Effect of Extracellular Matrix Derived from Porcine Tissue on Stemness of Porcine Spermatogonial Stem Cells
Abstract
1. Introduction
2. Results
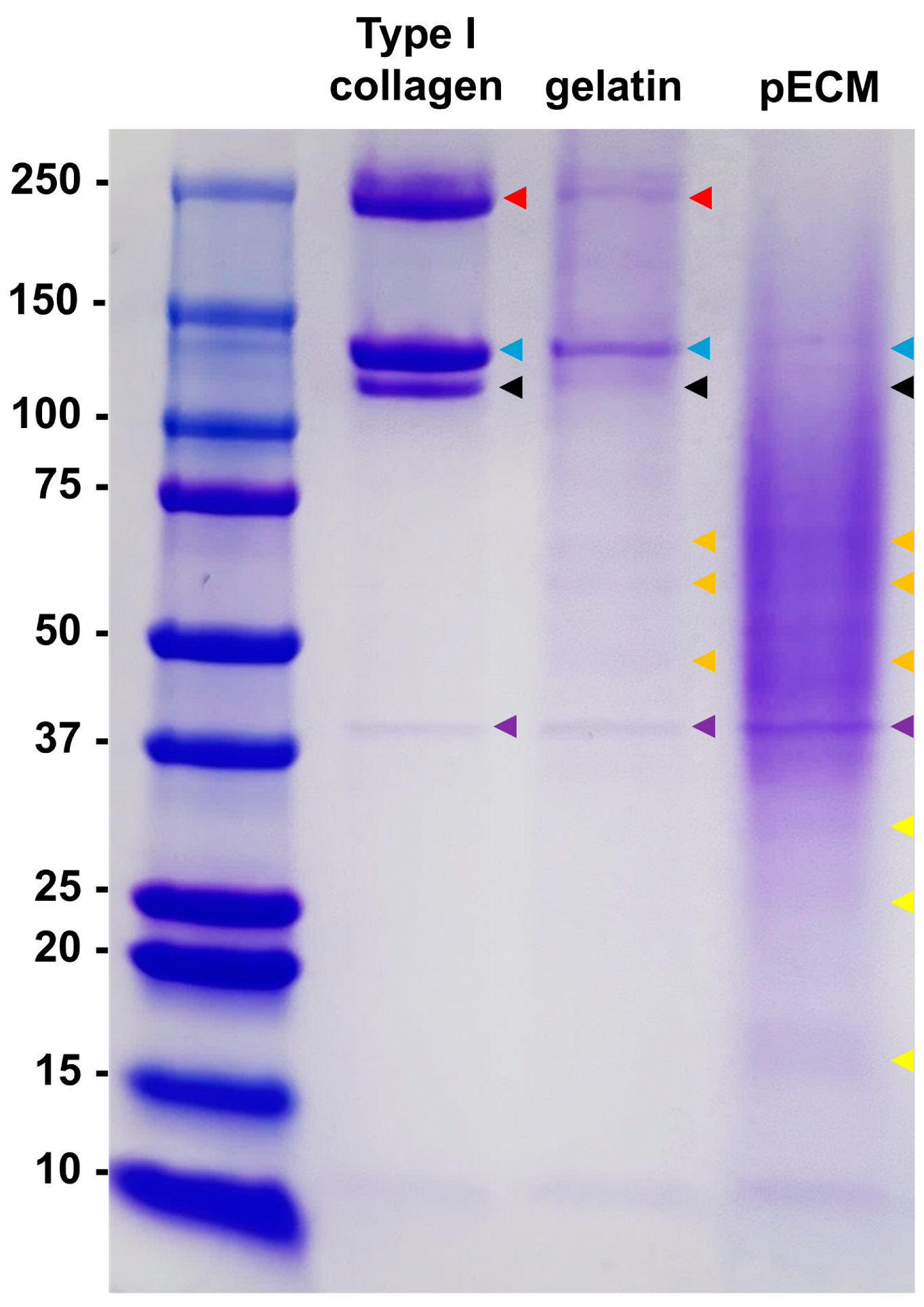
2.1. Molecular Weight Distribution and Composition of pECM
2.2. Surface Roughness of Coating Materials
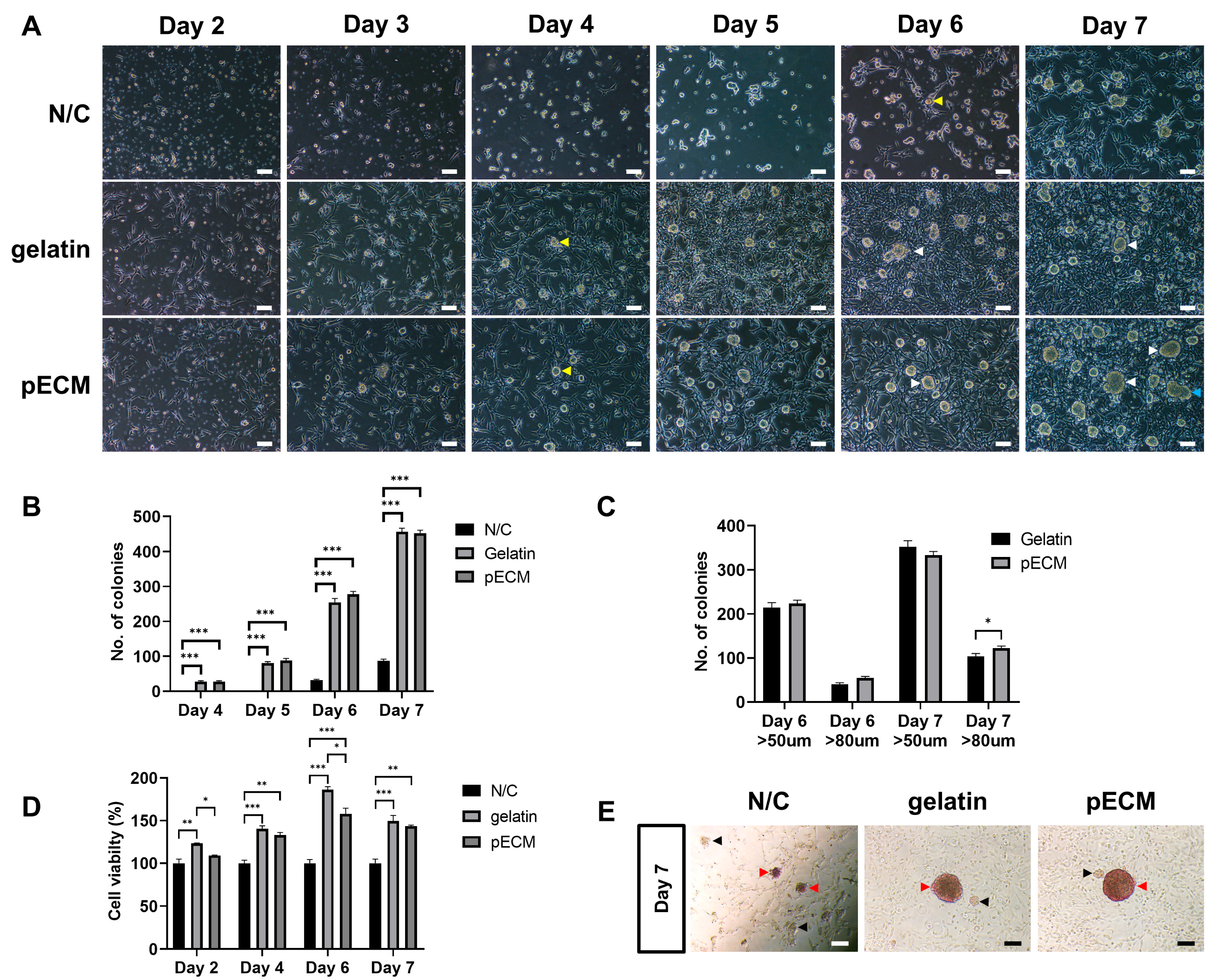
2.3. Comparison of Morphology, Proliferation, and Alkaline Phosphatase (AP) Activity of Cells Under Different Coating Conditions
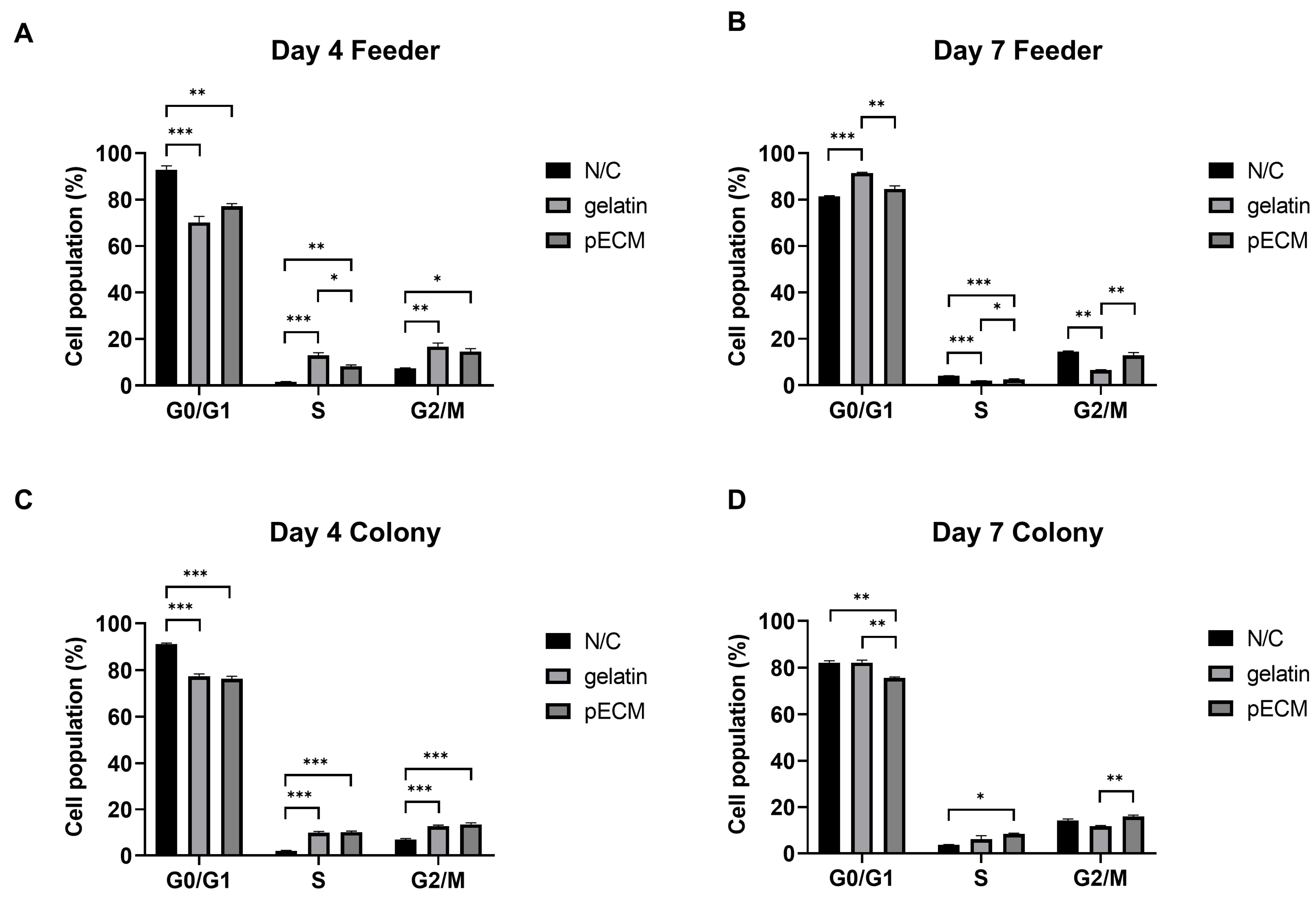
2.4. Cell Cycle Analysis of pSSC Colonies and pFeeders Under Different Coating Conditions
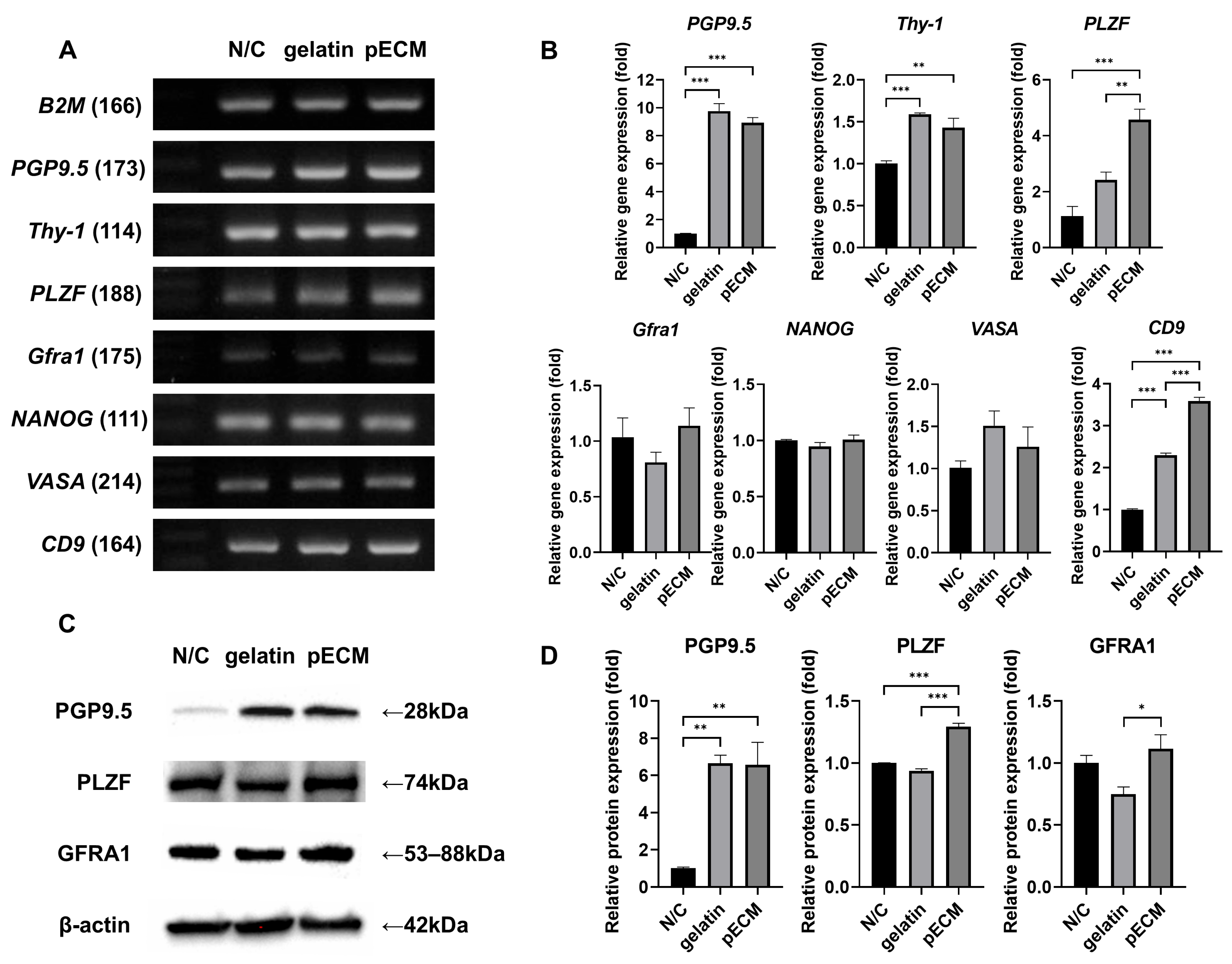
2.5. Gene and Protein Expression Analysis of Day 7 Colonies
2.6. Gene and Protein Expression Analysis of Day 13 Colonies
3. Discussion
3.1. Implications of pECM for SSC Culture Systems
3.2. Microenvironment Patterns for pSSC Culture
3.3. Regulation of Cellular Dynamics in pSSCs and Feeder Cells by pECM
3.4. Modulation of SSC-Associated Marker Expression by pECM
3.5. Further Applications of pECM
3.6. Limitations
4. Materials and Methods
4.1. Extraction of pECM from Porcine Foot Tissue
4.2. Proximate Composition and Protein Analysis of pECM
4.3. Isolation of pTTCs from Porcine Testes
4.4. pSSC Culture on Different Coating Environments
4.5. Surface Morphology of Coating Conditions Analyzed by Scanning Electron Microscopy
4.6. Surface Topography Analyzed by AFM
4.7. WST-1 Cell Viability Assay
4.8. Colony Counting and AP Staining
4.9. Colony and pFeeder Isolation
4.10. Cell Cycle Analysis
4.11. Total RNA Extraction, RT-PCR and qPCR
4.12. Western Blotting
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubota, H.; Brinster, R.L. Spermatogonial stem cells. Biol. Reprod. 2018, 99, 52–74. [Google Scholar] [CrossRef]
- Enders, G.C.; Kahsai, T.Z.; Lian, G.; Funabiki, K.; Killen, P.D.; Hudson, B.G. Developmental changes in seminiferous tubule extracellular matrix components of the mouse testis: α3 (IV) collagen chain expressed at the initiation of spermatogenesis. Biol. Reprod. 1995, 53, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Park, J.E.; Kim, M.S.; Lee, K.Y.; Hwang, J.Y.; Im Yun, J.; Choi, J.H.; Lee, E.; Lee, S.T. Effects of extracellular matrix protein-derived signaling on the maintenance of the undifferentiated state of spermatogonial stem cells from porcine neonatal testis. Asian-Australas. J. Anim. Sci. 2016, 29, 1398. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Park, H.-J.; Lee, R.; Lee, K.-H.; Kim, Y.-H.; Ryu, B.-Y.; Kim, N.-H.; Kim, J.-H.; Kim, J.-H.; Moon, S.-H. Establishment and in vitro culture of porcine spermatogonial germ cells in low temperature culture conditions. Stem Cell Res. 2013, 11, 1234–1249. [Google Scholar] [CrossRef]
- Hadley, M.A.; Byers, S.W.; Suárez-Quian, C.A.; Kleinman, H.K.; Dym, M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985, 101, 1511–1522. [Google Scholar] [CrossRef]
- Tung, P.S.; Fritz, I.B. Extracellular matrix promotes rat Sertoli cell histotypic expression in vitro. Biol. Reprod. 1984, 30, 213–229. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, W.Y.; Do, J.T.; Park, C.K.; Kim, N.H.; Kim, J.H.; Chung, H.J.; Kim, D.W.; Song, H. In vitro ectopic behavior of porcine spermatogonial germ cells and testicular somatic cells. Cell. Reprogram. 2016, 18, 246–255. [Google Scholar] [CrossRef]
- Lee, W.-Y.; Do, J.T.; Park, C.; Kim, J.H.; Chung, H.-J.; Kim, K.-W.; Gil, C.-H.; Kim, N.-H.; Song, H. Identification of putative biomarkers for the early stage of porcine spermatogonial stem cells using next-generation sequencing. PLoS ONE 2016, 11, e0147298. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Park, H.J.; Lee, W.Y.; Choi, Y.; Song, H. Nanoscale level gelatin-based scaffolds enhance colony formation of porcine testicular germ cells. Theriogenology 2023, 202, 125–135. [Google Scholar] [CrossRef] [PubMed]
- DeQuach, J.A.; Mezzano, V.; Miglani, A.; Lange, S.; Keller, G.M.; Sheikh, F.; Christman, K.L. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS ONE 2010, 5, e13039. [Google Scholar] [CrossRef]
- Hoshiba, T.; Chen, G.; Endo, C.; Maruyama, H.; Wakui, M.; Nemoto, E.; Kawazoe, N.; Tanaka, M. Decellularized extracellular matrix as an in vitro model to study the comprehensive roles of the ECM in stem cell differentiation. Stem Cells Int. 2016, 2016, 6397820. [Google Scholar] [CrossRef]
- Sawyer, A.A.; Hennessy, K.M.; Bellis, S.L. The effect of adsorbed serum proteins, RGD and proteoglycan-binding peptides on the adhesion of mesenchymal stem cells to hydroxyapatite. Biomaterials 2007, 28, 383–392. [Google Scholar] [CrossRef]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Madeira, M.S.; Pestana, J.; Coelho, D.; Lopes, P.A.; Toldrá, F.; Prates, J.A. Pork Byproducts. In Byproducts from Agriculture and Fisheries; Wiley: Hoboken, NJ, USA, 2019; pp. 19–41. [Google Scholar]
- Limeneh, D.Y.; Tesfaye, T.; Ayele, M.; Husien, N.M.; Ferede, E.; Haile, A.; Mengie, W.; Abuhay, A.; Gelebo, G.G.; Gibril, M. A comprehensive review on utilization of slaughterhouse by-product: Current status and prospect. Sustainability 2022, 14, 6469. [Google Scholar] [CrossRef]
- Choi, D.; Min, S.G.; Jo, Y.J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for liposomal delivery system. J. Food Biochem. 2018, 42, e12464. [Google Scholar] [CrossRef]
- Taubenberger, A.V.; Woodruff, M.A.; Bai, H.; Muller, D.J.; Hutmacher, D.W. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation. Biomaterials 2010, 31, 2827–2835. [Google Scholar] [CrossRef]
- Choi, H.-G.; Choi, H.-S.; Choi, Y.-S.; Jung, M.-O.; Choi, J.-S.; Choi, Y.-I. Effects of mixed bone and brisket meat on physico-chemical characteristics of shank bone and rib extracts from Hanwoo. Korean J. Food Sci. Anim. Resour. 2016, 36, 61. [Google Scholar] [CrossRef] [PubMed]
- Kort-Mascort, J.; Flores-Torres, S.; Peza-Chavez, O.; Jang, J.H.; Pardo, L.A.; Tran, S.D.; Kinsella, J. Decellularized ECM hydrogels: Prior use considerations, applications, and opportunities in tissue engineering and biofabrication. Biomater. Sci. 2023, 11, 400–431. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.Y.; Park, Y.S.; Ryu, J.-S.; Lee, H.J.; Araúzo-Bravo, M.J.; Ko, K.; Han, D.W.; Schöler, H.R.; Ko, K. A novel feeder-free culture system for expansion of mouse spermatogonial stem cells. Mol. Cells 2014, 37, 473–479. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Djourabtchi, A.; Golestaneh, N. Long-term culture of human SSEA-4 positive spermatogonial stem cells (SSCs). J. Stem Cell Res. Ther. 2011, 2, 2488. [Google Scholar] [CrossRef] [PubMed]
- David, S.; Orwig, K.E. Spermatogonial stem cell culture in oncofertility. Urol. Clin. North Am. 2020, 47, 227. [Google Scholar] [CrossRef] [PubMed]
- Naeemi, S.; Eidi, A.; Khanbabaee, R.; Sadri-Ardekani, H.; Kajbafzadeh, A.-M. Differentiation and proliferation of spermatogonial stem cells using a three-dimensional decellularized testicular scaffold: A new method to study the testicular microenvironment in vitro. Int. Urol. Nephrol. 2021, 53, 1543–1550. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Q.; Zhou, C.; Li, Q.; Li, Z.; Cao, Z.; Liang, J.; Li, H.; Mei, J.; Zhang, Q. A testis-derived hydrogel as an efficient feeder-free culture platform to promote mouse spermatogonial stem cell proliferation and differentiation. Front. Cell Dev. Biol. 2020, 8, 250. [Google Scholar] [CrossRef] [PubMed]
- Murdock, M.H.; David, S.; Swinehart, I.T.; Reing, J.E.; Tran, K.; Gassei, K.; Orwig, K.E.; Badylak, S.F. Human testis extracellular matrix enhances human spermatogonial stem cell survival in vitro. Tissue Eng. Part A 2019, 25, 663–676. [Google Scholar] [CrossRef]
- Salem, M.; Feizollahi, N.; Jabari, A.; Golmohammadi, M.G.; Shirinsokhan, A.; Ghanami Gashti, N.; Bashghareh, A.; Nikmahzar, A.; Abbasi, Y.; Naji, M. Differentiation of human spermatogonial stem cells using a human decellularized testicular scaffold supplemented by platelet-rich plasma. Artif. Organs 2023, 47, 840–853. [Google Scholar] [CrossRef]
- Fu, Y.; Fan, X.; Tian, C.; Luo, J.; Zhang, Y.; Deng, L.; Qin, T.; Lv, Q. Decellularization of porcine skeletal muscle extracellular matrix for the formulation of a matrix hydrogel: A preliminary study. J. Cell. Mol. Med. 2016, 20, 740–749. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Lin, C.; Yu, M.; Chen, S.; Guo, J.; Rao, P.; Miao, S.; Liu, S. Heat-induced structural changes in fish muscle collagen related to texture development in fish balls: Using eel ball as a study model. Food Sci. Nutr. 2022, 10, 329–341. [Google Scholar] [CrossRef]
- Brandao-Rangel, M.A.R.; Oliveira, C.R.; da Silva Olímpio, F.R.; Aimbire, F.; Mateus-Silva, J.R.; Chaluppe, F.A.; Vieira, R.P. Hydrolyzed collagen induces an anti-inflammatory response that induces proliferation of skin fibroblast and keratinocytes. Nutrients 2022, 14, 4975. [Google Scholar] [CrossRef]
- Boateng, S.Y.; Lateef, S.S.; Mosley, W.; Hartman, T.J.; Hanley, L.; Russell, B. RGD and YIGSR synthetic peptides facilitate cellular adhesion identical to that of laminin and fibronectin but alter the physiology of neonatal cardiac myocytes. Am. J. Physiol. -Cell Physiol. 2005, 288, C30–C38. [Google Scholar] [CrossRef]
- Norde, W. Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid Interface Sci. 1986, 25, 267–340. [Google Scholar] [CrossRef]
- Martin, J.; Dean, D.; Cochran, D.; Simpson, J.; Boyan, B.; Schwartz, Z. Proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63) cultured on previously used titanium surfaces. Clin. Oral Implant. Res. 1996, 7, 27–37. [Google Scholar] [CrossRef]
- Martin, J.; Schwartz, Z.; Hummert, T.; Schraub, D.; Simpson, J.; Lankford Jr, J.; Dean, D.; Cochran, D.; Boyan, B. Effect of titanium surface roughness on proliferation, differentiation, and protein synthesis of human osteoblast-like cells (MG63). J. Biomed. Mater. Res. 1995, 29, 389–401. [Google Scholar] [CrossRef]
- Lampin, M.; Warocquier-Clérout, R.; Legris, C.; Degrange, M.; Sigot-Luizard, M. Correlation between substratum roughness and wettability, cell adhesion, and cell migration. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997, 36, 99–108. [Google Scholar] [CrossRef]
- Calore, A.R.; Srinivas, V.; Anand, S.; Albillos-Sanchez, A.; Looijmans, S.F.; van Breemen, L.C.; Mota, C.; Bernaerts, K.; Harings, J.A.; Moroni, L. Shaping and properties of thermoplastic scaffolds in tissue regeneration: The effect of thermal history on polymer crystallization, surface characteristics and cell fate. J. Mater. Res. 2021, 36, 3914–3935. [Google Scholar] [CrossRef]
- Shiva, R.; Ghasem, S.; Masoud, H.; Sadat, K.L.; Ali, K.; Reza, D.M. Comparison of colony formation of human spermatogonial stem cells (SSCs) with and without collagen. JPMA J. Pak. Med. Assoc. 2016, 66, 285–291. [Google Scholar]
- Bauwens, C.L.; Peerani, R.; Niebruegge, S.; Woodhouse, K.A.; Kumacheva, E.; Husain, M.; Zandstra, P.W. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells 2008, 26, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Spradling, A.; Drummond-Barbosa, D.; Kai, T. Stem cells find their niche. Nature 2001, 414, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.-r.; Wang, J.-j.; Li, X.-x.; Zhang, D.-c.; Zou, K. Effects of inhibition of porcine sertoli cells exosomes on self-renewal and differentiation of porcine spermatogonial stem cells. J. Nanjing Agric. Univ. 2023, 46, 898–906. [Google Scholar]
- Mogilner, A.; Keren, K. The shape of motile cells. Curr. Biol. 2009, 19, R762–R771. [Google Scholar] [CrossRef]
- DiMilla, P.A.; Barbee, K.; Lauffenburger, D.A. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys. J. 1991, 60, 15–37. [Google Scholar] [CrossRef]
- Lutter, A.-H.; Scholka, J.; Richter, H.; Anderer, U. Applying XTT, WST-1, and WST-8 to human chondrocytes: A comparison of membrane-impermeable tetrazolium salts in 2D and 3D cultures. Clin. Hemorheol. Microcirc. 2017, 67, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Goldbeter, A. The balance between cell cycle arrest and cell proliferation: Control by the extracellular matrix and by contact inhibition. Interface Focus 2014, 4, 20130075. [Google Scholar] [CrossRef]
- Costoya, J.A.; Hobbs, R.M.; Barna, M.; Cattoretti, G.; Manova, K.; Sukhwani, M.; Orwig, K.E.; Wolgemuth, D.J.; Pandolfi, P.P. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 2004, 36, 653–659. [Google Scholar] [CrossRef]
- Hobbs, R.M.; Seandel, M.; Falciatori, I.; Rafii, S.; Pandolfi, P.P. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell 2010, 142, 468–479. [Google Scholar] [CrossRef]
- Kokkinaki, M.; Lee, T.-L.; He, Z.; Jiang, J.; Golestaneh, N.; Hofmann, M.-C.; Chan, W.-Y.; Dym, M. The molecular signature of spermatogonial stem/progenitor cells in the 6-day-old mouse testis. Biol. Reprod. 2009, 80, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Nagao, K.; Nakajima, K.; Miura, K.; Ishii, N. Thy-1+ cells isolated from adult human testicular tissues express human embryonic stem cell genes OCT3/4 and NANOG and may include spermatogonial stem cells. Reprod. Med. Biol. 2009, 8, 71–77. [Google Scholar] [CrossRef]
- Abbasi, H.; Tahmoorespur, M.; Hosseini, S.M.; Nasiri, Z.; Bahadorani, M.; Hajian, M.; Nasiri, M.R.; Nasr-Esfahani, M.H. THY1 as a reliable marker for enrichment of undifferentiated spermatogonia in the goat. Theriogenology 2013, 80, 923–932. [Google Scholar] [CrossRef]
- Castrillon, D.H.; Quade, B.J.; Wang, T.; Quigley, C.; Crum, C.P. The human VASA gene is specifically expressed in the germ cell lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 9585–9590. [Google Scholar] [CrossRef] [PubMed]
- Kanatsu-Shinohara, M.; Toyokuni, S.; Shinohara, T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol. Reprod. 2004, 70, 70–75. [Google Scholar] [CrossRef]
- Luo, J.; Megee, S.; Dobrinski, I. Asymmetric distribution of UCH-L1 in spermatogonia is associated with maintenance and differentiation of spermatogonial stem cells. J. Cell. Physiol. 2009, 220, 460–468. [Google Scholar] [CrossRef]
- Orozco-Fuentes, S.; Neganova, I.; Wadkin, L.E.; Baggaley, A.W.; Barrio, R.A.; Lako, M.; Shukurov, A.; Parker, N.G. Quantification of the morphological characteristics of hESC colonies. Sci. Rep. 2019, 9, 17569. [Google Scholar] [CrossRef] [PubMed]
- Reyes, R.; Cardeñes, B.; Machado-Pineda, Y.; Cabañas, C. Tetraspanin CD9: A key regulator of cell adhesion in the immune system. Front. Immunol. 2018, 9, 863. [Google Scholar] [CrossRef]
- Menezes, R.; Vincent, R.; Osorno, L.; Hu, P.; Arinzeh, T.L. Biomaterials and tissue engineering approaches using glycosaminoglycans for tissue repair: Lessons learned from the native extracellular matrix. Acta Biomater. 2023, 163, 210–227. [Google Scholar] [CrossRef]
- Wolf, M.T.; Daly, K.A.; Brennan-Pierce, E.P.; Johnson, S.A.; Carruthers, C.A.; D’Amore, A.; Nagarkar, S.P.; Velankar, S.S.; Badylak, S.F. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials 2012, 33, 7028–7038. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Vermeulen, M.; Del Vento, F.; Kanbar, M.; Pyr dit Ruys, S.; Vertommen, D.; Poels, J.; Wyns, C. Generation of organized porcine testicular organoids in solubilized hydrogels from decellularized extracellular matrix. Int. J. Mol. Sci. 2019, 20, 5476. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Josey, B.; Chou, C.J.; Tan, Y.; Zhang, N.; Wen, X. Short laminin peptide for improved neural stem cell growth. Stem Cells Transl. Med. 2014, 3, 662–670. [Google Scholar] [CrossRef]
- Lara, N.L.; Goldsmith, T.; Rodriguez-Villamil, P.; Ongaratto, F.; Solin, S.; Webster, D.; Ganbaatar, U.; Hodgson, S.; Corbière, S.M.; Bondareva, A. DAZL knockout pigs as recipients for spermatogonial stem cell transplantation. Cells 2023, 12, 2582. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Lee, W.-Y.; Park, C.; Hong, K.; Song, H. CD14 is a unique membrane marker of porcine spermatogonial stem cells, regulating their differentiation. Sci. Rep. 2019, 9, 9980. [Google Scholar] [CrossRef] [PubMed]
- Mendibil, U.; Ruiz-Hernandez, R.; Retegi-Carrion, S.; Garcia-Urquia, N.; Olalde-Graells, B.; Abarrategi, A. Tissue-specific decellularization methods: Rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 2020, 21, 5447. [Google Scholar] [CrossRef] [PubMed]
- Hoshiba, T.; Yunoki, S. Comparison of decellularization protocols for cultured cell-derived extracellular matrix—Effects on decellularization efficacy, extracellular matrix retention, and cell functions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Jang, H. The effect of gelatin-coating on embryonic stem cells as assessed by measuring Young’s modulus using an atomic force microscope. J. Anim. Reprod. Biotechnol. 2023, 38, 121–130. [Google Scholar] [CrossRef]
- Alcaraz, J.; Otero, J.; Jorba, I.; Navajas, D. Bidirectional mechanobiology between cells and their local extracellular matrix probed by atomic force microscopy. In Seminars in Cell & Developmental Biology; Elsevier: New York, NY, USA, 2018; pp. 71–81. [Google Scholar]


| Gene | Sequence | Tm (°C) | Product Size (bp) | GeneBank Accession |
|---|---|---|---|---|
| B2M | F 5′-TTCACACCGCTCCAGTAG-3′ | 55 | 166 | NM_213978.1 |
| R 5′-CCAGATACATAGCAGTTCAG-3′ | 55 | |||
| PGP9.5 | F 5′-GATGCCTTTTCCGGTGAACC-3′ | 59 | 173 | NM_213763.2 |
| R 5′-GAACGGGGATAAAGCGAAGG-3′ | 59 | |||
| Thy-1 | F 5’-GCTAACAGTCTTGCAGGTGG-3′ | 59 | 114 | NM_001146129.1 |
| R 5′-ATGGGCAGGTTGGTGGTATT-3′ | 57 | |||
| PLZF | F 5′-GGCTCGGTATCTCAAGAACATC-3′ | 60 | 188 | XM_021062870.1 |
| R 5′-ACTGCCCTATGGTCATCAAACT-3′ | 58 | |||
| Gfra1 | F 5′-GCCACCAAGCGTTGTCTTTT-3′ | 57 | 175 | XM_013983780.2 |
| R 5′-GAAGAGAGGGCCACATGTCC-3′ | 61 | |||
| NANOG | F 5′-ACCACTGGCCAAGGAATAGC-3′ | 59 | 111 | NM_001129971.2 |
| R 5′-GCAGGTTTCCAGAAGCGTTC-3′ | 59 | |||
| VASA | F 5′-CCAAGTTGGCCAGTACTCAAAA-3′ | 58 | 214 | NM_001291682.1 |
| R 5′-ACACTTCCCACAGCGAAAATC-3′ | 57 | |||
| CD9 | F 5′-TTCATCTTCTGGCTCGCTGG-3′ | 59 | 164 | NM_214006.1 |
| R 5′-AAGCCCACCACCATCATGAG-3′ | 59 | |||
| WT1 | F 5′-CTGAAGACCCACACCAGGAC-3′ | 61 | 124 | NM_001001264.1 |
| R 5′-GGTGCATGTTGTGATGACGG-3′ | 59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Han, M.-G.; Jeon, Y.; Maeng, H.; Choi, Y.; Hong, K.; Do, J.T.; Song, H. Effect of Extracellular Matrix Derived from Porcine Tissue on Stemness of Porcine Spermatogonial Stem Cells. Int. J. Mol. Sci. 2025, 26, 9937. https://doi.org/10.3390/ijms26209937
Kim D, Han M-G, Jeon Y, Maeng H, Choi Y, Hong K, Do JT, Song H. Effect of Extracellular Matrix Derived from Porcine Tissue on Stemness of Porcine Spermatogonial Stem Cells. International Journal of Molecular Sciences. 2025; 26(20):9937. https://doi.org/10.3390/ijms26209937
Chicago/Turabian StyleKim, Donghyeon, Min-Gi Han, Yoseop Jeon, Hyoyoung Maeng, Youngseok Choi, Kwonho Hong, Jeong Tae Do, and Hyuk Song. 2025. "Effect of Extracellular Matrix Derived from Porcine Tissue on Stemness of Porcine Spermatogonial Stem Cells" International Journal of Molecular Sciences 26, no. 20: 9937. https://doi.org/10.3390/ijms26209937
APA StyleKim, D., Han, M.-G., Jeon, Y., Maeng, H., Choi, Y., Hong, K., Do, J. T., & Song, H. (2025). Effect of Extracellular Matrix Derived from Porcine Tissue on Stemness of Porcine Spermatogonial Stem Cells. International Journal of Molecular Sciences, 26(20), 9937. https://doi.org/10.3390/ijms26209937







