Plant Cuticles Exhibit Significant Mid-Infrared Emissivity in the Atmospheric Windows †
Abstract
1. Introduction
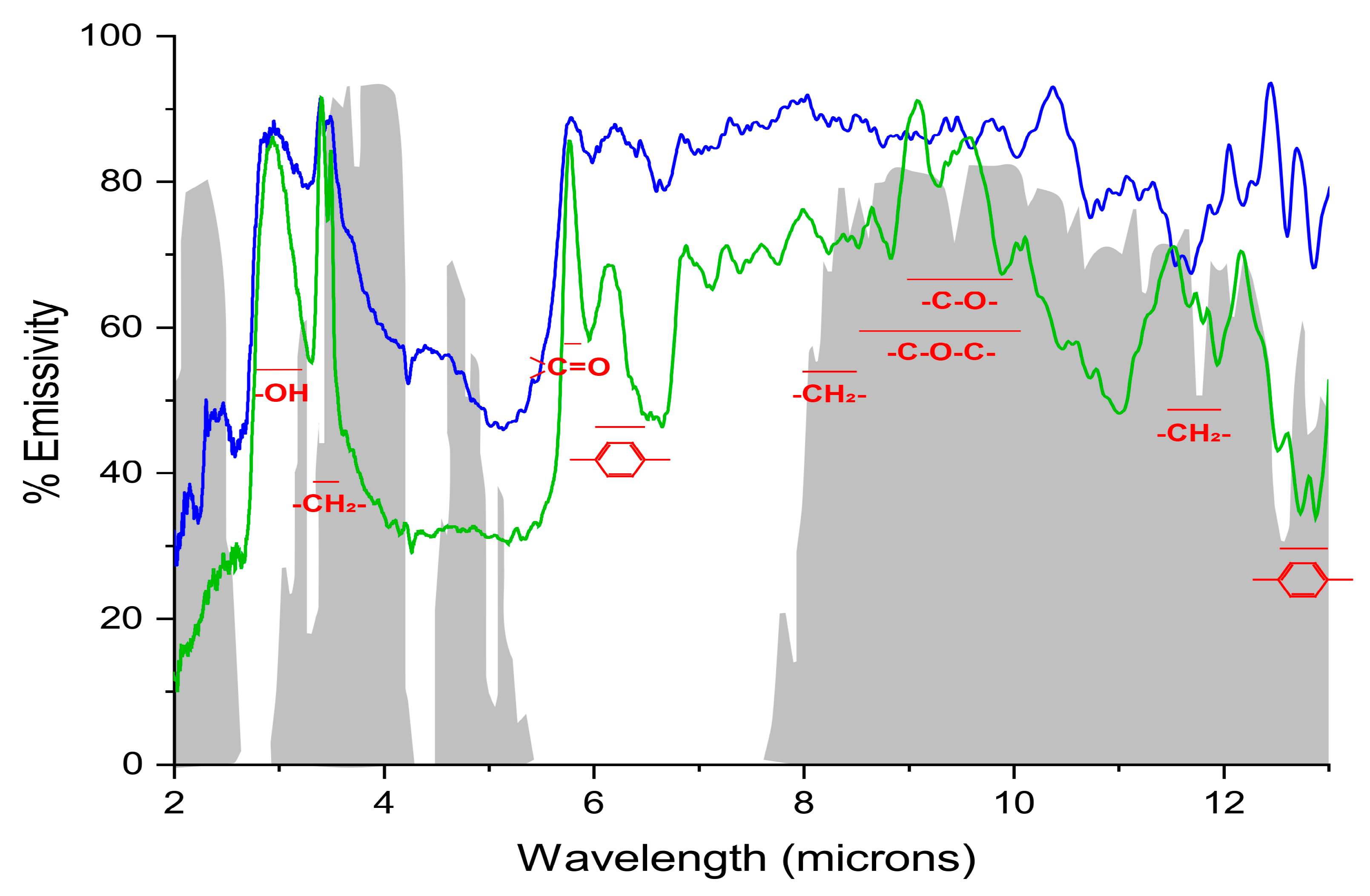
2. Results and Discussion
3. Materials and Methods
3.1. Cuticle Extraction
3.2. Tissue Sectioning and Microscopy
3.3. Transmittance, Absorbance and Reflectance Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liao, M.; Banerjee, D.; Hallberg, T.; Akerlind, C.; Alam, M.M.; Zhang, Q.; Kariis, H.; Zhao, D.; Jonsson, M.P. Cellulose-based radiative cooling and solar heating powers ionic thermoelectrics. Adv. Sci. 2023, 10, 2206510. [Google Scholar] [CrossRef]
- Liu, R.; Wang, S.; Zhou, Z.; Zhang, K.; Wang, C.; Chen, C.; Long, Y. Materials in radiative cooling technologies. Adv. Mater. 2025, 37, 2401577. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Luz, B. Attenuated total reflectance spectroscopy of plant leaves: A tool for ecological and botanical studies. New Phytol. 2006, 172, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro da Luz, B.; Crowley, J.K. Spectral reflectance and emissivity features of broad leaf plants: Prospects for remote sensing in the thermal infrared (8.0–14.0 μm). Remote Sens. Environ. 2007, 109, 393–405. [Google Scholar] [CrossRef]
- Richardson, A.D.; Aubrecht, D.M.; Basler, D.; Hufkens, K.; Muir, K.D.; Hanssen, L. Developmental changes in the reflectance spectra of temperate deciduous tree leaves and implications for thermal emissivity and leaf temperature. New Phytol. 2020, 229, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Heredia, A.; Benítez, J.J.; González-Moreno, A.; Domínguez, E. Revisiting plant cuticle biophysics. New Phytol. 2024, 244, 65–73. [Google Scholar] [CrossRef]
- Heredia, A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2002, 1620, 1–7. [Google Scholar] [CrossRef]
- González-Moreno, A.; de Cózar, A.; Prieto, P.; Domínguez, E.; Heredia, A. Radiationless mechanism of UV deactivation by cuticle phenolics in plants. Nat. Commun. 2022, 13, 1786. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Domínguez, E.; Bayer, I.S.; Cingolani, R.; Athanassiou, A.; Heredia, A. Infrared and Raman spectroscopic features of plant cuticles: A review. Front. Plant Sci. 2014, 5, 305. [Google Scholar] [CrossRef]
- Bi, H.; Kovalchuk, N.; Langridge, P.; Tricker, P.J.; Lopato, S. The impact of drought on wheat leaf cuticle properties. BMC Plant Biol. 2017, 17, 85. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Guzmán-Puyol, S.; Benítez, J.J.; Athanassiou, A.; Heredia, A.; Domínguez, E. Plant cuticle under global change: Biophysical implications. Glob. Change Biol. 2018, 24, 2749–2751. [Google Scholar] [CrossRef] [PubMed]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lü, S.; Joubès, J.; Jenks, M.A. The impact of water deficiency on leaf cuticle lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, Y.; Xu, D.; Busta, L.; Xiao, Y.; Jetter, R.; Guo, Y. Integrative analysis of the cuticular lipidome and transcriptome of Sorghum bicolor reveals cultivar differences in drought tolerance. Plant Physiol. Biochem. 2021, 163, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Pfündel, E.E.; Agati, G.; Cerovic, Z.G. Optical properties of plant surfaces. In Biology of Plant Cuticle; Riederer, M., Müller, C., Eds.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Bianchi, G.; Vlahov, G.; Anglani, C.; Murelli, C. Epicuticular wax of olive leaves. Phytochemistry 1992, 32, 49–52. [Google Scholar] [CrossRef]
- Vogg, G.; Fischer, S.; Leide, J.; Emmanuel, E.; Jetter, R.; Levy, A.A.; Riederer, M. Tomato fruit cuticular waxes and their effects on transpiration barrier properties: Functional characterization of a mutant deficient in a very-long-chain fatty acid β-ketoacyl-CoA synthase. J. Exp. Bot. 2004, 55, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Schulte, E.; Their, T.P. Composition of the surface waxes from bell pepper and eggplant. Eur. Food Res. Technol. 2005, 220, 5–10. [Google Scholar] [CrossRef]
- Jetter, R.; Riederer, M. Localization of the transpiration barrier in the epi- and intracuticular waxes of eight plant species: Water transport resistances are associated with fatty acyl rather than alicyclic components. Plant Physiol. 2016, 170, 921–934. [Google Scholar] [CrossRef]
- Zeisler-Diehl, V.; Müller, Y.; Schreiber, L. Epicuticular wax on leaf cuticles does not establish the transpiration barrier, which is essentially formed by intracuticular wax. J. Plant Physiol. 2018, 227, 66–74. [Google Scholar] [CrossRef]
- Arand, K.; Bieler, E.; Dürrenberger, M.; KassemeyerI, H.H. Developmental pattern of grapevine (Vitis vinifera L.) berry cuticular wax: Differentiation between epicuticular crystals and underlying wax. PLoS ONE 2021, 16, e0246693. [Google Scholar] [CrossRef]
- Benítez, J.J.; González-Moreno, A.; Guzmán-Puyol, S.; Heredia-Guerrero, J.A.; Heredia, A.; Domínguez, E. The response of tomato fruit cuticles against heat and light. Front. Plant Sci. 2021, 12, 807723. [Google Scholar] [CrossRef]
- Gamage, S.; Banerjee, D.; Mehebub, M.D.; Hallberg, T.; Akerlind, C.; Sultana, A.; Shanker, R.; Berggren, M.; Crispin, X.; Kariis, H.; et al. Reflective and transparent cellulose-bases passive radiative coolers. Cellulose 2021, 28, 9383–9393. [Google Scholar] [CrossRef]
- Bueno, A.; Alonso-Forn, D.; Peguero-Pina, J.J.; Xavier de Souza, A.; Ferrio, J.P. Minimum leaf conductance (gmin) is higher in the treeline of Pinus uncinata Ram. in the pyrenees: Michaelis’ hypothesis revisited. Front. Plant Sci. 2022, 12, 786933. [Google Scholar] [CrossRef]
- Trivedi, P.; Klavins, L.; Hykkerud, A.L.; Kviesis, J.; Elferts, D.; Martinussen, I.; Klavins, M.; Karppinen, K.; Häggmann, H.; Jaakola, L. Temperature has a major effect on the cuticular wax composition of bilberry (Vaccinium myrtillus L.) fruit. Front. Plant Sci. 2022, 13, 980427. [Google Scholar] [CrossRef]
- Li, C.; Jian, Q.; Jian, D. Influence of thickness on resistivity and infrared emissivity of TiN films. J. Mater. Sci. Mater. Electron. 2022, 33, 3606–3616. [Google Scholar] [CrossRef]
- Guo, J.; Guo, X.; Zeng, J.; Nie, L.; Dong, J.; Peng, L.; Ding, W. Effective strategy for improving infrared emissivity of Zn-Ni porous coating. Appl. Surf. Sci. 2019, 485, 92–100. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; de Leeuw, J.W.; Tegelaar, E.W.; Hatcher, P.G.; Kerp, H. A lignin-like polymer in the cuticle of spruce needles: Implications for the humification of spruce litter. Org. Geochem. 1994, 21, 1219–1228. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia, A.; González-Moreno, A.; Benítez, J.J.; Domínguez, E. Plant Cuticles Exhibit Significant Mid-Infrared Emissivity in the Atmospheric Windows. Int. J. Mol. Sci. 2025, 26, 9917. https://doi.org/10.3390/ijms26209917
Heredia A, González-Moreno A, Benítez JJ, Domínguez E. Plant Cuticles Exhibit Significant Mid-Infrared Emissivity in the Atmospheric Windows. International Journal of Molecular Sciences. 2025; 26(20):9917. https://doi.org/10.3390/ijms26209917
Chicago/Turabian StyleHeredia, Antonio, Ana González-Moreno, José J. Benítez, and Eva Domínguez. 2025. "Plant Cuticles Exhibit Significant Mid-Infrared Emissivity in the Atmospheric Windows" International Journal of Molecular Sciences 26, no. 20: 9917. https://doi.org/10.3390/ijms26209917
APA StyleHeredia, A., González-Moreno, A., Benítez, J. J., & Domínguez, E. (2025). Plant Cuticles Exhibit Significant Mid-Infrared Emissivity in the Atmospheric Windows. International Journal of Molecular Sciences, 26(20), 9917. https://doi.org/10.3390/ijms26209917





