Targeting Ferroptosis as the Achilles’ Heel of Breast Cancer: Mechanisms and Therapeutic Opportunities from a Comprehensive Systematic Review
Abstract
1. Introduction
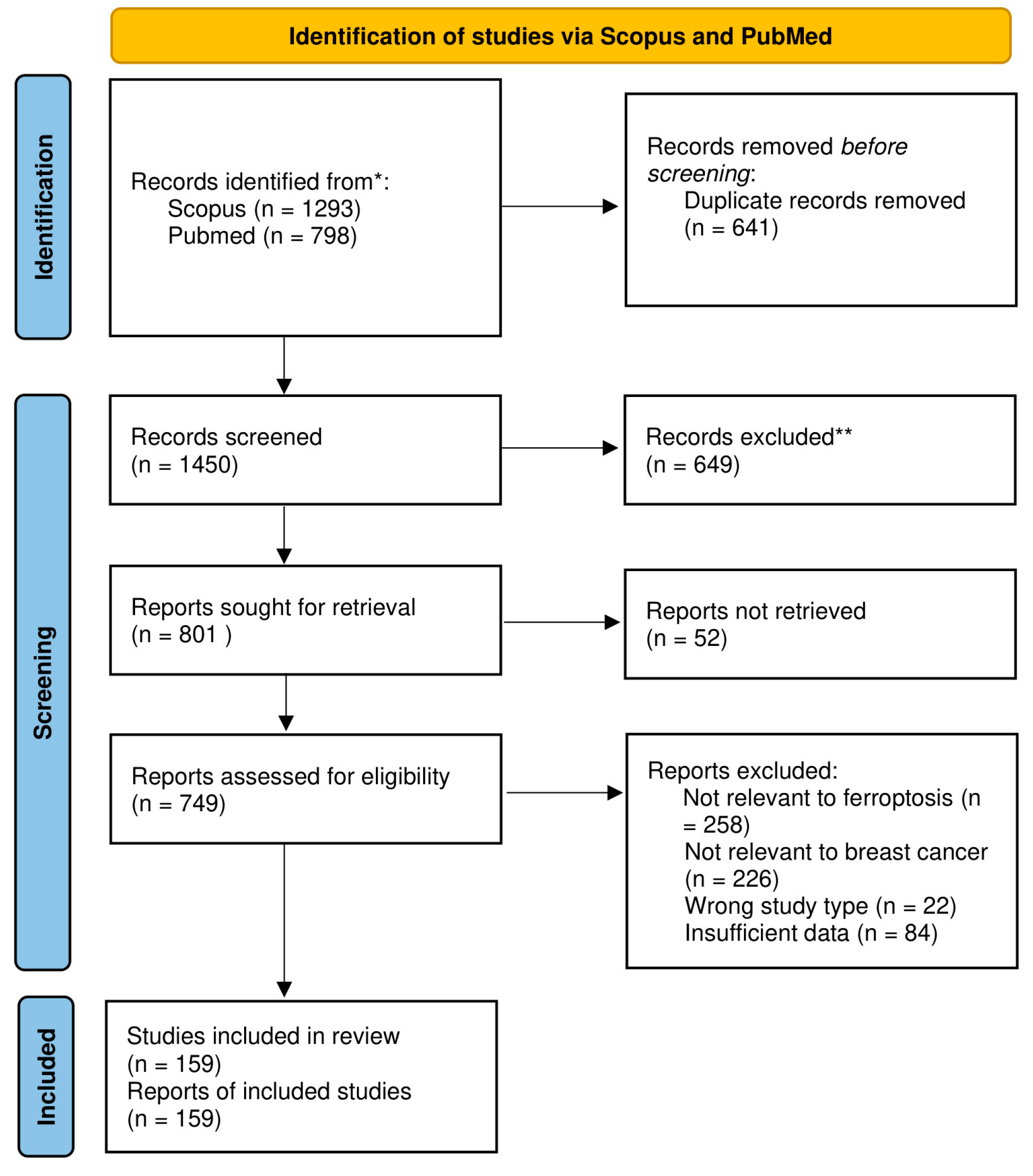
2. Materials and Methods
3. Results and Discussion
3.1. Tumor Microenvironment
3.2. Iron Metabolism and Ferroptosis in Breast Cancer
3.2.1. Defense Mechanisms of Breast Cancer Against Ferroptosis
3.2.2. Achilles’ Heel of Breast Cancer in Ferroptosis
3.3. Compounds Targeting Ferroptosis in Breast Cancer
3.3.1. Exploring Clinical Potential
3.3.2. Insights from In Vivo Studies
3.3.3. Insights from In Vitro Studies
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 3 March 2025).
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zheng, S.; Yang, A.; Zhang, X.; Zou, Y.; Tang, H.; Xie, X. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: A population-based study. Cancer Manag. Res. 2018, 10, 5329–5338. [Google Scholar] [CrossRef]
- Nolan, E.; Lindeman, G.J.; Visvader, J.E. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Loprinzi, C.L.; Haddad, T.C. Updates in the evaluation and management of breast cancer. Mayo Clin. Proc. 2018, 93, 794–807. [Google Scholar] [CrossRef]
- Lee, E.; Song, C.H.; Bae, S.J.; Ha, K.T.; Karki, R. Regulated cell death pathways and their roles in homeostasis, infection, inflammation, and tumorigenesis. Exp. Mol. Med. 2023, 55, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef]
- Wu, Q.; Siddharth, S.; Sharma, D. Triple-negative breast cancer: A mountain yet to be scaled despite the triumphs. Cancers 2021, 13, 3697. [Google Scholar] [CrossRef]
- Engmann, N.J.; Golmakani, M.K.; Miglioretti, D.L.; Sprague, B.L.; Kerlikowske, K. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017, 3, 1228–1236, Erratum in JAMA Oncol. 2019, 5, 1643. [Google Scholar] [CrossRef]
- Berg, W.A.; Rafferty, E.A.; Friedewald, S.M.; Hruska, C.B.; Rahbar, H. Screening algorithms in dense breasts: AJR expert panel narrative review. AJR Am. J. Roentgenol. 2021, 216, 275–294. [Google Scholar] [CrossRef]
- Boyd, N.F.; Guo, H.; Martin, L.J.; Sun, L.; Stone, J.; Fishell, E.; Jong, R.; Hislop, G.; Chiarelli, A.; Minkin, S.; et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007, 356, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, S.P.; Slanetz, P.J.; Lewin, A.A.; Battaglia, T.; Chagpar, A.B.; Dayaratna, S.; Dibble, E.H.; Goel, M.S.; Hayward, J.H.; Kubicky, C.D.; et al. ACR Appropriateness Criteria® Supplemental Breast Cancer Screening Based on Breast Density. J. Am. Coll. Radiol. 2021, 18, S456–S473. [Google Scholar] [CrossRef] [PubMed]
- Mandelson, M.T. Breast density as a predictor of mammographic detection: Comparison of interval- and screen-detected cancers. J. Natl. Cancer Inst. 2000, 92, 1081–1087. [Google Scholar] [CrossRef]
- Leek, R.D.; Landers, R.J.; Harris, A.L.; Lewis, C.E. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br. J. Cancer 1999, 79, 991–995. [Google Scholar] [CrossRef]
- Fisher, E.R.; Sass, R.; Fisher, B. Pathologic findings from the national surgical adjuvant project for breast cancers (protocol no. 4) X. Discriminants for tenth year treatment failure. Cancer 1984, 53, 712–723. [Google Scholar] [CrossRef]
- Jimenez, R.E.; Wallis, T.; Visscher, D.W. Centrally necrotizing carcinomas of the breast: A distinct histologic subtype with aggressive clinical behavior. Am. J. Surg. Pathol. 2001, 25, 331–337. [Google Scholar] [CrossRef]
- Li, X.F.; Carlin, S.; Urano, M.; Russell, J.; Ling, C.C.; O’Donoghue, J.A. Visualization of hypoxia in microscopic tumors by immunofluorescent microscopy. Cancer Res. 2007, 67, 7646–7653. [Google Scholar] [CrossRef]
- Fleisher, T.A. Apoptosis. Ann. Allergy Asthma Immunol. 1997, 78, 245–249. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The hallmarks of ferroptosis. Annu. Rev. Cancer Biol. 2019, 3, 35–54. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the crossroads of lipid homeostasis and ferroptosis. Proteomics 2019, 19, 1800311. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Gao, M.; Monian, P.; Quadri, N. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef]
- Okazaki, S.; Umene, K.; Yamasaki, J. Glutaminolysis-related genes determine sensitivity to xCT-targeted therapy in head and neck squamous cell carcinoma. Cancer Sci. 2019, 110, 3453–3463. [Google Scholar] [CrossRef]
- Gao, M.; Yi, J.; Zhu, J. Role of mitochondria in ferroptosis. Mol. Cell 2019, 73, 354–363.e3. [Google Scholar] [CrossRef]
- Tonnus, W.; Belavgeni, A.; Beuschlein, F.; Eisenhofer, G.; Fassnacht, M.; Kroiss, M.; Krone, N.P.; Reincke, M.; Bornstein, S.R.; Linkermann, A. The role of regulated necrosis in endocrine diseases. Nat. Rev. Endocrinol. 2021, 17, 497–510. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.; Barbosa, D.J.; Benfeito, S.; Silva, V.; Chavarria, D.; Borges, F.; Remião, F.; Silva, R. Molecular mechanisms of ferroptosis and their involvement in brain diseases. Pharmacol. Ther. 2023, 244, 108373. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Upadhyay, M.; Samal, J.; Kandpal, M.; Singh, O.V.; Vivekanandan, P. The Warburg effect: Insights from the past decade. Pharmacol. Ther. 2013, 137, 318–330. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Szulc, A.; Woźniak, M. Targeting pivotal hallmarks of cancer for enhanced therapeutic strategies in triple-negative breast cancer treatment—In vitro, in vivo and clinical trials literature review. Cancers 2024, 16, 1483. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Decock, J. Lactate metabolism and immune modulation in breast cancer: A focused review on triple-negative breast tumors. Front. Oncol. 2020, 10, 598626. [Google Scholar] [CrossRef]
- Ackerman, D.; Simon, M.C. Hypoxia, lipids, and cancer: Surviving the harsh tumor microenvironment. Trends Cell Biol. 2014, 24, 472–478. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. The two faces of reactive oxygen species in cancer. Annu. Rev. Cancer Biol. 2017, 1, 79–98. [Google Scholar] [CrossRef]
- Dirat, B.; Bochet, L.; Dabek, M.; Daviaud, D.; Dauvillier, S.; Majed, B.; Wang, Y.Y.; Meulle, A.; Salles, B.; Le Gonidec, S.; et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011, 71, 2455–2465. [Google Scholar] [CrossRef]
- Choi, J.; Cha, Y.J.; Koo, J.S. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog. Lipid Res. 2018, 69, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Attané, C.; Milhas, D.; Dirat, B.; Dauvillier, S.; Guerard, A.; Gilhodes, J.; Lazar, I.; Alet, N.; Laurent, V.; et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2017, 2, e87489. [Google Scholar] [CrossRef]
- Lee, Y.; Jung, W.H.; Koo, J.S. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res. Treat. 2015, 153, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Lapeire, L.; Hendrix, A.; Lambein, K.; Van Bockstal, M.; Braems, G.; Van Den Broecke, R.; Limame, R.; Mestdagh, P.; Vandesompele, J.; Vanhove, C.; et al. Secretome analysis of breast cancer-associated adipose tissue to identify paracrine regulators of breast cancer growth. Oncotarget 2017, 8, 47239–47249. [Google Scholar] [CrossRef][Green Version]
- Ayob, A.Z.; Ramasamy, T.S. Cancer stem cells as key drivers of tumour progression. J. Biomed. Sci. 2018, 25, 20. [Google Scholar] [CrossRef]
- Zaoui, M.; Morel, M.; Ferrand, N.; Fellahi, S.; Bastard, J.P.; Lamazière, A.; Larsen, A.K.; Béréziat, V.; Atlan, M.; Sabbah, M. Breast-associated adipocytes secretome induce fatty acid uptake and invasiveness in breast cancer cells via CD36 independently of body mass index, menopausal status and mammary density. Cancers 2019, 11, 2012. [Google Scholar] [CrossRef]
- Balaban, S.; Shearer, R.F.; Lee, L.S.; van Geldermalsen, M.; Schreuder, M.; Shtein, H.C.; Cairns, R.; Thomas, K.C.; Fazakerley, D.J.; Grewal, T.; et al. Adipocyte lipolysis links obesity to breast cancer growth: Adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Wei, X.H.; Li, S.J.; Liu, Y.; Hu, H.L.; Li, Z.Z.; Kuang, X.H.; Wang, L.; Shi, X.; Yuan, S.T.; et al. Adipocyte-derived IL-6 and leptin promote breast cancer metastasis via upregulation of lysyl hydroxylase-2 expression. Cell Commun. Signal. 2018, 16, 100. [Google Scholar] [CrossRef]
- Dias, A.S.; Almeida, C.R.; Helguero, L.A.; Duarte, I.F. Metabolic crosstalk in the breast cancer microenvironment. Eur. J. Cancer 2019, 121, 154–171. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y. CD36 tango in cancer: Signaling pathways and functions. Theranostics 2019, 9, 4893–4908. [Google Scholar] [CrossRef]
- Ozretic, P.; Alvir, I.; Sarcevic, B.; Vujaskovic, Z.; Rendic-Miocevic, Z.; Roguljic, A.; Beketic-Oreskovic, L. Apoptosis regulator Bcl-2 is an independent prognostic marker for worse overall survival in triple-negative breast cancer patients. Int. J. Biol. Markers 2018, 33, 109–115. [Google Scholar] [CrossRef]
- Oakes, S.R.; Vaillant, F.; Lim, E.; Lee, L.; Breslin, K.; Feleppa, F.; Deb, S.; Ritchie, M.E.; Takano, E.; Ward, T.; et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc. Natl. Acad. Sci. USA 2012, 109, 2766–2771. [Google Scholar] [CrossRef]
- Villar, E.; Redondo, M.; Rodrigo, I.; García, J.; Avila, E.; Matilla, A. Bcl-2 expression and apoptosis in primary and metastatic breast carcinomas. Tumour Biol. 2001, 22, 137–145. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef] [PubMed]
- White, E.; Mehnert, J.M.; Chan, C.S. Autophagy, Metabolism, and Cancer. Clin. Cancer Res. 2015, 21, 5037–5046. [Google Scholar] [CrossRef] [PubMed]
- Kimmelman, A.C.; White, E. Autophagy and Tumor Metabolism. Cell Metab. 2017, 25, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Xie, Y.; Song, X.; Sun, X.; Lotze, M.T.; Zeh, H.J.; Kang, R.; Tang, D. Autophagy Promotes Ferroptosis by Degradation of Ferritin. Autophagy 2016, 12, 1425–1428. [Google Scholar] [CrossRef]
- Martinez, T.A.; Zeybek, N.D.; Müftüoğlu, S. Evaluation of the Cytotoxic and Autophagic Effects of Atorvastatin on MCF-7 Breast Cancer Cells. Balkan Med. J. 2018, 35, 256–262. [Google Scholar] [CrossRef]
- Chung, S.J.; Nagaraju, G.P.; Nagalingam, A.; Muniraj, N.; Kuppusamy, P.; Walker, A.; Woo, J.; Gyorffy, B.; Gabrielson, E.; Saxena, N.K.; et al. ADIPOQ/Adiponectin Induces Cytotoxic Autophagy in Breast Cancer. Oncogenesis 2017, 6, e312. [Google Scholar]
- Salnikow, K. Role of iron in cancer. Semin. Cancer Biol. 2021, 76, 189–194. [Google Scholar] [CrossRef]
- Buss, J.L.; Torti, F.M.; Torti, S.V. The role of iron chelation in cancer therapy. Curr. Med. Chem. 2003, 10, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Cairo, G.; Bernuzzi, F.; Recalcati, S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006, 1, 25–39. [Google Scholar] [CrossRef]
- Faulk, W.P.; Hsi, B.L.; Stevens, P.J. Transferrin and transferrin receptors in carcinoma of the breast. Lancet 1980, 2, 390–392. [Google Scholar] [CrossRef]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef]
- Leng, X.; Ding, T.; Lin, H.; Wang, Y.; Hu, L.; Hu, J.; Feig, B.; Zhang, W.; Pusztai, L.; Symmans, W.F.; et al. Inhibition of lipocalin 2 impairs breast tumorigenesis and metastasis. Cancer Res. 2009, 69, 8579–8584. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Asimakopoulou, A.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 (LCN2) Expression in Hepatic Malfunction and Therapy. Front. Physiol. 2016, 7, 430. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Vela, D.; Vela-Gaxha, Z. Differential Regulation of Hepcidin in Cancer and Non-Cancer Tissues and Its Clinical Implications. Exp. Mol. Med. 2018, 50, e436. [Google Scholar] [CrossRef]
- Blanchette-Farra, N.; Kita, D.; Konstorum, A.; Tesfay, L.; Lemler, D.; Hegde, P.; Claffey, K.P.; Torti, F.M.; Torti, S.V. Contribution of Three-Dimensional Architecture and Tumor-Associated Fibroblasts to Hepcidin Regulation in Breast Cancer. Oncogene 2018, 37, 4013–4032. [Google Scholar] [CrossRef]
- Pinnix, Z.K.; Miller, L.D.; Wang, W.; D’Agostino, R., Jr.; Kute, T.; Willingham, M.C.; Hatcher, H.; Tesfay, L.; Sui, G.; Di, X.; et al. Ferroportin and Iron Regulation in Breast Cancer Progression and Prognosis. Sci. Transl. Med. 2010, 2, 43ra56. [Google Scholar] [CrossRef] [PubMed]
- Thelander, L.; Gräslund, A.; Thelander, M. Continual Presence of Oxygen and Iron Required for Mammalian Ribonucleotide Reduction: Possible Regulation Mechanism. Biochem. Biophys. Res. Commun. 1983, 110, 859–865. [Google Scholar] [CrossRef]
- Thelander, M.; Gräslund, A.; Thelander, L. Subunit M2 of Mammalian Ribonucleotide Reductase. Characterization of a Homogeneous Protein Isolated from M2-Overproducing Mouse Cells. J. Biol. Chem. 1985, 260, 2737–2741. [Google Scholar] [CrossRef] [PubMed]
- Hassannia, B.; Vandenabeele, P.; Vanden Berghe, T. Targeting Ferroptosis to Iron Out Cancer. Cancer Cell 2019, 35, 830–849. [Google Scholar] [CrossRef] [PubMed]
- Dufrusine, B.; Di Francesco, A.; Oddi, S.; Scipione, L.; Angelucci, C.B.; D’Addario, C.; Di Francesco, A.; Catacuzzeno, L.; Franciolini, F.; Dainese, E.; et al. Iron-Dependent Trafficking of 5-Lipoxygenase and Impact on Human Macrophage Activation. Front. Immunol. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the Clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef]
- Shpyleva, S.I.; Tryndyak, V.P.; Kovalchuk, O.; Starlard-Davenport, A.; Chekhun, V.F.; Beland, F.A.; Pogribny, I.P. Role of Ferritin Alterations in Human Breast Cancer Cells. Breast Cancer Res. Treat. 2011, 126, 63–71. [Google Scholar] [CrossRef]
- Arosio, P.; Ingrassia, R.; Cavadini, P. Ferritins: A Family of Molecules for Iron Storage, Antioxidation and More. Biochim. Biophys. Acta 2009, 1790, 589–599. [Google Scholar] [CrossRef]
- Huang, X. Does Iron Have a Role in Breast Cancer? Lancet Oncol. 2008, 9, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.C.; Jiang, X.; Elliott, R.L.; Head, J.F. Antisense Ferritin Oligonucleotides Inhibit Growth and Induce Apoptosis in Human Breast Carcinoma Cells. Anticancer Res. 2002, 22, 1513–1524. [Google Scholar] [PubMed]
- Alonso García, J.; Turiel Fernández, D.; Añón Álvarez, E.; Blanco González, E.; Montes-Bayón, M.; Sanz-Medel, A. Iron Speciation, Ferritin Concentrations and Fe:Ferritin Ratios in Different Malignant Breast Cancer Cell Lines: On the Search for Cancer Biomarkers. Metallomics 2016, 8, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy. Nat. Rev. Cancer 2016, 16, 619–634, Erratum in Nat. Rev. Cancer 2016, 16, 773. [Google Scholar] [CrossRef]
- van Geldermalsen, M.; Wang, Q.; Nagarajah, R.; Marshall, A.D.; Thoeng, A.; Gao, D.; Ritchie, W.; Feng, Y.; Bailey, C.G.; Deng, N.; et al. ASCT2/SLC1A5 Controls Glutamine Uptake and Tumour Growth in Triple-Negative Basal-Like Breast Cancer. Oncogene 2016, 35, 3201–3208. [Google Scholar] [CrossRef]
- El Ansari, R.; Craze, M.L.; Miligy, I.; Diez-Rodriguez, M.; Nolan, C.C.; Ellis, I.O.; Rakha, E.A.; Green, A.R. The Amino Acid Transporter SLC7A5 Confers a Poor Prognosis in the Highly Proliferative Breast Cancer Subtypes and Is a Key Therapeutic Target in Luminal B Tumours. Breast Cancer Res. 2018, 20, 21. [Google Scholar] [CrossRef]
- DeMichele, A.; Harding, J.; Telli, M.; Munster, P.; McKay, R.; Iliopoulos, O.; Orford, K.; Bennett, M.; Mier, J.; Owonikoko, T.; et al. Phase 1 Study of CB-839, a Small Molecule Inhibitor of Glutaminase (GLS) in Combination with Paclitaxel (Pac) in Patients (Pts) with Triple-Negative Breast Cancer (TNBC). J. Clin. Oncol. 2016, 34, 1011. [Google Scholar] [CrossRef]
- Cao, M.D.; Lamichhane, S.; Lundgren, S.; Bofin, A.; Fjøsne, H.; Giskeødegård, G.F.; Bathen, T.F. Metabolic Characterization of Triple-Negative Breast Cancer. BMC Cancer 2014, 14, 941. [Google Scholar] [CrossRef]
- Timmerman, L.A.; Holton, T.; Yuneva, M.; Louie, R.J.; Padro, M.; Daemen, A.; Hu, M.; Chan, D.A.; Ethier, S.P.; van ’t Veer, L.J.; et al. Glutamine Sensitivity Analysis Identifies the xCT Antiporter as a Common Triple-Negative Breast Tumor Therapeutic Target. Cancer Cell 2013, 24, 450–465. [Google Scholar] [CrossRef]
- Rather, G.M.; Pramono, A.A.; Szekely, Z.; Bertino, J.R.; Tedeschi, P.M. In Cancer, All Roads Lead to NADPH. Pharmacol. Ther. 2021, 226, 107864. [Google Scholar] [CrossRef]
- Song, X.; Wang, X.; Liu, Z.; Yu, Z. Role of GPX4-Mediated Ferroptosis in the Sensitivity of Triple-Negative Breast Cancer Cells to Gefitinib. Front. Oncol. 2020, 10, 597434. [Google Scholar] [CrossRef]
- Li, H.; Yang, P.; Wang, J.; Zhang, J.; Ma, Q.; Jiang, Y.; Wu, Y.; Han, T.; Xiang, D. HLF Regulates Ferroptosis, Development and Chemoresistance of Triple-Negative Breast Cancer by Activating Tumor Cell-Macrophage Crosstalk. J. Hematol. Oncol. 2022, 15, 2. [Google Scholar] [CrossRef]
- Gan, B. Mitochondrial Regulation of Ferroptosis. J. Cell Biol. 2021, 220, e202105043. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 Dictates Ferroptosis Sensitivity by Shaping Cellular Lipid Composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Hao, S.; Liang, B.; Huang, Q.; Dong, S.; Wu, Z.; He, W.; Shi, M. Metabolic Networks in Ferroptosis. Oncol. Lett. 2018, 15, 5405–5411. [Google Scholar] [CrossRef]
- Kalinina, E. Glutathione-Dependent Pathways in Cancer Cells. Int. J. Mol. Sci. 2024, 25, 8423. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wu, H.T.; Chen, W.J.; Xu, Y.; Ye, Q.Q.; Shen, J.X.; Liu, J. Involvement of Glutathione Peroxidases in the Occurrence and Development of Breast Cancers. J. Transl. Med. 2020, 18, 247. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, C.K.; Wu, J.; Sjol, J.; Wardell, S.; Spasojevic, I.; George, D.; McDonnell, D.P.; Hsu, D.S.; Chang, J.T.; et al. Cystine Addiction of Triple-Negative Breast Cancer Associated with EMT Augmented Death Signaling. Oncogene 2017, 36, 4235–4242, Erratum in Oncogene 2017, 36, 4379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, N.; Ding, C.; Zhang, H.; Liu, D.; Liu, S. Ferroptosis and EMT Resistance in Cancer: A Comprehensive Review of the Interplay. Front. Oncol. 2024, 14, 1344290. [Google Scholar] [CrossRef] [PubMed]
- Hangauer, M.J.; Viswanathan, V.S.; Ryan, M.J.; Bole, D.; Eaton, J.K.; Matov, A.; Galeas, J.; Dhruv, H.D.; Berens, M.E.; Schreiber, S.L.; et al. Drug-Tolerant Persister Cancer Cells Are Vulnerable to GPX4 Inhibition. Nature 2017, 551, 247–250. [Google Scholar] [CrossRef]
- Gkouvatsos, K.; Papanikolaou, G.; Pantopoulos, K. Regulation of Iron Transport and the Role of Transferrin. Biochim. Biophys. Acta 2012, 1820, 188–202. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing Out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In Vivo Delivery, Pharmacokinetics, Biodistribution and Toxicity of Iron Oxide Nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef]
- Fontecave, M.; Pierre, J.L. Iron: Metabolism, Toxicity and Therapy. Biochimie 1993, 75, 767–773. [Google Scholar] [CrossRef]
- Xie, P.; Yang, S.T.; Huang, Y.; Zeng, C.; Xin, Q.; Zeng, G.; Yang, S.; Xia, P.; Tang, X.; Tang, K. Carbon Nanoparticles-Fe(II) Complex for Efficient Tumor Inhibition with Low Toxicity by Amplifying Oxidative Stress. ACS Appl. Mater. Interfaces 2020, 12, 29094–29102. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06048367 (accessed on 25 April 2025).
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of Human Triple-Negative Breast Cancer Subtypes and Preclinical Models for Selection of Targeted Therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Tian, J.; Raffa, F.A.; Dai, M.; Moamer, A.; Khadang, B.; Hachim, I.Y.; Bakdounes, K.; Ali, S.; Jean-Claude, B.; Lebrun, J.J. Dasatinib Sensitizes Triple-Negative Breast Cancer Cells to Chemotherapy by Targeting Breast Cancer Stem Cells. Br. J. Cancer 2018, 119, 1495–1507. [Google Scholar] [CrossRef]
- Ocana, A.; Gil-Martin, M.; Antolín, S.; Atienza, M.; Montaño, Á.; Ribelles, N.; Urruticoechea, A.; Falcón, A.; Pernas, S.; Orlando, J.; et al. Efficacy and Safety of Dasatinib with Trastuzumab and Paclitaxel in First-Line HER2-Positive Metastatic Breast Cancer: Results from the Phase II GEICAM/2010-04 Study. Breast Cancer Res. Treat. 2019, 174, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, B.; Lu, W.; Cheng, Y.; Zhu, J.; Ai, G.; Zhang, X.; Liu, X.; Cheng, Z. Senolytic Drugs Dasatinib and Quercetin Combined with Carboplatin or Olaparib Reduced the Peritoneal and Adipose Tissue Metastasis of Ovarian Cancer. Biomed. Pharmacother. 2024, 174, 116474. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Hu, M. Quercetin Promotes TFEB Nuclear Translocation and Activates Lysosomal Degradation of Ferritin to Induce Ferroptosis in Breast Cancer Cells. Comput. Intell. Neurosci. 2022, 2022, 5299218. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT06355037 (accessed on 26 April 2025).
- Li, H.; Wang, J.; Wu, C.; Wang, L.; Chen, Z.S.; Cui, W. The Combination of Disulfiram and Copper for Cancer Treatment. Drug Discov. Today 2020, 25, 1099–1108. [Google Scholar] [CrossRef]
- Ren, X.; Li, Y.; Zhou, Y.; Hu, W.; Yang, C.; Jing, Q.; Zhou, C.; Wang, X.; Hu, J.; Wang, L.; et al. Overcoming the Compensatory Elevation of NRF2 Renders Hepatocellular Carcinoma Cells More Vulnerable to Disulfiram/Copper-Induced Ferroptosis. Redox Biol. 2021, 46, 102122. [Google Scholar] [CrossRef]
- McMahon, A.; Chen, W.; Li, F. Old Wine in New Bottles: Advanced Drug Delivery Systems for Disulfiram-Based Cancer Therapy. J. Control. Release 2020, 319, 352–359. [Google Scholar] [CrossRef]
- Chu, M.; An, X.; Fu, C.; Yu, H.; Zhang, D.; Li, Q.; Man, X.; Dai, X.; Li, Z. Disulfiram/Copper Induce Ferroptosis in Triple-Negative Breast Cancer Cell Line MDA-MB-231. Front. Biosci. 2023, 28, 186. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03323346 (accessed on 27 April 2025).
- Fleig, W.E.; Laudage, G.; Sommer, H.; Wellmann, W.; Stange, E.F.; Riemann, J. Prospective, Randomized, Double-Blind Comparison of Benzalazine and Sulfasalazine in the Treatment of Active Ulcerative Colitis. Digestion 1988, 40, 173–180. [Google Scholar] [CrossRef]
- Combe, B.; Codreanu, C.; Fiocco, U.; Gaubitz, M.; Geusens, P.P.; Kvien, T.K.; Pavelka, K.; Sambrook, P.N.; Smolen, J.S.; Khandker, R.; et al. Efficacy, Safety and Patient-Reported Outcomes of Combination Etanercept and Sulfasalazine versus Etanercept Alone in Patients with Rheumatoid Arthritis: A Double-Blind Randomised 2-Year Study. Ann. Rheum. Dis. 2009, 68, 1146–1152. [Google Scholar] [CrossRef]
- Gout, P.W.; Buckley, A.R.; Simms, C.R.; Bruchovsky, N. Sulfasalazine, a Potent Suppressor of Lymphoma Growth by Inhibition of the x(c)- Cystine Transporter: A New Action for an Old Drug. Leukemia 2001, 15, 1633–1640. [Google Scholar] [CrossRef]
- Seidlitz, E.P.; Sharma, M.K.; Saikali, Z.; Ghert, M.; Singh, G. Cancer Cell Lines Release Glutamate into the Extracellular Environment. Clin. Exp. Metastasis 2009, 26, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Seidlitz, E.P.; Singh, G. Cancer Cells Release Glutamate via the Cystine/Glutamate Antiporter. Biochem. Biophys. Res. Commun. 2010, 391, 91–95. [Google Scholar] [CrossRef] [PubMed]
- García-Gaytán, A.C.; Hernández-Abrego, A.; Díaz-Muñoz, M.; Méndez, I. Glutamatergic System Components as Potential Biomarkers and Therapeutic Targets in Cancer in Non-Neural Organs. Front. Endocrinol. 2022, 13, 1029210. [Google Scholar] [CrossRef] [PubMed]
- Ungard, R.G.; Seidlitz, E.P.; Singh, G. Inhibition of Breast Cancer-Cell Glutamate Release with Sulfasalazine Limits Cancer-Induced Bone Pain. Pain 2014, 155, 28–36. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT03847311 (accessed on 27 April 2025).
- Beckwitt, C.H.; Brufsky, A.; Oltvai, Z.N.; Wells, A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018, 20, 144. [Google Scholar] [CrossRef]
- Ahern, T.P.; Pedersen, L.; Tarp, M.; Cronin-Fenton, D.P.; Garne, J.P.; Silliman, R.A.; Sørensen, H.T.; Lash, T.L. Statin prescriptions and breast cancer recurrence risk: A Danish nationwide prospective cohort study. J. Natl. Cancer Inst. 2011, 103, 1461–1468. [Google Scholar] [CrossRef]
- Taylor, J. Joint societies CVD prevention guidelines launched in May 2012. Eur. Heart J. 2012, 33, 1539. [Google Scholar]
- Warner, G.J.; Huang, W.; Wright, D.E.; Tallman, M.S.; Cohen, R.J.; Larsen, P.R. Inhibition of selenoprotein synthesis by selenocysteine tRNA[Ser]Sec lacking isopentenyladenosine. J. Biol. Chem. 2000, 275, 28110–28119. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Longo, J.; van Leeuwen, J.E.; Mullen, P.J.; Ba-Alawi, W.; Haibe-Kains, B.; Penn, L.Z. Statin-induced cancer cell death can be mechanistically uncoupled from prenylation of RAS family proteins. Cancer Res. 2018, 78, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef]
- Yu, R.; Longo, J.; van Leeuwen, J.E.; Zhang, C.; Branchard, E.; Elbaz, M.; Cescon, D.W.; Drake, R.R.; Dennis, J.W.; Penn, L.Z. Mevalonate pathway inhibition slows breast cancer metastasis via reduced N-glycosylation abundance and branching. Cancer Res. 2021, 81, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05550415 (accessed on 28 April 2025).
- Pernicova, I.; Korbonits, M. Metformin—Mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex I of the mitochondrial respiratory chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, Y.; Xie, S.; Wang, J.; Li, Z.; Chen, L.; Mao, M.; Chen, C.; Huang, A.; Chen, Y.; et al. Metformin induces ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 206. [Google Scholar] [CrossRef]
- Sonnenblick, A.; Agbor-Tarh, D.; Bradbury, I.; Di Cosimo, S.; Azim, H.A., Jr.; Fumagalli, D.; Sarp, S.; Wolff, A.C.; Andersson, M.; Kroep, J.; et al. Impact of diabetes, insulin, and metformin use on the outcome of patients with human epidermal growth factor receptor 2-positive primary breast cancer: Analysis from the ALTTO phase III randomized trial. J. Clin. Oncol. 2017, 35, 1421–1429. [Google Scholar] [CrossRef]
- Lynn, J.V.; Urlaub, K.M.; Ranganathan, K.; Donneys, A.; Nelson, N.S.; Subramanian, C.; Cohen, M.S.; Buchman, S.R. The role of deferoxamine in irradiated breast reconstruction: A study of oncologic safety. Plast. Reconstr. Surg. 2019, 143, 1666–1676. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Milnes, K. The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents II: The mechanism of action of ligands derived from salicylaldehyde benzoyl hydrazone and 2-hydroxy-1-naphthylaldehyde benzoyl hydrazone. Blood 1997, 89, 3025–3038. [Google Scholar] [CrossRef] [PubMed]
- Brodie, C.; Siriwardana, G.; Lucas, J.; Schleicher, R.; Terada, N.; Szepesi, A.; Gelfand, E.; Seligman, P. Neuroblastoma sensitivity to growth inhibition by deferrioxamine: Evidence for a block in G1 phase of the cell cycle. Cancer Res. 1993, 53, 3968–3975. [Google Scholar] [PubMed]
- Zhang, Y.; Fan, B.Y.; Pang, Y.L.; Shen, W.Y.; Wang, X.; Zhao, C.X.; Li, W.X.; Liu, C.; Kong, X.H.; Ning, G.Z.; et al. Neuroprotective effect of deferoxamine on erastin-induced ferroptosis in primary cortical neurons. Neural Regen. Res. 2020, 15, 1539–1545. [Google Scholar]
- Zeng, X.; An, H.; Yu, F.; Wang, K.; Zheng, L.; Zhou, W.; Bao, Y.; Yang, J.; Shen, N.; Huang, D. Benefits of iron chelators in the treatment of Parkinson’s disease. Neurochem. Res. 2021, 46, 1239–1251. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.; Jiang, Y.; Bu, J.; Gu, X. Targeting ferroptosis, the Achilles’ heel of breast cancer: A review. Front. Pharmacol. 2022, 13, 1036140. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT05300958 (accessed on 29 April 2025).
- Gross, M.I.; Demo, S.D.; Dennison, J.B.; Chen, L.; Chernov-Rogan, T.; Goyal, B.; Janes, J.R.; Laidig, G.J.; Lewis, E.R.; Li, J.; et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther. 2014, 13, 890–901. [Google Scholar] [CrossRef]
- Choi, H.; Gupta, M.; Hensley, C.; Lee, H.; Lu, Y.T.; Pantel, A.; Mankoff, D.; Zhou, R. Disruption of redox balance in glutaminolytic triple-negative breast cancer by inhibition of glutamate export and glutaminase. bioRxiv 2023. [Google Scholar] [CrossRef]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Commun. Signal. 2023, 21, 61. [Google Scholar] [CrossRef]
- Kümler, I.; Tuxen, M.K.; Nielsen, D.L. A systematic review of dual targeting in HER2-positive breast cancer. Cancer Treat. Rev. 2014, 40, 259–270. [Google Scholar] [CrossRef]
- Chow, L.W.-C.; Xu, B.; Gupta, S.; Freyman, A.; Zhao, Y.; Abbas, R.; Van, M.-L.V.; Bondarenko, I. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br. J. Cancer 2013, 108, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neratinib-metastatic-her2-positive-breast-cancer (accessed on 5 May 2025).
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: Phase III NALA trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Gijsen, M.; Mullooly, M.; Bennett, R.; Bouguern, N.; Pedersen, K.; O’Brien, N.A.; Roxanis, I.; Li, J.L.; Bridge, E.; et al. Neratinib overcomes trastuzumab resistance in HER2-amplified breast cancer. Oncotarget 2013, 4, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, A.; Redvers, R.P.; Ling, X.; Ayton, S.; Fuentes, M.; Tavancheh, E.; Diala, I.; Lalani, A.; Loi, S.; David, S.; et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2+ breast cancer metastasis. Breast Cancer Res. 2019, 21, 94. [Google Scholar] [CrossRef]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug Des. Devel. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef]
- Mazrouei, R.; Raeisi, E.; Lemoigne, Y.; Heidarian, E. Activation of p53 gene expression and synergistic antiproliferative effects of 5-fluorouracil and beta-escin on MCF7 cells. J. Med. Signals Sens. 2019, 9, 196–203. [Google Scholar]
- Akar, S.; Donmez-Altuntas, H.; Hamurcu, Z. Beta-escin reduces cancer progression in aggressive MDA-MB-231 cells by inhibiting glutamine metabolism through downregulation of c-myc oncogene. Mol. Biol. Rep. 2022, 49, 7409–7415. [Google Scholar] [CrossRef]
- Li, C.; He, Z.; Yao, F.; Liao, S.; Sun, K.; Sun, S.; Li, Z.; Wang, Z. Role of escin in breast cancer therapy: Potential mechanism for inducing ferroptosis and synergistic antitumor activity with cisplatin. Apoptosis 2023, 28, 1154–1167. [Google Scholar] [CrossRef]
- Yang, H.C.; Stern, A.; Chiu, D.T. G6PD: A hub for metabolic reprogramming and redox signaling in cancer. Biomed. J. 2021, 44, 285–292. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, X.; Guan, Y.; Su, T.; Xie, Z.; Wu, Z.; Long, Y.; Zhu, H.; Shao, J.; Mai, X.; et al. K2FeO4-enhanced photodynamic therapy of breast cancer via in situ synthesis of Fe2O3 and O2. Adv. Healthc. Mater. 2025, 14, e2402827. [Google Scholar] [CrossRef]
- Rusnak, D.W.; Lackey, K.; Affleck, K.; Wood, E.R.; Alligood, K.J.; Rhodes, N.; Keith, B.R.; Murray, D.M.; Knight, W.B.; Mullin, R.J.; et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol. Cancer Ther. 2001, 1, 85–94. [Google Scholar]
- Ma, S.; Henson, E.S.; Chen, Y.; Gibson, S.B. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016, 7, e2307. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Dielschneider, R.F.; Henson, E.S.; Xiao, W.; Choquette, T.R.; Blankstein, A.R.; Chen, Y.; Gibson, S.B. Ferroptosis and autophagy-induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS ONE 2017, 12, e0182921. [Google Scholar] [CrossRef]
- Sun, C.; Liu, P.; Pei, L.; Zhao, M.; Huang, Y. Propofol inhibits proliferation and augments the anti-tumor effect of doxorubicin and paclitaxel partly through promoting ferroptosis in triple-negative breast cancer cells. Front. Oncol. 2022, 12, 837974. [Google Scholar] [CrossRef]
- Louandre, C.; Ezzoukhry, Z.; Godin, C.; Barbare, J.C.; Mazière, J.C.; Chauffert, B.; Galmiche, A. Iron-dependent cell death of hepatocellular carcinoma cells exposed to sorafenib. Int. J. Cancer 2013, 133, 1732–1742. [Google Scholar] [CrossRef] [PubMed]
- Badgley, M.A.; Kremer, D.M.; Maurer, H.C.; DelGiorno, K.E.; Lee, H.J.; Purohit, V.; Sagalovskiy, I.R.; Ma, A.; Kapilian, J.; Firl, C.E.M.; et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 2020, 368, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Targeting ferroptosis in pancreatic cancer: A double-edged sword. Trends Cancer 2021, 7, 891–901. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Chen, Y.H.; Tan, Q.Q.; Liu, X.B.; Tan, C. Clusterin is upregulated by erastin, a ferroptosis inducer, and exerts cytoprotective effects in pancreatic adenocarcinoma cells. Anticancer Drugs 2024, 35, 227–236. [Google Scholar] [CrossRef]
- Minami, J.K.; Morrow, D.; Bayley, N.A.; Fernandez, E.G.; Salinas, J.J.; Tse, C.; Zhu, H.; Su, B.; Plawat, R.; Jones, A.; et al. CDKN2A deletion remodels lipid metabolism to prime glioblastoma for ferroptosis. Cancer Cell 2023, 41, 1048–1060.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; He, Y.; Chen, K.; Sun, J.; Zhang, L.; He, Y.; Yu, H.; Li, Q. RSL3 drives ferroptosis through NF-κB pathway activation and GPX4 depletion in glioblastoma. Oxid. Med. Cell Longev. 2021, 2021, 2915019. [Google Scholar] [CrossRef]
- Chen, X.; Yu, Y.; Liang, S.; Guan, Z.; Shi, H.; Zhu, Q.; Lin, J. Ferroptosis induction improves the sensitivity of docetaxel in prostate cancer. Oxid. Med. Cell Longev. 2022, 2022, 4930643. [Google Scholar] [CrossRef]
- Vinik, Y.; Maimon, A.; Dubey, V.; Raj, H.; Abramovitch, I.; Malitsky, S.; Itkin, M.; Ma’ayan, A.; Westermann, F.; Gottlieb, E.; et al. Programming a ferroptosis-to-apoptosis transition landscape revealed ferroptosis biomarkers and repressors for cancer therapy. Adv. Sci. 2024, 11, e2307263. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Shen, Y.C.; Wu, C.Y.; Tsai, Y.Y.; Yang, Y.H.; Lin, Y.Y.; Kuan, F.C.; Lu, C.N.; Chang, G.H.; Tsai, M.S.; et al. Danshen improves survival of patients with breast cancer and dihydroisotanshinone I induces ferroptosis and apoptosis of breast cancer cells. Front. Pharmacol. 2019, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
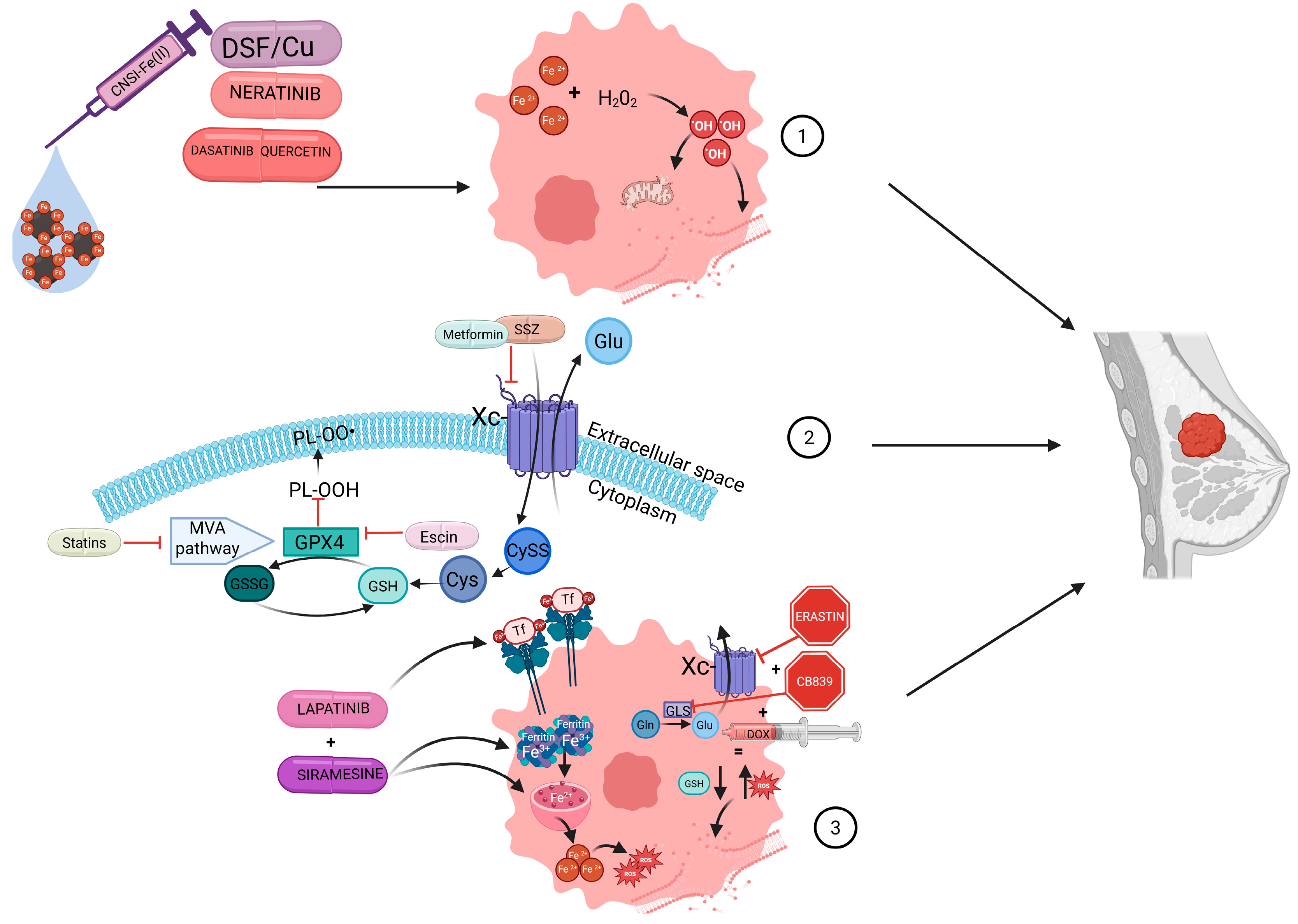
| Drug | Mechnism of Action | Study Type | Reference |
|---|---|---|---|
| CNSI-Fe(II) | Increases intracellular iron levels, triggering the Fenton reaction leading to ROS production. | Clinical trial | Clinical trial NCT06048367 [107] |
| Dasatinib + Quercetin | Targets breast cancer stem cells; induces ferritinophagy, increasing intracellular iron levels. intracellular iron levels. | Clinical trial | Clinical trial NCT06355037 [113] |
| Disulfiram + Copper | Enhances heme degradation, increasing intracellular iron levels; reduces GSH and GPX4 levels. | Clinical trial | Clinical trial NCT03323346 [118] |
| Sulfasalazine | Inhibits system Xc-, reducing intracellular GSH levels. | Clinical trial | Clinical trial NCT03847311 [126] |
| Simvastatin/Fluvastatin | Inhibits the mevalonate pathway, impairing GPX4 function. | Clinical trial | Clinical trial NCT05550415 [134] |
| Metformin | Disrupts mitochondrial function and inhibits system Xc-. | Clinical trial | Sonnenblick et al. [138] |
| Doxorubicin + CB839 + Erastin | Inhibits glutaminase and system Xc-, reducing intracellular GSH levels | In vivo | Choi et al. [147] |
| Neratinib | Disrupts iron homeostasis, leading to iron accumulation and lipid peroxidation. | In vivo | Nagpal et al. [154] |
| Escin | Reduces GSH/GSSG ratio and increases ROS, leading to lipid peroxidation; inhibits the pentose phosphate pathway impairing GPX4 function. | In vivo | Li et al. [158] |
| K2FeO4 following Ce6-mediated PDT | Promotes ROS generation, induces lipid peroxidation and suppresses GSH and GPX4. | In vivo and in vitro | Sun et al. [160] |
| Lapatinib + Siramesine | Increases intracellular iron levels, promoting ROS accumulation and lipid peroxidation. | In vitro | Ma et al. [162] Ma et al. [163] |
| Propofol | Increases intracellular iron levels, promoting ROS accumulation and lipid peroxidation | In vitro | Sun et al. [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc, A.; Woźniak, M. Targeting Ferroptosis as the Achilles’ Heel of Breast Cancer: Mechanisms and Therapeutic Opportunities from a Comprehensive Systematic Review. Int. J. Mol. Sci. 2025, 26, 9902. https://doi.org/10.3390/ijms26209902
Szulc A, Woźniak M. Targeting Ferroptosis as the Achilles’ Heel of Breast Cancer: Mechanisms and Therapeutic Opportunities from a Comprehensive Systematic Review. International Journal of Molecular Sciences. 2025; 26(20):9902. https://doi.org/10.3390/ijms26209902
Chicago/Turabian StyleSzulc, Anna, and Marta Woźniak. 2025. "Targeting Ferroptosis as the Achilles’ Heel of Breast Cancer: Mechanisms and Therapeutic Opportunities from a Comprehensive Systematic Review" International Journal of Molecular Sciences 26, no. 20: 9902. https://doi.org/10.3390/ijms26209902
APA StyleSzulc, A., & Woźniak, M. (2025). Targeting Ferroptosis as the Achilles’ Heel of Breast Cancer: Mechanisms and Therapeutic Opportunities from a Comprehensive Systematic Review. International Journal of Molecular Sciences, 26(20), 9902. https://doi.org/10.3390/ijms26209902






