Correlation of Amine Concentrations in Blood and Cerebrospinal Fluid in Healthy Volunteers and Migraineurs
Abstract
1. Introduction
2. Results
2.1. Amines in Plasma and CSF of Healthy Volunteers
2.1.1. Concentrations of Amines
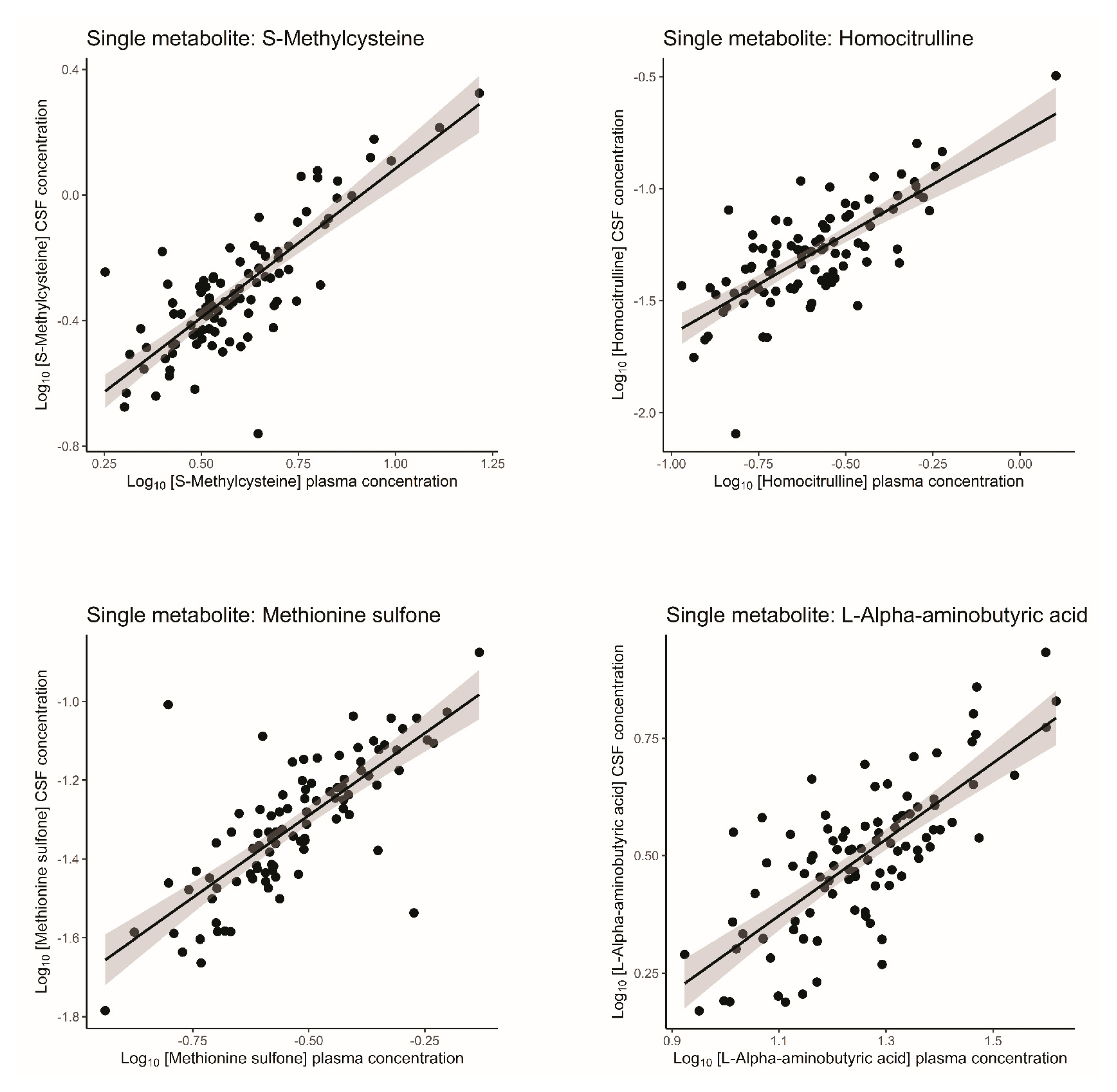
2.1.2. Correlations Between Single-Metabolite Levels in Plasma and CSF in Healthy Volunteers
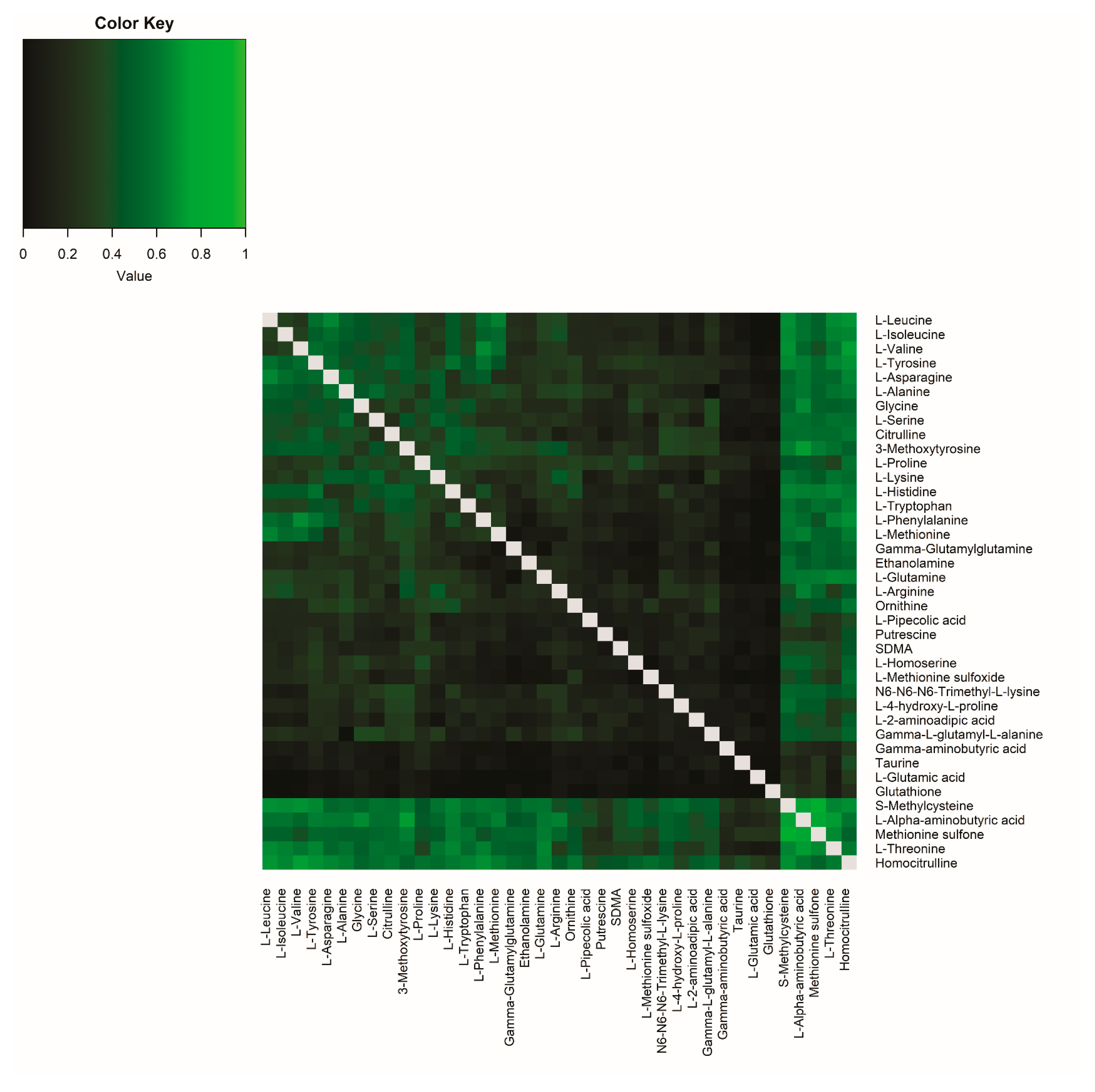
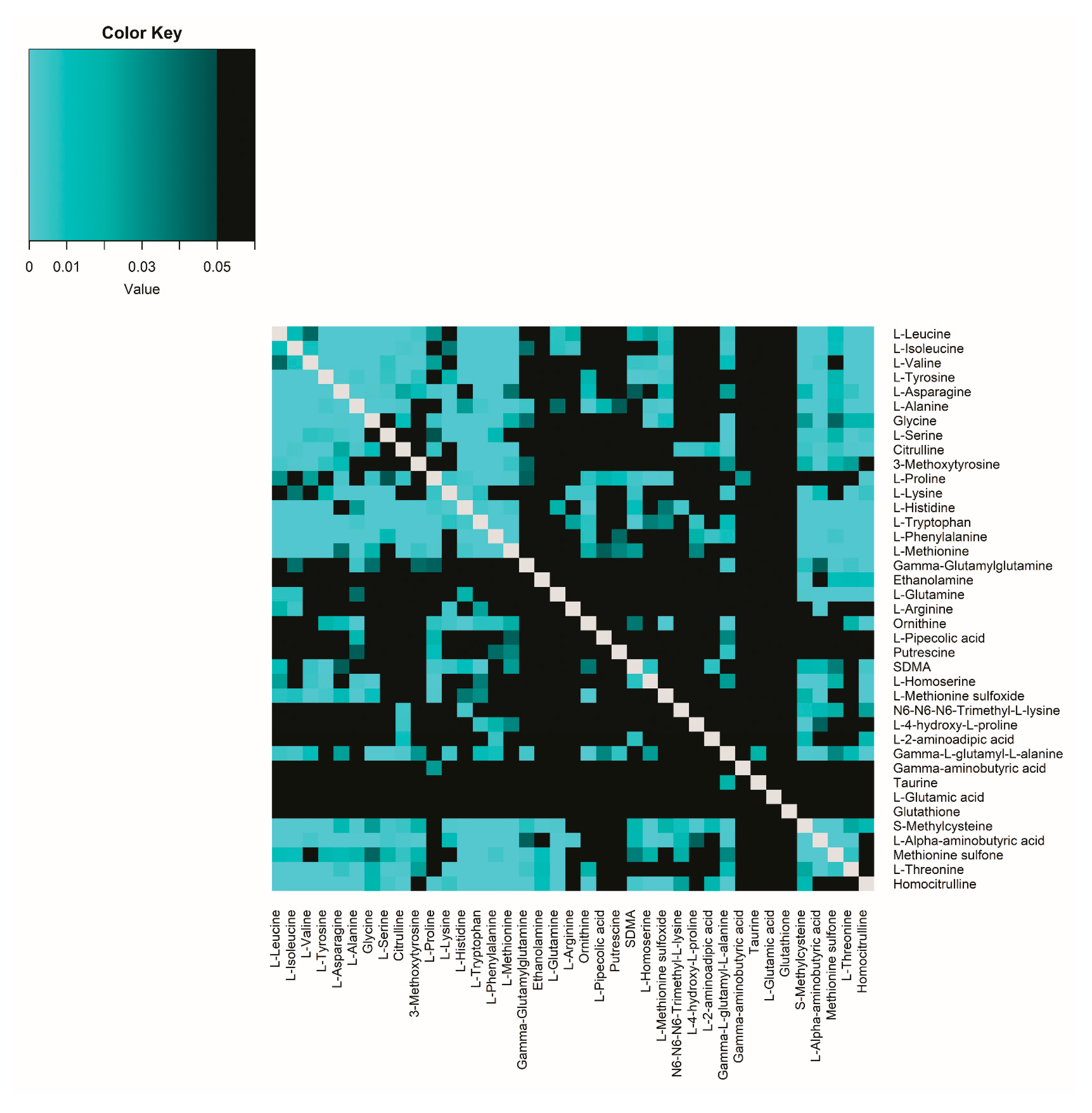
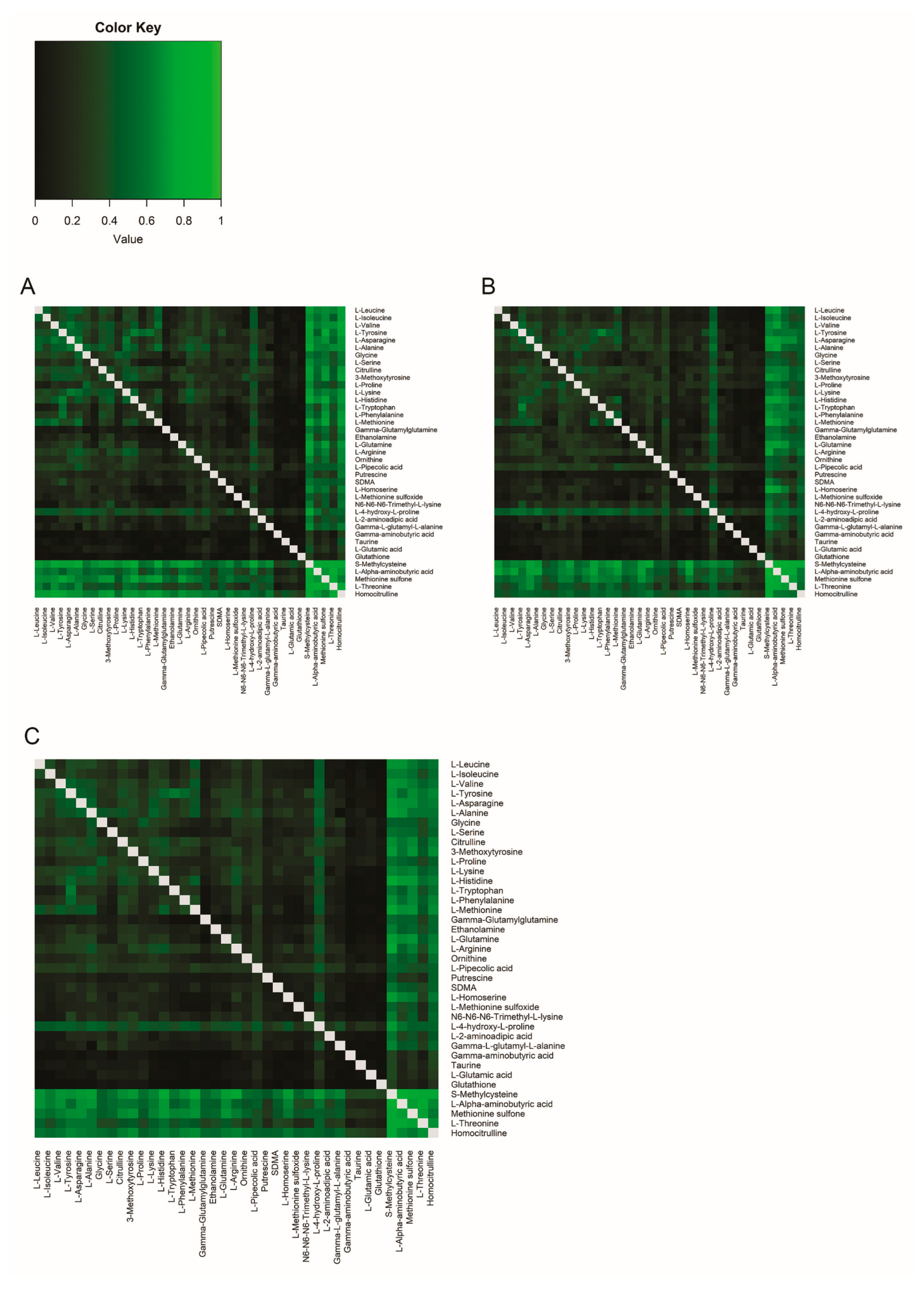
2.1.3. Correlations Between Amine Metabolite Ratios in Plasma and CSF in Healthy Volunteers
2.2. Amines in Plasma and CSF of Participants with Migraine
2.2.1. Concentrations of Amines in Participants with Migraine
2.2.2. Correlations Between Single-Metabolite Levels in Plasma and CSF in Participants with Migraine
2.2.3. Correlations Between Amine Metabolite Ratios in Plasma and CSF in Participants with Migraine
2.3. Comparison Between Healthy Volunteers and Participants with Migraine
2.3.1. Comparison of Single-Metabolite Levels in Plasma and CSF in Healthy Volunteers and Participants with Migraine
2.3.2. Comparison of Correlations Between Amine Metabolite Ratios in Healthy Volunteers and Participants with Migraine
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Study Population
4.3. Sample Collection
4.4. Amine Measurements
4.5. Statistical Analysis
4.5.1. Correlations Between Single-Metabolite Levels in Plasma and CSF
4.5.2. Correlations Between Amine Metabolite Ratios in Plasma and CSF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CSF | Cerebrospinal fluid |
| UPLC-MS | Ultra-performance liquid chromatography mass spectrometry |
| CNS | Central nervous system |
| GABA | γ-aminobutyric acid |
| QC | Quality control |
| PCA | Principal component analysis |
| MO | Migraine without aura |
| MA | Migraine with aura |
| ESI | Electrospray ionization |
| MRM | Multiple Reaction Monitoring |
| NAAs | Neutral amino acids |
| LAT/L1 | L amino acid transport system |
| eNOS | Nitric oxide synthase |
References
- Mokdad, A.H.; Mensah, G.A.; Krish, V.; Glenn, S.D.; Miller-Petrie, M.K.; Lopez, A.D.; Murray, C.J.L. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259, Erratum in: Lancet 2017, 390, e38. [Google Scholar] [CrossRef] [PubMed]
- GDB 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Sadlon, A.; Takousis, P.; Ankli, B.; Alexopoulos, P.; Perneczky, R. Association of Chronic Pain with Biomarkers of Neurodegeneration, Microglial Activation, and Inflammation in Cerebrospinal Fluid and Impaired Cognitive Function. Ann. Neurol. 2023, 95, 195–206. [Google Scholar] [CrossRef]
- Bjurström, M.F.; Bodelsson, M.; Montgomery, A.; Harsten, A.; Waldén, M.; Janelidze, S.; Hall, S.; Hansson, O.; Irwin, M.R.; Mattsson-Carlgren, N. Differential expression of cerebrospinal fluid neuroinflammatory mediators depending on osteoarthritis pain phenotype. Pain 2020, 161, 2142–2154. [Google Scholar] [CrossRef]
- Lajtha, A.; Galoyan, A.; Besedovsky, H. Handbook of Neurochemistry and Molecular Neurobiology: Neuroimmunology; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- van Dongen, R.M.; Zielman, R.; Noga, M.; Dekkers, O.M.; Hankemeier, T.; van den Maagdenberg, A.M.; Terwindt, G.M.; Ferrari, M.D. Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia 2017, 37, 49–63. [Google Scholar] [CrossRef]
- Yam, M.F.; Loh, Y.C.; Tan, C.S.; Khadijah Adam, S.; Abdul Manan, N.; Basir, R. General Pathways of Pain Sensation and the Major Neurotransmitters Involved in Pain Regulation. Int. J. Mol. Sci. 2018, 19, 2164. [Google Scholar] [CrossRef]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Monserrate, A.E.; Ryman, D.C.; Ma, S.; Xiong, C.; Noble, J.M.; Ringman, J.M.; Morris, J.C.; Danek, A.; Muller-Sarnowski, F.; Clifford, D.B.; et al. Factors associated with the onset and persistence of post-lumbar puncture headache. JAMA Neurol. 2015, 72, 325–332. [Google Scholar] [CrossRef]
- van Oosterhout, W.P.; van der Plas, A.A.; van Zwet, E.W.; Zielman, R.; Ferrari, M.D.; Terwindt, G.M. Postdural puncture headache in migraineurs and nonheadache subjects: A prospective study. Neurology 2013, 80, 941–948. [Google Scholar] [CrossRef]
- van Dongen, R.M.; Onderwater, G.L.J.; Pelzer, N.; Zielman, R.; van Oosterhout, W.P.J.; van Zwet, E.W.; Ferrari, M.D.; Terwindt, G.M. The effect of needle size on cerebrospinal fluid collection time and post-dural puncture headache: A retrospective cohort study. Headache 2021, 61, 329–334. [Google Scholar] [CrossRef]
- Otto, C.; Kalantzis, R.; Kübler-Weller, D.; Kühn, A.A.; Böld, T.; Regler, A.; Strathmeyer, S.; Wittmann, J.; Ruprecht, K.; Heelemann, S. Comprehensive analysis of the cerebrospinal fluid and serum metabolome in neurological diseases. J. Neuroinflamm. 2024, 21, 234. [Google Scholar] [CrossRef] [PubMed]
- Hagenfeldt, L.; Bjerkenstedt, L.; Edman, G.; Sedvall, G.; Wiesel, F.A. Amino acids in plasma and CSF and monoamine metabolites in CSF: Interrelationship in healthy subjects. J. Neurochem. 1984, 42, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Kruse, T.; Reiber, H.; Neuhoff, V. Amino acid transport across the human blood-CSF barrier. An evaluation graph for amino acid concentrations in cerebrospinal fluid. J. Neurol. Sci. 1985, 70, 129–138. [Google Scholar] [CrossRef]
- Noga, M.J.; Dane, A.; Shi, S.; Attali, A.; van Aken, H.; Suidgeest, E.; Tuinstra, T.; Muilwijk, B.; Coulier, L.; Luider, T.; et al. Metabolomics of cerebrospinal fluid reveals changes in the central nervous system metabolism in a rat model of multiple sclerosis. Metabolomics 2012, 8, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Goadsby, P.J.; Burstein, R.; Kurth, T.; Ayata, C.; Charles, A.; Ashina, M.; van den Maagdenberg, A.; Dodick, D.W. Migraine. Nat. Rev. Dis. Prim. 2022, 8, 2. [Google Scholar] [CrossRef]
- Onderwater, G.L.J.; van Dongen, R.M.; Harms, A.C.; Zielman, R.; van Oosterhout, W.P.J.; van Klinken, J.B.; Goeman, J.J.; Terwindt, G.M.; van den Maagdenberg, A.; Hankemeier, T.; et al. Cerebrospinal fluid and plasma amine profiles in interictal migraine. Ann. Neurol. 2023, 93, 715–728. [Google Scholar] [CrossRef]
- Zanatta, A.; Viegas, C.M.; Tonin, A.M.; Busanello, E.N.; Grings, M.; Moura, A.P.; Leipnitz, G.; Wajner, M. Disturbance of redox homeostasis by ornithine and homocitrulline in rat cerebellum: A possible mechanism of cerebellar dysfunction in HHH syndrome. Life Sci. 2013, 93, 161–168. [Google Scholar] [CrossRef]
- Viegas, C.M.; Zanatta, A.; Knebel, L.A.; Schuck, P.F.; Tonin, A.M.; Ferreira Gda, C.; Amaral, A.U.; Dutra Filho, C.S.; Wannmacher, C.M.; Wajner, M. Experimental evidence that ornithine and homocitrulline disrupt energy metabolism in brain of young rats. Brain Res. 2009, 1291, 102–112. [Google Scholar] [CrossRef]
- Khovarnagh, N.; Seyedalipour, B. Antioxidant, histopathological and biochemical outcomes of short-term exposure to acetamiprid in liver and brain of rat: The protective role of N-acetylcysteine and S-methylcysteine. Saudi Pharm. J. 2021, 29, 280–289. [Google Scholar] [CrossRef]
- Westergren, I.; Nyström, B.; Hamberger, A.; Nordborg, C.; Johansson, B.B. Concentrations of amino acids in extracellular fluid after opening of the blood-brain barrier by intracarotid infusion of protamine sulfate. J. Neurochem. 1994, 62, 159–165. [Google Scholar] [CrossRef]
- O’Kane, R.L.; Hawkins, R.A. Na+-dependent transport of large neutral amino acids occurs at the abluminal membrane of the blood-brain barrier. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1167–E1173. [Google Scholar] [CrossRef]
- del Amo, E.M.; Urtti, A.; Yliperttula, M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur. J. Pharm. Sci. 2008, 35, 161–174. [Google Scholar] [CrossRef]
- Hawkins, R.A.; O’Kane, R.L.; Simpson, I.A.; Vina, J.R. Structure of the blood-brain barrier and its role in the transport of amino acids. J. Nutr. 2006, 136, 218S–226S. [Google Scholar] [CrossRef]
- Schweikhard, E.S.; Ziegler, C.M. Amino acid secondary transporters: Toward a common transport mechanism. Curr. Top. Membr. 2012, 70, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Nałęcz, K.A. Solute Carriers in the Blood-Brain Barier: Safety in Abundance. Neurochem. Res. 2017, 42, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Li, J.Y.; Nagaya, M.; Zhang, C.; Pardridge, W.M. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 12079–12084. [Google Scholar] [CrossRef] [PubMed]
- Poncet, N.; Mitchell, F.E.; Ibrahim, A.F.; McGuire, V.A.; English, G.; Arthur, J.S.; Shi, Y.B.; Taylor, P.M. The catalytic subunit of the system L1 amino acid transporter (slc7a5) facilitates nutrient signalling in mouse skeletal muscle. PLoS ONE 2014, 9, e89547. [Google Scholar] [CrossRef]
- Zaragozá, R. Transport of Amino Acids Across the Blood-Brain Barrier. Front. Physiol. 2020, 11, 973. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Physiological regulation by plasma neutral amino acids. Science 1972, 178, 414–416. [Google Scholar] [CrossRef]
- O’Kane, R.L.; Vina, J.R.; Simpson, I.; Zaragoza, R.; Mokashi, A.; Hawkins, R.A. Cationic amino acid transport across the blood-brain barrier is mediated exclusively by system y+. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E412–E419. [Google Scholar] [CrossRef]
- McDonald, K.K.; Zharikov, S.; Block, E.R.; Kilberg, M.S. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the “arginine paradox”. J. Biol. Chem. 1997, 272, 31213–31216. [Google Scholar] [CrossRef]
- Olesen, J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol. Ther. 2008, 120, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Bagdy, G.; Riba, P.; Kecskemeti, V.; Chase, D.; Juhasz, G. Headache-type adverse effects of NO donors: Vasodilation and beyond. Br. J. Pharmacol. 2010, 160, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Neeb, L.; Reuter, U. Nitric oxide in migraine. CNS Neurol. Disord. Drug Targets 2007, 6, 258–264. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 209–218. [Google Scholar] [CrossRef]
- Noga, M.J.; Zielman, R.; van Dongen, R.M.; Bos, S.; Harms, A.; Terwindt, G.M.; van den Maagdenberg, A.; Hankemeier, T.; Ferrari, M.D. Strategies to assess and optimize stability of endogenous amines during cerebrospinal fluid sampling. Metabolomics 2018, 14, 44. [Google Scholar] [CrossRef]
- van der Kloet, F.M.; Bobeldijk, I.; Verheij, E.R.; Jellema, R.H. Analytical error reduction using single point calibration for accurate and precise metabolomic phenotyping. J. Proteome Res. 2009, 8, 5132–5141. [Google Scholar] [CrossRef]
- Steiger, J.H. Tests for comparing elements of a correlation matrix. Psychol. Bull. 1980, 87, 245–251. [Google Scholar] [CrossRef]

| Healthy Volunteers | |
|---|---|
| Number of participants | 95 |
| Subject characteristics | |
| Females (n, (%)) | 56 (58.9) |
| Years of age (±SD ‡) | 38.8 (±14.5) |
| BMI † (±SD ‡) | 23.7 (±2.8) |
| Smoking (n, (%)) | 20 (21.1) |
| Overnight fasting | |
| Fasting time in h (±SD ‡) | 11.6 (±2.4) |
| Sampling characteristics | |
| Opening pressure in mmH2O (±SD ‡) | 19.1 (±4.4) |
| CSF characteristics | |
| Erythrocytes count/3 µL (±SD ‡) | 154 (±934) |
| Leukocytes count/3 µL (±SD ‡) | 6 (±6) |
| Protein concentration in g/L (±SD ‡) | 0.35 (±0.13) |
| Glucose in mmol/L (±SD ‡) | 3.2 (±0.3) |
| Metabolite | Plasma (µM) | CSF † (µM) | Plasma/CSF Ratio | ||||
|---|---|---|---|---|---|---|---|
| M | Q1 | Q3 | M | Q1 | Q3 | M | |
| Ethanolamine | 12.088 | 11.004 | 13.338 | 22.679 | 20.239 | 24.404 | 0.55 |
| Putrescine | 0.105 | 0.083 | 0.13 | 0.161 | 0.123 | 0.198 | 0.67 |
| L-Glutamine | 665.43 | 600.158 | 714.841 | 591.26 | 538.944 | 637.634 | 1.12 |
| N6-N6-N6-Trimethyl-L-lysine | 0.468 | 0.39 | 0.568 | 0.404 | 0.365 | 0.447 | 1.17 |
| Gamma-aminobutyric acid | 0.146 | 0.123 | 0.169 | 0.099 | 0.071 | 0.121 | 1.51 |
| L-Homoserine | 0.134 | 0.12 | 0.15 | 0.06 | 0.053 | 0.066 | 2.25 |
| Gamma-Glutamylglutamine | 4.693 | 4.109 | 5.715 | 1.98 | 1.778 | 2.226 | 2.36 |
| L-Arginine | 78.796 | 66.678 | 94.937 | 24.216 | 21.507 | 26.295 | 3.4 |
| L-Threonine | 135.285 | 117.762 | 152.372 | 36.591 | 31.929 | 42.588 | 3.63 |
| SDMA | 0.61 | 0.549 | 0.68 | 0.163 | 0.139 | 0.199 | 3.65 |
| L-Serine | 108.619 | 92.113 | 121.252 | 28.161 | 25.711 | 30.606 | 3.78 |
| L-Histidine | 60.536 | 55.485 | 65.125 | 15.007 | 13.649 | 16.842 | 4.11 |
| Homocitrulline | 0.258 | 0.186 | 0.331 | 0.051 | 0.037 | 0.073 | 4.81 |
| L-Phenylalanine | 55.817 | 51.336 | 62.03 | 10.273 | 9.267 | 11.781 | 5.41 |
| L-Alpha-aminobutyric acid | 18.218 | 14.484 | 21.797 | 3.242 | 2.518 | 3.757 | 5.72 |
| L-Methionine | 23.506 | 21.018 | 26.282 | 3.904 | 3.544 | 4.455 | 5.8 |
| L-Tyrosine | 50.099 | 42.364 | 55.329 | 7.791 | 6.986 | 9.175 | 5.97 |
| 3-Methoxytyrosine | 0.089 | 0.077 | 0.106 | 0.015 | 0.012 | 0.017 | 6.11 |
| Methionine sulfone | 0.285 | 0.242 | 0.376 | 0.047 | 0.037 | 0.063 | 6.17 |
| L-Lysine | 186.795 | 167.22 | 204.311 | 29.905 | 26.963 | 32.829 | 6.22 |
| L-Asparagine | 52.278 | 47.707 | 58.596 | 8.114 | 7.484 | 9.141 | 6.47 |
| L-Methionine sulfoxide | 0.736 | 0.648 | 0.828 | 0.099 | 0.082 | 0.114 | 7.49 |
| S-Methylcysteine | 3.64 | 3.106 | 4.86 | 0.461 | 0.369 | 0.622 | 7.92 |
| L-2-aminoadipic acid | 0.699 | 0.583 | 0.906 | 0.082 | 0.072 | 0.1 | 8.22 |
| L-Leucine | 277.057 | 244.39 | 318.336 | 33.685 | 29.966 | 39.018 | 8.45 |
| L-Alanine | 359.072 | 317.329 | 421.116 | 34.465 | 29.743 | 43.473 | 10.06 |
| L-Isoleucine | 116.684 | 103.751 | 132.595 | 11.702 | 10.009 | 13.24 | 10.46 |
| Ornithine | 65.305 | 55.212 | 74.791 | 5.359 | 4.799 | 6.309 | 11.6 |
| L-Valine | 242.729 | 212.533 | 277.108 | 20.728 | 17.63 | 23.222 | 11.88 |
| L-4-hydroxy-L-proline | 7.569 | 5.951 | 10.286 | 0.664 | 0.474 | 0.838 | 11.97 |
| Gamma-L-glutamyl-L-alanine | 0.507 | 0.411 | 0.61 | 0.041 | 0.036 | 0.046 | 12.01 |
| Citrulline | 29.169 | 25.484 | 33.029 | 2.168 | 1.836 | 2.638 | 12.93 |
| Taurine | 97.824 | 71.535 | 127.601 | 7.397 | 6.461 | 8.244 | 13.03 |
| Glutathione | 5.577 | 4.595 | 6.355 | 0.337 | 0.275 | 0.428 | 15.83 |
| L-Tryptophan | 56.353 | 49.209 | 63.568 | 2.385 | 2.146 | 2.703 | 23.28 |
| Glycine | 296.343 | 237.466 | 333.362 | 8.686 | 7.506 | 10.336 | 33.39 |
| L-Pipecolic acid | 11.26 | 9.687 | 13.694 | 0.34 | 0.258 | 0.41 | 34.28 |
| L-Glutamic acid | 44.036 | 34.697 | 63.195 | 0.399 | 0.35 | 0.447 | 110.32 |
| L-Proline | 178.184 | 140.29 | 218.478 | 0.922 | 0.751 | 1.233 | 183.31 |
| Metabolite | r | R2 | 95% c.i. | FDR |
|---|---|---|---|---|
| Homocitrulline | 0.75 | 0.56 | 0.41–0.68 | 6.16 × 10−17 |
| S-Methylcysteine | 0.75 | 0.56 | 0.41–0.68 | 6.16 × 10−17 |
| Methionine sulfone | 0.75 | 0.56 | 0.41–0.68 | 6.16 × 10−17 |
| L-Alpha-aminobutyric acid | 0.73 | 0.54 | 0.39–0.67 | 2.71 × 10−16 |
| L-Threonine | 0.69 | 0.48 | 0.32–0.62 | 5.03 × 10−14 |
| 3-Methoxytyrosine | 0.64 | 0.40 | 0.24–0.55 | 3.11 × 10−11 |
| L-Arginine | 0.57 | 0.32 | 0.17–0.48 | 1.33 × 10−8 |
| L-Glutamine | 0.47 | 0.22 | 0.09–0.38 | 5.97 × 10−6 |
| L-Lysine | 0.46 | 0.21 | 0.08–0.37 | 1.11 × 10−5 |
| L-Serine | 0.46 | 0.21 | 0.08–0.37 | 1.20 × 10−5 |
| L-Tyrosine | 0.45 | 0.21 | 0.08–0.36 | 1.37 × 10−5 |
| L-Proline | 0.44 | 0.20 | 0.07–0.35 | 2.27 × 10−5 |
| Citrulline | 0.44 | 0.19 | 0.07–0.35 | 2.58 × 10−5 |
| Glycine | 0.43 | 0.18 | 0.06–0.34 | 4.56 × 10−5 |
| L-Asparagine | 0.42 | 0.17 | 0.05–0.33 | 6.85 × 10−5 |
| Ethanolamine | 0.41 | 0.17 | 0.05–0.32 | 9.12 × 10−5 |
| L-Alanine | 0.41 | 0.17 | 0.05–0.32 | 9.96 × 10−5 |
| N6-N6-N6-Trimethyl-L-lysine | 0.40 | 0.16 | 0.05–0.32 | 1.04 × 10−4 |
| L-4-hydroxy-L-proline | 0.36 | 0.13 | 0.03–0.28 | 6.05 × 10−4 |
| L-Isoleucine | 0.35 | 0.12 | 0.03–0.27 | 8.95 × 10−4 |
| L-Leucine | 0.35 | 0.12 | 0.02–0.26 | 1.12 × 10−3 |
| Ornithine | 0.34 | 0.12 | 0.02–0.26 | 1.29 × 10−3 |
| L-Valine | 0.33 | 0.11 | 0.02–0.25 | 1.70 × 10−3 |
| Gamma-Glutamylglutamine | 0.33 | 0.11 | 0.02–0.25 | 2.09 × 10−3 |
| Putrescine | 0.32 | 0.10 | 0.02–0.25 | 2.13 × 10−3 |
| L-2-aminoadipic acid | 0.30 | 0.09 | 0.01–0.23 | 4.59 × 10−3 |
| L-Pipecolic acid | 0.29 | 0.08 | 0.01–0.21 | 7.34 × 10−3 |
| L-Histidine | 0.25 | 0.06 | 0.00–0.18 | 2.31 × 10−2 |
| Taurine | 0.18 | 0.03 | 0.00–0.14 | 1.01 × 10−1 |
| L-Tryptophan | 0.17 | 0.03 | 0.00–0.13 | 1.38 × 10−1 |
| L-Homoserine | 0.16 | 0.03 | 0.00–0.13 | 1.40 × 10−1 |
| L-Methionine | 0.16 | 0.02 | 0.00–0.12 | 1.59 × 10−1 |
| Gamma-L-glutamyl-L-alanine | 0.15 | 0.02 | 0.00–0.12 | 1.64 × 10−1 |
| SDMA | 0.12 | 0.01 | 0.00–0.10 | 2.97 × 10−1 |
| L-Glutamic acid | 0.09 | 0.01 | 0.00–0.08 | 4.56 × 10−1 |
| L-Phenylalanine | 0.08 | 0.01 | 0.00–0.08 | 4.56 × 10−1 |
| Gamma-aminobutyric acid | 0.08 | 0.01 | 0.00–0.08 | 4.80 × 10−1 |
| L-Methionine sulfoxide | 0.06 | 0.00 | 0.00–0.07 | 5.58 × 10−1 |
| Glutathione | 0.02 | 0.00 | 0.00–0.05 | 8.63 × 10−1 |
| MO § Participants | MA ¶ Participants | |
|---|---|---|
| Number of participants | 98 | 99 |
| Subject characteristics | ||
| Females (n, (%)) | 60 (61.2) | 66 (66.7) |
| Years of age (±SD ‡) | 42.0 (±12.9) | 41.7 (±13.5) |
| BMI † (±SD ‡) | 23.6 (±2.5) | 24.0 (±2.8) |
| Smoking (n, (%)) | 13 (13.3) | 13 (13.1) |
| Overnight fasting | ||
| Fasting time in hours (±SD ‡) | 11.9 (±1.6) | 11.7 (±1.6) |
| Sampling characteristics | ||
| Opening pressure in mmH2O (±SD ‡) | 18.0 (±4.7) | 18.8 (±4.1) |
| CSF characteristics | ||
| Erythrocytes count/3 µL (±SD ‡) | 130 (±505) | 2276 (±20,577) |
| Leukocytes count/3 µL (±SD ‡) | 5.0 (±7) | 21.0 (±89) |
| Protein concentration in g/L (±SD ‡) | 0.35 (±0.10) | 0.36 (±0.25) |
| Glucose in mmol/L (±SD ‡) | 3.1 (±0.2) | 3.2 (±0.3) |
| Metabolite | Plasma (µM) | CSF † (µM) | Plasma/CSF Ratio | ||||
|---|---|---|---|---|---|---|---|
| Median | Q1 | Q3 | Median | Q1 | Q3 | Median | |
| Ethanolamine | 12.297 | 11.175 | 13.553 | 21.821 | 18.822 | 23.871 | 0.58 |
| Putrescine | 0.100 | 0.077 | 0.125 | 0.144 | 0.115 | 0.206 | 0.62 |
| L-Glutamine | 674.580 | 595.338 | 752.180 | 589.130 | 515.600 | 637.313 | 1.19 |
| O-Phosphoethanolamine | 10.010 | 6.639 | 13.745 | 7.752 | 6.437 | 8.995 | 1.22 |
| N6-N6-N6-Trimethyl-L-lysine | 0.513 | 0.418 | 0.610 | 0.384 | 0.343 | 0.454 | 1.28 |
| Gamma-aminobutyric acid | 0.141 | 0.127 | 0.163 | 0.100 | 0.074 | 0.129 | 1.41 |
| L-Homoserine | 0.132 | 0.119 | 0.144 | 0.058 | 0.053 | 0.064 | 2.31 |
| Gamma-Glutamylglutamine | 5.014 | 4.231 | 5.832 | 2.091 | 1.833 | 2.386 | 2.49 |
| L-Arginine | 80.671 | 60.536 | 94.243 | 23.307 | 20.515 | 26.424 | 3.50 |
| L-Threonine | 134.514 | 116.573 | 156.030 | 37.440 | 32.806 | 44.239 | 3.62 |
| SDMA | 0.600 | 0.540 | 0.680 | 0.152 | 0.131 | 0.190 | 3.79 |
| L-Serine | 104.086 | 95.945 | 119.437 | 27.274 | 24.831 | 31.276 | 3.95 |
| L-Histidine | 60.834 | 57.208 | 66.340 | 14.658 | 13.246 | 16.115 | 4.33 |
| Homocitrulline | 0.242 | 0.170 | 0.332 | 0.049 | 0.037 | 0.074 | 4.83 |
| L-Phenylalanine | 56.070 | 52.176 | 60.344 | 10.093 | 8.901 | 11.138 | 5.77 |
| L-Alpha-aminobutyric acid | 18.149 | 15.071 | 22.688 | 3.119 | 2.453 | 3.855 | 6.00 |
| L-Tyrosine | 49.079 | 43.117 | 55.010 | 8.053 | 6.849 | 9.219 | 6.20 |
| L-Methionine | 23.253 | 21.219 | 25.536 | 3.905 | 3.331 | 4.257 | 6.22 |
| 3-Methoxytyrosine | 0.093 | 0.083 | 0.108 | 0.015 | 0.013 | 0.017 | 6.31 |
| Methionine sulfone | 0.304 | 0.220 | 0.381 | 0.045 | 0.035 | 0.060 | 6.37 |
| L-Asparagine | 51.826 | 46.566 | 57.312 | 7.849 | 6.839 | 9.082 | 6.66 |
| L-Lysine | 200.450 | 175.718 | 217.740 | 29.707 | 24.925 | 34.051 | 6.68 |
| L-Methionine sulfoxide | 0.723 | 0.619 | 0.806 | 0.097 | 0.081 | 0.117 | 7.22 |
| S-Methylcysteine | 3.744 | 3.037 | 4.854 | 0.464 | 0.371 | 0.647 | 8.14 |
| L-Leucine | 280.280 | 255.741 | 320.453 | 32.286 | 28.673 | 39.077 | 8.59 |
| L-2-aminoadipic acid | 0.775 | 0.648 | 0.997 | 0.092 | 0.080 | 0.104 | 8.61 |
| L-Alanine | 374.163 | 326.391 | 429.543 | 35.577 | 30.439 | 41.706 | 10.84 |
| L-Isoleucine | 118.753 | 104.690 | 133.144 | 11.132 | 9.200 | 13.814 | 11.03 |
| Taurine | 92.333 | 69.557 | 116.352 | 7.763 | 6.767 | 8.755 | 12.16 |
| L-Valine | 245.782 | 223.515 | 277.096 | 20.551 | 17.680 | 24.981 | 12.36 |
| Gamma-L-glutamyl-L-alanine | 0.532 | 0.441 | 0.672 | 0.044 | 0.040 | 0.051 | 12.60 |
| L-4-hydroxy-L-proline | 8.812 | 6.417 | 12.451 | 0.685 | 0.554 | 0.938 | 12.73 |
| Ornithine | 65.407 | 51.951 | 79.256 | 5.250 | 4.162 | 6.138 | 12.83 |
| Citrulline | 28.087 | 23.808 | 35.088 | 2.151 | 1.697 | 2.619 | 13.21 |
| Glutathione | 5.661 | 4.636 | 6.485 | 0.400 | 0.312 | 0.493 | 14.56 |
| L.Tryptophan | 57.474 | 51.837 | 61.938 | 2.284 | 2.000 | 2.700 | 24.75 |
| Glycine | 289.064 | 235.273 | 334.399 | 8.261 | 7.287 | 9.859 | 32.34 |
| L-Pipecolic acid | 10.778 | 8.670 | 14.545 | 0.323 | 0.257 | 0.403 | 35.21 |
| L-Glutamic acid | 40.028 | 32.485 | 54.316 | 0.387 | 0.355 | 0.478 | 107.70 |
| L-Proline | 171.004 | 140.167 | 209.404 | 0.984 | 0.805 | 1.235 | 181.27 |
| Metabolite | Plasma (µM) | CSF † (µM) | Plasma/CSF Ratio | ||||
|---|---|---|---|---|---|---|---|
| Median | Q1 | Q3 | Median | Q1 | Q3 | Median | |
| Ethanolamine | 11.578 | 10.470 | 12.732 | 20.911 | 18.792 | 22.715 | 0.55 |
| Putrescine | 0.096 | 0.079 | 0.119 | 0.157 | 0.122 | 0.198 | 0.63 |
| L-Glutamine | 653.450 | 586.971 | 711.809 | 567.820 | 516.139 | 615.613 | 1.15 |
| N6-N6-N6-Trimethyl-L-lysine | 0.469 | 0.395 | 0.564 | 0.396 | 0.364 | 0.433 | 1.18 |
| O-Phosphoethanolamine | 9.092 | 6.046 | 13.232 | 6.789 | 6.028 | 7.943 | 1.24 |
| Gamma-aminobutyric acid | 0.141 | 0.126 | 0.162 | 0.098 | 0.073 | 0.123 | 1.46 |
| L-Homoserine | 0.132 | 0.117 | 0.147 | 0.060 | 0.053 | 0.065 | 2.28 |
| Gamma-Glutamylglutamine | 5.079 | 4.119 | 5.593 | 1.968 | 1.682 | 2.271 | 2.52 |
| L-Arginine | 72.078 | 60.850 | 81.213 | 22.499 | 19.179 | 24.445 | 3.22 |
| SDMA | 0.575 | 0.511 | 0.640 | 0.155 | 0.135 | 0.182 | 3.58 |
| L-Threonine | 131.388 | 114.403 | 146.662 | 37.090 | 30.923 | 41.640 | 3.61 |
| L-Serine | 109.030 | 93.263 | 124.880 | 28.032 | 24.813 | 32.959 | 3.86 |
| L-Histidine | 60.959 | 55.442 | 65.949 | 14.569 | 13.181 | 16.302 | 4.12 |
| Homocitrulline | 0.221 | 0.183 | 0.321 | 0.049 | 0.039 | 0.066 | 4.78 |
| L-Phenylalanine | 55.540 | 50.081 | 60.634 | 10.001 | 8.832 | 11.155 | 5.47 |
| L-Alpha-aminobutyric acid | 18.415 | 15.313 | 21.751 | 3.141 | 2.386 | 4.094 | 5.78 |
| L-Tyrosine | 49.443 | 42.673 | 56.030 | 8.247 | 6.846 | 9.526 | 5.81 |
| L-Methionine | 22.550 | 20.440 | 24.734 | 3.704 | 3.175 | 4.290 | 5.87 |
| 3-Methoxytyrosine | 0.088 | 0.077 | 0.105 | 0.014 | 0.013 | 0.017 | 6.05 |
| Methionine sulfone | 0.290 | 0.230 | 0.363 | 0.048 | 0.036 | 0.061 | 6.21 |
| L-Asparagine | 49.761 | 46.959 | 54.779 | 7.571 | 6.818 | 8.638 | 6.57 |
| L-Lysine | 188.289 | 171.233 | 214.701 | 29.191 | 25.893 | 32.633 | 6.64 |
| L-Methionine sulfoxide | 0.730 | 0.624 | 0.828 | 0.098 | 0.079 | 0.120 | 7.51 |
| S-Methylcysteine | 3.529 | 2.837 | 4.965 | 0.491 | 0.364 | 0.640 | 7.56 |
| L-2-aminoadipic acid | 0.716 | 0.593 | 0.899 | 0.088 | 0.074 | 0.102 | 8.26 |
| L-Leucine | 264.337 | 240.759 | 307.927 | 32.152 | 27.056 | 37.816 | 8.44 |
| L-Isoleucine | 112.672 | 99.368 | 130.394 | 10.764 | 9.395 | 12.632 | 10.49 |
| L-Alanine | 379.229 | 326.339 | 442.442 | 34.173 | 30.171 | 40.508 | 10.74 |
| Taurine | 83.719 | 64.708 | 113.254 | 7.117 | 6.093 | 8.013 | 11.14 |
| L-Valine | 235.246 | 213.624 | 269.877 | 21.665 | 16.751 | 24.563 | 11.86 |
| L-4-hydroxy-L-proline | 7.770 | 6.020 | 10.363 | 0.644 | 0.485 | 0.832 | 12.10 |
| Gamma-L-glutamyl-L-alanine | 0.540 | 0.462 | 0.651 | 0.042 | 0.037 | 0.048 | 12.55 |
| Citrulline | 27.375 | 22.837 | 31.007 | 2.021 | 1.758 | 2.428 | 12.69 |
| Ornithine | 62.759 | 51.411 | 78.293 | 5.143 | 4.161 | 6.015 | 13.09 |
| Glutathione | 5.404 | 4.490 | 6.696 | 0.327 | 0.267 | 0.445 | 15.62 |
| L-Tryptophan | 54.546 | 47.679 | 59.223 | 2.338 | 2.098 | 2.608 | 23.17 |
| Glycine | 286.608 | 235.756 | 352.980 | 8.037 | 7.021 | 10.223 | 34.24 |
| L-Pipecolic acid | 10.626 | 8.529 | 12.854 | 0.313 | 0.258 | 0.397 | 34.61 |
| L-Glutamic acid | 39.946 | 28.893 | 58.014 | 0.391 | 0.359 | 0.458 | 99.13 |
| L-Proline | 168.383 | 133.030 | 213.782 | 0.910 | 0.749 | 1.177 | 174.50 |
| Metabolite | MO | MA | ||||||
|---|---|---|---|---|---|---|---|---|
| r | R2 | 95% c.i. | FDR | r | R2 | 95% c.i. | FDR | |
| S-Methylcysteine | 0.84 | 0.71 | 0.60–0.80 | 4.44 × 10−26 | 0.86 | 0.74 | 0.64–0.82 | 1.04 × 10−28 |
| Methionine sulfone | 0.79 | 0.63 | 0.50–0.74 | 2.94 × 10−21 | 0.79 | 0.62 | 0.49–0.73 | 3.25 × 10−21 |
| Homocitrulline | 0.78 | 0.61 | 0.47–0.72 | 2.86 × 10−20 | 0.72 | 0.51 | 0.36–0.64 | 8.16 × 10−16 |
| L-Alpha-aminobutyric acid | 0.73 | 0.54 | 0.39–0.66 | 9.66 × 10−17 | 0.80 | 0.63 | 0.50–0.74 | 1.43 × 10−21 |
| L-Threonine | 0.68 | 0.47 | 0.31–0.61 | 6.85 × 10−14 | 0.62 | 0.39 | 0.23–0.53 | 4.40 × 10−11 |
| L-4-hydroxy-L-proline | 0.63 | 0.40 | 0.24–0.55 | 1.86 × 10−11 | 0.64 | 0.41 | 0.26–0.56 | 6.02 × 10−12 |
| L-Arginine | 0.56 | 0.32 | 0.17–0.47 | 9.56 × 10−9 | 0.45 | 0.21 | 0.08–0.36 | 7.27 × 10−6 |
| 3-Methoxytyrosine | 0.55 | 0.30 | 0.15–0.45 | 2.93 × 10−8 | 0.60 | 0.36 | 0.20–0.51 | 3.77 × 10−10 |
| Citrulline | 0.54 | 0.29 | 0.15–0.45 | 3.71 × 10−8 | 0.48 | 0.23 | 0.09–0.38 | 2.25 × 10−6 |
| L-Alanine | 0.54 | 0.29 | 0.14–0.45 | 3.71 × 10−8 | 0.44 | 0.19 | 0.07–0.34 | 1.84 × 10−5 |
| L-Lysine | 0.53 | 0.28 | 0.14–0.44 | 5.99 × 10−8 | 0.35 | 0.13 | 0.03–0.27 | 5.73 × 10−4 |
| L-Pipecolic acid | 0.52 | 0.27 | 0.12–0.42 | 1.57 × 10−7 | 0.56 | 0.32 | 0.17–0.47 | 6.38 × 10−9 |
| L-Tyrosine | 0.48 | 0.23 | 0.10–0.39 | 1.55 × 10−6 | 0.51 | 0.26 | 0.12–0.41 | 3.72 × 10−7 |
| Ornithine | 0.46 | 0.21 | 0.08–0.37 | 4.85 × 10−6 | 0.33 | 0.11 | 0.02–0.24 | 1.66 × 10−3 |
| L-Asparagine | 0.45 | 0.21 | 0.08–0.36 | 7.05 × 10−6 | 0.37 | 0.14 | 0.03–0.28 | 2.96 × 10−4 |
| Glycine | 0.41 | 0.17 | 0.05–0.32 | 7.11 × 10−5 | 0.36 | 0.13 | 0.03–0.27 | 4.58 × 10−4 |
| L-Proline | 0.38 | 0.15 | 0.04–0.29 | 2.27 × 10−4 | 0.47 | 0.22 | 0.09–0.38 | 2.78 × 10−6 |
| L-Valine | 0.37 | 0.14 | 0.03–0.28 | 3.74 × 10−4 | 0.38 | 0.14 | 0.04–0.29 | 2.23 × 10−4 |
| N6-N6-N6-Trimethyl-L-lysine | 0.36 | 0.13 | 0.03–0.28 | 5.13 × 10−4 | 0.43 | 0.18 | 0.06–0.34 | 2.53 × 10−5 |
| L-Glutamine | 0.35 | 0.12 | 0.02–0.26 | 9.34 × 10−4 | 0.40 | 0.16 | 0.05–0.31 | 8.91 × 10−5 |
| L-Histidine | 0.32 | 0.11 | 0.02–0.24 | 2.03 × 10−3 | 0.25 | 0.06 | 0.00–0.19 | 1.54 × 10−2 |
| L-Isoleucine | 0.32 | 0.10 | 0.02–0.24 | 2.04 × 10−3 | 0.22 | 0.05 | 0.00–0.16 | 4.06 × 10−2 |
| Gamma-L-glutamyl-L-alanine | 0.31 | 0.09 | 0.01–0.23 | 3.78 × 10−3 | 0.21 | 0.04 | 0.00–0.15 | 4.76 × 10−2 |
| L-2-aminoadipic acid | 0.30 | 0.09 | 0.01–0.22 | 4.49 × 10−3 | 0.41 | 0.16 | 0.05–0.31 | 8.31 × 10−5 |
| Putrescine | 0.29 | 0.08 | 0.01–0.21 | 6.09 × 10−3 | 0.19 | 0.04 | 0.00–0.14 | 7.30 × 10−2 |
| L-Serine | 0.28 | 0.08 | 0.01–0.21 | 7.59 × 10−3 | 0.40 | 0.16 | 0.05–0.31 | 8.41 × 10−5 |
| L-Leucine | 0.25 | 0.06 | 0.00–0.18 | 1.94 × 10−2 | 0.29 | 0.08 | 0.01–0.21 | 5.45 × 10−3 |
| SDMA | 0.23 | 0.05 | 0.00–0.17 | 2.88 × 10−2 | 0.10 | 0.01 | 0.00–0.09 | 3.79 × 10−1 |
| L-Methionine | 0.21 | 0.04 | 0.00–0.15 | 5.37 × 10−2 | 0.30 | 0.09 | 0.01–0.22 | 4.12 × 10−3 |
| L-Methionine sulfoxide | 0.20 | 0.04 | 0.00–0.15 | 6.20 × 10−2 | 0.19 | 0.04 | 0.00–0.14 | 6.49 × 10−2 |
| Gamma-Glutamylglutamine | 0.13 | 0.02 | 0.00–0.11 | 2.42 × 10−1 | 0.23 | 0.05 | 0.00–0.17 | 2.79 × 10−2 |
| Ethanolamine | 0.12 | 0.01 | 0.00–0.10 | 3.11 × 10−1 | 0.40 | 0.16 | 0.05–0.31 | 8.79 × 10−5 |
| Gamma-aminobutyric acid | −0.11 | 0.01 | 0.00–0.09 | 3.29 × 10−1 | 0.04 | 0.00 | 0.00–0.06 | 7.21 × 10−1 |
| Taurine | −0.11 | 0.01 | 0.00–0.09 | 3.35 × 10−1 | 0.20 | 0.04 | 0.00–0.15 | 6.29 × 10−2 |
| L-Homoserine | 0.11 | 0.01 | 0.00–0.09 | 3.37 × 10−1 | 0.32 | 0.10 | 0.02–0.24 | 2.19 × 10−3 |
| L-Phenylalanine | −0.05 | 0.00 | 0.00–0.06 | 6.95 × 10−1 | 0.33 | 0.11 | 0.02–0.25 | 1.59 × 10−3 |
| L-Glutamic acid | 0.04 | 0.00 | 0.00–0.06 | 7.15 × 10−1 | 0.05 | 0.00 | 0.00–0.06 | 6.31 × 10−1 |
| Glutathione | 0.03 | 0.00 | 0.00–0.05 | 7.67 × 10−1 | 0.09 | 0.01 | 0.00–0.08 | 3.91 × 10−1 |
| L-Tryptophan | −0.03 | 0.00 | 0.00–0.05 | 7.67 × 10−1 | 0.08 | 0.01 | 0.00–0.08 | 4.36 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harder, A.V.E.; van Klinken, J.B.; van Dongen, R.M.; Onderwater, G.L.J.; Ferrari, M.D.; Harms, A.C.; Hankemeier, T.; Terwindt, G.M.; van den Maagdenberg, A.M.J.M. Correlation of Amine Concentrations in Blood and Cerebrospinal Fluid in Healthy Volunteers and Migraineurs. Int. J. Mol. Sci. 2025, 26, 9899. https://doi.org/10.3390/ijms26209899
Harder AVE, van Klinken JB, van Dongen RM, Onderwater GLJ, Ferrari MD, Harms AC, Hankemeier T, Terwindt GM, van den Maagdenberg AMJM. Correlation of Amine Concentrations in Blood and Cerebrospinal Fluid in Healthy Volunteers and Migraineurs. International Journal of Molecular Sciences. 2025; 26(20):9899. https://doi.org/10.3390/ijms26209899
Chicago/Turabian StyleHarder, Aster V. E., Jan B. van Klinken, Robin M. van Dongen, Gerrit L. J. Onderwater, Michel D. Ferrari, Amy C. Harms, Thomas Hankemeier, Gisela M. Terwindt, and Arn M. J. M. van den Maagdenberg. 2025. "Correlation of Amine Concentrations in Blood and Cerebrospinal Fluid in Healthy Volunteers and Migraineurs" International Journal of Molecular Sciences 26, no. 20: 9899. https://doi.org/10.3390/ijms26209899
APA StyleHarder, A. V. E., van Klinken, J. B., van Dongen, R. M., Onderwater, G. L. J., Ferrari, M. D., Harms, A. C., Hankemeier, T., Terwindt, G. M., & van den Maagdenberg, A. M. J. M. (2025). Correlation of Amine Concentrations in Blood and Cerebrospinal Fluid in Healthy Volunteers and Migraineurs. International Journal of Molecular Sciences, 26(20), 9899. https://doi.org/10.3390/ijms26209899







