Role of Lipoprotein(a) and Blood Cells Ratios in Peripheral Artery Disease
Abstract
1. Introduction
2. Results
2.1. Biomarkers of Stenotic Atherosclerosis of Lower Limbs
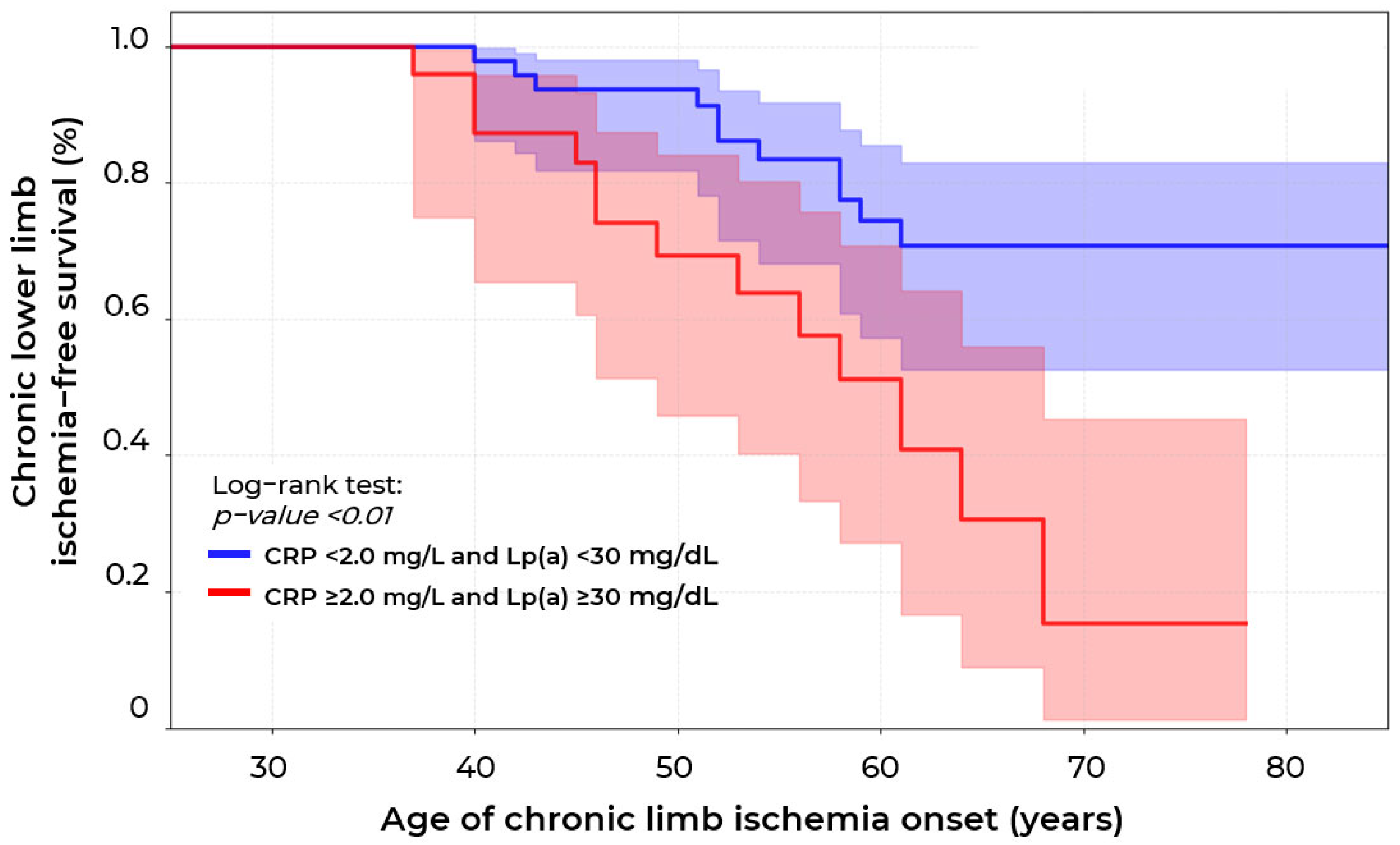
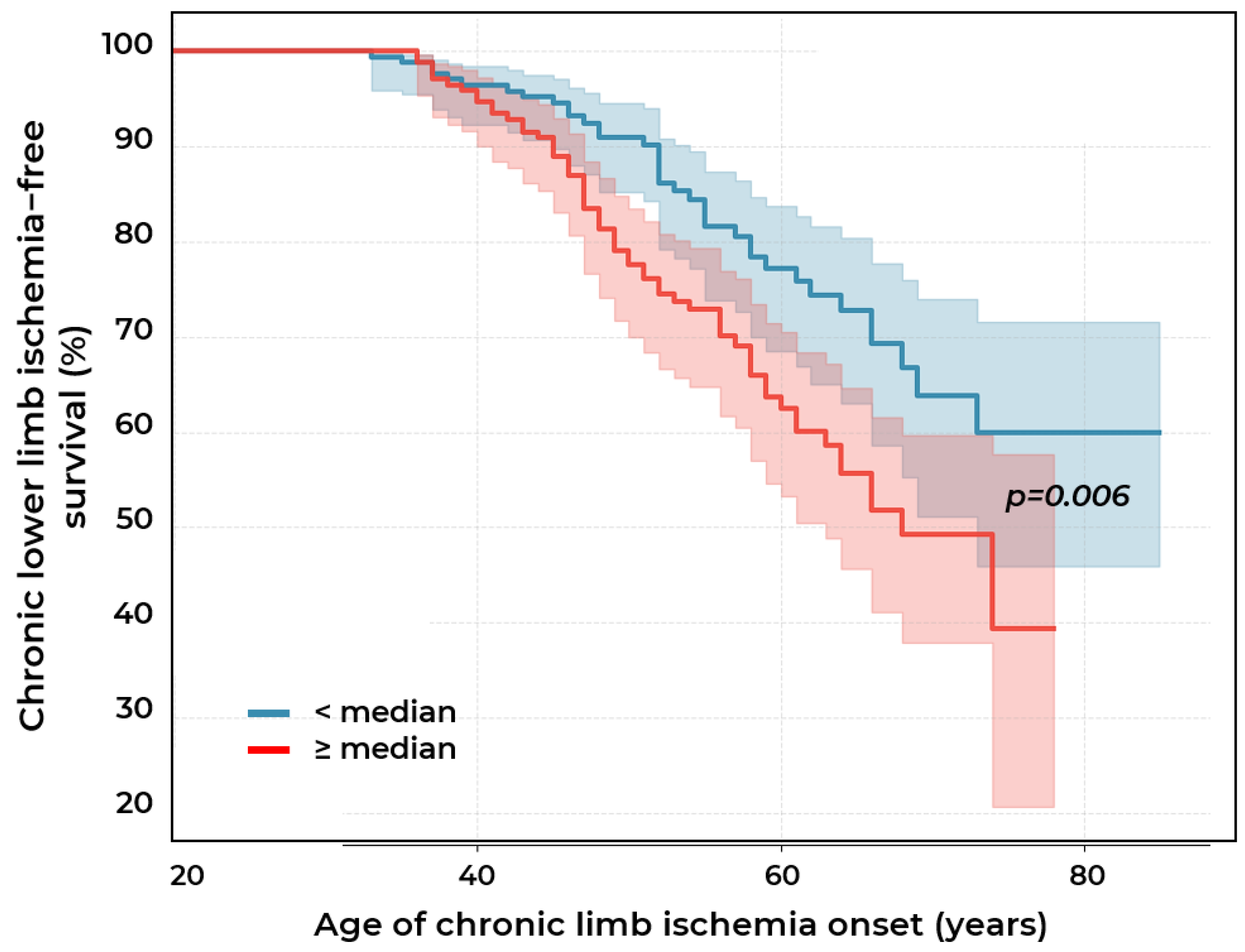
2.2. Biomarkers of Chronic Lower Limb Ischemia
3. Discussion
3.1. Absolute Monocyte Count
3.2. Monocyte-to-HDL-C Ratio
3.3. Neutrophils and Neutrophil-to-Lymphocyte Ratio
3.4. Neutrophil-to-HDL-C Ratio
3.5. Lymphocyte-to-Monocyte Ratio
3.6. Platelet-to-Lymphocyte Ratio
3.7. Platelet-to-HDL-C Ratio
3.8. Lipoprotein(a)-to-HDL-C Ratio
3.9. Composite Index [0.3 × Lp(a) Concentration (mg/dL) + 0.7 × Absolute Monocyte Count (×109/L)]
4. Materials and Methods
5. Conclusions
6. Study Limitations
- The design of the second visit involved selective participant selection: not all participants from the first visit were invited to the second visit. Of the 238 patients with initially diagnosed PAD, 158 were enrolled on the second visit after responding to the invitation and meeting no exclusion criteria. This subgroup for re-examination was formed randomly and was not based on any specific clinical or laboratory parameters. The sample size for the stenotic atherosclerosis group (n = 158) was determined based on statistical power calculations. All 123 patients without stenotic atherosclerosis of the lower limb arteries diagnosed at visit one were invited to the second visit.
- Despite the fact that the exclusion criteria from the study were diagnosed malignancies, and autoimmune, inflammatory or infectious diseases, we recognize that inflammatory markers are subject to internal variability and may depend on various factors, including subclinical infectious processes, which could affect the results.
- The relatively small sample size of patients with atherosclerosis first detected at visit two may limit the statistical power of the study when analyzing weak associations between certain markers and disease risk.
- The absence of data on smoking history and the degree of arterial hypertension control among participants limits the ability to assess the contribution of this risk factor to disease development.
- The prognostic stability of the developed composite index in various clinical subgroups was not evaluated in this study, which limits its potential for widespread clinical application. Multicenter prospective cohort studies are needed to validate the index in different patient populations, optimize threshold values, and assess its stability in different clinical scenarios.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CABG | Coronary artery bypass graft | MHR | Monocyte-to-HDL-cholesterol ratio |
| CAD | Coronary artery disease | MI | Myocardial infarction |
| CI | Confidence interval | NNH | Number needed to harm |
| CLLI | Chronic lower limb ischemia | NHR | Neutrophil-to-HDL-cholesterol ratio |
| CRP | C-reactive protein | NLR | Neutrophil-to-lymphocyte ratio |
| DM | Diabetes mellitus | OR | Odds ratio |
| DUS | Duplex ultrasonography | PAD | Peripheral artery disease |
| HDL-C | High-density lipoprotein cholesterol | PCI | Percutaneous coronary intervention |
| HR | Hazard ratio | PHR | Platelet-to-HDL-cholesterol ratio |
| IQR | Interquartile range | PLR | Platelet-to-lymphocyte ratio |
| LDL-C | Low-density lipoprotein cholesterol | PSV | Peak systolic velocity |
| LMR | Lymphocyte-to-monocyte ratio | RR | Relative risk |
| Lp(a) | Lipoprotein(a) | TC | Total cholesterol |
| LLA | Lower limb arteries | TG | Triglycerides |
| Me | Median |
References
- Kim, M.S.; Hwang, J.; Yon, D.K.; Lee, S.W.; Jung, S.Y.; Park, S.; Johnson, C.O.; Stark, B.A.; Razo, C.; Abbasian, M. Global burden of peripheral artery disease and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Glob. Health 2023, 11, e1553–e1565. [Google Scholar] [CrossRef] [PubMed]
- Allison, M.A.; Armstrong, D.G.; Goodney, P.P.; Hamburg, N.M.; Kirksey, L.; Lancaster, K.J.; Mena-Hurtado, C.I.; Misra, S.; Treat-Jacobson, D.J.; White Solaru, K.T. Health Disparities in Peripheral Artery Disease: A Scientific Statement From the American Heart Association. Circulation 2023, 148, 286–296. [Google Scholar] [CrossRef]
- Apresyan, A.Y. Assessment of the prevalence of peripheral artery diseases in the adult population of the Russian Federation. Bull. Ivanovo Med. Acad. 2022, 27, 25–30. [Google Scholar]
- Horne, B.D.; Anderson, J.L.; John, J.M.; Weaver, A.; Bair, T.L.; Jensen, K.R.; Renlund, D.G.; Muhlestein, J.B. Which white blood cell subtypes predict increased cardiovascular risk? J. Am. Coll. Cardiol. 2005, 45, 1638–1643. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 2013, 339, 161–166. [Google Scholar] [CrossRef]
- Gary, T.; Pichler, M.; Belaj, K.; Eller, P.; Hafner, F.; Gerger, A.; Brodmann, M. Lymphocyte-to-monocyte ratio: A novel marker for critical limb ischemia in PAOD patients. Int. J. Clin. Pract. 2014, 68, 1483–1487. [Google Scholar] [CrossRef]
- Selvaggio, S.; Abate, A.; Brugaletta, G.; Musso, C.; Di Guardo, M.; Di Guardo, C.; Vicari, E.; Romano, M.; Luca, S.; Signorelli, S. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as markers of peripheral artery disease in elderly patients. Int. J. Mol. Med. 2020, 4, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Selvaggio, S.; Brugaletta, G.; Abate, A.; Musso, C.; Romano, M.; Di Raimondo, D.; Pirera, E.; Dattilo, G.; Santo Signorelli, S. Platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio and monocyte-to-HDL cholesterol ratio as helpful biomarkers for patients hospitalized for deep vein thrombosis. Int. J. Mol. Med. 2023, 51, 52. [Google Scholar] [CrossRef]
- Wei, L.; Le, Y.; Xianying, F.; Baohua, H. Correlation of the Monocyte/High-Density Lipoprotein Ratio, Red Blood Cell, Hemoglobin and Combined Lower Extremity Atherosclerosis in Patients with Essential Hypertension. Int. J. Front. Med. 2024, 6. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Y.; Shu, Y.; Zhang, L.; Cheng, W.; Wang, L.; Shu, M.; Xue, B.; Wang, R.; Feng, Z.; et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: A cross-sectional study. Front. Endocrinol. 2023, 14, 1100453. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Xu, Y.J.L.i.H. The association of the platelet/high-density lipoprotein cholesterol ratio with self-reported stroke and cardiovascular mortality: A population-based observational study. Lipids Health Dis. 2024, 23, 121. [Google Scholar] [CrossRef]
- Bradley, N.A.; Roxburgh, C.S.; McMillan, D.C.; Guthrie, G.J. A systematic review of the neutrophil to lymphocyte and platelet to lymphocyte ratios in patients with lower extremity arterial disease. Vasa 2024, 53, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Ezhov, M.V.; Tmoyan, N.A.; Afanasieva, O.I.; Afanasieva, M.I.; Pokrovsky, S.N. Lipoprotein(a) and cardiovascular outcomes after revascularization of carotid and lower limbs arteries. Biomolecules 2021, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Tyurina, A.V.; Afanasieva, O.I.; Ezhov, M.V.; Klesareva, E.A.; Balakhonova, T.V.; Pokrovsky, S.N. Lipoprotein(a) and Blood Monocytes as Factors for Progression of Carotid Atherosclerosis in Patients with Premature Coronary Heart Disease. Diseases 2025, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Wildgruber, M.; Aschenbrenner, T.; Wendorff, H.; Czubba, M.; Glinzer, A.; Haller, B.; Schiemann, M.; Zimmermann, A.; Berger, H.; Eckstein, H.-H. The “Intermediate” CD14++ CD16+ monocyte subset increases in severe peripheral artery disease in humans. Sci. Rep. 2016, 6, 39483. [Google Scholar] [CrossRef]
- Tabas, I.; Lichtman, A.H. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017, 47, 621–634. [Google Scholar] [CrossRef]
- Jiang, M.; Yang, J.; Zou, H.; Li, M.; Sun, W.; Kong, X. Monocyte-to-high-density lipoprotein-cholesterol ratio (MHR) and the risk of all-cause and cardiovascular mortality: A nationwide cohort study in the United States. Lipids Health Dis. 2022, 21, 30. [Google Scholar] [CrossRef]
- Irawan, B.; Hariawan, H. Association between monocyte-high density lipoprotein ratio (MHR) and severity level of lower extremity artery disease (LEAD). J. Med. Sci. 2020, 52. [Google Scholar] [CrossRef]
- Gary, T.; Pichler, M.; Belaj, K.; Hafner, F.; Gerger, A.; Froehlich, H.; Eller, P.; Pilger, E.; Brodmann, M. Neutrophil-to-lymphocyte ratio and its association with critical limb ischemia in PAOD patients. PLoS ONE 2013, 8, e56745. [Google Scholar] [CrossRef]
- Ye, M.; Qian, X.; Guo, X.; Wang, H.; Ni, Q.; Zhao, Y.; Xue, G.; Deng, H.; Zhang, L. Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predict severity and prognosis of lower limb arteriosclerosis obliterans. Ann. Vasc. Surg. 2020, 64, 221–227. [Google Scholar] [CrossRef]
- Gao, J.; Lu, J.; Sha, W.; Xu, B.; Zhang, C.; Wang, H.; Xia, J.; Zhang, H.; Tang, W.; Lei, T. Relationship between the neutrophil to high-density lipoprotein cholesterol ratio and severity of coronary artery disease in patients with stable coronary artery disease. Front. Cardiovasc. Med. 2022, 9, 1015398. [Google Scholar] [CrossRef]
- Cosarca, M.C.; Hălmaciu, I.; Muresan, A.V.; Suciu, B.A.; Molnar, C.; Russu, E.; Horvath, E.; Niculescu, R.; Puscasiu, L.; Bacalbaşa, N. Neutrophil-to-lymphocyte, platelet-to-lymphocyte and lymphocyte-to-monocyte ratios are associated with amputation rates in patients with peripheral arterial disease and diabetes mellitus who underwent revascularization: A Romanian regional center study. Exp. Ther. Med. 2022, 24, 703. [Google Scholar] [CrossRef]
- Kurtul, A.; Ornek, E. Platelet to lymphocyte ratio in cardiovascular diseases: A systematic review. Angiology 2019, 70, 802–818. [Google Scholar] [CrossRef]
- Varım, C.; Varım, P.; Acar, B.A.; Vatan, M.B.; Uyanık, M.S.; Kaya, T.; Acar, T.; Akdemir, R. Usefulness of the platelet-to-lymphocyte ratio in predicting the severity of carotid artery stenosis in patients undergoing carotid angiography. Kaohsiung J. Med. Sci. 2016, 32, 86–90. [Google Scholar] [CrossRef]
- Wu, W.; Jia, C.; Xu, X.; He, Y.; Xie, Y.; Zhou, Y.; Lu, H.; Liu, J.; Chen, J.; Liu, Y. Impact of platelet-to-HDL-cholesterol ratio on long-term mortality in coronary artery disease patients with or without type 2 diabetes: Insights from a Chinese multicenter cohort. J. Inflamm. Res. 2024, 17, 2731–2744. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jia, W.; Liu, Y.; Cui, M.; Zhang, H.; Wu, H.; Li, J.; Wei, L.; Qi, X. Association of platelet to high density lipoprotein cholesterol ratio with coronary lesion severity in middle aged and elderly adults. Sci. Rep. 2025, 15, 27336. [Google Scholar] [CrossRef] [PubMed]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Afanas’eva, O.I.; Adamova, I.Y.; Benevolenskaya, G.; Pokrovskii, S. Enzyme immunoassay of lipoprotein (a). Bull. Exp. Biol. Med. 1995, 120, 1030–1033. [Google Scholar] [CrossRef]
- Filatova, A.Y.; Afanasieva, O.I.; Arefieva, T.I.; Potekhina, A.V.; Tyurina, A.V.; Klesareva, E.A.; Razova, O.A.; Ezhov, M.V.; Pokrovsky, S.N. The Concentration of PCSK9-Lp(a) Complexes and the Level of Blood Monocytes in Males with Coronary Atherosclerosis. J. Pers. Med. 2023, 13, 1077. [Google Scholar] [CrossRef]
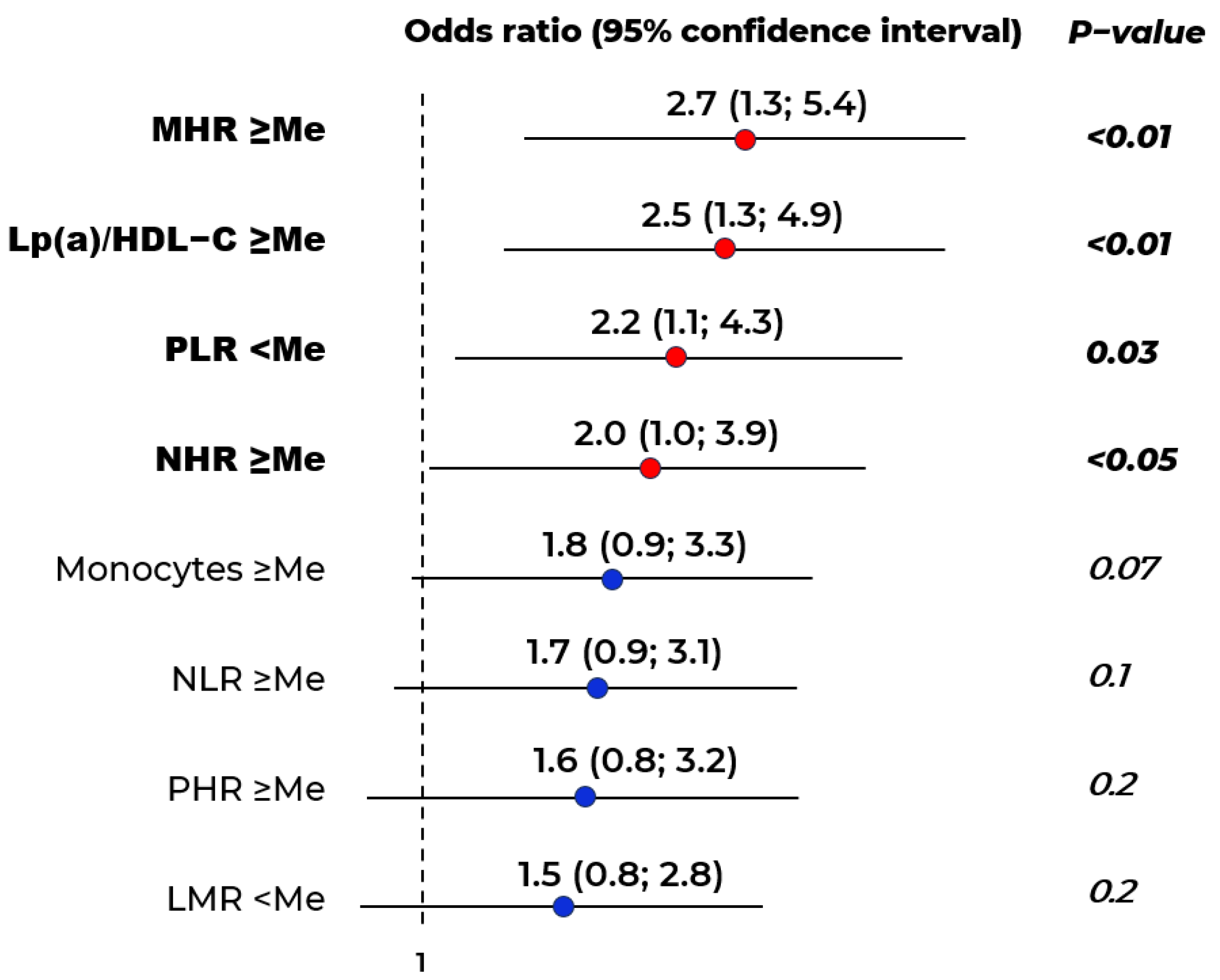


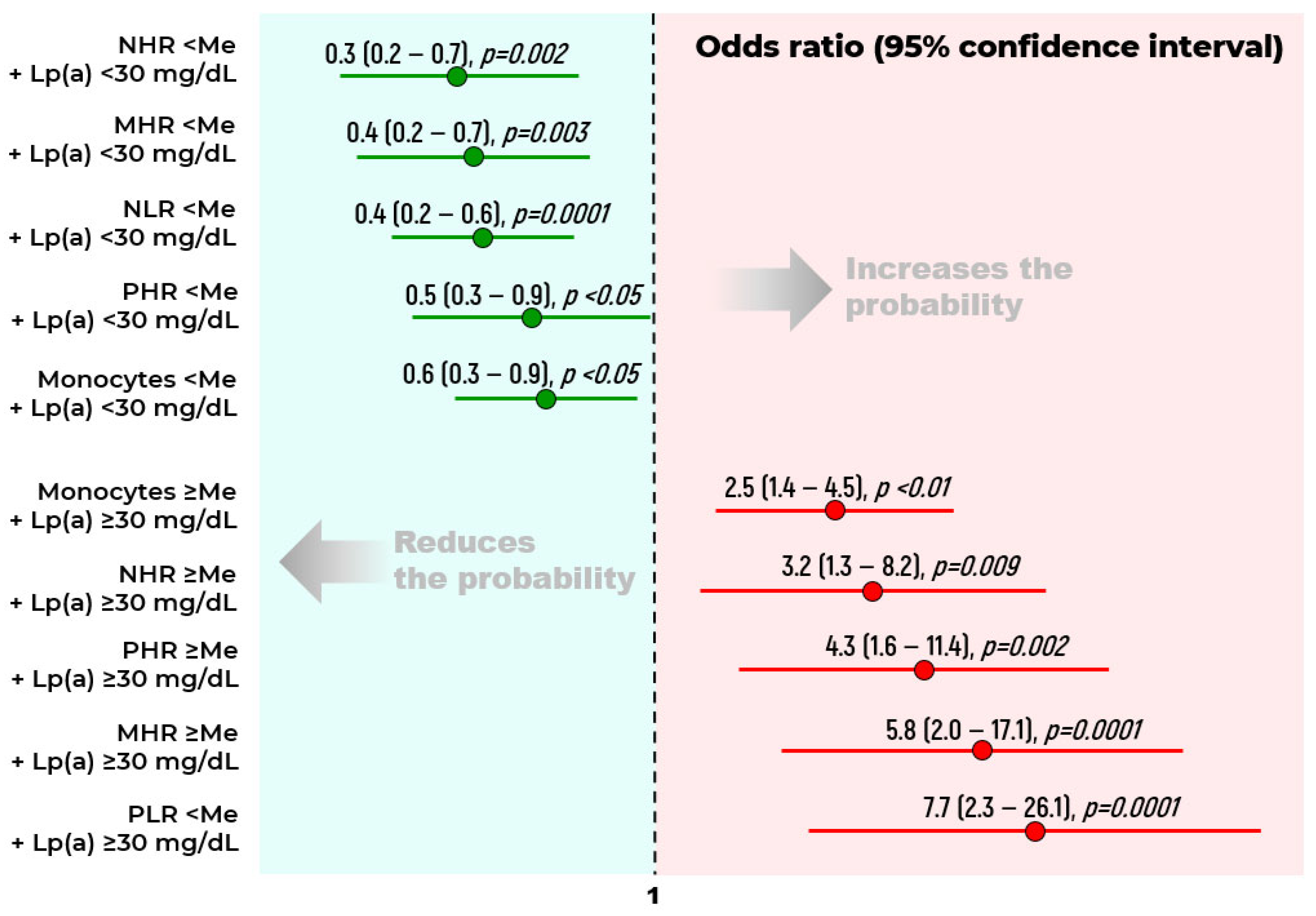
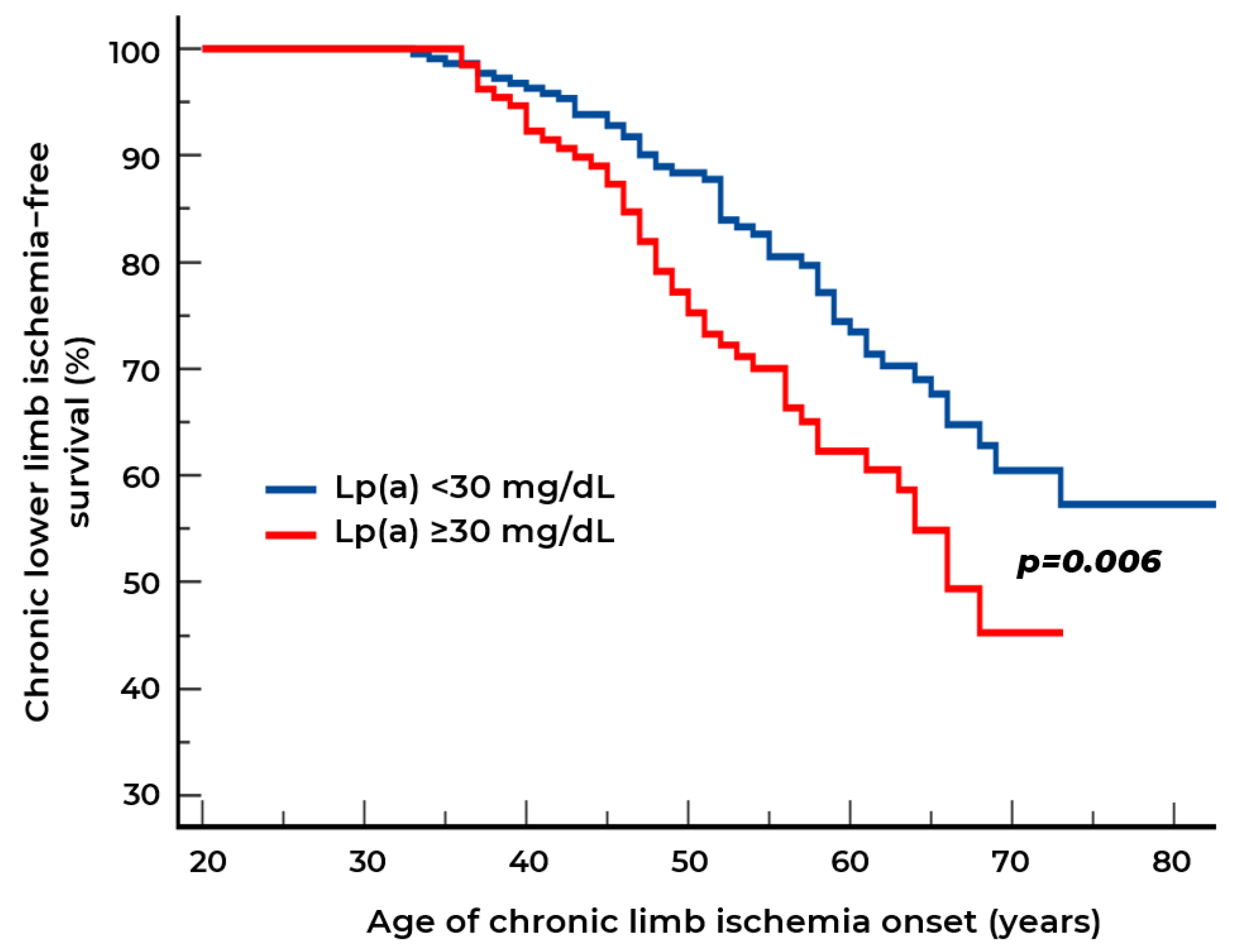
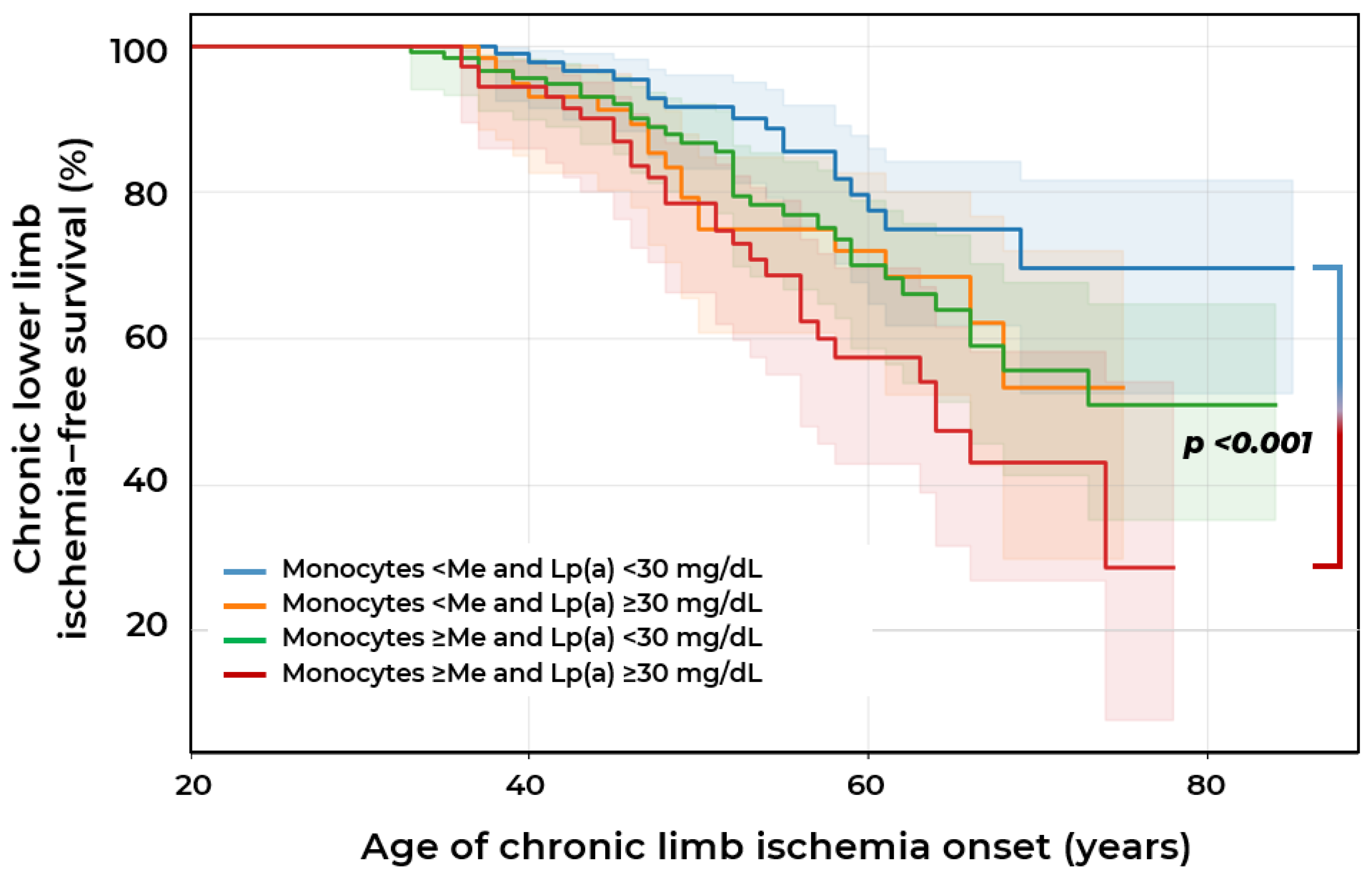
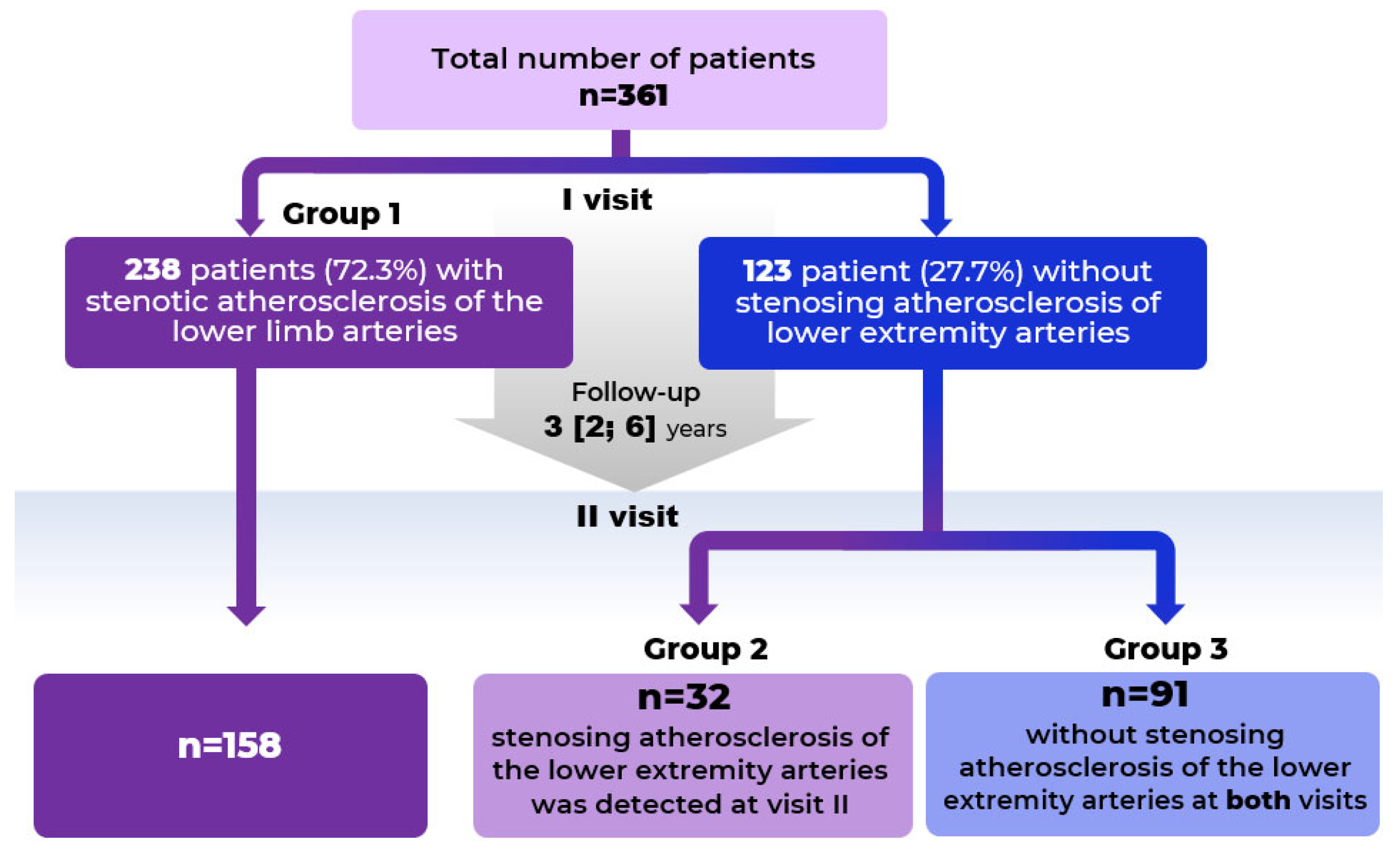
| Indicators | Stenotic Atherosclerosis at Visit I n = 238 (Group 1) | Stenotic Atherosclerosis at Visit II n = 32 (Group 2) | Stenotic Atherosclerosis Is Not Detected at Both Visits n = 91 (Group 3) | p |
|---|---|---|---|---|
| Male sex | 194 (81.5%) | 25 (78.1%) | 59 (64.8%) | 0.006 * p3−1 = 0.004 |
| CAD | 192 (80.7%) | 15 (46.9%) | 38 (41.8%) | <0.001 * p3−1 <0.001 p1−2 < 0.001 |
| Age | 62.0 [54.0; 68.0] | 59.0 [50.8; 70.5] | 56.0 [51.0; 65.0] | 0.1 |
| MI | 122 (51.3%) | 12 (37.5%) | 18 (19.8%) | <0.001 * p3−1 < 0.001 |
| PCI | 128 (53.8%) | 11 (34.4%) | 24 (26.4%) | <0.001 * p3−1 < 0.001 |
| CABG | 28 (11.8%) | 1 (3.1%) | 1 (1.1%) | 0.004 * p3−1 = 0.007 |
| Arterial hypertension | 189 (79.4%) | 21 (65.6%) | 73 (80.2%) | 0.2 |
| Obesity | 171 (71.8%) | 30 (93.8%) | 70 (76.9%) | 0.02 * p1−2 = 0.02 |
| Type 2 DM | 50 (21.0%) | 4 (12.5%) | 12 (13.2%) | 0.2 |
| Statins | 117 (49.2) | 10 (31.2%) | 45 (49.5) | 0.1 |
| The dose of statins equivalent to atorvastatin | 40.0 [20.0; 60.0] | 40.0 [20.0; 40.0] | 40.0 [20.0; 60.0] | 0.9 |
| Complete Blood Count | ||||
| Leukocytes, 109/L | 6.8 [5.0; 9.0] | 6.3 [4.7; 7.8] | 6.0 [5.0; 7.7] | 0.1 |
| Lymphocytes, 109/L | 2.3 [1.8; 2.7] | 2.2 [1.9; 2.5] | 2.1 [1.8; 2.6] | 0.5 |
| Lymphocytes, % | 30.4 ± 8.2 | 32.2 ± 8.5 | 33.26 ± 10.33 | 0.09 |
| Monocytes, 109/L | 0.58 [0.45; 0.71] | 0.57 [0.38; 0.70] | 0.52 [0.40; 0.60] | 0.05 |
| Monocytes, % | 7.92 ± 2.01 | 7.75 ± 2.28 | 7.79 ± 2.07 | 0.9 |
| Neutrophils, 109/L | 4.2 [3.4; 5.4] | 3.9 [3.0; 4.9] | 3.6 [3.0; 4.6] | 0.04 * p1−3 = 0.04 |
| Neutrophils, % | 57.9 ± 8.6 | 56.3 ± 8.6 | 56.0 ± 10.4 | 0.4 |
| Platelets, 109/L | 217.5 [187.5; 262.5] | 241.0 [217.0; 279.0] | 230.0 [199.0; 284.0] | 0.1 |
| Biochemical parameters | ||||
| CRP, mg/L | 1.1 [0.4; 2.4] | 0.7 [0.3; 1.1] | 0.4 [0.1; 0.7] | 0.01 * p1−3 = 0.02 |
| TC, mmol/L | 4.9 [3.9; 6.1] | 5.7 [4.6; 6.4] | 5.4 [4.4; 6.1] | 0.2 |
| HDL-C, mmol/L | 1.07 [0.89; 1.26] | 1.40 [1.02; 1.67] | 1.15 [1.0; 1.5] | 0.003 * p1−3 = 0.02 p2−1 = 0.03 |
| LDL-C, mmol/L | 2.9 [2.1; 4.0] | 3.50 [2.34; 4.11] | 3.2 [2.3; 4.2] | 0.2 |
| TG, mmol/L | 1.51 [1.19; 2.18] | 1.33 [0.99; 1.95] | 1.70 [1.16; 2.29] | 0.4 |
| Lp(a), mg/dL | 20.2 [6.7; 88.5] | 16.7 [7.5; 58.7] | 12.1 [5.2; 26.8] | 0.007 * p1−3 = 0.005 |
| Lp(a) ≥ 30 mg/dL | 103 (43.3%) | 13 (40.6%) | 22 (24.2%) | 0.006 * p3−1 = 0.004 |
| Investigated Indices | ||||
| LMR | 3.9 [3.1; 4.8] | 4.3 [3.0; 5.4] | 4.3 [3.5; 5.1] | 0.3 |
| NLR | 1.8 [1.5; 2.5] | 1.6 [1.3; 2.4] | 1.7 [1.4; 2.3] | 0.2 |
| MHR | 0.54 [0.40; 0.73] | 0.46 [0.25; 0.66] | 0.39 [0.30; 0.55] | 0.002 * p1−3 = 0.002 |
| NHR | 3.9 [2.9; 5.5] | 3.2 [1.9; 3.96] | 3.3 [2.3; 4.1] | 0.002 * p1−3 = 0.007 |
| Lp(a)/HDL-C | 20.9 [6.2; 71.8] | 11.5 [5.6; 54.3] | 8.8 [4.4; 21.4] | 0.003 * p1−3 = 0.002 |
| PHR | 207.2 [162.0; 260.8] | 200.9 [154.6; 246.1] | 192.7 [154.6; 255.6] | 0.6 |
| PLR | 98.1 [74.3; 124.1] | 111.8 [92.2; 122.0] | 115.2 [90.4; 147.8] | 0.03 * p1−3 = 0.03 |
| Indicators | Stenotic Atherosclerosis at Visit I (n = 158) Group 1 | Stenotic Atherosclerosis is Not Detected at Both Visits (n = 91) Group 3 | Stenotic Atherosclerosis at Visit II (n = 32) Group 2 | p |
|---|---|---|---|---|
| Age, years | 62.5 [52.0; 67.8] | 62.0 [55.0; 69.0] | 63.0 [54.3; 71.8] | 0.4 * |
| Male sex | 130 (82.3%) | 59 (64.8%) | 25 (78.1%) | 0.008 p1−3 = 0.006 |
| CAD | 145 (95.4%) | 61 (79.2%) | 27 (90.0%) | <0.001 * p1−3 < 0.001 |
| MI | 111 (70.3%) | 35 (50.0%) | 17 (63.0%) | 0.01 * p1−3 = 0.01 |
| PCI | 76 (59.4%) | 31 (55.4%) | 13 (72.2%) | 0.4 * |
| CABG | 6 (4.7%) | 7 (12.5%) | 1 (5.6%) | 0.1 * |
| Arterial hypertension | 112 (87.5%) | 48 (85.7%) | 16 (88.9%) | 0.9 * |
| Obesity | 42 (32.8%) | 14 (25.0%) | 7 (38.9%) | 0.4 * |
| Type 2 DM | 30 (23.4%) | 14 (25.0%) | 6 (33.3%) | 0.6 * |
| Complete Blood Count | ||||
| Leukocytes, 109/L | 6.6 [5.2; 8.2] | 6.9 [5.6; 8.6] | 6.8 [5.6; 7.8] | 0.7 * |
| Lymphocytes, 109/L | 2.1 [1.7; 2.6] | 2.3 [1.8; 2.8] | 2.2 [1.9; 2.5] | 0.5 * |
| Lymphocytes, % | 30.8 [25.9; 35.8] | 33.9 [28.4; 38.9] | 31.0 [25.3; 34.8] | 0.02 * p1−3 = 0.04 |
| Monocytes, 109/L | 0.6 [0.4; 0.7] | 0.6 [0.5; 0.7] | 0.6 [0.5; 0.7] | 0.8 * |
| Monocytes, % | 7.8 [6.6; 9.2] | 7.9 [6.5; 9.3] | 8.2 [7.1; 10.1] | 0.8 * |
| Neutrophils, 109/L | 4.0 [3.0; 5.3] | 3.9 [3.0; 4.6] | 3.9 [3.3; 4.9] | 0.6 * |
| Neutrophils, % | 56.3 [51.3; 62.8] | 54.8 [51.1; 58.9] | 56.9 [50.4; 63.5] | 0.2 * |
| Platelets, 109/L | 231.5 [192.8; 269.5] | 221.0 [199.0; 274.0] | 227.5 [199.5; 262.3] | 0.9 * |
| Investigated Indices | ||||
| LMR | 4.0 [3.1; 4.7] | 4.0 [3.2; 5.0] | 3.6 [3.0; 4.8] | 0.6 * |
| NLR | 1.8 [1.4; 2.4] | 1.6 [1.3; 2.2] | 1.9 [1.4; 2.5] | 0.07 * |
| MHR | 0.4 [0.3; 0.6] | 0.5 [0.3; 0.6] | 0.6 [0.4; 0.7] | 0.5 * |
| NHR | 3.2 [2.4; 4.5] | 3.5 [2.7; 4.6] | 3.8 [3.1; 4.1] | 0.7 * |
| Lp(a)/HDL-C | 17.8 [5.1; 75.2] | 11.4 [5.7; 27.3] | 39.5 [10.4; 97.8] | 0.2 * |
| PHR | 199.4 [158.1; 247.8] | 192.6 [173.3; 239.5] | 177.3 [164.0; 239.0] | 0.8 * |
| PLR | 102.4 [87.6; 135.7] | 101.7 [79.4; 127.5] | 94.1 [83.9; 120.4] | 0.3 * |
| Biochemical parameters | ||||
| TC, mmol/L | 4.0 [3.4; 5.1] | 4.1 [3.5; 5.1] | 4.0 [3.2; 5.0] | 0.9 * |
| HDL-C, mmol/L | 1.1 [0.9; 1.4] | 1.1 [0.9; 1.3] | 1.0 [0.9; 1.3] | 0.7 * |
| LDL-C, mmol/L | 2.1 [1.6; 2.9] | 2.0 [1.6; 3.0] | 2.5 [1.6; 2.9] | 0.9 * |
| TG, mmol/L | 1.3 [1.0; 1.8] | 1.6 [1.2; 2.3] | 1.5 [1.2; 2.5] | 0.08 * |
| CRP | 1.5 [0.4; 7.7] | 4.6 [0.5; 8.1] | 3.8 [1.4; 10.0] | 0.5 * |
| Lp(a), mg/dL | 27.2 [6.9; 103.2] | 12.1 [5.2; 26.8] | 16.7 [7.5; 58.7] | <0.001 * p1−3 < 0.001 |
| Lp(a) ≥ 30 mg/dL | 75 (47.5%) | 22 (24.2%) | 13 (40.6%) | 0.001 * p1−3 < 0.001 |
| Lp(a) ≥ 50 mg/dL | 59 (38.1%) | 14 (15.6%) | 9 (28.1%) | <0.001 * p1−3 < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyurina, A.V.; Afanasieva, O.I.; Ezhov, M.V.; Tmoyan, N.A.; Balakhonova, T.V.; Pokrovsky, S.N. Role of Lipoprotein(a) and Blood Cells Ratios in Peripheral Artery Disease. Int. J. Mol. Sci. 2025, 26, 9918. https://doi.org/10.3390/ijms26209918
Tyurina AV, Afanasieva OI, Ezhov MV, Tmoyan NA, Balakhonova TV, Pokrovsky SN. Role of Lipoprotein(a) and Blood Cells Ratios in Peripheral Artery Disease. International Journal of Molecular Sciences. 2025; 26(20):9918. https://doi.org/10.3390/ijms26209918
Chicago/Turabian StyleTyurina, Alexandra V., Olga I. Afanasieva, Marat V. Ezhov, Narek A. Tmoyan, Tatiana V. Balakhonova, and Sergei N. Pokrovsky. 2025. "Role of Lipoprotein(a) and Blood Cells Ratios in Peripheral Artery Disease" International Journal of Molecular Sciences 26, no. 20: 9918. https://doi.org/10.3390/ijms26209918
APA StyleTyurina, A. V., Afanasieva, O. I., Ezhov, M. V., Tmoyan, N. A., Balakhonova, T. V., & Pokrovsky, S. N. (2025). Role of Lipoprotein(a) and Blood Cells Ratios in Peripheral Artery Disease. International Journal of Molecular Sciences, 26(20), 9918. https://doi.org/10.3390/ijms26209918







