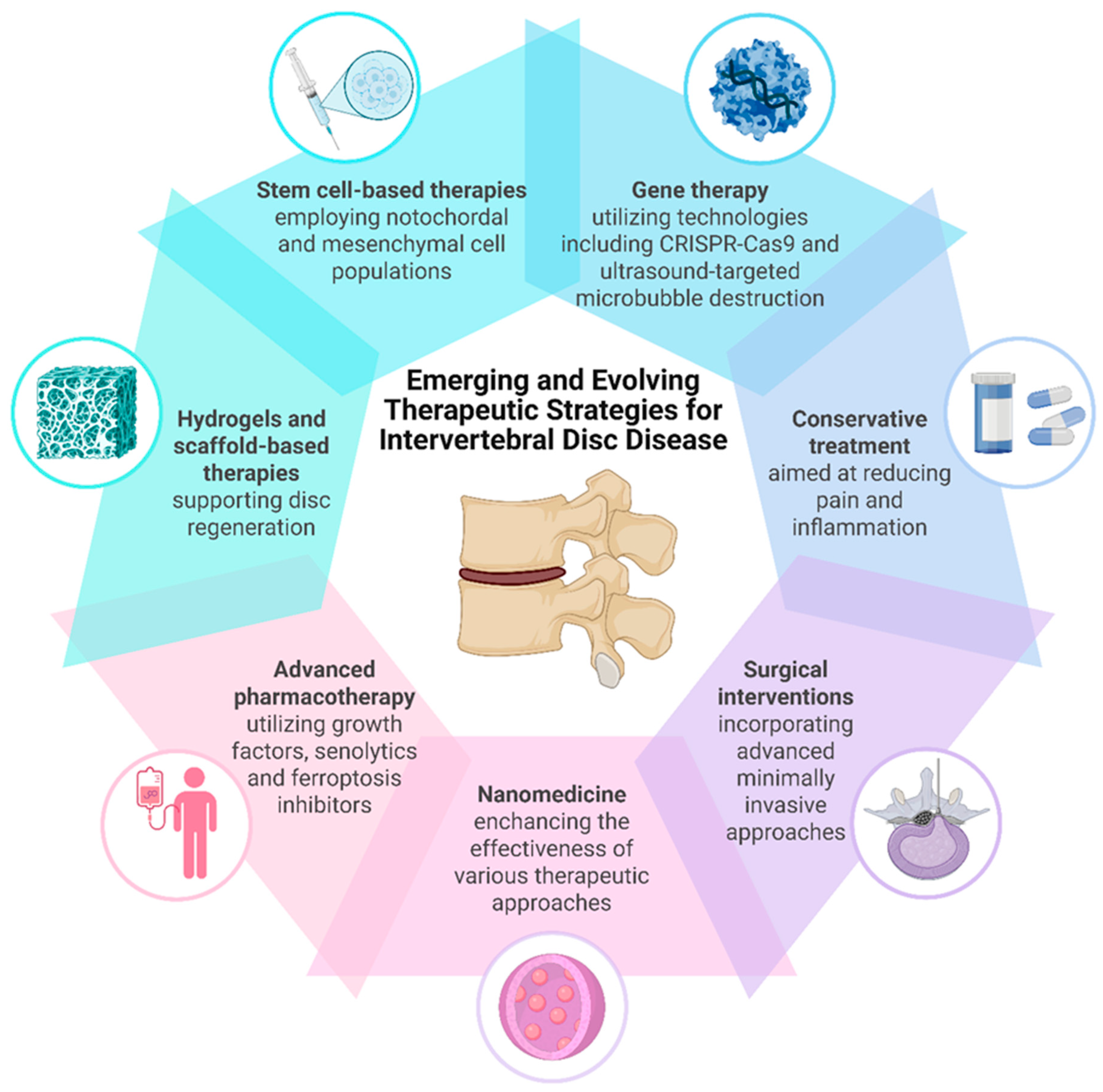
6. Treatment Approaches for Intervertebral Disc Degeneration
6.1. Conservative Management
6.2. Surgical Interventions
- Spinal fusion: Interbody fusion is achieved using cage implants, usually supported with bone graft or other materials enhancing bone fusion. The stability of the motion segment is often further improved with the addition of transpedicular stabilization devices. Although fusion can relieve pain and improve stability, it eliminates motion at the treated level and may predispose adjacent segments to accelerated degeneration [148,149,150].
6.3. Cell-Based Therapies
6.4. Growth Differentiation Factors
6.5. Senolytics
6.6. Ferroptosis Inhibition
6.7. Gene Therapy
6.8. Hydrogels and Scaffold-Based Therapies
6.9. Nanomedicine Approaches
7. Discussion
8. Conclusions
9. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAV | Adeno-associated viruses |
| ACSL4 | Acyl-CoA synthetase long chain family member 4 |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| AF | Annulus fibrosus |
| AGE | Advanced glycation end-product |
| AIM2 | Absent in melanoma 2 |
| AKAP150 | A-kinase anchoring protein 150 |
| AMPK | AMP-activated protein kinase |
| ANGPTL4 | Angiopoietin-like 4 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| Atg | Autophagy-related protein |
| BDNF | Brain-derived neurotrophic factor |
| BMP | Bone morphogenetic protein |
| BMP-7 | Bone morphogenetic protein-7 |
| β-NGF | β-nerve growth factor |
| CA HIF-1α | Constitutively active form of HIF-1α |
| CCL | Chemokine ligand |
| CEP | Cartilage endplates |
| C/EBP | CCAAT-enhancer-binding protein |
| CGRP | Calcitonin gene-related peptide |
| COX-2 | Cyclooxygenase-2 |
| DAMPs | Damage-associated molecular patterns |
| DFO | Deferoxamine |
| DFP | Deferiprone |
| DFX | Deferasirox |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| ER | Endoplasmic reticulum |
| ESCRT | Endosomal sorting complex required for transport |
| FAK | Focal adhesion kinase |
| FasL | Fas ligand |
| Fe2+ | Ferrous iron |
| Fer-1 | Ferrostatin-1 |
| FPN/SLC40A1 | Ferroportin |
| GDF | Growth differentiation factor |
| GF | Growth factor |
| Glut-1 | Glucose transporter 1 |
| Glut-3 | Glucose transporter 3 |
| GM-CSF | Granulocyte-macrophage colony stimulating factor |
| GPX4 | Glutathione peroxidase 4 |
| GSDMD | Gasdermin D |
| GSDMD-NT | N-terminal pore-forming GSDMD fragment |
| GSDME | Gasdermin E |
| GSH | Glutathione |
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| HIF-2α | Hypoxia inducible factor 2α |
| HO-1 | Heme oxygenase 1 |
| HTRA1 | High-temperature requirement protein A1 |
| IFN-γ | Interferon-γ |
| IGF-1 | Insulin-like growth factor-1 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| IRF1 | Interferon regulatory factor 1 |
| IVD | Intervertebral disc |
| IVDD | Intervertebral disc degeneration |
| LC3-II | LC3-phosphatidylethanolamine conjugate |
| Lip-1 | Liproxstatin-1 |
| LOX | Lipoxygenase |
| LPCAT3 | Lysophosphatidylcholine acyltransferase 3 |
| MAPK | Mitogen-activated protein kinase |
| miRNA | Micro ribonucleic acid |
| MLKL | Mixed lineage kinase domain-like protein |
| MMP | Matrix metalloproteinase |
| MSC | Mesenchymal stem cell |
| mTOR | Mammalian/Mechanistic target of rapamycin |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| NC | Notochordal cell |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve growth factor |
| NK | Natural killer |
| NLRC4 | NLR family CARD domain-containing protein 4 |
| NLRP3 | NLR family pyrin domain containing 3 |
| NO | Nitric oxide |
| NP | Nucleus pulposus |
| NR4A1 | Nuclear receptor 4A1 |
| NSAID | Non-steroidal anti-inflammatory drug |
| OPN | Osteopontin |
| PAMP | Pathogen-associated molecular pattern |
| PDGF | Platelet-derived growth factor |
| PGE2 | Prostaglandin E2 |
| PRP | Platelet-rich plasma |
| PUFA | Polyunsaturated fatty acid |
| Rb | Retinoblastoma protein |
| RIPK1 | Receptor-interacting serine/threonine-protein kinase 1 |
| RIPK3 | Receptor-interacting serine/threonine-protein kinase 3 |
| RNAi | RNA interference |
| ROS | Reactive oxygen species |
| SASP | Senescence-associated secretory phenotype |
| SA-β-gal | Senescence-associated β-galactosidase |
| SIPS | Stress-induced premature senescence |
| SLRP | Small leucine-rich proteoglycan |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| sTNF-α | Soluble tumor necrosis factor-alpha |
| TACE | Tumor necrosis factor-alpha converting enzyme |
| TAZ | Transcriptional co-activator with PDZ-binding motif |
| TDR | Total disc replacement |
| TFR1 | Transferrin receptor |
| TGF-β | Transforming growth factor-beta |
| TIMP | Tissue inhibitor of metalloproteinase |
| TLR4 | Toll-like receptor 4 |
| tmTNF-α | Transmembrane tumor necrosis factor-alpha |
| TNFR1 | Tumor necrosis factor receptor 1 |
| TNFR2 | Tumor necrosis factor receptor 2 |
| TNF-α | Tumor necrosis factor-alpha |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| ULK1 | Unc-51-like kinase 1 |
| UTMD | Ultrasound-targeted microbubble destruction |
| VEGF | Vascular endothelial growth factor |
| YAP | Yes-associated protein |
References
- Kirnaz, S.; Capadona, C.; Wong, T.; Goldberg, J.L.; Medary, B.; Sommer, F.; McGrath, L.B.; Härtl, R. Fundamentals of Intervertebral Disc Degeneration. World Neurosurg. 2022, 157, 264–273. [Google Scholar] [CrossRef]
- Taylor, W.; Erwin, W.M. Intervertebral Disc Degeneration and Regeneration: New Molecular Mechanisms and Therapeutics: Obstacles and Potential Breakthrough Technologies. Cells 2024, 13, 2103. [Google Scholar] [CrossRef]
- Teraguchi, M.; Yoshimura, N.; Hashizume, H.; Yamada, H.; Oka, H.; Minamide, A.; Nagata, K.; Ishimoto, Y.; Kagotani, R.; Kawaguchi, H.; et al. Progression, Incidence, and Risk Factors for Intervertebral Disc Degeneration in a Longitudinal Population-Based Cohort: The Wakayama Spine Study. Osteoarthr. Cartil. 2017, 25, 1122–1131. [Google Scholar] [CrossRef]
- Weber, K.T.; Jacobsen, T.D.; Maidhof, R.; Virojanapa, J.; Overby, C.; Bloom, O.; Quraishi, S.; Levine, M.; Chahine, N.O. Developments in Intervertebral Disc Disease Research: Pathophysiology, Mechanobiology, and Therapeutics. Curr. Rev. Musculoskelet. Med. 2015, 8, 18–31. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, Y.; Dong, H.; Mao, Q.; Zhu, L.; Xia, J.; Weng, Z.; Liao, W.; Hu, Z.; Yi, J.; et al. Progress in the Study of Molecular Mechanisms of Intervertebral Disc Degeneration. Biomed. Pharmacother. 2024, 174, 116593. [Google Scholar] [CrossRef] [PubMed]
- Ruffilli, A.; Viroli, G.; Neri, S.; Traversari, M.; Barile, F.; Manzetti, M.; Assirelli, E.; Ialuna, M.; Vita, F.; Faldini, C. Mechanobiology of the Human Intervertebral Disc: Systematic Review of the Literature and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 2728. [Google Scholar] [CrossRef] [PubMed]
- Kadow, T.; Sowa, G.; Vo, N.; Kang, J.D. Molecular Basis of Intervertebral Disc Degeneration and Herniations: What Are the Important Translational Questions? Clin. Orthop. Relat. Res. 2015, 473, 1903–1912. [Google Scholar] [CrossRef]
- Scarcia, L.; Pileggi, M.; Camilli, A.; Romi, A.; Bartolo, A.; Giubbolini, F.; Valente, I.; Garignano, G.; D’Argento, F.; Pedicelli, A.; et al. Degenerative Disc Disease of the Spine: From Anatomy to Pathophysiology and Radiological Appearance, with Morphological and Functional Considerations. J. Pers. Med. 2022, 12, 1810. [Google Scholar] [CrossRef] [PubMed]
- Kos, N.; Gradisnik, L.; Velnar, T. A Brief Review of the Degenerative Intervertebral Disc Disease. Med. Arch. 2019, 73, 421–424. [Google Scholar] [CrossRef]
- Roughley, P.J. Biology of Intervertebral Disc Aging and Degeneration. Spine 2004, 29, 2691–2699. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. Intervertebral Disk Degeneration and Repair. Neurosurgery 2017, 80, S46–S54. [Google Scholar] [CrossRef] [PubMed]
- Vergroesen, P.-P.A.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.W.; Welting, T.J.; van Royen, B.J.; van Dieën, J.H.; Smit, T.H. Mechanics and Biology in Intervertebral Disc Degeneration: A Vicious Circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef]
- Waxenbaum, J.A.; Reddy, V.; Futterman, B. Discs. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ohshima, H.; Tsuji, H.; Hirano, N.; Ishihara, H.; Katoh, Y.; Yamada, H. Water Diffusion Pathway, Swelling Pressure, and Biomechanical Properties of the Intervertebral Disc During Compression Load. Spine 1989, 14, 1234–1244. [Google Scholar] [CrossRef]
- Bezci, S.E.; Nandy, A.; O’Connell, G.D. Effect of Hydration on Healthy Intervertebral Disk Mechanical Stiffness. J. Biomech. Eng. 2015, 137, 101007. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Notochordal Cells in the Adult Intervertebral Disc: New Perspective on an Old Question. Crit. Rev. Eukaryot. Gene Expr. 2011, 21, 29–41. [Google Scholar] [CrossRef]
- Lin, H.; Tian, S.; Peng, Y.; Wu, L.; Xiao, Y.; Qing, X.; Shao, Z. IGF Signaling in Intervertebral Disc Health and Disease. Front. Cell Dev. Biol. 2022, 9, 817099. [Google Scholar] [CrossRef]
- Than, K.D.; Rahman, S.U.; Vanaman, M.J.; Wang, A.C.; Lin, C.-Y.; Zhang, H.; La Marca, F.; Park, P. Bone Morphogenetic Proteins and Degenerative Disk Disease. Neurosurgery 2012, 70, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, S.; Ma, K.; Zhao, L.; Lin, H.; Shao, Z. TGF-β Signaling in Intervertebral Disc Health and Disease. Osteoarthr. Cartil. 2019, 27, 1109–1117. [Google Scholar] [CrossRef]
- Ashley, J.W.; Enomoto-Iwamoto, M.; Smith, L.J.; Mauck, R.L.; Chan, D.; Lee, J.; Heyworth, M.F.; An, H.; Zhang, Y. Intervertebral Disc Development and Disease-Related Genetic Polymorphisms. Genes. Dis. 2016, 3, 171–177. [Google Scholar] [CrossRef]
- Torre, O.M.; Mroz, V.; Bartelstein, M.K.; Huang, A.H.; Iatridis, J.C. Annulus Fibrosus Cell Phenotypes in Homeostasis and Injury: Implications for Regenerative Strategies. Ann. N. Y Acad. Sci. 2019, 1442, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Pattappa, G.; Li, Z.; Peroglio, M.; Wismer, N.; Alini, M.; Grad, S. Diversity of Intervertebral Disc Cells: Phenotype and Function. J. Anat. 2012, 221, 480–496. [Google Scholar] [CrossRef]
- Hayes, A.; Benjamin, M.; Ralphs, J. Extracellular Matrix in Development of the Intervertebral Disc. Matrix Biol. 2001, 20, 107–121. [Google Scholar] [CrossRef]
- Molladavoodi, S.; DeWitte-Orr, S.J.; Gregory, D.E. An in Vitro 3D Annulus Fibrosus Cell Culture Model with Type I Collagen: An Examination of cell–Matrix Interactions. JOR Spine 2022, 5, e1193. [Google Scholar] [CrossRef]
- Tomaszewski, K.A.; Saganiak, K.; Gładysz, T.; Walocha, J.A. The Biology behind the Human Intervertebral Disc and Its Endplates. Folia Morphol. 2015, 74, 157–168. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family with Structural and Functional Diversity. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Zheng-wei, S.; Yuan, T.; Chao-shuai, F.; Lei, Z.; Zong-rang, S.; Tuan-jiang, L.; Ding-jun, H. Roles of Hippo–YAP/TAZ Signalling in Intervertebral Disc Degeneration. Biomed. Pharmacother. 2023, 159, 114099. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, T.; Zhong, Y.; Chen, M.; Yao, Q.; Chen, D.; Shao, Z.; Xiao, G. Roles of Focal Adhesion Proteins in Skeleton and Diseases. Acta Pharm. Sin. B 2023, 13, 998–1013. [Google Scholar] [CrossRef]
- De Geer, C.M. Intervertebral Disk Nutrients and Transport Mechanisms in Relation to Disk Degeneration: A Narrative Literature Review. J. Chiropr. Med. 2018, 17, 97–105. [Google Scholar] [CrossRef]
- Grunhagen, T.; Shirazi-Adl, A.; Fairbank, J.C.T.; Urban, J.P.G. Intervertebral Disk Nutrition: A Review of Factors Influencing Concentrations of Nutrients and Metabolites. Orthop. Clin. N. Am. 2011, 42, 465–477. [Google Scholar] [CrossRef]
- Di Pauli von Treuheim, T.; Torre, O.M.; Ferreri, E.D.; Nasser, P.; Abbondandolo, A.; Delgado Caceres, M.; Lin, D.; Docheva, D.; Iatridis, J.C. Tenomodulin and Chondromodulin-1 Are Both Required to Maintain Biomechanical Function and Prevent Intervertebral Disc Degeneration. Cartilage 2021, 13, 604S–614S. [Google Scholar] [CrossRef] [PubMed]
- Iatridis, J.C.; Nicoll, S.B.; Michalek, A.J.; Walter, B.A.; Gupta, M.S. Role of Biomechanics in Intervertebral Disc Degeneration and Regenerative Therapies: What Needs Repairing in the Disc and What Are Promising Biomaterials for Its Repair? Spine J. 2013, 13, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Erwin, W.M.; Islam, D.; Inman, R.D.; Fehlings, M.G.; Tsui, F.W. Notochordal Cells Protect Nucleus Pulposus Cells from Degradation and Apoptosis: Implications for the Mechanisms of Intervertebral Disc Degeneration. Arthritis Res. Ther. 2011, 13, R215. [Google Scholar] [CrossRef]
- Erwin, W.M.; Hood, K.E. The Cellular and Molecular Biology of the Intervertebral Disc: A Clinician’s Primer. J. Can. Chiropr. Assoc. 2014, 58, 246–257. [Google Scholar] [PubMed]
- Rodrigues-Pinto, R.; Ward, L.; Humphreys, M.; Zeef, L.A.H.; Berry, A.; Hanley, K.P.; Hanley, N.; Richardson, S.M.; Hoyland, J.A. Human Notochordal Cell Transcriptome Unveils Potential Regulators of Cell Function in the Developing Intervertebral Disc. Sci. Rep. 2018, 8, 12866. [Google Scholar] [CrossRef]
- Mohanty, S.; Pinelli, R.; Pricop, P.; Albert, T.J.; Dahia, C.L. Chondrocyte-like Nested Cells in the Aged Intervertebral Disc Are Late-stage Nucleus Pulposus Cells. Aging Cell 2019, 18, e13006. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.; Darling, E.M.; Chen, J.; Setton, L.A. Extracellular Matrix Ligand and Stiffness Modulate Immature Nucleus Pulposus Cell-Cell Interactions. PLoS ONE 2011, 6, e27170. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the Intervertebral Disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.; Su, Z.; So, K.K.H.; Lu, Q.; Lyu, M.; Zuo, J.; Huang, Y.; Guan, Z.; Cheung, K.M.C.; et al. Fibrocyte Enrichment and Myofibroblastic Adaptation Causes Nucleus Pulposus Fibrosis and Associates with Disc Degeneration Severity. Bone Res. 2025, 13, 10. [Google Scholar] [CrossRef]
- Sun, Y.; Lyu, M.; Lu, Q.; Cheung, K.; Leung, V. Current Perspectives on Nucleus Pulposus Fibrosis in Disc Degeneration and Repair. Int. J. Mol. Sci. 2022, 23, 6612. [Google Scholar] [CrossRef]
- Castro, A.L.; Ribeiro-Machado, C.; Oliveira, C.M.; Teixeira, G.Q.; Neidlinger-Wilke, C.; Pereira, P.; Vaz, R.; Barbosa, M.A.; Gonçalves, R.M. Fibrotic Alterations in Human Annulus Fibrosus Correlate with Progression of Intervertebral Disc Herniation. Arthritis Res. Ther. 2022, 24, 25. [Google Scholar] [CrossRef]
- Bogduk, N. Functional Anatomy of the Spine. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 136, pp. 675–688. [Google Scholar]
- Lama, P.; Tiwari, J.; Mutreja, P.; Chauhan, S.; Harding, I.J.; Dolan, T.; Adams, M.A.; Le Maitre, C. Cell Clusters in Intervertebral Disc Degeneration: An Attempted Repair Mechanism Aborted via Apoptosis. Anat. Cell Biol. 2023, 56, 382–393. [Google Scholar] [CrossRef]
- Tavakoli, J.; Diwan, A.D.; Tipper, J.L. Advanced Strategies for the Regeneration of Lumbar Disc Annulus Fibrosus. Int. J. Mol. Sci. 2020, 21, 4889. [Google Scholar] [CrossRef]
- Grant, M.; Epure, L.; Bokhari, R.; Roughley, P.; Antoniou, J.; Mwale, F. Human Cartilaginous Endplate Degeneration Is Induced by Calcium and the Extracellular Calcium-Sensing Receptor in the Intervertebral Disc. Eur. Cell Mater. 2016, 32, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.G.; Smith, S.; Fairbank, J.C.T. Nutrition of the Intervertebral Disc. Spine 2004, 29, 2700–2709. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.R.; Huang, C.-Y.; Gu, W.Y. Effect of Endplate Calcification and Mechanical Deformation on the Distribution of Glucose in Intervertebral Disc: A 3D Finite Element Study. Comput. Methods Biomech. Biomed. Engin 2011, 14, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Venkatadass, K.; Naresh Babu, J.; Ganesh, K.; Shetty, A.P. Pharmacological Enhancement of Disc Diffusion and Differentiation of Healthy, Ageing and Degenerated Discs. Eur. Spine J. 2008, 17, 626–643. [Google Scholar] [CrossRef]
- Natarajan, R.N.; Ke, J.H.; Andersson, G.B.J. A Model to Study the Disc Degeneration Process. Spine 1994, 19, 259–265. [Google Scholar] [CrossRef]
- Aigner, T.; Greskötter, K.-R.; Fairbank, J.C.T.; von der Mark, K.; Urban, J.P.G. Variation with Age in the Pattern of Type X Collagen Expression in Normal and Scoliotic Human Intervertebral Discs. Calcif. Tissue Int. 1998, 63, 263–268. [Google Scholar] [CrossRef]
- Roberts, S.; Bains, M.A.; Kwan, A.; Menage, J.; Eisenstein, S.M. Type X Collagen in the Human Invertebral Disc: An Indication of Repair or Remodelling? Histochem. J. 1998, 30, 89–95. [Google Scholar] [CrossRef]
- Yurube, T.; Takeoka, Y.; Kanda, Y.; Kuroda, R.; Kakutani, K. Intervertebral Disc Cell Fate during Aging and Degeneration: Apoptosis, Senescence, and Autophagy. N. Am. Spine Soc. J. (NASSJ) 2023, 14, 100210. [Google Scholar] [CrossRef]
- Silwal, P.; Nguyen-Thai, A.M.; Mohammad, H.A.; Wang, Y.; Robbins, P.D.; Lee, J.Y.; Vo, N.V. Cellular Senescence in Intervertebral Disc Aging and Degeneration: Molecular Mechanisms and Potential Therapeutic Opportunities. Biomolecules 2023, 13, 686. [Google Scholar] [CrossRef]
- Roberts, S.; Evans, E.H.; Kletsas, D.; Jaffray, D.C.; Eisenstein, S.M. Senescence in Human Intervertebral Discs. Eur. Spine J. 2006, 15, 312–316. [Google Scholar] [CrossRef]
- Feng, C.; Liu, H.; Yang, M.; Zhang, Y.; Huang, B.; Zhou, Y. Disc Cell Senescence in Intervertebral Disc Degeneration: Causes and Molecular Pathways. Cell Cycle 2016, 15, 1674–1684. [Google Scholar] [CrossRef]
- Wang, F.; Cai, F.; Shi, R.; Wang, X.-H.; Wu, X.-T. Aging and Age Related Stresses: A Senescence Mechanism of Intervertebral Disc Degeneration. Osteoarthr. Cartil. 2016, 24, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Nakamura, Y.; Nakai, T.; Mishima, T.; Kato, S.; Grad, S.; Alini, M.; Risbud, M.V.; Chan, D.; Cheah, K.S.E.; et al. Exhaustion of Nucleus Pulposus Progenitor Cells with Ageing and Degeneration of the Intervertebral Disc. Nat. Commun. 2012, 3, 1264. [Google Scholar] [CrossRef]
- Patil, P.; Niedernhofer, L.J.; Robbins, P.D.; Lee, J.; Sowa, G.; Vo, N. Cellular Senescence in Intervertebral Disc Aging and Degeneration. Curr. Mol. Biol. Rep. 2018, 4, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kritschil, R.; Scott, M.; Sowa, G.; Vo, N. Role of Autophagy in Intervertebral Disc Degeneration. J. Cell Physiol. 2022, 237, 1266–1284. [Google Scholar] [CrossRef] [PubMed]
- Madhu, V.; Guntur, A.R.; Risbud, M.V. Role of Autophagy in Intervertebral Disc and Cartilage Function: Implications in Health and Disease. Matrix Biol. 2021, 100–101, 207–220. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Yu, P.; Zhu, Y.; Dai, F.; Ma, Z.; Shen, X.; Jiang, H.; Liu, J. Role of Autophagy and Pyroptosis in Intervertebral Disc Degeneration. J. Inflamm. Res. 2024, 17, 91–100. [Google Scholar] [CrossRef]
- Gong, C.; Zhang, H. Autophagy as a Potential Therapeutic Target in Intervertebral Disc Degeneration. Life Sci. 2021, 273, 119266. [Google Scholar] [CrossRef]
- Bahar, M.E.; Hwang, J.S.; Ahmed, M.; Lai, T.H.; Pham, T.M.; Elashkar, O.; Akter, K.-M.; Kim, D.-H.; Yang, J.; Kim, D.R. Targeting Autophagy for Developing New Therapeutic Strategy in Intervertebral Disc Degeneration. Antioxidants 2022, 11, 1571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, X.; Shen, H.; Zhang, C. Molecular Mechanisms of Cell Death in Intervertebral Disc Degeneration (Review). Int. J. Mol. Med. 2016, 37, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Shao, Z.; Xiong, L. Cell Death in Intervertebral Disc Degeneration. Apoptosis 2013, 18, 777–785. [Google Scholar] [CrossRef]
- Zhang, Q.-C.; Zou, Y.-P.; Hu, S.-Q.; Zhang, T.-W.; Zhou, H.; Liang, B.; Zhuang, C.-Y.; Wang, H.-R.; Jiang, L.-B.; Li, X.-L. TNF-α-Stimulated Nucleus Pulposus Cells Induce Cell Apoptosis through the Release of Exosomal MiR-16 Targeting IGF-1 and IGF-1R in Rats. Ann. Transl. Med. 2021, 9, 1376. [Google Scholar] [CrossRef]
- Chen, S.; Lei, L.; Li, Z.; Chen, F.; Huang, Y.; Jiang, G.; Guo, X.; Zhao, Z.; Liu, H.; Wang, H.; et al. Grem1 Accelerates Nucleus Pulposus Cell Apoptosis and Intervertebral Disc Degeneration by Inhibiting TGF-β-Mediated Smad2/3 Phosphorylation. Exp. Mol. Med. 2022, 54, 518–530. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Z.; Zhang, G.; Ma, C.; Qiu, X.; Wang, Y.; Liu, M.; Guo, X.; Chen, H.; Deng, Q.; et al. Periostin Promotes Nucleus Pulposus Cells Apoptosis by Activating the Wnt/Β-catenin Signaling Pathway. FASEB J. 2022, 36, e22369. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Kim, J.-H.; Hwang, B.-J.; Kang, J.-K.; Quan, M.; Kim, Y.-Y. Role of Necroptosis in Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2023, 24, 15292. [Google Scholar] [CrossRef]
- Ran, R.; Zhang, S.; Shi, Y.; Dong, H.; Song, W.; Dong, Y.; Zhou, K.; Zhang, H. Spotlight on Necroptosis: Role in Pathogenesis and Therapeutic Potential of Intervertebral Disc Degeneration. Int. Immunopharmacol. 2024, 138, 112616. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, X.; Wang, W.; Li, Y.; Cui, P.; Wang, P.; Kong, C.; Chen, X.; Lu, S. Understanding Necroptosis and Its Therapeutic Target for Intervertebral Disc Degeneration. Int. Immunopharmacol. 2023, 121, 110400. [Google Scholar] [CrossRef]
- Zhou, K.; Wu, S.; Wu, Z.; Ran, R.; Song, W.; Dong, H.; Zhang, H. Integrating Bioinformatics and Experimental Validation to Investigate IRF1 as a Novel Biomarker for Nucleus Pulposus Cells Necroptosis in Intervertebral Disc Degeneration. Sci. Rep. 2024, 14, 30138. [Google Scholar] [CrossRef]
- Fan, H.; Chen, Z.; Tang, H.-B.; Shan, L.-Q.; Chen, Z.-Y.; Liu, S.-C.; Zhang, Y.-Y.; Guo, X.-Y.; Yang, H.; Hao, D.-J. Necroptosis of Nucleus Pulposus Cells Involved in Intervertebral Disc Degeneration through MyD88 Signaling. Front. Endocrinol. 2022, 13, 994307. [Google Scholar] [CrossRef] [PubMed]
- Imre, G. Pyroptosis in Health and Disease. Am. J. Physiol. -Cell Physiol. 2024, 326, C784–C794. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of Pyroptosis in Inflammation and Cancer. Cell Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Zhou, K.; Ran, R.; Gong, C.; Zhang, S.; Ma, C.; Lv, J.; Lei, Z.; Ren, Y.; Zhang, H. Roles of Pyroptosis in Intervertebral Disc Degeneration. Pathol. Res. Prac. 2023, 248, 154685. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yang, Y.; Wang, X.; Chang, X.; Fu, S. Role of Pyroptosis in Intervertebral Disc Degeneration and Its Therapeutic Implications. Biomolecules 2022, 12, 1804. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Mei, Y.; Wang, L.; Chen, T.; Song, C.; Cheng, K.; Cai, W.; Zhou, D.; Gao, S.; Jiang, F.; Liu, S.; et al. Ferroptosis: A New Direction in the Treatment of Intervertebral Disc Degeneration. Cell Biochem. Biophys. 2024, 83, 33–42. [Google Scholar] [CrossRef]
- Liu, X.-W.; Xu, H.-W.; Yi, Y.-Y.; Zhang, S.-B.; Wang, S.-J. Role of Ferroptosis and Immune Infiltration in Intervertebral Disc Degeneration: Novel Insights from Bioinformatics Analyses. Front. Cell Dev. Biol. 2023, 11, 1170758. [Google Scholar] [CrossRef]
- Zhou, L.-P.; Zhang, R.-J.; Jia, C.-Y.; Kang, L.; Zhang, Z.-G.; Zhang, H.-Q.; Wang, J.-Q.; Zhang, B.; Shen, C.-L. Ferroptosis: A Potential Target for the Intervention of Intervertebral Disc Degeneration. Front. Endocrinol. 2022, 13, 1042060. [Google Scholar] [CrossRef]
- Fan, C.; Chu, G.; Yu, Z.; Ji, Z.; Kong, F.; Yao, L.; Wang, J.; Geng, D.; Wu, X.; Mao, H. The Role of Ferroptosis in Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2023, 11, 1219840. [Google Scholar] [CrossRef]
- Chen, J.-W.; Li, B.; Yang, Y.-H.; Jiang, S.-D.; Jiang, L.-S. Significance of Hypoxia in the Physiological Function of Intervertebral Disc Cells. Crit. Rev. Eukaryot. Gene Expr. 2014, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kwon, W.-K.; Moon, H.J.; Kwon, T.-H.; Park, Y.-K.; Kim, J.H. The Role of Hypoxia in Angiogenesis and Extracellular Matrix Regulation of Intervertebral Disc Cells During Inflammatory Reactions. Neurosurgery 2017, 81, 867–875. [Google Scholar] [CrossRef]
- LA Binch, A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.; Chiverton, N.; Cross, A.K.; Le Maitre, C.L. Expression and Regulation of Neurotrophic and Angiogenic Factors during Human Intervertebral Disc Degeneration. Arthritis Res. Ther. 2014, 16, 416. [Google Scholar] [CrossRef]
- Rätsep, T.; Minajeva, A.; Asser, T. Relationship between Neovascularization and Degenerative Changes in Herniated Lumbar Intervertebral Discs. Eur. Spine J. 2013, 22, 2474–2480. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, J.; Wang, Y.; Sun, X.; Zhu, J.; Xu, X.; Sun, J.; Shi, J. The Role of Nerve Fibers and Their Neurotransmitters in Regulating Intervertebral Disc Degeneration. Ageing Res. Rev. 2022, 81, 101733. [Google Scholar] [CrossRef] [PubMed]
- Purmessur, D.; Freemont, A.J.; Hoyland, J.A. Expression and Regulation of Neurotrophins in the Nondegenerate and Degenerate Human Intervertebral Disc. Arthritis Res. Ther. 2008, 10, R99. [Google Scholar] [CrossRef]
- Binch, A.L.A.; Cole, A.A.; Breakwell, L.M.; Michael, A.L.R.; Chiverton, N.; Creemers, L.B.; Cross, A.K.; Le Maitre, C.L. Nerves Are More Abundant than Blood Vessels in the Degenerate Human Intervertebral Disc. Arthritis Res. Ther. 2015, 17, 370. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, H.; Wang, T.; Zhang, K.; Zhang, Y.; Kang, X. Oxidative Stress in Intervertebral Disc Degeneration: Molecular Mechanisms, Pathogenesis and Treatment. Cell Prolif. 2023, 56, e13448. [Google Scholar] [CrossRef]
- Li, Y.; Chen, L.; Gao, Y.; Zou, X.; Wei, F. Oxidative Stress and Intervertebral Disc Degeneration: Pathophysiology, Signaling Pathway, and Therapy. Oxid. Med. Cell Longev. 2022, 2022, 1984742. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, A.; Zhao, K.; Gao, H.; Shi, P.; Chen, Y.; Cheng, Z.; Zhou, W.; Zhang, Y. The Role of Oxidative Stress in Intervertebral Disc Degeneration: Mechanisms and Therapeutic Implications. Ageing Res. Rev. 2024, 98, 102323. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yang, H.; Cheng, Y.; Liu, Y.; Hai, Y.; Zhang, Y. The Role of Oxidative Stress in Intervertebral Disc Cellular Senescence. Front. Endocrinol. 2022, 13, 1038171. [Google Scholar] [CrossRef]
- Le Maitre, C.L.; Hoyland, J.A.; Freemont, A.J. Catabolic Cytokine Expression in Degenerate and Herniated Human Intervertebral Discs: IL-1β and TNFα Expression Profile. Arthritis Res. Ther. 2007, 9, R77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Che, M.; Xin, J.; Zheng, Z.; Li, J.; Zhang, S. The Role of IL-1β and TNF-α in Intervertebral Disc Degeneration. Biomed. Pharmacother. 2020, 131, 110660. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of Cytokines in Intervertebral Disc Degeneration: Pain and Disc Content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.G.; Weiler, C.; Paesold, G.; Jochum, M.; Boos, N. Analysis of Tissue Distribution of TNF-α, TNF-α-Receptors, and the Activating TNF-α–Converting Enzyme Suggests Activation of the TNF-α System in the Aging Intervertebral Disc. Ann. N. Y Acad. Sci. 2007, 1096, 44–54. [Google Scholar] [CrossRef]
- Pan, H.; Li, H.; Guo, S.; Wang, C.; Long, L.; Wang, X.; Shi, H.; Zhang, K.; Chen, H.; Li, S. The Mechanisms and Functions of TNF-α in Intervertebral Disc Degeneration. Exp. Gerontol. 2023, 174, 112119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Pan, H.; Xiao, C.; Wang, C.; Guo, S.; Long, L.; Shi, H.; Chen, H.; Li, S. The Mechanisms and Functions of IL-1β in Intervertebral Disc Degeneration. Exp. Gerontol. 2023, 177, 112181. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, S.; Wang, M.; Jin, X.; Lv, C.; Deng, Y.; Wang, J. Enhanced NLRP3, Caspase-1, and IL- 1β Levels in Degenerate Human Intervertebral Disc and Their Association with the Grades of Disc Degeneration. Anat. Rec. 2015, 298, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, A.; Touli, E.; Hitzl, W.; Greutert, H.; Ferguson, S.J.; Wuertz-Kozak, K.; Hausmann, O.N. Inflammaging in Cervical and Lumbar Degenerated Intervertebral Discs: Analysis of Proinflammatory Cytokine and TRP Channel Expression. Eur. Spine J. 2018, 27, 564–577. [Google Scholar] [CrossRef]
- Chen, J.; Mei, Z.; Huang, B.; Zhang, X.; Liu, J.; Shan, Z.; Wang, J.; Wang, X.; Zhao, F. IL-6/YAP1/Β-catenin Signaling Is Involved in Intervertebral Disc Degeneration. J. Cell Physiol. 2019, 234, 5964–5971. [Google Scholar] [CrossRef]
- Khan, A.N.; Jacobsen, H.E.; Khan, J.; Filippi, C.G.; Levine, M.; Lehman, R.A.; Riew, K.D.; Lenke, L.G.; Chahine, N.O. Inflammatory Biomarkers of Low Back Pain and Disc Degeneration: A Review. Ann. N. Y. Acad. Sci. 2017, 1410, 68–84. [Google Scholar] [CrossRef]
- Molinos, M.; Almeida, C.R.; Caldeira, J.; Cunha, C.; Gonçalves, R.M.; Barbosa, M.A. Inflammation in Intervertebral Disc Degeneration and Regeneration. J. R. Soc. Interface 2015, 12, 20141191. [Google Scholar] [CrossRef]
- Liu, X.-G.; Hou, H.-W.; Liu, Y.-L. Expression Levels of IL-17 and TNF-α in Degenerated Lumbar Intervertebral Discs and Their Correlation. Exp. Ther. Med. 2016, 11, 2333–2340. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, Y.; Liu, Y.; Sun, X.; Liu, X.; Li, B.; Yang, Q. Role of Macrophage in Intervertebral Disc Degeneration. Bone Res. 2025, 13, 15. [Google Scholar] [CrossRef]
- Xu, H.; Li, J.; Fei, Q.; Jiang, L. Contribution of Immune Cells to Intervertebral Disc Degeneration and the Potential of Immunotherapy. Connect. Tissue Res. 2023, 64, 413–427. [Google Scholar] [CrossRef]
- Song, C.; Zhou, D.; Cheng, K.; Liu, F.; Cai, W.; Mei, Y.; Chen, J.; Huang, C.; Liu, Z. Bioinformatics-based Discovery of Intervertebral Disc Degeneration Biomarkers and Immune-inflammatory Infiltrates. JOR Spine 2024, 7, e1311. [Google Scholar] [CrossRef]
- Bosco, M.C. Macrophage Polarization: Reaching across the Aisle? J. Allergy Clin. Immunol. 2019, 143, 1348–1350. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Wu, W.; Lin, H.; Qing, X.; Liu, S.; Wang, B.; Xiao, Y.; Shao, Z.; Peng, Y. Nucleus Pulposus Cells Regulate Macrophages in Degenerated Intervertebral Discs via the Integrated Stress Response-Mediated CCL2/7-CCR2 Signaling Pathway. Exp. Mol. Med. 2024, 56, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Karchevskaya, A.E.; Poluektov, Y.M.; Korolishin, V.A. Understanding Intervertebral Disc Degeneration: Background Factors and the Role of Initial Injury. Biomedicines 2023, 11, 2714. [Google Scholar] [CrossRef]
- Ye, F.; Lyu, F.; Wang, H.; Zheng, Z. The Involvement of Immune System in Intervertebral Disc Herniation and Degeneration. JOR Spine 2022, 5, e1196. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, X.; Zhou, C.; Ren, Q.; Zhang, Y. Roles of Large Aggregating Proteoglycans in Human Intervertebral Disc Degeneration. Connect. Tissue Res. 2019, 60, 209–218. [Google Scholar] [CrossRef]
- Sivan, S.S.; Wachtel, E.; Roughley, P. Structure, Function, Aging and Turnover of Aggrecan in the Intervertebral Disc. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 3181–3189. [Google Scholar] [CrossRef]
- Liang, H.; Luo, R.; Li, G.; Zhang, W.; Song, Y.; Yang, C. The Proteolysis of ECM in Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2022, 23, 1715. [Google Scholar] [CrossRef] [PubMed]
- Kibble, M.J.; Domingos, M.; Hoyland, J.A.; Richardson, S.M. Importance of Matrix Cues on Intervertebral Disc Development, Degeneration, and Regeneration. Int. J. Mol. Sci. 2022, 23, 6915. [Google Scholar] [CrossRef] [PubMed]
- Roughley, P.J.; Alini, M.; Antoniou, J. The Role of Proteoglycans in Aging, Degeneration and Repair of the Intervertebral Disc. Biochem. Soc. Trans. 2002, 30, 869–874. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, W.; Chen, S.; Wang, B.; Wang, P.; Hu, B.; Lv, X.; Shao, Z. Extracellular Matrix in Intervertebral Disc: Basic and Translational Implications. Cell Tissue Res. 2022, 390, 1–22. [Google Scholar] [CrossRef]
- Inkinen, R.I.; Lammi, M.J.; Lehmonen, S.; Puustjärvi, K.; Kääpä, E.; Tammi, M.I. Relative Increase of Biglycan and Decorin and Altered Chondroitin Sulfate Epitopes in the Degenerating Human Intervertebral Disc. J. Rheumatol. 1998, 25, 506–514. [Google Scholar] [PubMed]
- Molière, S.; Jaulin, A.; Tomasetto, C.-L.; Dali-Youcef, N. Roles of Matrix Metalloproteinases and Their Natural Inhibitors in Metabolism: Insights into Health and Disease. Int. J. Mol. Sci. 2023, 24, 10649. [Google Scholar] [CrossRef]
- Ohnishi, T.; Novais, E.J.; Risbud, M.V. Alterations in ECM Signature Underscore Multiple Sub-Phenotypes of Intervertebral Disc Degeneration. Matrix Biol. Plus 2020, 6–7, 100036. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Pathak, Z.; Kumar, H. Extra Cellular Matrix Remodeling: An Adjunctive Target for Spinal Cord Injury and Intervertebral Disc Degeneration. Neurospine 2022, 19, 632–645. [Google Scholar] [CrossRef]
- Basaran, R.; Senol, M.; Ozkanli, S.; Efendioglu, M.; Kaner, T. Correlation of Matrix Metalloproteinase (MMP)-1, -2, -3, and -9 Expressions with Demographic and Radiological Features in Primary Lumbar Intervertebral Disc Disease. J. Clin. Neurosci. 2017, 41, 46–49. [Google Scholar] [CrossRef]
- Xu, H.; Mei, Q.; Xu, B.; Liu, G.; Zhao, J. Expression of Matrix Metalloproteinases Is Positively Related to the Severity of Disc Degeneration and Growing Age in the East Asian Lumbar Disc Herniation Patients. Cell Biochem. Biophys. 2014, 70, 1219–1225. [Google Scholar] [CrossRef]
- Aripaka, S.S.; Bech-Azeddine, R.; Jørgensen, L.M.; Mikkelsen, J.D. The Expression of Metalloproteinases in the Lumbar Disc Correlates Strongly with Pfirrmann MRI Grades in Lumbar Spinal Fusion Patients. Brain Spine 2022, 2, 100872. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, X.; Han, S.; Wei, L.; Zheng, Z.; Wang, Y.; Xin, J.; Zhang, S. Pathogenesis and Therapeutic Implications of Matrix Metalloproteinases in Intervertebral Disc Degeneration: A Comprehensive Review. Biochimie 2023, 214, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Caterson, B.; Menage, J.; Evans, E.H.; Jaffray, D.C.; Eisenstein, S.M. Matrix Metalloproteinases and Aggrecanase. Spine 2000, 25, 3005–3013. [Google Scholar] [CrossRef]
- Vo, N.V.; Hartman, R.A.; Yurube, T.; Jacobs, L.J.; Sowa, G.A.; Kang, J.D. Expression and Regulation of Metalloproteinases and Their Inhibitors in Intervertebral Disc Aging and Degeneration. Spine J. 2013, 13, 331–341. [Google Scholar] [CrossRef]
- Wang, W.-J.; Yu, X.-H.; Wang, C.; Yang, W.; He, W.-S.; Zhang, S.-J.; Yan, Y.-G.; Zhang, J. MMPs and ADAMTSs in Intervertebral Disc Degeneration. Clin. Chim. Acta 2015, 448, 238–246. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Nerlich, A.; Mittermaier, N.; Weiler, C.; Lumenta, C.; Wuertz, K.; Boos, N. Matrix Metalloproteinase Expression Levels Suggest Distinct Enzyme Roles during Lumbar Disc Herniation and Degeneration. Eur. Spine J. 2009, 18, 1573–1586. [Google Scholar] [CrossRef]
- Patel, K.P.; Sandy, J.D.; Akeda, K.; Miyamoto, K.; Chujo, T.; An, H.S.; Masuda, K. Aggrecanases and Aggrecanase-Generated Fragments in the Human Intervertebral Disc at Early and Advanced Stages of Disc Degeneration. Spine 2007, 32, 2596–2603. [Google Scholar] [CrossRef]
- Mead, T.J.; Apte, S.S. ADAMTS Proteins in Human Disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar] [CrossRef]
- Pockert, A.J.; Richardson, S.M.; Le Maitre, C.L.; Lyon, M.; Deakin, J.A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Modified Expression of the ADAMTS Enzymes and Tissue Inhibitor of Metalloproteinases 3 during Human Intervertebral Disc Degeneration. Arthritis Rheum. 2009, 60, 482–491. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, Y.; Jiang, S.; Li, H.; Jiang, L.; Dai, L. ADAMTS-5 and Intervertebral Disc Degeneration: The Results of Tissue Immunohistochemistry and In Vitro Cell Culture. J. Orthop. Res. 2011, 29, 718–725. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Gao, F.; Li, X.; An, Y.; Wang, J.; Jin, A. Resistin Promotes Intervertebral Disc Degeneration by Upregulation of ADAMTS-5 Through P38 MAPK Signaling Pathway. Spine 2016, 41, 1414–1420. [Google Scholar] [CrossRef]
- Deng, B.; Ren, J.Z.; Meng, X.Q.; Pang, C.G.; Duan, G.Q.; Zhang, J.X.; Zou, H.; Yang, H.Z.; Ji, J.J. Expression Profiles of MMP-1 and TIMP-1 in Lumbar Intervertebral Disc Degeneration. Genet. Mol. Res. 2015, 14, 19080–19086. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Han, X.; Mao, C.; Zhang, K.; Zhao, T.; Zhao, J. The Imbalance between TIMP3 and Matrix-Degrading Enzymes Plays an Important Role in Intervertebral Disc Degeneration. Biochem. Biophys. Res. Commun. 2016, 469, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.W.; Sze, K.L.; Samartzis, D.; Leung, V.Y.L.; Chan, D. Structure and Biology of the Intervertebral Disk in Health and Disease. Orthop. Clin. N. Am. 2011, 42, 447–464. [Google Scholar] [CrossRef]
- Xin, J.; Wang, Y.; Zheng, Z.; Wang, S.; Na, S.; Zhang, S. Treatment of Intervertebral Disc Degeneration. Orthop. Surg. 2022, 14, 1271–1280. [Google Scholar] [CrossRef]
- Lyu, F.-J.; Cui, H.; Pan, H.; MC Cheung, K.; Cao, X.; Iatridis, J.C.; Zheng, Z. Painful Intervertebral Disc Degeneration and Inflammation: From Laboratory Evidence to Clinical Interventions. Bone Res. 2021, 9, 7. [Google Scholar] [CrossRef]
- Mitchell, U.H.; Helgeson, K.; Mintken, P. Physiological Effects of Physical Therapy Interventions on Lumbar Intervertebral Discs: A Systematic Review. Physiother. Theory Prac. 2017, 33, 695–705. [Google Scholar] [CrossRef]
- Ogutluler Ozkara, G.; Ozgen, M.; Ozkara, E.; Armagan, O.; Arslantas, A.; Atasoy, M.A. Assesment of the Effectiveness of Physical Thraphy and Rehabilitation Programme Starts Immediately after Lumbar Disc Surgery. Turk. Neurosurg. 2015, 25, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Enthoven, W.T.; Roelofs, P.D.; Deyo, R.A.; van Tulder, M.W.; Koes, B.W. Non-Steroidal Anti-Inflammatory Drugs for Chronic Low Back Pain. Cochrane Database Syst. Rev. 2016, 2, CD012087. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, R.M.; Manchikanti, L.; Parr, A.T.; Diwan, S.; Singh, V.; Falco, F.J.E.; Datta, S.; Abdi, S.; Hirsch, J.A. The Effectiveness of Lumbar Interlaminar Epidural Injections in Managing Chronic Low Back and Lower Extremity Pain. Pain Physician 2012, 15, E363–E404. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Hashimoto, R.; Friedly, J.; Fu, R.; Bougatsos, C.; Dana, T.; Sullivan, S.D.; Jarvik, J. Epidural Corticosteroid Injections for Radiculopathy and Spinal Stenosis. Ann. Intern. Med. 2015, 163, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Blamoutier, A. Surgical Discectomy for Lumbar Disc Herniation: Surgical Techniques. Orthop. Traumatol. Surg. Res. 2013, 99, S187–S196. [Google Scholar] [CrossRef]
- Vangen-Lønne, V.; Madsbu, M.A.; Salvesen, Ø.; Nygaard, Ø.P.; Solberg, T.K.; Gulati, S. Microdiscectomy for Lumbar Disc Herniation: A Single-Center Observational Study. World Neurosurg. 2020, 137, e577–e583. [Google Scholar] [CrossRef]
- Buttermann, G.R. Anterior Cervical Discectomy and Fusion Outcomes over 10 Years. Spine 2018, 43, 207–214. [Google Scholar] [CrossRef]
- Gum, J.L.; Reddy, D.; Glassman, S. Transforaminal Lumbar Interbody Fusion (TLIF). JBJS Essent. Surg. Tech. 2016, 6, e22. [Google Scholar] [CrossRef]
- Rathbone, J.; Rackham, M.; Nielsen, D.; Lee, S.M.; Hing, W.; Riar, S.; Scott-Young, M. A Systematic Review of Anterior Lumbar Interbody Fusion (ALIF) versus Posterior Lumbar Interbody Fusion (PLIF), Transforaminal Lumbar Interbody Fusion (TLIF), Posterolateral Lumbar Fusion (PLF). Eur. Spine J. 2023, 32, 1911–1926. [Google Scholar] [CrossRef]
- Othman, Y.A.; Verma, R.; Qureshi, S.A. Artificial Disc Replacement in Spine Surgery. Ann. Transl. Med. 2019, 7, S170. [Google Scholar] [CrossRef]
- Eskandar, T.; Ahmed, Z.; Pan, J.K.; Agrawal, D. The Decline of Lumbar Artificial Disc Replacement. J. Spine Res. Surg. 2024, 6, 86–92. [Google Scholar] [CrossRef]
- Drossopoulos, P.N.; Ononogbu-uche, F.C.; Tabarestani, T.Q.; Huang, C.-C.; Paturu, M.; Bardeesi, A.; Ray, W.Z.; Shaffrey, C.I.; Goodwin, C.R.; Erickson, M.; et al. Evolution of the Transforaminal Lumbar Interbody Fusion (TLIF): From Open to Percutaneous to Patient-Specific. J. Clin. Med. 2024, 13, 2271. [Google Scholar] [CrossRef]
- Butler, A.J.; Alam, M.; Wiley, K.; Ghasem, A.; Rush III, A.J.; Wang, J.C. Endoscopic Lumbar Surgery: The State of the Art in 2019. Neurospine 2019, 16, 15–23. [Google Scholar] [CrossRef]
- Munda, M.; Velnar, T. Stem Cell Therapy for Degenerative Disc Disease: Bridging the Gap between Preclinical Promise and Clinical Potential. Biomol. Biomed. 2024, 24, 210–218. [Google Scholar] [CrossRef]
- Richardson, S.M.; Kalamegam, G.; Pushparaj, P.N.; Matta, C.; Memic, A.; Khademhosseini, A.; Mobasheri, R.; Poletti, F.L.; Hoyland, J.A.; Mobasheri, A. Mesenchymal Stem Cells in Regenerative Medicine: Focus on Articular Cartilage and Intervertebral Disc Regeneration. Methods 2016, 99, 69–80. [Google Scholar] [CrossRef]
- Urits, I.; Capuco, A.; Sharma, M.; Kaye, A.D.; Viswanath, O.; Cornett, E.M.; Orhurhu, V. Stem Cell Therapies for Treatment of Discogenic Low Back Pain: A Comprehensive Review. Curr. Pain Headache Rep. 2019, 23, 65. [Google Scholar] [CrossRef]
- Sakai, D.; Mochida, J.; Iwashina, T.; Watanabe, T.; Nakai, T.; Ando, K.; Hotta, T. Differentiation of Mesenchymal Stem Cells Transplanted to a Rabbit Degenerative Disc Model. Spine 2005, 30, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Crevensten, G.; Walsh, A.J.L.; Ananthakrishnan, D.; Page, P.; Wahba, G.M.; Lotz, J.C.; Berven, S. Intervertebral Disc Cell Therapy for Regeneration: Mesenchymal Stem Cell Implantation in Rat Intervertebral Discs. Ann. Biomed. Eng. 2004, 32, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; McConnell, J.C.; Sherratt, M.J.; Derby, B.; Richardson, S.M.; Hoyland, J.A. Growth Differentiation Factor 6 and Transforming Growth Factor-Beta Differentially Mediate Mesenchymal Stem Cell Differentiation, Composition, and Micromechanical Properties of Nucleus Pulposus Constructs. Arthritis Res. Ther. 2014, 16, R67. [Google Scholar] [CrossRef]
- Miguélez-Rivera, L.; Pérez-Castrillo, S.; González-Fernández, M.L.; Prieto-Fernández, J.G.; López-González, M.E.; García-Cosamalón, J.; Villar-Suárez, V. Immunomodulation of Mesenchymal Stem Cells in Discogenic Pain. Spine J. 2018, 18, 330–342. [Google Scholar] [CrossRef]
- Teixeira, G.Q.; Pereira, C.L.; Ferreira, J.R.; Maia, A.F.; Gomez-Lazaro, M.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Immunomodulation of Human Mesenchymal Stem/Stromal Cells in Intervertebral Disc Degeneration. Spine 2018, 43, E673–E682. [Google Scholar] [CrossRef]
- Steffen, F.; Smolders, L.A.; Roentgen, A.M.; Bertolo, A.; Stoyanov, J. Bone Marrow-Derived Mesenchymal Stem Cells as Autologous Therapy in Dogs with Naturally Occurring Intervertebral Disc Disease: Feasibility, Safety, and Preliminary Results. Tissue Eng. Part. C Methods 2017, 23, 643–651. [Google Scholar] [CrossRef]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sánchez, A.; García-Sancho, J. Intervertebral Disc Repair by Autologous Mesenchymal Bone Marrow Cells: A Pilot Study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Pettine, K.A.; Murphy, M.B.; Suzuki, R.K.; Sand, T.T. Percutaneous Injection of Autologous Bone Marrow Concentrate Cells Significantly Reduces Lumbar Discogenic Pain Through 12 Months. Stem Cells 2015, 33, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Williams, C.J.; Hyzy, M.; Ichim, T.; Freeman, M. Treatment of Lumbar Degenerative Disc Disease-Associated Radicular Pain with Culture-Expanded Autologous Mesenchymal Stem Cells: A Pilot Study on Safety and Efficacy. J. Transl. Med. 2017, 15, 197. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Ueda, Y.; Miyazaki, K.; Koizumi, M.; Takakura, Y. Disc Regeneration Therapy Using Marrow Mesenchymal Cell Transplantation. Spine 2010, 35, E475–E480. [Google Scholar] [CrossRef] [PubMed]
- Elabd, C.; Centeno, C.J.; Schultz, J.R.; Lutz, G.; Ichim, T.; Silva, F.J. Intra-Discal Injection of Autologous, Hypoxic Cultured Bone Marrow-Derived Mesenchymal Stem Cells in Five Patients with Chronic Lower Back Pain: A Long-Term Safety and Feasibility Study. J. Transl. Med. 2016, 14, 253. [Google Scholar] [CrossRef]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef]
- Wuertz, K.; Godburn, K.; Neidlinger-Wilke, C.; Urban, J.; Iatridis, J.C. Behavior of Mesenchymal Stem Cells in the Chemical Microenvironment of the Intervertebral Disc. Spine 2008, 33, 1843–1849. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Zhu, D.; Yang, F.; Wang, Z.; Wei, Z.; Yang, Z.; Jia, J.; Kang, X. Notochordal Cells: A Potential Therapeutic Option for Intervertebral Disc Degeneration. Cell Prolif. 2024, 57, e13541. [Google Scholar] [CrossRef]
- Bach, F.C.; Poramba-Liyanage, D.W.; Riemers, F.M.; Guicheux, J.; Camus, A.; Iatridis, J.C.; Chan, D.; Ito, K.; Le Maitre, C.L.; Tryfonidou, M.A. Notochordal Cell-Based Treatment Strategies and Their Potential in Intervertebral Disc Regeneration. Front. Cell Dev. Biol. 2022, 9, 780749. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Shen, B.; Diwan, A.; Hoyland, J.A.; Richardson, S.M. Therapeutic Potential of Growth Differentiation Factors in the Treatment of Degenerative Disc Diseases. JOR Spine 2019, 2, e1045. [Google Scholar] [CrossRef]
- Wei, A.; Williams, L.A.; Bhargav, D.; Shen, B.; Kishen, T.; Duffy, N.; Diwan, A.D. BMP13 Prevents the Effects of Annular Injury in an Ovine Model. Int. J. Biol. Sci. 2009, 5, 388–396. [Google Scholar] [CrossRef]
- Miyazaki, S.; Diwan, A.D.; Kato, K.; Cheng, K.; Bae, W.C.; Sun, Y.; Yamada, J.; Muehleman, C.; Lenz, M.E.; Inoue, N.; et al. ISSLS PRIZE IN BASIC SCIENCE 2018: Growth Differentiation Factor-6 Attenuated pro-Inflammatory Molecular Changes in the Rabbit Anular-Puncture Model and Degenerated Disc-Induced Pain Generation in the Rat Xenograft Radiculopathy Model. Eur. Spine J. 2018, 27, 739–751. [Google Scholar] [CrossRef]
- Walsh, A.J.L.; Bradford, D.S.; Lotz, J.C. In Vivo Growth Factor Treatment of Degenerated Intervertebral Discs. Spine 2004, 29, 156–163. [Google Scholar] [CrossRef]
- Heckmann, L.; Fiedler, J.; Mattes, T.; Dauner, M.; Brenner, R.E. Interactive Effects of Growth Factors and Three-dimensional Scaffolds on Multipotent Mesenchymal Stromal Cells. Biotechnol. Appl. Biochem. 2008, 49, 185–194. [Google Scholar] [CrossRef]
- Gulati, T.; Chung, S.A.; Wei, A.; Diwan, A.D. Localization of Bone Morphogenetic Protein 13 in Human Intervertebral Disc and Its Molecular and Functional Effects in Vitro in 3D Culture. J. Orthop. Res. 2015, 33, 1769–1775. [Google Scholar] [CrossRef]
- Zhou, F.Y.; Wei, A.-Q.; Shen, B.; Williams, L.; Diwan, A.D. Cartilage Derived Morphogenetic Protein-2 Induces Cell Migration and Its Chondrogenic Potential in C28/I2 Cells. Int. J. Spine Surg. 2015, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of Growth Differentiation Factor-5 on the Intervertebral Disc−In Vitro Bovine Study and In Vivo Rabbit Disc Degeneration Model Study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Kim, H.S.; Jang, I.-T. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int. J. Mol. Sci. 2020, 21, 2135. [Google Scholar] [CrossRef] [PubMed]
- Romaniyanto; Mahyudin, F.; Sigit Prakoeswa, C.R.; Notobroto, H.B.; Tinduh, D.; Ausrin, R.; Rantam, F.A.; Suroto, H. An Update of Current Therapeutic Approach for Intervertebral Disc Degeneration: A Review Article. Ann. Med. Surg. 2022, 77, 103619. [Google Scholar] [CrossRef]
- Samanta, A.; Lufkin, T.; Kraus, P. Intervertebral Disc Degeneration—Current Therapeutic Options and Challenges. Front. Public. Health 2023, 11, 1156749. [Google Scholar] [CrossRef]
- Yokozeki, Y.; Uchida, K.; Kawakubo, A.; Nakawaki, M.; Okubo, T.; Miyagi, M.; Inoue, G.; Itakura, M.; Sekiguchi, H.; Takaso, M. TGF-β Regulates Nerve Growth Factor Expression in a Mouse Intervertebral Disc Injury Model. BMC Musculoskelet. Disord. 2021, 22, 634. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.; Moore, C.A.; Kim, K.; Karim, A.; Smith, K.; Barbosa, Z.; Van Eps, J.; Rameshwar, P.; Weiner, B. Novel Therapeutic Strategies for Degenerative Disc Disease: Review of Cell Biology and Intervertebral Disc Cell Therapy. SAGE Open Med. 2018, 6, 2050312118761674. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, S.; Shi, Y.; Tian, N.; Zhou, Y.; Zhang, X. Senolytics: Eliminating Senescent Cells and Alleviating Intervertebral Disc Degeneration. Front. Bioeng. Biotechnol. 2022, 10, 823945. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Wang, B.; Shi, Y.; Xie, C.; Huang, C.; Chen, B.; Zhang, H.; Zeng, G.; Liang, H.; Wu, Y.; et al. Senolytic Agent Quercetin Ameliorates Intervertebral Disc Degeneration via the Nrf2/NF-ΚB Axis. Osteoarthr. Cartil. 2021, 29, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, C.; Chen, H.; Duan, X.; Li, J.; Zhou, Y.; Zeng, W.; Yang, L. Navitoclax (ABT263) Reduces Inflammation and Promotes Chondrogenic Phenotype by Clearing Senescent Osteoarthritic Chondrocytes in Osteoarthritis. Aging 2020, 12, 12750–12770. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; d’Adda di Fagagna, F. Cellular Senescence in Ageing: From Mechanisms to Therapeutic Opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Cherif, H.; Bisson, D.; Jarzem, P.; Weber, M.; Ouellet, J.; Haglund, L. Curcumin and O-Vanillin Exhibit Evidence of Senolytic Activity in Human IVD Cells In Vitro. J. Clin. Med. 2019, 8, 433. [Google Scholar] [CrossRef]
- Chong, S.J.F.; Marchi, S.; Petroni, G.; Kroemer, G.; Galluzzi, L.; Pervaiz, S. Noncanonical Cell Fate Regulation by Bcl-2 Proteins. Trends Cell Biol. 2020, 30, 537–555. [Google Scholar] [CrossRef]
- Novais, E.J.; Tran, V.A.; Johnston, S.N.; Darris, K.R.; Roupas, A.J.; Sessions, G.A.; Shapiro, I.M.; Diekman, B.O.; Risbud, M.V. Long-Term Treatment with Senolytic Drugs Dasatinib and Quercetin Ameliorates Age-Dependent Intervertebral Disc Degeneration in Mice. Nat. Commun. 2021, 12, 5213. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin Is a Senotherapeutic That Extends Health and Lifespan. EBioMedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Lim, S.; An, S.B.; Jung, M.; Joshi, H.P.; Kumar, H.; Kim, C.; Song, S.Y.; Lee, J.; Kang, M.; Han, I.; et al. Local Delivery of Senolytic Drug Inhibits Intervertebral Disc Degeneration and Restores Intervertebral Disc Structure. Adv. Healthc. Mater. 2022, 11, e2101483. [Google Scholar] [CrossRef]
- Liu, P.; Feng, Y.; Li, H.; Chen, X.; Wang, G.; Xu, S.; Li, Y.; Zhao, L. Ferrostatin-1 Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Inhibiting Ferroptosis. Cell Mol. Biol. Lett. 2020, 25, 10. [Google Scholar] [CrossRef]
- Skouta, R.; Dixon, S.J.; Wang, J.; Dunn, D.E.; Orman, M.; Shimada, K.; Rosenberg, P.A.; Lo, D.C.; Weinberg, J.M.; Linkermann, A.; et al. Ferrostatins Inhibit Oxidative Lipid Damage and Cell Death in Diverse Disease Models. J. Am. Chem. Soc. 2014, 136, 4551–4556. [Google Scholar] [CrossRef]
- Li, X.; Duan, L.; Yuan, S.; Zhuang, X.; Qiao, T.; He, J. Ferroptosis Inhibitor Alleviates Radiation-Induced Lung Fibrosis (RILF) via down-Regulation of TGF-Β1. J. Inflamm. 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues de Morais, T.; Gambero, A. Iron Chelators in Obesity Therapy—Old Drugs from a New Perspective? Eur. J. Pharmacol. 2019, 861, 172614. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Y.; Li, W.; Ward, R.; Abdelsaid, M.; Hafez, S.; Dong, G.; Jamil, S.; Wolf, V.; Johnson, M.H.; Fagan, S.C.; et al. Deferoxamine Treatment Prevents Post-Stroke Vasoregression and Neurovascular Unit Remodeling Leading to Improved Functional Outcomes in Type 2 Male Diabetic Rats: Role of Endothelial Ferroptosis. Transl. Stroke Res. 2021, 12, 615–630. [Google Scholar] [CrossRef]
- Jomen, W.; Ohtake, T.; Akita, T.; Suto, D.; Yagi, H.; Osawa, Y.; Kohgo, Y. Iron Chelator Deferasirox Inhibits NF-ΚB Activity in Hepatoma Cells and Changes Sorafenib-Induced Programmed Cell Deaths. Biomed. Pharmacother. 2022, 153, 113363. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin Alleviates Acute Kidney Injury by Inhibiting Ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; SteMarie, E.J.; et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell 2019, 177, 1262–1279.e25. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of Ferroptotic Cancer Cell Death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef]
- Mishima, E.; Sato, E.; Ito, J.; Yamada, K.; Suzuki, C.; Oikawa, Y.; Matsuhashi, T.; Kikuchi, K.; Toyohara, T.; Suzuki, T.; et al. Drugs Repurposed as Antiferroptosis Agents Suppress Organ Damage, Including AKI, by Functioning as Lipid Peroxyl Radical Scavengers. J. Am. Soc. Nephrol. 2020, 31, 280–296. [Google Scholar] [CrossRef]
- Roh, E.; Darai, A.; Kyung, J.; Choi, H.; Kwon, S.; Bhujel, B.; Kim, K.; Han, I. Genetic Therapy for Intervertebral Disc Degeneration. Int. J. Mol. Sci. 2021, 22, 1579. [Google Scholar] [CrossRef]
- Wehling, P.; Schulitz, K.-P.; Robbins, P.D.; Evans, C.H.; Reinecke, J.A. Transfer of Genes to Chondrocytic Cells of the Lumbar Spine. Spine 1997, 22, 1092–1097. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L.; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral Vectors for Gene Therapy: Translational and Clinical Outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, S.; Huang, H.; Fang, J.; Wei, H.; Xi, Y. In Vivo Delivery of MMP3-ShRNA and Sox9 Lentivirus Cocktail Enhances Matrix Synthesis to Prevent Lumbar Disc Degeneration. Adv. Clin. Exp. Med. 2020, 29, 639–647. [Google Scholar] [CrossRef]
- Seki, S.; Asanuma-Abe, Y.; Masuda, K.; Kawaguchi, Y.; Asanuma, K.; Muehleman, C.; Iwai, A.; Kimura, T. Effect of Small Interference RNA (SiRNA) for ADAMTS5on Intervertebral Disc Degeneration in the Rabbit Anular Needle-Puncture Model. Arthritis Res. Ther. 2009, 11, R166. [Google Scholar] [CrossRef] [PubMed]
- Bi, F.; Liu, W.; Wu, Z.; Ji, C.; Chang, C. Antiaging Factor Klotho Retards the Progress of Intervertebral Disc Degeneration through the Toll-Like Receptor 4-NF- κ B Pathway. Int. J. Cell Biol. 2020, 2020, 8319516. [Google Scholar] [CrossRef]
- Nishida, K.; Doita, M.; Takada, T.; Kakutani, K.; Miyamoto, H.; Shimomura, T.; Maeno, K.; Kurosaka, M. Sustained Transgene Expression in Intervertebral Disc Cells In Vivo Mediated by Microbubble-Enhanced Ultrasound Gene Therapy. Spine 2006, 31, 1415–1419. [Google Scholar] [CrossRef]
- Feng, G.; Zha, Z.; Huang, Y.; Li, J.; Wang, Y.; Ke, W.; Chen, H.; Liu, L.; Song, Y.; Ge, Z. Sustained and Bioresponsive Two-Stage Delivery of Therapeutic MiRNA via Polyplex Micelle-Loaded Injectable Hydrogels for Inhibition of Intervertebral Disc Fibrosis. Adv. Healthc. Mater. 2018, 7, e1800623. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zha, Z.; Li, H.; Toh, K.; Dirisala, A.; Matsumoto, Y.; Osada, K.; Kataoka, K.; Ge, Z. Ternary Polyplex Micelles with PEG Shells and Intermediate Barrier to Complexed DNA Cores for Efficient Systemic Gene Delivery. J. Control Release 2015, 209, 77–87. [Google Scholar] [CrossRef]
- Feng, G.; Chen, H.; Li, J.; Huang, Q.; Gupte, M.J.; Liu, H.; Song, Y.; Ge, Z. Gene Therapy for Nucleus Pulposus Regeneraaon by Heme Oxygenase-1 Plasmid DNA Carried by Mixed Polyplex Micelles with Thermo-Responsive Heterogeneous Coronas. Biomaterials 2015, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Cambria, E.; Arlt, M.J.E.; Wandel, S.; Krupkova, O.; Hitzl, W.; Passini, F.S.; Hausmann, O.N.; Snedeker, J.G.; Ferguson, S.J.; Wuertz-Kozak, K. TRPV4 Inhibition and CRISPR-Cas9 Knockout Reduce Inflammation Induced by Hyperphysiological Stretching in Human Annulus Fibrosus Cells. Cells 2020, 9, 1736. [Google Scholar] [CrossRef] [PubMed]
- Farhang, N.; Brunger, J.M.; Stover, J.D.; Thakore, P.I.; Lawrence, B.; Guilak, F.; Gersbach, C.A.; Setton, L.A.; Bowles, R.D. CRISPR-Based Epigenome Editing of Cytokine Receptors for the Promotion of Cell Survival and Tissue Deposition in Inflammatory Environments. Tissue Eng. Part A 2017, 23, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.D.; Farhang, N.; Berrett, K.C.; Gertz, J.; Lawrence, B.; Bowles, R.D. CRISPR Epigenome Editing of AKAP150 in DRG Neurons Abolishes Degenerative IVD-Induced Neuronal Activation. Mol. Ther. 2017, 25, 2014–2027. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yurube, T.; Kakutani, K.; Maeno, K.; Takada, T.; Terashima, Y.; Kakiuchi, Y.; Takeoka, Y.; Miyazaki, S.; Kuroda, R.; et al. Selective Interference of MTORC1/RAPTOR Protects against Human Disc Cellular Apoptosis, Senescence, and Extracellular Matrix Catabolism with Akt and Autophagy Induction. Osteoarthr. Cartil. 2017, 25, 2134–2146. [Google Scholar] [CrossRef]
- Kamali, A.; Ziadlou, R.; Lang, G.; Pfannkuche, J.; Cui, S.; Li, Z.; Richards, R.G.; Alini, M.; Grad, S. Small Molecule-Based Treatment Approaches for Intervertebral Disc Degeneration: Current Options and Future Directions. Theranostics 2021, 11, 27–47. [Google Scholar] [CrossRef]
- Ouyang, Z.-H.; Wang, W.-J.; Yan, Y.-G.; Wang, B.; Lv, G.-H. The PI3K/Akt Pathway: A Critical Player in Intervertebral Disc Degeneration. Oncotarget 2017, 8, 57870–57881. [Google Scholar] [CrossRef]
- Desai, S.U.; Srinivasan, S.S.; Kumbar, S.G.; Moss, I.L. Hydrogel-Based Strategies for Intervertebral Disc Regeneration: Advances, Challenges and Clinical Prospects. Gels 2024, 10, 62. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, Characterization, and Applications: A Review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Ligorio, C.; Hoyland, J.A.; Saiani, A. Self-Assembling Peptide Hydrogels as Functional Tools to Tackle Intervertebral Disc Degeneration. Gels 2022, 8, 211. [Google Scholar] [CrossRef]
- Yan, C.; Wang, X.; Xiang, C.; Wang, Y.; Pu, C.; Chen, L.; Jiang, K.; Li, Y. Applications of Functionalized Hydrogels in the Regeneration of the Intervertebral Disc. Biomed. Res. Int. 2021, 2021, 2818624. [Google Scholar] [CrossRef]
- Ying, Y.; Cai, K.; Cai, X.; Zhang, K.; Qiu, R.; Jiang, G.; Luo, K. Recent Advances in the Repair of Degenerative Intervertebral Disc for Preclinical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1259731. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.B.; Baranwal, G.; Vijayan, P.; Keyan, K.S.; Jayakumar, R. Composite Hydrogel of Chitosan–Poly(Hydroxybutyrate- Co -Valerate) with Chondroitin Sulfate Nanoparticles for Nucleus Pulposus Tissue Engineering. Colloids Surf. B Biointerfaces 2015, 136, 84–92. [Google Scholar] [CrossRef]
- Priyadarshani, P.; Li, Y.; Yao, L. Advances in Biological Therapy for Nucleus Pulposus Regeneration. Osteoarthr. Cartil. 2016, 24, 206–212. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Peng, P.; Cheng, R.; Lin, J.; Xu, C.; Yang, H.; Cui, W.; Mao, H.; Li, Y.; et al. Regulation of the Inflammatory Cycle by a Controllable Release Hydrogel for Eliminating Postoperative Inflammation after Discectomy. Bioact. Mater. 2021, 6, 146–157. [Google Scholar] [CrossRef]
- Chen, W.; Chen, H.; Zheng, D.; Zhang, H.; Deng, L.; Cui, W.; Zhang, Y.; Santos, H.A.; Shen, H. Gene-Hydrogel Microenvironment Regulates Extracellular Matrix Metabolism Balance in Nucleus Pulposus. Adv. Sci. 2020, 7, 1902099. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Qian, Q.; Makvandi, P.; Zare, E.N.; Chen, Q.; Chen, L.; Zhang, Z.; Zhou, H.; Zhou, W.; Wang, H.; et al. Engineered High-Strength Biohydrogel as a Multifunctional Platform to Deliver Nucleic Acid for Ameliorating Intervertebral Disc Degeneration. Bioact. Mater. 2023, 25, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, C.; Jin, S.; Ye, Y.; Fang, Y.; Xu, P.; Zhang, C. Salvianolic Acid B Combined with Bone Marrow Mesenchymal Stem Cells Piggybacked on HAMA Hydrogel Re-Transplantation Improves Intervertebral Disc Degeneration. Front. Bioeng. Biotechnol. 2022, 10, 950625. [Google Scholar] [CrossRef]
- Qingxin, S.; Kai, J.; Dandan, Z.; Linyu, J.; Xiuyuan, C.; Yubo, F.; Kun, W.; Yingchao, H.; Hao, C.; Jie, S.; et al. Programmable DNA Hydrogel Provides Suitable Microenvironment for Enhancing Autophagy-Based Therapies in Intervertebral Disc Degeneration Treatment. J. Nanobiotechnol. 2023, 21, 350. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, H.; Xia, J.; Zhu, Y.; Xia, C.; Hu, Z.; Jin, Y.; Wang, J.; He, Y.; Dai, J.; et al. High-Performance Multi-Dynamic Bond Cross-Linked Hydrogel with Spatiotemporal SiRNA Delivery for Gene–Cell Combination Therapy of Intervertebral Disc Degeneration. Adv. Sci. 2023, 10, e2206306. [Google Scholar] [CrossRef]
- Li, P.; Zhang, M.; Chen, Z.; Tian, B.; Kang, X. Tissue-Engineered Injectable Gelatin–Methacryloyl Hydrogel-Based Adjunctive Therapy for Intervertebral Disc Degeneration. ACS Omega 2023, 8, 13509–13518. [Google Scholar] [CrossRef]
- Dixon, D.T.; Gomillion, C.T. Conductive Scaffolds for Bone Tissue Engineering: Current State and Future Outlook. J. Funct. Biomater. 2021, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- van Uden, S.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Current Strategies for Treatment of Intervertebral Disc Degeneration: Substitution and Regeneration Possibilities. Biomater. Res. 2017, 21, 22. [Google Scholar] [CrossRef]
- Ding, Y.; Li, F.; Wang, Y.; Pan, W.; Fu, X.; Tan, S. Nanomedicine Approaches for Intervertebral Disc Regeneration: From Bench to Bedside. Pharmaceutics 2025, 17, 313. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What Low Back Pain Is and Why We Need to Pay Attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Sousa, M.J.; Vlieghe, H.; Yang, J.; León-Félix, C.M.; Amorim, C.A. Extracellular Vesicles in Nanomedicine and Regenerative Medicine: A Review over the Last Decade. Bioact. Mater. 2024, 36, 126–156. [Google Scholar] [CrossRef]
- Yang, W.; Li, K.; Pan, Q.; Huang, W.; Xiao, Y.; Lin, H.; Liu, S.; Chen, X.; Lv, X.; Feng, S.; et al. An Engineered Bionic Nanoparticle Sponge as a Cytokine Trap and Reactive Oxygen Species Scavenger to Relieve Disc Degeneration and Discogenic Pain. ACS Nano 2024, 18, 3053–3072. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ding, Y.; Zhang, W.; Wei, K.; Pei, Y.; Zou, C.; Zhang, C.; Ding, J.; Fang, H.; Tan, S. Oxymatrine Liposomes for Intervertebral Disc Treatment: Formulation, in Vitro and Vivo Assessments. Drug Des. Devel Ther. 2020, 14, 921–931. [Google Scholar] [CrossRef]
- Xiao, L.; Huang, R.; Zhang, Y.; Li, T.; Dai, J.; Nannapuneni, N.; Chastanet, T.R.; Chen, M.; Shen, F.H.; Jin, L.; et al. A New Formyl Peptide Receptor-1 Antagonist Conjugated Fullerene Nanoparticle for Targeted Treatment of Degenerative Disc Diseases. ACS Appl. Mater. Interfaces 2019, 11, 38405–38416. [Google Scholar] [CrossRef]
- Nguyen, K.; Pan, H.-Y.; Haworth, K.; Mahoney, E.; Mercado-Shekhar, K.P.; Lin, C.-Y.; Zhang, Z.C.; Park, Y. Multiple-Exposure Drug Release from Stable Nanodroplets by High-Intensity Focused Ultrasound for a Potential Degenerative Disc Disease Treatment. Ultrasound Med. Biol. 2019, 45, 160–169. [Google Scholar] [CrossRef]
- Shen, J.; Zhuo, N.; Xu, S.; Song, Z.; Hu, Z.; Hao, J.; Guo, X. Resveratrol Delivery By Ultrasound-Mediated Nanobubbles Targeting Nucleus Pulposus Cells. Nanomedicine 2018, 13, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Lian, M.; Han, Y.; Mo, X.; Jiang, W.; Qiao, Z.; Dai, K. A 3D-Bioprinted Dual Growth Factor-Releasing Intervertebral Disc Scaffold Induces Nucleus Pulposus and Annulus Fibrosus Reconstruction. Bioact. Mater. 2021, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Pereira, C.L.; Teixeira, G.Q.; Silva, R.V.; Caldeira, J.; Grad, S.; Gonçalves, R.M.; Barbosa, M.A. Poly(γ-Glutamic Acid) and Poly(γ-Glutamic Acid)-Based Nanocomplexes Enhance Type II Collagen Production in Intervertebral Disc. J. Mater. Sci. Mater. Med. 2017, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, Z.; Dang, M.; Zhang, X.; Doleyres, Y.; Song, Y.; Chen, D.; Ma, P.X. Injectable Nanofibrous Spongy Microspheres for NR4A1 Plasmid DNA Transfection to Reverse Fibrotic Degeneration and Support Disc Regeneration. Biomaterials 2017, 131, 86–97. [Google Scholar] [CrossRef]


| Cytokine/Chemokine | Source (Cells) | Expression in IVDD | Primary Role in IVDD | References |
|---|---|---|---|---|
| IL-1β |
|
|
| [94,95,96,98,99,100,101,103,106] |
| TNF-α |
|
|
| [94,95,96,97,98,101,103,105,106] |
| IL-6 |
|
|
| [95,96,98,101,102,103,104,106] |
| IL-8 |
|
|
| [95,98,101,103,104] |
| IL-17 |
|
|
| [95,96,98,105] |
| IFN-γ |
|
|
| [96,104] |
| IL-4 |
|
|
| [96,103] |
| CCL-3 |
|
|
| [95,98,104] |
| CCL-5 |
|
|
| [95,96,98,99,103] |
| β-NGF |
|
|
| [96,104] |
| MMP | Major Matrix Substrates | Primary Role in IVDD | Key Features | References |
|---|---|---|---|---|
| MMP-1 (Collagenase-1) | Intact interstitial collagen (type I-III, VII, X), aggrecan | Initiates collagen degradation |
| [122,123,124,125,126] |
| MMP-2 (Gelatinase A) | Denatured collagen of the basement membrane (collagen type IV-VI, X), gelatin, elastin, fibronectin | Degrades denatured collagens |
| [123,124,126] |
| MMP-3 (Stromelysin-1) | Collagen (type II, IV, IX), proteoglycans, elastin, fibronectin, aggrecan, laminin | Broad ECM degradation and MMP activation |
| [122,123,126,127] |
| MMP-7 (Matrilysin) | Gelatin (type I, II, IV, V), fibronectin, proteoglycans, aggrecan, collagen (type II, IV–X) | Extensive matrix remodeling |
| [126,129] |
| MMP-8 (Neutrophil collagenase) | Collagen (type I–III) | Collagen degradation |
| [127] |
| MMP-9 (Gelatinase B) | Gelatin, denatured collagen (type IV, V, VII, X, XIV), aggrecan, elastin, fibrillin, osteonectin | Basement membrane and ECM degradation |
| [126] |
| MMP-10 | Collagen (type III-V), gelatin, aggrecan, elastin | ECM degradation |
| [126] |
| MMP-12 (Metalloelastase) | Aggrecan, elastin, collagen (type I, IV), gelatin, fibronectin, laminin, vitronectin, proteoglycan | ECM degradation |
| [126] |
| MMP-13 (Collagenase-3) | Fibrillar collagens (esp. type II, also I and III) | Late-stage ECM degradation |
| [121,122,125,126] |
| MMP-14 (MT1-MMP) | Collagen (type I-III), gelatin, fibronectin, laminin, aggrecan | Cell-surface protease activator |
| [124,126] |
| MMP-19 | Basement membrane collagen type IV, gelatin type I, aggrecan, fibronectin, laminin | Inhibits angiogenesis |
| [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ząbek, Z.; Wyczałkowska-Tomasik, A.; Poboży, K.; Adamus, J.P.; Turek, G.; Ząbek, M.; Pączek, L. Understanding the Microenvironment of Intervertebral Disc Degeneration: A Comprehensive Review of Pathophysiological Insights and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 9938. https://doi.org/10.3390/ijms26209938
Ząbek Z, Wyczałkowska-Tomasik A, Poboży K, Adamus JP, Turek G, Ząbek M, Pączek L. Understanding the Microenvironment of Intervertebral Disc Degeneration: A Comprehensive Review of Pathophysiological Insights and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(20):9938. https://doi.org/10.3390/ijms26209938
Chicago/Turabian StyleZąbek, Zuzanna, Aleksandra Wyczałkowska-Tomasik, Kamil Poboży, Jakub Piotr Adamus, Grzegorz Turek, Mirosław Ząbek, and Leszek Pączek. 2025. "Understanding the Microenvironment of Intervertebral Disc Degeneration: A Comprehensive Review of Pathophysiological Insights and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 20: 9938. https://doi.org/10.3390/ijms26209938
APA StyleZąbek, Z., Wyczałkowska-Tomasik, A., Poboży, K., Adamus, J. P., Turek, G., Ząbek, M., & Pączek, L. (2025). Understanding the Microenvironment of Intervertebral Disc Degeneration: A Comprehensive Review of Pathophysiological Insights and Therapeutic Implications. International Journal of Molecular Sciences, 26(20), 9938. https://doi.org/10.3390/ijms26209938







